Abstract
Phytochrome-mediated perception of the ratio of red to far-red wavelengths in the ambient light environment is fundamental to plant growth and development. Such monitoring enables plants to detect neighboring vegetation and initiate avoidance responses, thus conferring considerable selective advantage. The shade avoidance syndrome in plants is characterized by elongation growth and early flowering, responses that are fully induced by end-of-day far-red light treatments. Elucidating the roles of individual phytochromes in mediating responses to red to far-red has however always been confounded by synergistic and mutually antagonistic coactions between family members. The creation of triple and quadruple mutants in Arabidopsis, deficient in multiple phytochromes, has revealed functional redundancy between phyB, D, and E in controlling flowering time, leaf development, and regulation of the homeobox gene, ATHB-2. In addition, mutant analysis suggests a possible novel role for phyC in suppressing ATHB-2 transcription in the light.
In addition to its role as an energy source, light signals serve to provide plants with information about the surrounding environment. The efficient perception, interpretation, and transduction of such signals allow plants to synchronize their development with seasonal changes and to minimize the adverse effects of environmental perturbations. The ability to monitor the intensity, quality, and direction of incident light enables plants to modulate a number of physiological responses, including seed germination, shoot architecture, and the onset of flowering. Three principle families of photoreceptors have been identified for light perception in higher plant tissues, the red/far-red (R/FR) light-absorbing phytochromes (PHY) and the UV-A/blue light (B)-absorbing cryptochromes and phototropins (for review, see Quail, 2002).
PHY exist as a homodimer of two independently reversible subunits. Each subunit consists of a polypeptide (approximately 124 kD) attached to a linear tetrapyrrole via a thioether linkage. In Arabidopsis, five discrete apo-PHY-encoding genes, PHYA-PHYE, have been isolated and sequenced (Mathews and Sharrock, 1997). PHY A, B, C, and E are evolutionarily divergent proteins, sharing only 46% to 53% sequence identity, whereas PHYD encodes an apoprotein that shares 80% sequence identity with PHYB (Clack et al., 1994). Molecular phylogenetic analysis supports the occurrence of four major duplication events in the evolution of PHY genes. An initial duplication is believed to have separated PHYA (light labile in the FR light-absorbing [Pfr] form) and PHYC (light stable in the Pfr form) from PHYB/D/E (all light stable in the Pfr form). The subsequent separation of PHYA from PHYC and PHYB/D from PHYE resulted in three subfamilies: A/C, B/D, and E (Smith, 2000).
PHY regulate two principle adaptive phenomena in light-grown plants. These are proximity perception, leading to shade avoidance responses, and photoperiodic perception, leading to floral induction in some species. Fluctuations in the spectral quality of daylight are detected via perception of the ratio of R to FR wavelengths (R:FR). Such fluctuations can occur daily (dusk and dawn) and under vegetation canopies. Early reaction to the threat of impending shade is triggered by the localized drop in R:FR, reflected from surrounding vegetation (Ballare et al., 1990). Selective attenuation of R wavelengths by chlorophyllous tissue results in a significant decrease in the R:FR quantum ratio of reflected light detected by plants lower in the canopy. Neighbor detection initiates shade avoidance responses, enabling plants to compete for light. Such responses include enhanced internode and petiole extension growth, increased apical dominance, retarded leaf development, and an acceleration of flowering (Halliday et al., 1994; Smith and Whitelam, 1997). These physiological adaptations are accompanied by changes in the distribution of assimilates between leaves, stems, and roots (Keiller and Smith, 1989), and confer a considerable selective advantage. The ability to respond to the perceived threat of shading and therefore initiate responses before canopy closure is a crucial competitive strategy in rapidly growing populations (Ballare et al., 1990).
The roles of individual PHY in regulating these responses have been largely inferred from studies of mutant plants. Mutants of tomato (Lycopersicon esculentum) and Arabidopsis, deficient in phyA, display a phenotype almost indistinguishable from wild-type (WT) plants when grown in white light (Whitelam et al., 1993; van Tuinen et al., 1995). Such phenotypes suggest that multiple PHY regulate mature plant morphology in a functionally redundant manner. Deficiency of phyB has been shown to result in plants displaying phenotypes comparable with those of the shade avoidance syndrome (Robson et al., 1993; Whitelam and Devlin, 1997). Reduction of Pfr by an end-of-day (EOD) FR treatment results in a qualitatively similar response to lowering of the Pfr level during the light period by reduction of the R:FR photon ratio. The retention of shade avoidance and EOD FR responses in Arabidopsis phyB null mutants suggested the involvement of other PHY in mediating responses to R:FR, roles subsequently assigned to phyD and E (Robson et al., 1993; Devlin et al., 1998, 1999).
