Abstract
Tetrahydrofolate (THF) is a central cofactor for one-carbon transfer reactions in all living organisms. In this study, we analyzed the expression of dihydropterin pyrophosphokinase-dihydropteroate synthase (HPPK-DHPS) in pea (Pisum sativum) organs during development, and so the capacity to synthesize dihydropteroate, an intermediate in the de novo THF biosynthetic pathway. During seedling development, all of the examined organs/tissues contain THF coenzymes, collectively termed folate, and express the HPPK-DHPS enzyme. This suggests that each organ/tissue is autonomous for the synthesis of THF. During germination, folate accumulates in cotyledons and embryos, but high amounts of HPPK-DHPS are only observed in embryos. During organ differentiation, folate is synthesized preferentially in highly dividing tissues and in photosynthetic leaves. This is associated with high levels of the HPPK-DHPS mRNA and protein, and a pool of folate 3- to 5-fold higher than in the rest of the plant. In germinating embryos and in meristematic tissues, the high capacity to synthesize and accumulate folate correlates with the general resumption of cell metabolism and the high requirement for nucleotide synthesis, major cellular processes involving folate coenzymes. The particular status of folate synthesis in leaves is related to light. Thus, when illuminated, etiolated leaves gradually accumulate the HPPK-DHPS enzyme and folate. This suggests that folate synthesis plays an important role in the transition from heterotrophic to photoautotrophic growth. Analysis of the intracellular distribution of folate in green and etiolated leaves indicates that the coenzymes accumulate mainly in the cytosol, where they can supply the high demand for methyl groups.
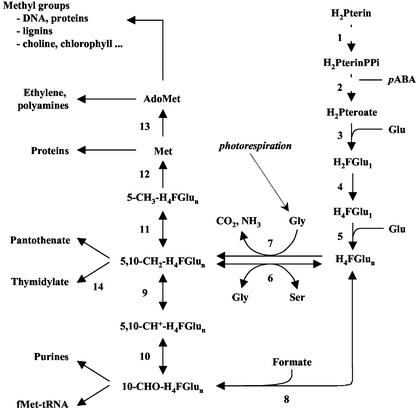
The synthesis of numerous biological compounds and the regulation of many metabolic processes require the addition or removal of one-carbon units (C1 metabolism). Tetrahydrofolate (THF) coenzymes mediate these C1 transfer reactions that are involved in several major cellular processes, including the synthesis of purines and thymidylate, amino acid metabolism, pantothenate synthesis, mitochondrial and chloroplastic protein biogenesis, and Met synthesis (Fig. 1). Met is the direct precursor of S-adenosyl-Met (Ado-Met), which in turn is the source of methyl units for the synthesis of a myriad of molecules such as choline, chlorophyll, or lignin (for reviews, see Cossins, 2000; Scott et al., 2000; Hanson and Roje, 2001). In plants, THF is also involved in the photorespiratory cycle, a specific pathway that occurs at very high rates in green leaves from C3 plants. Photorespiration relies on two THF-dependent enzymes present in the matrix space of leaf mitochondria, the Gly decarboxylase complex (GDC) and Ser hydroxymethyltransferase (SHMT; for reviews, see Oliver, 1994; Douce et al., 2001).
Figure 1.
Overview of key reactions in THF synthesis and one-carbon metabolism in plant cells. Enzymes involved in THF biosynthesis are 1, dihydropterin pyrophosphokinase; 2, dihydropteroate synthase; 3, dihydrofolate synthetase; 4, dihydrofolate reductase; and 5, folylpoly-Glu synthetase. Enzymes involved in the transfer of C1 units are 6, SHMT; 7, Gly decarboxylase; 8, 10-formyl H4F synthetase; 9, methylene H4F dehydrogenase; 10, methenyl H4F cyclohydrolase; 11, methylene H4F reductase; 12, Met synthase; 13, Ado-Met synthetase; and 14, thymidylate synthase. Reactions 1–2 (gray squares), 4–14, and 9–10 are catalyzed by bifunctional enzymes. H2Pterin(PPi), Dihydropterin(pyrophosphate); H2Pteroate, dihydropteroate; H2F-Glu1, dihydrofolate mono-Glu; H4F-Glu1(n), THF mono(poly) Glu; pABA, p-aminobenzoate; f-Met-tRNA, formylmethionyl-tRNA; Ado-Met, S-adenosyl Met.
THF is composed of three distinct parts, namely a pterin ring, a p-aminobenzoic acid, and a poly-Glu chain of variable length (1–8 residues). Its function is to bind, transport, and donate C1 units that differ in their oxidation state (methyl, methylene, methenyl, or 10-formyl, from the most reduced to the most oxidized; Fig. 1). Thus, the cofactor exists with diverse chemical forms, and these various derivatives are collectively termed folate or vitamin B9 (Cossins, 2000; Scott et al., 2000; Hanson and Roje, 2001). From dihydropterin and p-aminobenzoic acid, the biosynthesis of THF in plants and microorganisms requires the sequential operation of five reactions (Fig. 1, reactions 1–5), the first three being absent in animals (for reviews, see Scott et al., 2000; Hanson and Gregory, 2002). In plants, mitochondria play a central role in this synthesis (Neuburger et al., 1996; Rébeillé et al., 1997; Ravanel et al., 2001). Leaf mitochondria contain all of the required enzymes, and the first three steps are presumably exclusively localized in this compartment. In contrast, the last step involved in the formation of the poly-Glu tail is present in the cytosol and in the chloroplasts in addition to the mitochondria (Ravanel et al., 2001).
Despite its low concentration in plant tissues (Cossins, 1984), folate is likely to be of major importance during seedling development due to the housekeeping functions mediated by folate coenzymes (Fig. 1). In this regard, it is noteworthy that the pool of folate in pea (Pisum sativum) cotyledons increased during germination and that the inhibition of de novo synthesis of THF using folate analogs blocked seedling development (Roos and Cossins, 1971; Gambonnet et al., 2001). Also, a continuous synthesis of THF is essential to maintain high rates of Ser synthesis through the mitochondrial activities of GDC and SHMT in Arabidopsis (Prabhu et al., 1996).
