Abstract
The interaction of tropisms is important in determining the final growth form of the plant body. In roots, gravitropism is the predominant tropistic response, but phototropism also plays a role in the oriented growth of roots in flowering plants. In blue or white light, roots exhibit negative phototropism that is mediated by the phototropin family of photoreceptors. In contrast, red light induces a positive phototropism in Arabidopsis roots. Because this red-light-induced response is weak relative to both gravitropism and negative phototropism, we used a novel device to study phototropism without the complications of a counteracting gravitational stimulus. This device is based on a computer-controlled system using real-time image analysis of root growth and a feedback-regulated rotatable stage. Our data show that this system is useful to study root phototropism in response to red light, because in wild-type roots, the maximal curvature detected with this apparatus is 30° to 40°, compared with 5° to 10° without the feedback system. In positive root phototropism, sensing of red light occurs in the root itself and is not dependent on shoot-derived signals resulting from light perception. Phytochrome (Phy)A and phyB were severely impaired in red-light-induced phototropism, whereas the phyD and phyE mutants were normal in this response. Thus, PHYA and PHYB play a key role in mediating red-light-dependent positive phototropism in roots. Although phytochrome has been shown to mediate phototropism in some lower plant groups, this is one of the few reports indicating a phytochrome-dependent phototropism in flowering plants.
Plants have evolved selective and sensitive mechanisms to deal with the constant sensory input they receive from the environment. In roots, gravity is the most critical signal for growth and development, and, thus, gravitropism has been well-characterized in this organ (Sack, 1991; Kiss, 2000). However, it has become increasingly clear that gravitropism interacts with a number of other tropistic responses including phototropism, thigmotropism, and hydrotropism in determining the final growth form of the primary root and the entire root system (Hangarter, 1997; Correll and Kiss, 2002).
Phototropism in roots was extensively reviewed in a classical paper by Hubert and Funke (1937) but has received increased attention since the report by Okada and Shimura (1992), who isolated mutants in root phototropism that were later shown to be deficient in the blue-light receptor PHOT1 (Briggs and Christie, 2002). Roots are typically negatively phototropic in response to white and blue light (Okada and Shimura, 1992; Vitha et al., 2000) and use the same photoreceptors that are involved in phototropism in stems and stem-like organs (Sakai et al., 2000). Furthermore, similar to root gravisensing (Blancaflor et al., 1998), sensing of blue light for phototropism occurs in the root cap (Mullen et al., 2002).
We have recently identified a red-light-induced positive phototropism in primary roots of Arabidopsis (Ruppel et al., 2001). This tropistic response appears to be relatively weak compared with other root tropisms but is readily apparent in mutants that are impaired in gravisensing. This red-light-induced positive phototropism also occurs in the lateral roots of Arabidopsis (Kiss et al., 2002). The role of root phototropism is unknown, but it may serve in optimization of the orientation of the entire root system, especially in soils through which light can readily penetrate (Mandoli et al., 1990).
Because root phototropism is relatively weak, we used a novel apparatus that combines high-resolution image analysis of root growth and a computer-controlled rotatable stage that allows phototropism to be studied without the complications of a counteracting and constantly changing gravitational stimulus (Mullen et al., 2000). This device has recently been used to determine signaling pathways involved in gravitropism (Wolverton et al., 2002) and to describe the spatial separation of blue-light perception and growth response in negative root phototropism (Mullen et al., 2002).
In this paper, we provide a more detailed characterization of the red-light-induced phototropic response in Arabidopsis roots, including determining the location of light sensing and the response and the nature of the photoreceptor(s). Our results show specifically that PHYTOCHROME (PHY)A and PHYB mediate the red-light-induced positive phototropic response in roots, whereas other phytochromes do not appear to be involved.
RESULTS
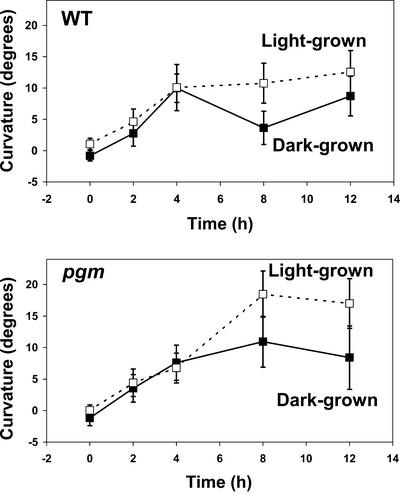
Red-light-induced positive root phototropism was first reported in light-grown Arabidopsis seedlings (Kiss et al., 2001; Ruppel et al., 2001), whereas much of the published work on root phototropism has been done with dark-grown seedlings (e.g., Vitha et al., 2000). Because the red response is weak, we examined the response in light- or dark-grown plants to determine whether light-grown seedlings would have a stronger response. This was done with both wild type (WT) and a starchless phosphoglucomutase (pgm) mutant, which was previously shown (Ruppel et al., 2001) to have a more robust photoresponse because of its impaired graviresponse. Our data show that roots of light-grown seedlings exhibited a greater magnitude of phototropic curvature in response to red light in both the WT and pgm (Fig. 1), so light-grown plants were used in the subsequent studies.
