Abstract
As a catalytic cofactor, biotin has a critical role in the enzymological mechanism of a number of enzymes that are essential in both catabolic and anabolic metabolic processes. In this study we demonstrate that biotin has additional non-catalytic functions in regulating gene expression in plants, which are biotin autotrophic organisms. Biotin controls expression of the biotin-containing enzyme, methylcrotonyl-coenzyme A (CoA) carboxylase by modulating the transcriptional, translational and/or posttranslational regulation of the expression of this enzyme. The bio1 mutant of Arabidopsis, which is blocked in the de novo biosynthesis of biotin, was used to experimentally alter the biotin status of this organism. In response to the bio1-associated depletion of biotin, the normally biotinylated A-subunit of methylcrotonyl-CoA carboxylase (MCCase) accumulates in its inactive apo-form, and both MCCase subunits hyperaccumulate. This hyperaccumulation occurs because the translation of each subunit mRNA is enhanced and/or because the each protein subunit becomes more stable. In addition, biotin affects the accumulation of distinct charge isoforms of MCCase. In contrast, in response to metabolic signals arising from the alteration in the carbon status of the organism, biotin modulates the transcription of the MCCase genes. These experiments reveal that in addition to its catalytic role as an enzyme cofactor, biotin has multiple roles in regulating gene expression.
Biotin is a water-soluble vitamin biosynthesized by plants, some fungi, and most bacteria and is required by all living organisms for normal cellular functions and growth. Extensive genetic and biochemical studies of prokaryotic organisms have established that biotin is biosynthesized from pimeloyl-CoA and Ala via a four-reaction biosynthetic pathway (DeMoll, 1996). Less extensive studies indicate that plants biosynthesize biotin via an analogous pathway (Shellhammer and Meinke, 1990; Weaver et al., 1995; Patton et al., 1996; Patton et al., 1998; Alban et al., 2000). Of the four enzymes required for biotin biosynthesis, only the one catalyzing the terminal reaction has been molecularly characterized in plants. This enzyme, called biotin synthase, is encoded by the BIO2 gene of Arabidopsis and is a mitochondrial protein (Weaver et al., 1995; Patton et al., 1996; Baldet et al., 1997; Patton et al., 1998). Hence, in plants, biotin is biosynthesized in the mitochondria.
Biotin acts as a coenzyme, covalently bound to a Lys residue of a group of enzymes that catalyze carboxylation, decarboxylation or transcarboxylation reactions (Moss and Lane, 1971). The reactions catalyzed by these enzymes are involved in diverse metabolic processes including lipogenesis (acetyl-CoA carboxylase [ACCase]), gluconeogenesis (pyruvate carboxylase), and amino acid metabolism (methylcrotonyl-CoA carboxylase [MCCase] and propionyl-CoA carboxylase). These enzymes share a common biochemical reaction mechanism, in which the biotin prosthetic group acts as an intermediate carrier of the carboxyl group that is used as the substrate in the reaction. The carboxyl group specifically is first transferred from the donor substrate (D-CO2−) to the enzyme-bound biotin (reaction 1) and then to the final acceptor substrate (A; reaction 2).
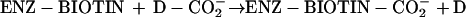 |
1 |
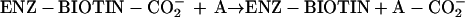 |
2 |
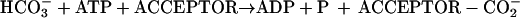 |
3 |
Different organisms contain different complements of biotin-containing proteins. Bacteria and archaea have one to three biotin-containing proteins; for example, Escherichia coli contains only the biotin carboxyl carrier subunit of ACCase. Eukaryotic organisms have four or five such proteins. For example, animals contain ACCase, MCCase, pyruvate carboxylase, and propionyl-CoA carboxylase; Brewer's yeast (Saccharomyces cerevisiae), contains ACCase, pyruvate carboxylase, and urea carboxylase; and plants contain two isozymes of ACCase (Sasaki et al., 1993; Yanai et al., 1995; Ke et al., 2000), MCCase (Alban et al., 1993; Chen et al., 1993; Diez et al., 1994; Song et al., 1994; Wang et al., 1994; McKean et al., 2000), geranoyl-CoA carboxylase (Guan et al., 1999), and a seed-specific biotin protein that may function to store biotin (Duval et al., 1994; Hsing et al., 1998).
In addition to its catalytic function as an enzyme-bound prosthetic group, biotin may have a role in regulating gene expression. For example, in bacteria, biotin biosynthesis is regulated by the biotin status of the organism, and the activated form of biotin, biotinyl-AMP, acts as a corepressor to control the transcription of the biotin-biosynthetic operon (bio) and thus regulate biotin content of the cell (Cronan, 1989). A nonenzymological function(s) for biotin has also been reported in animals. For example, biotin affects the expression of several genes, including those coding for glucokinase (Chauhan and Dakshinamurti, 1991), phosphoenolpyruvate carboxykinase (Dakshinamurti and Li, 1994), holocarboxylase synthetase, ACCase, and propionyl-CoA carboxylase (Solorzano-Vargas et al., 2002). In addition, several studies have demonstrated that enhanced dietary biotin intake has beneficial effects on the growth of farm animals, which appear to be independent of biotin's cofactor function (Whitehead et al., 1976; Whitehead and Bannister, 1981; Lischer et al., 2002). Although mechanistically not well understood, these latter observations indicate that biotin may have more extensive roles in biology than its enzymological function as an enzyme cofactor.
We report here that in Arabidopsis, a the eukaryotic biotin autotrophic organism biotin regulates the expression of MCCase subunit genes via complex mechanisms that are independent of the role of the molecule as a cofactor in the carboxylation reaction catalyzed by this enzyme.
