Abstract
The actin cytoskeleton has been proposed to be a major player in plant gravitropism. However, understanding the role of actin in this process is far from complete. To address this problem, we conducted an analysis of the effect of Latrunculin B (Lat B), a potent actin-disrupting drug, on root gravitropism using various parameters that included detailed curvature kinetics, estimation of gravitropic sensitivity, and monitoring of curvature development after extended clinorotation. Lat B treatment resulted in a promotion of root curvature after a 90° reorientation in three plant species tested. More significantly, the sensitivity of maize (Zea mays) roots to gravity was enhanced after actin disruption, as determined from a comparison of presentation time of Lat B-treated versus untreated roots. A short 10-min gravistimulus followed by extended rotation on a 1-rpm clinostat resulted in extensive gravitropic responses, manifested as curvature that often exceeded 90°. Application of Lat B to the cap or elongation zone of maize roots resulted in the disruption of the actin cytoskeleton, which was confined to the area of localized Lat B application. Only roots with Lat B applied to the cap displayed the strong curvature responses after extended clinorotation. Our study demonstrates that disrupting the actin cytoskeleton in the cap leads to the persistence of a signal established by a previous gravistimulus. Therefore, actin could function in root gravitropism by providing a mechanism to regulate the proliferation of a gravitropic signal originating from the cap to allow the root to attain its correct orientation or set point angle.
Plants respond to an array of environmental and developmental stimuli with gravity being one of the more significant cues to which plants must adapt to survive. Under the Earth's gravitational field, shoots normally grow upward to maximize light absorption for photosynthesis, whereas roots grow down for optimal water and nutrient acquisition. This directional growth response of plant organs to gravity or gravitropism has been conveniently divided into a series of events consisting of gravity sensing, signal transduction, signal transmission, and the growth response (Kiss, 2000). In roots, gravity sensing is believed to occur in the root cap (Sack, 1997), but recent evidence suggests that roots may have dual gravity sensors, one of which may be located in a region outside the cap (Wolverton et al., 2002a, 2002b).
Despite recent proposals of alternative gravity-sensing sites in roots (Wolverton et al., 2002a), there is a great deal of cell biological and physiological evidence demonstrating that starch-containing amyloplasts in the columella region of the root cap are significant for gravity sensing (for review, see Kiss, 2000; Boonsirichai et al., 2002). Popularly known as the starch-statolith hypothesis, the sedimentation of amyloplasts within the columella cells is proposed to constitute one of the initial acts of gravity sensing in roots (Sack, 1997; Kiss, 2000). In shoots, sedimentable amyloplasts are located in the endodermal cell layer and like in roots, there is strong evidence for their importance in the gravitropic response of above ground plant organs (Tasaka et al., 1999; Weise et al., 2000). However, the mechanisms by which the plant transforms the physical signal resulting from amyloplast sedimentation into a physiological or biochemical signal to initiate the bending response has been elusive and remains an area of intense research (Boonsirichai et al., 2002; Chen et al., 2002).
Molecular genetics approaches have led to an increased understanding of the role of polar auxin transport in higher plant gravitropism (for review, see Muday, 2001). Moreover, several factors are emerging as possible players in gravity-related signaling events including dynamic pH and calcium fluxes (Monshausen et al., 1996; Scott and Allen, 1999; Fasano et al., 2001; Johannes et al., 2001; Plieth and Trewavas, 2002), modulation of potassium channel activity (Philippar et al., 1999), changes in inositol 1,4,5-triphosphate levels (Perera et al., 1999, 2001), and the activation of reactive oxygen species (Joo et al., 2001). Root gravitropism also has been shown to be partly regulated by protein phosphatases (Rashotte et al., 2001), and evidence implicating vacuoles in shoot gravitropism (Kato et al., 2002) adds to the list of cellular structures that include the endoplasmic reticulum (ER) and plastids as contributors to plant gravity responses (Sack, 1997).
Another cellular component that often has been proposed to modulate gravitropism in higher plants is the cytoskeleton (Baluška and Hasenstein, 1997; Sedbrook et al., 1999; Volkmann and Baluška, 2000; Blancaflor, 2002). Two major constituents of the cytoskeleton are microtubules and actin filaments (F-actin). The organization and function of these important structural elements in plants are under tight regulation by a variety of environmental and endogenous factors (Kost et al., 1999). Although the involvement of microtubules in gravitropism has been investigated primarily at the growth response phase (Blancaflor and Hasenstein, 1993, 1995; Bao et al., 2001; Himmelspach and Nick, 2001), the actin cytoskeleton is generally believed to be involved in gravity sensing with at least two models proposed to explain its role (for review, see Blancaflor, 2002). In the restrained gravity-sensing model, amyloplasts are postulated to be anchored to elements of the actin cytoskeleton. Amyloplast sedimentation could induce tensional changes within the actin network, leading to the activation of the downstream signaling cascades responsible for the gravitropic growth response (Sievers et al., 1991; Baluška and Hasenstein, 1997). Amyloplast sedimentation could alternatively contact other cellular structures such as the ER and trigger downstream gravity-related signaling events (Sack, 1997). A more recent model referred to as the tensegrity model states that signaling in the columella is activated by sedimenting amyloplasts that locally disrupt the actin cytoskeleton (Yoder et al., 2001). A major component of the tensegrity model is the recently described nodal ER network located exclusively in the columella cells. These nodal ER networks have been proposed to shield local plasma membrane receptors from sedimenting amyloplasts, thereby providing a directional cue to the sensing system (Zheng and Staehelin, 2001).
A simple but major approach to elucidating the function of the actin cytoskeleton in gravitropism has been to use cytoskeletal-disrupting compounds. Although actin disruption with various inhibitors has been shown to affect the polarized distribution and sedimentation of amyloplasts (Hensel, 1985; Sievers et al., 1989; Baluška et al., 1997; Zheng and Staehelin, 2001) and the morphology of the columella-specific nodal ER networks (Zheng and Staehelin, 2001), several studies have shown that these inhibitors do not abolish gravitropism (Blancaflor and Hasenstein, 1997; Staves et al., 1997; Yamamoto and Kiss, 2002; Yamamoto et al., 2002). However, there are also reports demonstrating that actin inhibitors such as cytochalasins cause a delay in the onset of root curvature (Wendt et al., 1987), which is concomitant with an alteration in the pattern of gravity-related ion fluxes (Sievers et al., 1995; Monshausen et al., 1996).
Further complicating the interpretation of the inhibitor work are some recent studies employing Latrunculin B (Lat B), a potent actin-depolymerizing drug, to study gravitropism in Arabidopsis. These studies demonstrate that disruption of the actin cytoskeleton in Arabidopsis can promote gravitropic curvature in hypocotyls and inflorescence stems but has minimal or no effect on root gravitropism (Yamamoto and Kiss, 2002; Yamamoto et al., 2002). Although, this promotive effect of actin disruption on gravitropism has been alluded to in previous reports (Nick et al., 1997; Wang and Nick, 1998) including roots (see Blancaflor and Hasenstein, 1997), its significance remains unclear. To further investigate this interesting phenomenon, we conducted an analysis of gravitropism in roots treated with cytoskeletal inhibitors. In this paper, we report that disruption of the actin cytoskeleton can also promote gravitropism in roots. These observations were extended by monitoring the development of gravitropic bending on a slowly rotating clinostat, and we report, for the first time to our knowledge, the induction of extensive curvature responses in roots with a disrupted actin cytoskeleton. Such responses could be induced by specific disruption of actin in the root cap. Our results have significant implications for models on root gravitropism because this is the first report demonstrating that an altered root actin cytoskeleton leads to the persistence of a gravitropic signal induced by short periods of gravistimulation. Therefore, in addition to modulating amyloplast mobility and position (Sievers et al., 1991), actin could be involved in regulating the timing and duration of a signal originating from the cap that allows the root to resume normal vertical growth.
RESULTS
Lat B But Not Oryzalin Enhances the Curvature and Gravity Sensitivity of Roots
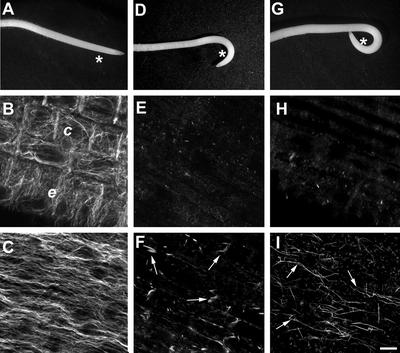
The effects of the actin-depolymerizing drug Lat B and the microtubule-depolymerizing drug oryzalin on the curvature of maize (Zea mays) roots were examined with a concentration and incubation period previously determined to cause significant disruption of the cytoskeleton (Hasenstein et al., 1999; Blancaflor, 2000). Incubating roots for 1 h in 1 μm oryzalin or Lat B induced the depolymerization of microtubules and actin filaments, respectively, in various regions of the root including the elongation zone (Fig. 1, A–D) and columella cells (Fig. 1, E–H).
