Abstract
During spore germination, arbuscular mycorrhizal (AM) fungi show limited hyphal development in the absence of a host plant (asymbiotic). In the presence of root exudates, they switch to a new developmental stage (presymbiotic) characterized by extensive hyphal branching. Presymbiotic branching of the AM fungus Gigaspora rosea was induced in liquid medium by a semipurified exudate fraction from carrot (Daucus carota) root organ cultures. Changes in RNA accumulation patterns were monitored by differential display analysis. Differentially appearing cDNA fragments were cloned and further analyzed. Five cDNA fragments could be identified that show induced RNA accumulation 1 h after the addition of root exudate. Sequence similarities of two fragments to mammalian Nco4 and mitochondrial rRNA genes suggested that root exudates could influence fungal respiratory activity. To support this hypothesis, additional putative mitochondrial related-genes were shown to be induced by root exudates. These genes were identified after subtractive hybridization and putatively encode a pyruvate carboxylase and a mitochondrial ADP/ATP translocase. The gene GrosPyc1 for the pyruvate carboxylase was studied in more detail by cloning a cDNA and by quantifying its RNA accumulation. The hypothesis that respiratory activity of AM fungi is stimulated by root exudates was confirmed by physiological and cytological analyses in G. rosea and Glomus intraradices. Oxygen consumption and reducing activity of both fungi was induced after 3 and 2 h of exposition with the root factor, respectively, and the first respiration activation was detected in G. intraradices after approximately 90 min. In addition, changes in mitochondrial morphology, orientation, and overall biomass were detected in G. rosea after 4 h. In summary, the root-exuded factor rapidly induces the expression of certain fungal genes and, in turn, fungal respiratory activity before intense branching. This defines the developmental switch from asymbiosis to presymbiosis, first by gene activation (0.5–1 h), subsequently on the physiological level (1.5–3 h), and finally as a morphological response (after 5 h).
Arbuscular mycorrhizal (AM) fungi are obligate biotrophic root symbionts that cannot be propagated in pure culture. Therefore, they are difficult to study, and analyses concerning the structure and the function of their genes are rare and mainly based on PCR techniques (Franken and Requena, 2001). Establishment of the symbiosis after spore germination includes hyphal branching, appressorium development after contacting the root, symbiotic colonization of the cortex, formation of the intracellular arbuscules, and, concomitantly, production of a sporulative extraradical mycelium (Bianciotto and Bonfante, 1998; Smith and Read, 1997). These developmental stages presumably require molecular communication between the fungus and the plant. Signals should be exchanged between the partners, leading to stage-specific patterns of gene expression. The corresponding gene products in turn would be responsible for the morphological and physiological changes necessary for the progressive integration of the two partners into one unit called arbuscular mycorrhiza.
Signaling from the plant to the fungus has only been investigated during the stages before the contact with the root. In the absence of a host, fungal spores are able to germinate, but hyphal growth is limited in duration (a few days or weeks depending on the fungus). Hyphal elongation ranges from a few millimeters to a few centimeters. During this asymbiotic stage, the fungus seems to consume a minimum of its stored carbon and energy (Bécard and Piché, 1989a; Bago et al., 1999, 2000). In the presence of a host plant, but still before physical contact with the root, fungal growth pattern changes and intense presymbiotic branching can be observed. This developmental switch can also be induced by root exudates alone (Elias and Safir, 1987; Gianinazzi-Pearson et al., 1989; Tawaraya et al., 1996; Vierheilig et al., 1998) or synergistically with CO2 (Bécard and Piché, 1989b). Further investigations showed that the root factors that elicited hyphal branching were smaller than 500 D and only produced by host roots (Giovannetti et al., 1996). The chemical structure of the exuded root compounds, responsible for the stimulation of hyphal branching, is still unknown (Franken and Requena, 2000).
Recent studies have attempted to isolate the active root fraction responsible for stimulation of hyphal branching. A new experimental system was developed (Nagahashi and Douds, 1999) that allowed the rapid testing of fractions of root exudates introduced into small holes made in the gelled medium near the tips of fungal hyphae. Hyphal proliferation (branching) was the main fungal response studied. Morphological and growth responses could be scored within 24 h in a very reproducible and sensitive manner (Buée et al., 2000; Nagahashi and Douds, 2000). A lipophilic fraction (branching factor) with a strong stimulatory activity on Glomus intraradices and several Gigaspora spp. was isolated from carrot (Daucus carota) root organ cultures and partially purified. The branching factor is believed to be present in all mycotrophic plants because it was found in root exudates of host plants representing several families and not in root exudates of non-hosts (Buée et al., 2000). The chemical nature of the factor is not known. However, assays with various compounds such as abietic acid, brassinolide, jasmonic acid, salicylic acid, and quercetin were all negative. Flavonoids in general were also excluded as branching factor candidates because root exudates from maize mutants, deficient in chalcone synthase (necessary for flavonoid synthesis), contained the same branching activity as the wild type (Buée et al., 2000). The branching factor is believed to be a signal rather than a nutrient because it is active irrespective of the culture medium used, including when germinating fungal spores are growing in pure water. In addition, preliminary HPLC and mass spectroscopy analyses indicate that it is active at extremely low concentrations (S. Roy, C. Roux, and G. Bécard, unpublished data).
In contrast to investigations into the plant factors, there are only a few studies on the fungal response. After stimulating germinating spores of Gigaspora rosea with CO2 and root exudates, uptake of phosphate was elevated and plasmalemma-ATPase activity increased (Lei et al., 1991). In addition, the cytosolic pH was more alkaline when G. rosea was growing in the vicinity of a host root (Jolicoeur et al., 1998). In Gigaspora margarita, transmembrane electric potential difference became more negative when plant root extracts were added to the medium (Ayling et al., 2000).
The morphological response of AM fungi to the branching factor in the lipophilic fraction of root exudates from carrot root organ cultures cannot be recorded before 6 h after the root stimulus (Buée et al., 2000). The present work characterizes the response of G. rosea that occurs between 1 and 4 h. Gene expression analysis was carried out by differential RNA display and suppressive subtractive hybridization. cDNA fragments were identified and transcript accumulation of selected genes was quantified by reverse northern hybridization and reverse transcriptase (RT)-PCR. A number of induced cDNA fragments were found to be putatively associated with mitochondrial activity. One gene encoding a pyruvate carboxylase was studied in more detail. Physiological and cytological investigations were carried out with two AM fungi, G. rosea and G. intraradices, to confirm that respiration is a primary target of fungal metabolism induced by the root factor.
