Abstract
Galactomannans [(1→6)-α-d-galactose (Gal)-substituted (1→4)-β-d-mannans] are major cell wall storage polysaccharides in the endosperms of some seeds, notably the legumes. Their biosynthesis in developing legume seeds involves the functional interaction of two membrane-bound glycosyltransferases, mannan synthase (MS) and galactomannan galactosyltransferase (GMGT). MS catalyzes the elongation of the mannan backbone, whereas GMGT action determines the distribution and amount of Gal substitution. Fenugreek (Trigonella foenum-graecum) forms a galactomannan with a very high degree of Gal substitution (Man/Gal = 1.1), and its GMGT has been characterized. We now report that the endosperm cell walls of the tobacco (Nicotiana tabacum) seed are rich in a galactomannan with a very low degree of Gal substitution (Man/Gal about 20) and that its depositional time course is closely correlated with membrane-bound MS and GMGT activities. Furthermore, we demonstrate that seeds from transgenic tobacco lines that express fenugreek GMGT constitutively in membrane-bound form have endosperm galactomannans with increased average degrees of Gal substitution (Man/Gal about 10 in T1 generation seeds and about 7.5 in T2 generation seeds). Membrane-bound enzyme systems from transgenic seed endosperms form galactomannans in vitro that are more highly Gal substituted than those formed by controls under identical conditions. To our knowledge, this is the first report of structural manipulation of a plant cell wall polysaccharide in transgenic plants via a biosynthetic membrane-bound glycosyltransferase.
The endosperm cell walls of many seeds are relatively thick due to deposits of mannan-type cell wall storage polysaccharides (Reid, 1985). These have the common structural feature of a linear (1→4)-β-linked glycan backbone consisting of residues of Man (mannans) or Man and Glc (glucomannans). The backbone may carry single-unit side chains of Gal residues linked (1→6)-α to Man (galactomannans and galactoglucomannans). Although these related seed polysaccharides all have a storage role, their rheological properties are also relevant. If Gal substitution is absent or low (less than about 10%), the molecules self-associate and the cell walls that contain them are hard. They may protect the seed against mechanical damage (Reid, 1989). The commercial processing of such hard seeds can be difficult, and their nutritional value may be limited. Leguminous seeds that retain an endosperm in the mature state (the endospermic legumes) always have endosperm cell walls that consist almost entirely of galactomannans that are relatively highly Gal substituted (Man/Gal between 1.1 and about 3.5; Meier and Reid, 1982). These molecules have hydrophilic properties that are important to the germinative ecology of the seed (Reid and Bewley, 1979), and also form the basis of numerous industrial applications of galactomannans (Dea and Morrison, 1975; Reid and Edwards, 1995).
Galactomannan biosynthesis has been studied extensively in the endospermic legumes (Campbell and Reid, 1982; Edwards et al., 1989, 1992, 1999, 2002; Reid et al., 1995). Two tightly membrane-bound glycosyltransferases together catalyze the polymerization of galactomannans—a GDP-Man (and Mg2+)-dependent (1→4)-β-d-mannosyltransferase or “mannan synthase” (MS), and a UDP-Gal (and Mn2+)-dependent mannan-specific (1→6)-α-d-galactosyltransferase (galactomannan galactosyltransferase [GMGT]). In microsomal membrane preparations from developing legume seed endosperms, the observed interaction of MS and GMGT in galactomannan biosynthesis in vitro conforms to a model (Reid et al., 1995; Edwards et al., 2002) whereby Gal is transferred only to an acceptor Man residue at or near the elongating nonreducing end of the mannan backbone, and the transfer properties of the GMGT are important in determining the statistical distribution of galactosyl residues along the mannan backbone and the Man/Gal value.
The fenugreek (Trigonella foenum-graecum) seed contains a galactomannan that is almost fully Gal substituted (Man/Gal = 1.1), and its GMGT has been characterized (Edwards et al., 1999). Once detergent solubilized from the membrane, the enzyme has an absolute requirement for added manno-oligosaccharide or galactomannan acceptor substrates. It has a principal acceptor substrate recognition sequence of six (1→4)-β-linked d-Man residues, with transfer occurring at the third Man residue from the nonreducing end of the sequence (Edwards et al., 2002). The fenugreek GMGT protein has a single transmembrane α-helix near the N terminus that is believed to specify Golgi membrane-localization.
We now report that the tobacco (Nicotiana tabacum) seed endosperm contains a galactomannan with very low Gal content, and that its biosynthesis in developing seeds is closely correlated with MS and GMGT. We further demonstrate that tobacco transgenic lines transformed with fenugreek GMGT cDNA under the control of the 2x35S “constitutive” promoter express membrane-bound GMGT activity in their leaf tissues, and that seeds derived from these transgenic lines have endosperm galactomannans with significantly increased Gal substitution due to altered galactomannan biosynthesis.
RESULTS
Tobacco Endosperm Cell Walls Are Rich in a Galactomannan with Very Low Gal Substitution
It was known that tobacco seeds are endospermic and that the endosperm cell walls are thickened (Avery, 1933). Although the wall thickenings suggested the presence of cell wall storage polysaccharides, there was no information on the composition of these walls. However, the endosperm cell walls of tomato (Lycopersicon esculentum), also in the Solanaceae, are known to be rich in residues of Man (Nonogaki et al., 2000). Therefore, tobacco seeds were dissected into tissue fractions consisting mainly of testa, endosperm, and embryo, respectively, and total cell wall material was prepared from each. For comparison, total cell wall material was prepared also from tobacco leaf midrib, the cell walls of which have been reported to contain a galactoglucomannan (Eda et al., 1984). The wall preparations were subjected to complete acid hydrolysis and quantitative analysis of the monosaccharides released. The results (Table I) showed that the endosperm was distinctly different in its cell wall composition from the other tissues. It contained nearly 60% Man, whereas Man was only a minor component of the other tissues. The cell walls of the testa were unusually rich in Xyl residues.
Table I.