Figure 1.
Time course of phototropic curvature of roots of WT and pgm seedlings illuminated with continuous unilateral red light. For both genotypes, white-light-grown seedlings exhibited a greater magnitude of curvature compared with dark-grown plants. Bars represent se, and n = 107 to 121 for WT; n = 65 to 99 for pgm.
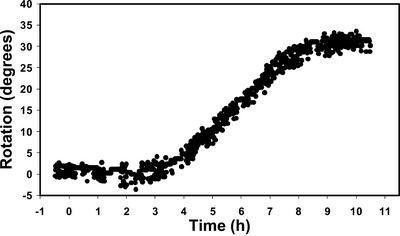
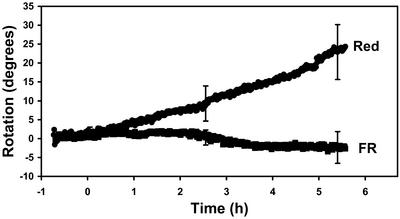
Because the photoresponse in roots is weak relative to the graviresponse, we performed studies with a new technique to assay tropistic responses. This involved using a feedback system and a rotating stage that can keep the root tip constrained to a particular angle (Mullen et al., 2000). With this apparatus, the rotation of the stage, which is necessary to constrain the orientation of the root tip, corresponds to the curvature response in the roots. In our studies, we constrained the root apex to 0° (vertical), which allowed us to study root phototropism without the complications of a constantly changing gravitational stimulus. Under these conditions, roots exhibited a vigorous red-induced positive phototropism that is illustrated in Figure 2. Furthermore, the response obtained with the feedback system (Fig. 2) was greater in magnitude compared with results obtained with unconstrained roots (Fig. 1). After a latent period of 1 to 2 h, root curvature developed in response to the red-light stimulus and maintained a constant rate of curvature for several hours, which was followed by a plateau phase (approximately 30°) in the response curve (Fig. 2). Illumination with continuous far-red failed to induce a curvature response (Fig. 3).
Figure 2.
Kinetics of the positive phototropic response of a typical WT root in response to continuous unilateral red illumination as measured with the feedback system (Mullen et al., 2000). The plot shows the rotation of the stage necessary to keep the root tip constrained at 0° (vertical). In all figures, 0 h represents the time at which the seedlings are exposed to unilateral red illumination. This experiment was repeated 20 times with similar results.
Figure 3.
Mean response of WT roots (n = 10) to continuous unilateral red or far-red (FR) illumination as measured with the feedback system. The plot shows the rotation of the stage necessary to keep the root tip constrained at 0° (vertical). Bars represent se.
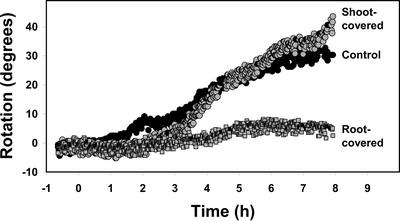
By selectively illuminating the root and shoot, we were able to determine that the root is the site of perception for red-light-induced positive root phototropism and that this phototropism did not involve light or signal transfer from the shoot (Fig. 4). In these experiments, the mean curvature (± se) of six roots after 8 h of red illumination was 25.9° ± 4.8°, 24.9° ± 7.1°, and 0.1° ± 6.0° for the shoot-covered, control (uncovered), and root-covered seedlings, respectively. In terms of statistical significance as determined by an ANOVA (P = 0.014) and Dunnett's post-test (P < 0.05), roots of the shoot-covered seedlings exhibited a curvature that was not significantly different from control seedlings, whereas those of the root-covered seedlings were significantly different from the uncovered control seedlings.
Figure 4.
Kinetics of the positive phototropic response of individual WT roots in response to localized unilateral red illumination as measured with the feedback system. In these experiments, either the root or shoot was blocked from the unilateral light source by inserting black foil in the agar adjacent to the seedling. The control seedlings were left uncovered. This experiment was repeated 12 times with similar results.