RESULTS
Hyphal Growth Stimulation by Root Exudates and Identification of Fungal Genes
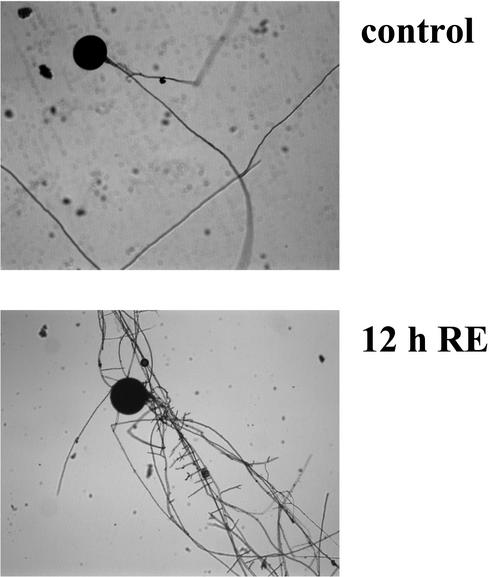
After germination and initial growth of the mycelium for 5 d in liquid medium, spores of G. rosea were exposed to methanol/water (control) or to purified exudates from carrot roots. Five hours after the addition of root exudates, the first morphological responses were visible and after 12 h, hyphae exhibited strong branching activity (Fig. 1) as it has already been described on solid medium (Buée et al., 2000). To investigate the molecular events associated with this developmental switch, fungal genes, specifically induced during the first hours after addition of the root stimulus, were identified and further analyzed. Fungal RNA was extracted from the mycelium at the time points 0, 0.5, 1, and 4 h. Differential display analysis using six combinations of anchored oligo(dT)s and arbitrary decameric primers showed that no new cDNA fragments appeared, nor did certain fragments disappear in controls treated with methanol/water alone, as has been described before (Franken et al., 2000). In contrast, samples challenged with root exudates revealed first responses in banding patterns already 0.5 h after stimulation, but most differences were detected at 1 h, indicating activation of gene expression at that time point. For further analysis, 14 fragments, which showed a clear difference, were excised from the polyacrylamide gels, re-amplified, and cloned into a plasmid vector. Sequencing showed that seven different cDNA fragments represented in one to three copies were obtained belonging to six different genes (Table I). One type of fragment (DD1) was nearly identical to the 28S ribosomal RNA gene of G. rosea (Van Tuinen et al., 1998). Fragment DD2 showed no similarity to known DNA sequences. Fragment DD3 was similar to a gene encoding an adrenal gland protein and the peptide sequence deduced from the fragment DD4 revealed similarity to a cytochrome P450 monooxygenase. Fragment DD5 showed similarity to the mammalian gene Nco4. This gene possesses the same promoter as Cox4, a nuclear gene, encoding a cytochrome C oxidase subunit. Nco4 is transcribed in the opposite direction but is coregulated with Cox4 in humans (Bachman et al., 1999). The function of the Nco4 corresponding protein is unknown. Two fragments (DD6 and DD7) showed strong homologies with 24S mitochondrial rRNA genes.
Figure 1.
Presymbiotic development. Germinating spores of G. rosea in liquid minimal medium 12 h after the addition of methanol/water (control) or of root exudates (RE).
Table I.
Root exudate-induced genes in G. rosea
| Clone | bp | Putative Gene Function | Significance of Similarity | Accession No. | Experiment
1a
|
Experiment
2b
|
Experiment 3b
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 4 | − | + | − | + | |||||
| Gros(RE)-DD1 | 291 | 28S rRNA gene | e−131 | AJ419662 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Gros(RE)-DD2 | 335 | No similarity | – | AJ419663 | 0.19 | 0.32 | 0.43 | 0.88 | ndc | nd | nd | nd |
| Gros(RE)-DD3 | 581 | Adrenal gland protein | 2e−15 | AJ419664 | 0 | 0.25 | 0.39 | 1.01 | nd | nd | nd | nd |
| Gros(RE)-DD4 | 212 | P450-monooxygenase | e−07 | AJ419665 | 0.23 | 0.62 | 0.89 | 1.02 | nd | nd | nd | nd |
| Gros(RE)-DD5 | 626 | NCO4 | 9e−16 | AJ419666 | 0 | 0 | 0.05 | 0.47 | 1.18 | 3.04 | 0.22 | 0.65 |
| Gros(RE)-DD6 | 252 | 24S mt rRNA | 8e−19 | AJ419667 | 0.02 | 0.16 | 0.33 | 0.13 | nd | nd | nd | nd |
| Gros(RE)-DD7 | 462 | 24S mt-rRNA | 6e−64 | AJ419668 | 0.35 | 0.9 | 0.98 | 0.53 | nd | nd | nd | nd |
| Gros(RE)-60d | 435 | mt ATP/ADP translocase | e−14 | AJ419669 | nd | nd | nd | nd | 0.41 | 1.21 | 0.19 | 2.74 |
| Gros(RE)-92d | 501 | Pyruvate carboxylase | e−51 | AJ419670 | nd | nd | nd | nd | 0.63 | 1.31 | 0.40 | 0.98 |
Size and putative functions of fragments isolated by differential display or by subtractive hybridization are shown as well as the percentage of identical amino acids over length of the alignment, the accession nos., and the relative levels of expression measured in different reverse northern-blot experiments.
Hours after addition of root exudates.
One hour of growth with water/methanol in the absence (−) or in the presence (+) of root exudates.
nd, Not determined.
Expressed sequence tags obtained from the subtractive library.
The inserts of all clones were analyzed by reverse northern blot. cDNA probes, made of RNA extracted from non-stimulated and stimulated spores (at different time points after the addition of root exudate or methanol/water), were used. No differences in expression were detected for DD1, the fragment similar to the 28S rRNA gene. Fragments of fungal 28S rRNA have already been isolated before as false positives in other differential display experiments (Martin-Laurent et al., 1997; Lapopin et al., 1999). Therefore, the values for the signal intensities of the other fragments were normalized using DD1 as reference and are shown in Table I (Exp. 1). Earliest induction (0.5 h) was observed for DD2, DD3, DD4, DD6, and DD7. RNA accumulation for fragments DD6 and DD7 representing mt rRNA remained constant after induction at 1 h and decreased after 4 h. The transcripts belonging to the two other fragments (DD2 and DD4), however, steadily accumulated to higher levels during the period of observation. A similar accumulation pattern was observed for DD5, but not before 1 h of stimulation.
Early accumulation of DD5 (GrosNco4) transcript and of DD6 and DD7 (24S mt rRNA) suggested that mitochondrial activity could be specifically and rapidly activated in the fungus. To obtain data from more fungal genes specifically induced by root exudates, a subtractive hybridization was carried out using RNA extracted from stimulated and non-stimulated hyphae. Among others, two cDNAs displayed homologies with genes encoding a pyruvate carboxylase and a mitochondrial ADP/ATP translocase (Table I). Together with the Nco4 fragment, they were hybridized in two independent experiments with cDNA probes obtained from total RNA of induced and noninduced hyphae, 1 h after the addition of root exudates or methanol/water (Table I, Experiments 2 and 3). Expression of all three genes putatively involved in mitochondrial activity was 2.1 to 14 times activated after the addition of root exudates.
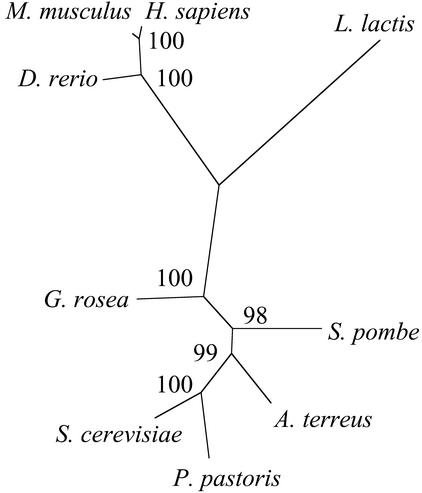
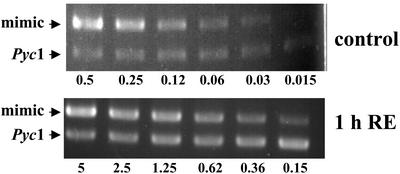
One of the genes putatively encoding a pyruvate carboxylase (GrosPyc1) was chosen for further analysis, because this enzyme plays a central role in the mitochondrial metabolism. The fragment from the subtractive library covered the 5′-untranslated region and the first part of the open reading frame. Therefore, 3′-RACE experiments were carried out to obtain more sequence information. After assembling of the different cDNA fragments, alignment of the deduced amino acid sequence with pyruvate carboxylases from other organisms showed a clear grouping with the true fungi (Fig. 2). Closest similarity with 70% identical residues was obtained to the pyruvate carboxylase of A. terreus. Based on the sequence, gene-specific primers were designed to quantify RNA accumulation by RT-PCR with an internal standard for calibration. RNA was extracted from hyphae 1 h after stimulation with root exudates and from the corresponding control hyphae treated with methanol/water. After cDNA synthesis, an internal standard was added in different concentrations and PCR amplifications were carried out with specific primers. PCR products were separated on an agarose gel (Fig. 3). Similar amounts of product from the MIMIC DNA and the genes were obtained with 0.62 nm for the induced hyphae and 0.06 nm for the controls. This suggests an approximately 10-fold increase of Pyc1 after stimulation with root exudates. This is more than observed by hybridization of the fragments with labeled cDNA probes. However, such an underestimation of induction levels by reverse northern blots or on cDNA arrays has been observed before (U. Grunwald and P. Franken, unpublished data) and was also obtained for the Nco4 gene after stimulation of G. rosea hyphae with the branching factor (data not shown).