Neutral monosaccharide composition of cell wall materials from tobacco tissues
| Tissue | Araa | Gal | Glc | Xyl | Man |
|---|---|---|---|---|---|
| mol % | |||||
| Leaf midrib | 5 | 5 | 68 | 17 | 5 |
| Seed testa | 19 | 9 | 23 | 46 | 2 |
| Seed endosperm | 15 | 9 | 16 | 3 | 58 |
| Seed embryo | 29 | 7 | 41 | 23 | <1 |
Includes Rha.
To investigate the structure of the Man-containing polymers in the endosperm cell walls, total cell wall material from hand-isolated endosperms visibly free of embryo and testa was treated with the pure endo-(1→4)-β-d-mannanase from Aspergillus niger, the action of which has been described in detail by McCleary and Matheson (1983). The optimum substrate subsite-binding requirement of this enzyme is a stretch of five (1→4)-β-linked d-mannosyl residues, although mannotetraose is nonetheless hydrolyzed slowly. Thus, the products of digestion of an unsubstituted (1→4)-β-mannan are mannobiose (M2) and mannotriose (M3), usually accompanied by some Man. Gal substitution at the Man residues occupying the second and/or the fourth position within the binding sequence prevents hydrolysis, with the result that only certain well-defined fragment oligosaccharides are released from galactomannans by the action of the enzyme (McCleary, 1979; McCleary and Matheson, 1983). The smallest of these allowed oligosaccharides are M2, M3, and the three Gal-substituted manno-oligosaccharides galactosylmannobiose (M2G), galactosylmannotriose (M3G), and digalactosylmannopentaose (M5G2), the molecular structures of which are illustrated by Edwards et al. (2002). After treatment of the endosperm cell wall material with the mannanase, the resultant fragment oligosaccharides were separated from undigested polymers by extraction with hot 70% (v/v) methanol. Both the oligosaccharide and undigested polymer fractions were then subjected to complete acid hydrolysis and compositional analysis. The analytical data (Table II) show that the material that became soluble in 70% (v/v) methanol after treatment with the enzyme was very rich in Man residues (87%–89%) alongside much lower amounts of Gal (3.7%–4.1%), Glc (5.6%–6.2%), and Ara (1.2%–2.7%) residues. When further samples of endosperm cell wall materials were digested with the mannanase, and the spun digest was subjected directly to thin-layer chromatography (TLC), the only TLC-mobile compounds detected were M2 and M3 and M, alongside much smaller amounts of M2G and M3G, and traces of M5G2 and higher (1→6)-α-Gal-substituted manno-oligosaccharides. These are the oligosaccharide fragments that would be expected on endo-mannanase digestion of a galactomannan with a very low degree of Gal substitution. If all the Gal residues in the cell wall material that became 70% (v/v) methanol-soluble on mannanase digestion were linked to Man, then the tobacco endosperm mannan had a Man/Gal value of about 20.
Table II.
Treatment of tobacco endosperm cell wall material with A. niger endo-(1→4)-β-mannanase
| Composition (mol % of Fraction)
|
||||||
|---|---|---|---|---|---|---|
| Digestion Time | Fraction (D or R) | % of Total | Ara | Gal | Glc | Man |
| h | ||||||
| 1 | D | 36 | 1.2 | 3.8 | 5.9 | 89 |
| 1 | R | 64 | 28 | 14 | 5.5 | 53a |
| 4 | D | 49 | 2.3 | 3.7 | 5.6 | 88 |
| 4 | R | 51 | 36 | 16 | 5.6 | 42a |
| 16 | D | 57 | 2.7 | 4.1 | 6.2 | 87 |
| 16 | R | 43 | 34 | 15 | 7.1 | 44a |
Relative amounts and compositions of the cell wall fractions depolymerized by (D) and resistant to (R) enzyme digestion.
Contained some Xyl (<10%).
The amount of the 70% (v/v) methanol-soluble fraction that was obtained depended on the length of the enzyme treatment, but its monosaccharide composition varied little (Table II). Thus, it seems likely that the 15% to 30% of total cell wall Man remaining in the 70% (v/v) methanol-insoluble material after 16 h of enzyme treatment was present also as galactomannan, but resistant to digestion because of strong self-association or (more likely) restricted diffusion of the enzyme into relatively large particles.
The Glc residues present in the fraction brought into 70% (v/v) methanol solution after enzyme digestion may have been present as occasional (1→4)-β-linked Glc residues within the mannan chain. These would have given rise to some glucomanno-oligosaccharides on endo-β-mannanase digestion (McCleary and Matheson, 1983), although possibly in such small amounts as to elude TLC detection. On the other hand, the Glc residues may have originated in a low-Mr glucan that became partially soluble in 70% (v/v) methanol once the cell wall matrix had been loosened by the endo-β-mannanase-catalyzed depolymerization of the predominant galactomannan component. The Ara residues were almost certainly present in a low-Mr arabinan released in this way, because some pectin-associated arabinans are known to be soluble in hot, 70% (v/v) ethanol (Aspinall, 1970).
Galactomannan Biosynthesis in Developing Tobacco Seeds
To investigate the deposition of Man residues in the cell walls of tobacco seed endosperm, developing fruits (capsules) were harvested from a single plant at various times after anthesis. Between the first appearance of endosperm tissue in the developing seeds and the beginning of capsule desiccation, the fruits were scored for developmental markers (capsule and seed fresh weight; appearance of capsule, seed, and residual flower parts). Samples of the developing seeds within the capsules were used to prepare total cell wall materials for compositional analysis and to obtain microsomal membrane preparations to investigate galactomannan biosynthesis in vitro. Some developmental markers subsequently found to be reliable indicators of mannan deposition are correlated with days after anthesis and cell wall Man content in Table III. On the assumption (reasonable from Table I) that the Man content of whole-seed cell wall preparations originates almost exclusively in the endosperm, the period of mannan deposition appeared to start at about d 17 and to continue at least until d 23 after anthesis. In subsequent experiments the descriptors in Table III were found to be more reliable indicators of developmental stage than days after anthesis.
Table III.
