Abstract
We have investigated the consequences of endogenous limitations in oxygen delivery for phloem transport in Ricinus communis. In situ oxygen profiles were measured directly across stems of plants growing in air (21% [v/v] oxygen), using a microsensor with a tip diameter of approximately 30 μm. Oxygen levels decreased from 21% (v/v) at the surface to 7% (v/v) in the vascular region and increased again to 15% (v/v) toward the hollow center of the stem. Phloem sap exuding from small incisions in the bark of the stem was hypoxic, and the ATP to ADP ratio (4.1) and energy charge (0.78) were also low. When 5-cm stem segments of intact plants were exposed to zero external oxygen for 90 min, oxygen levels within the phloem decreased to approximately 2% (v/v), and ATP to ADP ratio and adenylate energy charge dropped further to 1.92 and 0.68, respectively. This was accompanied by a marked decrease in the phloem sucrose (Suc) concentration and Suc transport rate, which is likely to be explained by the inhibition of retrieval processes in the phloem. Germinating seedlings were used to analyze the effect of a stepwise decrease in oxygen tension on phloem transport and energy metabolism in more detail. Within the endosperm embedding the cotyledons—next to the phloem loading sites—oxygen decreased from approximately 14% (v/v) in 6-d-old seedlings down to approximately 6% (v/v) in 10-d-old seedlings. This was paralleled by a similar decrease of oxygen inside the hypocotyl. When the endosperm was removed and cotyledons incubated in a 100 mm Suc solution with 21%, 6%, 3%, or 0.5% (v/v) oxygen for 3 h before phloem sap was analyzed, decreasing oxygen tensions led to a progressive decrease in phloem energy state, indicating a partial inhibition of respiration. The estimated ratio of NADH to NAD+ in the phloem exudate remained low (approximately 0.0014) when oxygen was decreased to 6% and 3% (v/v) but increased markedly (to approximately 0.008) at 0.5% (v/v) oxygen, paralleled by an increase in lactate and ethanol. Suc concentration and translocation decreased when oxygen was decreased to 3% and 0.5% (v/v). Falling oxygen led to a progressive increase in amino acids, especially of alanine, γ-aminobutyrat, methionine, and isoleucine, a progressive decrease in the C to N ratio, and an increase in the succinate to malate ratio in the phloem. These results show that oxygen concentration is low inside the transport phloem in planta and that this results in adaptive changes in phloem metabolism and function.
In contrast to animals, plants lack specialized systems for oxygen distribution. Oxygen moves by diffusion from the surrounding air (21% [v/v] oxygen) through apertures in the epidermis and intercellular air spaces within the tissue (Drew, 1997). The absence of specialized systems for oxygen delivery is not generally considered to be a problem for plant growth and metabolism because most plant organs have a relatively high surface-to-volume ratio, and their respiration rates per unit volume of tissue are usually lower than in animals because plant cells usually have large vacuoles. However, tissues with high metabolic activity do become hypoxic, especially when they lack large intercellular air spaces and contain cells that are poorly vacuolated or are located in the center of organs remote from the sites where oxygen enters the plant.
In bulky storage organs, including apples (Malus domestica; Magness, 1920), bananas (Musa spp; Banks, 1983), avocados (Persea americana; Ke et al., 1995), carrots (Daucus carota; Lushuk and Salveit, 1991), and potato (Solanum tuberosum) tubers (Stiles, 1960; Geigenberger et al., 2000), internal oxygen concentrations can be quite low. In growing potato tubers, oxygen concentrations are significantly decreased in the outer zones of the tubers, and there is a further decline between the outer zones and the center, where oxygen concentrations fall below 5% (Geigenberger et al., 2000). This is accompanied by adaptive changes in metabolism including a partial inhibition of respiration, a decrease in the cellular energy status, and a parallel inhibition of a wide range of energy-consuming metabolic processes. Pyrophosphate (PPi), which is utilized as an alternative energy donor in plants, is maintained at a high level under hypoxic conditions, in contrast to the progressive decrease of the energy status of the adenine, uridine, and guanine nucleotide systems. It has been proposed that these metabolic acclimations allow ATP and oxygen consumption to be decreased and prevent the tissue of becoming anoxic (Geigenberger et al., 2000).