Figure 2.
Dendrogram of pyruvate carboxylases. An amino acid sequence was deduced from different cDNA fragments and aligned using the program ClustalW with the pyruvate carboxylases from the ascomycetes Aspergillus terreus (AAC69197), Saccharomyces cerevisiae (AAA34843), Schizosaccharomyces pombe (BAA11239), and Pichia pastoris (AAL69566), with the vertebrates Danio rerio (NP 571625), Mus musculus (NP 032823), and humans (Homo sapiens; P11498). The prokaryote Lactococcus lactis (AAF09095) was taken as an outgroup. Phylogenetic analysis was carried by the program PUZZLE, and the dendrogram was constructed by the program TREEVIEW. Quartet puzzling support values of 1,000 replicates are indicated. A similar dendrogram was obtained using the program package PHYLIP.
Figure 3.
RT-PCR analysis of RNA accumulation. PCR products were obtained with GrosPyc1-specific primers after adding different dilutions of MIMIC DNA to cDNA from G. rosea spores treated for 1 h with methanol/water (control) or with root exudates (1h RE).
Measurement of Fungal Respiratory Activity
Our finding that several genes associated with mitochondrial activity were rapidly induced after the addition of root exudate led us to look for potential correlative activation of fungal respiration. Therefore, O2 consumption of stimulated and non-stimulated spores was measured using a Clark-type electrode. Assays were carried out with G. rosea, as well as with G. intraradices, two highly divergent AM fungal species belonging to two different orders of the Glomeromycota (Schüssler et al., 2001). The O2 consumption rate of the non-germinated spores, used as a negative control, was nearly zero (data not shown), indicating that the polarograph was not sensitive enough to detect respiration of non-germinating spores. After germination for 3 d, O2 consumption rate was 57% to 88% higher for G. rosea and around 30% higher for G. intraradices, 3 h after the addition of root exudates (Table II). For further confirmation, cytological measurements of reduction of tetrazolium salts were carried out. These compounds are positively charged yellow dyes and enter the cell where they are reduced to a lipid-insoluble purple formazan by cleavage of the tetrazolium ring due to the activity of dehydrogenase enzymes in the cytosol and the mitochondria (Altman, 1976). Tetrazolium salts are used for cell respiration measurement (Stowe et al., 1995) or more generally as indicators of active cell metabolism (Bernas and Dobrucki, 1999). In a first experiment, three different substances (3-methylthiazolyldiphenyltetrazolium bromide [MTT], triphenyltetrazolium chloride [TTC], and nitroblue tetrazolium [NBT]) were tested under comparable conditions. Formazan deposition could not be observed with NBT, and only very slowly with TTC (data not shown). In contrast, MTT resulted in high amounts of formazan deposits 6 h after application in hyphae cultivated on solid agar medium, as well as in liquid culture. The deposits stayed inside the hyphae and were clearly visible by light microscopy (Fig. 4A). No formazan deposits were observed when the spores were pretreated with 4% (v/v) formaldehyde, showing that the presence of formazan precipitates was dependent on living hyphae. For the three independent experiments carried out with G. rosea and the two experiments carried out with G. intraradices, the ratio of area covered with precipitate to total area (Sp:Sh) was always significantly different (P < 0.01 or 0.05) when comparing stimulated and control hyphae (Table III). The metabolic activity of stimulated hyphae, related to formazan production, was 20% to 70% higher at the time point of measurement. Moreover, histograms that represent the frequency distribution of different Sp:Sh values show a shift toward higher classes for exudate-treated hyphae (Fig. 4B). The results from the two experiments show that root exudates significantly increased the respiratory level of G. rosea and G. intraradices and that this fungal response occurred within 3 h after the stimulus, i.e. before branching was visible.
Table II.
O2 consumption in G. rosea and G. intraradices
| Experiments | Increase of O2 Consumption | Slope Values
|
|
|---|---|---|---|
| Control | RE | ||
| % | |||
| G. rosea 1 | 88 | 0.25 | 0.47 |
| G. rosea 2 | 57 | 0.50 | 0.78 |
| G. intraradices 1 | 27 | 1.22 | 1.55 |
| G. intraradices 2 | 31 | 0.80 | 1.05 |
| G. intraradices 3 | 36 | 0.27 | 0.37 |
Relative differences in O2 consumption were measured by polarography in germinating spores treated for 3 h with methanol/water (control) or with root exudates (RE).
Figure 4.
Formazan precipitation in hyphae. A, Image of a hyphal segment of G. rosea with black formazan precipitates (arrow). Total hyphal areas (Sh) and areas covered by formazan precipitates (Sp) were measured. B, Frequency (%) distribution of the different ratio Sp:Sh is shown for controls treated with methanol/water (white columns) or root exudate-stimulated hyphae (black columns) for G. rosea or G. intraradices.
Table III.
Relative hyphal areas with formazan precipitates in G. rosea and G. intraradices
| Experiments | Control | na | RE | n | P< |
|---|---|---|---|---|---|
| G. rosea 1 | 17.62 ± 0.20 | 53 | 29.59 ± 0.18 | 69 | 0.01 |
| G. rosea 2 | 23.61 ± 0.15 | 56 | 31.54 ± 0.07 | 110 | 0.01 |
| G. rosea 3 | 30.26 ± 0.16 | 76 | 45.40 ± 0.24 | 72 | 0.01 |
| G. intraradices 1 | 14.24 ± 0.27 | 27 | 24.30 ± 0.23 | 44 | 0.05 |
| G. intraradices 2 | 30.31 ± 0.21 | 61 | 36.73 ± 0.27 | 53 | 0.05 |
The ratio of hyphal area with formazan precipitates to total hyphal area in percentage Sp:Sh was calculated after 2 h of treatment with methanol/water (control) or with root exudates (RE) and further 6-h incubation with tetrazolium salt.
No. of analyzed images.