RESULTS
The Effects of Biotin Depletion on MCCase Expression
To ascertain the effect of biotin on gene expression, tissues that contain low endogenous levels of biotin, optimally no biotin, are needed. Although it is simple to experimentally manipulate biotin levels in biotin heterotrophic organisms, this is more problematic in biotin autotrophs such as plants and bacteria. Because the bio1 mutant of Arabidopsis cannot biosynthesize biotin (Shellhammer and Meinke, 1990), it is ideally suited for investigations into the effect of biotin. The BIO1 gene is thought to encode for 7,8-diaminopelargonic acid aminotransferase, the second enzyme required in the conversion of pimeloyl-CoA and Ala to biotin (Patton et al., 1996). Plants homozygous for the bio1 mutation show an embryonic-lethal phenotype, which can be rescued by the exogenous supply of biotin (Shellhammer and Meinke, 1990). Hence, homozygous bio1 seeds germinate and grow on maternally supplied biotin. When this store of biotin is depleted, bio1 seedlings stop growing (at the cotyledon stage), but these seedlings can be rescued with exogenously provided biotin. From such biotin-rescued plants, homozygous bio1 plants can be grown, and seeds of this genotype can be recovered (Shellhammer and Meinke, 1990).
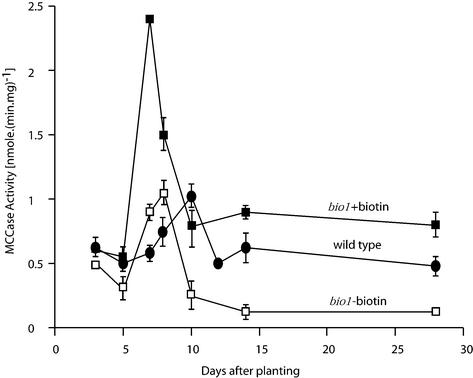
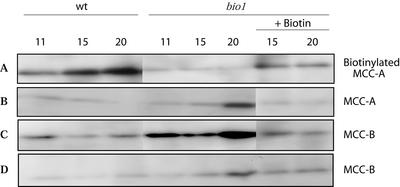
As an initial step to ascertain the effect of biotin depletion in the bio1 mutant, MCCase activity was compared between bio1 and wild-type Arabidopsis seedlings. As shown in Figure 1, MCCase activity increases during seedling development, peaking at 8 d after planting (DAP) in bio1 seedlings and at 10 DAP in wild-type seedlings. Within 2 d after this peak, MCCase activity declines to lower levels, but in the bio1 mutant, this activity is 3-fold lower than in the wild-type. The addition of exogenous biotin to bio1 seedlings does not alter the pattern of MCCase expression, but elevates MCCase activity even above wild-type levels. These data indicate that in the bio1 plants, maternally derived biotin is depleted from MCCase by 10 DAP. Consistent with this conclusion, parallel western-blot analyses with streptavidin indicates that the biotin content on the MCC-A subunit is reduced as biotin is depleted from the bio1 seedlings; but when exogenous biotin is provided to these seedlings, the biotin content on this subunit increases (Fig. 2A).
Figure 1.
The effect of plant growth on MCCase activity. MCCase specific activity was determined in extracts of wild-type or bio1 mutant seedlings between 3 and 28 d after sowing. Seedlings were grown either with or without the exogenous addition of 1 mm biotin. Data are the mean ± se from four replicates.
Figure 2.
The effect of biotin on the biotinylation status and accumulation of the MCCase subunits. Protein extracts were prepared from seedlings (A–C) or excised cotyledons (D) of wild-type and bio1 Arabidopsis seedlings at the indicated DAP. Aliquots of extracts containing equal amounts of protein (150 μg) were subjected to SDS-PAGE, followed by western-blot analysis with either 125I-streptavidin to detect the biotinylated MCC-A subunit (A) or immunological detection with antibodies to MCC-A (B) or MCC-B (C and D). Where indicated, exogenous biotin (0.25 mm) was provided to the bio1 seedlings 2 d before harvest. The data presented were gathered from a single experiment; five replicates of this experiment, with two different batches of bio1 seeds, gave similar results.
In contrast, immunological detection of MCC-A indicates that the accumulation of this subunit is induced as biotin is depleted (Fig. 2B). This induction is paralleled by a similar increase in the accumulation of the non-biotinylated MCC-B subunit (Fig. 2C). Thus, in response to biotin depletion, the accumulation of both the MCC-A and MCC-B subunits is induced 5- to 10-fold, but the MCC-A subunit accumulates in the non-biotinylated apo-form.
Because bio1 mutant seedlings are developmentally arrested at the cotyledon stage, whereas wild-type plants develop to the 4-leaf stage by 20 DAP, it was necessary to ensure that the observed induction of MCC-A and MCC-B accumulation was directly due to biotin depletion and not to the developmental arrest of the seedling. This question was addressed by comparing the accumulation of these subunits in the cotyledons of wild-type and bio1 seedlings, which are morphologically indistinguishable between these two genotypes. These analyses indicate that the accumulation of the MCC-A (data not shown) and MCC-B (Fig. 2D) subunits are induced in the cotyledons of bio1 seedlings relative to the cotyledons of wild-type seedlings. Furthermore, the induction of the accumulation of the MCC-A and MCC-B subunits is reversed when exogenous biotin is provided to the seedlings (Fig. 2, B–D), indicating that MCC-A and MCC-B accumulation is a direct consequence of biotin depletion. These findings demonstrate for the first time, to our knowledge, that biotin plays a role in the regulation of MCCase gene expression. Specifically, the accumulation of MCC-A and MCC-B proteins is inversely related to the biotin content of the Arabidopsis seedling.
Biotin Regulation of MCCase Expression Is Controlled at the Translational and/or Posttranslational Level
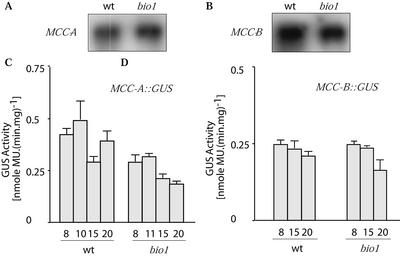
To further investigate the mechanism by which biotin depletion enhances the accumulation of the MCCase subunits, we compared the accumulation of the MCC-A and MCC-B mRNAs between wild-type and bio1 plants. As shown in Figure 3A, the abundance of the MCC-A and MCC-B mRNAs is similar in both wild-type and bio1 plants at 20 DAP. Furthermore, MCC-A- and MCC-B-promoter-mediated GUS expression is similar or slightly reduced when these transgenes are in the bio1 background as compared with the wild-type genetic background (Fig. 3, C and D).