Recent studies also document low oxygen tensions within seeds of Arabidopsis (Porterfield et al., 1999; Gibon et al., 2002), Vicia faba, and Pisum sativum (Rolletscheck et al., 2002). In the latter case, optical sensors were used to analyze detailed oxygen profiles across developing seeds showing that oxygen decreases sharply to approximately 1% (v/v) within the seed coat, which suggests that oxygen entry from the surrounding gas space into the seed is strongly restricted by the seed coat. There is an increase in oxygen tension upon illumination, indicating that photosynthesis significantly contributes to internal oxygen levels in these green seeds (Rolletscheck et al., 2002).
Based on indirect evidence, it has been suggested frequently that phloem tissue might be hypoxic. First, specific biochemical and morphological characteristics could lead to oxygen deficiency within the phloem. The phloem represents a specialized transport tissue with high metabolic activity and high respiration rates (Willenbrink, 1957; Geigenberger et al., 1993; van Bel and Knoblauch, 2000) to provide ATP for active import and transport of Suc (Komor, 1977; Bouché-Pillon et al., 1994; DeWitt and Sussman, 1995; Stadler et al., 1995; Kühn et al., 1996), and this will result in high local rates of oxygen consumption. Oxygen access might be restricted because it is a rather dense tissue with little intercellular spaces or vacuoles (Parthasarathy, 1975; Behnke and Sjolund, 1990). Second, high ethanol concentrations and alcohol dehydrogenase activities have been reported in the vascular cambium of trees (Kimmerer and Stringer, 1988), indicating anaerobic conditions in tissue near the phloem. Third, in root steles, in which the vascular tissue is embedded in, low oxygen concentrations have been reported (Thomson and Greenway, 1991; Ober and Sharp, 1996; Drew, 1997), and a decrease in external oxygen leads to an inhibition of ion transport into the xylem (Gibbs et al., 1998). However, roots differ from shoots in the fact that the external oxygen concentration is often low, phloem and xylem are located in the stele in the center of the root, rather than in the periphery, and there is no oxygen supply by photosynthesizing cells. Therefore, it remains unclear whether a similar situation exists in the vascular bundles of shoots.
Previous studies indicated that low external oxygen concentration can have an inhibitory effect on phloem translocation, but results were contradictory because other workers failed to observe any significant effect of external anoxia on phloem transport (for review, see Milburn and Kallarackal, 1989). The possible reason(s) for this discrepancy could not be resolved due to the lack of knowledge concerning the impact on the internal oxygen levels and energy metabolism inside the phloem.
In the following study, the effect of the internal oxygen concentration on phloem function is investigated and related to a detailed study on phloem energy metabolism. For this study, Ricinus communis plants were used. This plant is specifically suitable because pure phloem sap is easily sampled because the phloem continues bleeding after it has been incised. Also, seedlings have hypogeous cotyledons that, until approximately 10 d after germination (DAG), take up Suc from the surrounding endosperm and load it into the phloem, where it is transported to the growing hypocotyl and root. The endosperm can be easily removed and replaced by a Suc solution, whereas the cotyledons remain to function as active source, thus enabling controlled manipulation of phloem loading, metabolism, and transport (Kallarackal et al., 1989; Geigenberger et al., 1993).
Several direct approaches to analyze oxygen levels and phloem energy metabolism were used. (a) By using microsensors, in situ oxygen concentrations were analyzed in stem transects and in phloem exudates of adult R. communis plants. In exudates samples taken in parallel, adenylate energy charge and ATP to ADP ratios were analyzed. These minimal invasive techniques allowed us to investigate oxygen tensions and energy status in the phloem of an intact plant. (b) To assess the effect of external anoxia on phloem energy metabolism and transport, we exposed a stem segment of the plant with gaseous nitrogen using a plastic cuvette and analyzed phloem sap collected below the nitrogen girdle. (c) In a complementary approach, we used germinating R. communis seedlings to study phloem metabolism and transport in response to oxygen in more detail. The in situ oxygen concentration inside the endosperm and in hypocotyl transects were determined by using oxygen microsensors. To manipulate oxygen tensions, the endosperm was removed and the cotyledons exposed to solutions with various oxygen concentrations (0.5%, 3%, 6%, and 21% [v/v]). After 3 h, the hypocotyl was severed and phloem sap was collected to analyze the concentrations of Suc, amino acids, fermentation products, glycolytic intermediates, nucleotides, inorganic phosphate (Pi), and PPi in the sieve tube sap. The data were used to calculate changes in Suc translocation rates, C to N ratios, adenylate energy charge, ATP, UTP and PPi phosphorylation potentials, and NAD+ reduction state in response to a stepwise decrease in oxygen. The results show that metabolism and phloem function are modified at the oxygen levels found in the neighborhood of the phloem in intact plants.