Development of tobacco fruits in relation to mannan deposition
| Days after Anthesis | Capsule Fresh Wt | Capsule Color | Calyx Color | Seed Fresh Wt | Seed Color | Man as % of Seed Cell Walls |
|---|---|---|---|---|---|---|
| g | g | |||||
| 14 | 0.34 | gr. | l. gr. | 0.04 | Colorless | 0 |
| 15 | 0.56 | gr. | l. gr. | 0.06 | Colorless | 0 |
| 17 | 0.96 | d. gr. | gr. | 0.19 | y. | 5 |
| 19 | 1.13 | d. gr. | gr. | 0.21 | y./br. | 23 |
| 20 | 1.20 | y./gr. | y./gr. | 0.20 | br./d. br | 28 |
| tip br. | ||||||
| 21 | 1.23 | y./gr. | y./gr. | 0.22 | br./d. br. | 29 |
| tip br. | ||||||
| 22 | 1.29 | y./gr. | y./gr. | 0.26 | d. br. | 34 |
| tip br. | ||||||
| 23 | 1.08 | y./gr. | y. | 0.19 | d. br (dry) | 38 |
| tip br. |
gr, Green; y, yellow; br, brown. d, dark; l, light.
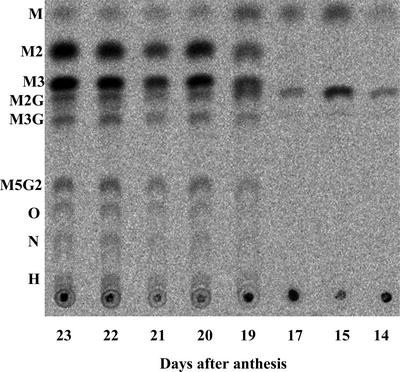
From d 14 onwards, microsomal membrane preparations from developing seeds catalyzed the incorporation of label from GDP-(14C)Man (with and without UDP-Gal) into radioactive products that were insoluble in hot 70% (v/v) methanol. Incorporation rates were low at first, increasing sharply from d 19 (data not shown). Enzymatic fragmentation analyses of the labeled polymeric products using the A. niger (1→4)-β-mannanase revealed that it was only from d 19 onwards that they consisted mainly of mannan and galactomannan, as witnessed by the release of diagnostic manno- and galactomanno-oligosaccharides. The products formed in the presence of labeled GDP-Man alone were mannans, releasing M2 and M3. Those formed from labeled GDP-Man and unlabeled UDP-Gal were galactomannans, releasing the labeled Gal-substituted manno-oligosaccharides M2G, M3G, M5G2, octasaccharides, nonasaccharides, and higher saccharides in addition to M2 and M3 (Fig. 1).
Figure 1.
Autoradiogram of TLC-separated, labeled products of endo-β-mannanase digestion of 70% (v/v) methanol-insoluble products formed on incubating microsomal membrane preparations from developing tobacco seeds with GDP-(14C)-Man (80 μm) and unlabeled UDP-Gal (0.8 mm). M, Man; O, galactomannan octasaccharides; N, galactomannan nonasaccharides; H, higher galactomannan oligosaccharides.
Seed Populations (T1 and T2 Generations) from Transgenic Tobacco Plants Expressing Fenugreek GMGT Constitutively Have Endosperm Galactomannans with Increased Gal Substitution
The fenugreek GMGT protein encoded by the full-length cDNA sequence includes a hydrophobic membrane-spanning α-helical domain near the N terminus (Edwards et al., 1999) believed to specify and to bring about membrane localization. The full-length cDNA sequence was inserted into a GPTV binary plasmid vector (Becker et al., 1992) behind a 2x35S strong “constitutive” promoter (Kay et al., 1987). The plasmid was amplified in Escherichia coli and transferred to Agrobacterium tumefaciens for transformation of tobacco leaf discs. Kanamycin-resistant plantlets regenerated from tissue culture were screened initially by genomic PCR of leaf tissues. Microsomal membranes from the leaves of control plants were unable to catalyze the synthesis of mannan or galactomannan in the presence of labeled GDP-Man and UDP-Gal, nor did spun homogenates or detergent-treated microsomal membranes from the leaves of control plants contain GMGT activity as assayed by the transfer of label from UDP-(14C)Gal to locust bean (Ceratonia siliqua) galactomannan (Man/Gal = 3.5; Edwards et al., 1999). In contrast, most of the PCR-positive putative transformants displayed GMGT activity in their leaf tissues, present only in microsomal membranes, and detectable only after detergent treatment of the membranes. Thus, the full-length fenugreek GMGT cDNA open reading frame encodes a protein that is exclusively membrane localized.
Selected transformants were allowed to flower (self-pollination) and set seed, and seeds from individual plants were pooled. Seedlings from these seeds (T1 generation plantlets) generally showed 3:1 segregation of kanamycin resistance, as would be expected from a single transgene insertion into the allotetraploid genome of tobacco, although one individual (not investigated further) appeared to have a double insertion. T1 generation seeds from six primary transformants were germinated on kanamycin and resistant plantlets (carrying one or two alleles of the fenugreek GMGT transgene) were grown on to maturity, flowering (self-pollination), and seeding. Seeds (T2 generation) from individual plants were pooled and germinated on kanamycin, allowing those T1 generation plants with two alleles of the transgene (duplex state) to be identified on the basis of apparently 100% kanamycin resistance in the seedlings (theoretical segregation of 35 resistant seedlings to one susceptible). All the T2 generation seeds used in subsequent experiments were from these duplex T1 generation plants.