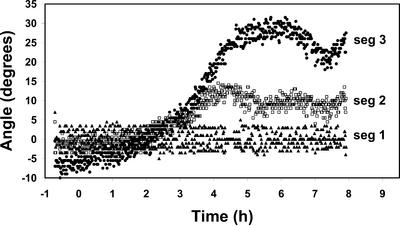
We also carried out high-resolution analysis of the curvature of root segments while the tip was constrained by the feedback system. In Figure 5, the most apical portion of the root is segment 1, and each segment is 330 μm in length. Thus, segment 1 includes the root cap and the distal elongation zone (DEZ), segment 2 consists of the central elongation zone (CEZ), and segment 3 is the base of the elongation zone. On the basis of a comparison of the deviation of the curvature of these segments, most of the phototropic curvature of the entire root results from differential growth of segment 3. Considering this result and the images of roots during positive phototropism (Fig. 6), we conclude that positive curvature induced by red light occurs at the basal edge of the CEZ in contrast to gravicurvature, which occurs in the DEZ (Mullen et al., 2000).
Figure 5.
Localized changes in root orientation following illumination with unilateral red light. The data show the angles of orientation of different regions of a representative root when the apical segment was constrained at 0° (vertical). The apical most portion of the root is segment 1, and each segment (seg) is 330 μm in length. Most of the photocurvature can be seen to result from curvature of segment 3. This experiment was repeated 12 times with similar results.
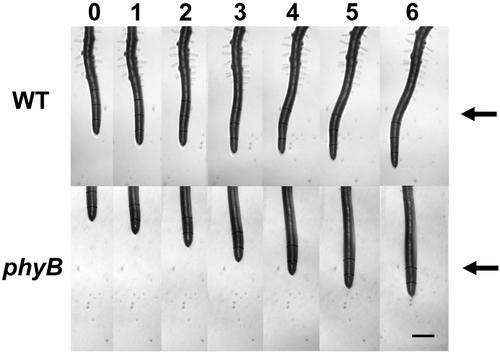
Figure 6.
Images of roots of WT and phyB seedlings following illumination with unilateral red light. In this experiment, red light was applied from the direction indicated (arrow) at time 0, and the root tip was constrained at 0° (vertical) by the feedback system. Images were taken hourly as indicated along the top of the figure. Note the obvious curvature in the WT root while the root of the phyB mutant grew straight, without a response to the red light. Scale bar = 500 μm.
Most red-light responses in plants are mediated by one or more of the phytochrome photoreceptors. Because red-light-induced positive phototropism in roots has only recently been discovered (Ruppel et al., 2001), we wanted to determine which, if any, of the five-member phytochrome gene family participates in controlling this response. To this end, root phototropism was studied in Arabidopsis mutants deficient in various phytochromes (Figs. 6 and 7). Figure 6 illustrates a WT root tip that exhibited positive phototropism of approximately 30° during red-light illumination when the tip was vertically constrained (0°) by the rotating stage apparatus. In contrast, a root of the phyB mutant lacked positive phototropism in response to red light and exhibited straight growth (Fig. 6).
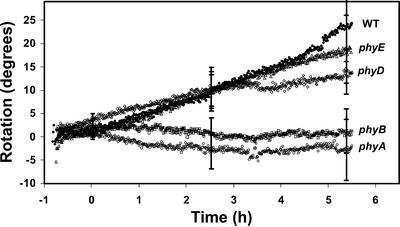
Figure 7.
Mean response of WT and phytochrome mutant roots (n = 12) to unilateral red illumination as measured with the feedback system. The responses of the phyA and phyB mutants were significantly less than that of the WT as determined by an ANOVA (P = 0.016) and Dunnett's post-test (P < 0.05). In contrast, there was no significant difference in the response of phyD and phyE compared with the WT (P > 0.05). Bars represent se.
Figure 7 shows the results obtained with the rotating stage apparatus for four available phytochrome single mutants (phyA, phyB, phyD, and phyE) in comparison with WT. From this figure, it is clear that roots of phyA and phyB were inhibited in the photoresponse compared with the WT roots. In contrast, roots of phyD and phyE exhibited a response similar to that of WT roots. In terms of statistical significance as determined by an ANOVA (P = 0.016) and Dunnett's post-test (P < 0.05), phyA and phyB exhibited significantly less curvature relative to the WT, whereas the curvature of the phyD and phyE mutants was not significantly different from that of WT seedlings. The red-light photoresponse was studied in the double and triple mutants phyAB, phyABD, and phyBE; and roots of all three were inhibited in the response compared with WT roots (not shown).
