Abstract
Fluorescence differential display was used to isolate the gibberellin (GA)-responsive gene, CsAGP1, from cucumber (Cucumis sativus) hypocotyls. A sequence analysis of CsAGP1 indicated that the gene putatively encodes a “classical” arabinogalactan protein (AGP) in cucumber. Transgenic tobacco (Nicotiana tabacum) plants overexpressing CsAGP1 under the control of the cauliflower mosaic virus 35S promoter produced a Y(βGlc)3-reactive proteoglycan in addition to AGPs present in wild-type tobacco plants. Immuno-dot blotting of the product, using anti-AGP antibodies, showed that the CsAGP1 protein had the AGP epitopes common to AGP families. The transcription level of CsAGP1 in cucumber hypocotyls increased in response not only to GA but also to indole-3-acetic acid. Although CsAGP1 is expressed in most vegetative tissues of cucumber, including the shoot apices and roots, the GA treatment resulted in an increase in the mRNA level of CsAGP1 only in the upper part of the hypocotyls. Y(βGlc)3, which selectively binds AGPs, inhibited the hormone-promoted elongation of cucumber seedling hypocotyls. Transgenic plants ectopically expressing CsAGP1 showed a taller stature and earlier flowering than the wild-type plants. These observations suggest that CsAGP1 is involved in stem elongation.
Stem elongation is governed by cell division and cell elongation. Cell elongation is controlled by the turgor pressure and cell wall extensibility in a particular direction, which is regulated by the orientation of both cellulose microfibrils and the cell wall matrix containing polysaccharides and proteins, and by the viscoelastic properties of the matrix macromolecules (Cosgrove, 1999; for review, see Shibaoka, 1994). Moreover, the process of cell elongation in a plant requires loosening of the cell wall structure and the deposition of new materials. The signals leading to these conditions directly involved in regulating stem elongation are transduced from various plant hormones. Auxin, GAs, and brassinosteroids promote stem elongation, whereas cytokinins, ethylene, and abscisic acid have a growth-inhibiting effect (for review, see Phillips, 1998). Although researchers have provided information on the signal mediators transmitting signals from plant hormones for cell elongation, the mechanism for regulating cell elongation is still poorly understood at the molecular level.
We screened for cDNAs with expression that was responsive to GA4 in cucumber (Cucumis sativus) hypocotyls by using the fluorescent differential display (FDD) method to identify new members involved in cell elongation. The deduced peptide sequence of one of those genes was predicted to be an arabinogalactan protein (AGP).
AGPs are a class of proteoglycans with broad taxonomic distribution throughout the plant kingdom (Serpe and Nothnagel, 1999). Although the precise function of AGPs currently remains speculative, evidence is accumulating to suggest that AGPs play an important role in plant growth and development (Fincher et al., 1983; Kreuger and van Holst, 1996; Nothnagel, 1997).
Experiments with monoclonal antibodies against particular AGP epitopes have demonstrated that the expression of AGPs was localized in specific tissue types, e.g. in pea (Pisum sativum) flowers (Pennell and Roberts, 1990), carrot (Daucus carota) cell suspension cultures (Thompson and Knox, 1998), and in the developing carrot root (Knox et al., 1991).
The specific interaction of AGPs with the β-glucosyl Yariv reagent [Y(β-Glc)3], an artificial carbohydrate antigen (Yariv et al., 1962), has been extensively used for isolating and identifying AGPs from plant tissues and cultured cells (Clarke et al., 1978; Fincher et al., 1983; van Holst and Clarke, 1986; Komalavilas et al., 1991; Zhu et al., 1993). In addition, Y(β-Glc)3 has been used to disrupt the AGP function in living systems and has provided insight into the role of AGPs in planta (Serpe and Nothnagel, 1994; Jauh and Lord, 1996; Willats and Knox, 1996; Langan and Nothnagel, 1997). For example, Y(β-Glc)3 have inhibited the proliferation of suspension-cultured rose (Rosa spp.) cells, which suggested that AGPs function in cell division (Serpe and Nothnagel, 1994). The involvement of AGPs in the phytohormone function has also been suggested by the observation that Y(β-Glc)3 inhibited GA-promoted induction of α-amylase in barley (Hordeum vulgare) aleurone protoplasts (Suzuki et al., 2002). The use of the Yariv reagent has also indicated that AGPs may play a role in cell elongation. Y(β-Glc)3 added to carrot suspension-cultured cells that had been induced to elongate rather than proliferate resulted in the inhibition of cell elongation (Willats and Knox, 1996). Y(β-Glc)3 also caused inhibition of the root growth and bulging of root epidermal cells in Arabidopsis seedlings (Willats and Knox, 1996; Ding and Zhu, 1997).
Although these approaches represent promising leads to the function of AGPs, the exact role of these molecules remains to be ascertained. Cloning of cDNAs encoding the core polypeptide of various AGPs has provided additional approaches for analyzing the function. DNA probes could give more specific detection of AGP gene expression than anti-AGP antibodies for AGP detection; therefore, the tissue specificity of AGP gene expression has been examined (Mau et al., 1995; Du et al., 1996; Schultz et al., 2000). This approach has supported the idea that the expression of AGP is tightly regulated in a tissue-specific manner. An analysis of transgenic plants producing sense or antisense mRNAs for the AGP core polypeptide has provided an alternative route to determine the AGP function. For example, an AGP (known as the tobacco [Nicotiana tabacum] stylar transmitting tissue [TTS] protein) that occurs in TTS has been implicated in pollen tube growth, based on an analysis of the growth rate in transgenic plants that had a reduced level of TTS protein resulting from either antisense suppression or sense cosuppression (Cheung et al., 1995).
This paper describes the cloning and characterization of the GA-responsive gene (CsAGP1) from cucumber hypocotyls that encodes a protein core characteristic of AGPs. Here, we discuss the notion that AGP was involved in stem elongation based on an analysis of transgenic plants overexpressing CsAGP1 and the inhibitory effect of Y(β-Glc)3 on hypocotyl elongation in cucumber seedlings.
RESULTS
CsAGP1 from the Cucumber Hypocotyl Encodes a Classical AGP
The fluorescence differential display method was used to isolate a cDNA whose transcriptional level increased in the hypocotyls of cucumber seedlings within 1 and 3 h after their treatment with GA4. The 908-bp full-length cDNA (GenBank accession no. AB029092) was cloned, and the gene was designated as CsAGP1. A BLAST search of the protein databases identified a number of basic Pro-rich cell wall proteins that had significant similarity to the predicted amino acid sequence of the CsAGP1 protein. LeAGP1 encoded by cDNA isolated from tomato (Lycopersicon esculentum; Li and Showalter, 1996) showed the highest identity (48%).