In a previous study, we analyzed the distribution of folate during pea development and showed that folate coenzymes accumulate in green leaves, most probably to enable high fluxes of C1 units that are necessary for the accomplishment of photosynthesis and photorespiration (Gambonnet et al., 2001). In this paper, we examined the expression of the enzyme dihydropterin pyrophosphokinase-dihydropteroate synthase in pea organs during development, and so the enzymatic capacity for the synthesis of dihydropteroate, an intermediate in the de novo THF biosynthetic pathway. During germination and early stages of development, we found that folate accumulates in the whole seed but that de novo synthesis of THF was stimulated only in the embryos. During later stages of development, folate synthesis and accumulation were found greatest in highly dividing tissues and in photosynthetic leaves. The regulation of de novo THF synthesis in these particular physiological situations is discussed in relation to the involvement of folate coenzymes in DNA synthesis, photorespiration, and methylation cycle.
RESULTS
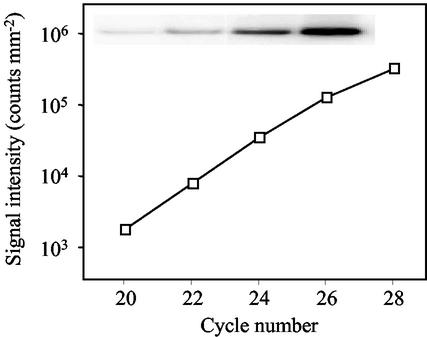
Dihydropterin pyrophosphokinase-dihydropteroate synthase (HPPK-DHPS) is a bifunctional enzyme that catalyzes the first two reactions specific to de novo THF synthesis (Fig. 1, reactions 1 and 2). In pea, Southern-blot analysis indicated that a single-copy gene codes for this mitochondrial enzyme (Rébeillé et al., 1997). The relative abundance of the mRNA coding pea HPPK-DHPS is determined by semiquantitative reverse transcription (RT)-PCR analysis (Fig. 2). Results are normalized to the amount of total RNA used in the RT step because, to our knowledge, no gene has been demonstrated to be constitutively expressed in pea during the examined period of growth (see “Materials and Methods” section). The expression of the HPPK-DHPS protein is monitored by western blotting using specific antibodies, and the amount of soluble proteins is used as a reference.
Figure 2.
Quantification of the HPPK-DHPS mRNA by semiquantitative RT-PCR. Total RNA was isolated from 6-d-old green pea leaves using the RNeasy Plant Mini Kit (Qiagen USA, Valencia, CA), reverse transcribed, and PCR-amplified with primers specific for HPPK-DHPS. Templates amplified for 20 to 28 cycles were analyzed by Southern blot, and signal intensity was quantified using a phosphor imager (background values are around 103 counts mm−2). The insert corresponds to the scanned image obtained in the log-linear range of amplification of the target gene.
Folate Synthesis during Germination and Early Stages of Development
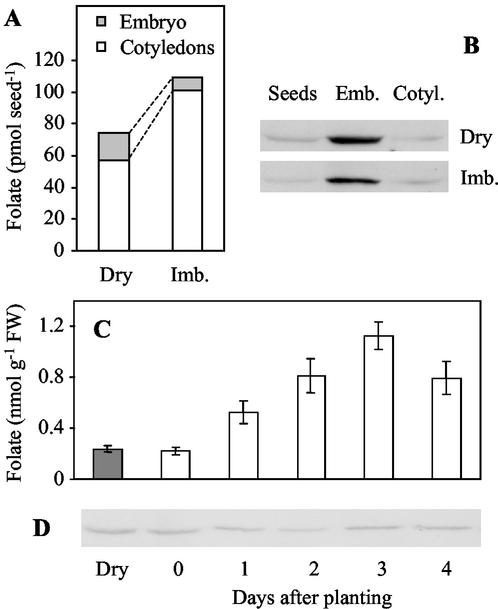
In dry pea seeds, folate is present in very low concentration (0.35 ± 0.03 nmol g−1 fresh weight) and is distributed unequally between the embryos and the cotyledons. Dry embryos contain 7.5 ± 0.37 nmol folate g−1 fresh weight and cotyledons contain only 0.27 ± 0.03 nmol folate g−1 fresh weight. Although it represents only 1% of the dry seed fresh weight, the embryo contains 23% of the seed folate (Fig. 3A). In dry seeds, the amount of the HPPK-DHPS protein in the embryos and the cotyledons matches the distribution of folate (Fig. 3B). The enzyme is present in high amount in dry embryos, whereas it is present in trace amount in the cotyledons. During the imbibition process, the folate pool increases from 74 ± 5 to 110 ± 9 pmol seed−1, which is suggestive of a de novo synthesis of THF (Fig. 3A). Only the cotyledons are concerned by the increase in folate content (1.8-fold as compared with dry tissues), whereas the folate concentration in the embryos decreases by nearly 50%. Thus, 90% of the folate pool is localized in the two large cotyledons of the imbibed seeds (Fig. 3A). The evolution of folate distribution during imbibition is not accompanied by a significant change in the HPPK-DHPS protein levels in the embryos or in the cotyledons (Fig. 3B).
Figure 3.
Changes in folate content and HPPK-DHPS protein expression during germination. Folate distribution (A) and HPPK-DHPS protein expression (B) in dry and imbibed pea seeds. Folate accumulation (C) and HPPK-DHPS protein expression (D) in cotyledons during germination and the early stages of development. Dry mature pea seeds were imbibed for 18 h at room temperature in circulating water and then planted in vermiculite (d 0). Growth was conducted at 22°C with a photoperiod of 12 h. Folate was determined using a microbiological assay with Lactobacillus casei. Values are means ± se of three separate experiments, each performed in triplicate. The amount of the HPPK-DHPS protein was analyzed by western blotting using 60 μg of soluble proteins for each sample. Quantification of the mRNA coding HPPK-DHPS was not realized because the extraction of total RNA from dry seeds and cotyledons using the RNeasy Plant Mini Kit (Qiagen USA) results in low amounts of RNA, which are not suitable for RT-PCR experiments in our standard conditions.