The molecular mechanisms underlying physiological responses to perceived R:FR are poorly understood. Identification of the homeobox gene ATHB-2 (also known as HAT4; Ruberti et al., 1991; Schena and Davis, 1992) provided the first evidence of a gene reversibly regulated by changes in R:FR (Carabelli et al., 1993, 1996). Overexpression of ATHB-2 has been shown to result in a pleiotropic phenotype, some aspects of which resemble the shade avoidance phenotype in Arabidopsis (Steindler et al., 1997, 1999). Expression analysis revealed transcript abundance to be controlled by phyA and phyB in addition to another, as yet unidentified, PHY (Steindler et al., 1997). The synergistic, and in some cases mutually antagonistic, action of phytochrome species in multiple physiological responses has made elucidation of the roles of individual family members problematic in existing mutants. Therefore, the creation of triple and quadruple mutant combinations has provided a unique insight into functional redundancy between PHY in mediating responses to R:FR. In addition, the creation of a phyABDE quadruple mutant has revealed a unique function for the least well characterized of the PHY, phyC.
RESULTS
Effect of EOD FR and Reduced R:FR on Internode Elongation
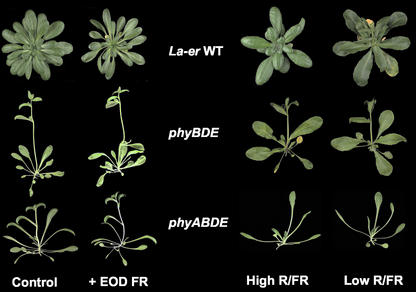
The promotion of internode elongation after growth under low R:FR and EOD FR treatment is well documented in Arabidopsis grown at temperatures above 21°C (Robson et al., 1993; Devlin et al., 1996, 1998). When grown under low R:FR at 16°C, such responses were absent in WT, phyBDE triple and phyABDE quadruple mutants (Fig. 1.). A similar lack of response was observed in WT and phyBDE triple mutant plants grown under 8-h light/16-h dark cycles, with and without EOD FR treatment. However, elongation of internodes and loss of a rosette habit were clearly visible in phyABDE quadruple mutants grown under identical conditions. Such elongation was displayed in control and EOD FR-treated plants, suggesting an important role for phyA in maintaining rosette habit in Arabidopsis.
Figure 1.
La-er, phyBDE, and phyABDE mutants (in La-er background) were grown for 36 d at 16°C under 8-h light and 16-h dark cycles (control), the same conditions with 15-min EOD FR treatments (+EOD FR), and under continuous irradiation of high and low R:FR.
Effect of EOD FR and Reduced R:FR on Leaf Morphology
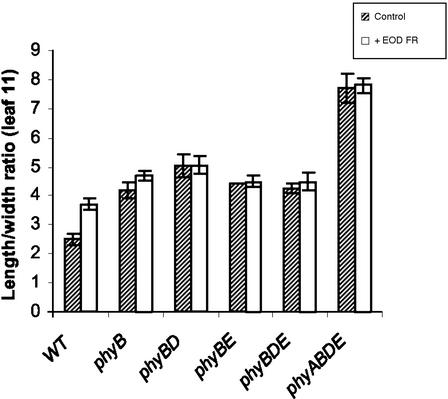
In response to EOD FR light, WT plants displayed increased petiole elongation and reduced leaf area, phenotypes consistent with the characterized shade avoidance syndrome (Fig. 1; Smith and Whitelam, 1997). However, when grown under continuous light of low R:FR, an increased leaf area was observed in WT and phyBDE triple mutant plants (Fig. 1). Consonant with visible changes in leaf morphology, WT plants treated with EOD FR displayed an increase in leaf length/width ratio (Fig. 2). This response was severely reduced in the phyB mutant and was absent in the phyBD double mutant, phyBE double mutant, phyBDE triple mutant, and the phyABDE quadruple mutant, all of which displayed an elongated leaf phenotype under control conditions. A pronounced increase in leaf length/width ratio was observed in phyABDE mutant plants compared with phyBDE plants grown under 8-h light/16-h dark cycles (Fig. 2). A similar increase was recorded in plants grown under continuous irradiation (high and low R:FR, data not shown), suggesting a significant role for phyA in the regulation of leaf morphology.
Figure 2.