The deduced amino acid sequence of CsAGP1 (243 amino acids) is shown in Figure 1. The classical AGPs are characterized by the presence of three domains: an N-terminal signal sequence; a domain rich in Pro/Hyp (hyp), Ala, Ser, and Thr; and a C-terminal GPI anchor signal sequence. The pSORT program (http://psort.nibb.ac.jp/) predicted the presence in the deduced CsAGP1 peptide sequence of a signal peptide (the cleavage site at Gly-21) and a GPI anchor signal at the N- and C- terminal ends, respectively. There was a central region between these two signals that was rich in Pro (33.5%), Ala (19.8%), and Ser (16.8%). The C-terminal signal contained a GPI anchor attachment site (Ser-218) predicted by the ω/ω + 2 rule (Udenfriend and Kodukula, 1995), followed by a short spacer, a conserved basic amino acid (Lys-225), and terminated in a hydrophobic transmembrane domain. CsAGP1 could be categorized as a classical AGP based on these domain properties.
Figure 1.
Deduced amino acid sequence of CsAGP1. The putative signal peptide and C-terminal transmembrane domain predicted by pSORT (Prediction of Protein Localization Sites, version 6.4) are underlined and in italics, respectively. The Pro (P) residues in the central region between the two hydrophobic regions are shown in bold type. The potential cleavage site predicted for glycosylphosphatidylinositol (GPI) anchoring is shown with an arrowhead. The conserved Lys basic residue (K) immediately before the C-terminal transmembrane domain is double underlined.
CsAGP1 Is Reactive to Y(β-Glc)3 and Recognized by Anti-AGP Antibodies
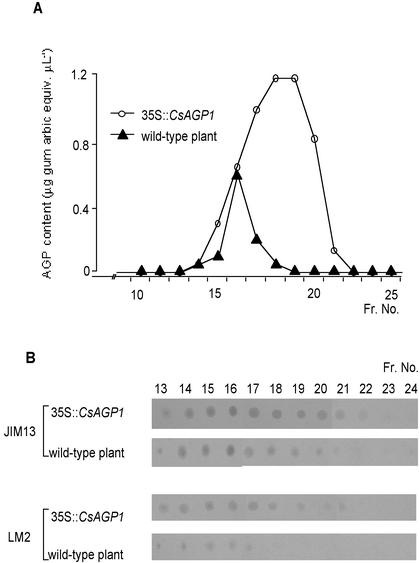
AGPs have been defined by their ability to bind Y(β-Glc)3 (Yariv et al., 1962; Fincher et al., 1983; Baldwin et al., 1993; Bosch et al., 2001). To confirm whether the product of CsAGP1 had this AGP-like property, CsAGP1 was expressed in tobacco under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The expression of the transgene was confirmed by a northern-blot analysis with T1 transgenic tobacco (data not shown). AGPs in either transgenic or wild-type leaf tissue were purified by coprecipitation with Y(β-Glc)3 and reverse-phase (RP)-HPLC, fractionated further by gel permeation chromatography (GPC), and quantified with a single radial diffusion assay to monitor the binding capacity with Y(β-Glc)3. As shown in Figure 2A, the fractions from the transgenic tobacco extract showed a prominent Y(β-Glc)3-reactive peak that was clearly larger than and had a different retention time from that in wild-type tobacco, indicating that the Y(β-Glc)3-reactive component eluted in fraction (fr.) numbers 17 to 20 was CsAGP1 produced in tobacco. AGPs in those fractions could also be detected by immuno-dot blotting on nitrocellulose with the anti-AGP antibodies, LM2 and JIM13, which are reactive to a wide range of AGPs (Fig. 2B; Knox et al., 1991; Smallwood et al., 1996). Fr. numbers 17 to 21 from the transgenic plant gave darker staining than those from the wild-type plant, indicating that the CsAGP1 product in tobacco carried epitopes recognized by these antibodies. Although fr. numbers 17 to 20 showed much higher reactivity to Y(β-Glc)3 than fr. number 16, which is likely to have contained intrinsic tobacco AGPs, immunostaining of fr. number 16 was almost equal to or even darker than that of fr. numbers 17 to 20. These results indicate that the reactivity of CsAGP1 to the antibodies was lower than that of tobacco AGPs.
Figure 2.
HPLC profiles of AGPs in wild-type tobacco and transformants overexpressing CsAGP1. AGPs extracted and purified by Y(β-Glc)3 precipitation and RP-HPLC were separated by gel permeation HPLC. AGPs in each fraction were detected by a single radial gel diffusion assay (A) and dot-blot analysis with the anti-AGP antibodies, LM2 and JIM13 (B).
Expression of CsAGP1 in Cucumber Seedlings
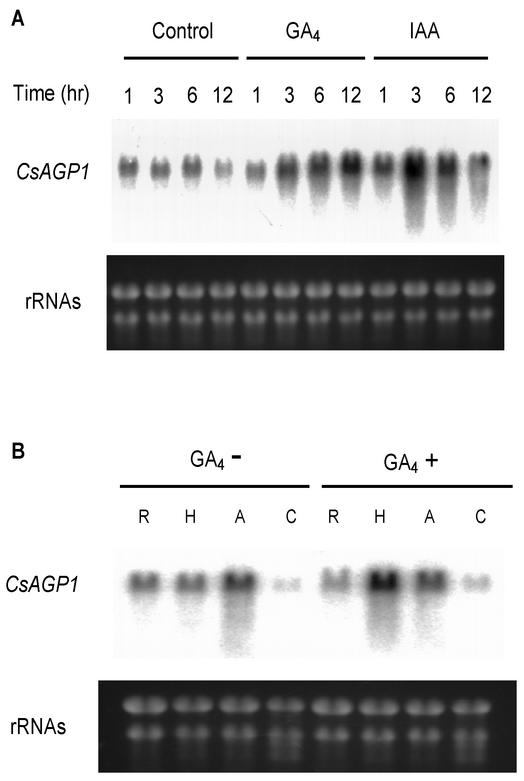
The expression properties of the CsAGP1 gene in cucumber were studied by a northern-blot analysis. Total RNA was isolated from cucumber hypocotyls that had been harvested 1, 3, 6, and 12 h after being respectively treated with GA4 and indole-3-acetic acid (IAA). The expression level of CsAGP1 in cucumber hypocotyls was increased not only by GA4 but also by IAA (Fig. 3A). The level of CsAGP1 mRNA was increased within 1 and 3 h (becoming maximal 3 and 12 h) after the respective treatment with IAA and GA4.
Figure 3.