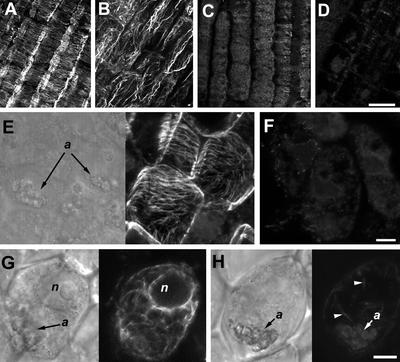
Figure 1.
Disruption of actin filaments and microtubules in roots of maize treated with Lat B and oryzalin. In cortical cells of maize, microtubules are oriented transversely to the longitudinal axis of the roots (A), whereas actin is predominantly oriented as longitudinal bundles (B). Treatment of roots with 1 μm oryzalin results in the depolymerization of microtubules (C), whereas Lat B treatment induces extensive disruption of the actin filament network (D). E, Brightfield and corresponding fluorescent image of cortical microtubules in columella cells of maize roots. Like the elongation zone, cortical microtubules in the columella cells are oriented perpendicular to the longitudinal axis of the root. F, Columella cells of roots treated with oryzalin display diffuse fluorescence, which is indicative of microtubule depolymerization. G, Brightfield and corresponding fluorescence image of a columella cell. For labeling actin in the columella cells of maize, aldehyde fixation was avoided and roots were processed following the methods of Collings et al. (2001). Using this method, a weakly fluorescent but filamentous actin network could be detected in single columella cells. Columella cells typically contain randomly organized actin filaments and a ring of fluorescence surrounding the nucleus (n). H, The filamentous actin network from a columella cell of a root treated with Lat B is disrupted as evident from the weaker fluorescent signal and fewer filamentous structures (arrowheads). Furthermore, a weak fluorescent signal appears to be associated with the amyloplasts (a, arrow). In A through D, bar = 50 μm; in E through H, bar = 10 μm.
Because of the difficulty in labeling actin in the root columella region using aldehyde fixatives, we employed a modified glycerol permeabilization and 3-maleimidobenzoil-N-hydroxy-succinimide ester (MBS) fixation method previously used to image actin filaments in the columella (Collings et al., 2001). Using this technique, we were able to visualize a network of distinct actin filaments in maize columella cells. However, actin labeling was not uniform throughout the columella region but was confined to single columella cells (Fig. 1G; see also Collings et al., 2001). In roots treated with Lat B, we could not detect any columella cell with filamentous labeling. Lat B-treated columella cells either were completely devoid of filamentous labeling or displayed only a few weakly fluorescing actin strands. Moreover, diffuse and weak labeling was observed to be associated with amyloplasts (Fig. 1H). The reduction in filamentous labeling and lower fluorescence intensity indicates that Lat B was capable of depolymerizing the actin network in columella cells.
Having determined that 1 μm Lat B or oryzalin was exerting its desired effect on the cytoskeleton, we used this concentration for most of the growth and curvature measurements described throughout this study. Roots subjected to inhibitor treatment showed a significant inhibition of elongation growth. Whereas control roots elongated at a rate of 1.31 mm h−1, the growth rate of oryzalin and Lat B-treated roots was reduced to 0.95 and 0.51 mm h−1, respectively (Fig. 2A). Oryzalin had no significant effect on root curvature compared with DMSO controls despite a significant reduction in growth rate. These results are similar to previous reports showing that microtubule disruption does not alter root gravitropism (Baluška et al., 1996; Hasenstein et al., 1999). On the other hand, Lat B treatment consistently induced stronger root gravitropic responses compared with controls and oryzalin-treated roots. The difference in curvature became most apparent 90 min after horizontal reorientation wherein Lat B-treated roots curved at a faster rate. The rate of curvature 90 min after reorientation was 45° h−1 for Lat B-treated roots, whereas the rate of curvature of control and oryzalin-treated roots was 24° h−1 and 29° h−1, respectively. The faster rate of curvature of Lat B-treated roots within the first 3 h of gravistimulation led to a larger final angle of curvature 10 h after reorientation. Whereas Lat B-treated roots had a final angle of curvature of 68°, the final angle of curvature of oryzalin-treated and control roots were 58° and 55°, respectively (Fig. 2B).
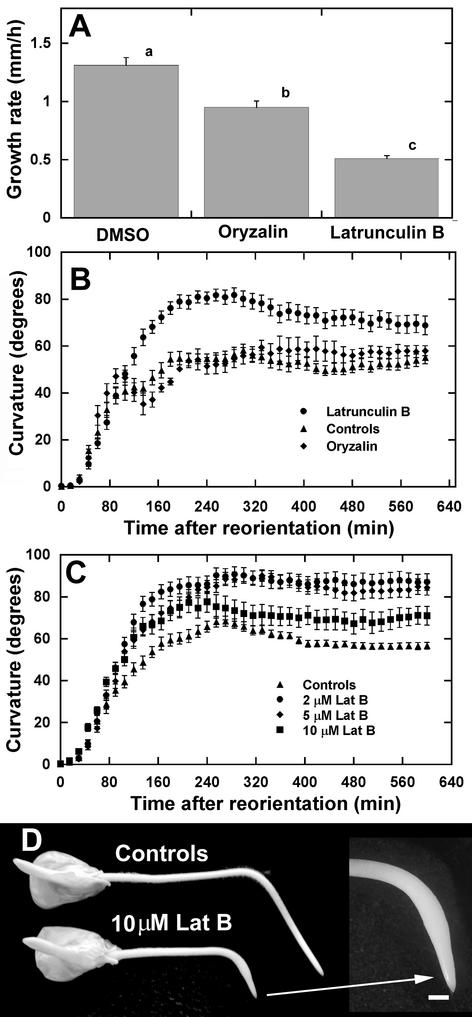
Figure 2.
Effects of cytoskeletal disruption on the growth and curvature kinetics of maize primary roots. A, Growth rates of vertically grown roots treated with 1 μm oryzalin and Lat B. Values are means of at least 10 roots + se in each group. Means with different letters are significantly different (P < 0.05, Tukey's test). B, Time course of curvature of maize roots treated with 1 μm Lat B, 1 μm oryzalin, and corresponding dimethyl sulfoxide (DMSO) controls after a 90° horizontal reorientation. Data are means ± se, n = 15 roots for each treatment. Note the enhanced gravitropic curvature of maize roots treated with Lat B but not with oryzalin. C, Higher Lat B concentrations still promoted root curvature. However, the promotive effect of Lat B on root curvature was not as extensive at a concentration of 10 μm. D, Comparison of a control root and a root treated with 10 μm Lat B 12 h after gravistimulation. Curvature was not inhibited despite the eventual swelling of the root apex (arrow). Bar = 1 mm.
Higher concentrations of Lat B resulted in stronger inhibitory effects on root growth. At 2 and 5 μm Lat B, growth rate was reduced to 0.49 and 0.34 mm h−1, respectively. Despite the stronger growth inhibitory effect of these Lat B concentrations, root curvature was still promoted to a similar extent as roots treated with 1 μm Lat B (Fig. 2C). At 10 μm Lat B, root growth rate was reduced to 0.32 mm h−1 but was not significantly different from the growth rate of roots treated with 5 μm Lat B. However, at this concentration, there was still a slight promotion of root curvature (Fig. 2C) despite the eventual swelling of the root apex (Fig. 2D).
To further investigate the promotive effect of Lat B on root curvature, we conducted a series of experiments employing a clinostat. A clinostat is a mechanical device that rotates a biological specimen usually around a horizontal axis. By doing so, unilateral gravistimulation is effectively eliminated by exposing the specimen to a succession of gravitropic stimuli (Brown et al., 1976; Dedolph and Dipert, 1971). The clinostat has routinely been used to estimate the presentation time because it can serve as an indicator of gravisensitivity (Perbal et al., 1997). Presentation time analysis was conducted by giving roots a brief 90° horizontal stimulation (gravistimulation), and the curvature that developed after rotating roots for 2 h on a 1-rpm clinostat was measured. The resulting curvature was plotted against the logarithm of the stimulation time. From a regression analysis, presentation time was calculated based on the intercept of the extrapolated regression line with the x axis taken as an estimate of the presentation time (Kiss et al., 1989, 1996; Blancaflor et al., 1998). The presentation times calculated for the growth conditions employed in this study were 2.45 min for controls, 3.02 min for oryzalin, and 1.58 min for Lat B-treated roots (Fig. 3A). Although the calculated presentation time of oryzalin-treated roots was greater than controls, the curvature that developed at each induction point for both treatments was not significantly different. Lat B-treated roots, on the other hand, consistently exhibited larger curvatures at each induction point that were significantly different from the curvature values of oryzalin-treated and control roots (Fig. 3A).
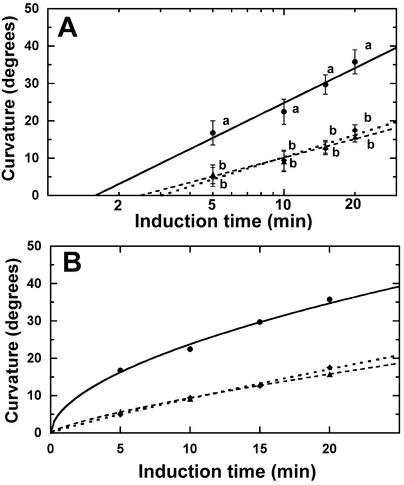
Figure 3.
