Abstract
trans-Zeatin is a major and ubiquitous cytokinin in higher plants. cis-Zeatin has traditionally been viewed as an adjunct with low activity and rare occurrence. Recent reports of cis-zeatin and its derivatives as the predominant cytokinin components in some plant tissues may call for a different perspective on cis-isomers. The existence of a maize (Zea mays) gene (cisZOG1) encoding an O-glucosyltransferase specific to cis-zeatin (R.C. Martin, M.C. Mok, J.E. Habben, D.W.S. Mok [2001] Proc Natl Acad Sci USA 98: 5922–5926) lends further support to this view. Results described here include the isolation of a second maize cisZOG gene, differential expression of cisZOG1 and cisZOG2, and identification of substantial amounts of cis-isomers in maize tissues. The open reading frame of cisZOG2 has 98.3% identity to cisZOG1 at the nucleotide level and 97.8% at the amino acid level. The upstream regions contain common and unique segments. The recombinant enzymes have similar properties, Km values of 46 and 96 μm, respectively, for cis-zeatin and a pH optimum of 7.5. Other cytokinins, including N6-(Δ2-isopentenyl)adenine, trans-zeatin, benzyladenine, kinetin, and thidiazuron inhibited the reaction. Expression of cisZOG1 was high in maize roots and kernels, whereas cisZOG2 expression was high in roots but low in kernels. cis-Zeatin, cis-zeatin riboside, and their O-glucosides were detected in all maize tissues, with immature kernels containing very high levels of the O-glucoside of cis-zeatin riboside. The results are a clear indication that O-glucosylation of cis-zeatin is a natural metabolic process in maize. Whether cis-zeatin serves as a precursor to the active trans-isomer or has any other unique function remains to be demonstrated.
Cytokinins are plant hormones regulating cell division and a range of developmental events such as bud formation, leaf expansion, senescence, seed germination, and chloroplast formation (Mok, 1994). trans-Zeatin is a major and ubiquitous cytokinin in higher plants. Earlier cytokinin analyses detected cis-zeatin and its derivatives in trace amounts in some plants, but due to their low activity (Schmitz et al., 1972), cis-isomers were viewed as adjunct to trans-isomers. Recent analyses, however, showed that the cis-isomers can be the dominant cytokinins at particular stages of development in plants such as chickpea (Cicer arietinum) and lupine (Lupinus albus; Emery et al., 1998, 2000). Moreover, the presence of cis-isomers was associated with male sterility in Mercurialis spp. flowers (Louis et al., 1990; Durand and Durand, 1994). These are indications that cis-isomers may have unique physiological functions. The ability to regulate the levels of cis-zeatin is evidenced by the maize (Zea mays) cisZOG1 gene, encoding an O-gluco-syltransferase with specificity to cis-zeatin (Martin et al., 2001).
O-Glucosylation is a major step in the metabolism of trans-zeatin (Mok and Mok, 2001). The resulting O-glucosides seem to serve as storage compounds and are resistant to degradation by cytokinin oxidases (Armstrong, 1994). O-Glucosides are found in xylem sap and are likely also transport forms of zeatin (Letham, 1994). Phaseolus spp. enzymes and genes involved in conversion of trans-zeatin to its O-glucoside and O-xyloside have been characterized in our laboratories (Turner et al., 1987; Dixon et al., 1989; Martin et al., 1999a, 1999b). These bean enzymes have either no or very low affinity to cis-zeatin. The discovery of the maize cisZOG1 gene is indicative of the existence of similar conversions for trans- and cis-zeatin, but mediated by isomer-specific enzymes. This idea is compatible with findings reported here, the isolation of a second cisZOG gene, the preference of both enzymes for cis-zeatin, and substantial levels of cis-zeatin and its O-glucoside in maize tissues.
RESULTS
Isolation of cisZOG2
Screening of a genomic library of the maize inbred B73 with cisZOG1 led to identification of two different clones. One clone contained a partial sequence of the open reading frame (ORF) of cisZOG1 and an upstream region of 3.5 kb. The other genomic fragment contained a gene designated as cisZOG2 plus a 2.5-kb upstream fragment. The sequences have been deposited in the GenBank database (AF318075 for cisZOG1 and AY082660 for cisZOG2). The upstream sequence of cisZOG1 can be found under AF466203. Neither cisZOG1 nor cisZOG2 have any introns (see GenBank sequences). The amino acid sequence of cisZOG2 is 98% identical to that of cisZOG1, but the protein is shorter by four amino acids (Fig. 1A). However, the upstream regions of the two genes are different, with large gaps in cisZOG2, although there are also highly similar regions (Fig. 1B).
Figure 1.
Comparison of the cisZOG1 and cisZOG2 genes. A, Alignment of the deduced amino acid sequences. B, Diagrammatic presentation of the upstream sequences. Alignment was performed with gcg software (Genetics Computer Group, Madison, WI).