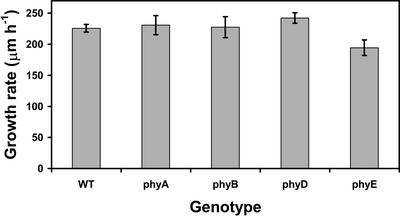
The growth rates for roots of all strains were similar (Fig. 8) except for roots of phyE, which had a slightly reduced growth rate as determined by an ANOVA (P = 0.049) and Dunnett's post-test (P < 0.05). However, the reduced growth rate did not affect the phototropic response of phyE roots. Moreover, growth did not contribute to the attenuation of the root phototropic responses of phyA and phyB relative to the WT, because their growth rates were similar to WT.
Figure 8.
Mean rates (n = 13–20) of root elongation of WT and phytochrome mutant seedlings. The only difference among growth rates was that phyE was significantly less than the WT as determined by an ANOVA (P = 0.49) and Dunnett's post-test (P < 0.05), and bars represent se.
DISCUSSION
In a previous study (Ruppel et al., 2001), we presented evidence for a positive phototropic response to unilateral red illumination in Arabidopsis roots. The data presented here provide a robust characterization of the response, including fluence rate characteristics, the localization of light sensing for this root response, and the identification of the phytochromes involved in red-light-induced phototropism.
In preliminary studies, we found that the positive phototropic response could be observed at fluence rates from 0.01 to 100 μmol m−2 s−1 (data not shown). The response increased with increasing fluence rate up to about 10 μmol m−2 s−1, with no reversal of curvature detected at any of the fluence rates tested. Except for the different direction, the fluence rate dependence for the positive phototropic response elicited by red light was similar to those reported for negative blue-light-induced phototropism in roots of Arabidopsis by Sakai et al. (2000), who found maximal curvature in the range of 10 to 100 μmol m−2 s−1. In addition, Mullen et al. (2002) reported saturation of the blue-light phototropic response in maize (Zea mays) roots at 10 μmol m−2 s−1.
Because the gravitropic response in roots overwhelms the phototropic response (Hubert and Funke, 1937; Okada and Shimura, 1992), root phototropism has been largely unstudied for decades. This is particularly true for red-light phototropism. In terms of the relative strength of tropistic responses in roots, we find the following order: gravitropism > negative phototropism (induced by blue light) > positive phototropism (induced by red light). The comparatively weak nature of red-light-induced positive phototropism in roots is evident by comparing the results of the present study to previously published papers on tropisms in roots. For example, numerous reports have shown that after reorientation of seedlings from vertical to horizontal, roots will exhibit a vigorous graviresponse and achieve a 90° curvature (for review, see Sack, 1991; Kiss, 2000) such that the root tips regain a vertical orientation. The negative blue-light phototropic response in roots is generally viewed as a competition between the gravity and light stimuli in that roots exposed to unilateral illumination will reach an intermediate curvature of about 45° (Okada and Shimura, 1992; Sakai et al., 2000). In contrast, roots will curve only 5° to 10° in response to unilateral red illumination (Fig. 1), although the gravitropically impaired starchless mutant will curve up to 15° under these conditions (Fig. 1; Ruppel et al., 2001).
To study the relatively weak red-light-induced phototropic response, we used a custom-built apparatus that allows phototropism to be measured in the absence of a change in the gravity vector (Mullen et al., 2000, 2002). Using this feedback system, which effectively eliminates competition with gravitropism, we were able to obtain red-light-induced curvature response in WT roots as large as 30° to 40° (Figs. 2–4), compared with 5° to 10° without use of the feedback computer-induced system (Figs. 1 and 2; Ruppel et al., 2001). The initial rate of curvature for roots presented with a constant phototropic stimulus on the feedback system was approximately 5° h−1, which was only one-half the rate for responses to even small gravitropic stimulations (e.g. 20° reorientations). Therefore, even if the phototropic and gravitropic interactions are additive, the equilibrium orientation will be near-vertical in unilateral red illumination. Furthermore, the red-light phototropic response attenuates after several hours of curvature and is subject to adaptation (Fig. 2), unlike the gravitropic response, which maintains a near-constant rate of curvature throughout gravistimulation (Mullen et al., 2000). That differential growth continues indefinitely during gravitropism suggests that the adaptation in the red-light phototropic response is upstream from differential growth.