Length/width ratios of rosette leaf 11 in La-er, phyB, phyBD, phyBE, phyBDE, and phyABDE mutants (in La-er background) at flowering. Plants were grown at 16°C under 8-h light and 16-h dark cycles (control) and under the same conditions with 15-min EOD FR treatments (+EOD FR).
PHY Regulation of Cotyledon Development
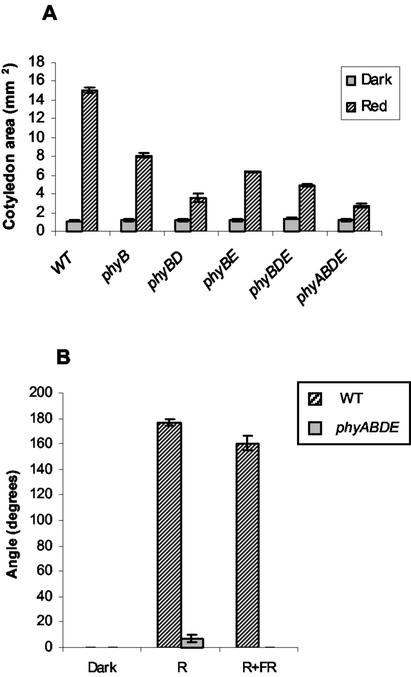
When grown under Rc, sequential removal of PHY species resulted in reduced cotyledon area (Fig. 3A). Monogenic mutants for phyA, phyD, and phyE displayed no such reduction (data not shown). However, the considerable reduction observed in phyB mutants suggests a significant role for this PHY in cotyledon expansion. The additional removal of phyD and E resulted in further decreases in cotyledon area, suggesting interactions between PHY family members in regulating cotyledon size. The smallest cotyledons were observed in the phyABDE quadruple mutant, confirming, as with leaf morphology, a role for phyA. The observation that phyABDE quadruple mutants displayed some cotyledon unfolding and expansion under Rc provides the first evidence that phyC can function as a weak red light sensor independently from other PHY. Such data is supported by the R/FR reversible promotion of cotyledon opening observed in phyABDE mutants (Fig. 3B). Cotyledon opening was not observed in plants treated with pulses of R (data not shown).
Figure 3.
A, Cotyledon areas in La-er, phyB, phyBD, phyBE, phyBDE, and phyABDE mutants (in La-er background) grown for 5 d in the dark and Rc at a fluence rate of 50 μmol m−2 s−1. B, Cotyledon angles in WT (La-er) and phyABDE mutants grown in the dark, Rc (10 μmol m−2 s−1) and Rc + FRc (50:50, 20 μmol m−2 s−1) for 5 d.
Effect of EOD FR and Reduced R:FR on Flowering Time
An acceleration of flowering (as measured by number of rosette leaves at bolting) in response to growth under low R:FR and EOD FR treatment is a well-characterized component of the shade avoidance syndrome (Smith and Whitelam, 1997). The early flowering response of phyB mutants grown at temperatures above 21°C mimics that of shade-avoiding plants and has resulted in phyB being universally regarded as a repressor of flowering (Simpson et al., 1999). Observations that phyBD and phyBE double mutants flower earlier than either monogenic mutant infer additional roles for phyD and E in the control of flowering time in Arabidopsis (Devlin et al., 1998, 1999). A retention of flowering response to EOD FR treatment in phyABD, but not phyABE triple mutants suggested that phyE performs a more dominant role than phyD (Devlin et al., 1998). The work described here investigates the interaction of phyB, D, and E in regulating flowering response to R:FR in multiple PHY mutant combinations grown at 16°C.
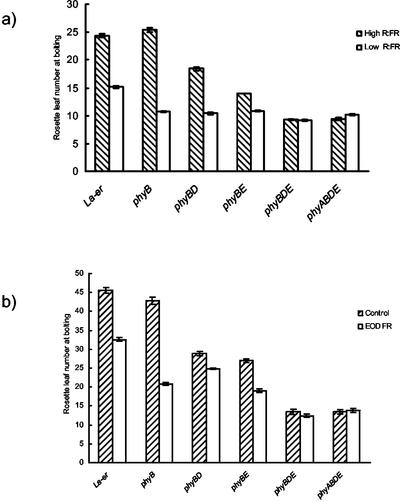
As described previously under long- and short-day conditions (Halliday et al., 2002), when grown at 16°C under continuous irradiation, the phyB mutant flowers with the same number of leaves as WT (Fig. 4A). However, an acceleration of flowering was observed in phyBD and phyBE double mutants grown under continuous irradiation and short days, with a further acceleration being observed in phyBDE triple mutants (Fig. 4, A and B). Such data suggest repression of flowering to be mediated by phyB, D, and E in a functionally redundant manner. An acceleration of flowering was observed in monogenic phyB mutants grown under low R:FR or treated with EOD FR. This acceleration was reduced in phyBD and phyBE double mutants and was absent in phyBDE triple mutants, suggesting functional redundancy between phyB, D, and E in regulating flowering response to R:FR. A quantitatively similar response was observed in phyBDE triple and phyABDE quadruple mutants, proposing little role for phyA in mediating flowering response to R:FR under these conditions.