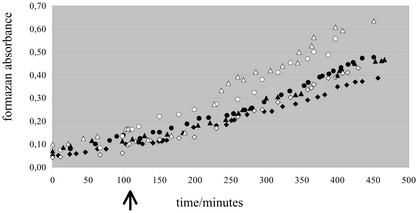
The minimum time required for the fungal response could not be measured with the two above techniques. This was possible with the small spore-producing fungus G. intraradices by using the soluble tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2-H-tetrazolium (MTS), which allowed nondestructive measurement of respiration for several hours by absorbance. Independent time course experiments were carried out to follow the MTS absorbance before and after adding methanol/water or root exudates in methanol/water in the spore suspension (Fig. 5). At first, MTS absorbance increased linearly, and this linear increase continued for further with the same slope after subjecting the spores to either treatment (a slightly higher optical density was observed immediately after adding the root exudates, indicating that the fraction contained light-absorbing compounds). After this “lag phase,” the rate of absorbance increased. However, this rate increase was significantly more pronounced for the root exudates than for methanol/water alone in the three independent experiments and could be detected approximately 90 min after the treatment. Negative controls with dead spores (glutaraldehyde treated) or with spores treated with valinomycin exhibited no increase at all (data not shown).
Figure 5.
Formazan solubilized in the medium. G. intraradices spores were germinated in water in the presence of the tetrazolium salt MTS, and absorbance of formazan solubilized in the medium was measured every 15 min after homogenization. In each of three independent experiments (squares, circles, or triangles), water/methanol (black symbols) or the root exudate fraction in water/methanol (white symbols) was added 110 min after the start of the measuring (black arrow). Note that about 90 min after the addition of the root exudates, the slopes of the white symbol curves become stiffer.
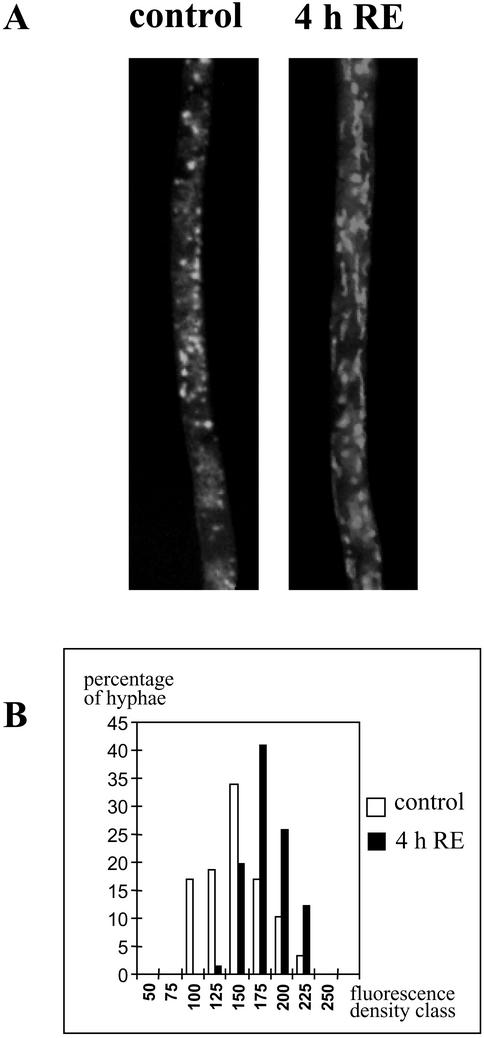
Increased respiration could be the result of an increased mitochondrial activity and/or an increase of mitochondrial biogenesis. In an attempt to answer this question, a new set of stimulation experiments was carried out using the AM fungus G. rosea. Hyphae were stained with MitoTracker green, a specific dye for mitochondria (Funk et al., 1999) and monitored by fluorescence microscopy. Without root exudates, mitochondria appeared randomly distributed and spherical in shape, whereas no fluorescence was visible in KCN-treated hyphae (data not shown). In contrast, in the presence of the root exudate fraction for 4 h, morphology of mitochondria changed to a thread-like shape and the organelles aligned parallel to the major axis of the hyphae (Fig. 6A). A comparison of total biomass of mitochondria between treated and untreated hyphae was made by measuring the fluorescence density (see “Materials and Methods”) within the hyphae. The percentage of hyphal segments with high fluorescence density was higher in the presence of root exudates (Fig. 6B). The mean values increased from 135 (135 ± 31, n = 59) without root exudate to 171 (171 ± 23, n = 66) in the presence of root exudate. These values were significantly different (P < 0.01). Correlatively, the number of bright spots increased significantly (P < 0.05) from 0.125 μm2 for the controls to 0.158 μm2 for the stimulated hyphae. Altogether, these data indicate that the increased mitochondrial activity was at least partly associated with an increased biomass of mitochondria, implying mitochondrial reorganization and biogenesis. The relative activity of organelles could also be stimulated, but our investigation method was not designed to detect it.
Figure 6.
Development of mitochondria. Germinated spores of G. rosea were treated for 4 h with (4h RE) or without (control) root exudates, washed, and stained with MitoTracker Green. A, Micrographs were taken in epifluorescence microscopy. Note the modification of mitochondria shape and number. B, The percentage of hyphae with a given fluorescence density was plotted against classes of fluorescence density from 50 to 255 (arbitrary scale), in the presence (black columns) or absence (white columns) of root exudates.
DISCUSSION
To identify early responses to the addition of an active root exudate fraction in the AM fungus G. rosea, RNA accumulation patterns were analyzed at time points before the emergence of the first hyphal branches. By using different techniques, 10 genes were identified that all show increased transcript levels, already 1 h after stimulation. One of the cDNA fragments revealed no similarity to any known sequence. A second fragment was similar to a gene that was found to be expressed in the human adrenal gland. The function of the encoded protein is unknown, but it shows a putative central coiled-coiled region and is thought to be located in the nucleus (O'Brien et al., 2000). The peptide encoded by the third fragment was highly similar to a putative cytochrome-P450-monooxygenase in plants. Fungal enzymes of the P450 complex are involved in many bioconversion processes (Van den Brink et al., 1998); for example, in the degradation of phytoalexins (Weltring et al., 1988). It is tempting to speculate that such an enzyme contributes to the modification of specific compounds for detoxification. Alternatively, the enzyme could convert certain compounds of the root exudates, resulting in the production or removal of specific signals.
Some of the induced genes are putatively involved in mitochondrial activity. One fragment could correspond to a gene encoding a mitochondrial translocase. A function of these translocases is the transport of ADP into the organelle (Cozens et al., 1989). ADP is an important regulator of the respiratory chain (Tiivel et al., 2000). Another cDNA fragment showed high similarity to genes for pyruvate carboxylase. The corresponding gene was called GrosPyc1 and was chosen for a more detailed analysis. Interestingly, although its GC content was very low (about 36%), a molecular phylogeny analysis grouped the deduced protein clearly to the true fungi. This low GC content has been also detected in other AM fungal genes (Stommel et al., 2001) and is probably due to the overall AT richness of the genomes of these organisms (Hosny et al., 1997). The gene GrosPyc1 showed in the RT-PCR experiments a 10-fold induced RNA level after addition of the root exudate fraction from carrot. Using parsley (Petroselinum crispum) root exudates (Franken and Gnaedinger, 1994) showed only a 4-fold induction of GrosPyc1 expression, which was correlated to a lower level of branching (data not shown). The Bacillus subtilis strain, which was shown to influence presymbiotic development and gene expression in Glomus mosseae (Requena et al., 1999), did not promote the development of G. rosea, and a Streptomycete orientalis being described as inducing development of certain AM fungal strains (Tylka et al., 1991) also failed. Therefore, they were not used for the analysis of gene expression.