Biochemical Characterization of cisZOG1 and cisZOG2
The ORFs of both genes were cloned into the modified expression vector Ptrc99A producing recombinant proteins with a His tag at the N terminus. Enzymes were purified based on their poly-his tag on a Ni affinity column and used to study the biochemical properties. The enzymes have a theoretical pI of 5.4 and mass of 52 kD. The recombinant proteins catalyze the formation of cis-zeatin-O-glucoside with a pH optimum of 7.5. The Km for cis-zeatin is about 46 μm for cisZOG1 and 96 μm for cisZOG2. UDP-Glc is the sugar donor with Km values of 0.11 and 0.59 mm for cisZOG1 and cisZOG2, respectively. When UDP-Xyl was used in place of UDP-Glc, no product was formed. This is another property distinguishing these enzymes from the trans-zeatin O-glucosyl-transferases of beans, which can transfer the Xyl from UDP-Xyl albeit at a lower affinity than UDP-Glc (Dixon et al., 1989; Martin et al., 1999a,1999b). Using a larger quantity of purified enzyme, formation of trans-[3H]zeatin-O-glucoside was mediated by both maize enzymes under standard reaction conditions. However, conversion of cis-zeatin was much higher than that of trans-zeatin under the same conditions. For instance, under assay conditions that resulted in 44% conversion of cis-zeatin to its glucoside, only 4% of trans-zeatin was glucosylated.
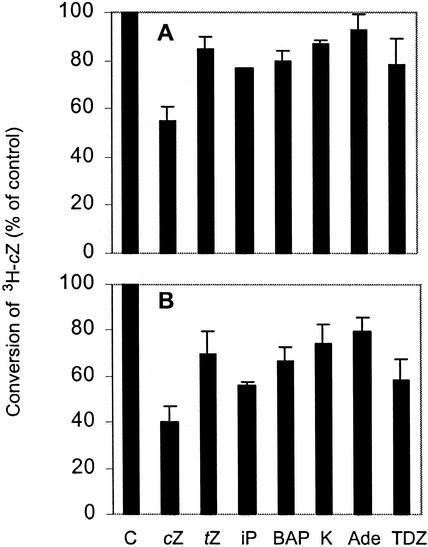
To examine possible affinity to or interference by other cytokinins, competition experiments were conducted. Conversion of cis-zeatin to its glucoside was lowered by all adenine-type cytokinins tested (Fig. 2). Although the inhibition seemed to be very moderate for the large excess of unlabeled cis-zeatin, this would be expected for an enzyme with Km for cis-zeatin of 46 μm. Interestingly, thidiazuron, a very active phenylurea-type cytokinin (Mok et al., 1982), also decreased conversion of cis-[3H]zeatin. Thidiazuron also inhibits cytokinin oxidase activity (Armstrong, 1994) and was shown to be a competitive inhibitor of the maize oxidase (Bilyeu et al., 2000). Whether the inhibition of glucosyltransferase activity observed here is competitive or noncompetitive remains to be determined, but it appears that thidiazuron interferes with several cytokinin-specific but distinct enzymatic reactions.
Figure 2.
Effects of various cytokinins on conversion of cis-[3H]zeatin to its glucoside by cisZOG1. Enzyme activity was assayed by incubating 0.75 μm cis-[3H]zeatin (specific activity 3.1 Ci mmol−1), 200 μm (A) or 400 μm (B) cytokinin, 4 mm UDP-Glc, 10 μL of recombinant protein, and 0.17 m MgCl2 in 150 μL 0.17 m Tris, pH 7.5, for 30 min at 27°C. C, Control (no cytokinin added); cZ, cis-zeatin; tZ, trans-zeatin; iP, N6-(Δ2-isopentenyl) adenine; BAP, N6-benzyladenine; K, kinetin; Ade, adenine; and TDZ, thidiazuron.
Expression of cisZOG1 and cisZOG2 in Maize Tissues
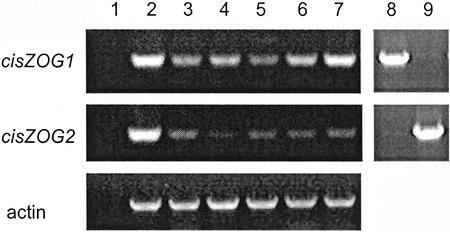
To examine whether there is differential expression of the two genes in maize tissues, mRNA levels of cisZOG1 and cisZOG2 were compared by reverse transcriptase (RT)-PCR (Fig. 3). Gene-specific primers were used, as shown by the differential amplification of the cisZOG1 and cisZOG2 plasmid controls (Fig. 3, lanes 8 and 9), and amounts were adjusted using the actin gene message as control. Both cisZOG genes were highly expressed in roots (Fig. 3, lane 2) and very weakly expressed in stems and leaves (Fig. 3, lanes 3 and 4). Differential expression was observed in developing kernels, with high expression of cisZOG1, particularly in large seeds, and low expression of cisZOG2 in all kernel sizes sampled (Fig. 3, lanes 5 through 7). These results indicate a divergence in gene expression, which may be reflected in the differences in the upstream sequences (Fig. 1) harboring the promoter.