RESULTS
Oxygen Concentrations across the Stem and in the Phloem Sap Exuding from Normoxic Plants
To determine in situ oxygen concentrations in the phloem, a microsensor was impaled into the stem of adult R. communis plants growing in normoxic air (21% [v/v]). Oxygen levels across the transect decreased markedly from 21% (v/v) at the surface to 7% (v/v) in the vascular region (Fig. 1). Further toward the center of the stem, in the inner parenchyma tissue, the oxygen concentration increased to 15% (v/v), which is also the oxygen tension in the inner cavity of the stem. These measurements show that the cells of transport phloem tissue are exposed to an environment with relatively low oxygen.
Figure 1.
Oxygen profile across the stem of intact 4-week-old flowering R. communis plants growing under normoxic conditions as revealed by using an oxygen microsensor with a tip diameter of 30 μm. The graph overlays an image of a representative stem slice through which profile was measured. The inset on the lower right shows the stem anatomy in more detail (10× magnification) and reveals the vascular tissue, which is embedded in parenchymous tissue. Results are the mean ± se (n = 4). vb, Vascular bundle; p, parenchyma; c, inner cavity.
To determine oxygen concentrations within the phloem symplast, phloem sap exuding from an incision in the bark of the stem was analyzed. Depending on the size of the droplet and the speed of exudation, O2 tensions varied between 15% and 19% (v/v; n = 17 measurements). Because oxygen will diffuse into the exudate droplet directly after exposure to air, it must be expected that all these values are overestimations of the actual oxygen concentration in intact phloem. Small droplets seemed to be affected more by diffusion of ambient oxygen then bigger droplets were.
Energy Status and Suc Concentration in the Phloem of Normoxic Plants and in Plants after Application of External Anoxia
To determine the energy status in the phloem of normoxic R. communis plants, the concentrations of ATP, ADP, and AMP were measured in the phloem sap (Fig. 2, A–C). The ATP to ADP ratio was 4.1 (Fig. 2D), the total amount of AdN was 860 μm (Fig. 2E), and the adenylate energy charge [ATP + 1/2ADP/(ATP + ADP + AMP)] was 0.78 (Fig. 2F).
Figure 2.
Concentrations of adenine nucleotides, Suc and Gluc-6-P in the sieve tube sap collected from 4-week-old flowering R. communis plants living in a normoxic environment (21% [v/v] oxygen) or from plants after exposing a stem segment to gaseous nitrogen (zero oxygen). A, ATP; B, ADP; C, AMP; D, ATP/ADP; E, total adenine nucleotide level; F, adenylate energy charge (EC); G, Suc; H, Glucose-6-Phosphate. Results are the mean ± se (n = 4).
To investigate the consequences of a decrease in oxygen for energy metabolism and Suc transport in the phloem, we exposed a 5-cm stem segment of intact replicate plants to a continuous stream of gaseous nitrogen (0% [v/v] oxygen) for 90 min. The oxygen concentration inside the N2-treated stem segment decreased to 2.0% ± 0.34% (v/v) in the peripheral stem tissue and to 3.6% ± 0.24% (v/v) in the hollow central part of the stem (mean ± se, n = 4). Oxygen concentration also decreased to 10% to 15% (v/v) in the phloem exudate that was collected approximately 3 cm below the treated segment. As pointed out above, this is likely to be an overestimate of the true concentration. The decrease in oxygen led to a decrease in the energy state of the phloem. In the phloem exudate collected below the N2 girdle, there was a 2-fold decrease of ATP (Fig. 2A), ATP/ADP (Fig. 2D), and total adenine nucleotides (Fig. 2E), and a decrease in the adenylate energy charge down to 0.68 (Fig. 2F). The decrease in adenylate energy state was accompanied by a marked decrease in the Suc concentration in the phloem sap (Fig. 2G). The concentration of Glc6P decreased only slightly (Fig. 2H). In phloem exudate collected 3 cm above the treated segment, oxygen and Suc concentrations remained high (data not shown).