After imbibition, the accumulation of folate in the cotyledons continues during the first 3 d of growth (5-fold increase, Fig. 3C). It is surprising to note that the amount of the HPPK-DHPS protein remains low and constant during this period (Fig. 3D). The folate concentration remains below 1.5 nmol g−1 fresh weight and slowly decreases back to the initial value (0.3 nmol g−1 fresh weight) after 1 week. The initial rise corresponds to a de novo synthesis of THF because it is not observed when the seeds are imbibed and watered in presence of 100 μm asulam (methyl sulfanilylcarbamate, Asulox, Rhône-Poulenc, France), a sulfonamide that inhibits the DHPS reaction and thus blocks THF synthesis.
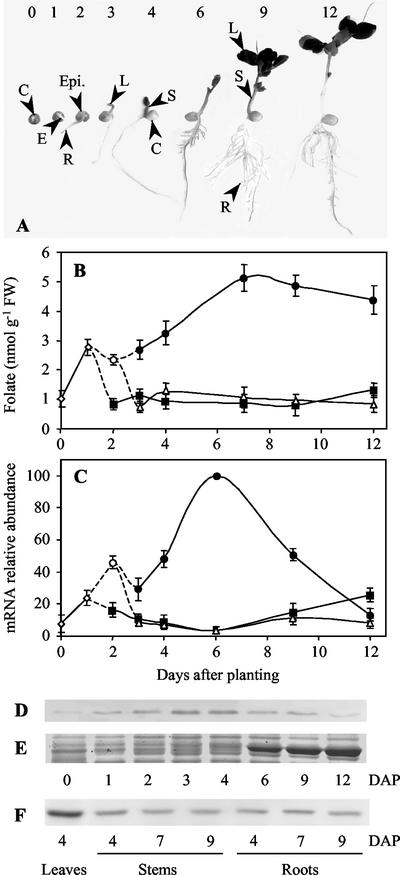
During the 1st d of growth, de novo synthesis of folate is also observed in developing embryos where the pool of folate increases approximately 3-fold (Fig. 4B). Contrary to the situation observed in cotyledons, this accumulation of folate is concomitant with a rise in the abundance of the HPPK-DHPS mRNA and protein (Fig. 4, C and D). At d 2, the steady-state levels of the mRNA and protein for HPPK-DHPS increase in the epicotyl, the part of the embryo located above the cotyledons, i.e. that produces the aerial plant organs. Regarding the central position of folate and C1 transfer reactions in plant cell metabolism (Fig. 1), the strong stimulation of THF synthesis in the embryos during germination and the early stages of seedling development is not surprising because this period is characterized by a transition from a quiescent to an active metabolic state and a resumption of cell cycle activity (Bewley, 1997).
Figure 4.
Changes in folate content and HPPK-DHPS mRNA and protein expression in pea organs during development in the light. A, Pea seedlings at different stages of development. Imbibed seeds were planted in vermiculite at d 0 and grown at 22°C with a photoperiod of 12 h. At d 0 and 1, embryos (E) could be analyzed apart from the cotyledons (C). From d 2, the embryos could be separated into the epicotyls (Epi.) and the roots (R). The separation of stems (S) and leaves (L) occurred at d 3. The first leaves to appear are followed during all of the examined period of growth. B, Changes in folate content in pea organs during development. Folate was determined in embryos (⋄), epicotyls (○), roots (▪), leaves (●), and stems (▵) using a microbiological assay with L. casei. C, HPPK-DHPS mRNA expression in pea organs during development. The amount of HPPK-DHPS mRNA was estimated by semiquantitative RT-PCR using total RNA isolated from the different organs (symbols are similar to B). The amount of mRNA detected in 6-d-old leaves was the highest and was used as a reference for comparative analysis. D through F, HPPK-DHPS protein expression in pea organs during development. D, Thirty micrograms of soluble proteins extracted from embryos (d 0 and 1), epicotyls (d 2), and leaves (d 3–12) were analyzed by western blot with antiserum to HPPK-DHPS. E, Staining with Coomassie Brilliant Blue (polypeptides in the 50–60 kD range) illustrates the accumulation of the large subunit of Rubisco (about 55 kD) during leaf development. This polypeptide migrates just above HPPK-DHPS (52 kD), thus packing this protein when it accumulates. Therefore, from d 6, the amount of HPPK-DHPS is probably underestimated. F, Proteins (30 μg) from 4-d-old leaves, stems, and roots (d 4–9) are analyzed by western blotting with HPPK-DHPS antiserum. The data shown in this figure are means ± se from at least two determinations using three independent cultures of pea seedlings. DAP, Days after planting.
Folate Synthesis in Meristematic Tissues
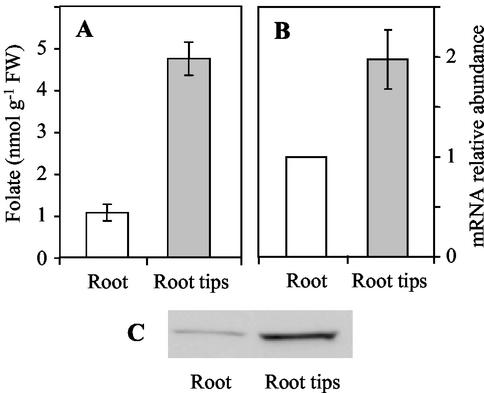
To examine the relationship between folate synthesis and accumulation and the proliferating status of cells, we used root tips as a source of meristematic tissue. Folate measurements indicate that root tips contain, on a fresh weight basis, 5-fold more coenzymes than the mature root (Fig. 5A). Also, the strong difference in folate content can be paralleled with the high relative abundance of the HPPK-DHPS mRNA and protein detected in root tips as compared with the entire differentiated organ (Fig. 5, B and C). These results fit well with the high requirement for nucleotide synthesis in actively dividing tissues and the use of methylene-THF and 10-formyl-THF for the synthesis of thymidylate and purines (Fig. 1). They suggest that proliferating tissues have a high capacity to synthesize and accumulate folate coenzymes.
Figure 5.