Figure 3.
Gene expression analysis of cisZOG1, cisZOG2, and actin (control) in maize tissues as determined by RT-PCR with gene-specific primers. Lane 1, Water control; lane 2, roots of 2-week-old seedlings; lanes 3 and 4, stems and leaves of 2-week-old plants; lanes 5 through 7, kernels 1, 2, and 3 weeks after pollination; lane 8, cisZOG1 plasmid; and lane 9, cisZOG2 plasmid.
Identification of Cytokinins in Maize Tissues
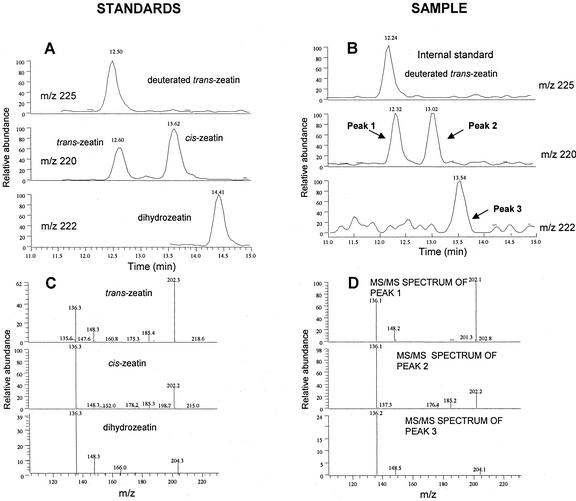
Cytokinins were purified from maize tissues and quantified by liquid chromatography-mass spectrometry (LC-MS) with equal attention to trans- and cis-zeatin and their derivatives. Cytokinins with cis-hydroxylated side chains have not been reported previously for maize and are usually not part of the repertoire of traditional analyses. The methodologies can clearly discriminate between the two isomers, as shown in Figure 4. The LC method causes clean separation of the two compounds (standards as well as samples), and their mass spectra show significant differences in the relative intensities of fragments. The analyses revealed that cis-zeatin was present in roots, stems, leaves, unfertilized cobs, and kernels (Table I) along with its riboside and nucleotide. The O-glucosides of cis-isomers were found in roots, young cobs, and kernels, which is compatible with the expression of cisZOG1 and cisZOG2 in maize tissues (Fig. 3). Comparing the two groups of cytokinins, cis-isomers were more prevalent in roots, stems, and leaves, whereas trans-isomers were more abundant in the kernels. The levels of other types of cytokinins, dihydrozeatin and isopentenyladenine derivatives, were relatively low.
Figure 4.
Chromatographical profiles and mass spectra of standards (A and C) and a representative biological sample obtained from LC-MS/MS (B and D). The mass spectra of peaks 1, 2, and 3 correspond to those of standards of trans-zeatin, cis-zeatin, and dihydrozeatin, respectively.
Table I.
Cytokinin concentrationsain roots, stems, leaves, unfertilized cobs, and kernels of maize
| Cytokinin | Concentration
|
||||
|---|---|---|---|---|---|
| Roots | Stems | Leaves | Cobs | Kernels | |
| pmol g−1 fresh wt | |||||
| Zb | <DLc | <DL | 4.5 | 5.5 | 25.3 |
| ZR | <DL | 1.1 | 1.9 | 13.4 | 180.0 |
| ZRP | 2.6 | <DL | 1.0 | 13.9 | 589.0 |
| Z-9G | 4.6 | <DL | <DL | 23.5 | 31.9 |
| Z-OG | <DL | <DL | <DL | 2.7 | 10.5 |
| ZR-OG | <DL | <DL | <DL | <DL | 16.0 |
| cZ | 2.5 | 28.1 | 26.6 | 9.7 | 5.3 |
| cZR | 6.5 | 39.5 | 26.5 | 21.4 | 25.3 |
| cZRP | 1.2 | 7.0 | 6.1 | 7.1 | 25.8 |
| cZ-OG | 55.2 | 7.6 | 8.2 | 10.0 | 23.5 |
| cZR-OG | 18.8 | <DL | <DL | 12.2 | 226.9 |
| DZ | <DL | <DL | <DL | <DL | <DL |
| DZR | <DL | <DL | 1.9 | 0.8 | 8.9 |
| DZRP | <DL | <DL | <DL | <DL | 6.3 |
| DZ-9G | <DL | <DL | <DL | <DL | <DL |
| DZ-OG | <DL | <DL | <DL | <DL | <DL |
| DZR-OG | 1.0 | <DL | 2.1 | 0.4 | 34.2 |
| iP | 0.9 | <DL | 0.8 | 2.1 | 6.9 |
| iPR | 1.2 | 3.4 | 2.2 | 2.9 | 34.4 |
| iPRP | <DL | <DL | 4.2 | <DL | 34.3 |
| iP-9G | 10.4 | <DL | <DL | <DL | <DL |
Averages of two replicate samples except for cobs (one sample).