Calculations of the latent period before response to a stimulus have been used as another indication of the relative strength of tropisms. Estimates using the feedback system showed that the latent period for gravitropism is approximately 10 min (Mullen et al., 2000), the latent period for blue-light phototropism is 40 min (Mullen et al., 2002), and, as reported here, this value for red-light phototropism is 1 to 2 h (Fig. 2). These differences in latent period may be indicative of the relative strengths of the different responses. However, we have also found that the location of the growth response to these stimuli occurred in different parts of the root. In the present study, we found that the precise position of curvature in red-light-induced phototropism was at the basal edge of the CEZ (Figs. 5 and 6). In contrast to this observation, gravicurvature occurred primarily in the DEZ (Mullen et al., 2000) and blue-light-induced photocurvature occurred directly in the CEZ (Mullen et al., 2002). The differences in latent periods may be due to these differences in the location of these tropistic responses. For instance, the response for red-light-induced phototropism occurs in a more basal portion of the root (basal edge of CEZ) compared with the response for gravicurvature (DEZ), so the delay in the red photoresponse may be due to a longer transmission distance of a signal from the root cap, where perception is likely to occur.
The root cap has long been implicated in mechanisms of gravitropism (for review, see Sack, 1991) and has been shown to be the primary site of gravity perception (Blancaflor et al., 1998; Kiss, 2000). In addition to its role in gravisensing, the root cap also appears to play a role in the sensing of blue light in negative phototropism in roots because maize roots failed to develop negative phototropism when the root cap was surgically removed or when the blue light was applied (by fiber optics) to parts of the root other than the cap (Mullen et al., 2002). In the present study, although it was not possible to remove the root cap in Arabidopsis roots, we performed experiments in which either the entire root or shoot was covered by black foil. When the shoot was covered, the root exhibited a typical positive phototropism in response to red light, but there was no photoresponse when the root was covered (Fig. 4). Thus, these results indicate that sensing of the red light occurs in the root itself and that the phototropic response in roots is not a result of light perception in the shoot, transmittance of light through the plant to the root, or production and delivery of a red-light-induced transmissible signal from the shoot.
The mechanistic reasons for roots to exhibit a positive phototropism are unknown. In a previous study, we speculated that this type of root phototropism may serve a role in orienting lateral roots near the surface zone or that it may represent a nonadaptive characteristic in roots and has been retained as a consequence of other red-light responses that are essential for other aspects of plant development (Ruppel et al., 2001). The former hypothesis is supported by a recent paper which showed that lateral roots also exhibit red-light-induced positive phototropism (Kiss et al., 2002). However, it is also possible that the responses we are observing in roots are evolutionary remnants and may not actually have an adaptive role. For example, because many blue-light responses in aboveground plant parts show co-action with phytochrome, the root responses we have identified may be a result of some mechanistic codependency between the photosensory systems.
Our results also show that PHYA and PHYB mediate the red-light-induced phototropic response in roots (Figs. 6 and 7). Single mutants that lack PHYA or PHYB (and double and triple mutants which lacked at least one of these phytochromes) were severely impaired in red-light-induced phototropism, whereas the phyD and phyE mutants responded as well as WT. The lack of response in the phyA and phyB mutant lines was not due to phytochrome-dependent growth limitations because the growth rates of roots among all the mutants tested (except for phyE) were not significantly different (P > 0.05) from that of the WT (Fig. 8).
Phytochrome has been shown to mediate phototropism in some lower plants (e.g. mosses; Esch et al., 1999), but there are few reports of a direct phytochrome-regulated phototropism in flowering plants (Iino et al., 1984; Parker et al., 1989). In addition, Ballare et al. (1992) reported a phytochrome-dependent phototropism in cucumber (Cucumis sativus) shoots by showing that a far-red-dependent negative phototropic response was at least partly mediated by phyB.
The data presented in this paper show that both phyA and phyB are required for the red-light-dependent positive phototropic response in Arabidopsis roots. In most phytochrome responses that are regulated by both phyA and phyB, the photoreceptors appear to act redundantly (Nagy and Schäfer, 2002; Quail, 2002). However, some phytochrome responses require the action of at least two phytochromes. For example, far-red high-irradiance response-induced seed germination is absent in phyA and phyE mutants, indicating a requirement of phyE for phyA-mediated far-red high-irradiance response (Hennig et al., 2002). At this time, it is not clear why the root phototropic response shows codependency for phyA and phyB. It is possible that this curvature response requires coordination between growth inhibition and stimulation on opposite sides of the root and that phyA and phyB control one or the other. Thus, in the absence of either phyA or phyB, curvature may not develop. Furthermore, the curvature induced by red light is relatively weak, and it may be difficult to establish it with input from only one component. It is also possible that phyA and phyB interact directly to carry out the response. Whatever the exact mechanism, recent evidence that phyA is expressed in the root cap of Arabidopsis (Hall et al., 2001), where light sensing occurs, supports our observations that phyA plays a role in the root phototropic response. Furthermore, whereas the blue-light receptor family of phototropins (Briggs and Christie, 2002) function in light perception mechanisms of phototropism in stems and stem-like organs, phyA (Parks et al., 1996) and phyB (Janoudi and Poff, 1997) have been shown to regulate red- and far-red induction of phototropic enhancement in hypocotyls.