Folate accumulation, HPPK-DHPS mRNA and protein expression in meristematic tissues. A, Folate content in roots (whole organs) and root tips collected from 7-d-old pea seedlings grown in the light. B, Relative abundance of the mRNA for HPPK-DHPS in roots and root tips. The amount of mRNA coding HPPK-DHPS was measured by semiquantitative RT-PCR using total RNA isolated from whole root and root tips. The amount of mRNA detected in differentiated roots was used as a reference. C, Accumulation of the HPPK-DHPS protein in roots and root tips. Soluble proteins (30 μg) were analyzed by western blotting with antiserum to HPPK-DHPS. Values in A and B are the means ± se of three separate experiments, each performed in triplicate.
Folate Synthesis in Differentiating Organs during Development
The results presented in Figure 4 indicate that there is a good correlation between the abundance of the mRNA and protein for HPPK-DHPS and the concentration of folate in differentiating pea organs. Roots and stems that contain low concentrations of folate (≤ 1.5 nmol g−1 fresh weight) also exhibit low amounts of the mRNA and protein for the first enzyme involved in THF synthesis. In leaves, the concentration of folate increases gradually to reach a maximum after 7 d (Fig. 4B), i.e. a stage at which the photosynthetic apparatus builds up (Vauclare et al., 1996). High concentration of the vitamin (4–5 nmol g−1 fresh weight) is then maintained in the mature leaf, which finally contains about three times more folate than other organs. Between d 3 to 6 the abundance of the HPPK-DHPS mRNA increases to reach the highest steady-state level observed in any organ or stage of development (Fig. 4B). Then the amount of the transcript progressively decreases to reach the basal level observed in roots or stems. Interestingly, the changes in the level of the HPPK-DHPS mRNA measured during the first 7 d of development match the pattern previously observed for the small subunit of the Rubisco enzyme and the Gly cleavage system (Vauclare et al., 1996). This observation suggests that de novo THF biosynthesis and folate accumulation in leaves are related to photosynthesis. To examine this possibility, we compared folate synthesis and accumulation in green and etiolated leaves.
Folate Synthesis in Green versus Etiolated Leaves
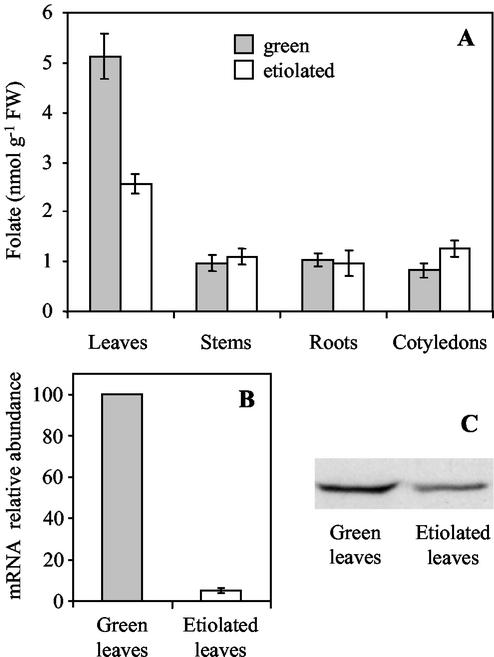
Pea seedlings were grown in the dark and folate was determined in the different organs. Folate concentrations are similar to that measured in green seedling organs except for leaves (Fig. 6A). Between d 4 to 12, the folate concentration in etiolated leaves is maintained at about 2.5 nmol g−1 fresh weight, which is one-half the value measured in green leaves. In agreement with this result, the steady-state levels of the HPPK-DHPS mRNA and protein measured in 6-d-old leaves are lower in etiolated than in green leaves (Fig. 6, B and C). These observations indicate that the particular status of folate metabolism in leaves is related to light. Thus, they raise the questions of (a) the intracellular distribution of folate coenzymes in green versus etiolated leaves, and in particular their accumulation in a specific compartment during greening; and (b) the behavior of folate and THF-synthesizing enzymes during de-etiolation.
Figure 6.
Comparison of folate accumulation, HPPK-DHPS mRNA and protein expression in light- and dark-grown pea seedlings. Pea seedlings were grown at 22°C in the light (with a photoperiod of 12 h) or in the dark for 7 d before sample harvesting and analysis. A, Folate content in the organs of light- and dark-grown pea seedlings. B, Relative abundance of the mRNA for HPPK-DHPS in green and etiolated leaves. The amount of mRNA coding HPPK-DHPS was measured by semiquantitative RT-PCR using total RNA isolated from green and etiolated leaves. The amount of mRNA detected in green leaves was used as a reference. C, Accumulation of the HPPK-DHPS protein in green and etiolated leaves. Soluble proteins (30 μg) were analyzed by western blot with antiserum to HPPK-DHPS. In A and B, values ± se are means of triplicate determinations for at least three independent experiments.
Subcellular Distribution of Folate in Green and Etiolated Leaves
Previous studies described the folate content of pea leaf organelles (Neuburger et al., 1996; Gambonnet et al., 2001), but the data were not sufficient to establish and compare the subcellular distribution of folate in green and etiolated leaves. To obtain a complete set of data, we measured folate concentrations in whole-leaf extracts and in organelles (mitochondria and plastids) purified from both green and etiolated leaves using Percoll gradients. As shown in Table I, green leaf mitochondria have double the folate (on a protein basis) of etiolated leaf mitochondria. No major difference can be observed, however, when comparing folate contents in purified chloroplasts (35 pmol mg−1 protein) and etioplasts (45 pmol mg−1 protein). In leaf extracts, the amount of proteins originating from organelles was estimated through various markers (see “Materials and Methods”). From these data we calculated the distribution of folate within mitochondria, plastids, and the remaining compartments (“cytosolic” fraction that comprised cytosol, nucleus, and vacuole; Table I). Mitochondria are the subcellular compartment exhibiting the highest folate concentration under the two physiological conditions examined (light- and dark-grown seedlings), an observation that may be related to the fact that mitochondria are the unique site for de novo synthesis of THF. The highest concentration measured in mitochondria from green leaves could be explained by the presence of two folate-dependent enzymes, SHMT (Fig. 1, reaction 6) and the T-protein of GDC (Fig. 1, reaction 7), that accumulate during greening and represent up to 40% of soluble proteins in mitochondria from mature pea leaves (Oliver et al., 1990; Bardel et al., 2002). However, mitochondria represent a small cellular compartment (4%–6% of the leaf soluble proteins) and thus cannot account for the 2-fold difference in folate concentration between green and etiolated leaves. The part of plastid folate in the cellular pool of the vitamin increases more than 2-fold between etiolated and green leaves (11 versus 24 pmol mg−1 leaf soluble proteins, respectively). Because plastids contain only 9% to 10% of total folate in the two types of leaves, the high value observed in chloroplasts versus etioplasts cannot explain the whole-folate increase in green leaves. Altogether, the data presented in Table I indicate that 60% of the folate present in green leaves is recovered in the so-called cytosolic fraction. The contribution of this fraction to the total folate pool increases by approximately 2.5-fold between etiolated and green leaves, and represents 70% of the total difference (84 out of 115 pmol mg−1 protein).