Pyruvate carboxylases catalyze the reaction of pyruvate and CO2 to oxaloacetate. Previous reports showed an active CO2 dark fixation in G. intraradices germinating spores (Bago et al., 1999) and a synergistic action of CO2 with root exudates on growth stimulation of G. rosea (Bécard and Piché, 1989b). If the fungus needs CO2 for its metabolism besides its own reserves, the 10 times up-regulation of the pyruvate carboxylase as detected by MIMIC RT-PCR is necessary to incorporate more effectively this additional carbon source into oxaloacetate. This oxaloacetate in turn would be needed for various processes: gluconeogenesis for cell wall or ribonucleotide synthesis, amino acid biosynthesis, etc., important to sustain fungal growth activation and branching phenomenon.
The fact that mitochondrial genes (mt rRNAs) and several genes involved in mitochondrial function were similarly and rapidly induced within 1 h by the branching factor led to the hypothesis that the fungal mitochondrial activity was one primary target of stimulation. Three methods were used to measure in vivo physiological and cytological parameters related to respiratory activity. O2 consumption clearly showed higher fungal respiratory activity 3 h after the addition of root exudates. This was confirmed by measuring the reducing power of hyphae, i.e. their capacity to reduce formazan into a colored precipitate, and by mitochondria staining with MitoTracker Green, showing an increase and reorganization of mitochondrial biomass. Similar results were obtained with the AM fungus G. intraradices, which also exhibited a higher O2 consumption and production of reducing power (formazan precipitates) after being stimulated for 3 h with the root exudates. The fact that the same fraction of root exudates, whose activity has been found in several plant species (Buée et al., 2000), similarly activated respiration of two phylogenetically distant AM fungi suggests that this is a general phenomenon in AM fungus-root relationships. The time point of induced respiration could be more precisely defined for G. intraradices because hyphae of the fungus are able to export the formazan derived from the novel tetrazolium compound MTS. Measuring the absorbance of the medium over time showed the earliest response approximately 90 min after the stimulus. No morphogenetic fungal response is yet observed at this time point, indicating that the stimulation of fungal respiration clearly precedes the phenomenon of intense hyphal branching.
Induction of respiratory activity has also been observed in other plant-microbe interactions. However, this induction was detected in the plant and not in the microbial partner. An elicitor of Phytophthora megasperma induces respiratory CO2 production in parsley cell cultures in 20 min (Norman et al., 1994). In the symbiotic interaction between alfalfa (Medicago sativa) and Rhizobium meliloti, O2 uptake by the roots was higher after 4 h in the presence of the bacteria (Volpin and Phillips, 1998). The responsible bacterial factor was identified to be a common break down product of riboflavin, named lumichrome (Phillips et al., 1999). Interestingly, it not only induces respiratory activity of the roots, but also increases shoot growth through an unknown mechanism.
Higher respiration of AM fungi at the presymbiotic stage might depend upon a general regulatory process that affects mitochondrial activity involving highly coordinated expression of mitochondrial and nuclear genes (Poyton and McEwen, 1996). Regulation of fungal respiration may also be more specific and involve the components of the respiratory chain itself. In contrast with animals, fungi, like plants, possess branched respiratory chains (Joseph-Horne, 2001). In addition to the core pathway, these consist of alternative NADH dehydrogenase and/or alternative oxidase. These alternative paths of electron transfer generate less proton motive force and ATP, but they also generate lower amounts of reactive oxygen species, partly responsible for senescence processes (Dufour et al., 2000). We speculate that germinating spores of AM fungi exhibit a low respiratory activity during asymbiotic growth, perhaps by using alternative electron transports, to minimize C consumption from their own resources like trehalose, glycogen, and lipids and from the incorporation of additional carbon in the form of CO2 (Bago et al., 1999). When they come into contact with specific root factors, they activate their metabolism to more efficiently use these C sources. As a result of this activated catabolism, biosynthetic activity can take place to sustain the growth and hyphal ramification required for root colonization. We can speculate further and propose that obligate biotrophic organisms such as AM fungi, which germinate spontaneously, need a regulatory mechanism to prevent them from consuming their sporal reserves until they perceive the presence (root signal) of a host. In this hypothesis, respiratory activity is an appropriate, upstream, metabolic target for efficient growth control. We do not know the precise regulatory mechanism, but we suggest that the plant is involved in the developmental switch from asymbiotic to presymbiotic fungal growth.
Although root exudates of non mycotrophic plants do not stimulate growth or branching of AM fungi (Giovannetti et al., 1993; Buée et al., 2000), we still lack unequivocal genetic evidence that the inducing root factor is an essential symbiotic signal: Plant mutants not producing the inducing factor and exhibiting an Myc− phenotype have not yet been isolated. This, and the fact that the chemical nature of the inducing factor is not yet known, make it possible that the observed fungal responses are not symbiosis specific, but a more general phenomenon occurring in the rhizosphere between plant roots and also other (non-mycorrhizal) microbes. Whether or not the roots have stimulated fungal growth through a symbiosis-specific signal, this stimulation corresponds to an important developmental switch for the fungus: The activation of certain genes (molecular response) leads to a boost in respiratory activity and energy status (physiological response), which creates the physiological state required for intense hyphal branching (morphological response).
MATERIALS AND METHODS
Biological Materials
Roots of carrots (Daucus carota) transformed by the Ri T-DNA of Agrobacterium rhizogenes were routinely cultivated according to Bécard and Fortin (1988) on a minimal medium gelled with 0.4% (w/v) gellan gum (Phytagel, Sigma, Steinheim, Germany).
Spores of the AM fungus Gigaspora rosea Nicolson & Schenck (BEG 9) were provided by Biorize (Dijon, France). They were washed in a 0.05% (v/v) Tween 20 solution, soaked with 2% (w/v) chloramine T (Sigma) solution for 10 min, washed again three times for 30 s in sterile water, and stored in an antibiotic solution containing 100 mg L−1 gentamycin and 200 mg L−1 streptomycin. After 5 d at 4°C, a second treatment with chloramine T was carried out under the same conditions. To initiate in vitro mycorrhizal culture, three surface-sterilized germinated spores were placed with a single transformed root in a square petri dish on minimal medium and incubated vertically at 25°C (Diop et al., 1992). Dual cultures were maintained for 5 to 6 months at 25°C to produce axenic spores. The development of extraradical hyphae and sporulation was monitored using dissecting and inverted microscopes. After spore production, Phytagel was dissolved in a citrate buffer (pH 6) at 37°C (Doner and Bécard, 1991), and spores were filtered and rinsed with sterile water on an analytical 63-μm sieve (Retsch, Haan, Germany).
Production and purification of root exudates from transformed carrot roots have been carried out as described (Buée et al., 2000). In brief, 500 mg of equivalent dry weight of hairy roots was incubated and oxygenated for 2 d in 100 mL of sterile water in the dark. Crude exudates were filtered and fractionated with ethyl acetate/water. The lipophilic phase was dried and redissolved in 1 mL of methanol. An insoluble fraction was removed by centrifugation, and the methanol phase was diluted with 1 volume of water before use.
For measurements of respiratory activity, surface-sterilized spores of G. rosea were used, as well as spores of Glomus intraradices. Spores of the latter species were produced in vitro on carrot root organ cultures as described by St-Arnaud et al. (1996) and isolated from the Phytagel medium with the citrate buffer method (Doner and Bécard, 1991). They germinated in 1 mL of liquid minimal medium at 2% (v/v) CO2 and 30°C.