Effects of GA4 and IAA on the mRNA expression of CsAGP1. A, Total RNA was isolated from cucumber hypocotyls harvested after being treated with GA4 (1 μg plant−1) or IAA (10 μg plant−1) for 1, 3, 6, and 12 h. B, Total RNA was isolated from the roots, hypocotyls, shoot apices, and cotyledons (harvested 2 h after being treated with GA4 [1 μg plant−1]). R, Roots; H, hypocotyls; A, shoot apices; C, cotyledons. Fifteen micrograms of total RNA was separated on 1% (w/v) agarose gel, and ethidium bromide staining of ribosomal RNAs (lower panels) was used to confirm the equivalent loading.
The expression of CsAGP1 mRNA was detected in all vegetative tissues of cucumber seedlings, including the roots, hypocotyls, shoot apices, and cotyledons. Although the effect of exogenous GA4 on the mRNA level in the roots could not be detected because GA4 had been applied to the shoot apices of the seedlings, the transcriptional level in the hypocotyls was increased by GA4 (Fig. 3B). These results suggest that CsAGP1 might have been involved in stem elongation.
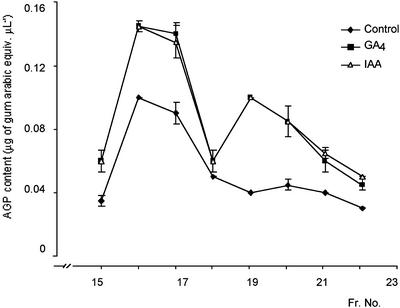
The AGP level in cucumber hypocotyls was compared among the GA4- and IAA-treated and non-treated seedlings. RP-HPLC gave three Y(β-Glc)3 peaks, one major and two minor ones. The AGP content in each of these fractions was about 1.5-fold higher in the GA4- and IAA-treated hypocotyls than in the control tissue (Fig. 4).
Figure 4.
Effects of GA4 and IAA on the AGP contents of cucumber hypocotyls. AGPs were extracted from cucumber hypocotyls that had been GA, IAA, or mock treated as control. AGPs purified by Y(β-Glc)3 precipitation were separated by RP-HPLC. AGPs in each fraction were detected by a single radial gel diffusion assay. Vertical bars represent ses from two independent experiments.
Effect of Y(β-Glc)3 on Cucumber Hypocotyl Elongation
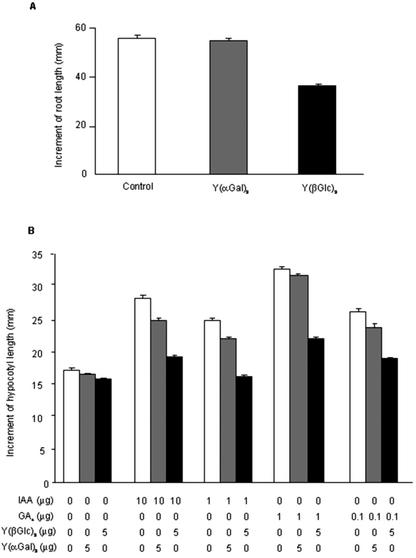
Because CsAGP1 showed some AGP-like characteristics (Figs. 1 and 2), we studied the role of AGP in cell elongation by determining the effect of the Yariv reagents on tissues of cucumber seedlings undergoing cell elongation. The root growth was less in seedlings grown in a medium containing Y(β-Glc)3 than in a medium containing Y(α-Gal)3 and less than in those seedlings that had not been treated (Fig. 5A). This result was not unexpected, because the inhibitory effect of Y(β-Glc)3 on root elongation has already been reported (Willats and Knox, 1996; Ding and Zhu, 1997). In our experiment, we focused on the possible role of AGP in stem elongation. The Yariv reagents were applied to shoot apices of GA4- and IAA-treated cucumber seedlings. Y(β-Glc)3 significantly inhibited the elongation of hypocotyls either non-treated or treated with GA4 or IAA. The inhibitory effect of Y(β-Glc)3 was much more obvious on the hypocotyl elongation promoted by hormone treatments. On the other hand, Y(α-Gal)3, which does not bind AGPs, showed smaller effect than Y(β-Glc)3 (Fig. 5B), indicating that the inhibitory effect of Y(β-Glc)3 was expressed through disruption of the AGP function. These results strongly suggest that AGPs are essential components for stem elongation.
Figure 5.
Effects of Yariv reagents on the elongation of cucumber seedlings. A, Four-day-old seedlings grown on sterile moist filter paper were transferred on to an agar medium with or without 50 μm of a Yariv reagent, and the primary root tips were marked on petri dishes. The increase in root length was measured 42 h after the treatment. Vertical bars represent ses from 10 determinations. B, Hypocotyls of 6-d-old seedlings were marked with ink 15 mm below the cotyledonary node. Cucumber seedlings were treated with GA4 (1 or 0.1 μg) or IAA (10 or 1 μg), and with or without Yariv reagents (5 μg). The 15-mm portion of each hypocotyl was measured 3 d after the treatment. Vertical bars represent ses from 50 determinations. The data are representative of two independent experiments with similar results.
Phenotypes of Transgenic Tobacco Overexpressing CsAGP1
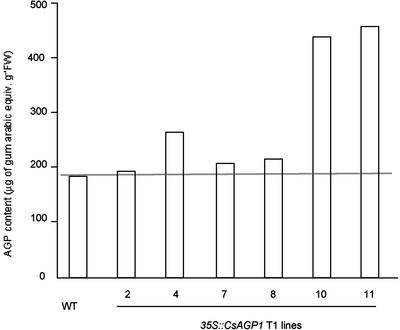
The biological function of CsAGP1 was examined by an analysis of transgenic tobacco plants overexpressing CsAGP1 under the control of the CaMV 35S promoter. Ten kanamycin-resistant T0 plants were obtained and shown to be derived from independent transformation events by a Southern-blot analysis (data not shown). At first sight, two lines, 10 and 11, showed a taller stature than that of wild-type plants, whereas the other transformed lines (2, 4, 7, and 8) showed no clear phenotypes. Quantification of the endogenous level of AGPs showed that only lines 10 and 11 contained a higher amount of AGPs than the wild type (Fig. 6). These data suggested that the taller stature of lines 10 and 11 was related to the AGP content. Thus, lines 10 and 11 were selected for a detailed analysis of the possible role of CsAGP1 in stem elongation.
Figure 6.
AGP contents in wild-type tobacco and transformants overexpressing CsAGP1. AGPs were extracted from the leaves of wild-type tobacco (WT) and transformants overexpressing CsAGP1, and purified by Y(β-Glc)3 precipitation. The amount of total AGPs was determined by a single radial gel diffusion assay.