Figure 4.
Rosette leaf number in La-er, phyB, phyBD, phyBE, phyBDE, and phyABDE mutants (in La-er background) at bolting. Plants were grown at 16°C under a) Continuous irradiation of high and low R:FR and b) 8 h light/16 h dark cycles, with and without EOD FR treatments.
Phytochrome Regulation of ATHB-2 Transcript Abundance
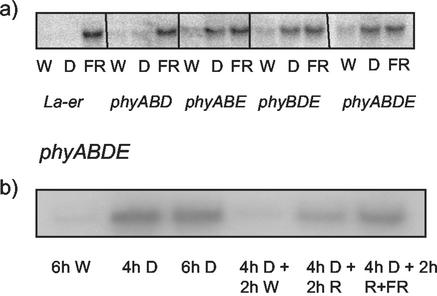
Previous observations suggest transcript abundance of the homeobox gene ATHB-2 to be regulated by several members of the PHY family (Carabelli et al., 1996). Transcript levels of ATHB-2 are low in tissues that have been maintained in white light, but are rapidly elevated in response to EOD FR treatment before transfer to darkness (Fig. 5A). Response to EOD FR was retained in phyABD triple mutant plants, suggesting the additional action of phyC and/or phyE in mediating this phenomenon. Analysis of phyABE triple and phyABDE quadruple mutants revealed a significant increase in ATHB-2 transcript upon transfer from white light to darkness (Fig. 5A). This increase was not affected by EOD FR treatment, suggesting regulation of ATHB-2 transcript by EOD FR to be mediated by phyB and phyE in a functionally redundant manner. Further investigation of the phyABDE quadruple mutant showed significant repression of ATHB-2 transcript abundance upon transfer of dark-adapted plants to white light. The partial repression observed upon transfer to R light suggests a novel role for phyC. Because of the rapid accumulation of ATHB-2 transcript in darkness, the reversibility of R-mediated repression was demonstrated by irradiating plants simultaneously with R and FR. The subsequent attenuation of the response suggests that phyC can, at least, partially mediate repression of ATHB-2 transcript abundance in an R/FR reversible manner. No effects on transcript accumulation were observed after 2 h of FR (data not shown).
Figure 5.
PHY regulation of ATHB-2 expression. A, RNA gel-blot showing ATHB-2 transcript accumulation in La-er, phyABD, phyABE, phyBDE, and phyABDE mutant plants grown under continuous white light (Wc) for 3 weeks, then transferred to white light (W) for 1 h, dark (D) for 1 h, or EOD FR (15 min) and then dark (45 min; labeled FR). B, RNA gel-blot showing ATHB-2 transcript accumulation in phyABDE mutant plants grown under Wc for 3 weeks, then transferred to different light regimes. W, White light; D, dark. Repeated experiments showed identical results.
DISCUSSION
PHY B, D, and E Act Redundantly to Control Leaf Morphology and Flowering Time in Response to R:FR
The ability to modulate plant architecture and flowering time in response to perceived threat of shading remains one of the most radical adaptive strategies available to higher plants. The most pronounced phenotypes of the shade avoidance syndrome are extension of internode and leaf growth and an acceleration of flowering (Smith and Whitelam, 1997). Such adaptations are believed to elevate leaves to a better-lit stratum in the canopy and promote seed set at a time when resources may be limiting. Anticipatory avoidance responses upon perception of neighboring vegetation also represent a crucial survival strategy, particularly in fast-developing stands (Ballare et al., 1990). The analysis of PHY-deficient mutants has been fundamental to the elucidation of roles for individual PHY in these responses. Studies have revealed that different PHY play distinct and overlapping roles within the spectrum of plant photomorphogenesis (McCormac et al., 1993; Whitelam et al., 1993; Whitelam and Harberd, 1994; Smith, 1995; Whitelam and Devlin, 1997). The involvement of phyB in mediating responses to R:FR is widely documented in a variety of species (Somers et al., 1991; Devlin et al., 1992; López-Juez et al., 1992; Reed et al., 1993), although the detection of shade avoidance responses in phyB null mutants have indicated the involvement of additional PHY (Whitelam and Smith, 1991; Robson et al., 1993; Halliday et al., 1994). Subsequent investigations have revealed the involvement of phyD and phyE, although, until now, a functional role for other phytochromes could not be unequivocally excluded (Devlin et al., 1996, 1998, 1999).