Z, (trans-)zeatin; ZR, zeatin riboside; ZRP, zeatin riboside phosphate; Z-9G, zeatin-9-glucoside; Z-OG, zeatin-O-glucoside; ZR-OG, ribosylzeatin-O-glucoside; cZ, cis-zeatin; cZR, cis-zeatin riboside; cZRP, cis-zeatin riboside phosphate; cZ-OG, cis-zeatin-O-glucoside; cZR-OG, cis-ribosylzeatin-O-glucoside; DZ, dihydrozeatin; DZR, dihydrozeatin riboside; DZRP, dihydrozeatin riboside phosphate; DZ-9G, dihydrozeatin-9-glucoside; DZ-OG, dihydrozeatin-O-glucoside; DZR-OG, dihydroribosylzeatin-O-glucoside; iP, N6-(Δ2-isopentenyl)adenine; iPR, N6-(Δ2-isopentenyl)adenosine; iPRP, N6-(Δ2-isopentenyl)adenosine phosphate; iP-9G, N6-(Δ2-isopentenyl)adenine-9-glucoside.
<DL, Concentration is smaller than the detection limit.
DISCUSSION
The clear preference of the two glucosyltransferases for cis-zeatin and the presence of O-glucosides of cis-zeatin and its riboside in maize lead to the obvious conclusion that cis-zeatin O-glucosylation is a natural metabolic process in maize. The Km values for cis-zeatin and UDP-Glc are well within the range expected of cytokinin metabolic enzymes. For example, the recombinant cytokinin oxidase of maize has Km values of 46 μm for cis-zeatin and 14 μm for trans-zeatin (Bilyeu et al., 2000), whereas other native cytokinin oxidases have reported Km values for trans-zeatin ranging from 25 to 33 μm (Armstrong, 1994). The Km of the native zeatin O-glucosyltransferase of P. lunatus is 28 μm for trans-zeatin and 0.2 mm for UDP-Glc (Dixon et al., 1989). The difference in Km between cisZOG1 and cisZOG2 may be related to the gap of four amino acids in cisZOG2 or in the additional amino acid differences between the two enzymes, resulting in slight differences in folding. With larger amounts of recombinant enzyme available, trans-zeatin was also found to be a substrate, even though in previous tests with less pure enzyme conversion was found to be negligible (Martin et al., 2001).
The O-glycosyltransferases we have identified thus far have high substrate specificity, differentiating between trans- and cis-zeatin and between sugar donors UDP-Glc and UDP-Xyl (Dixon et al., 1989; Martin et al., 1999a, 1999b). The genes encoding these enzymes have higher homology to each other than to any other sequences in the GenBank. Among glycosyltransferase genes, the cytokinin O-glycosyltransferases constitute a distinct branch in the evolutionary tree (Li et al., 2001). The upstream regions of the cisZOG1 and cisZOG2 ORFs have stretches with high identity but also unique segments. These unique sequences may have contributed to the differences in gene expression in maize kernels (Fig. 3).
It was somewhat surprising to find high levels of cis-zeatin derivatives in maize tissues because maize has been the subject of numerous cytokinin analyses in the past and the occurrence of cis-zeatin has, to our knowledge, never been reported. However, in rice (Oryza sativa), another monocot, O-glucosyl-cis-zeatin and its riboside were found (Takagi et al., 1989). In fact, the number of species in which cis-zeatin was detected has increased steadily over the years and now includes potato (Solanum tuberosum; Mauk and Langille, 1978; Suttle and Banowetz, 2000), hops (Watanabe et al., 1981), sweet potato (Ipomoea batatas; Hashizumi et al., 1982), rice (Takagi et al., 1985), wheat (Triticum aestivum; Parker et al., 1989), oats (Avena sativa; Parker et al., 1989), Mercurialis spp. (Durand and Durand, 1994), chickpea (Emery et al., 1998), lupins (Emery et al., 2000), and tobacco (Nicotiana tabacum; Dobrev et al., 2002). Although many other studies did not report the presence of cis-isomers, that is more likely due to their exclusion from cytokinin analyses rather than to their absence from the tissues.