In addition to their effect on the red-light-induced positive phototropism described in this paper, phytochromes have been shown to play a role in gravitropism (Liscum and Hangarter, 1993). In particular, light-induced reduction of negative gravitropism was shown to be controlled by both phyA and phyB (Poppe et al., 1996). It has also been proposed that a function of PHYA and PHYB in developing seedlings in the presence of light is to switch off negative gravitropism to allow for phototropic stimuli to determine the orientation of growth (Robson and Smith, 1996; Hangarter, 1997). In addition, we have observed that root gravitropism is impaired in phyAB double mutants (J.Z. Kiss and J.L. Mullen, unpublished observations). Thus, it is becoming increasing clear that these two forms of phytochrome play key roles in integrating multiple environmental stimuli throughout the course of plant development.
MATERIALS AND METHODS
Plant Material and Culture Conditions
In these experiments, we used WT and mutant lines of Arabidopsis. The starchless mutant, which is deficient in pgm, was isolated from the Wassilewskija ecotype and has been described by Kiss et al. (1996). All of the phytochrome mutants were in the Landsberg erecta ecotype background. The mutant strains used were phyA-201, phyB-1, phyD-1, phyE-1, and double and triple mutants, and all of these are summarized by Hennig et al. (2002). PhyA and phyB were provided by Prof. R. Sharrock (Montana State University, Bozeman), and phyD and phyE were from Prof. G. Whitelam (University of Leicester, UK).
Seeds were surface-sterilized in 30% (v/v) commercial bleach and 0.002% (v/v) Triton X-100 for 20 min. After four to five rinses in sterile distilled water, seeds were sown onto a presterilized cellophane that was placed on top of a growth medium (described by Kiss et al. [1996]) with 1% (w/v) Suc in 1.2% (w/v) agar in square (100- × 15-mm) petri dishes. In the experiments done with the rotating stage system, the agar medium contained one-half-strength Murashige and Skoog (1962) salts with 1% (w/v) Suc and 1 mm MES (pH 5.8) in 1.0% (w/v) agar in 60- × 15-mm petri dishes. In all experiments, the petri dishes were sealed with laboratory film (Parafilm, American National Can, Greenwich, CT) and placed on edge in a rack so that the surface of the agar was vertical. Seedlings were grown in white light of 70 to 90 μmol m−2 s−1obtained from 34 W “cool light” fluorescent lamps, but in some cases, seedlings were grown in darkness as a control. Fluence rates where measured with a quantum radiometer photometer (LI-189, LI-COR, Lincoln, NE) equipped with an LI-190SA Quantum sensor. Seedlings were used in experiments when the roots were approximately 1 cm in length, which was typically 3 to 4 d after the seeds were sown and incubated at 20°C to 22°C under the indicated light conditions.
Light Sources
In experiments to determine whether light- or dark-grown seedlings exhibited a maximal response, red illumination was obtained by passing light from fluorescent bulbs through Plexiglas filters. The fluence rate through the red filter (Rohm and Haas no. 2423, Dayton Plastics, Columbus, OH) was 12 to 14 μmol m−2 s−1 with a transmission maximum of 630 nm as determined with a LI-COR LI-1800 spectroradiometer. In the fluence rate response experiments, the same red Plexiglas filter was used in conjunction with fluorescent bulbs or halogen bulbs (for the higher fluence rates). In experiments that involved the feedback system, a red-light-emitting diode, LED (catalog no. 276–309, Radio Shack, Fort Worth, TX) of 660 nm was used at 10 to 20 μmol m−2 s−1. For experiments with far red illumination, light from a far red LED lamp (QBeam 2200 with lamp QB1310CS, Quantum Devices, Barneveld, WI) was passed through one layer of far red Plexiglas (FRF 700, Westlake Plastics Co., Lenni, PA). The fluence rate was approximately 8 μmol m−2 s−1 at wavelengths between 700 and 750 nm, as measured by a spectroradiometer.
Computer Digitizer and Feedback System to Measure Growth and Rotation
The seedling to be observed was first repositioned so that its root tip was at the center of the petri dish (center of rotation). Repositioning was accomplished by placing forceps under the hypocotyl or cotyledons, lifting slightly, and sliding the plant along the agar surface. Repositioned seedlings were allowed to equilibrate12 to 15 h before red-light stimulation. In all figures, 0 h represents the time at which the seedlings are exposed to unilateral red illumination.