Estimation of gravitropic sensitivity in maize roots treated with cytoskeletal-disrupting drugs. A, Presentation time analysis of maize roots treated with 1 μm Lat B or oryzalin. The intercept of the regression line with the x axis provided an estimate of the presentation time. Presentation time was 2.45 min for controls (▴), 3.02 min for oryzalin (♦), and 1.58 min for Lat B-treated roots (●). The smaller presentation time value of Lat B-treated roots indicates increased gravisensitivity. Correlation coefficients for the regression lines are 0.97 (Lat B and oryzalin treated) and 0.98 (controls). Each data point represents a mean ± se, n = 30 roots. Means at each induction point with different letters are significantly different (P < 0.05, Tukey's test). B, Fitting of the hyperbolic (H) model to the experimental data shows correlation coefficients of 0.98 (Lat B) and 0.99 (oryzalin and controls). Gravisensitivity (S) was estimated according to the methods of Perbal et al. (2002) where S = a/b and S corresponds to an angle per unit dose. The values for S were 1.21° g−1 min−1 for controls, 3.96° g−1 min−1 for Lat B-treated and 0.98° g−1 min−1 for oryzalin-treated roots. The larger S value for Lat B-treated roots indicates increased sensitivity to low doses of stimulation (Perbal et al., 2002).
In a recent study, the use of presentation time as an indicator of gravitropic sensitivity was re-examined, and it was shown that hyperbolic functions (referred to as the H model) better fit the dose response data than the logarithmic functions (L model) commonly used to estimate presentation time (Perbal et al., 2002). To determine whether the H model is a better fit than the L model, the data were re-analyzed following the methods of Perbal et al. (2002). Fitting of the hyperbolic model to the experimental data of drug-treated and control roots is shown in Figure 3B. The higher correlation coefficients in the H model indicate that this model provides a better fit for the experimental data. Therefore, we estimated gravitropic sensitivity (S) using the H model where S = a/b. S corresponds to an angle (α) per unit dose; a is the maximal angle of curvature; and b is the dose of stimulation that gives rise to an angle of α/2 (for details, see Perbal et al., 2002). The values obtained using the H model were 1.21° g−1 min−1 for controls, 3.96 ° g−1 min−1 for Lat B-treated, and 0.98° g−1 min−1 for oryzalin-treated roots (Fig. 3B).
Interestingly, Lat B-treated roots maintained on the clinostat beyond 2 h continued to bend past the vertical. Induction times as short as 10 min followed by 12 to 15 h of continued growth on the clinostat resulted in root curvature often exceeding 90° despite a significant reduction in growth rate. These extensive curvature responses after prolonged clinorotation were not observed in oryzalin-treated or control roots (Fig. 4).
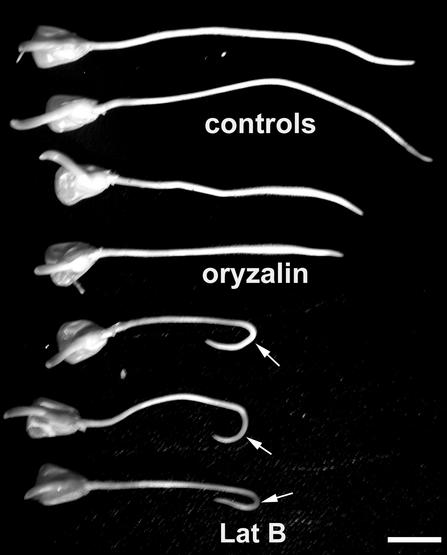
Figure 4.
Curvature responses of representative maize roots after disruption of the cytoskeleton. Roots were treated with 1 μm Lat B, 1 μm oryzalin, or corresponding DMSO control for 1 h, given a brief 10-min horizontal gravity stimulus and rotated on a clinostat for 15 h. Note the extensive curvature responses of Lat B-treated roots (arrows) but not oryzalin-treated or DMSO solvent controls. The enhanced curvature responses in Lat B-treated roots occurred despite significant inhibition of root growth rate. Bar = 5 mm.
Kinetics of Actin Filament Reformation during Growth Reorientation on a Clinostat
The dramatic growth reorientation (i.e. curvature) of Lat B-treated roots maintained on the clinostat for extended periods (Fig. 4) is indicative of continued polar cell growth, which is dependent on an intact cytoskeleton (Kost et al., 1999; Wasteneys, 2000). To determine whether the strong curvature of roots on a clinostat is correlated with the reformation of the actin cytoskeleton upon removal of Lat B, we imaged F-actin during periods of extensive root curvature. We focused our analysis on the elongation zone because this is the region of the root where gravitropic curvature is strongly manifested (Ishikawa and Evans, 1993). In control roots, a 10-min gravistimulus followed by 12 h of rotation on a clinostat did not produce significant curvature (Fig. 5A). Confocal microscopy of Alexa Fluor-phalloidin-labeled root cells in the epidermal, cortical (Fig. 5B), and vascular parenchyma cells of the stele (Fig. 5C) revealed filamentous staining typical of an intact actin cytoskeleton (see Blancaflor and Hasenstein, 1997). Lat B-treated roots given a 10-min gravistimulus followed by a 4-h rotation on a clinostat exhibited extensive curvature (Fig. 5D). However, cells in the cortex and stele of the elongation zone were almost completely devoid of actin filaments (Fig. 5, E and F). Lat B-treated roots continued to bend during clinorotation and after 12 h, some roots had curved extensively resulting in the formation of a loop (Fig. 5G). However, cortical cells of roots showing such strong curvature responses still lacked filamentous labeling (Fig. 5H). Only cells in the stele showed a clear reformation of actin filaments, but these were not as extensive as the actin network in the stele cells of controls (Fig. 5, compare I with C).
Figure 5.
Kinetics of actin filament reformation in the elongation zone of roots grown on a clinostat. A through C, Controls; D through F, 4 h after Lat B treatment; and G through I, 12 h after Lat B treatment. A, Root tip of an untreated root gravistimulated for 10 min and rotated on a clinostat for 12 h. Asterisks indicate the region of the root where actin filaments were imaged. B, The actin filament network in epidermal (e) and cortical (c) cells of the elongation zone. Actin is oriented transversely to the longitudinal axis of the root in the epidermal cells, whereas random to longitudinal actin is characteristic of cortical cells. C, A dense array of thick longitudinal actin cables are evident in cells of the stele. D, 4 h after clinorotation, Lat B-treated roots show extensive curvature after an initial 10-min gravistimulus. E, Despite the strong curvature, cortical cells in the elongation zone are devoid of actin filaments. F, In the stele, only short and thick brightly fluorescing fragments are detected (arrows). G, 12 h after clinorotation, the curvature of Lat B-treated roots became even more pronounced. H, Cells in the elongation zone were still devoid of actin filaments. I, Thick actin cables in the stele begin to form 12 h after removal of the root from Lat B but the filaments remained highly fragmented (arrows). Bar = 20 μm.
Specific Disruption of Actin in the Cap Results in Enhanced Curvature Responses of Clinostat-Grown Roots
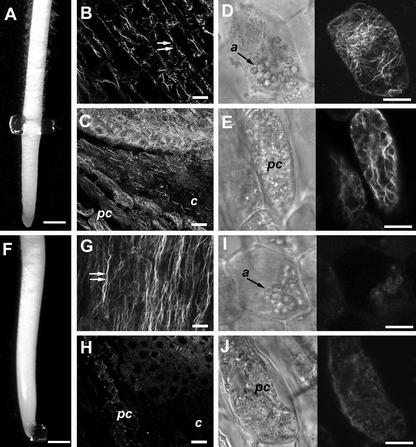
Although we observed the hypergravitropic responses of clinostat-grown roots in other plant species including Medicago truncatula and Linum usitatissimum (data not shown), the larger roots of maize allowed us to design experiments wherein we could locally apply cytoskeletal-disrupting drugs to specific regions in the root. Because the cap has been proposed to be significant for root gravitropism (Sack, 1997; Kiss, 2000), we attempted to confine actin disruption to the root cap by localized Lat B application and to determine whether such treatments could also induce the strong curvature responses observed when a larger area of the root was treated with Lat B. This was done by carefully positioning agar blocks previously incubated in 1 μm Lat B so that only the elongation zone or cap of the root was in contact with the block (e.g. Fig. 6, A and F). After 1 h, the blocks were removed and roots were processed for actin labeling to determine if actin disruption was confined to the area of block application. Application of Lat B-loaded agar blocks to the elongation zone (Fig. 6A) induced the fragmentation of the longitudinal F-actin bundles in the region where the block was applied (Fig. 6B). However, the state of actin in the cap appeared not to be affected. Labeling the cap actin cytoskeleton of roots fixed using aldehydes typically produces intense fluorescence from the peripheral cap region and a weaker more diffuse fluorescence pattern in the columella (Fig. 6C; see also Baluška et al., 1997; Baluška and Hasenstein, 1997). Because it is difficult to discern filamentous actin in the columella using aldehyde fixatives, we employed the glycerol permeabilization and MBS fixation method described above (see Fig. 1, G and H; Collings et al., 2001) to determine whether localized application of Lat B-imbibed agar blocks affected actin in the columella. Localized application of Lat B-imbibed agar blocks to the elongation zone did not disrupt the fine actin filament network in the columella (Fig. 6D) and the thicker longitudinal bundles in the peripheral cap cells (Fig. 6E). Lat B-loaded agar blocks applied to the cap (Fig. 6F) did not disrupt the pattern of longitudinal actin bundles in the elongation zone (Fig. 6G) or meristematic region (data not shown). However, fluorescence in the cap region was significantly reduced, and only short filamentous actin fragments were observed in the peripheral cap region (Fig. 6H). A closer examination of actin organization in the cap using MBS fixation revealed the lack of actin filaments in both the columella (Fig. 6I) and peripheral cap cells (Fig. 6J). In the columella cells, weak fluorescence associated with the amyloplast was detected (Fig. 6I), which was similar to the fluorescence pattern observed when the entire root was treated with Lat B (see Fig. 1H). Peripheral cap cells, on the other hand, displayed diffuse fluorescence throughout the cell (Fig. 6J).