The work described here shows leaf elongation and acceleration of flowering in response to low R:FR and EOD FR (i.e. perceived threat of shading) to be mediated solely by phyB, D, and E in a functionally redundant manner. These represent the most recently evolved members of the PHY family, forming a distinct subgroup (Mathews and Sharrock, 1997). It has been previously speculated that shade avoidance may have provided the selective pressure for the evolution of these PHY (Devlin et al., 1998). Earlier studies have reported an EOD FR flowering response in phyABD triple but not phyABE triple mutants, thus implicating a greater role for phyE in mediating this response (Devlin et al., 1998). The apparent discrepancy between previous and current data may reflect the higher growth temperatures used by other authors. Growth at 16°C also results in a surprising increase in leaf area in WT plants grown under low R:FR. Such a response is in apparent contradiction to previously described shade avoidance phenotypes. Together with emerging data showing aberrant developmental responses in phy mutants grown at lower temperatures (Halliday et al., 2003), it must be questioned whether laboratory experiments using temperatures in excess of 21°C truly represent the adaptive phenomena of plants grown natively in more temperate conditions. Such observations question the true nature of the shade avoidance syndrome and will require further investigation.
PHY A Plays a Predominant Role in Modulating Internode and Leaf Elongation Growth in Light-Grown Plants
The role of phyA, a light-labile phytochrome, in modulating mature plant architecture was indicated in earlier studies after an observed reduction in biomass in the phyAB double mutant compared with either monogenic mutant (Devlin et al., 1996). Internode elongation and loss of rosette habit has been previously observed in phyAB double and phyABD triple mutant plants after EOD FR treatment (Devlin et al., 1996, 1999). The constitutive elongation phenotype observed in phyABE mutants grown under control conditions was the basis on which the mutant was isolated and led to the proposal that inhibition of internode elongation in mature plants is regulated, redundantly, by phyA, B, and E (Devlin et al., 1998). The caulescent appearance of phyABDE quadruple mutants grown under short days, a phenotype not displayed in phyBDE triple mutants, thereby supports such a proposal. In addition, the pronounced increase in leaf length/width ratio in phyABDE quadruple mutants grown under short days (Fig. 2) and continuous irradiation (data not shown) suggests a significant role for phyA in inhibiting leaf elongation in light-grown plants, independent of R:FR ratio.
PHY A, B, C, D, and E Act Redundantly to Modulate Cotyledon Development under Rc.
The synergistic coactions between phyA and phyB in regulating cotyledon expansion have been previously recorded (Neff and Vanvolkenburgh, 1994; Reed et al., 1994; Neff and Chory, 1998). Through the creation of triple and quadruple mutant combinations, additional roles for phyD and phyE have been revealed in this study. The partial cotyledon opening and expansion, observed in Rc-grown phyABDE quadruple mutant seedlings, also suggest that phyC can weakly mediate these responses in isolation. The reversibility of cotyledon opening by FR provides support for such a conclusion. Reporter gene studies by Tóth et al. (2001) revealed significant PHYC promoter activity in light-grown cotyledons. Therefore, it can be speculated that the weak response observed in phyABDE quadruple mutants does not result from reduced PHYC transcription in cotyledon tissue. However, it is plausible that the phyABDE quadruple mutant contains reduced levels of phyC, a phenotype previously observed in phyB-deficient plants (Hirschfeld et al., 1998). Such a deficiency would exacerbate an already reduced response and will be investigated in future experiments. Taken together, the data presented here reveal functionally redundant roles for all five PHY in regulating cotyledon development under Rc. In addition, cotyledon opening experiments have provided the first evidence of a R/FR reversible function for phyC.
PHY C Mediates the Repression of ATHB-2 Transcript in the Light
The ATHB-2 gene encodes a homeodomain-Leu zipper protein thought to be involved in cotyledon expansion, growth of the vascular system, and lateral root formation (Steindler et al., 1999). The light-regulation of ATHB-2 transcript abundance is well documented and is believed to be predominantly controlled by changes in R:FR (Carabelli et al., 1993, 1996). Mutant studies have suggested the involvement of a PHY other than phyA or phyB in mediating this response (Steindler et al., 1997). This work reveals regulation of ATHB-2 transcript abundance in response to EOD FR to be mediated redundantly by phyB and phyE. However, analysis of the phyABDE quadruple mutant has revealed a possible function for phyC in the partial suppression ATHB-2 transcription in the light. Future experiments should ascertain whether the increased repression in white light results from the increased fluence rate used or represents an additional cryptochrome-mediated repression of gene expression. The reduced repression of ATHB-2 transcription observed by supplementing R with FR provides evidence of another R:FR reversible function for phyC, although future mutant analyses will ultimately be required to ascertain whether phyC performs a similar function in planta.