After equilibration, the dish containing the seedling was attached to a vertical stage in the dark, and growth was analyzed with a digital imaging system described by Mullen et al. (1998). In brief, roots were imaged using infrared illumination (940 nm LED, Radio Shack) and a CCD camera interfaced to a PC computer with a frame grabber board (Imagenation, Beaverton, OR).
In addition, a computer feedback system was used to constrain the root tip angle to the vertical during unilateral red-light stimulation perpendicular to the root axis, as described by Mullen et al. (2000). In brief, roots were positioned a rotatable vertical stage (catalog no. RT-3S17, Nutec Components, Deer Park, NY), with individual steps of the motor corresponding to 0.17°. The stage was connected to a PC computer, and the stepper motor was controlled by custom software. Roots were imaged every 45 s by using infrared illumination and a CCD camera. The software analyzed the images, and if the root tip deviated from 0°, the software activated the stepper motor to make corrections to the rotating stage to constrain the tip segment of the root to 0o (vertical). The software also recorded the angle of the root segments and the rotation of the vertical stage, and this value is termed “rotation” in the text and figures in this paper. As controls, either the shoot or root was blocked from receiving the unilateral light stimulus by inserting small pieces of black foil in the agar adjacent to the appropriate part of the plant to block the light path.
Measurement of Curvature
In all experiments, seedlings were illuminated 90o from the vertical, and roots that grew toward the light source were assigned positive angles and those that grew away from the light were assigned negative angles. Roots that deviated more than 10° to 15° from the original gravity vector (at the start of the experimental treatments) were excluded from these studies (Kiss et al., 1989, 1997). Root curvature was defined as the change in angle of the root apex. In fluence rate experiments, curvature was measured after a 48-h exposure to unilateral red light according to the methods of Sakai et al. (2000).
In light- and dark-grown and fluence rate response experiments, seedlings were photographed with a 35-mm camera equipped with a macro lens using Kodak Technical Pan film (no. 2415, Eastman Kodak, Rochester, NY) at ASA 50. Images were digitally captured from the film, and measurements of curvature and growth were made with the image analysis program Image-Pro Plus (v4.5, Media Cybernetics, Silver Spring, MD) on a Pentium PC computer. Seedlings were excluded from measurement if their roots contacted neighboring plants. These experiments were repeated at least three times, and values are reported as the means ± se.
All phototropism and growth experiments with the phytochrome mutants were repeated a minimum of 10 times. Statistical significance was determined by using a one-way ANOVA test (P < 0.05), and if necessary, this was followed by Dunnett's post-test (P < 0.05) performed with a PC using Sigma Stat software (v2.0, SPSS, Chicago).
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NCC2–1200).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.013847.
LITERATURE CITED
- Ballare CL, Scopel AL, Radosevich SR, Kendrick RE. Phytochrome-mediated phototropism in de-etiolated seedlings: occurrence and ecological significance. Plant Physiol. 1992;100:170–177. doi: 10.1104/pp.100.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM. Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci. 2002;7:204–210. doi: 10.1016/s1360-1385(02)02245-8. [DOI] [PubMed] [Google Scholar]
- Correll MJ, Kiss JZ. Interactions between gravitropism and phototropism in plants. J Plant Grow Regul. 2002;21:89–101. doi: 10.1007/s003440010056. [DOI] [PubMed] [Google Scholar]
- Esch H, Hartmann E, Cove D, Wada M, Lamparter T. Phytochrome-controlled phototropism of protonemata of the moss Ceratodon purpureus: physiology of the wild type and class 2 ptr-mutants. Planta. 1999;209:290–298. doi: 10.1007/s004250050635. [DOI] [PubMed] [Google Scholar]
- Hall A, Kozma-Bognár L, Tóth R, Nagy F, Millar AJ. Conditional circadian regulation of PHYTOCHROME A gene expression. Plant Physiol. 2001;127:1808–1818. [PMC free article] [PubMed] [Google Scholar]
- Hangarter RP. Gravity, light and plant form. Plant Cell Environ. 1997;20:796–800. doi: 10.1046/j.1365-3040.1997.d01-124.x. [DOI] [PubMed] [Google Scholar]
- Hennig L, Stoddart WM, Dieterle M, Whitelam GC, Schäfer E. Phytochrome E controls light-induced germination of Arabidopsis. Plant Physiol. 2002;128:194–200. [PMC free article] [PubMed] [Google Scholar]
- Hubert B, Funke GL. The phototropism of terrestrial roots. Biol Jaarboek. 1937;4:286–315. [Google Scholar]