Figure 6.
Specific disruption of F-actin in the cap of maize roots by localized Lat B application. Roots with Lat B applied locally to the elongation zone (A) resulted in the fragmentation of actin filaments in the elongation zone (double arrows, B) but not in the cap. C, Peripheral cap (pc) cells contained brightly fluorescing thick longitudinal bundles, while a more diffuse pattern was observed in the columella region (c). D, To preserve the actin network in the cap region, agar block-treated roots were processed following the methods of Collings et al. (2001). Brightfield and corresponding fluorescence image of a maize columella cell reveals an extensive network of fine and randomly oriented actin filaments. a, Amyloplasts. E, Peripheral cap cells are characterized by thicker longitudinally oriented actin bundles. Roots with Lat B-loaded agar bocks applied to the cap region (F) showed an intact longitudinally oriented actin network in the elongation zone (double arrows, G). H, However, the actin network in the cap region was disrupted as was evident from the weaker fluorescence originating from the peripheral cap (pc) and columella region (c). A single columella (I) or peripheral cap cell (J) labeled using the methods of Collings et al. (2001) reveals the absence of filamentous structures, which is indicative that agar block application of Lat B was sufficient to induce depolymerization of actin filaments in these cells. Bars in A and F = 1 mm; in B, C, G, and H = 30 μm; and in D, E, I, and J = 10 μm.
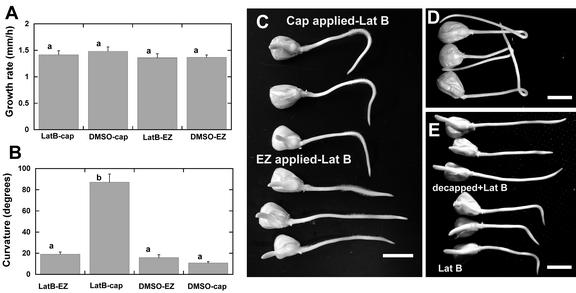
Having established that we could confine disruption of the actin cytoskeleton to cells at the tip of the root without affecting actin organization in elongation zone, we then analyzed whether these specific patterns of actin disruption could affect the graviresponse of clinostat-grown roots. In contrast to the strong growth inhibitory effect of immersing the terminal 5 to 10 mm of the root in Lat B (see Fig. 2A), localized Lat B application to either the cap or elongation zone did not significantly affect root growth rate (Fig. 7A). However, clinostat-grown roots with Lat B applied to the cap after a 10-min gravistimulus showed a larger final angle of curvature compared with roots with Lat B applied to the elongation zone (Fig. 7, B and C). The curvature that developed when Lat B was applied to the elongation zone was not significantly different from the curvature of roots treated with control blocks (i.e. equivalent concentrations of DMSO applied to the cap or elongation zone; Fig. 7B). Although a majority of the roots with Lat B applied to the cap would exhibit a 90° curvature and slight overshoot from the vertical (Fig. 7C), some roots with cap applied Lat B would exhibit extreme bending responses wherein the root would form complete loops (Fig. 7D). This was reminiscent of the type of curvature response observed when the entire root was treated with Lat B (see Fig. 5). Moreover, decapping of the root before Lat B treatment prevented the development of these strong curvature responses (Fig. 7E).
Figure 7.
Effects of local actin disruption on the growth and gravicurvature of maize roots grown on a clinostat. A, Growth rate of maize roots with Lat B applied to the cap or elongation zone (EZ). Positional application of 1 μm Lat B to the cap or elongation zone for 1 h did not significantly affect root growth rate (P > 0.05, Tukey's test). Bars represent mean growth rate + se, n = 9 roots for each group. B, Lat B was applied by the agar block method described in Figure 6. Roots were given a 10-min horizontal stimulus and rotated axially on a 1 rpm clinostat, and curvature was measured after 15 h. Note the larger angle of curvature of roots with Lat B applied to the cap. Data are means for 25 to 30 roots + se. Means with similar letters are not significantly different (P > 0.05, Tukey's test). C, Representative maize roots grown on a clinostat for 15 h after a 10-min induction time. Roots with Lat B applied to the cap typically bend to about 90° or overshoot the vertical, whereas roots with Lat B applied to the elongation zone show only slight curvature. D, In some cases, cap application of Lat B resulted in roots that would grow in a loop. E, Roots that were decapped before Lat B treatment did not curve and occasionally would bend opposite the gravity vector. Gravity vector is toward the bottom of the page. Bar = 10 mm.
Inhibition of Polar Auxin Transport Abolishes the Hypergravitropic Responses of Lat B-Treated Roots Grown on a Clinostat
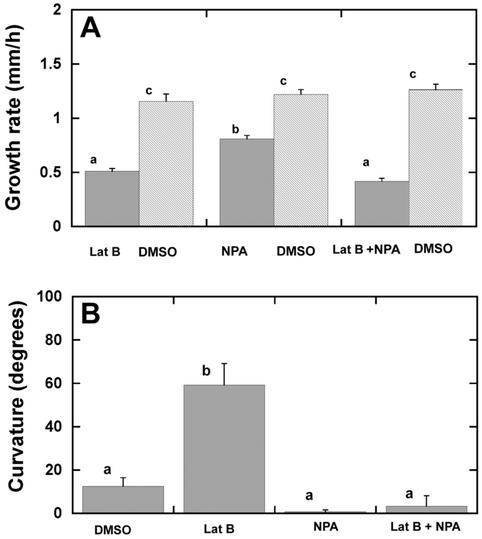
The polar auxin transport inhibitor N-(1-napthyl) phthalamic acid (NPA) is known to block gravitropic curvature in roots (Blancaflor and Hasenstein, 1997; Hasenstein et al., 1999). To determine whether the hypergravitropic responses induced by Lat B treatment are connected to auxin transport, we treated roots simultaneously with Lat B and NPA for 1 h, and roots were given a brief 10-min horizontal stimulus before clinorotation. Lat B, NPA, or simultaneous application of NPA and Lat B caused significant reductions in root growth rate compared with solvent controls (Fig. 8A). After 15 h of rotation on a clinostat, Lat B-treated roots displayed the characteristic strong curvature responses described above (see Figs. 4, 5, and 7), whereas roots treated with NPA alone showed less curvature compared with DMSO controls. Simultaneous application of NPA and Lat B resulted in a significantly smaller final angle of curvature compared with roots treated with Lat B alone (Fig. 8B).
Figure 8.
NPA treatment abolishes the hypergravitropic responses of maize roots induced by Lat B. A, Growth rate measurements of maize roots treated with Lat B, NPA, Lat B + NPA, and DMSO controls. Bars are means + se and the same letter indicate that they are not statistically different (P > 0.05, Tukey's test), n = 9 for each group. B, The terminal 5- to 10-mm region of maize roots was incubated in Lat B, NPA, Lat B + NPA, or DMSO for 1 h. All the roots treated were given a 10-min horizontal stimulus and rotated axially for 12 h on a clinostat before measurement of curvature. Group of Lat B only treated roots was significantly different from all other three groups (P < 0.05, Tukey's test). Data are means for 20 roots + se.