In a further experiment, phloem sap was collected 10 cm below and 10 cm above the N2 girdle to analyze exudation rate and Suc concentration in parallel (Table I). There was a 3–5-fold decrease in exudation rate and a 1.3-fold decrease in Suc concentration below the nitrogen girdle, compared with the values monitored above the girdle or in normoxic plants. The data were used to estimate the absolute rates of Suc translocation (calculated by multiplying exudation rate and Suc concentration). In stems treated with gaseous nitrogen, Suc transport rates decreased by 73% and 82% below the girdle, compared with the rates above the girdle or in control plants exposed to air respectively (Table I).
Table I.
Exudation rates and sucrose concentrations in the phloem sap of adult R. communis plants after treatment of a 5-cm stem segment with gaseous nitrogen
| Treatment | Exudation Rate | Sucrose in Sieve Tube Sap | Suc Transport Rate |
|---|---|---|---|
| μL min−1 | mm | nmol min−1 | |
| Normoxic Control Plants | |||
| Exudate sampled above cuvette | 1.35 ± 0.08 | 343 ± 30 | 473 ± 48 |
| Exudate sampled below cuvette | 1.33 ± 0.11 | 351 ± 38 | 479 ± 92 |
| After application of gaseous N2 | |||
| Exudate sampled above cuvette | 0.88 ± 0.06 | 361 ± 7 | 315 ± 21 |
| Exudate sampled below cuvette | 0.28 ± 0.07 | 267 ± 35 | 84 ± 29 |
A 5-cm zone of the second stem segment of 4-week-old intact R. communis plants was exposed to a continuous stream of gaseous nitrogen for 90 min before phloem sap was collected 10 cm below and 10 cm above the treated segment. As a control, phloem sap was also collected from normoxic plants exposed to 21% (v/v) oxygen. Data are the mean ± se (n = 3–4).
Interestingly, substantial levels of oxygen remained inside stem segments that were exposed to an atmosphere containing zero oxygen. The oxygen gradient was reversed, increasing from 0% (v/v) at the surface to 2% (v/v) in the cortex to 3.6% (v/v) in the hollow center of the stem.
Internal Oxygen Tensions and Phloem Energy Status in Seedlings
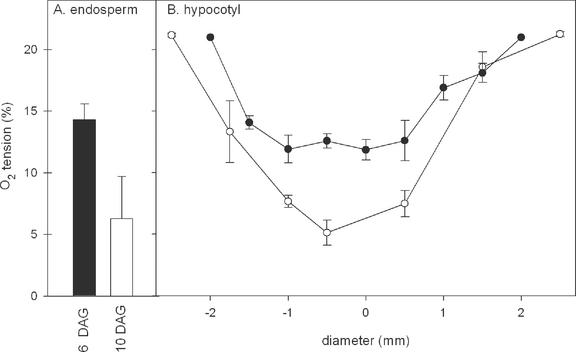
Germinating R. communis seedlings were used to analyze the influence of oxygen tensions on transport and energy metabolism in the phloem in more detail. To analyze the in situ oxygen tension in the vicinity of the cotyledons where phloem loading occurs, an oxygen microsensor was introduced into the endosperm of both 6- and 10-d-old seedlings germinating in the dark. The oxygen concentration measured in the endosperm facing the cotyledons depended on the developmental stage of the seedling. At 6 DAG, the lowest oxygen tension measured in the endosperm was 14.3% ± 1.3 (mean ± se, n = 5), whereas 4 d later, this value significantly decreased to 6.3% ± 3.4% (Fig. 3A). This may be related to the higher rates of respiration as the endosperm is mobilized. In situ oxygen tensions were also measured in hypocotyl transects. The oxygen concentration in the inner part of the stem was 11.9% ± 0.8 and 5.0% ± 1.0 at 6 and 10 DAG, respectively (Fig. 3B). This suggests that developmental changes in oxygen tension within the endosperm affect the oxygen tension inside the hypocotyl.
Figure 3.
Oxygen concentrations across the endosperm (A) and hypocotyl (B) of R. communis seedlings, 6 (black) and 10 (white) DAG respectively. Plants germinated in a normoxic environment (21% [v/v]) in the dark. Results are the mean ± se of at least four measurements. The hypocotyl diameter were 5 and 6 mm for seedlings 6 and 10 DAG, respectively. There was no hollow center inside hypocotyls.