The creation of mutants in Arabidopsis, null for multiple PHY, has provided new insights into functional redundancy between family members in controlling responses to R:FR. Analysis has revealed phyB, D, and E, the most recently evolved group of phytochromes, to solely mediate leaf elongation and flowering responses to R:FR ratio in a functionally redundant manner. The creation of a quadruple mutant, possessing only a functional phyC, has exposed significant roles for phyA in controlling internode elongation and leaf morphology in mature plants, independent of R:FR. Analysis of cotyledon development in phyABDE quadruple mutants has also provided the first evidence of a R/FR-reversible phyC function. Growth of plants at 16°C has revealed leaf phenotypes at variance with the characterized shade avoidance syndrome, implicating the interaction of light- and temperature-sensing mechanisms. Regulation of the homeobox gene, ATHB-2, by EOD FR treatment has been shown to be mediated redundantly by phyB and E, with a possible role for phyC in suppressing transcript abundance in the light. Redundant interplay between PHY B and E has been previously observed in R/FR-reversible seed germination (Hennig et al., 2002) and maintenance of rosette habit (Devlin et al., 1998), responses with no identifiable role for phyD. However, functional interaction with cryptochrome 1 has been shown to be mediated redundantly by phyB and D, with no apparent role for phyE (Hennig et al., 1999). Therefore, it can be concluded that phyB, D, and E are functionally unique photoreceptors, but act redundantly to regulate multiple responses during Arabidopsis development. Taken together, the data presented here have confirmed previously speculated functional divisions between PHY groups in addition to revealing new roles for family members and casting incertitude on the true nature of the shade avoidance syndrome under natural conditions.
MATERIALS AND METHODS
Plant Material
All experiments were performed using Arabidopsis, ecotype La-er. The phytochrome mutant alleles used in this study were phyB-1 (Koornneef et al., 1980), phyBD (Devlin et al., 1999), phyBE (Devlin et al., 1998), phyABD (Devlin et al., 1999), phyABE (Devlin et al., 1998), and phyBDE (Shalitin et al., 2002). The phyABDE quadruple mutant was created from existing mutants using standard screening procedures.
Growth Conditions
Seeds were surface sterilized in 10% (v/v) commercial bleach and were sown directly onto Lehle media (Lehle Seeds, Round Rok, TX) supplemented with 0.8% (w/v) agar. After 4 d of stratification in darkness at 4°C, germination was synchronized by treating seeds with a light pulse and returning to the dark for 24 h. Seedlings were germinated under 8-h light/16-h-dark cycles at 16°C. After an additional 9 d, uniformly sized seedlings were transplanted to 5- × 5- × 5-cm pots containing a 3:1 mixture of compost:horticultural silver sand. After an additional 12 d of growth under the same conditions, plants were transferred to experimental light regimes at 16°C. For cotyledon area measurements, seeds were germinated on Hoagland no. 2 basal salt mixture (0.8% [w/v] agar) as above before transfer to Rc at a fluence rate of 50 μmol m−2 s−1 at 22°C. Cotyledons were excised after 5 d of growth and were measured using scan pro5 (SPSS UK, Surrey, UK). For cotyledon angle measurements, seeds were germinated as above before transfer to Rc at a fluence rate of 10 μmol m−2 s−1 or Rc + FRc (50:50) at a fluence rate of 20 μmol m−2 s−1, both at 22°C. Seedlings were photographed and angles were measured with a protractor.
Light Sources
For R:FR ratio experiments, plants were grown under continuous irradiation in controlled cabinets described previously (Keiller and Smith, 1989). The high R:FR cabinet provided a photon irradiance, 400 to 700 nm, of 130 μmol m−2 s−1 and an R:FR of 4.7. The low R/FR cabinet provided the same photon irradiance, but an R:FR of 0.089. EOD FR experiments were carried out under controlled conditions comprising of 8 h of warm-white fluorescent light (photon irradiance 400–700 nm, 100 μmol m−2 s−1). Plants treated with EOD FR received 15 min of FR light (photon irradiance 700–800 nm, 57 μmol m−2 s−1) obtained by filtering the output of 500-W tungsten halogen lamps (Haloline; Osram-Sylvania, Towanda, PA) through 10 mm of flowing water and one layer of black Plexiglas (FRF 700; West Lakes Plastics, Lenni, PA). R and FR light was provided by light-emitting diodes at λmax665 and 735 nm, respectively. All light measurements were performed using a photosystem II spectroradiometer (LI-3000; LI-COR, Lincoln, NE).