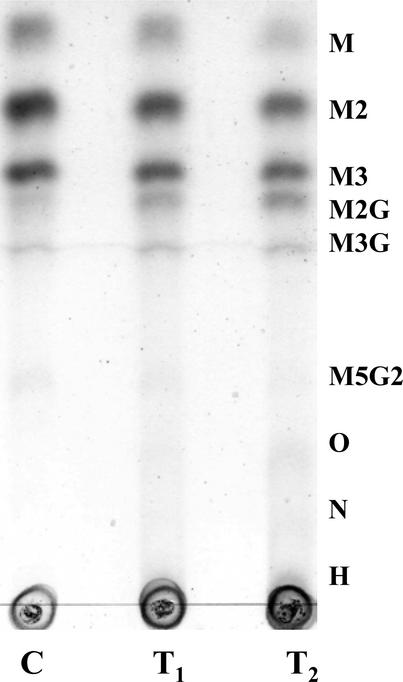
Endosperms from T1 generation and T2 generation seeds from independent transgenic lines and from controls, were dissected free of testa and embryo, and used to prepare total cell wall materials. Statistically, the T1 generation seeds would comprise 75% transgenic individuals. The T2 generation seeds were from duplex T1 plants and would on the basis of theoretical segregation ratios comprise over 97% transgenic individuals. Complete acid hydrolysis and compositional analysis of these showed that the endosperm cell walls from the transgenic seeds had a consistently higher content of Gal residues relative to Man residues than did endosperm cell walls from seeds of control plants. Furthermore, the difference was more marked in the T2 samples (Table IV). An estimation of the (average) Man/Gal values of the galactomannans in endosperm cell wall materials from the transgenic seeds and from control plants was obtained as before by digesting them with the A. niger endo-β-mannanase and carrying out a compositional analysis of the 70% (v/v) methanol-soluble oligosaccharide fraction released by the action of the enzyme. The results (Table V) showed that the endo-(1→4)-β-d-mannanase depolymerized similar proportions of the endosperm cell wall materials from controls and transgenic seeds. The depolymerized materials from control endosperms had compositions closely similar to those in Table II, whereas those derived from the transgenics contained very significantly more residues of Gal relative to Man (Man/Gal about 7.5 in T2 generation seeds, 9.5–11.5 in T1 generation seeds, and 20–22 in controls). When the 70% (v/v) methanol-soluble fractions obtained on endo-β-mannanase digestion were analyzed by TLC, the proportion of Gal-containing galactomannan oligosaccharides (notably M2G) relative to M, M2, and M3 increased visibly in the series control → T1 generation → T2 generation (Fig. 2). This provided direct confirmation that the (1→4)-β-d-mannans in the transgenic endosperms were more highly (1→6)-α-Gal substituted than those in the endosperms of controls.
Table IV.
Compositions of total cell wall materials from endosperm tissues of T1 and T2 generation seeds from transgenic tobacco plants expressing fenugreek GMGT
| Transgenic or Control Line | Composition
|
Man/Gal Valueb | ||||
|---|---|---|---|---|---|---|
| Ara | Gal | Glc | Xyla | Man | ||
| mol % | ||||||
| T2 generation | ||||||
| FGT 1 | 17.0 | 17.0 | 4.4 | – | 61.7 | 3.5 |
| 16.1 | 15.1 | 4.4 | – | 64.3 | 4.1 | |
| FGT 6 | 20.1 | 16.0 | 4.2 | 2.8 | 56.8 | 3.6 |
| 19.8 | 16.1 | 4.0 | 2.4 | 57.7 | 3.6 | |
| FGT AA | 20.1 | 15.9 | 3.6 | 2.4 | 58.0 | 3.6 |
| 18.6 | 14.5 | 12.0 | 2.2 | 52.6 | 3.6 | |
| T1 generation | ||||||
| FGT 1 | 17.3 | 10.8 | 4.8 | 2.7 | 64.5 | 6.0 |
| 17.7 | 11.7 | 4.8 | – | 65.8 | 5.4 | |
| FGT 3 | 17.3 | 11.5 | 5.4 | – | 65.8 | 5.5 |
| 17.6 | 11.7 | 5.3 | – | 65.4 | 5.4 | |
| FGT 6 | 16.7 | 11.6 | 5.8 | – | 65.9 | 5.5 |
| 18.2 | 12.4 | 5.9 | 2.9 | 60.6 | 4.9 | |
| FGT O | 16.9 | 11.9 | 5.4 | – | 65.8 | 5.3 |
| 18.8 | 14.3 | 5.0 | – | 61.9 | 4.2 | |
| FGT L | 18.2 | 12.3 | 5.0 | – | 64.5 | 5.0 |
| 17.6 | 11.8 | 5.3 | – | 65.4 | 5.3 | |
| FGT AA | 18.6 | 14.9 | 3.8 | – | 62.6 | 4.0 |
| 18.9 | 15.2 | 4.8 | – | 61.0 | 3.8 | |
| Controls | ||||||
| CTR A | 18.4 | 10.2 | 3.5 | – | 67.9 | 6.4 |
| 19.5 | 10.0 | 4.0 | 2.4 | 64.2 | 6.4 | |
| CTR B | 18.4 | 9.2 | 4.5 | – | 67.8 | 7.1 |
| 17.8 | 9.3 | 5.4 | – | 67.5 | 7.0 | |
| CTR C | 19.0 | 10.2 | 3.8 | 2.4 | 64.5 | 6.3 |
| 17.7 | 10.2 | 3.7 | – | 68.3 | 6.4 | |
The T1 generation seeds include 25% azygous individuals without the transgene, whereas the T2 generation seeds include less than 3% of such individuals. Transgenics were independent lines, and control plants had been regenerated from tissue culture. Duplicate analyses are of independent batches of 50 seeds.
Where not listed, included in Man total.
Where independent figure for Xyl not given, 2.6 mol % Xyl assumed
Table V.