Differential RNA Display and Suppressive Subtractive Hybridization
Five hundred G. rosea axenic spores per experiment were germinated at 24°C in the dark under 2% (v/v) CO2 atmosphere in 200 μL of sterile water. Three days after germination, hyphae were stimulated with 5 μL of the root exudate preparation. For controls, 5 μL of water/methanol (v/v) were injected. Germinating spores were harvested 0.5, 1, and 4 h after stimulation. RNA extraction and DNase treatment were carried out on spin columns (Qiagen, Hilden, Germany) following the protocol of Requena et al. (1999). RNA amount and quality were controlled by measuring absorbance and by northern-blot analysis (Stommel et al., 2001). For differential display analysis, 20 ng of DNA-free total RNA was reverse transcribed and amplified with Moloney murine leukemia virus-RT (Promega, Mannheim, Germany) and recombinant Taq polymerase (GibcoBRL, Karlsruhe, Germany) as described by Martin-Laurent et al. (1997). Six primer combinations were used with the degenerate anchored primer (dT11) GC and (dT11) CC and the arbitrary primers AGTCAGCCAC, GGGTAACGCC, and TCGGCGATAG. Five of 25 μL of PCR products was displayed on a 7% (w/v) denaturing polyacrylamide gel, transferred onto Whatman paper (Whatman, Clifton, NJ), dried, and exposed overnight to an x-ray-Omat film (Eastman-Kodak, Rochester, NY) at −70°C. Bands of interest were excised, eluted, and re-amplified as described by Martin-Laurent et al. (1995). The reaction products were separated on 2% (w/v) agarose gels. Single bands were cut out and purified using the Qiaex kit (Qiagen). One-tenth of the purified PCR reaction products were directly used for cloning into the TOPO vector (Invitrogen, Groningen, The Netherlands) following the instructions of the supplier. Recombinant clones were tested by PCR and plasmid DNA was extracted by the alkali lysis method (Sambrook et al., 1989). After sequencing (MWG-Biotech, Ebersberg, Germany), similarity searches were carried out by BlastN (Altschul et al., 1990) and BlastX (Gish and States, 1993).
For suppressive subtractive hybridization (Diatchenko et al., 1996), doubled-stranded cDNAs were obtained by using the SMART-PCR cDNA Synthesis Kit (CLONTECH, Heidelberg) from 1 μg of total RNA of G. rosea spores treated for 1 h with methanol/water (driver) or with root exudates (tester). After two rounds of subtraction of the tester by the driver following the manufacturer's instructions (PCR-Select cDNA Subtraction Kit, CLONTECH), the remaining cDNA was amplified and cloned into the TOPO vector as described above.
Gene Cloning and Analysis
Double-stranded cDNA from root exudates-stimulated spores was used as template for a 3′-RACE experiment with the CDS oligonucleotide of the SMART PCR cDNA Synthesis kit and the two nested primers: Pyc1.for (TGG TCC TAC ACC TGA TGT TG, AT: 59°C) and Pyc2.for (TGC GGT GTT CCA GTA GTT CCA GG, AT: 64°C). Amplification products were cloned and sequenced as described. The deduced amino acid sequence was aligned with pyruvate carboxylases from the SWISSPROT database using ClustalW (Thompson et al., 1994). Based on the alignment, phylogenetic analyses were carried out with the program PUZZLE (version 4.0.2; Strimmer and von Haeseler, 1996) and the program package PHYLIP (version 3.573c; Felsenstein, 1993). Based on the results, dendrograms were constructed by the program TREEVIEW (version 1.5.3; Page, 1996).
RNA Accumulation Analyses
Inserts of clones were amplified using plasmid forward and reverse M13 primers under standard conditions and separated on 1.4% (w/v) agarose gels. After capillary transfer onto Hybond N+ membranes (Amersham Pharmacia Biotech, Freiburg, Germany), DNA was cross linked in a 2400 UV Stratalinker (Stratagene, La Jolla, CA). Prehybridization, hybridization, and washing of the membranes were carried out in digoxigenin (DIG) standard hybridization buffer at 65°C following the protocol of Roche Diagnostics (Penzberg, Germany). Probes were PCR products from an independent differential RNA display experiment or synthesized with the SMART cDNA system (CLONTECH) under incorporation of DIG (DIG-11-dUTP to dTTP 1/1). Probes were analyzed for their quality and calibrated by gel electrophoresis by measuring their A260 and by dot blot and subsequent DIG detection. The alkaline phosphatase activity of the DIG antibody was tested with the substrate CPD Star (Roche Diagnostics) for autoradiography. Signal intensities were evaluated with the program ImageQuant (version 5.0, Amersham Pharmacia Biotech).
Quantitative RT-PCR was carried out as described by Lapopin et al. (1999) using the MIMIC construction system (CLONTECH). One microgram of DNA-free total RNA was reverse transcribed with the SMART cDNA system. For calibration of the single-stranded cDNA, it was amplified at different cycle numbers with the general PCR primers that bind to the adapters. Six l:1 (v/v) dilutions of the PCR MIMICs (10 nm, 5 nm, etc.) were added to the cDNA samples and amplified for 30 cycles. Sequences of primer pairs were GiroPyc1.for (GTC ATG TAT CAT GAT CAT ACT GA) and GiroPyc1.rev (CCT GGA ACT ACT GGA ACA CCG CA). Products were separated by 1.6% (w/v) agarose gel electrophoresis, stained with ethidium bromide, photographed, and signal intensities were compared.
Respiratory Activity
For polarographic measurements, germinated spores were incubated for 3 h with 10 μL of water:methanol (l:1 [v/v]) alone (control) or supplemented with root exudates in 1 mL of liquid minimal medium at 2% (v/v) CO2 and 30°C. Twenty spores of G. rosea or 200 spores of G. intraradices were used for each experiment with the polarograph. O2 consumption was measured using a Clark-type electrode (Hansatech Ltd, Hardwick Industrial, Norfolk, UK) that was calibrated between 0% and 100% with atmospheric oxygen. Spores were added to the chamber, and the temperature was maintained at 30°C using a circulating water bath. Relative differences of O2 consumption were read directly from the chart recording for 15 min. Increase of O2 consumption in treated spores was calculated by comparison of the slope with the control one. Two and three independent experiments were carried out with G. rosea or G. intraradices, respectively.
For cytological analyses using tetrazolium salts, the germinated spores were stimulated with water/methanol root exudates or pure water/methanol as control. Six to 12 spores were used for each treatment. Two hours after stimulation, the liquid medium ± root exudates was replaced by 500 μL of a 50 mm phosphate solution (pH 7.4) containing 1‰ (v/v) Tween 20 and 1% (w/v) MTT, TTC, or NBT. The staining preparations were incubated for 6 h. For negative controls with dead spores, spores were pre-incubated with 4% (v/v) formaldehyde. Samples were washed with the phosphate buffer, mounted on a glass slide, and observed using an inverted microscope (Leitz, DMIRBE, Leica, Rueil-Malmaison, France). Images were acquired by a CCD camera (Color Coolview, Photonic Science, Robertsbridge, UK) with the 63× objective and processed by image analysis (Image Pro-Plus, Media Cybernetics, Silver Spring, MD). Fifty to 110 images for G. rosea in three and 25 to 65 for G. intraradices in two independent experiments, equally distributed along germinating hyphae, were acquired per experiment. Total area of the optically sampled hyphal segments (Sh) and area covered with precipitates (Sp) were measured. Results were expressed as: (a) the mean value of the ratio Sp:Sh, which was considered as an estimation of the respiratory level of the fungus; and (b) a histogram showing the frequency distribution of the Sp:Sh classes. Mean values were compared using the standard Student's t test.