Leaf and Flowering Measurements
All physiological measurements were performed after bolting. Data represent the means ± se from at least 10 plants. Leaf measurements were determined using a ruler. Flowering time was recorded as the number of rosette leaves present when plants displayed a 1-cm inflorescence stem.
RNA Gel-Blot Analysis
Seeds were germinated as described previously, and plants were grown under white light (8-h day and 16-h night cycles) at 16°C for 3 weeks. Plants were then transferred to continuous white light at 22°C for 24 h before experimental light treatments, all at 22°C. R and R:FR (50:50) were provided by light-emitting diodes at a fluence rate of 6 and 12 μmol m−2 s−1, respectively. Total RNA was extracted from whole plants using the method of Logemann et al. (1987). Samples of 20 μg of RNA were heat denatured (65°C for 15 min) in the presence of 50% (w/v) formamide, separated on a denaturing 1.5% (w/v) agarose gel (Sambrook et al., 1989), and blotted onto Hybond-N nylon membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). Equal loadings of RNA were confirmed by ethidium bromide staining of gels before blotting. Prehybridization and hybridization were performed in the presence of 50% (w/v) formamide at 42°C (Sambrook et al., 1989). Washings were carried out to a final stringency of 0.2× SSC and 0.1% (w/v) SDS at 42°C. An ATHB-2 fragment was isolated by PCR amplification of La-er genomic DNA using the following primers: forward: 5′-GAAAGACGATCTGGGTCTAAGCTTAGG-3′ and reverse: 5′-CAACCTCAGGCTGCTACGTCAGCG-3′. Probes were labeled with [α-32P] dCTP using random hexanucleotide priming (Rediprime; Amersham Pharmacia Biotech), and blots were exposed onto x-ray film (Kodak Biomax MS; Amersham Pharmacia Biotech).
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (UK) (grant no. 91/P15700 to K.A.F., 91/P08472 to U.P., and 91/P09944 to K.J.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.015487.
LITERATURE CITED
- Ballare CL, Scopel AL, Sanchez RA. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science. 1990;247:329–332. doi: 10.1126/science.247.4940.329. [DOI] [PubMed] [Google Scholar]
- Carabelli M, Morelli G, Whitelam GC, Ruberti I. Twilight-zone and canopy shade induction of the ATHB-2 homeobox gene in green plants. Proc Natl Acad Sci USA. 1996;93:3530–3535. doi: 10.1073/pnas.93.8.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabelli M, Sessa G, Ruberti I, Morelli G. The Arabidopsis ATHB-2 and -4 genes are strongly induced by far-red-rich light. Plant J. 1993;4:469–479. doi: 10.1046/j.1365-313x.1993.04030469.x. [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Halliday KJ, Harberd NP, Whitelam GC. The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: novel phytochromes control internode elongation and flowering time. Plant J. 1996;10:1127–1134. doi: 10.1046/j.1365-313x.1996.10061127.x. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1487. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock RA, Whitelam GC. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Rood SB, Somer SE, Quail PH, Whitelam GC. Photophysiology of the elongated internode (ein) mutant of Brassica rapa: The ein mutant lacks a detectable phytochrome B-like polypeptide. Plant Physiol. 1992;100:1442–1447. doi: 10.1104/pp.100.3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Koorneef M, Whitelam GC. Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana to low red/far-red ratio. Plant Physiol. 1994;104:1311–1315. doi: 10.1104/pp.104.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes MG, Whitelam GC (2003) The phyB-controlled flowering pathway is temperature sensitive and is mediated by the floral integrator FT. Plant J (in press) [DOI] [PubMed]
- Hennig L, Funk M, Whitelam GC, Schäfer E. Functional interaction of cryptochrome 1 and phytochrome D. Plant J. 1999;20:289–294. doi: 10.1046/j.1365-313x.1999.t01-1-00599.x. [DOI] [PubMed] [Google Scholar]
- Hennig L, Stoddart WM, Dieterle M, Whitelam GC, Schäfer E. Phytochrome E controls light-induced germination of Arabidopsis. Plant Physiol. 2002;128:194–200. [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock RA. Coordination of phytochrome levels in phy B mutants of Arabidopsis as revealed by apoprotein specific monoclonal antibodies. Genetics. 1998;149:523–535. doi: 10.1093/genetics/149.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiller D, Smith H. Control of carbon partitioning by light quality mediated by phytochrome. Plant Sci. 1989;63:25–29. [Google Scholar]