Amounts and compositions of cell wall materials dissolved by the action of endo-(1→4)-β-d-mannanase on endosperm tissues of T1 and T2 generation seeds from transgenic tobacco plants expressing fenugreek GMGT
| Compositiona
|
|||||||
|---|---|---|---|---|---|---|---|
| Transgenic or Control Line | Ara | Gal | Glc | Man | Man/Gal Value | % of Wall Dissolved | % of Man Dissolved |
| mol % | |||||||
| T2 generation | |||||||
| FGT 1 | 3.3 | 10.3 | 6.6 | 79.8 | 7.7 | 54 | 70 |
| 4.2 | 10.4 | 8.1 | 77.3 | 7.4 | 51 | 69 | |
| FGT 6 | 4.8 | 9.9 | 11.1 | 74.1 | 7.5 | 58 | 78 |
| 5.0 | 10.1 | 9.0 | 76.0 | 7.5 | 60 | 80 | |
| FGT AA | 3.9 | 10.4 | 6.2 | 79.6 | 7.7 | 56 | 75 |
| 4.3 | 10.7 | 6.1 | 78.8 | 7.4 | 55 | 77 | |
| T1 generation | |||||||
| FGT 1 | 4.4 | 7.0 | 6.9 | 81.7 | 11.7 | 62 | 80b |
| 4.2 | 7.1 | 6.5 | 82.1 | 11.5 | 63 | 85b | |
| FGT 3 | 4.1 | 7.4 | 6.7 | 81.8 | 11.1 | 61 | 80b |
| 4.0 | 7.8 | 7.1 | 81.1 | 10.4 | 63 | 80b | |
| FGT 6 | 4.0 | 7.5 | 6.9 | 81.5 | 10.9 | 58 | 75b |
| 3.6 | 7.3 | 7.0 | 82.1 | 11.2 | 60 | 80b | |
| FGT O | 3.6 | 7.4 | 7.5 | 81.5 | 11.0 | 58 | 75 |
| 4.3 | 7.2 | 7.2 | 81.2 | 11.2 | 57 | 74 | |
| FGT L | 3.7 | 8.3 | 7.1 | 80.8 | 9.7 | 52 | 71 |
| 5.0 | 8.1 | 6.4 | 80.6 | 10.0 | 62 | 79 | |
| FGT AA | 4.2 | 8.3 | 6.0 | 81.5 | 9.8 | 54 | 74 |
| 4.4 | 8.8 | 5.9 | 80.9 | 9.2 | 51 | 70b | |
| Controls | |||||||
| CTR A | 2.7 | 4.4 | 6.7 | 86.2 | 19.6 | 58 | 80b |
| 2.8 | 4.2 | 5.6 | 87.4 | 20.8 | 62 | 84 | |
| CTR B | 3.3 | 4.1 | 5.9 | 86.8 | 21.2 | 69 | 87 |
| 2.9 | 4.0 | 5.6 | 87.6 | 21.9 | 63 | 85b | |
| CTR C | 3.1 | 4.2 | 5.2 | 87.5 | 20.8 | 61 | 82 |
| 3.7 | 4.5 | 5.8 | 86.1 | 19.1 | 60 | 82 | |
The T1 generation seeds include 25% azygous individuals without the transgene, whereas the T2 generation seeds include less than 3% of such individuals. Transgenics were independent lines, and control plants had been regenerated from tissue culture. Duplicate analyses are of independent lots of 50 endosperms.
Xyl absent from dissolved cell wall material.
Less precise value due to poor separation of Xyl from Man in hydrolysate of undissolved cell wall material.
Figure 2.
TLC separation of the saccharides released on treating total endosperm cell wall materials with the pure endo-(1→4)-β-mannanase from A. niger. C, Endosperms from control seeds (CTR B). T1, T1 generation seeds (self-pollination) from primary transformant FGT 6. T2, T2 generation seeds derived from FGT 6. Statistically, the T1 generation seeds will include 25% azygous individuals lacking the transgene, and the T2 generation seeds will include less than 3% azygous individuals. M, Man; O, galactomannan octasaccharides; N, galactomannan nonasaccharides; H, higher galactomannan oligosaccharides.
Galactomannan Biosynthesis in Developing Endosperms of Seeds (T1 Generation) from Transgenic Tobacco Plants
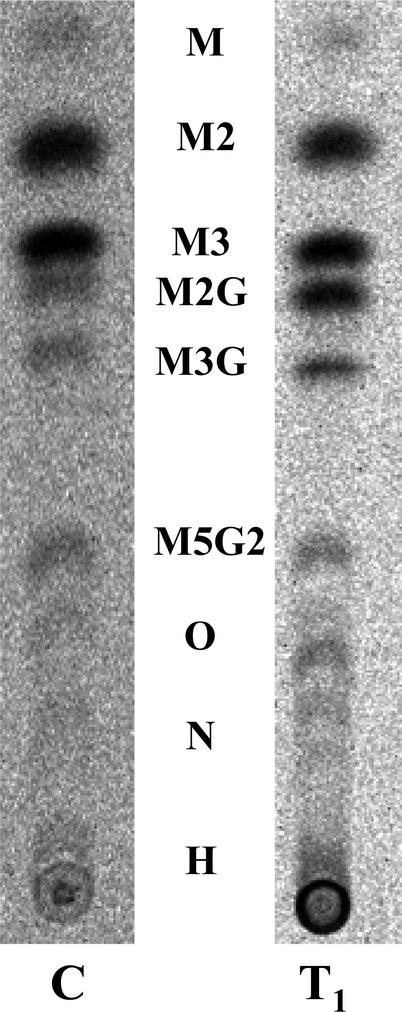
The transgenic tobacco plants did not differ from controls either in appearance or in speed of development. Mannan deposition in the endosperms of developing T1 transgenic seeds from one transgenic tobacco line (FGT 3) was mapped and found to follow virtually the same time course as in control seeds (data not shown). However, it was observed consistently that the galactomannans formed in vitro from GDP-(14C)Man and unlabeled UDP-Gal in the presence of microsomal membranes from the T1 seeds were more highly Gal-substituted than those formed by membranes from control plants under the same experimental conditions. This difference is clear on comparing the manno- and galactomanno-oligosaccharides released from the radiolabeled in vitro galactomannan products on digestion with the A. niger endo-β-mannanase (Fig. 3). The mannanase digests from the T1 galactomannans contained a visibly higher proportion of labeled M2G, M3G, and M5G2 and higher Gal-substituted manno-oligosaccharides relative to M, M2, and M3 than did those from controls. Thus, the observed increase in Gal substitution in the endosperm galactomannans of the T1 generation seeds was accompanied by a parallel change in the Man/Gal value of the biosynthetic galactomannan product formed in vitro. This change was almost certainly attributable to the expression of fenugreek GMGT in the developing endosperms of the transgenic seeds in the T1 population.
Figure 3.