- Iino M, Briggs WR, Schäfer E. Phytochrome-mediated phototropism in maize seedling shoots. Planta. 1984;160:41–51. doi: 10.1007/BF00392464. [DOI] [PubMed] [Google Scholar]
- Janoudi A-K, Poff KL. Multiple phytochromes are involved in red light-induced enhancement of first positive curvature in Arabidopsis thaliana. Plant Physiol. 1997;113:975–979. doi: 10.1104/pp.113.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ. Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997;38:518–525. doi: 10.1093/oxfordjournals.pcp.a029199. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- Kiss JZ, Miller KM, Ogden LA, Roth KK. Phototropism and gravitropism in lateral roots of Arabidopsis. Plant Cell Physiol. 2002;43:35–43. doi: 10.1093/pcp/pcf017. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Ruppel NJ, Hangarter RP. Phototropism in Arabidopsis roots is mediated by two sensory systems. Adv Space Res. 2001;27:877–885. doi: 10.1016/s0273-1177(01)00154-5. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Wright JB, Caspar T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant. 1996;97:237–244. doi: 10.1034/j.1399-3054.1996.970205.x. [DOI] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP. Genetic evidence that the Pr form of phytochrome B plays a role in Arabidopsis thaliana gravitropism. Plant Physiol. 1993;103:15–19. doi: 10.1104/pp.103.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli DF, Ford GA, Waldron LJ, Nemson JA, Briggs WR. Some spectral properties of several soil types: implications for photomorphogenesis. Plant Cell Environ. 1990;13:287–294. [Google Scholar]
- Mullen JL, Turk E, Johnson K, Wolverton C, Ishikawa H, Simmons C, Söll D, Evans ML. Root-growth behavior of the Arabidopsis mutant rgr1: roles of gravitropism and circumnutation in the waving/coiling phenomenon. Plant Physiol. 1998;118:1139–1145. doi: 10.1104/pp.118.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JL, Wolverton C, Ishikawa H, Evans ML. Kinetics of constant gravitropic stimulus responses in Arabidopsis roots using a feedback system. Plant Physiol. 2000;123:665–670. doi: 10.1104/pp.123.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JL, Wolverton C, Ishikawa H, Hangarter RP, Evans ML. Spatial separation of light perception and growth response in maize root phototropism. Plant Cell Environ. 2002;25:1191–1196. doi: 10.1046/j.1365-3040.2002.00899.x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nagy F, Schäfer E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu Rev Plant Biol. 2002;53:329–355. doi: 10.1146/annurev.arplant.53.100301.135302. [DOI] [PubMed] [Google Scholar]
- Okada K, Shimura Y. Mutational analysis of root gravitropism and phototropism of Arabidopsis thaliana seedlings. Aust J Plant Physiol. 1992;19:439–448. [Google Scholar]
- Parker K, Baskin TI, Briggs WR. Evidence for a phytochrome-mediated phototropism in etiolated pea seedlings. Plant Physiol. 1989;89:493–497. doi: 10.1104/pp.89.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH, Hangarter RP. Phytochrome A regulates the induction of phototropic enhancement in Arabidopsis thaliana. Plant Physiol. 1996;110:155–162. doi: 10.1104/pp.110.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Hangarter RP, Sharrock RA, Nagy F, Schäfer E. The light-induced reduction of the gravitropic growth-orientation of seedlings of Arabidopsis thaliana (L.) Heynh is a photomorphogenic response mediated synergistically by the far-red-absorbing forms of phytochromes A and B. Planta. 1996;199:511–514. doi: 10.1007/BF00195180. [DOI] [PubMed] [Google Scholar]
- Quail PH. Phytochrome photosensory signaling networks. Nat Rev Mol Cell Biol. 2002;3:85–93. doi: 10.1038/nrm728. [DOI] [PubMed] [Google Scholar]
- Robson PRH, Smith H. Genetic and transgenic evidence that phytochromes A and B act to modulate the gravitropic orientation of Arabidopsis thaliana hypocotyls. Plant Physiol. 1996;110:211–216. doi: 10.1104/pp.110.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppel NJ, Hangarter RP, Kiss JZ. Red-light-induced positive phototropism in Arabidopsis roots. Planta. 2001;212:424–430. doi: 10.1007/s004250000410. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plant gravity sensing. Int Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K. RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell. 2000;12:225–236. doi: 10.1105/tpc.12.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitha S, Zhao L, Sack FD. Interaction of root gravitropism and phototropism in Arabidopsis wild-type and starchless mutants. Plant Physiol. 2000;122:453–461. doi: 10.1104/pp.122.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolverton C, Mullen JL, Ishikawa H, Evans ML. Root gravitropism in response to a signal originating outside of the cap. Planta. 2002;215:153–157. doi: 10.1007/s00425-001-0726-9. [DOI] [PubMed] [Google Scholar]