- Koornneff M, Rolff E, Spruitt CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana L Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- López-Juez E, Nagatani A, Tomizawa K-I, Deak M, Kern R, Kendrick RE, Furuya M. The cucumber long hypocotyl mutant lacks a light-stable PHYB-like phytochrome. Plant Cell. 1992;4:241–251. [PMC free article] [PubMed] [Google Scholar]
- McCormac A, Wagner D, Boylan MT, Quail PH, Whitelam GC. Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B-encoding cDNAs: evidence that PHYA and PHYB have distinct photoregulatory functions. Plant J. 1993;4:19–27. [Google Scholar]
- Mathews S, Sharrock RA. Phytochrome gene diversity. Plant Cell Environ. 1997;20:666–671. [Google Scholar]
- Neff MM, Chory J. Genetic interaction between phytochrome A, phytochrome B and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–36. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Vanvolkenburgh E. Light-stimulated cotyledon expansion in Arabidopsis seedlings: the role of phytochrome B. Plant Physiol. 1994;104:1027–1032. doi: 10.1104/pp.104.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail PH. Photosensory perception and signalling in plant cells: new paradigms? Curr Opin Plant Biol. 2002;14:180–188. doi: 10.1016/s0955-0674(02)00309-5. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich T, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson PRH, Whitelam GC, Smith H. Selected components of the shade-avoidance syndrome are displayed in a normal manner in mutants of Arabidopsis thaliana and Brassica rapa deficient in phytochrome B. Plant Physiol. 1993;102:1179–1184. doi: 10.1104/pp.102.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberti I, Sessa G, Lucchetti S, Morelli G. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 1991;10:1787–1791. doi: 10.1002/j.1460-2075.1991.tb07703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Frisch EF, Maniatis F. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schena M, Davis RW. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc Natl Acad Sci USA. 1992;89:3894–3898. doi: 10.1073/pnas.89.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin D, Yang H, mockler TC, Maymon M, Guo H, Whitelam GC, Lin C. Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature. 2002;417:763–767. doi: 10.1038/nature00815. [DOI] [PubMed] [Google Scholar]
- Simpson GG, Gendall AR, Dean C. When to switch to flowering. Annu Rev Cell Dev Biol. 1999;99:519–550. doi: 10.1146/annurev.cellbio.15.1.519. [DOI] [PubMed] [Google Scholar]
- Smith H. Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Smith H. Phytochromes and light signal perception by plants: an emerging synthesis. Nature. 2000;407:585–591. doi: 10.1038/35036500. [DOI] [PubMed] [Google Scholar]
- Smith H, Whitelam GC. The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997;20:840–844. [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell. 1991;3:1263–1274. doi: 10.1105/tpc.3.12.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindler C, Carabelli M, Borello U, Morelli G, Ruberti I. Phytochrome A, phytochrome B and other phytochromes regulate ATHB-2 expression in etiolated and green Arabidopsis plants. Plant Cell Environ. 1997;20:759–763. [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development. 1999;126:4235–4245. doi: 10.1242/dev.126.19.4235. [DOI] [PubMed] [Google Scholar]
- Tóth R, Hall A, Miller AJ, Nagy F, Kozma-Bognár L. Circadian clock-regulated expression of phytochrome and cryptochrome genes in Arabidopsis. Plant Physiol. 2001;127:1607–1616. doi: 10.1104/pp.010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuinen A, Kerckhoffs LHJ, Nagatani A, Kendrick RE, Koorneef M. Far-red light insensitive, phytochrome A-deficient mutants of tomato. Mol Gen Genet. 1995;246:133–141. doi: 10.1007/BF00294675. [DOI] [PubMed] [Google Scholar]
- Whitelam GC, Devlin PF. Roles for different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 1997;20:752–758. [Google Scholar]
- Whitelam GC, Harberd NP. Action and function of phytochrome family members revealed through the study of mutant and transgenic plants. Plant Cell Environ. 1994;17:615–625. [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson MC, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Smith H. Retention of phytochrome-mediated shade avoidance responses in phytochrome-deficient mutants of Arabidopsis, cucumber and tomato. J Plant Physiol. 1991;139:119–125. [Google Scholar]