Autoradiogram of TLC-separated, labeled products of endo-β-mannanase digestion of 70% (v/v) methanol-insoluble products formed on incubating microsomal membrane preparations from developing tobacco seeds with GDP-(14C)-Man (80 μm) and unlabeled UDP-Gal (0.8 mm). C, Membranes from developing seeds of control plant (CTR A, self pollination) 20 d after anthesis. T1, Membranes from developing seeds of primary transformant FGT 3 (T1 generation, self-pollination) 20 d after anthesis. M, Man; O, galactomannan octasaccharides; N, galactomannan nonasaccharides; H, higher galactomannan oligosaccharides.
DISCUSSION
The thickened endosperm cell walls of tobacco seeds are rich in a galactomannan (or possibly a low-Glc galactoglucomannan) with a very low degree of Gal substitution (Man/Gal about 20; over 70% of the tobacco galactomannan is solubilized from the endosperm cell walls by the action of a pure endo-[1→4]-β-d-mannanase, and the Man/Gal values quoted here and below are inferred from the compositions of the solubilized materials). The time course of Man deposition in developing seeds is closely correlated with the presence in endosperm tissues of endogenous membrane-bound MS and GMGT that interact as in the legume seed systems to catalyze galactomannan biosynthesis in vitro.
The endosperms of T1 generation seed populations (self-pollination) from primary transformant tobacco plants with a single transgene insertion that express fenugreek GMGT constitutively contain galactomannans with very significantly increased average degrees of galactosyl substitution. Furthermore, the galactomannans formed in vitro in the presence of membrane preparations from developing T1 seeds are correspondingly more Gal substituted than those formed in the presence of membranes from developing control seeds under the same conditions. Thus, the observed increase in Gal substitution in the endosperm galactomannans of the T1 generation seeds is due to a change in the Man/Gal value of the biosynthetic product brought about by the expression of fenugreek GMGT in the developing endosperms of the transgenic seeds in the T1 population.
The T1 seed population is a heterogeneous mixture, expected on the basis of a single transgene insertion into the allotetraploid genome of tobacco to contain 25% azygous individuals lacking the transgene and 75% individuals with one or two alleles of the fenugreek GMGT (simplex and duplex states, respectively). Thus, the compositional data reported for T1 generation seeds must be viewed as average values. The transgenic seeds within the population would be expected to have more extreme changes in their galactomannan structures. Analysis of T2 seed populations containing over 97% transgenic individuals confirmed this. The endosperm galactomannans in the T2 generation seeds derived from three primary transformants all exhibited a Man/Gal value of about 7.6. This compares with about 11 for the corresponding T1 seed population and about 21 for control lines (Table V).
The current experimental model for galactomannan biosynthesis in microsomal membrane preparations from legume systems requires a functional interaction between MS and GMGT. The nascent mannan backbone is exposed to GMGT action as it emerges from the MS, whereas the transfer specificity of the GMGT determines the statistical distribution of Gal residues according to a second order Markov chain model (Reid et al., 1995; Edwards et al., 2002). It is not yet clear whether the functional interaction requires any physical association between the MS and GMGT proteins, or if it is dependent on the repetitive transfer of Gal residues to a nascent mannan chain by a single GMGT molecule (Edwards et al., 2002).
Our observation that tobacco plants expressing fenugreek GMGT under the control of a strong promoter have endosperm galactomannans with Gal contents that are increased demonstrates that GMGT amount is a factor that limits Gal substitution in tobacco. However, the increase in Gal content across the various independent transgenic lines is remarkably constant (about 2.8 times increase), suggesting that a limiting degree of Gal substitution may have been reached due to factors other than GMGT amount. One possibility is that further increase in Gal substitution is limited by the supply of UDP-Gal, as regulated either by the rate of its synthesis in the cytosol or by the rate of its transport across the Golgi membrane. However, a further interesting possibility is that the degree of Gal substitution achieved in the tobacco transgenic lines is the maximum achievable without any specific association between the tobacco MS and fenugreek GMGT proteins other than their proximity on the Golgi membrane. The much higher degrees of Gal substitution observed in the legume seed systems (e.g. Man/Gal = 1.1 in fenugreek) would then be the result of cooperativity between the MS and GMGT proteins, probably involving an enzyme complex. Thus, it will be interesting to investigate further the nature of the interaction between endogenous tobacco MS and the fenugreek GMGT transgene in developing tobacco endosperms. Is a second order Markov chain rule followed in transgenic tobacco as in the legume seed systems? If so, are the Markov probability values that determine the statistical distribution of Gal residues along the mannan backbone the same as in fenugreek? Will tobacco plants expressing GMGTs from other legume species that form galactomannans with higher Man/Gal values than fenugreek exhibit galactomannan biosynthesis altered in different ways? Transgenic seed populations will be used to investigate these questions. They will be used also to investigate how increased levels of Gal substitution in tobacco endosperm galactomannans affect their rheological properties, the mechanical properties of the endosperm tissues, and the germinative properties of the seeds.
It has been reported recently that expression of a fungal endo-(1→5)-α-arabinanase in the Golgi compartment of potato (Solanum tuberosum) interfered with the biosynthesis of the arabinan side chains of the rhamnogalacturonan I component of pectin in the tubers (Skjøt et al., 2002). This was the first example of the manipulation of the biosynthesis of a plant cell wall polysaccharide by the targeting of a glycosylhydrolase to the Golgi. To our knowledge, the present paper reports the first demonstration that the molecular structure of a plant cell wall matrix polysaccharide can be altered by adjusting the level in planta of a Golgi membrane-bound glycosyltransferase involved in the biosynthetic process.
MATERIALS AND METHODS
Materials and General Methods
(14C) Man-labeled GDP-Man was purchased from DuPont-NEN (Stevenage, UK). Specialized biochemicals were from Sigma (Poole, Dorset, UK). Media for plant propagation were from Duchefa (Haarlem, The Netherlands). General laboratory chemicals were at least of analytical quality. The preparation of Aspergillus niger endo-(1→4)-β-d-mannanase was the one described by Edwards et al. (1989).