A segregation analysis of T1 generation in these lines showed a single insertion site for T-DNA, i.e. kanamycin resistant:kanamycin sensitive = 3:1 with probabilities above 0.05 (kanamycin resistant:kanamycin sensitive = 63:23 and 42:18 for lines 10 and 11, respectively). The expression of the transgene was confirmed by a northern-blot analysis (data not shown). A segregation analysis of the T2 progenies enabled homozygous lines 10.1 and 11.4 to be selected and used for a phenotypic analysis, including that of stem elongation.
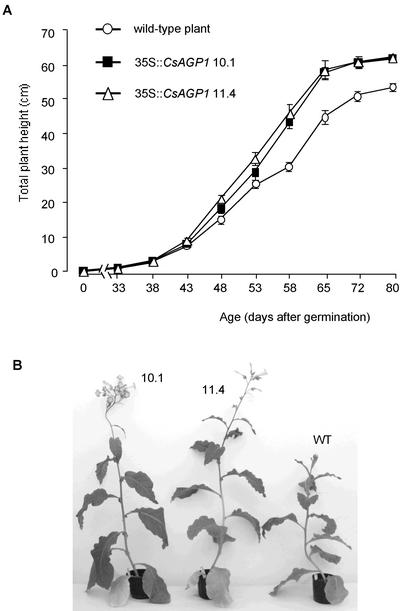
Lines 10.1 and 11.4 both showed greater shoot elongation than that of the wild-type plant, especially in the late growth stage (Fig. 7A). Up to 43 d after germination, no significant difference in plant height could be detected between the transgenic and wild-type plants, but the growth difference gradually became clear thereafter. These lines also showed an early flowering phenotype. The transgenic plants started opening flowers an average of 7 (line 10.1) and 9 (line 11.4) d earlier than the control plants (Table I). The final plant height and node number of the aerial part including the inflorescence stem were compared among the transformants and wild-type plants 80 d after germination when all the plants had already flowered and stopped growing (Table I). The transgenic plants showed a taller stature than the wild-type plants, although they did not have any significant difference in the node numbers, indicating that the increased final plant height was due to promoted internodes elongation. This phenotype was correlated with the increased AGP contents in elongating internodes of transgenic plants (Table I). The appearance of representatives from the transgenic lines 63 d after germination is shown with that of a wild-type plant of the same age in Figure 7B.
Figure 7.
Phenotypes of transgenic tobacco plants overexpressing CsAGP1. The phenotypic differences between the wild-type and transgenic tobacco plants are shown by the growth curve of total plant height (centimeters; A), and by the appearance of representative plants 63 d after germination (B). 10.1 and 11.4, Progeny from independent homozygous transformants; WT, wild-type plant. Vertical bars in A represent ses from 10 determinations.
Table I.
Phenotypes and AGP contents of transgenic tobacco plants (lines 10.1 and 11.4) overexpressing CsAGP1
| Line | Final Plant Height | No. of Nodes | Flower Opening Time | AGP Contents in Internodes |
|---|---|---|---|---|
| cm | d after germination | μg of gum arabic eq. g fresh wt−1 | ||
| WT | 53.0 ± 0.9 | 13.8 ± 0.1 | 64.5 ± 1.2 | 38 |
| 10.1 | 61.7 ± 1.0 | 13.5 ± 0.2 | 57.5 ± 1.2 | 126 |
| 11.4 | 61.9 ± 0.5 | 13.3 ± 0.2 | 55.8 ± 1.3 | 120 |
Final plant height and node no. of aerial part including the inflorescence stem were measured at 80 d after germination. Each value is the mean of 10 replicates ± se. 10.1 and 11.4, Progeny from independent homozygous transformants; WT, wild-type plant.
As shown in Figure 2A, AGPs were more abundant in the transgenic leaf tissue than in the wild-type leaf tissue due to the overexpression of CsAGP1. This higher AGP level in the stem tissue of transgenic plants was also confirmed by single radial diffusion. The amount of total AGPs, including ectopically expressed CsAGP1 in the transgenic lines (T2 10.1 and 11.4), was 3 times higher than that of the wild-type plants (Table I). The elution profiles of Y(β-Glc)3-reactive AGPs on GPC from the transgenic and wild-type tobacco plants were similar to those from the leaves shown in Figure 2A (data not shown). These results suggest that the accumulation of AGPs in the stem tissue was responsible for the increased stem growth of the transgenic plants.
DISCUSSION
We cloned CsAGP1 as a GA4-responsive gene from cucumber hypocotyls. The deduced amino acid sequence showed the presence of three domains, an N-terminal hydrophobic region as a signal peptide for targeting to endoplasmic reticulum, a central Pro-rich region, and a C-terminal hydrophobic region. At the molecular level, AGP core polypeptides can be classified into “classical” and “nonclassical” forms (Mau et al., 1995; Du et al., 1996). Classical AGP sequences generally encode a polypeptide with at least three distinct domains, an N-terminal signal sequence, a central Pro/Hyp-rich region and a C-terminal hydrophobic transmembrane domain that, in mature AGP, could be replaced by a GPI anchor (Schultz et al., 1998; Youl et al., 1998; Oxley and Bacic, 1999; Majewska-Sawka and Nothnagel, 2000). Although the GPI moiety has only been chemically determined from a couple of AGP molecules (Youl et al., 1998; Oxley and Bacic, 1999; Svetek et al., 1999), an examination of the protein backbones deduced from cDNA clones of putative classical AGPs shows that GPI anchoring may be a common feature of this class of AGPs. On the other hand, “nonclassical” AGPs that have been studied to date do not have the required features for GPI anchor attachment (Youl et al., 1998). In the deduced peptide sequence of CsAGP1, there was a Ser-218-Gly-219-Ala-220 sequence before the C-terminal hydrophobic region that, as a cleavage site for GPI anchoring, fits well with the ω/ω + 2 rule (Udenfriend and Kodukula, 1995). A Lys-225 was also found as a well-conserved basic amino acid residue immediately before the putative transmembrane domain. With these characteristics, particularly the GPI anchor signal, CsAGP1 could be classified as a “classical” AGP.