Figure 3.
The effect of biotin-depletion on MCCase gene transcription. Northern-blot analysis of MCC-A (A) and MCC-B (B) mRNA accumulation in wild-type and bio1 Arabidopsis seedlings. RNA was isolated from wild-type and bio1 seedlings grown to 20 DAP in the absence of exogenous biotin. Equal amounts of isolated RNA (50 μg) were subjected to electrophoresis in formaldehyde-containing agarose gels, and MCC-A or MCC-B mRNAs were detected by hybridization with respective 32P-labeled probes. Reporter gene expression studies of the MCC-A and MCC-B genes. GUS activity was determined in protein extracts from transgenic Arabidopsis seedlings of either wild-type (wt) or bio1 genetic background and carrying an MCC-A::GUS (C) or MCC-B::GUS (D) reporter transgene. Seedlings were grown without exogenous biotin to the indicated DAP. Data are the means ± se from three replicates.
We interpret these results to indicate that the enhanced accumulation of MCC-A and MCC-B proteins in response to biotin depletion is not caused by enhanced transcription of the respective genes or increased accumulation of the respective mRNAs. Instead, the enhanced accumulation of the MCC-A and MCC-B proteins is due to either enhanced translation of the respective mRNAs or reduced turnover of these proteins.
Biotin Is Required for Metabolic Control of MCCase Gene Expression
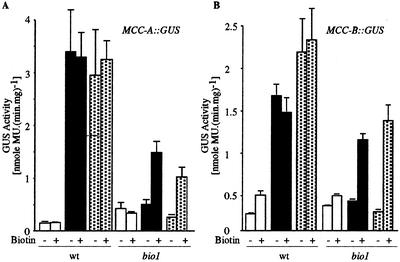
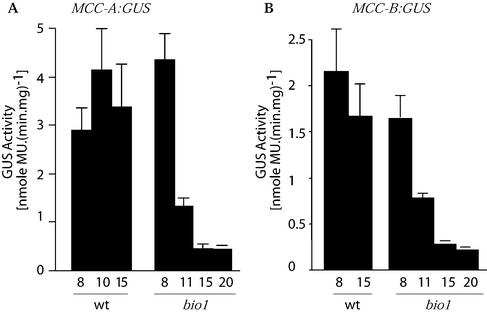
In Arabidopsis, the expression of MCC-A and MCC-B genes respond to the carbon status of the organism (Che et al., 2002). Carbon starvation specifically increases MCCase activity, and the accumulation of MCC-A and MCC-B mRNAs, proteins, and this is the consequence of increased transcription of the MCC-A and MCC-B genes (Che et al., 2002). This conclusion was based upon experiments in which the carbon status of Arabidopsis seedlings was manipulated by altering the illumination of seedlings or by growing seedlings in a CO2-free atmosphere. Darkness and CO2 deprivation induce the expression of MCC-A::GUS and MCC-B::GUS transgenes. To test whether biotin plays any role in this regulation, wild-type or bio1 seedlings carrying either the MCC-A::GUS or MCC-B::GUS transgenes were grown under constant illumination for 13 d and were then either maintained in continuous illumination or transferred to darkness for 2 additional d. In parallel experiments, 13-d-old seedlings grown under continuous illumination were transferred to a CO2-free atmosphere for 2 additional d without any change in the illumination status. As shown in Figure 4, A and B, in the wild-type background, these environmental manipulations (darkness or CO2-free atmosphere) induced the expression of the MCC-A::GUS and MCC-B::GUS transgenes 10- to 20-fold. However, in the bio1 genetic background, this induction is suppressed. That this failure to induce MCC-A- and MCC-B-mediated GUS activity in the bio1 mutant background is due to the lowered biotin status of the seedlings is evidenced by the fact that the addition of exogenous biotin partially reverses the suppression. These results indicate that biotin is required for the metabolic regulation of the transcription of the MCCase subunit genes.
Figure 4.
The effect of biotin-depletion on the metabolic regulation of MCC-A and MCC-B gene transcription. GUS activity was determined in protein extracts from wild-type (wt) or bio1 Arabidopsis seedling carrying an MCC-A::GUS (A) or MCC-B::GUS (B) reporter transgene. Seedlings were grown to 13 DAP on Murashige and Skoog agar medium without biotin, followed by 2 d of additional growth either in the absence (−) or presence (+) of exogenous biotin. In these last 2 d of growth, seedlings were grown either under constant illumination (white bars), or transferred to darkness (black bars), or CO2-free air (dotted bars). Data are the means ± se from three replicates.
To further characterize this interaction between biotin and carbon status in the regulation of MCCase gene transcription, we studied the timing of the induction of the MCC-A- and MCC-B-mediated GUS expression in response to light deprivation. In this experiment, seedlings containing the MCC-A::GUS and MCC-B::GUS transgenes, carried in either the wild-type or bio1 mutant background, were grown under continuous illumination for 6, 9, 13, and 18 d, and then transferred to complete darkness for an additional 2 d of growth, at which stage GUS activity was determined. As shown in Figure 5, A and B, at 8 DAP, MCC-A- and MCC-B-mediated GUS activity is similarly induced by darkness in both bio1 and wild-type seedlings. However, by 11 DAP the induction of the transgenes in the bio1 plants declines, and by 15 DAP, they are at 15% to 20% of the wild-type levels. This reduction in the ability of the MCC-A::GUS and MCC-B-GUS transgenes to be dark-induced at 11 DAP, coincides with the timing of the loss of biotin from the MCC-A subunit (Figs. 1 and 2), consistent with a role for biotin in regulating MCCase gene transcription.