TLC separations of saccharides and the detection of separated compounds were carried out as before (Edwards et al., 1989). After the TLC separation of 14C-labeled saccharides, the dried plates were subjected to quantitative autoradiography using the Fujifilm Bio-imaging Analyzer BAS-1500, with BAS-IIIs imaging plate (Raytec Scientific Ltd., Sheffield, UK). This allowed the precise localization of radioactive zones on plates, and the quantitative evaluation of the relative amounts of radioactivity in separated zones.
Preparation of Cell Wall Materials
Endosperm, embryo, and testa tissues were obtained from tobacco (Nicotiana tabacum SR1, Petit Havana) seeds by dissection. The seeds were first heated in 70% (v/v) ethanol for 3 min at 95°C, and dissection was carried out in a drop of water under an MZ6 microscope (Leica Microsystems, Milton Keys, UK) equipped with directional cold-light illumination. Dissected individual tissues from 50 seeds were ground in a small glass/glass Potter homogenizer with water (2 × 200 μL). Methanol was added to the homogenized tissue to give a final methanol concentration of 70% (v/v), and the mixture was heated to 70°C for 10 min. This dissolved sugars and other low-Mr compounds. After centrifugation (13,000g for 10 min) the supernatant was discarded and the pellet was re-extracted twice with 70% (v/v) methanol (500 μL) in the same way. The residual, washed pellet was resuspended in water and freeze dried to give the total cell wall material. Tobacco leaf midrib was excised with a razor blade, sliced, ground in a mortar, and pre-extracted twice with chloroform:methanol (1:1 [v/v]) to remove chlorophyll before being treated with 70% (v/v) methanol as above.
Analysis of Cell Wall Materials
For compositional analysis, total cell wall materials and other incompletely water-soluble polysaccharide materials were hydrolyzed to their constituent monosaccharides by dissolving them in 72% (w/v) H2SO4 at 30°C and completing the hydrolysis in 4% (w/v) H2SO4 at 105°C in a sealed tube (Saeman et al., 1945). The 72% (w/v) H2SO4 pretreatment was omitted for samples that were completely water soluble. Hydrolysates were neutralized using the calculated amount of NaOH and analyzed for their neutral monosaccharide composition by high-performance anion-exchange chromatography as described elsewhere (Edwards et al., 1992). In the case of some endosperm samples, an estimate of amount was required in addition to composition. A known amount of l-Fuc, a monosaccharide not present in the endosperm cell walls, was added to these samples before 4% (w/v) H2SO4 treatment, as an internal standard.
The digestion of tobacco endosperm total cell wall material with the A. niger endo-β-mannanase was carried out as follows. Wall material from 50 endosperms was rehydrated by heating to 100°C in 50 mm ammonium acetate buffer (pH 5.0; 80 μL), and endo-β-mannanase (20 μL) was added. The suspension was incubated for 16 h, and then heated at 100°C for 2 min to deactivate the enzyme. Methanol was then added to the suspension to give a final methanol concentration of 70% (v/v). The mixture was heated at 70°C for 10 min and then centrifuged (40,000g for 20 min). The supernatant was retained and the pellet washed twice with 70% (v/v) methanol with centrifugation as above. The methanolic supernatant was evaporated to dryness in a stream of nitrogen, and the pellet was resuspended in water and freeze dried. The pellet and the 70% (v/v) methanol-soluble fraction were hydrolyzed and subjected to compositional analysis.
Galactomannan Biosynthesis in Developing Tobacco Seeds
Flowers on the main central inflorescence of an individual plant (control or transgenic) were tagged at anthesis, and capsules were harvested when a range of maturity stages were available. Changes in the appearance of capsules and of the seeds contained within them were noted, as were the fresh weights of capsules and seeds. From each capsule, 50 seeds were used to prepare total cell wall material for compositional analysis. The remaining seeds were used immediately for the preparation of microsomal membranes.
Microsomal membranes were prepared from developing tobacco seeds by grinding the remaining seeds from a single capsule in a mortar with isolation buffer (8.0 mL) as described by Edwards et al. (1989) but containing 1 mm EDTA, spinning the suspension at 5,000g (10 min), and further spinning the supernatant at 100,000g (1 h). The 100,000g microsomal pellet was resuspended in the same buffer (0.5 mL), and this suspension was used as particulate enzyme for incubation with GDP-Man and UDP-Gal. Membranes (50 μL) were incubated at 30°C exactly as described by Reid et al. (1995) Two series of incubations were carried out, one in the presence of GDP-Man (80 μm) labeled with 14C in the Man moiety, and one with labeled GDP-Man (80 μm) plus unlabeled UDP-Gal (800 μm). Labeled polysaccharide products were isolated and fragmented using the A. niger endo-β-mannanase exactly as before (Reid et al., 1995).
Plant Transformation and Propagation
Tobacco plants were cultivated under a 16-h-light/8-h-dark regime at 25°C. The light intensity at table level was 200 μmol m−2 s−1 of photosynthetically active radiation. Surface-sterilized (hypochlorite) seeds were germinated on Murashige and Skoog medium supplemented with 0.8% (w/v) agar and 3% (w/v) Suc. Seedlings were transferred to a commercial compost (Levington Multi-purpose, The Scotts Company, Godalming, UK). Mineral nutrition was with “Phostrogen” (pbi Home and Garden, Enfield, UK), applied once weekly via capillary matting. To ensure self-pollination, each developing inflorescence was enclosed in a paper bag with a transparent panel.
A transformation vector was constructed that placed a cDNA encoding the full-length fenugreek (Trigonella foenum-graecum) GMGT (Edwards et al., 1999) between the double 35S promoter from cauliflower mosaic virus (2x35S, Kay et al., 1987) and the NOS poly(A+) signal in the plant transformation vector pGPTV-KAN (Becker et al., 1992). The plasmid was amplified in Escherichia coli, and introduced into Agrobacterium tumefaciens LBA 4404. A. tumefaciens clones containing the plasmid were used to transform tobacco leaf discs essentially as described by Horsch et al. (1985), except that nurse cells were not used and A. tumefaciens was suppressed using Cephotaxime (250 μg mL−1). To ensure that transformations were independent, no more than one plant regenerated from a single explant was processed further. Control plants were regenerated from tissue culture without kanamycin selection.