The origin of high level of cis-zeatin is not known. Hydrolysis of cytokinin-containing tRNAs (Skoog and Armstrong, 1970) is a potential source, but it is probably insufficient to account for the substantial amounts detected in more recent analyses (for more detailed discussion, see Mok and Mok, 2001). Another possibility is direct synthesis, either through cis-hydroxylation of isopentenyladenine (riboside or nucleotide) by an enzyme similar to the trans-hydroxylase of cauliflower microsomes (Chen and Leisner, 1984), or through attachment of a cis-hydroxylated precursor side chain to AM(D,T)P. Formation of trans-zeatin riboside 5′-phosphate was demonstrated by incubation of the Tzs protein of Agrobacterium tumefaciens in the presence of AMP and 4-hydroxy-3-methyl-2-(E)-butenyl (Krall et al., 2002), an intermediate of the methylerythritol phosphate pathway (Lichtenthaler, 1999; Wolff et al., 2002). Although a similar side chain hydroxylated in the cis-configuration could possibly occur, thus far it has not been reported as a natural compound. In addition, plant isopentenyltransferases (Kakimoto, 2001; Takei et al., 2001) have not been shown to mediate the direct conversion to hydroxylated cytokinins. In any case, it is likely that substrate specificity governs the reactions, similar to O-glucosylation, with separate enzymes mediating the formation of cis- and trans-zeatin.
The presence of high levels of cis-isomers is puzzling considering that cis-zeatin is only weakly active in standard cytokinin bioassays and no clear function has been assigned to this cytokinin. It is possible that at specific stages of development cis-zeatin is converted to the more active isomer by a cis-trans zeatin isomerase (Bassil et al., 1993). In this context, early abundance of cis-isomers in developing chickpea seed followed by an increase in trans-isomers (Emery et al., 1998) could indicate that cis-zeatin contributes to the highly active cytokinin pool. However, direct conversion from cis- to trans-isomers has yet to be demonstrated. cis-Zeatin could possibly also be converted to dihydrozeatin, by a reductase similar to the trans-zeatin reductase of Phaseolus spp. (Martin et al., 1989). cis-Zeatin may also have some specialized function as yet unidentified. For instance, the presence of cis-zeatin is associated with male sterility in Mercurialis spp. (Louis et al., 1990; Durand and Durand, 1994). cis-Zeatin may alternatively be an active cytokinin in certain plant species or at specific stages of development. In support of this hypothesis is the recent finding that the maize cytokinin receptor ZmHK1 is responsive to cis-zeatin as well as trans-zeatin, whereas the corresponding Arabidopsis receptor, CRE1, responds only to trans-zeatin (K. Yonekura-Sakakibara, T. Yamaya, and H. Sakakibara, personal communication), indicating that cis-zeatin may be an active cytokinin in maize. In any event, the new findings on endogenous cytokinins, metabolic enzymes, and receptors suggest a need to re-examine the role of cis-zeatin and its derivatives in cytokinin biology.
MATERIALS AND METHODS
Isolation of cisZOG2
A λ DASH II (Stratagene, La Jolla, CA) genomic library of maize (Zea mays inbred B73) was obtained from Pioneer Hi-Bred International, Inc. and screened with 32P-labeled cisZOG1. Ready-To-Go DNA labeling beads (Amersham Biosciences, Piscataway, NJ) were used to label the cisZOG1 gene with 32P according to the manufacturer's protocol. Positive clones were plaque purified, and λ DNA was digested with various restriction enzymes (KpnI, XbaI, EcoRI, HindIII, and XmnI). Digestion products were separated by electrophoresis on a 1.1% (w/v) agarose gel, blotted to a Zeta-Probe GT membrane (Bio-Rad, Hercules, CA) following the manufacturer's protocol, and probed with 32P-labeled cisZOG1. Positive fragments of approximately 3.5 kb were identified from the EcoRI- and XbaI-digested DNA. These fragments were subcloned into pUC18 digested with either EcoRI or XbaI and then sequenced by the Central Services Laboratory (Center for Gene Research and Biotechnology, Oregon State University, Corvallis) with sequence analyzers (Applied Biosystems, Foster City, CA). On the basis of the sequence comparison to cisZOG1, a variant of this gene, cisZOG2, was identified.
Generation of Recombinant Protein
The ORF of cisZOG2 was amplified by PCR with a forward primer specific to this gene containing an NcoI site (GAG CTC CAT GGC GGT TGA CAC GAT GGA) and a backward primer containing an XbaI site (GAA TTC ACT CTA GAT TAC CTT GTG ATG TAG CCA ATG). The PCR product was digested with NcoI and XbaI and ligated into the Ptrc99A vector (Amersham Biosciences), which was modified to contain a translational fusion with seven His residues at the N terminus of the protein. This vector was transformed into the XL1 Blue cell line. The same procedures as used for generating crude cisZOG1 enzyme (Martin et al., 2001) were used to obtain cisZOG2 protein. Both cisZOG1 and cisZOG2 enzymes were purified by incubation with Ni column material (Novagen, Madison, WI) for 2 h at 4°C, followed by sequential elution with 100 mm and 500 mm imidazole. The 500 mm imidazole fraction contained the purified enzyme, which was concentrated by ultrafiltration in Centriprep 10 (Millipore, Bedford, MA) and desalted on an Econo-Pac10 DG column (Bio-Rad).