In addition to the structural characteristics, AGPs are often classified by their ability to bind Y(β-Glc)3 (Yariv et al., 1962; Fincher et al., 1983; Baldwin et al., 1993; Bosch et al., 2001). The observation that Y(β-Glc)3 bound to the CsAGP1 product in transgenic tobacco plants (Fig. 2) supports the notion of CsAGP1 being an AGP. We further analyzed the carbohydrate epitopes of CsAGP1 by using anti-AGP antibodies. Much of the evidence to date relating to the AGP function has been based on the use of monoclonal antibodies that react with carbohydrate epitopes on AGPs (Knox, 1997; McCabe et al., 1997). We employed the anti-AGP antibodies, LM2 and JIM13, which have been extensively used in studies with cultured cells and root tissues, and have been shown to have relatively broad spectra for AGP recognition (Knox et al., 1991; Smallwood et al., 1996). Both LM2 and JIM13 recognized CsAGP1 and other intrinsic AGPs, which were detected as Y(β-Glc)3-reactive components in the fractions from HPLC (Fig. 2B), suggesting that CsAGP1 had AGP epitopes common to many other AGPs. Taken together, we conclude from these results that CsAGP1 is a classical AGP.
The northern-blot analysis showed that the transcript level of CsAGP1 in cucumber hypocotyls was increased not only by GA4 but also by IAA. The effect of auxin on shoot elongation generally appears earlier than that of GA. Cucumber seedlings also have shown an elongation response more rapidly to IAA than to GA4 when those phytohormones were applied in the same manner as that in the northern-blotting analysis (Chono et al., 1998). As shown in Figure 3A, the level of CsAGP1 mRNA was increased within 1 h after the treatment with IAA, and 3 h after the treatment with GA4. This expression pattern corresponds well with the elongation response to those phytohormones. This suggests that the induction of CsAGP1 gene expression was not a specific event for either GA action or IAA action, but was one of the conditions necessary for stem elongation. Although the expression of CsAGP1 was observed in other vegetative tissues, including shoot apices and roots (Fig. 3B), the GA4 treatment increased the transcriptional level of CsAGP1 only in the hypocotyls where GA-promoted cell elongation occurred. These expression properties of the CsAGP1 gene suggest its involvement in stem elongation.
To further investigate the role of CsAGP1 on growth, we prepared transgenic tobacco overexpressing CsAGP1 sense RNA. Those transformants with a higher content of AGP showed taller stature with longer internodes and earlier flowering than the wild-type plants (Fig. 7; Table I). In many cases, it is true that the ectopic overexpression of specific gene products causes early flowering, dwarfism, bushy phenotype, etc., probably as a consequence of stress responses. In that sense, it is possible that the early flowering phenotype observed with the CsAGP1 transformants was likewise caused by the stress effect of CsAGP1 overexpression. On the other hand, the promotion of shoot elongation is likely to imply the original function of CsAGP1. The difference in plant height between the transgenic and wild type gradually appeared during shoot development and became clear in the late growth stage, particularly later than 58 d after germination. This result suggests that CsAGP1 was not the causal agent for shoot elongation like phytohormones and their early signal transmitters, but was a regulator functioning in the later step of signal transduction arising from phytohormones and resulting in stem elongation, e.g. maintaining cell wall extensibility in the late growth stage. This proposal is supported by the result that CsAGP1 gene expression was responsive to both GA4 and IAA.
The idea that AGPs are involved in stem elongation was supported by the results with the Yariv reagents. Yariv treatment has indicated that AGPs function in cell elongation in a range of systems, e.g. Arabidopsis root elongation (Ding and Zhu, 1997), pollen tube elongation (Roy et al., 1998), and cultured cell elongation (Willats and Knox, 1996; Vissenberg et al., 2001). We have shown that Y(β-Glc)3-treated cucumber seedlings exhibited reduced root growth (Fig. 5A). In addition, Y(β-Glc)3 almost completely inhibited the hormone-induced growth of cucumber hypocotyls, whereas Y(α-Gal)3, which does not bind AGPs, only partially inhibited it (Fig. 5B). It has been suggested that both the Y(β-Glc)3 and Y(α-Gal)3 reagents bind to cellulose and other glucans that serve to hold the primary cell wall (Triplett and Timpa, 1997). The inhibitory effect of Y(α-Gal)3 on cell elongation could have been due to binding to cellulose and thereby led to only slight growth inhibition. On the other hand, the large inhibition of growth caused by Y(β-Glc)3 could be attributed to disruption of the AGP function. This indicates the functional significance of AGPs in cucumber stem growth.
Similar results were obtained with rice (Oryza sativa) seedlings and adzuki bean (Vigna angularis) epicotyl segments (data not shown). It has been shown recently that IAA-promoted elongation of cucumber hypocotyl segments was inhibited by a Y(β-Glc)3 treatment (Darley et al., 2001), the inhibitory effect being greater than that observed in our experiment with intact seedlings. We applied Y(β-Glc)3 to only the apical buds, whereas the segments were being incubated in a solution containing Y(β-Glc)3, and this difference in results might have been due to the accessibility of the reagent to those AGPs important for cell elongation. Our results, together with those of Darley et al. (2001), support the idea that AGPs are not specifically involved in either GA or auxin functions, but are involved in stem elongation that is cooperatively regulated by these phytohormones and other factors.
Cell elongation is mainly caused by turgor pressure, and fine control is provided by regulating loosening of the cell wall (Pritchard, 1994). Several studies have suggested that AGPs could be involved in cell expansion growth as a cell wall-loosening factor. Schopfer (1990) has suggested that AGPs may function as lubricating agents in cellulose microfibrils of the cell wall of maize (Zea mays) coleoptiles. Tobacco cells adapted to NaCl had lower levels of AGPs, and their walls were less extensible than those of unadapted cells (Zhu et al., 1993). Gao and Showalter (1999) have reported that binding of the Y(β-Glc)3 reagent to AGPs led to the aggregation of AGPs, which disrupted the normal interactions with other cell surface components. On the other hand, it is also critical for cell elongation to synthesize the cell wall components in a controlled manner. Previous reports have shown that AGPs were involved in cell wall assembly (Kieliszewski and Lamport, 1994; Roy et al., 1998). A recent report has provided direct evidence that Y(β-Glc)3 inhibited cellulose deposition on the protoplasts of cultured tobacco cells (Vissenberg et al., 2001), and they also showed that the reagent inhibited the elongation of these cells. Because the direction of expansion is controlled by the orientation of cellulose microfibrils, which itself is controlled by the alignment of cortical microtubules, AGPs might act on the linkage between these microtubules and cellulose microfibrils. Because GA and auxin are the major stimuli to control microtubule alignment, AGPs may also play a role in stem tissues for stabilizing the microtubules or for cellulose synthesis after the microtubule alignment.
An examination of the effect of Y(β-Glc)3 on the alignment of microtubules and on the synthesis of cellulose microfibrils will provide information on the possible mechanism of AGPs for stem elongation. A biophysical analysis of cell wall extensibility is also important, and an analysis of AGP mutants will be extremely useful for these studies to address the function of any specific AGP molecule.