Figure 5.
Time course of the biotin dependence of the metabolic regulation of MCC-A and MCC-B gene transcription. GUS activity was determined in protein extracts from wild-type (wt) or bio1 Arabidopsis seedlings carrying an MCC-A::GUS (A) or MCC-B::GUS (B) reporter transgene. Seedlings were grown in the absence of exogenous biotin to the indicated DAP. These seedlings were maintained in constant illumination until the last 2 d of growth, at which stage they were transferred to total darkness. Data are the means ± se from three replicates.
The Effect of Biotin on MCCase Subunit Stoichiometry
All MCCases investigated to date (from bacterial, plant, and animal sources) are composed of two subunits, a larger biotinylated subunit of about 80 kD (MCC-A) and a smaller non-biotinylated subunit of about 60 kD (MCC-B; Schiele et al., 1975; Lau et al., 1980; Wurtele and Nikolau, 2000). However, two types of MCCase differing in their subunit stoichiometries and hence molecular weights have been reported. The MCCase from animals (Lau et al., 1980), carrot (Daucus carota; Chen et al., 1993), maize (Zea mays; Diez et al., 1994), soybean (Glycine max; Song, 1993), and tomato (Lycopersicon esculentum; Wang, 1993) appear to have an A6B6 quaternary structure, with a molecular mass of about 850 kD. In contrast, MCCase from bacteria (Schiele et al., 1975), pea (Pisum sativum), and potato (Solanum tuberosum; Alban et al., 1993) appear to have an A4B4 quaternary structure, with a molecular mass of about 530 kD. Because the biotinylation of MCCase is an important mechanism for regulating this enzyme (Wang et al., 1995), we considered the possibility that the apparent differences in the quaternary structure of MCCase from different sources may reflect the biotinylation status of the enzyme. To test this hypothesis, we determined the Mr of the MCCase complexes from wild-type and the biotin-depleted bio1 mutant Arabidopsis seedlings and compared them with the soybean and pea MCCase.
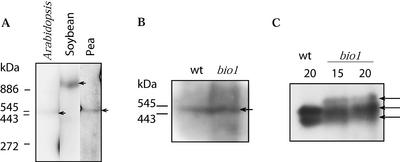
The Mr of MCCase was determined by subjecting extracts to exhaustive electrophoresis in gels consisting of a 5% to 30% (w/v) linear gradient of polyacrylamide (Hedrick and Smith, 1968; Diez et al., 1994). Under these conditions, migration of proteins becomes limited when the sieve size of the gel pores is similar to the Stokes radius of the protein (which is proportional to the protein's Mr). Thus, migration in this electrophoresis system is inversely proportional to the Mr of the protein. Hence, by comparing the migration of MCCase with the migration of standard proteins, it was possible to determine the apparent Mr of MCCase. As shown in Figure 6A, the molecular mass of MCCase in pea and Arabidopsis is about 500 kD, whereas soybean MCCase is about 900 kD. Furthermore, biotin-depleted MCCase from Arabidopsis bio1 seedlings migrated identically to the enzyme from wild-type seedlings, i.e. with a molecular mass of about 500 kD.
Figure 6.
Electrophoretic characterization of MCCase. Protein extracts from Arabidopsis, soybean, and pea seedlings (A) and wild-type (wt) and bio1 mutant Arabidopsis seedlings (B) were subjected to exhaustive electrophoresis (for 14,400 V h−1) in gels composed of a linear gradient of 5% to 30% polyacrylamide according to the method of Hedrick and Smith (1968). After western blotting, MCCase was immunologically detected by reacting the membranes with anti-MCC-B serum (identical results were obtained with anti-MCC-A serum; data not shown). The native molecular mass of MCCase was determined by comparing its migration to standard proteins (apoferritin dimer, 886 kD; apoferritin monomer, 443 kD; urease dimer, 545 kD; and urease monomer, 272 kD). The position of MCCase is indicated by arrows. C, Analysis of MCCase charge isoforms. Aliquots of protein extracts from wild-type (wt) and bio1 Arabidopsis seedlings at the indicated DAP, containing equal amounts of MCCase activity, were subjected to electrophoresis at 70 V for 17 h in a linear 5% to 20% gradient polyacrylamide gel (Lambin and Fine, 1979). After western blotting, MCCase was immunologically detected by reacting the membranes with anti-MCC-B serum (identical results were obtained with anti-MCC-A serum; data not shown).
These data indicate that the subunit stoichiometry of MCCase is unaffected by the biotinylation status of the enzyme. Furthermore, these results confirm the earlier studies, which implied that MCCase from different plant species have different subunit stoichiometries. Namely, soybean MCCase appears to have an A6B6 configuration, whereas the pea and Arabidopsis MCCase has an A4B4 configuration. However, the physiological significance of this difference is still unclear.
The Effect of Biotin on the Formation of MCCase Charge Isoforms
Fractionation of Arabidopsis extracts by non-denaturing PAGE identifies two distinct forms of MCCase that migrate at different rates during electrophoresis (Fig. 6C). The electrophoresis system used in this study (Lambin and Fine, 1979) separates proteins both on the basis of charge and size. Knowing that there is no size heterogeneity in the MCCase present in these extracts (see Fig. 6, A and B), we conclude that these electrophoretically separable forms of MCCase represent charge isoforms of this enzyme. These charge isoforms of MCCase were also detectable by isoelectric focusing of Arabidopsis extracts (data not shown). Furthermore, previous analyses indicated that charge isoforms of MCCase occur in soybean (Song, 1993).