Table I.
Intracellular distribution of folate in green and etiolated pea leaves
| Folate Content | Protein Content | Folate Distribution | |
|---|---|---|---|
| pmol mg−1 protein | % | pmol mg−1 leaf soluble protein | |
| Green leaves | |||
| Whole leaf | 240 ± 25 | 100 | 240 ± 25 |
| Mitochondria | 1,800 ± 300 | 4 ± 2 | 72 ± 6 |
| Chloroplasts | 35 ± 4 | 70 ± 8 | 24 ± 3 |
| Cytosolic fraction | 550 | 26 | 144 |
| Etiolated leaves | |||
| Whole leaf | 125 ± 25 | 100 | 125 ± 25 |
| Mitochondria | 900 ± 200 | 6 ± 2 | 54 ± 4 |
| Etioplasts | 45 ± 4 | 24 ± 5 | 11 ± 2 |
| Cytosolic fraction | 110 | 72 | 60 |
Mitochondria and plastids were purified from pea leaves after 9 d of growth in the light (12-h photoperiod) or in darkness. Folate was determined after osmotic lysis of organelles, and values are expressed on the basis of soluble proteins. The contribution of organelles proteins to whole cell soluble proteins was estimated through fumarase activity measurements for mitochondria (Hill and Bradshaw, 1969) and chlorophyll/carotenoids determinations for chloroplasts/etioplasts (Lichtenthaler, 1987). Values are an average of four different determinations ± se. To estimate the proteins/folate contents in the cytosolic fraction (which comprised cytosol, nucleus, and vacuole), the average values obtained for the organelles were subtracted from the values obtained for the whole-leaf organ. For each subcellular compartment, folate distribution is expressed as picomoles per milligram of protein with reference to total soluble proteins measured in the green/etiolated leaves.
Folate Synthesis in Leaf during De-Etiolation
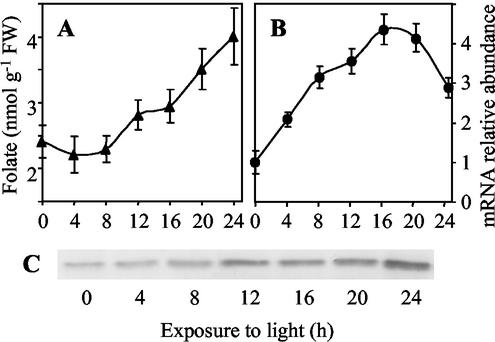
To analyze the relationship between light and the particular status of folate metabolism in leaves, 7-d-old etiolated seedlings were exposed to light. As shown in Figure 7A, the concentration of folate remains stable (approximately 2.5 nmol g−1 fresh weight) during the first 8 h of de-etiolation and then increases slowly to reach about 4 nmol g−1 fresh weight after 24 h of light exposure. This value is similar to the one measured in leaves collected from seedlings grown under light conditions (Fig. 4B). During the de-etiolation process, the amount of the HPPK-DHPS mRNA increases gradually up to 16 h after the onset of illumination (Fig. 7B). Western-blot analysis shows that the HPPK-DHPS protein accumulates during this period (Fig. 7C). Altogether, these results indicate that de novo THF synthesis and folate accumulation in leaves are stimulated by light.
Figure 7.
Folate accumulation, HPPK-DHPS mRNA and protein expression in leaves during de-etiolation. Pea seedlings were grown for 7 d in the dark and then transferred to continuous white light (100 μmol m−2 s−1). A, Folate content in leaves during de-etiolation. B, Steady-state level of the mRNA for HPPK-DHPS during de-etiolation. The mRNA coding HPPK-DHPS was measured by semiquantitative RT-PCR using total RNA isolated from leaves. The amount of mRNA detected in 7-d-old etiolated leaves was used as a reference. C, Accumulation of the HPPK-DHPS protein during de-etiolation. Soluble proteins (30 μg) were analyzed by western blot with antiserum specific to HPPK-DHPS. Values in A and B are the means ± se of triplicate determinations for a representative de-etiolation experiment.
DISCUSSION
Germination and subsequent post-germinative growth are characterized by major cellular events that involve C1 metabolism and the pool of folate coenzymes. The proteomic analysis of Arabidopsis seed germination indicated that Met synthase and two isoforms of Ado-Met synthetase, two enzymes participating in the methylation cycle (Fig. 1, reactions 12 and 13), accumulate during the first 2 d of imbibition (Gallardo et al., 2001, 2002). The accumulation of these housekeeping proteins strongly suggests that a huge demand for methyl groups, and thus for methyl-THF, must be fulfilled to ensure a myriad of methylation reactions as well as ethylene or polyamines syntheses (Fig. 1). It is not surprising therefore that folate and the HPPK-DHPS enzyme are present in high amounts in dry mature embryos to allow a rapid resumption of C1 metabolism upon seed imbibition. The reserve of folate that accumulates in embryos during seed maturation is sufficient to allow germination even in the presence of a sulfonamide that blocks de novo synthesis of THF. During seed imbibition and the first 3 d of germination, the pool of folate increases several times in the cotyledons (Fig. 3C). It was shown previously that the cotyledonary folate pool contains principally methylated derivatives (Roos and Cossins, 1971) and that the concentration of folylpoly-Glu derivatives increases gradually during germination (Chan et al., 1986). It is surprising therefore that the accumulation of highly conjugated folate derivatives is not accompanied by an increase in the amount of the HPPK-DHPS protein (Fig. 3) nor in the FPGS activity (Fig. 1, reaction 5; Chan et al., 1986). In the embryos, however, the increase in folate concentration during germination and early stages of plant growth corresponds to a rise in the abundance of the HPPK-DHPS mRNA and protein. Thus, the embryos are autonomous for the synthesis of folate during this period and apparently do not rely on a transport of cotyledonary folate. This assumption is supported by the observation that folate accumulation in embryos during the first 3 d of growth is not affected by a physical separation from the cotyledons (not shown).