MATERALS AND METHODS
Plant Materials
Cucumber (Cucumis sativus L. Spacemaster 80) seeds were sown and grown in vermiculite for 6 d at 25°C under continuous white light (approximately 3.2 W m−2). The 6-d-old seedlings were used for FDD, northern-blot, and AGP analyses after being treated with GA4, IAA, and/or the Yariv reagents as described in the subsequent sections.
Tobacco (Nicotiana tabacum cv Petit Havana SR1) seeds were cultured on a Murashige and Skoog medium, and leaf sections isolated from 3-week-old plants were used for the transformation experiment. Leaf tissues and stem segments were harvested from 72-d-old plants for the AGP analysis.
Isolation and Cloning of CsAGP1
The GA-responsive gene, CsAGP1, was isolated by the FDD method with an FDD kit (Takara, Tokyo) according to the manufacturer's specifications. Poly(A+) RNA was prepared from cucumber hypocotyls 1 or 3 h after their treatment with either 1 μg of GA4 in 50% (v/v) acetone or a mock solution on the shoot apices of 6-d-old seedlings. Reverse transcription of mRNA was carried out by using nine anchor primers (T15V: mixture of A, C, and G). The single-stranded cDNA mixture was used as a template to perform PCR with nine fluorescein-labeled primers and 24 different 10-mer arbitrary primers. A fragment of CsAGP1 was amplified with 5′-T15AC-3′ as an anchor primer and 5′-GATCCAGTAC-3′ as a 10-mer arbitrary primer. 5′-RACE and 3′-RACE were performed with a Marathon cDNA amplification kit (CLONTECH Laboratories, Palo Alto, CA) to determine the sequence of full-length CsAGP1 (DSQ-1000L, Shimadzu, Kyoto). Full-length CsAGP1 was cloned by PCR with the 5′-CTAGCAAGAAGAAGTCAAA GAAGCAC-3′ and 5′-CTAAAAGATGATGCTGACGGCGAC-3′ primers, and subcloned into the pGEM-T plasmid to give pGEM-CsAGP1 (Promega, Madison, WI).
Transformation of Tobacco
The pGEM-CsAGP1 was digested with EcoRI, and the insert cDNA was cloned in sense orientation into the EcoRI site of the binary vector, pBI-PL, which had been constructed based on the pBI vector (Jefferson et al., 1987) to contain a polylinker site. Details of the vector construction will be published elsewhere, and are available from the corresponding author. The resulting plasmid, pBI-CsAGP1, contained the transformation marker gene, nptII, and the coding sequence of CsAGP1 under the control of the CaMV 35S promoter. pBI-CsAGP1 was transferred from Escherichia coli to Agrobacterium tumefaciens strain GV3010 (pMP90) by the triparental method (Koncz and Schell, 1986). Transformation was performed with the A. tumefaciens -mediated leaf disc method (Horsch et al., 1985), the transformed plants being selected on a Murashige and Skoog medium supplemented with 0.2% (w/v) gellan gum (Wako Pure Chemicals, Osaka), 1 mg L−1 of N6-benzylaminopurine, 0.1 mg L−1 of 1-naphthaleneacetic acid, 100 mg L−1 of kanamycin (Meiji Seika, Tokyo), and 500 mg L−1 of claforan (Hoechst, Frankfurt). Shoots that were regenerated on the selective medium were transferred to vermiculite after roots had formed. The progeny from each transgenic line was obtained by self-fertilization. The plants were then grown under continuous fluorescent light at 25°C.
Northern-Blot Analysis
One microgram of GA4 or 10 μg of IAA in 10 μL of 50% (v/v) acetone or the mock solution were applied to the shoot apices of 6-d-old seedlings. Total RNA was extracted from each plant material by the phenol-SDS method as described by Ausubel et al. (1995). Fifty micrograms of RNA per sample was analyzed by the standard blotting technique (Sambrook et al., 1989). Full-length cDNA of CsAGP1 was labeled with 32P and used as a hybridization probe. After hybridization, the membranes were washed in 0.1× SSC containing 0.1% (w/v) SDS at 65°C. Radioactive signals were detected with a BAS 2000II radio-imaging analyzer (Fujix, Tokyo).
Extraction and Partial Purification of AGPs
AGPs were purified according to the method described by Schultz et al. (2000) with a slight modification. AGP was extracted from 1 g of the leaf or stem tissue of transgenic or wild-type tobacco plants. For cucumber AGP, 1 g of 10-mm-long hypocotyl segments below the cotyledonary node was harvested 12 h after being treated with GA4, the IAA solution, and the mock solution, respectively, as described for the northern-blot analysis.
The plant material was homogenized in liquid nitrogen and added to 1.5 mL of an extraction buffer (50 mm Tris-HCl [pH 8.0], 10 mm EDTA, 0.1% [v/v] β-mercaptoethanol, and 1% [w/v] Triton X-100). Each sample was incubated at 4°C for 3 h and then centrifuged at 14,000g for 10 min. The resulting supernatant was mixed with 5 volumes of ethanol and incubated overnight at 4°C. A precipitate was recovered by centrifugation at 14,000g for 10 min. The resulting pellet was resuspended in 1 mL of 50 mm Tris-HCl (pH 8.0) and then centrifuged at 14,000g for 10 min. After this centrifugation, the supernatant was transferred to a 2-mL tube. The residue was re-suspended in 0.8 mL of the same buffer. After centrifugation, the resulting supernatant was combined with the first extract, and Y(β-Glc)3 and NaCl, respectively, were added to 1 mm and 1% (w/v). After an overnight incubation at 4°C, the Y(β-Glc)3-AGP complex was collected by centrifugation at 14,000g for 1 h. The pellet was washed three times with 1% (w/v) NaCl and twice in methanol before being dried at room temperature. The pellet was then dissolved in a minimum volume of dimethyl sulfoxide, and appropriate amounts of solid sodium dithionate and water were added to give a clear pale-yellow solution. The protein fraction was obtained with an NAP-10 column (Amersham-Pharmacia Biotech, Uppsala) and then concentrated in vacuo. The concentration of AGP was determined with a single radial gel diffusion assay (van Holst and Clarke, 1985).
HPLC
RP-HPLC was performed with an R-type column (Pegasil-300 C8, 4.6 × 150 mm, Senshu Scientific, Tokyo). The AGP extract (50 μL) was loaded into the column, which had been equilibrated previously with 0.1% (v/v) trifluoroacetic acid (TFA). The column was eluted by a three-step linear gradient: from 0 to 30 min to 0.1% (v/v) TFA in 10% (v/v) acetonitrile, from 30 to 40 min to 0.1% (v/v) TFA in 30% (v/v) acetonitrile, and from 40 to 72 min to 0.1% (v/v) TFA in 100% (v/v) acetonitrile at a flow rate of 1 mL min−1.