Further cytological analyses were carried out using MitoTracker Green (Molecular Probes, Leiden, The Netherlands) as a probe to visualize mitochondria in G. rosea hyphae. Germinated spores in liquid minimal medium were incubated with root exudates in water/methanol or with water/methanol alone as control. After 4 h, they were washed with liquid minimal medium and then incubated for 5 h in the same medium supplemented with 4 mm of MitoTracker Green. For negative controls, spores were pre-incubated with 1 mm of KCN for 4 h to inhibit the respiratory activity before staining. Samples were washed with the minimal medium, mounted on a glass slide, and observed using the same inverted microscope as above, equipped with an immersion oil objective lens (63× numerical aperture 1.4). Images equally distributed along germinating hyphae were acquired and processed like above. In each image, from the value distribution of pixels (histogram of the gray levels of the green channel), we determined the fluorescence mean value or “fluorescence density.” Because the incubation time with MitoTracker Green was long enough to saturate fluorescence intensity within hyphae, the fluorescence densities obtained served to determine a mitochondrial biomass rather than a mitochondrial activity. We also calculated in each image the number of bright spots per micrometer to estimate the number of mitochondria. Data (mean values ± se) were analyzed using the standard Student's t test.
Time course measurements of fungal respiration were carried out by using a novel tetrazolium compound, MTS, coupled with phenazine ethosulfate. It is used for cell proliferation assays (CellTiter 96, Promega, Madison, WI). The MTS formazan product is soluble in culture medium and was directly titrated with a spectrophotometer at 490 nm. MTS (0.095 mg mL−1) was added to 400 pregerminated spores of G. intraradices grown in 1 mL of liquid minimal medium and incubated at 25°C in a spectrophotometer cuvette. After 3 h of incubation, absorbance measurements were carried out every 15 min for 8 h. Before each measurement, spore suspensions were carefully stirred with a micropipette to homogenize formazan solution, and the spores were allowed to drop to the bottom of the cuvette.
ACKNOWLEDGMENT
We thank Regine Kahmann for critical reading and helpful discussions.
Footnotes
This work was supported by the German Research Council (Deutsche Forschungsgemeinschaft; grant no. SFB 395) and by the French Ministère de l'Education Nationale et de la Recherche Technologique.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.012898.
LITERATURE CITED
- Altman FP. Tetrazolium salts and formazan. Prog Histochem Cytochem. 1976;9:1–51. doi: 10.1016/s0079-6336(76)80015-0. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ayling SM, Smith SE, Smith FA. Transmembrane electric potential difference of germ tubes of arbuscular mycorrhizal fungi responds to external stimuli. New Phytol. 2000;147:631–639. doi: 10.1046/j.1469-8137.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Bachman NJ, Wu W, Schmidt TR, Grossman LI, Lomax MI. The 5′ region of the COX4 gene contains a novel overlapping gene, NOC4. Mamm Genome. 1999;10:506–512. doi: 10.1007/s003359901031. [DOI] [PubMed] [Google Scholar]
- Bago B, Pfeffer PE, Douds DD, Brouillette J, Bécard G, Shachar-Hill Y. Carbon metabolism in spores of the arbuscular mycorrhizal fungus Glomus intraradicesas revealed by nuclear magnetic resonance spectroscopy. Plant Physiol. 1999;121:263–271. doi: 10.1104/pp.121.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago B, Pfeffer PE, Shachar-Hill Y. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 2000;124:949–957. doi: 10.1104/pp.124.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bécard G, Fortin JA. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988;108:211–218. doi: 10.1111/j.1469-8137.1988.tb03698.x. [DOI] [PubMed] [Google Scholar]
- Bécard G, Piché Y. New aspects on the acquisition of biotropic status by a vesicular-arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytol. 1989a;112:77–83. [Google Scholar]
- Bécard G, Piché Y. Fungal growth stimulation by CO2and root exudates in vesicular-arbuscular mycorrhizal symbiosis. Appl Environ Microbiol. 1989b;55:2320–2322. doi: 10.1128/aem.55.9.2320-2325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernas T, Dobrucki JW. Reduction of a tetrazolium salt, CTC, by intact HepG2 human hepatoma cells: subcellular localisation of reducing systems. Biochim Biophys Acta Mol Cell Res. 1999;1451:73–81. doi: 10.1016/s0167-4889(99)00071-3. [DOI] [PubMed] [Google Scholar]
- Bianciotto V, Bonfante P. Presymbiotic versus symbiotic phase in arbuscular endomycorrhizal fungi. In: Varma A, Hock B, editors. Mycorrhiza. Heidelberg: Springer-Verlag; 1998. pp. 229–251. [Google Scholar]
- Buée M, Rossignol M, Jauneau A, Ranjeva R, Bécard G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol Plant-Microbe Interact. 2000;13:693–698. doi: 10.1094/MPMI.2000.13.6.693. [DOI] [PubMed] [Google Scholar]
- Cozens AL, Runswick MJ, Walker JE. DNA sequences of two expressed nuclear genes for human mitochondrial ADP/ATP translocase. J Mol Biol. 1989;206:261–280. doi: 10.1016/0022-2836(89)90477-4. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Lau Y-FC, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED et al. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop TA, Bécard G, Piché Y. Long term in vitro culture of an endomycorrhizal fungus, Gigaspora margarita, on Ri T-DNA transformed roots of carrot. Symbiosis. 1992;12:249–259. [Google Scholar]
- Doner LW, Bécard G. Solubilization of gellan gels by chelation of cations. Biotechnol Technol. 1991;5:25–28. [Google Scholar]
- Dufour E, Boulay J, Rincheval V, Sainsard-Chanet A. A causal link between respiration and senescence in Podospora anserina. Proc Natl Acad Sci USA. 2000;97:4138–4143. doi: 10.1073/pnas.070501997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias KS, Safir GR. Hyphal elongation of Glomus fasciculatusin response to root exudates. Appl Environ Microbiol. 1987;53:1928–1933. doi: 10.1128/aem.53.8.1928-1933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP (Phylogeny Inference Package), Version 3.5c. Seattle: University of Washington; 1993. [Google Scholar]
- Franken P, Gnaedinger F. Analysis of parsley arbuscular endomycorrhiza: infection development and mRNA levels of defense-related genes. Mol Plant-Microbe Interact. 1994;7:612–620. [Google Scholar]
- Franken P, Requena N. Molecular approaches to arbuscular mycorrhiza functioning. In: Hock B, editor. The Mycota. Berlin: Springer Verlag; 2000. pp. 19–28. [Google Scholar]
- Franken P, Requena N. Analysis of gene expression in arbuscular mycorrhiza: new approaches and challenges. New Phytol. 2001;150:431–439. [Google Scholar]
- Franken P, Requena N, Bütehorn B, Krajinski F, Kuhn G, Lapopin L, Mann P, Rhody D, Stommel M. Molecular analysis of the arbuscular mycorrhiza symbiosis. Arch Agron Soil Sci. 2000;45:271–286. [Google Scholar]