The completion of germination is accompanied by cell division. In mature seeds, most of the embryonic cells are arrested in the G1-phase of the cell cycle, and the transition from quiescent to proliferating status that occurs during seed imbibition is characterized by DNA synthesis (de Castro et al., 2000). Root tips also provide a good source of meristematic cells. We found that these proliferating tissues contain high levels of the HPPK-DHPS mRNA and protein as well as an elevated concentration of folate, as compared with quiescent tissues. In maize (Zea mays), the transcripts for dihydrofolate reductase thymidylate synthase were shown to accumulate to high levels in root tips and in developing kernels, when endosperm cells are undergoing endoreplication (Cox et al., 1999). This singular expression pattern was attributed only to a requirement for DNA synthesis and to the involvement of the bifunctional dihydrofolate reductase thymidylate synthase enzyme in thymidylate synthesis (Fig. 1, reaction 14). Our results indicate that actively dividing cells not only require a higher rate of nucleotide synthesis but also a higher rate of THF synthesis. This suggests that folate turnover could be a potential limiting factor in proliferating cells.
In addition to actively dividing tissues, folate synthesis and accumulation were found to be elevated in photosynthetic leaves. During leaf development in the light, the expression pattern of the mRNA for HPPK-DHPS follows the accumulation of the transcripts coding proteins involved in photosynthesis and photorespiration, e.g. the small subunit of Rubisco and the constituents of the Gly cleavage system (Vauclare et al., 1996). Also, the HPPK-DHPS protein and folate accumulate gradually during de-etiolation (Fig. 7). Upon light exposure, the HPPK-DHPS mRNA fails to exhibit a strong induction, suggesting that the observed accumulation is an indirect response to illumination and most probably is a consequence of the stimulation of photosynthesis and photorespiration by light (Oliver, 1994). These observations suggest that a huge demand for folate and thus for C1-transfer reactions is associated with leaf development in the light. Comparison of folate distribution in green and etiolated mesophyll cells indicates that the 2-fold increase of the folate pool observed during greening is mainly due to the accumulation of the coenzymes in the cytosolic fraction (Table I). This finding may be unexpected because plastids and mitochondria are basically the cellular compartments undergoing the most dramatic structural and physiological modifications during transition from darkness to light (building of the photosynthetic and photorespiratory apparatus). C1 transfer reactions that are unique to the cytosol consist in the reduction of methylene-THF into methyl-THF (Fig. 1, reaction 11; Roje et al., 1999) and in the synthesis of Met and Ado-Met (Fig. 1, reactions 12 and 13; Ravanel et al., 1998). Thus, the high cytosolic pool of folate in green tissues may reflect a high demand for C1 units for the accomplishment of the methylation cycle. This assumption is supported by a previous analysis, indicating that methyl-THF is the major form of the coenzyme found in the cytosol of green pea leaves (Chen et al., 1997). The importance of methylation reactions during de-etiolation can be also estimated by simply measuring the accumulation of chlorophyll, whose synthesis requires one Ado-Met-dependent methylation step (von Wettstein et al., 1995). A flux of methyl groups through chlorophyll synthesis of approximately 40 nmol h−1 g−1 fresh weight was measured during the 24 h after the onset of illumination. Assuming a methyl-THF pool of 0.6 to 0.7 nmol g−1 fresh weight in etiolated leaves (48% of folate in etiolated leaves is located in the cytosol as indicated in Table I and 53% of folate in a cytosolic-enriched fraction of pea leaf corresponds to the methyl derivative; Chen et al., 1997), one can estimate that chlorophyll synthesis alone will deplete the cytosolic pool of methyl-THF in about 1 min.
In this study, we obtained new information concerning the regulation of de novo THF synthesis in various physiological situations. However, these data alone cannot explain the maintenance of typical folate concentrations in organs or tissues, which results from the balance between synthesis and breakdown routes. Our knowledge concerning folate breakdown in plants is limited (for a review, see Hanson and Gregory, 2002). Yet, it is established that THF is sensitive to chemical oxidation, and green leaves produce reactive oxygen species in the light (Scott et al., 2000). As a result, the elevated capacity of green leaves to synthesize folate at a high rate may also be required to compensate for breakdown of folate coenzymes. Thus, future work is needed to determine how plants sense their folate status and thus regulate the synthesis and breakdown of THF to meet the cellular demands for C1 units.
MATERIALS AND METHODS
Plant Material
Pea (Pisum sativum L. var Douce Provence) seeds were imbibed during 18 h in circulating tap water and planted in moist vermiculite. Plants were grown for 12 d under a 12-h photoperiod (140 μmol m−2 s−1) at 22°C (day) and 20°C (night). Etiolated pea plants were grown in complete darkness at 20°C, and organs were collected under a green safelight. In de-etiolation experiments, 7-d-old etiolated seedlings were transferred to continuous white light (100 μmol m−2 s−1), and leaves were harvested every 4 h during a 24-h period. For folate, RNA, and protein analyses, organs and tissues were collected and immediately ground in liquid N2 with mortar and pestle. Samples were stored at −80°C until use.