Time Course of Curvature Development under Extended Clinorotation
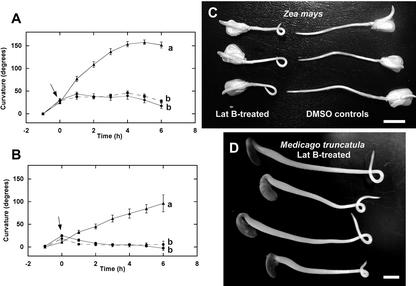
Previous reports have described a phenomenon wherein gravitropically curved organs straighten upon removal of the constant gravistimulus (for review, see Stankovic et al., 1998a). A detailed analysis of this autonomic straightening phenomenon using cress roots revealed that despite the curvature that developed after 1 h of constant gravistimulation, subsequent clinorotation resulted in root straightening after 5 h (Stankovic et al., 1998b). The extensive root-bending responses we observed in this study after prolonged clinorotation could be indicative of an impaired ability of the root to resume straight growth after withdrawal of the gravistimulus (Stankovic et al., 1998a, 1998b). To determine the kinetics of curvature development on a clinostat, maize and M. truncatula roots treated with either oryzalin or Lat B were given a 1-h 90° gravistimulus followed by rotation on a 1-rpm clinostat. The curvature of the roots was recorded at 1-h intervals for up to 6 h. In both plant species examined, bending of the roots was observed after 1 h of continuous gravistimulation, but the extent of curvature was not significantly different among treatments (Fig. 9, A and B). Although maize roots in all treatments continued to bend 1 h after clinorotation, the rate of curvature was higher in Lat B-treated roots. Although this rapid rate of curvature persisted in Lat B-treated roots, controls and oryzalin-treated roots showed no further development of curvature within the 6-h period of clinorotation (Fig. 9A). Similar observations were made in M. truncatula (Fig. 9B) and L. usitatissimum roots (data not shown). The absence of additional curvature development in oryzalin-treated and control roots eventually led to new growth parallel to the original vertical position, whereas the continued curvature of Lat B-treated roots resulted in the formation of loops (Fig. 9, C and D), similar to what we observed in roots subjected to low doses (i.e. 10 min) of gravi-stimulation (see Fig. 5).
Figure 9.
Time course of curvature development of maize and M. truncatula roots on a clinostat after 1 h of gravistimulation. Maize (A and C) and M. truncatula (B and D) roots were treated with 1 μm oryzalin (●), Lat B (▴), or DMSO solvent controls (▪) for 1 h and given a 1-h 90° horizontal reorientation followed by rotation on a 1-rpm clinostat. The curvature of the roots was recorded at 1-h intervals for a total of 6 h. Clinorotation commenced after roots were given a 1-h gravistimulus (arrows) and defined as time 0. Data points are means for 10 roots ± se. One hour of gravistimulation resulted in detectable curvature in all treatments. However, upon the withdrawal of the gravistimulus (beginning of clinorotation, arrows), only Lat B-treated roots continued to curve. At time −1 and 0, root curvatures for all three treatments were not significantly different (P > 0.05, Tukey's test). One to 6 h after clinorotation, Lat B-treated roots (both maize [A] and M. truncatula [B]) exhibited significantly stronger curvature than oryzalin-treated or DMSO-treated roots (P < 0.05, Tukey's test). Fifteen hours of continuous rotation on a clinostat would eventually result in Lat B-treated roots forming complete loops in both maize (C) and M. truncatula (D) roots. Bar in C = 10 mm and in D = 3 mm.
DISCUSSION
The actin cytoskeleton has been proposed to be a major player in plant gravity responses, and there has been a flurry of recent studies that have attempted to define its role in this process (Driss-Ecole et al., 2000; Collings et al., 2001; Yoder et al., 2001; Yamamoto and Kiss, 2002; Yamamoto et al., 2002). The use of actin-depolymerizing compounds has been the method of choice to probe the function of actin in gravitropism because of the rapidity and simplicity of the technique, and in many cases, such pharmacological approaches have been instrumental in deciphering the role of the cytoskeleton in other plant cellular processes (Kost et al., 1999). However, the application of actin inhibitors to gravitropism research has often been contradictory with reports showing no effects, inhibitory effects, or promotive effects on gravitropism (for review, see Blancaflor, 2002). To clarify this issue, we re-investigated gravitropism in roots treated with microtubule and F-actin inhibitors using additional parameters that included presentation time analysis and monitoring of curvature development after prolonged clinorotation.
After a 90° reorientation, roots with disrupted microtubules or actin filaments were still capable of bending (Fig. 2A). These observations are consistent with previous studies showing that disruption of either component of the cytoskeleton does not abolish gravitropism (Baluška et al., 1996; Blancaflor and Hasenstein, 1997; Staves et al., 1997; Hasenstein et al., 1999; Yamamoto and Kiss, 2002; Yamamoto et al., 2002). However, a detailed kinetic analysis of the curvature response of maize roots revealed stronger and faster curvature in Lat B-treated roots compared with oryzalin-treated roots (Fig. 2B). Moreover, higher doses of Lat B also promoted gravitropism despite a strong inhibitory effect on root growth (Fig. 2, C and D).
Enhanced curvature responses have been previously reported in maize roots and rice (Oryza sativa) coleoptiles treated with cytochalasin B, another actin-disrupting drug (Blancaflor and Hasenstein, 1997; Nick et al., 1997; Wang and Nick, 1998). Moreover, Lat B was recently shown to promote gravitropism in hypocotyls and inflorescence stems of Arabidopsis (Yamamoto and Kiss, 2002; Yamamoto et al., 2002). Although several studies have now reported on the promotive effect of actin disruption on gravitropism, such studies focused only on monitoring curvature development after continuous gravistimulation (i.e. 90° horizontal reorientation). In our work, we have employed the use of clinorotation as a tool to further understand the significance of the enhanced gravitropic responses of roots resulting from an altered actin cytoskeleton. In addition to confirming the promotive effect of actin disruption on gravitropism (Fig. 2), our studies revealed a significantly reduced presentation time of Lat B-treated roots compared with oryzalin-treated or control roots (Fig. 3A). Presentation time is defined as the minimum exposure time in a 1g field to elicit a gravity response (Perbal et al., 2002) and often has been used to estimate the sensitivity of plants to gravity under various conditions and treatments (Kiss et al., 1989, 1996; Blancaflor et al., 1998; Vitha et al., 1998).
We extended our analysis of clinostat-grown roots to consider other models used in estimating gravitropic sensitivity. Perbal et al. (2002) recently reported that dose response data from published literature better fit a hyperbolic function (H model) than the logarithmic function (L model) that we used to measure presentation time. Therefore, we estimated gravitropic sensitivity based on the H model according to the methods of Perbal et al. (2002). On the basis of this analysis, roots treated with Lat B were three times more sensitive than controls and oryzalin-treated roots (Fig. 3B). The reduction in presentation time of Lat B-treated roots using the L model and increased S value using the H model (Perbal et al., 2002) point to an alteration in some aspect of the gravity-sensing mechanism of roots and could explain the stronger gravitropic responses documented in this study.
In their work on Arabidopsis shoot gravitropism, Yamamoto and Kiss (2002) proposed that increased plastid movement due to depolymerization of actin could account for the promotive effect of Lat B on hypocotyl curvature. Such conclusions were based on the observation that Lat B treatment enhanced the gravitropic responses of mutants with altered plastid morphology, which normally have reduced rates of curvature (Yamamoto et al., 2002). In roots, columella cells with the fastest rate of amyloplast sedimentation are those that contribute most to gravity sensing (Blancaflor et al., 1998). Furthermore, wild-type Arabidopsis roots, which are more sensitive to gravity, have a faster rate of amyloplast sedimentation than the less gravisensitive starchless mutants (MacCleary and Kiss, 1999).
In further support of the above argument is evidence showing that increased amyloplast sedimentation in roots occurs in response to actin inhibitors (Sievers et al., 1989; Baluška et al., 1997). A recent study of amyloplast sedimentation kinetics in living maize columella cells revealed that amyloplasts that moved through the central part of the cell (via side to side sedimentation) had lower average velocities than amyloplasts that traversed the cell periphery (via side to distal sedimentation; Yoder et al., 2001). Cytochalasin D treatment enhanced sedimentation velocity along the central part of the cell suggesting that the lower rate of amyloplast sedimentation through the cell center is due to an actin-based cytoskeletal network that pervades the central columella cytoplasm. The localized disruption of this central actin network by sedimenting amyloplasts could facilitate gravity sensing in roots (Yoder et al., 2001). The lateral movement of amyloplasts upon gravi-stimulation may alternatively modify the tensile forces within the columella actin network, and this information is subsequently transmitted to peripheral membranes where a signal that leads to the graviresponse is initiated (Sievers et al., 1991). A detailed study in cress roots using intermittent stimulation revealed that amyloplasts require only short distance movement for gravity perception to occur and the only cellular structure that could sense such small displacements is the cytoskeleton (Hejnowicz et al., 1998).
How could the enhanced gravitropic bending resulting from actin disruption be incorporated into any of the existing models? On the basis of the models detailed above, disruption of the actin network should consequently lead to an inhibition of gravitropism because the tensile forces within the cell that change in response to amyloplast movement are dissipated (Sievers et al., 1991). However, the enhanced gravitropic behavior reported in this study leads to the possibility that the actin cytoskeleton may also function in gravitropism by regulating the intensity and/or duration of the initial gravity signal resulting from displacement of amyloplasts. Without such a mechanism in place (i.e. an intact actin cytoskeleton), the continuous proliferation of the initial gravity signal due to unregulated sedimentation of amyloplasts (Yoder et al.2001) could lead to the extensive curvature responses and a failure to attain the correct orientation or gravitropic set point angle (GSA; Firn and Digby, 1997; see below). These assumptions are supported by the fact that Lat B-treated roots maintained on a clinostat continued to bend despite short periods of gravistimulation (Figs. 4, 5, and 7). The identity of the gravity-induced signal that persist upon actin disruption is currently unknown but could include the transient cytoplasmic calcium and pH changes that occur immediately after gravistimulation (Scott and Allen, 1999; Fasano et al., 2001; Johannes et al., 2001; Plieth and Trewavas, 2002). In Arabidopsis, roots of starchless mutants that are less responsive to gravity (Kiss et al., 1989) display lower rates of amyloplast sedimentation (MacCleary and Kiss, 1999) and a markedly reduced gravity-dependent columella pH change (Fasano et al., 2001). Studies are under way to determine whether an altered cytoskeleton modifies the duration and intensity of pH fluxes in graviresponding roots.