Tobacco AGPs that had been purified by RP-HPLC were fractionated by GPC in a Shodex Protein KW-803 column (8 × 300 mm, Showa Denko, Tokyo). The column was eluted with 0.1 m NaCl in 20 mm Tris-HCl (pH 8.0) at a flow rate of 0.5 mL min−1. The AGP fractions were quantified by a single radial gel diffusion assay (van Holst and Clarke, 1985).
Immuno-Dot Blotting
The procedure for immuno-dot blotting followed the method of Smallwood et al. (1996). A sample was adjusted to 2 μg L−1 of protein and spotted on to a nitrocellulose membrane (Trans blot transfer medium, 0.45 μm, Bio-Rad Laboratories, Hercules, CA). The nitrocellulose membrane blot was blocked with 5% (w/v) milk protein in phosphate-buffered saline (PBS; pH 7.3) for 1 h at room temperature. The membrane was then washed with PBS and incubated with either LM2 or JIM13 (Knox et al., 1991; Smallwood et al., 1996), respectively, diluted 1:100 (v/v) in PBS for 1 h at room temperature. After washing in PBS, the membrane was incubated with an anti-rat IgM-HRP conjugate (Cappel Products, Durham, NC) diluted 1:500 (v/v) in PBS for 1 h at room temperature. The membrane was then washed with PBS and incubated in the substrate solution (0.2 mg mL−1 of 3,3-diamino benzidine tetrahydrochloride in 50 mm Tris-HCl [pH 7.4] containing 0.03% [v/v] hydrogen peroxide).
Cucumber Bioassay
The cucumber hypocotyl bioassay followed the method of Katsumi et al. (1965) with a small modification. Cucumber seedlings were grown in vermiculite under continuous white light at 25°C. The hypocotyls of 6-d-old seedlings were marked with ink 15 mm below the cotyledonary node. The Yariv regents dissolved in water (10 μL per plant) or distilled water alone were applied to the shoot apices of the seedlings. A solution of GA4 or IAA in 50% (v/v) aqueous acetone (10 μL per plant) was then likewise applied. Seedlings treated with 50% (v/v) aqueous acetone (10 μL per plant) were used as controls. The length of the marked region was measured 3 d after the treatment.
To evaluate the effect of the Yariv reagents on root growth, cucumber seeds were surface sterilized and sown on sterile moist filter paper. Four-day-old seedlings were transferred to 0.8% (w/v) agar with or without 50 μm of a Yariv reagent, and the primary root tips were marked on petri dishes, which were placed vertically. The increase in root length was measured 42 h after the treatment.
Yariv Reagents
(β-Glc)3Y [1,3,5-tri-(p-β-d-glucosyloxyphenylazo)-2,4,6-trihydroxybenzene] and (α-Gal)3Y [1,3,5-tri-(p-α-d-galactosyloxyphenylazo)-2, 4, 6-trihydroxybenzene] were synthesized according to the method of Yariv et al. (1962) by the respective diazo-coupling reaction of phloroglucinol with p-aminophenyl β-d-glucoside and p-aminophenyl α-d-galactoside. These compounds dissolved in dimethyl sulfoxide were stored at −20°C as stock solutions.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.015628.
LITERATURE CITED
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1995. [Google Scholar]
- Baldwin TC, McCann MC, Roberts K. A novel hydroxyproline-deficient arabinogalactan protein secreted by suspension-cultured cells of Daucus carota: purification and partial characterization. Plant Physiol. 1993;103:115–123. doi: 10.1104/pp.103.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Knudsen JS, Derksen J, Mariani C. Class III pistil-specific extensin-like proteins from tobacco have characteristics of arabinogalactan proteins. Plant Physiol. 2001;125:2180–2188. doi: 10.1104/pp.125.4.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wang H, Wu HM. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell. 1995;82:383–393. doi: 10.1016/0092-8674(95)90427-1. [DOI] [PubMed] [Google Scholar]
- Chono M, Nemoto K, Yamane H, Yamaguchi I, Murofushi N. Characterization of a protein kinase gene responsive to auxin and gibberellin in cucumber hypocotyls. Plant Cell Physiol. 1998;39:958–967. doi: 10.1093/oxfordjournals.pcp.a029460. [DOI] [PubMed] [Google Scholar]
- Clarke AE, Gleeson PA, Jermyn MA, Knox RB. Characterization and localization of β-lectins in lower and higher plants. Aust J Plant Physiol. 1978;5:707–722. [Google Scholar]
- Cosgrove DJ. Enzymes and other agents that enhance cell wall extensiblity. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- Darley CP, Forrester AM, McQueen-Mason SJ. The molecular basis of plant cell wall extension. Plant Mol Biol. 2001;47:179–195. [PubMed] [Google Scholar]
- Ding L, Zhu JK. A role for arabinogalactan-proteins in root epidermal cell expansion. Planta. 1997;203:289–294. doi: 10.1007/s004250050194. [DOI] [PubMed] [Google Scholar]
- Du H, Simpson RJ, Clarke AE, Bacic A. Molecular characterization of a stigma-specific gene encoding an arabinogalactan-protein (AGP) from Nicotiana alata. Plant J. 1996;9:313–323. doi: 10.1046/j.1365-313x.1996.09030313.x. [DOI] [PubMed] [Google Scholar]
- Fincher GB, Stone BA, Clarke AE. Arabinogalactan-proteins: structure, biosynthesis, and function. Annu Rev Plant Physiol. 1983;34:47–70. [Google Scholar]
- Gao M, Showalter AM. Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J. 1999;19:321–331. doi: 10.1046/j.1365-313x.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Wallroth M, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Jauh GY, Lord EM. Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta. 1996;199:251–261. [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi M, Phinney BO, Purves WK. The roles of gibberellin and auxin in cucumber hypocotyl growth. Physiol Plant. 1965;18:462–473. [Google Scholar]
- Kieliszewski MJ, Lamport DT. Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J. 1994;5:157–172. doi: 10.1046/j.1365-313x.1994.05020157.x. [DOI] [PubMed] [Google Scholar]
- Knox JP. The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int Rev Cytol. 1997;171:79–120. doi: 10.1016/s0074-7696(08)62586-3. [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991;1:317–326. doi: 10.1046/j.1365-313X.1991.t01-9-00999.x. [DOI] [PubMed] [Google Scholar]