- Funk RHW, Nagel F, Wonka F, Krinke HE, Golfert F, Hofer A. Effects of heat shock on the functional morphology of cell organelles observed by video-enhanced microscopy. Anat Rec. 1999;255:458–464. doi: 10.1002/(SICI)1097-0185(19990801)255:4<458::AID-AR11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Gianinazzi-Pearson V, Branzanti B, Gianinazzi S. In vitroenhancement of spore germination and early hyphal growth of a vesicular-arbuscular mycorrhizal fungus by host root exudates and plant flavonoids. Symbiosis. 1989;7:243–255. [Google Scholar]
- Giovannetti M, Sbrana C, Avio L, Citernesi AS, Logi C. Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during pre-infection stages. New Phytol. 1993;125:587–593. doi: 10.1111/j.1469-8137.1993.tb03907.x. [DOI] [PubMed] [Google Scholar]
- Giovannetti M, Sbrana C, Citernesi AS, Avio L. Analysis of factors involved in fungal recognition responses to host-derived signals by arbuscular mycorrhizal fungi. New Phytol. 1996;133:65–71. [Google Scholar]
- Gish W, States DJ. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- Hosny M, Barros JP, Gianinazzi-Pearson V, Dulieu H. Base composition of DNA from glomalean fungi: high amount of methylated cytosine. Fung Genet Biol. 1997;22:103–111. doi: 10.1006/fgbi.1997.1008. [DOI] [PubMed] [Google Scholar]
- Jolicoeur M, Germette S, Gaudette M, Perrier M, Bécard G. Intracellular pH in arbuscular mycorrhizal fungi: a symbiotic physiological marker. Plant Physiol. 1998;116:1279–1288. doi: 10.1104/pp.116.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Horne T. Fungal respiration: a fusion of standard and alternative components. Biochim Biophys Acta Bioenerg. 2001;1504:179–195. doi: 10.1016/s0005-2728(00)00251-6. [DOI] [PubMed] [Google Scholar]
- Lapopin L, Gianinazzi-Pearson V, Franken P. Comparative differential display analysis of arbuscular mycorrhiza in Pisum sativumand a mutant defective in late stage development. Plant Mol Biol. 1999;41:669–677. doi: 10.1023/a:1006387523343. [DOI] [PubMed] [Google Scholar]
- Lei J, Bécard G, Catford JG, Piché Y. Root factor stimulate 32P uptake and plasmalemma ATPase activity in the vesicular-arbuscular mycorrhizal fungus, Gigaspora margarita. New Phytol. 1991;118:289–294. doi: 10.1111/j.1469-8137.1991.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Martin-Laurent F, Van Tuinen D, Dumas-Gaudot E, Gianinazzi-Pearson V, Gianinazzi S, Franken P. Differential display analysis of RNA accumulation in arbuscular mycorrhiza of pea and isolation of a novel symbiosis-regulated plant gene. Mol Gen Genet. 1997;256:37–44. doi: 10.1007/s004380050543. [DOI] [PubMed] [Google Scholar]
- Martin-Laurent FA, Franken P, Gianinazzi S. Screening of cDNA fragments generated by differential RNA display. Anal Biochem. 1995;228:182–184. doi: 10.1006/abio.1995.1337. [DOI] [PubMed] [Google Scholar]
- Nagahashi G, Douds DD. A rapid and sensitive bioassay with practical application for studies on interactions between root exudates and arbuscular mycorrhizal fungi. Biotechnol Technol. 1999;12:893–897. [Google Scholar]
- Nagahashi G, Douds DD. Partial separation of root exudate components and their effects upon the growth of germinated spores of AM fungi. Mycol Res. 2000;104:1453–1464. [Google Scholar]
- Norman EG, Walton AB, Turpin DH. Immediate activation of respiration in Petroselinum crispum L. in response to the Phytophthora megasperma f. sp. glycineaelicitor. Plant Physiol. 1994;106:1541–1546. doi: 10.1104/pp.106.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KP, Tapia-Paez I, Stahle-Backdahl M, Kedra D, Dumanski JP. Characterization of five novel human genes in the 11q13–q22 region. Biochem Biophys Res Commun. 2000;273:90–94. doi: 10.1006/bbrc.2000.2910. [DOI] [PubMed] [Google Scholar]
- Page RDM. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Phillips DA, Joseph CM, Yang GP, Martinez-Romero E, Sanborn JR, Volpin H. Identification of lumichrome as a Sinorhizobiumenhancer of alfalfa root respiration and shoot growth. Proc Natl Acad Sci USA. 1999;96:12275–12280. doi: 10.1073/pnas.96.22.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton RO, McEwen JE. Cross talk between nuclear and mitochondrial genomes. Annu Rev Biochem. 1996;65:563–607. doi: 10.1146/annurev.bi.65.070196.003023. [DOI] [PubMed] [Google Scholar]
- Requena N, Füller P, Franken P. Molecular characterisation of GmFOX2, an evolutionary highly conserved gene from the mycorrhizal fungus Glomus mosseae, down-regulated during interaction with rhizobacteria. Mol Plant-Microbe Interact. 1999;12:934–942. doi: 10.1094/MPMI.1999.12.10.934. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1989. [Google Scholar]
- Schüssler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res. 2001;105:1413–1421. [Google Scholar]
- Smith SE, Read DJ. Mycorrhizal Symbiosis. London: Academic Press; 1997. [Google Scholar]
- St-Arnaud M, Hamel C, Vimard B, Caron M, Fortin JA. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitrosystem in the absence of host roots. Mycol Res. 1996;100:328–332. [Google Scholar]
- Stommel M, Mann P, Franken P. Construction and analysis of an EST library using RNA from activated spores of the arbuscular mycorrhizal fungus Gigaspora rosea. Mycorrhiza. 2001;10:281–285. [Google Scholar]
- Stowe RP, Koenig DW, Mishra SK, Pierson DL. Nondestructive and continuous spectrophotometric measurement of cell respiration using a tetrazolium-formazan microemulsion. J Microbiol Methods. 1995;22:283–292. [Google Scholar]
- Strimmer K, von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–969. [Google Scholar]
- Tawaraya K, Watanabe S, Yoshida E, Wagatsuma T. Effect of onion (Allium cepa) root exudates on the hyphal growth of Gigaspora margarita. Mycorrhiza. 1996;6:57–59. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiivel T, Kadaya L, Kuznetsov A, Kaambre T, Peet N, Sikk P, Braun U, Ventura-Clapier R, Saks V, Seppet EK. Developmental changes in regulation of mitochondrial respiration by ADP and creatine in rat heart in vivo. Mol Cell Biol. 2000;208:119–128. doi: 10.1023/a:1007002323492. [DOI] [PubMed] [Google Scholar]
- Tylka GL, Hussey RS, Roncadori RW. Axenic germination of vesicular arbuscular mycorrhital fungi: effects of selected Streptomycesspecies. Phytopathol. 1991;81:754–759. [Google Scholar]
- Van den Brink HJM, Van Gorcom RFM, Van den Hondel C, Punt PJ. Cytochrome P450 enzyme systems in fungi. Fung Genet Biol. 1998;23:1–17. doi: 10.1006/fgbi.1997.1021. [DOI] [PubMed] [Google Scholar]
- Van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianinazzipearson V. Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol. 1998;7:879–887. doi: 10.1046/j.1365-294x.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Althug M, Engelstreitwolf R, Mader P, Wiemken A. Studies on the attractional effect of root exudates on hyphal growth of an arbuscular mycorrhizal fungus in a soil compartment-membrane System. Plant Soil. 1998;203:137–144. [Google Scholar]
- Volpin H, Phillips DA. Respiratory elicitors from Rhizobium melilotiaffect intact alfalfa roots. Plant Physiol. 1998;116:777–783. doi: 10.1104/pp.116.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltring K-M, Turgeon BG, Yoder OC, VanEtten HD. Isolation of a phytoalexin-detoxification gene from the plant pathogenic fungus Nectria haematococca by detecting its expression in Aspergillus nidulans. Gene. 1988;68:335–344. doi: 10.1016/0378-1119(88)90036-4. [DOI] [PubMed] [Google Scholar]