To investigate whether changes in the biotinylation status of MCCase affects the accumulation of these charge isoforms, we performed non-denaturing PAGE of seedling extracts from wild-type and biotin-depleted, bio1 plants. MCCase was detected by western-blot analysis of the resulting gels using anti-MCC-B serum. As shown in Figure 6C, two MCCase bands accumulate in extracts from wild-type seedlings, but an additional band is detected in extracts from bio1 seedlings. The origin of these MCCase charge isoforms is not clear. Because the two MCCase subunits are each encoded by a single gene in Arabidopsis (Weaver et al., 1995; McKean et al., 2000), the charge isoforms cannot represent products of different members of a gene family. However, there is evidence that the MCC-A gene can generate two alternatively spliced mRNAs (Che et al., 2002), which could generate isoforms of MCCase. Two MCC-A cDNAs have specifically been identified (GenBank accession nos. U12536 and AY070723) that differ from each other by the insertion of a 62-nucleotide sequence, which would be the result of alternative splicing of the initial transcript. An alternative explanation is that the charge isoforms are a direct effect of biotinylation. Namely, apo- and holo-MCC-A would be expected to differ from each other by a single charge as the Lys residue that becomes biotinylated contributes a single positive charge to apo-MCC-A, which is eliminated upon biotinylation and conversion to holo-MCC-A. Hence, MCCase that contains apo-MCC-A subunits would migrate more slowly during electrophoresis due to the increased positive charge associated with this form of the enzyme. The change in electrophoresis pattern of MCCase isoforms associated with the change in the biotinylation status of the enzyme is consistent with this explanation.
DISCUSSION
The biochemical function of biotin as an enzyme-bound cofactor was established with the purification and characterization of such biotin-containing enzymes as MCCase, ACCase, and propionyl-CoA carboxylase. Each of these enzymes catalyze reactions that are critical in primary metabolism. For example, MCCase is part of the mitochondrial Leu catabolic pathway, a function that in humans is essential for growth and development. To date, three mechanisms have been reported to regulate MCCase expression. First, during the development of Arabidopsis, the MCC-A and MCC-B mRNAs show a coordinated programmed accumulation pattern (McKean et al., 2000) that probably reflects the metabolic role of MCCase in Leu (Anderson et al., 1998) and possibly cytosolic mevalonate-derived isoprenoid metabolism (Nes and Bach, 1985). Second, a complex interplay between environmental and metabolic signals mediates the transcription of the Arabidopsis MCC-A and MCC-B genes (Che et al., 2002). This sensitive regulation of MCCase expression probably reflects the physiological demands for MCCase function in Leu catabolism in response to changes in the carbon status of the organism. The transcription of the MCCase subunit genes is induced when the carbon status of the plant is lowered, and this response appears to be mediated via a sugar-signaling pathway (Che et al., 2002). Finally, in tomato, MCCase activity in roots and leaves is regulated by differential biotinylation of the MCC-A subunit (Wang et al., 1995). Specifically, whereas the MCC-A subunit accumulates to near equal levels in both roots and leaves, leaves express only 10% of the MCCase activity found in roots, and this difference is due to the lower biotinylation status of the MCC-A subunit in the leaf.
The studies reported herein indicate that as is the case for another cofactor, vitamin A (Truckenmiller et al., 2001; Chang et al., 2002), biotin has additional biological roles, namely in regulating gene expression. We have used the bio1 mutant of Arabidopsis, which is blocked in the de novo biosynthesis of biotin, to elucidate the role of biotin in regulating MCCase expression. In response to biotin depletion (due to the bio1 mutation), the MCC-A subunit accumulates in its inactive apo-form, and the accumulation of both MCCase subunits is induced. This induction occurs either because the translation of each subunit mRNA is enhanced or because the turnover of each subunit protein is reduced. Because the accumulation of both MCCase subunits is similarly induced, the mechanism(s) that controls the expression of these subunits probably coordinately affects both subunits. In addition, biotin is required for the two MCCase subunit genes to respond to metabolic signals. Specifically, in environmental conditions that reduce the carbon status of seedlings (deprivation of CO2 or deprivation of illumination), transcription of the MCCase genes is normally induced (Che et al., 2002). However, this induction in gene transcription fails to occur in seedlings that are depleted of biotin. Finally, MCCase can accumulate as distinct isoforms that are separable by electrophoresis, and biotin influences the distribution of these isozymes. These charge isoforms may be due to alternatively spliced MCC-A mRNAs and/or to incomplete biotinylation, which leads to the accumulation of the differently charged apo-form of MCC-A.
It is interesting to consider how this complex regulation of MCCase expression by biotin is mediated. For example, does the organism sense the accumulation of the apo-subunit directly and alter MCCase expression, or does it respond to a decreased concentration of biotin, or does the organism detect the block in the metabolic function associated with MCCase (i.e. Leu catabolism) and alter MCCase expression? Although these questions cannot be addressed by the data presented herein, ongoing studies of MCC-A expression in an Arabidopsis mcc-B knockout mutant indicate that the organism is responding either to changes in the biotinylation status of MCCase or changes in the biotin status of the organism per se (P. Che and B.J. Nikolau, unpublished data). Namely, when Leu catabolism is blocked due to the mcc-B mutation, the accumulation of the MCC-A subunit is not induced, and the metabolic induction of MCC-A transcription still occurs. Hence, the experiments reported herein reveal that, in addition to its catalytic role as an enzyme cofactor, biotin may have a role in regulating MCCase gene expression. Therefore, previous findings, which indicate that biotin concentration differs among different cellular and subcellular compartments of a plant (Shellhammer and Meinke, 1990; Baldet et al., 1993; Wang et al., 1995) may manifest different patterns of gene expression among these compartments.