The enhanced curvature responses of roots to actin disruption could alternatively be indicative of a passive and unspecific role of the actin cytoskeleton in gravitropism. A disrupted actin network could simply facilitate the displacement of amyloplasts (Yoder et al., 2001), and subsequent reformation of the actin network upon removal of Lat B could explain the enhanced root gravitropic curvature that we observed in this study. Interestingly, low (nanomolar) concentrations of Lat B cause an even stronger promotive effect on root gravitropism (G. Hou and E.B. Blancaflor, unpublished data). Such low doses of Lat B could specifically disrupt the fine and dynamic actin filaments in the columella cells that have been proposed to function in gravity sensing (Volkmann et al., 1999). The rapid reformation of this population of dynamic actin upon withdrawal of Lat B could consequently lead to extended gravity responses by stabilizing amyloplasts displacement and preventing the sensing mechanism from resetting. Future studies will focus on examining the reformation of the actin network in the columella cells of clinostat-grown roots treated with Lat B to confirm this possibility.
Surprisingly, complete reformation of the actin cytoskeleton in the elongation zone 4 and 12 h after removing roots from Lat B did not occur. At these time points, extensive curvature responses were already apparent (Fig. 5). Furthermore, roots exposed to higher concentrations of Lat B exhibited significant swelling but were still capable of differential cellular growth (see Fig. 2D). These results indicate that whereas significant polarized (i.e. linear) cell growth is dependent on an intact actin cytoskeleton (Blancaflor, 2000; Wasteneys, 2000; Baluška et al., 2001), differential cellular growth during gravitropism could be regulated by a different mechanism that is independent of the actin cytoskeleton (Blancaflor and Hasenstein, 1997; Yamamoto and Kiss, 2002).
The enhanced gravitropic responses that we report in this study could also have important implications on gravity signal transmission for which there is accumulating molecular genetic evidence for auxin involvement (for review, see Muday, 2001; Chen et al., 2002). Auxin regulation of gravitropism has been explained primarily by the Cholodny-Went hypothesis, wherein asymmetric auxin redistribution results in the induction of differential cellular growth leading to the bending response (Chen et al., 2002). The identification of mutants altered in gravitropism has led to the cloning of several components of the auxin transport machinery including several members of the PIN gene family of auxin efflux carriers (Chen et al., 1998; Friml and Palme, 2002). Immunolocalization studies have demonstrated asymmetric distribution of auxin efflux carriers in roots and a rapid relocalization of PIN3 in the columella cells of Arabidopsis upon gravistimulation (Friml et al., 2002). The NPA-binding protein, a component of the polar auxin transport machinery, is associated with the actin cytoskeleton (Butler et al., 1998; Hu et al., 2001), and recent evidence demonstrates that the rapid recycling and asymmetric distribution of PIN is actin dependent (Geldner et al., 2001). Interestingly, a new class of Arabidopsis mutants (AtMDR) related to multidrug resistance (MDR) genes of animals is impaired in auxin transport (Noh et al., 2002). These mutants display faster and stronger hypocotyl gravitropic responses (Edgar Spalding [University of Wisconsin], personal communication), which have similar kinetics to Arabidopsis hypocotyls treated with Lat B (Yamamoto and Kiss, 2002). The inhibition of the strong curvature responses of Lat B-treated roots exposed to NPA (Fig. 8) further implicates the involvement of polar auxin transport in the regulation of gravitropism by the actin cytoskeleton (Muday, 2000). It would be interesting to know how polar auxin transport is modified in roots that exhibit hypergravitropic responses upon actin disruption.
In our experiments, untreated roots never reached an angle of 90° despite starting at the vertical position (Fig. 2). Similar results were observed in roots of Sinapsis spp. that were grown under comparable conditions (i.e. roots grown in moist air). However, when Sinapsis spp. roots were gravistimulated in soil, they would eventually reorient to the vertical (Bennet-Clark et al., 1959). These results could be explained based on the concept of GSA, which proposes that plant organs possess a mechanism that allows them to maintain a stable gravitropic position. The GSA of an organ could be regulated by both developmental and environmental factors (Digby and Firn, 1995), however, the exact mechanisms by which GSA is determined is unclear. Our results showing an enhancement of root curvature when actin is disrupted suggests that the cytoskeleton maybe an important factor in determining the variability in GSA displayed by different plant organs. The experimental approach presented in this study could form the basis for the formulation of cell-based models for incorporating the GSA concept into existing models of gravitropism (Firn and Digby, 1997).
An important result that we show in this study is that the localized application of Lat B to the cap region of maize roots was sufficient to induce the strong curvature responses of roots subjected to extended clinorotation. Such strong curvature responses were not observed when Lat B was applied to the elongation zone (see Fig. 7). These results further substantiate the importance of the root cap in processes related to directional root growth and points to the actin cytoskeleton in the cap as an important regulatory element in processes related to gravitropism. Although several reports have demonstrated the presence of actin networks in the root columella (White and Sack, 1990; Driss-Ecole et al., 2000; Collings et al., 2001; Yoder et al., 2001), the state of actin organization and its interaction with amyloplasts within the columella cells is still uncertain (Blancaflor, 2002). A challenge for the future will be to observe actin (re-)organization in columella cells and to correlate these changes with amyloplast sedimentation, auxin transport patterns, and curvature development.
In conclusion, our study reports on some interesting gravity-related responses in roots with a disrupted cap actin cytoskeleton. These responses were unexpectedly manifested as enhanced curvature that was exaggerated upon extended periods of clinorotation. These findings have important implications for current models on how the actin cytoskeleton is involved in the perception and transduction of gravity. Our results indicate that one function of the actin cytoskeleton in gravitropism lies in its control of the duration or amplitude of an early signaling event induced by gravistimulation. A major challenge will be to identify which component in the gravitropic signal transduction chain is modified by actin disruption. The experiments described here provide a simple but promising approach to help identify the cytoskeletal basis of gravitropic signaling in higher plants.
MATERIALS AND METHODS
Plant Material and Drug Treatments
Seeds of maize (Zea mays cv Merit), Medicago truncatula (ecotype Jemalong line A17), and Linum usitatissimum were germinated and grown vertically in opaque plastic trays at 22°C as previously described (Blancaflor and Hasenstein, 1993). Stock solutions of Lat B (5 mm; Calbiochem, La Jolla, CA) and oryzalin (10 mm; Chem Services, West Chester, PA) were prepared in 100% (v/v) DMSO (Sigma-Aldrich, St. Louis). Working solutions of Lat B and oryzalin were prepared by adding the appropriate volume of stock solution to deionized water. A corresponding amount of DMSO was used as solvent controls. Seedlings that were about 3 cm long were selected (3 d old for maize and 2 d old for M. truncatula and L. usitatissimum) and placed in 1.5-mL microfuge tubes containing working solutions of the drugs, with the terminal 15 mm of the roots immersed in the solutions. After 1 h, seedlings with straight roots were mounted in 9-cm petri dishes lined with two layers of wet filter paper and one layer of brown paper towel. Roots were allowed to grow vertically for an additional 30 min before performing the growth and microscopy analyses described below.
Growth, Curvature, and Analysis of Gravitropic Sensitivity
Growth of vertically oriented roots was monitored by capturing images of the roots every 10 min for 10 h using a C2400–75i camera (Hamamatsu, Tokyo) running the Metamorph 5.0 image acquisition software (Universal Imaging, West Chester, PA). For curvature measurements, the petri dish was rotated 90°, and images of the roots were captured every 15 min for up to 10 h. The length and angle of the roots were measured from digitized images using Metamorph 5.0. For presentation time determination, roots treated with Lat B and oryzalin were given a brief (5–20 min) horizontal stimulation and rotated on a 1-rpm clinostat. The resulting curvature of the roots at each induction time was measured after 2 h and plotted against the logarithm of the stimulation time. Presentation times were calculated as described previously (Kiss et al., 1996; Blancaflor et al., 1998). Gravisensitivity (S) using the hyperbolic model was estimated as detailed in Perbal et al. (2002).
All experiments were repeated at least three times to ensure reproducibility of measurements. One-way ANOVA was used to test statistical significance and Tukey's honestly significant difference test was used for multiple comparison of means. All statistical analysis including determination of ses was done with SPSS 11.5 software (SPSS, Chicago).