When putative transformants were 10 to 16 weeks old, genomic DNA was prepared from their leaf tissues (Edwards et al., 1991) and used as template in PCR reactions using primer pairs designed to the fenugreek GMGT cDNA sequence. PCR-positive plants were further screened for the expression of GMGT activity in their leaf tissues. Leaf tissue (2–3 g) was ground in a mortar with liquid nitrogen, added to 50 mm Tris-HCl buffer (pH 7.5) containing 1 mm EDTA, mixed for 30 s, and centrifuged (5,000g for 10 min). The supernatant was further spun (100,000g for 1 h). The pellet (microsomal membranes) was resuspended in 800 μL of the same buffer, containing 1% (w/v) Triton X-100, and left on ice for at least 20 min. The detergent-containing suspension (50 μL) was assayed for soluble GMGT exactly as described by Edwards et al. (1999).
ACKNOWLEDGMENT
As part of her undergraduate student project undertaken in the Spring semester of 2001, Katie Barr helped us to acquire the data on galactomannan biosynthesis in developing transgenic and control tobacco seeds. This contribution is gratefully acknowledged.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (UK; research grant).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.016840.
LITERATURE CITED
- Aspinall GO. Polysaccharides. Oxford: Pergamon; 1970. [Google Scholar]
- Avery GS. Structure and germination of tobacco seed and the developmental anatomy of the seedling plant. Am J Bot. 1933;23:309–327. [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol. 1992;20:1195–1197. doi: 10.1007/BF00028908. [DOI] [PubMed] [Google Scholar]
- Campbell JM, Reid JSG. Galactomannan formation and guanosine 5′-diphosphate-mannose: galactomannan mannosylyransferase in developing seeds of fenugreek (Trigonella foenum-graecum L., Leguminosae) Planta. 1982;155:105–111. doi: 10.1007/BF00392539. [DOI] [PubMed] [Google Scholar]
- Dea ICM, Morrison A. Chemistry and interactions of seed galactomannans. Adv Carbohydr Chem Biochem. 1975;31:241–312. [Google Scholar]
- Eda S, Akiyama Y, Kato K, Takahashi R, Kusakabe I, Ishizu A, Nakano J. Structural investigation of a galactoglucomannan from cell walls of tobacco (Nicotiana tabacum) midrib. Carbohydr Res. 1984;131:105–118. [Google Scholar]
- Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of genomic DNA for PCR analysis. Nucleic Acids Res. 1991;16:1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Bulpin PV, Dea ICM, Reid JSG. Biosynthesis of legume seed galactomannans in vitro. Planta. 1989;178:41–51. doi: 10.1007/BF00392525. [DOI] [PubMed] [Google Scholar]
- Edwards M, Scott C, Gidley MJ, Reid JSG. Control of mannose/galactose ratio during galactomannan formation in developing legume seeds. Planta. 1992;187:67–74. doi: 10.1007/BF00201625. [DOI] [PubMed] [Google Scholar]
- Edwards ME, Dickson CA, Chengappa S, Sidebottom C, Gidley MJ, Reid JSG. Molecular characterisation of a membrane-bound galactosyltransferase of plant cell wall matrix polysaccharide biosynthesis. Plant J. 1999;19:691–697. doi: 10.1046/j.1365-313x.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Edwards ME, Marshall E, Gidley MJ, Reid JSG. Transfer specificity of detergent-solubilized fenugreek galactomannan galactosyltransferase. Plant Physiol. 2002;129:1391–1397. doi: 10.1104/pp.002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Kay R, Chan A, Daly M, McPherson J. Duplication of CAMV-35S promoter sequences creates a strong enhancer for plant genes. Science. 1987;236:1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- McCleary BV. Modes of action of β-d-mannanase enzymes of diverse origin on legume seed galactomannans. Phytochemistry. 1979;18:757–763. [Google Scholar]
- McCleary BV, Matheson NK. Action patterns and substrate-binding requirements of β-mannanase with mannosaccharides and mannan-type polysaccharides. Carbohydr Res. 1983;119:191–219. [Google Scholar]
- Meier H, Reid JSG. Reserve polysaccharides other than starch in higher plants. In: Loewus FA, Tanner W, editors. Encyclopedia of Plant Physiology, New Series 13A: Plant Carbohydrates I. Berlin: Springer; 1982. pp. 418–471. [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ. A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol. 2000;123:1235–1245. doi: 10.1104/pp.123.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JSG. Cell wall storage carbohydrates in seeds: biochemistry of the seed “gums” and “hemicelluloses.”. Adv Bot Res. 1985;11:125–155. [Google Scholar]
- Reid JSG. Analysis of carbohydrates conferring hardness on seeds. In: Linskens HF, Jackson JF, editors. Modern Methods of Plant Analysis, New Series 10: Plant Fibers. Berlin: Springer; 1989. pp. 295–312. [Google Scholar]
- Reid JSG, Bewley JD. A dual role for the endosperm and its galactomannan reserves in the germinative physiology of fenugreek (Trigonella foenum-graecum L.), an endospermic leguminous seed. Planta. 1979;147:145–150. doi: 10.1007/BF00389515. [DOI] [PubMed] [Google Scholar]
- Reid JSG, Edwards M. Galactomannans and other cell wall storage polysaccharides in seeds. In: Stephen AM, editor. Food Polysaccharides and Their Applications. New York: Marcel Dekker; 1995. pp. 155–186. [Google Scholar]
- Reid JSG, Edwards M, Gidley MJ, Clark AH. Enzyme specificity in galactomannan biosynthesis. Planta. 1995;195:489–495. [Google Scholar]
- Saeman JF, Buhl JL, Harris EE. Quantitative saccharification of wood nad cellulose. Ind Eng Chem Anal Ed. 1945;17:35–37. [Google Scholar]
- Skjøt M, Pauly M, Bush MS, Borkhardt B, McCann MC, Ulvskov P. Direct interference with rhamnogalacturonan I biosynthesis in Golgi vesicles. Plant Physiol. 2002;129:95–102. doi: 10.1104/pp.010948. [DOI] [PMC free article] [PubMed] [Google Scholar]