Non-catalytic roles for biotin have previously been reported in bacteria, where biotin directly affects the transcription of the bio operon and thus autoregulates its own biosynthesis (Cronan, 1989). In this capacity, the activated form of biotin, biotinyl-AMP, binds to holocarboxylase synthetase and induces functional changes in that protein, which enable it to bind to the bio operator and to suppress the transcription of the bio operon. In addition, in animals, the expression of a number of genes is enhanced by biotin. Specifically, in rat, biotin induces the transcription of the glucokinase (Chauhan and Dakshinamurti, 1991) and phosphoenolpyruvate carboxykinase (Dakshinamurti and Li, 1994) genes. Furthermore, in multiple carboxylase deficiency syndromes of humans, holocarboxylase synthase is required for the biotin-dependent induction of the accumulation of mRNAs coding for holocarboxylase synthase, and biotin subunits of ACCase and propionyl-CoA carboxylase (Solorzano-Vargas et al., 2002). Although the mechanism by which biotin affects gene expression is unclear, the intriguing observation that biotinidase can catalyze the biotinylation of histones offers the possibility that this modification of histones may affect gene transcription (Hymes et al., 1995; Stanley et al., 2001).
Our characterization of the biotin-mediated regulation of MCCase expression demonstrates a non-catalytic function of biotin in plants, which are biotin autotrophic organisms. Furthermore, these studies indicate that biotin cannot only regulate gene expression by modulating transcription (as occurs in bacteria and animals), but also mediates regulation of gene expression at the translational and/or posttranslational level. These regulatory functions appear not to be confined only to MCCase, but may be part of a more complex regulatory pathway for controlling the biotin metabolic network. For example, we have found that the transcription of one of the two genes that code for the biotin subunit of the chloroplastic ACCase is induced by biotin (Che, 2000; P. Che and B.J. Nikolau, unpublished data).
MATERIALS AND METHODS
Plant Genetic Stocks and Plant Growth Conditions
The Arabidopsis bio1 mutant genetic stock (Shellhammer and Meinke, 1990) in the Columbia ecotype background was obtained from the Arabidopsis Biological Resource Center (Columbus, OH). The MCC-A::GUS and MCC-B::GUS transgenic stocks were generated in the Columbia ecotype, and the transgenes were made homozygous by propagating stocks to the T3 generation (Che et al., 2002). The MCC-A::GUS and MCC-B::GUS transgenes were moved into the bio1-mutant background by intercrossing. Homozygotes for each of the MCC-A::GUS, MCC-B::GUS transgenes (scored by the tightly linked KANR gene) in a homozygous mutant bio1 background (scored by the biotin-requirement phenotype) were identified at the F3 generation. Unless otherwise stated, plants were grown on Murashige and Skoog media either with or without biotin, in a controlled growth-room maintained at 22°C under continuous white light irradiation at 150 μmol m−2 s−1 provided by 40-W cold-white fluorescent bulbs.
Extraction and Analysis of Proteins
Arabidopsis protein extracts were prepared from 0.1 to 0.3 g of tissue using 3 volumes of 0.1 m HEPES-KOH, pH 7.0, 20 mm 2-mercaptoethanol, 0.1 mg mL−1 phenylmethylsulfonyl fluoride, 0.1% (v/v) Triton X-100, 1 mm EDTA, and 20% (v/v) glycerol, as described by Diez et al. (1994). Proteins were fractionated by electrophoresis in polyacrylamide gels either after denaturation with SDS (Laemmli, 1970), or in a non-denatured state (Hedrick and Smith, 1968; Lambin and Fine, 1979). Gels were either stained with Coomassie Brilliant Blue or subjected to western-blot analysis. The two MCCase subunits (MCC-A and MCC-B) were immunologically detected with subunit-specific antisera (McKean et al., 2000; Che et al., 2002). The biotin-containing proteins were detected by using 125I-streptavidin (Nikolau et al., 1985). Relative intensities of protein bands detected by western analysis were quantified by exposing membranes to a phosphor screen (Molecular Dynamics, Sunnyvale, CA), and the radioactivity associated with each band was quantified with a Storm 840 PhosphorImager (Molecular Dynamics).
Assays
MCCase activity was determined as the rate of methylcrotonyl-CoA-dependent incorporation of radioactivity from H14CO3− into an acid-stable product (Wurtele and Nikolau, 1990). GUS activity was determined in extracts with a fluorometric assay essentially as described by Jefferson (1987). Protein concentrations were determined by the Bradford (1976) method. All of the experiments were repeated three times using three independently transformed plant lines.
Isolation of RNA and Hybridization Analysis
RNA was isolated by the method of Logemann et al. (1987). Twenty micrograms of each RNA sample was subjected to electrophoresis in a 1.4% (w/v) agarose gel containing formaldehyde, and the RNA was blotted to nylon membrane by capillary transfer using 25 mm sodium phosphate buffer, pH 7.0. After baking the membrane at 90°C for 60 min, it was hybridized with 32P-labeled MCC-A or MCC-B cDNA fragments (Weaver et al., 1995; McKean et al., 2000). The blots were washed once with 1× SSC and 0.1% (w/v) SDS at 60°C for 15 min followed by 0.25× SSC and 0.1% (w/v) SDS at 60°C for 30 min.
Footnotes
This work was supported by the National Science Foundation (grant no. IBN–9982892 to E.S.W. and B.J.N.). This is journal paper no. J–19851 of the Iowa Agriculture and Home Economics Experiment Station (Ames; project nos. 6545 and 6546).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.013243.