Localized Drug Application and Extended Clinorotation
Localized application of oryzalin and Lat B was performed by immersing polymerized slabs of 1% (w/v) bactoagar in 5-cm petri dishes containing working solutions of the drugs for 2 h or more. After drug incubation, the agar slab was divided into smaller blocks (approximately 1 mm3) using a set of double-edged razor blades carefully mounted to give 1-mm spacing between the edges. Blocks were applied to either the cap or the elongation zone (3 mm from the root tip) and removed after 1 h (see Fig. 6). Roots were allowed to grow vertically for an additional 30 min before performing the curvature and microscopy experiments.
In a separate set of experiments, roots treated with either Lat B or oryzalin were given a 1-h horizontal stimulus and rotated axially on the clinostat. Images of the roots while rotating on the clinostat were captured every 1 h for up to 6 h with a CoolPix 990 digital camera (Nikon, Melville, NY).
Actin Filament and Microtubule Labeling
Labeling of actin filaments was performed as described in Blancaflor and Hasenstein (1997). In brief, the terminal 8 mm of the root were excised with a razor blade, fixed in 2% (v/v) formaldehyde in PME (50 mm PIPES, 4 mm MgSO4, and 10 mm EGTA) buffer, and sectioned at 70 μm with a Vibratome 1000 (Technical Products International, St. Louis). Sections were transferred to glass slides, incubated briefly with a cocktail of wall digesting enzymes, 1% (v/v) Triton X-100, and treated overnight with Alexa Fluor-phalloidin (Molecular Probes, Eugene, OR). After incubation, root sections were washed three times with PME buffer, mounted in glass slides with Mowiol 4–88 (Calbiochem), and immediately observed under a confocal microscope (see below).
To image actin filaments in maize columella cells, the methods described by Collings et al. (2001) were followed. In brief, the terminal 2 mm of the root was attached to a Vibratome, and median longitudinal sections were fixed in PME containing 2% (v/v) glycerol, 0.3 m mannitol, and 300 μm water-soluble MBS (Calbiochem) for 30 min. Sections were incubated in 0.1 μm Alexa Fluor phalloidin, and after 10 min, they were observed under a confocal microscope.
Labeling of microtubules was as described by Blancaflor and Hasenstein (1993). The terminal 8 mm of control and oryzalin-treated roots were placed in separate vials containing 4% (v/v) formaldehyde in PME buffer, pH 6.9 and 5% (v/v) DMSO for 2 h. After obtaining 70-μm median longitudinal sections with a Vibratome, sections were secured onto glass coverslips, digested in a cocktail of wall degrading enzymes, and incubated in 1% (v/v) Triton X-100. After an overnight incubation in monoclonal rat anti-yeast α-tubulin (clone YOL1/34, Accurate Chemicals, Westbury, NY) and a 2-h treatment in secondary antibody (goat anti-rat IgG conjugated to fluorescein isothiocyanate, Sigma-Aldrich), root sections were mounted in phosphate-buffered saline, pH 8.5, containing 20% (w/v) Mowiol 4–88 (CaLatBiochem, La Jolla, CA) and 0.1% (w/v) phenylenediamine before observation with a confocal microscope.
Confocal Microscopy
Microtubules and actin in the root sections were imaged with a confocal laser scanning microscope (1024ES, Bio-Rad Hercules, CA) equipped with a 63×, 1.2 numerical aperture (N.A.) water-immersion objective. Alexa-Fluor and fluorescein isothiocyanate were excited at 488 nm with emission detected at 522 nm. All images were assembled using Adobe Photoshop 5.0LE (Adobe Systems Inc. Mountain View, CA).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permission will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We thank Drs. Rujin Chen and Wayne Versaw (Noble Foundation) for critical comments on the manuscript. We also thank Dr. Gerald Perbal (Universite Pierre et Marie Curie, Paris France) for assistance in the analysis of gravisensitivity using the hyperbolic (H) model shown in Figure 3B.
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NAG 2–1518 to E.B.B.) and by the Noble Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.014423.
LITERATURE CITED
- Baluška F, Hasenstein KH. Root cytoskeleton: its role in perception of and response to gravity. Planta. 1997;203:S69–S78. doi: 10.1007/pl00008117. [DOI] [PubMed] [Google Scholar]
- Baluška F, Hauskrecht M, Barlow PW, Sievers A. Gravitropism of the primary root of maize: a complex pattern of differential cellular growth in the cortex independent of the microtubular cytoskeleton. Planta. 1996;198:310–318. doi: 10.1007/BF00206258. [DOI] [PubMed] [Google Scholar]
- Baluška F, Jasik J, Edelman HG, Salajova T, Volkmann D. Latrunculin B-induced plant dwarfism: Plant cell elongation is F-actin dependent. Dev Biol. 2001;231:113–124. doi: 10.1006/dbio.2000.0115. [DOI] [PubMed] [Google Scholar]
- Baluška F, Kreibaum A, Vitha S, Parker JS, Barlow PW, Sievers A. Central root cap cells are depleted of endoplasmic microtubules and actin microfilament bundles: implications for their role as gravity-sensing statocytes. Protoplasma. 1997;196:212–223. doi: 10.1007/BF01279569. [DOI] [PubMed] [Google Scholar]
- Bao Y, Kost B, Chua N-H. Reduced expression of α-tubulin genes in Arabidopsis thaliana specifically affects root growth and morphology, root hair development and root gravitropism. Plant J. 2001;28:145–157. doi: 10.1046/j.1365-313x.2001.01142.x. [DOI] [PubMed] [Google Scholar]
- Bennet-Clark TA, Younis AF, Esnault R. Geotropic behavior of roots. J Exp Bot. 1959;10:69–86. [Google Scholar]
- Blancaflor EB. Cortical actin filaments potentially interact with cortical microtubules in regulating polarity of cell expansion in primary roots of maize (Zea mays L.) J Plant Growth Regul. 2000;19:406–414. doi: 10.1007/s003440000044. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB. The cytoskeleton and gravitropism in higher plants. J Plant Growth Regul. 2002;21:120–136. doi: 10.1007/s003440010041. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH. Organization of cortical microtubules in graviresponding maize roots. Planta. 1993;191:231–237. doi: 10.1007/BF00199754. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH. Time course and auxin sensitivity of microtubule reorientation in maize roots. Protoplasma. 1995;185:72–82. doi: 10.1007/BF01272755. [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Hasenstein KH. The organization of the actin cytoskeleton in vertical and graviresponding primary roots of maize. Plant Physiol. 1997;113:1447–1455. doi: 10.1104/pp.113.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonsirichai K, Guan C, Chen R, Masson PH. Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu Rev Plant Biol. 2002;53:421–447. doi: 10.1146/annurev.arplant.53.100301.135158. [DOI] [PubMed] [Google Scholar]
- Brown AH, Dahl AO, Chapman DK. Limitation on the use of the horizontal clinostat as a gravity compensator. Plant Physiol. 1976;58:127–130. doi: 10.1104/pp.58.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JH, Hu S, Brady SR, Dixon MW, Muday GK. In vitro and in vivo evidence for actin association of the naphthylphthalamic acid-binding protein from zucchini hypocotyls. Plant J. 1998;13:291–301. doi: 10.1046/j.1365-313x.1998.00017.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Guan C, Boonsirichai K, Masson PH. Complex physiological and molecular processes underlying root gravitropism. Plant Mol Biol. 2002;49:305–317. [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC1 gene encodes a component of the polar auxin transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15122–15177. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Zsuppan G, Allen NS, Blancaflor EB. Demonstration of prominent actin filaments in the root columella. Planta. 2001;212:392–403. doi: 10.1007/s004250000406. [DOI] [PubMed] [Google Scholar]
- Dedolph RR, Dipert MH. The physical basis of gravity stimulus nullification by clinostat rotation. Plant Physiol. 1971;47:756–764. doi: 10.1104/pp.47.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby J, Firn RD. The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture. Plant Cell Environ. 1995;18:1434–1440. doi: 10.1111/j.1365-3040.1995.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Driss-Ecole D, Vassy J, Rembur J, Giuvarc'h A, Proteau M, Dewitte W, Perbal G. Immunolocalization of actin in root statocytes of Lens culinaris L. J Exp Bot. 2000;51:512–528. doi: 10.1093/jexbot/51.344.521. [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao T-h, Gilroy S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firn RD, Digby J. Solving the puzzle of gravitropism-has a lost piece been found? Planta. 1997;203:S159–S163. doi: 10.1007/pl00008104. [DOI] [PubMed] [Google Scholar]
- Friml J, Palme K. Polar auxin transport-old questions and new concepts? Plant Mol Biol. 2002;49:273–284. [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendger K, Palme K. Lateral relocation of auxin efflux regulator AtPIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof Y-D, Jurgens G, Palme K. Auxin transport inhibitors block PIN cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Hasenstein KH, Blancaflor EB, Lee JS. The microtubule cytoskeleton does not integrate auxin transport and gravitropism in maize roots. Physiol Plant. 1999;105:729–738. doi: 10.1034/j.1399-3054.1999.105418.x. [DOI] [PubMed] [Google Scholar]