- Komalavilas P, Zhu JK, Nothnagel EA. Arabinogalactan-proteins from the suspension culture medium and plasma membrane of rose cells. J Biol Chem. 1991;266:15956–15965. [PubMed] [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Kreuger M, van Holst GJ. Arabinogalactan proteins and plant differentiation. Plant Mol Biol. 1996;30:1077–1086. doi: 10.1007/BF00019543. [DOI] [PubMed] [Google Scholar]
- Langan KJ, Nothnagel EA. Cell surface arabinogalactan-proteins and their relation to cell proliferation and viability. Protoplasma. 1997;196:87–98. [Google Scholar]
- Li SX, Showalter AM. Cloning and developmental/stress-regulated expression of a gene encoding a tomato arabinogalactan protein. Plant Mol Biol. 1996;32:641–652. doi: 10.1007/BF00020205. [DOI] [PubMed] [Google Scholar]
- Majewska-Sawka A, Nothnagel EA. The multiple roles of arabinogalactan proteins in plant development. Plant Physiol. 2000;122:3–10. doi: 10.1104/pp.122.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau SL, Chen CG, Pu ZY, Moritz RL, Simpson RJ, Bacic A, Clarke AE. Molecular cloning of cDNAs encoding the protein backbones of arabinogalactan-proteins from the filtrate of suspension-cultured cells of Pyrus communis and Nicotiana alata. Plant J. 1995;8:269–281. doi: 10.1046/j.1365-313x.1995.08020269.x. [DOI] [PubMed] [Google Scholar]
- McCabe PF, Valentine TA, Forsberg LS, Pennell RI. Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell. 1997;9:2225–2241. doi: 10.1105/tpc.9.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel EA. Proteoglycans and related components in plant cells. Int Rev Cytol. 1997;174:195–291. doi: 10.1016/s0074-7696(08)62118-x. [DOI] [PubMed] [Google Scholar]
- Oxley D, Bacic A. Structure of the glycosylphosphatidylinositol anchor of an arabinogalactan protein from Pyrus communis suspension-cultured cells. Proc Natl Acad Sci USA. 1999;96:14246–14251. doi: 10.1073/pnas.96.25.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Roberts K. Sexual development in the pea is presaged by altered expression of arabinogalactan protein. Nature. 1990;344:547–549. [Google Scholar]
- Phillips A. Gibberellins in Arabidopsis. Plant Physiol Biochem. 1998;36:115–124. [Google Scholar]
- Pritchard J. The control of cell expansion in roots. New Phytol. 1994;127:3–26. doi: 10.1111/j.1469-8137.1994.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Roy S, Jauh GY, Hepler PK, Lord EM. Effects of Yariv phenylglycoside on cell wall assembly in the lily pollen tube. Planta. 1998;204:450–458. doi: 10.1007/s004250050279. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. Ed 2. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schopfer P. Cytochemical identification of arabinogalactan protein in the outer epidermal wall of maize coleoptiles. Planta. 1990;183:139–142. doi: 10.1007/BF00197578. [DOI] [PubMed] [Google Scholar]
- Schultz C, Gilson P, Oxley D, Youl J, Bacic A. GPI-anchors on arabinogalactan-proteins: implications for signalling in plants. Trends Plant Sci. 1998;3:426–431. [Google Scholar]
- Schultz CJ, Johnson KL, Currie G, Bacic A. The classical arabinogalactan protein gene family of Arabidopsis. Plant Cell. 2000;12:1751–1768. doi: 10.1105/tpc.12.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA. Effects of Yariv phenylglycosides on Rosa cell suspensions: evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta. 1994;193:542–550. [Google Scholar]
- Serpe MD, Nothnagel EA. Arabinogalactan-proteins in the multiple domains of the plant cell surface. Adv Bot Res. 1999;30:207–289. [Google Scholar]
- Shibaoka H. Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:527–544. [Google Scholar]
- Smallwood M, Yates EA, Willats WGT, Martin H, Knox JP. Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta. 1996;198:452–459. [Google Scholar]
- Suzuki Y, Kitagawa M, Knox JP, Yamaguchi I. A role for arabinogalactan proteins in gibberellin-induced alpha-amylase production in barley aleurone cells. Plant J. 2002;29:733–741. doi: 10.1046/j.1365-313x.2002.01259.x. [DOI] [PubMed] [Google Scholar]
- Svetek J, Yadav MP, Nothnagel EA. Presence of a glycosylphosphatidylinositol lipid anchor on rose arabinogalactan proteins. J Biol Chem. 1999;274:14724–14733. doi: 10.1074/jbc.274.21.14724. [DOI] [PubMed] [Google Scholar]
- Thompson HJM, Knox JP. Stage-specific responses of embryogenic carrot cell suspension cultures to arabinogalactan protein-binding β-glucosyl Yariv reagent. Planta. 1998;205:32–38. [Google Scholar]
- Triplett BA, Timpa JD. β-glucosyl and α-galactosyl Yariv reagents bind to cellulose and other glucans. J Agric Food Chem. 1997;45:4650–4654. [Google Scholar]
- Udenfriend S, Kodukula K. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. 1995;64:563–591. doi: 10.1146/annurev.bi.64.070195.003023. [DOI] [PubMed] [Google Scholar]
- van Holst G-J, Clarke AE. Quantification of arabinogalactan-protein in plant extracts by single radial gel diffusion. Anal Biochem. 1985;148:446–450. doi: 10.1016/0003-2697(85)90251-9. [DOI] [PubMed] [Google Scholar]
- van Holst G-J, Clarke AE. Organ-specific arabinogalactan-proteins of Lycopersicon peruvianum (Mill) demonstrated by crossed electrophoresis. Plant Physiol. 1986;80:786–789. doi: 10.1104/pp.80.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Feijo JA, Weisenseel MH, Verbelen JP. Ion fluxes, auxin and the induction of elongation growth in Nicotiana tabacum cells. J Exp Bot. 2001;52:2161–2167. doi: 10.1093/jexbot/52.364.2161. [DOI] [PubMed] [Google Scholar]
- Willats WG, Knox JP. A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of beta-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J. 1996;9:919–925. doi: 10.1046/j.1365-313x.1996.9060919.x. [DOI] [PubMed] [Google Scholar]
- Yariv J, Rapport MM, Graf L. The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochem J. 1962;85:383–388. doi: 10.1042/bj0850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youl JJ, Bacic A, Oxley D. Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphatidylinositol membrane anchors. Proc Natl Acad Sci USA. 1998;95:7921–7926. doi: 10.1073/pnas.95.14.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K, Bressan RA, Hasegawa PM. Loss of arabinogalactan-proteins from the plasma membrane of NaCl-adapted tobacco cells. Planta. 1993;190:221–226. [Google Scholar]