LITERATURE CITED
- Alban C, Baldet P, Axiotis S, Douce R. Purification and characterization of 3-methylcrotonyl-CoA carboxylase from higher plant mitochondria. Plant Physiol. 1993;102:957–965. doi: 10.1104/pp.102.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban C, Job D, Douce R. Biotin metabolism in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:17–47. doi: 10.1146/annurev.arplant.51.1.17. [DOI] [PubMed] [Google Scholar]
- Anderson MD, Che P, Song J, Nikolau BJ, Wurtele ES. 3-Methylcrotonyl-coenzyme A carboxylase is a component of the mitochondrial leucine catabolic pathway in plant. Plant Physiol. 1998;118:1127–1138. doi: 10.1104/pp.118.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldet P, Alban C, Axiotis S, Douce R. Biotin biosynthesis in plants: identification of intermediates. Eur J Biochem Arch Biochem Biophys. 1993;303:67–73. [Google Scholar]
- Baldet P, Alban C, Douce R. Biotin synthesis in higher plants: purification and characterization of bioB gene product equivalent from Arabidopsis thaliana overexpressed in Escherichia coli and its subcellular localization in pea leaf cells. FEBS Lett. 1997;419:206–210. doi: 10.1016/s0014-5793(97)01458-0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification for microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang WH, Reddy SP, Di YP, Yoneda K, Harper R, Wu R. Regulation of thioredoxin gene expression by vitamin A in human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:627–635. doi: 10.1165/ajrcmb.26.5.4276. [DOI] [PubMed] [Google Scholar]
- Chauhan J, Dakshinamurti K. The E. coli bio operon: transcriptional repression by an essential protein modification enzyme. J Biol Chem. 1991;266:10035–10038. [PubMed] [Google Scholar]
- Che P. Biochemical and molecular genetic studies of the metabolic role of methylcrotonyl-CoA carboxylase. PhD thesis. Ames: Iowa State University; 2000. [Google Scholar]
- Che P, Wurtele ES, Nikolau BJ. Metabolic and environmental regulation of 3-methylcrotonyl-coenzyme a carboxylase expression in Arabidopsis. Plant Physiol. 2002;129:625–637. doi: 10.1104/pp.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wurtele ES, Wang X, Nikolau BJ. Purification and characterization of 3-methylcrotonyl-CoA carboxylase from somatic embryos of Daucus carota. Arch Biochem Biophys. 1993;305:103–109. doi: 10.1006/abbi.1993.1398. [DOI] [PubMed] [Google Scholar]
- Cronan JE. The E. coli bio operon: transcriptional repression by an essential protein modification enzyme. Cell. 1989;58:427–429. doi: 10.1016/0092-8674(89)90421-2. [DOI] [PubMed] [Google Scholar]
- Dakshinamurti K, Li W. Transcriptional regulation of liver phosphoenolpyruvate carboxykinase by biotin in diabetic rats. Mol Cell Biochem. 1994;132:127–132. doi: 10.1007/BF00926921. [DOI] [PubMed] [Google Scholar]
- DeMoll E. Biosynthesis of biotin and lipoic acid. In: Neidhardt FC, editor. Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington, DC: ASM Press; 1996. pp. 704–709. [Google Scholar]
- Diez TA, Wurtele ES, Nikolau BJ. Purification and characterization of 3-methylcrotonyl-coenzyme-A carboxylase from leaves of Zea mays. Arch Biochem Biophys. 1994;310:64–75. doi: 10.1006/abbi.1994.1141. [DOI] [PubMed] [Google Scholar]
- Duval M, DeRose RT, Job C, Faucher D, Douce R, Job D. The major biotinyl protein from Pisum sativum seeds covalently binds biotin at a novel site. Plant Mol Biol. 1994;26:265–273. doi: 10.1007/BF00039537. [DOI] [PubMed] [Google Scholar]
- Guan X, Diez T, Prasad TK, Nikolau BJ, Wurtele ES. Geranoyl-CoA carboxylase: a novel biotin-containing enzyme in plants. Arch Biochem Biophys. 1999;362:12–21. doi: 10.1006/abbi.1998.1014. [DOI] [PubMed] [Google Scholar]
- Hedrick JL, Smith AJ. Size and charge isomer separation and estimation of molecular weights of protein by disc gel electrophoresis. Arch Biochem Biophys. 1968;126:155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hsing YC, Tsou CH, Hsu TF, Chen ZY, Hsieh KL, Hsieh JS, Chow TY. Tissue- and stage-specific expression of a soybean (Glycine max L.) seed-maturation, biotinylated protein. Plant Mol Biol. 1998;38:481–490. doi: 10.1023/a:1006079926339. [DOI] [PubMed] [Google Scholar]
- Hymes J, Fleischhauer K, Wolf B. Biotinylation of histones by human serum biotinidase: assessment of biotinyl-transferase activity in sera from normal individuals and children with biotinidase deficiency. Biochem Mol Med. 1995;56:76–83. doi: 10.1006/bmme.1995.1059. [DOI] [PubMed] [Google Scholar]
- Jefferson RA. Assay chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- Ke J, Wen T-N, Nikolau BJ, Wurtele ES. Coordinated regulation of the nuclear and plastidic genes coding for the subunits of the heteromeric acetyl-coenzyme A carboxylase. J Plant Physiol. 2000;122:1057–1071. doi: 10.1104/pp.122.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structure protein during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P, Fine JM. Molecular weight estimation of proteins by electrophoresis in linear polyacrylamide gradient gels in the absence of denaturing agents. Anal Biochem. 1979;98:160–168. doi: 10.1016/0003-2697(79)90721-8. [DOI] [PubMed] [Google Scholar]
- Lau EL, Cochran BR, Fall RR. Isolation of 3-methylcrotonyl-coenzyme A carboxylase from bovine kidney. Arch Biochem Biophys. 1980;205:352–359. doi: 10.1016/0003-9861(80)90117-4. [DOI] [PubMed] [Google Scholar]