- Hejnowicz Z, Sondag C, Alt W, Sievers A. Temporal course of graviperception in intermittently stimulated cress roots. Plant Cell Environ. 1998;21:1293–1300. doi: 10.1046/j.1365-3040.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Hensel W. Cytochalasin B affects the structural polarity of statocytes from cress roots Lepidium sativum L. Protoplasma. 1985;129:178–187. doi: 10.1007/BF01279915. [DOI] [PubMed] [Google Scholar]
- Himmelspach R, Nick P. Gravitropic microtubule reorientation can be uncoupled from growth. Planta. 2001;212:184–189. doi: 10.1007/s004250000378. [DOI] [PubMed] [Google Scholar]
- Hu S, Brady SR, Kovar DR, Staiger CJ, Clark GB, Roux SJ, Muday GK. Identification of plant actin-binding proteins by F-actin affinity chromatography. Plant J. 2001;24:127–137. doi: 10.1046/j.1365-313x.2000.00852.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 1993;102:1203–1210. doi: 10.1104/pp.102.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes E, Collings DA, Rink JC, Allen NS. Cytoplasmic pH dynamics in maize pulvinal cells induced by gravity vector changes. Plant Physiol. 2001;127:119–130. doi: 10.1104/pp.127.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HJ, Bae YS, Lee JS. Role of auxin induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Morita MT, Tasaka M. Role of endodermal cell vacuoles in shoot gravitropism. J Plant Growth Regul. 2002;21:113–119. doi: 10.1007/s003440010047. [DOI] [PubMed] [Google Scholar]
- Kiss JZ. Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Reduced gravitropic sensitivity in roots of a starch-deficient mutant of Nicotiana sylvestris. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- Kost B, Mathur J, Chua N-H. Cytoskeleton in plant development. Curr Opin Plant Biol. 1999;2:462–470. doi: 10.1016/s1369-5266(99)00024-2. [DOI] [PubMed] [Google Scholar]
- MacCleary SA, Kiss JZ. Plastid sedimentation kinetics in roots of wild-type and starch-deficient mutants of Arabidopsis. Plant Physiol. 1999;120:183–192. doi: 10.1104/pp.120.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Zieschang HE, Sievers A. Differential proton secretion in the apical elongation zone caused by gravistimulation is induced by a signal from the cap. Plant Cell Environ. 1996;19:1408–1414. doi: 10.1111/j.1365-3040.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Muday GK. Maintenance of asymmetric cellular localization of an auxin transport protein through interaction with the actin cytoskeleton. J Plant Growth Regul. 2000;19:385–396. doi: 10.1007/s003440000041. [DOI] [PubMed] [Google Scholar]
- Muday GK. Auxins and tropisms. J Plant Growth Regul. 2001;20:226–243. doi: 10.1007/s003440010027. [DOI] [PubMed] [Google Scholar]
- Nick P, Godbole R, Wang QY. Probing rice gravitropism with cytoskeletal drugs and cytoskeletal mutants. Biol Bull. 1997;192:141–143. doi: 10.2307/1542589. [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2002;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal G, Driss-Ecole D, Tewinkle M, Volkmann D. Statocyte polarity and gravisensitivity in seedling roots grown in microgravity. Planta. 1997;203:S57–S62. doi: 10.1007/pl00008115. [DOI] [PubMed] [Google Scholar]
- Perbal G, Jeune B, Lefranc A, Carnero-Diaz E, Driss-Ecole D. The dose-response curve of the gravitropic reaction: a re-analysis. Physiol Plant. 2002;114:336–342. doi: 10.1034/j.1399-3054.2002.1140302.x. [DOI] [PubMed] [Google Scholar]
- Perera I, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-triphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera I, Heilman I, Chang SC, Boss WF, Kaufman PB. A role for inositol 1,4,5-triphosphate in gravitropic signaling and retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiol. 2001;125:1499–1507. doi: 10.1104/pp.125.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Bottger M. Auxin induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Trewavas AJ. Reorientation of seedlings in the earth's gravitational field induces cytosolic calcium transients. Plant Physiol. 2002;129:786–796. doi: 10.1104/pp.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, De Long AM, Muday GK. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response and lateral root growth. Plant Cell. 2001;13:1683–1697. doi: 10.1105/TPC.010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Scott AC, Allen NS. Changes in cytosolic pH within Arabidopsis root columella cells play a role in the early signaling pathway for root gravitropism. Plant Physiol. 1999;121:1291–1298. doi: 10.1104/pp.121.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Chen R, Masson PH. ARG1 (Altered response to gravity) encodes a DNAJ-like protein that potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA. 1999;96:1140–1145. doi: 10.1073/pnas.96.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers A, Buchen B, Volkmann D, Hejnowicz Z. Role of cytoskeleton in gravity perception. In: Lloyd CW, editor. The Cytoskeletal Basis of Plant Growth and Form. London: Academic Press; 1991. pp. 169–182. [Google Scholar]
- Sievers A, Kruse S, Kuo-Huang L, Wendt M. Statoliths and microfilaments in plant cells. Planta. 1989;179:275–278. doi: 10.1007/BF00393699. [DOI] [PubMed] [Google Scholar]
- Sievers A, Sondag C, Trebacz K, Hejnowicz Z. Gravity induced changes in intracellular potentials in statocytes of cress roots. Planta. 1995;197:392–398. doi: 10.1007/BF00202662. [DOI] [PubMed] [Google Scholar]
- Stankovic B, Volkmann D, Sack FD. Autonomic straightening after gravitropic curvature of cress roots. Plant Physiol. 1998a;117:893–900. doi: 10.1104/pp.117.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic B, Volkmann D, Sack FD. Autotropism, automorphogenesis, and gravity. Physiol Plant. 1998b;102:328–335. doi: 10.1034/j.1399-3054.1998.1020222.x. [DOI] [PubMed] [Google Scholar]
- Staves MP, Wayne R, Leopold AC. Cytochalasin D does not inhibit gravitropism in roots. Am J Bot. 1997;84:1530–1535. [PubMed] [Google Scholar]
- Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999;4:103–107. doi: 10.1016/s1360-1385(99)01376-x. [DOI] [PubMed] [Google Scholar]
- Vitha S, Yang M, Kiss JZ, Sack FD. Light promotion of hypocotyls gravitropism of a starch-deficient tobacco mutant correlates with plastid enlargement and sedimentation. Plant Physiol. 1998;116:495–502. doi: 10.1104/pp.116.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann D, Baluška F. Actin cytoskeleton related to gravisensing in higher plants. In: Staiger C, Baluška F, Volkmann D, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 557–571. [Google Scholar]
- Volkmann D, Baluška F, Lichtscheidl IK, Driss-Ecole D, Perbal G. Statoliths motions in gravity-perceiving plant cells: Does actomyosin counteract gravity? FASEB J. 1999;13:S143–S147. doi: 10.1096/fasebj.13.9001.s143. [DOI] [PubMed] [Google Scholar]
- Wang QY, Nick P. The auxin response of actin is altered in the rice mutant Yin-yang. Protoplasma. 1998;200:154–162. doi: 10.1007/BF01282290. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO. The cytoskeleton and growth polarity. Curr Opin Plant Biol. 2000;3:503–511. doi: 10.1016/s1369-5266(00)00120-5. [DOI] [PubMed] [Google Scholar]
- Weise SE, Kuznetsov OA, Hasenstein KH, Kiss JZ. Curvature in Arabidopsis inflorescence stems is limited to the regions of amyloplasts displacement. Plant Cell Physiol. 2000;41:702–709. doi: 10.1093/pcp/41.6.702. [DOI] [PubMed] [Google Scholar]
- Wendt M, Kuo-Huang L, Sievers A. Gravitropic bending of cress roots without contact between amyloplasts and complexes of endoplasmic reticulum. Planta. 1987;172:321–329. doi: 10.1007/BF00398660. [DOI] [PubMed] [Google Scholar]
- White RG, Sack FD. Actin microfilaments in presumptive statocytes of roots caps and coleoptiles. Am J Bot. 1990;77:17–26. [PubMed] [Google Scholar]
- Wolverton C, Ishikawa H, Evans ML. The kinetics of root gravitropism: dual motors and sensors. J Plant Growth Regul. 2002a;21:102–112. doi: 10.1007/s003440010053. [DOI] [PubMed] [Google Scholar]
- Wolverton C, Mullen JL, Ishikawa H, Evans ML. Root gravitropism in response to a signal originating outside of the cap. Planta. 2002b;215:153–157. doi: 10.1007/s00425-001-0726-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kiss JZ. Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiol. 2002;128:669–681. doi: 10.1104/pp.010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Pyke KA, Kiss JZ. Reduced gravitropism in inflorescence stems and hypocotyls, but not roots, of Arabidopsis mutants with large plastids. Physiol Plant. 2002;114:627–636. doi: 10.1034/j.1399-3054.2002.1140417.x. [DOI] [PubMed] [Google Scholar]
- Yoder TL, Zheng H-Q, Todd P, Staehelin LA. Amyloplast sedimentation dynamics in maize columella cells support a new model for the gravity-sensing apparatus of roots. Plant Physiol. 2001;125:1045–1060. doi: 10.1104/pp.125.2.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H-Q, Staehelin LA. Nodal ER, a novel form of ER found exclusively in gravity-sensing columella cells. Plant Physiol. 2001;125:252–265. doi: 10.1104/pp.125.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]