Isolation of Plastids and Mitochondria
For the preparation of plastids and mitochondria, green or etiolated leaves were collected from 9-d-old seedlings. Green leaf mitochondria were isolated and purified as described by Douce et al. (1987) using a self-generating gradient of Percoll. Mitochondria from etiolated leaves were isolated using the procedure described for potato (Solanum tuberosum) tuber mitochondria (Douce et al., 1987). Chloroplasts were isolated and purified on a continuous Percoll gradient as described by Douce and Joyard (1982). To obtain etioplasts, etiolated pea leaves were briefly homogenized in chilled extracting buffer (25 mm MOPS, pH 7.2, 0.45 m Suc, 1 mm EDTA, 1 mm MgCl2, 0.1% [w/v] bovine serum albumin, and 0.2% [w/v] ascorbate) and then filtered on four layers of muslin and one of 78-μm nylon netting. The suspension was centrifuged for 10 min at 3,500 rpm (GS3 rotor, Sorvall, Newton, CT). Pellets were then resuspended in washing medium (25 mm MOPS, pH 7.2, 0.45 m Suc, 1 mm EDTA, and 1 mm MgCl2) and centrifuged for 10 min at 3,500 rpm (SS34 rotor, Sorvall). Pellets were resuspended in a small volume of washing medium, loaded onto a density gradient containing 30% to 80% (v/v) Percoll in washing medium, and performed for 50 min at 10,000 rpm (SS90 rotor, Sorvall). Gradients were centrifuged for 15 min at 8,000 rpm (SS90, Sorvall). Intact etioplasts were recovered from the gradient, diluted 10-fold in washing medium, and pelleted (10 min, 5,000 rpm in a SS34 rotor). Pellets were then resuspended in washing medium and centrifuged as before. Using these experimental procedures, mitochondria and plastids were found to be devoid of contamination from other cellular compartments (Douce and Joyard, 1982; Douce et al., 1987). The intactness of plastids and mitochondria was verified by measuring the latency of phosphogluconate dehydrogenase (Journet, 1987) and the oxidation of exogenous cytochrome c (Douce et al., 1987), respectively.
To estimate the relative contribution of the organelles to soluble proteins in whole-leaf tissues, fumarase and chlorophyll/carotenoids were used as markers for mitochondria and plastids, respectively. Fumarase activity was measured as described by Hill and Bradshaw (1969). Chlorophyll and carotenoids were measured in 80% (v/v) acetone using the coefficients of Lichtenthaler (1987).
Folate Measurements
Folate was extracted and determined using the microbiological assay with the folate heterotrophic bacteria Lactobacillus casei ATCC7469 (American Type Culture Collection, Manassas, VA), as described by Gambonnet et al. (2001). Folate contents were expressed on a fresh weight basis for organs and tissues, and on the basis of soluble proteins for organelles.
Semiquantitative RT-PCR Analysis
The relative abundance of the mRNA coding HPPK-DHPS was estimated by semiquantitative RT-PCR. Total RNA was extracted from the different organs using the RNeasy Plant Mini Kit (Qiagen USA). To allow comparison between the steady-state level of the HPPK-DHPS mRNA in the different organs or tissues during the examined growing period, cDNAs were synthesized from equal amounts of total RNA (2 μg) with Moloney murine leukemia virus-reverse transcriptase (Stratagene, La Jolla, CA) in the presence of oligo(dT)18. The amount of total RNA was used as criteria to normalize data because we found no “constitutive” gene during the examined period of development. We found that the actin (GenBank accession no. X68649) and ubiquitin (L81142) genes were expressed at similar steady-state levels between d 4 to 12 in the different organs, but their expressions varied considerably during germination and post-germinative events.
Total RNA was quantified spectrophotometrically and the integrity of 28S and 18S rRNA was checked by agarose gel electrophoresis and ethidium bromide staining. PCR reactions were then carried out using the Titanium Taq polymerase (BD Biosciences Clontech, Palo Alto, CA) with primers HPPKDHPS1, 5′-CTGCAGTAAGAGCGGATACG-3′, and HPPKDHPS2, 5′-GCCATTCTGGTGGACTAAATG-3′. Aliquots were analyzed on agarose gels and blotted onto nylon membranes according to Sambrook et al. (1989). Membranes were hybridized with 32P-labeled specific probes obtained by PCR using the primers described above. Relative hybridization intensities were quantified on a phosphor imager (Amersham Biosciences AB, Uppsala). To allow reliable quantification of the transcript, we determined the log-linear range of amplification with number of cycles varying from 20 to 26 (Fig. 2). Thus, this procedure was suitable to quantify the relative abundance of the HPPK-DHPS mRNA in samples with expression levels varying by 26 = 64 folds. For each sample, cDNAs were amplified for 22, 24, and 26 cycles, and quantification of the transcript was done in the log-linear range of amplification. Also, a negative control containing RNA instead of cDNA was performed to rule out genomic DNA contamination.
Immunoblot Analysis
Recombinant HPPK-DHPS from pea (Mouillon et al., 2002) was injected into Guinea pigs to raise antibodies (Centre Valbex, IUT de Biologie, Villeurbanne, France). Proteins were extracted from powdered samples by grinding in 50 mm Tris-HCl, pH 8.0, 10 mm 2-mercaptoethanol, 5% (v/v) glycerol, 1 mm phenylmethylsulfonylfluoride, and a cocktail of protease inhibitors (no. 1873580, Roche Diagnostics, Indianapolis). Samples were centrifuged at 16,000g for 20 min at 4°C, and the supernatant was used as a source of soluble proteins. Proteins were measured by the method of Lowry et al. (1951) using bovine serum albumin as standard. Proteins were resolved by SDS-PAGE and electroblotted to nitrocellulose membrane. The blots were probed using the HPPK-DHPS antibodies and horseradish peroxidase conjugated anti-Guinea pig IgGs, and detection was achieved by chemiluminescence.
ACKNOWLEDGMENTS
We thank Dr. M. Block, Dr. E. Maréchal, and Prof. J. Roberts for critical reading of the manuscript.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.016915.