Biochemical Characterization of Recombinant Enzymes
Enzyme activity was assayed by incubating 0.75 μm cis-[8-3H]zeatin (specific activity 3.1 Ci mmol−1; OlChemIm, Brno, Czech Republic), 4 mm UDP-Glc, 10 μL of recombinant protein, and 0.17 m MgCl2 in 150 μL of 0.17 m Tris, pH 7.5, for 30 min or 1 h at 27°C. The reaction was terminated by addition of 800 μL of cold 95% (v/v) ethanol, after which the mixture was centrifuged, and the supernatant was collected. Substrate and product were separated by HPLC (Dixon et al., 1989). For determination of the optimum pH for the reaction, the pH range of 6 to 8 was used. For Km determinations of cis-zeatin, various amounts of non-radiolabeled cis-zeatin were added and for Km of UDP-Glc, the cis-zeatin concentration was 100 μm. The reaction was linear over time (30 min) at the concentrations tested (data not shown). Km values were determined with Scientist Micromath version 2.0 from Micromath Inc. To determine possible inhibition by other cytokinins, 200 or 400 μm of the compound was added to the reaction mixture containing 10 μm cis-[3H]zeatin.
RT-PCR
Total RNA was isolated from roots, stems, leaves, and kernels of B73 using a modified cetyl-trimethyl-ammonium bromide procedure (Chang et al., 1993). The roots, stems, and leaves were taken from 2-week-old seedlings in the greenhouse. Kernels were from field-grown maize, taken 1, 2, and 3 weeks post-fertilization. The RNA was treated with RNase-free DNase (Qiagen USA, Valencia, CA) and further purified using an RNeasy Mini Kit (Qiagen USA). SuperScript II RNase H− reverse transcriptase (Invitrogen, Carlsbad, CA) was used to synthesize first strand cDNA using oligo(dT)15 as a primer following the manufacturer's instructions. Escherichia coli RNase H (Invitrogen) was used to remove RNA complementary to cDNA before PCR.
Primers were designed to amplify cisZOG1 (but not cisZOG2) and cisZOG2 (but not cisZOG1). The maize actin gene was used as an internal standard. Primer sequences were as follows: actin sense, GTGACAATGGCACTGGAATG; actin antisense, GACCTGACCATCAGGCATCT; cisZOG1 sense, CAGGGAGTTCGTGGACCTC, cisZOG1 antisense, GAGCCCTGCCTTGAAGTAC; cisZOG2 sense, CAGGGAGTTCGTCGACCTG, and cisZOG2 antisense, GAGCCCTGCCTTGAAGTAG. PCR conditions used to compare the cisZOG1 and cisZOG2 transcripts were as follows: 95°C for 5 min followed by 30 cycles of: 94°C for 1 min, 57°C for 45 s, 72°C for 1 min. This was followed by a 10-min extension at 72°C, after which samples were held at 4°C until they were analyzed. PCR products were separated by electrophoresis in a 1.2% (w/v) Tris-acetate-EDTA agarose gel. Products were stained with ethidium bromide and visualized with a gel documentation system (UVP, Upland, CA).
Identification of Cytokinins in Maize Tissues
Roots were obtained from 2-week-old B73 seedlings grown under aseptic conditions on filter paper in Magenta boxes in the dark at 27°C. Leaves and stems were obtained from 5-week-old seedlings grown in the greenhouse at 75°C/65°C (day/night). Immature cobs (unfertilized) and young fertilized cobs (10 and 16 d after anthesis) were taken from the field. All tissues were frozen in liquid nitrogen and stored at −80°C. Tissues (roots, stems, leaves, unfertilized cobs, and kernels from the fertilized cobs) were lyophylized for 72 h before cytokinin analyses.
For MS quantification, nine deuterium-labeled cytokinins ([2H5]Z, [2H5]ZR, [2H5]Z-7G, [2H5]Z-9G, [2H5]Z-OG, [2H5]ZR-OG, [2H6]iP, [2H6]iPR, [2H6]iP-9G; Apex Organics, Honiton, UK), each at 100 pmol per sample, were used as internal standards. Cytokinins were extracted from 1 g of tissue with a mixture of MeOH:water:formic acid (15:4:1, v/v) at −20°C overnight. After passing the sample through a Si-C18 cartridge (Waters, Milford, MA), cytokinins were trapped on an Oasis MCX mixed mode, cation-exchange, reverse-phase column (150 mg, Waters). Cytokinin nucleotides were eluted with 0.17 m NH4OH in water, and after dephosphorylation (with 1 mg of phosphatase per 1 g tissue fresh weight for 1 h at 37°C), they were analyzed as the corresponding ribosides. Cytokinin bases, ribosides, and glucosides were eluted from MCX with 0.17 m NH4OH in 60% (v/v) MeOH and concentrated using Si-C18 cartridges.