- Lischer CJ, Koller U, Geyer H, Mulling Ch, Schulze J, Ossent P. Effect of therapeutic dietary biotin on the healing of uncomplicated sole ulcers in dairy cattle: a double blinded controlled study. Vet J. 2002;163:51–60. doi: 10.1053/tvjl.2001.0627. [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- McKean AL, Ke J, Song J, Che P, Achenbach S, Nikolau BJ, Wurtele ES. Molecular characterization of the non-biotin-containing subunit of 3-methylcrotonyl-CoA carboxylase. J Biol Chem. 2000;275:5582–5590. doi: 10.1074/jbc.275.8.5582. [DOI] [PubMed] [Google Scholar]
- Moss J, Lane MD. The biotin-dependent enzymes. Adv Enzymol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- Nes WD, Bach TJ. Evidence for a mevalonate shunt in a tracheophyte. Proc R Soc Lond B Biol Sci. 1985;225:425–444. [Google Scholar]
- Nikolau BJ, Wurtele ES, Stumpf PK. Use of streptavidin to detect biotin-containing proteins in plants. Anal Biochem. 1985;149:448–453. doi: 10.1016/0003-2697(85)90596-2. [DOI] [PubMed] [Google Scholar]
- Patton DA, Schetter AL, Franzmann LH, Nelson K, Ward ER, Meinke DW. An embryo-defective mutant of Arabidopsis disrupted in the final step of biotin synthesis. Plant Physiol. 1998;116:935–946. doi: 10.1104/pp.116.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DA, Volrath S, Ward ER. Complementation of the bio 1 Arabidopsis biotin auxotroph with a bacterial biotin biosynthetic gene. Mol Gen Genet. 1996;251:261–266. doi: 10.1007/BF02172516. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hakamada K, Suams Y, Nagano Y, Furusawa I, Matsuno R. Chloroplast-encoded protein as a subunit of acetyl-CoA carboxylase in pea plant. J Biol Chem. 1993;268:25118–25123. [PubMed] [Google Scholar]
- Schiele U, Niedermeier R, Sturzer M, Lynen F. Investigations of the structure of 3-methylcrotonyl-CoA carboxylase from Achromobacter. Eur J Biochem. 1975;60:259–266. doi: 10.1111/j.1432-1033.1975.tb20998.x. [DOI] [PubMed] [Google Scholar]
- Shellhammer J, Meinke D. Arrested embryos from the bio 1 auxotroph of Arabidopsis thaliana contain reduced levels of biotin. Plant Physiol. 1990;93:1162–1167. doi: 10.1104/pp.93.3.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano-Vargas RS, Pacheco-Alvarez D, Leon-Del-Rio A. Holocarboxylase synthetase is an obligate participant in biotin-mediated regulation of its own expression and of biotin-dependent carboxylases mRNA levels in human cells. Proc Natl Acad Sci USA. 2002;99:5325–5330. doi: 10.1073/pnas.082097699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. Molecular cloning and characterization of 3-methylcrotonyl-CoA carboxylase from soy bean. PhD thesis. Ames: Iowa State University; 1993. [Google Scholar]
- Song J, Wurtele ES, Nikolau BJ. Molecular cloning and characterization of the cDNA coding for the biotin-containing subunit of 3-methylcrotonyl-CoA carboxylase: identification of the biotin carboxylase and biotin-carrier domains. Proc Natl Acad Sci USA. 1994;91:5779–5783. doi: 10.1073/pnas.91.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JS, Griffin JB, Zempleni J. Biotinylation of histones in human cells: effects of cell proliferation. Eur J Biochem. 2001;268:5424–5429. doi: 10.1046/j.0014-2956.2001.02481.x. [DOI] [PubMed] [Google Scholar]
- Truckenmiller ME, Vawter MP, Cheadle C, Coggiano M, Donovan DM, Freed WJ, Becker KG. Gene expression profile in early stage of retinoic acid-induced differentiation of human SH-SY5Y neuroblastoma cells. Restor Neurol Neurosci. 2001;18:67–80. [PubMed] [Google Scholar]
- Wang X. Characterization of β-methylcrotonyl-CoA carboxylase of tomato, a newly identified biotin enzyme in plants. PhD thesis. Ames: Iowa State University; 1993. [Google Scholar]
- Wang X, Wurtele ES, Keller G, McKean AL, Nikolau BJ. Molecular cloning of cDNAs and genes coding for β-methylcrotonyl-CoA carboxylase of tomato. J Biol Chem. 1994;269:11760–11769. [PubMed] [Google Scholar]
- Wang X, Wurtele ES, Nikolau BJ. Regulation of β-methylcrotonyl-coenzyme A carboxylase activity by biotinylation of the apoenzyme. Plant Physiol. 1995;108:1133–1139. doi: 10.1104/pp.108.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Lebrun L, Franklin A, Huang L, Hoffman N, Wurtele ES, Nikolau BJ. Molecular cloning of the biotinylated subunit of 3-methylcrotonyl-coenzyme A carboxylase of Arabidopsis thaliana. Plant Physiol. 1995;107:1013–1014. doi: 10.1104/pp.107.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead CC, Bannister DW. Aspects of metabolism related to the occurrence of skin lesions in biotin-deficient chicks. Br Poult Sci. 1981;22:467–472. doi: 10.1080/00071688108447911. [DOI] [PubMed] [Google Scholar]
- Whitehead CC, Bannister DW, Evans AJ, Siller WG, Weight PA. Biotin deficiency and fatty liver and kidney syndrome in chicks given purified diets containing different fat and protein levels. Br J Nutr. 1976;35:115–125. doi: 10.1079/bjn19760015. [DOI] [PubMed] [Google Scholar]
- Wurtele ES, Nikolau BJ. Plants contain multiple biotin enzymes: discovery of 3-methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase and pyruvate carboxylase in the plant kingdom. Arch Biochem Biophys. 1990;278:179–186. doi: 10.1016/0003-9861(90)90246-u. [DOI] [PubMed] [Google Scholar]
- Wurtele ES, Nikolau BJ. Characterization of 3-methylcrotonyl-CoA carboxylase from plants. Methods Enzymol. 2000;324:280–292. doi: 10.1016/s0076-6879(00)24238-9. [DOI] [PubMed] [Google Scholar]
- Yanai Y, Kawasaki T, Shimada H, Wurtele ES, Nikolau BJ. Genomic organization of 251 kDa acetyl-CoA carboxylase genes in Arabidopsis: Tandem gene organization has made two differentially expressed isozymes. Plant Physiol. 1995;36:779–787. doi: 10.1093/oxfordjournals.pcp.a078822. [DOI] [PubMed] [Google Scholar]