LITERATURE CITED
- Bardel J, Louwagie M, Jacquinod M, Jourdain A, Luche S, Rabilloud T, Macherel D, Garin J, Bourguignon J. A survey of the plant mitochondrial proteome in relation to development. Proteomics. 2002;2:880–898. doi: 10.1002/1615-9861(200207)2:7<880::AID-PROT880>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PY, Coffin JW, Cossins EA. In vitro synthesis of pteroylpoly-γ-glutamates by cotyledon extracts of Pisum sativum L. Plant Cell Physiol. 1986;27:431–441. [Google Scholar]
- Chen L, Chan S, Cossins EA. Distribution of folate derivatives and enzymes for synthesis of 10-formyltetrahydrofolate in cytosolic and mitochondrial fractions of pea leaves. Plant Physiol. 1997;115:299–309. doi: 10.1104/pp.115.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins EA. Folates in biological materials. In: Blakley RL, Benkovic SJ, editors. Folates and Pterins. Vol. 1. New York: Wiley (Interscience); 1984. pp. 1–59. [Google Scholar]
- Cossins EA. The fascinating world of folate and one-carbon metabolism. Can J Bot. 2000;78:691–708. [Google Scholar]
- Cox K, Robertson D, Fites R. Mapping and expression of a bifunctional thymidylate synthase, dihydrofolate reductase gene from maize. Plant Mol Biol. 1999;41:733–739. doi: 10.1023/a:1006324328355. [DOI] [PubMed] [Google Scholar]
- de Castro RD, van Lammeren AAM, Groot SPC, Bino RJ, Hilhorst HWM. Cell division and subsequent radicle protrusion in tomato seeds are inhibited by osmotic stress but DNA synthesis and formation of microtubular cytoskeleton are not. Plant Physiol. 2000;122:327–335. doi: 10.1104/pp.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R, Bourguignon J, Brouquisse R, Neuburger M. Isolation of plant mitochondria: general principles and criteria of integrity. Methods Enzymol. 1987;148:403–415. [Google Scholar]
- Douce R, Bourguignon J, Neuburger M, Rébeillé F. The glycine decarboxylase system: a fascinating complex. Trends Plant Sci. 2001;6:167–176. doi: 10.1016/s1360-1385(01)01892-1. [DOI] [PubMed] [Google Scholar]
- Douce R, Joyard J. Purification of the chloroplast envelope. In: Eldman M, Hallick R, Chua NH, editors. Method in Chloroplast Molecular Biology. Amsterdam: Elsevier/North-Holland Publishing; 1982. pp. 239–256. [Google Scholar]
- Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 2001;126:835–848. doi: 10.1104/pp.126.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D. Proteomics of Arabidopsis seed germination: a comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002;129:823–837. doi: 10.1104/pp.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambonnet B, Jabrin S, Ravanel S, Karan M, Douce R, Rébeillé F. Folate distribution during higher plant development. J Sci Food Agric. 2001;81:835–841. [Google Scholar]
- Hanson AD, Gregory JF., III Synthesis and turnover of folates in plants. Curr Opin Plant Biol. 2002;5:244–249. doi: 10.1016/s1369-5266(02)00249-2. [DOI] [PubMed] [Google Scholar]
- Hanson AD, Roje S. One-carbon metabolism in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:119–137. doi: 10.1146/annurev.arplant.52.1.119. [DOI] [PubMed] [Google Scholar]
- Hill RL, Bradshaw RA. Fumarase. Methods Enzymol. 1969;13:91–99. [Google Scholar]
- Journet EP. Isolation of plastids from buds of cauliflower (Brassica oleracea L.) Methods Enzymol. 1987;148:234–240. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mouillon JM, Ravanel S, Douce R, Rébeillé F. Folate synthesis in higher-plant mitochondria: coupling between the dihydropterin pyrophosphokinase and the dihydropteroate synthase activities. Biochem J. 2002;363:313–319. doi: 10.1042/0264-6021:3630313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M, Rébeillé F, Jourdain A, Nakamura S, Douce R. Mitochondria are a major site for folate and thymidylate synthesis in plants. J Biol Chem. 1996;271:9466–9472. doi: 10.1074/jbc.271.16.9466. [DOI] [PubMed] [Google Scholar]
- Oliver DJ. The glycine decarboxylase complex from plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:323–337. [Google Scholar]
- Oliver DJ, Neuburger M, Bourguignon J, Douce R. Interaction between the component enzymes of the glycine decarboxylase multienzyme complex. Plant Physiol. 1990;94:833–839. doi: 10.1104/pp.94.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu V, Chatson KB, Abrams GD, King J. 13C nuclear magnetic resonance detection of interactions of serine hydroxymethyltransferase with C1-tetrahydrofolate synthase and glycine decarboxylase complex activities in Arabidopsis. Plant Physiol. 1996;112:207–216. doi: 10.1104/pp.112.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanel S, Cherest H, Jabrin S, Grunwald D, Surdin-Kerjan Y, Douce R, Rébeillé F. Tetrahydrofolate biosynthesis in plants: molecular and functional characterization of dihydrofolate synthetase and three isoforms of folylpolyglutamate synthetase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2001;98:15360–15365. doi: 10.1073/pnas.261585098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanel S, Gakière B, Job D, Douce R. The specific features of methionine biosynthesis and metabolism in plants. Proc Natl Acad Sci USA. 1998;95:7805–7812. doi: 10.1073/pnas.95.13.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébeillé F, Macherel D, Mouillon JM, Garin J, Douce R. Folate biosynthesis in higher plants: purification and molecular cloning of a bifunctional 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase/7,8-dihydropteroate synthase localized in mitochondria. EMBO J. 1997;16:947–957. doi: 10.1093/emboj/16.5.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roje S, Wang H, McNeil SD, Raymond RK, Appling DR, Shachar-Hill Y, Bohnert HJ, Hanson AD. Isolation, characterization and functional expression of cDNAs encoding NADH-dependent methylenetetrahydrofolate reductase from higher plants. J Biol Chem. 1999;274:36089–36096. doi: 10.1074/jbc.274.51.36089. [DOI] [PubMed] [Google Scholar]
- Roos AJ, Cossins EA. Pteroylglutamate derivatives in Pisum sativum L.: biosynthesis of cotyledonary tetrahydropteroylglutamates during germination. Biochem J. 1971;125:17–26. doi: 10.1042/bj1250017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Scott J, Rébeillé F, Fletcher J. Folic acid and folates: the feasibility for nutritional enhancement in plant foods. J Sci Food Agric. 2000;80:795–824. [Google Scholar]
- Vauclare P, Diallo N, Bourguignon J, Macherel D, Douce R. Regulation of the expression of the glycine decarboxylase complex during pea leaf development. Plant Physiol. 1996;112:1523–1530. doi: 10.1104/pp.112.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein D, Gough S, Kannangara CG. Chlorophyll biosynthesis. Plant Cell. 1995;7:1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]