Cytokinins were quantified by HPLC linked to an Ion Trap mass spectrometer Finnigan MAT LCQ-MSn equipped with an electrospray interface using an RP-C8 column (Supersphere RP Select B, 2 × 250 mm, 4 μm; Merck, Darmstadt, Germany). Linear gradients of acetonitrile (B) in 0.001% (v/v) acetic acid in water (A), 14% B to 20% B in 14 min, to 80% B in 6 min, and to 100% B in 6 min, were used at a flow rate 0.2 mL min−1. Detection and quantification were carried out with a Finnigan LCQ operated in the positive ion, full-scan MS/MS mode, using a multilevel calibration graph with deuterated cytokinins as internal standards. Because deuterated standards of cis-zeatin and derivatives are not available, the levels of these compounds were calculated based on the recovery of deuterated standards of the corresponding trans compounds. The electrospray ionization probe was installed with a sheath and auxiliary gasses at 96 and 6 units, respectively. The heated metal capillary temperature was maintained at 250°C and capillary voltage at 2.5 V. Data were obtained from two samples, except for unfertilized cobs (one sample). The detection limit was calculated for each compound as 3.3ς/S, where ς is the sd of the response and S is the slope of the calibration curve.
Footnotes
This work was supported by the National Science Foundation (grant nos. IBN–9981974 and IBN–0086731), by the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (grant no. 01–02015), by the Nucleic Acids and Proteins Core Facility of the Oregon State Environmental Health Sciences Center (grant no. 01–02015), by Pioneer Hi-Bred International, and by the Czech Ministry of Education, Youth and Sports (grant no. Kontakt ME 406). This is paper no. 11,892 of the Oregon Agricultural Experiment Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.017210.
LITERATURE CITED
- Armstrong DJ. Cytokinin oxidase and the regulation of cytokinin degradation. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton, FL: CRC Press; 1994. pp. 139–154. [Google Scholar]
- Bassil NV, Mok DWS, Mok MC. Partial purification of a cis-trans-isomerase of zeatin from immature seed of Phaseolus vulgaris L. Plant Physiol. 1993;102:867–872. doi: 10.1104/pp.102.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyeu KD, Cole JL, Laskey JG, Riekhof WR, Esparza TJ, Kramer MD, Morris RO. Molecular and biochemical characterization of a cytokinin oxidase from Zea mays. Plant Physiol. 2000;125:378–386. doi: 10.1104/pp.125.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Chen C-M, Leisner SM. Modification of cytokinins by cauliflower microsomal enzymes. Plant Physiol. 1984;75:442–446. doi: 10.1104/pp.75.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SC, Martin RC, Mok MC, Shaw G, Mok DWS. Zeatin glycosylation enzymes in Phaseolus: isolation of O-glucosyltransferase from P. lunatus and comparison to O-xylosyltransferase from P. vulgaris. Plant Physiol. 1989;90:1316–1321. doi: 10.1104/pp.90.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev P, Motyka V, Gaudinová A, Malbeck J, Trávnícková A, Kamínek M, Vanková R. Transient accumulation of cis- and trans-zeatin type cytokinins and its relations to cytokinin oxidase activity during cell cycle of synchronized tobacco cells. Plant Physiol Biochem. 2002;40:333–337. [Google Scholar]
- Durand R, Durand B. Cytokinins and reproductive organogenesis in Mercurialis. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton, FL: CRC Press; 1994. pp. 295–304. [Google Scholar]
- Emery RJN, Leport L, Barton JE, Turner NC, Atkins CA. cis-Isomers of cytokinins predominate in chickpea seeds throughout their development. Plant Physiol. 1998;117:1515–1523. doi: 10.1104/pp.117.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery RJN, Ma Q, Atkins CA. The forms and sources of cytokinins in developing white lupine seeds and fruits. Plant Physiol. 2000;123:1593–1604. doi: 10.1104/pp.123.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizumi T, Suye S, Sugiyama T. Isolation and identification of cis-zeatin riboside from tubers of sweet potato (Ipomoea batatas L.) Agric Biol Chem. 1982;46:663–665. [Google Scholar]
- Kakimoto T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001;42:677–685. doi: 10.1093/pcp/pce112. [DOI] [PubMed] [Google Scholar]
- Krall L, Raschke M, Zenk MH, Baron C. The Tzs protein from Agrobacterium tumefaciens C58 produces zeatin riboside 5′-phosphate from 4-hydroxy-3-methyl-2-(E) butenyl riboside 5′phosphate and AMP. FEBS Lett. 2002;527:315–318. doi: 10.1016/s0014-5793(02)03258-1. [DOI] [PubMed] [Google Scholar]
- Letham DS. Cytokinins as phytohormones: sites of biosynthesis, translocation, and function of translocated cytokinin. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton, FL: CRC Press; 1994. pp. 57–80. [Google Scholar]
- Li Y, Baldauf S, Lim E-K, Bowles DJ. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. J Biol Chem. 2001;276:4338–4343. doi: 10.1074/jbc.M007447200. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Louis J-P, Augur C, Teller G. Cytokinins and differentiation processes in Mercurialis annua. Plant Physiol. 1990;94:1535–1541. doi: 10.1104/pp.94.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Habben JE, Mok DWS. A cytokinin gene from maize encoding an O-glucosyltransferase specific to cis-zeatin. Proc Natl Acad Sci USA. 2001;98:5922–5926. doi: 10.1073/pnas.101128798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Mok DWS. Isolation of a cytokinin gene, ZOG1, encoding zeatin O-glucosyltransferase of Phaseolus lunatus. Proc Natl Acad Sci USA. 1999a;96:284–289. doi: 10.1073/pnas.96.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Mok DWS. A gene encoding the cytokinin enzyme zeatin O-xylosyltransferase of Phaseolus vulgaris. Plant Physiol. 1999b;120:553–557. doi: 10.1104/pp.120.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Shaw G, Mok DWS. An enzyme mediating the conversion of zeatin to dihydrozeatin in Phaseolus embryos. Plant Physiol. 1989;90:1630–1635. doi: 10.1104/pp.90.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk CS, Langille AR. Physiology of tuberization in Solanum tuberosum L. Plant Physiol. 1978;62:438–442. doi: 10.1104/pp.62.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DWS, Mok MC. Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- Mok MC. Cytokinins and plant development: an overview. In: Mok DWS, Mok MC, editors. Cytokinins: Chemistry, Activity, and Function. Boca Raton, FL: CRC Press; 1994. pp. 155–166. [Google Scholar]
- Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y, Okamoto T. Cytokinin activity of N-phenyl-N′-1,2,3-thiadiazol-5-ylurea (thidiazuron) Phytochemistry. 1982;21:1509–1511. [Google Scholar]
- Parker CW, Badenoch-Jones J, Letham DS. Radioimmunoassay for quantifying the cytokinins cis-zeatin and cis-zeatin riboside and its application to xylem sap samples. J Plant Growth Regul. 1989;8:93–105. [Google Scholar]
- Schmitz RY, Skoog F, Playtis AJ, Leonard NJ. Cytokinins: synthesis and biological activity of geometric and position isomers of zeatin. Plant Physiol. 1972;50:702–705. doi: 10.1104/pp.50.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Armstrong DJ. Cytokinins. Annu Rev Plant Physiol. 1970;21:359–384. [Google Scholar]
- Suttle JC, Banowetz GM. Changes in cis-zeatin and cis-zeatin riboside levels and biological activity during tuber dormancy. Physiol Plant. 2000;109:68–74. [Google Scholar]
- Takagi M, Yokota T, Murofushi N, Ota Y, Takahashi N. Fluctuation of endogenous cytokinin contents in rice during its life cycle: quantification of cytokinins by selected ion monitoring using deuterium-labelled internal standards. Agric Biol Chem. 1985;49:3271–3277. [Google Scholar]
- Takagi M, Yokota T, Murofushi N, Saka H, Takahashi N. Quantitative changes of free-base, riboside, ribotide and glucoside cytokinins in developing rice grains. Plant Growth Regul. 1989;8:349–364. [Google Scholar]
- Takei K, Sakakibara H, Sugiyama T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J Biol Chem. 2001;276:26405–26410. doi: 10.1074/jbc.M102130200. [DOI] [PubMed] [Google Scholar]
- Turner JE, Mok DWS, Mok MC, Shaw G. Isolation and partial purification of an enzyme catalyzing the formation of O-xylosylzeatin in Phaseolus vulgaris embryos. Proc Natl Acad Sci USA. 1987;84:3714–3717. doi: 10.1073/pnas.84.11.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Yokota T, Takahashi N. Variations in the levels of cis- and trans-ribosylzeatins and other minor cytokinins during development and growth of cones of the hop plant. Plant Cell Physiol. 1981;22:489–500. [Google Scholar]
- Wolff M, Seemann M, Grosdemange-Billiard C, Tritsch D, Campos N, Rodríguez-Concepción M, Boronal A, Rohmer M. Isoprenoid biosynthesis via the methylerythritol phosphate pathway: (E)-4-hydroxy-3-methylbut-2-enyl diphosphate: chemical synthesis and formation from methylerythritol cyclodiphosphate by a cell-free system from Escherichia coli. Tetrahedron Lett. 2002;43:2555–2559. [Google Scholar]