After severing the hypocotyls from 6-d-old seedlings, phloem sap was collected from the residual stump and used for metabolic analysis. The concentrations of Suc, ATP, ADP, and AMP were 350 ± 8, 1.31 ± 0.06, 0.213 ± 0.02, and 0.08 ± 0.01 mm, respectively, yielding in an ATP to ADP ratio of 6.1 ± 0.43 and an adenylate energy charge of 0.88 ± 0.01 (mean ± se, n = 5 seedlings).
Influence of the Oxygen Tension on Exudation Rate and Suc Concentration in the Phloem Sap of Seedlings
To investigate the influence of a stepwise decrease in the external oxygen concentration on phloem transport and energy metabolism in more detail, we removed the endosperm and incubated cotyledons of 6-d-old seedlings in Suc solutions with different oxygen concentrations (0.5%, 3%, 6%, or 21% [v/v]). After 3 h, the hypocotyl was severed, and phloem exudate was collected for further analysis. Seedlings which were exposed to 100 mm Suc, and 21% (v/v) oxygen revealed an exudation rate of around 8 μL h−1 and a Suc concentration of approximately 300 mm in the tube sap (Fig. 4, A and B). This is in agreement with previous studies by Kallarackal et al. (1989). When the external oxygen tension was decreased, exudation rates decreased slightly at 6% (v/v) oxygen (by 20%) and more dramatically at 3% and 0.5% oxygen (v/v; by 60% and 75%, respectively; Fig. 4A). Suc concentration in the tube sap remained unaltered when oxygen was decreased to 6% (v/v), but decreased by 20% and 50% when external oxygen was decreased to 3% and 0.5% (v/v), respectively (Fig. 4B). The estimated absolute rate of Suc translocation decreased slightly (by 20%) in 6% (v/v) oxygen and more markedly in 3% and 0.5% (v/v) oxygen (by 64% and 84%, respectively; Fig. 4C). The results indicate a slight inhibition of phloem loading and translocation at 6% (v/v) oxygen and a marked and progressive inhibition at 3% and 0.5% (v/v) external oxygen.
Figure 4.
Suc concentration in the sieve tube sap and exudation rate of R. communis seedlings after incubation at different oxygen tensions. The endosperm of 6-d-old seedlings was removed, and cotyledons were supplied with 100 mm Suc at different external oxygen tensions (0.5%, 3%, 6%, or 21% [v/v], using premixed gases). After 3 h, the hypocotyl was cut, exudation monitored, and phloem exudate collected for metabolite analysis. A, Exudation rate; B, Suc concentration in tube sap; C, Suc translocation rate (exudation rate multiplied by the Suc concentration in the tube sap). Results are the mean ± se (n = 3).
Influence of the Oxygen Tension on the Concentrations of Nucleotides, Pi, and Inorganic PPi in the Phloem Sap of Seedlings
Phloem sap derives from a pure cytosolic compartment; therefore, metabolite analysis will reveal corresponding changes in cytosolic concentrations of various metabolites, organic acids, Pi, inorganic PPi, and nucleotides (Geigenberger et al., 1993). Decreasing oxygen tensions led to a progressive decrease of ATP (Fig. 5A), an increase of ADP (Fig. 5B) and AMP (Fig. 5C), and a marked decrease of the ATP to ADP ratio (Fig. 5D) and the adenylate energy charge in the phloem sap (Fig. 5E). The overall concentration of adenine nucleotides in the phloem declined, even though the incubation only lasted a few hours (Fig. 5F). There were already marked changes of adenine nucleotides when oxygen was decreased from 21% to 6% (v/v). Total adenylates decreased by 30%, the ATP to ADP ratio decreased from 8 to 4, and the adenylate energy charge decreased from 0.9 to 0.8. These parameters showed a further progressive decrease when oxygen was reduced to 3% and 0.5% (v/v). At 0.5% (v/v) oxygen, the ATP to ADP ratio was around 1.5, and the adenylate energy charge was approximately 0.64. Adenine nucleotide levels responded in a similar manner to low oxygen concentrations in seedlings and adult plants. The absolute values of total adenine nucleotide concentrations, ATP to ADP ratios, and adenylate energy charge in the phloem of normoxic adult plants (see Fig. 2) resembled those found in the phloem of seedlings incubated with 6% (v/v) oxygen (see Fig. 5). Based on our previous measurements (see Fig. 1), the oxygen concentrations in the transport tissue of intact adult plants would have been in this range.
Figure 5.
Concentrations and ratios of adenine and uridine nucleotides in the sieve tube sap of R. communis seedlings after incubation at different oxygen tensions (see legend to Fig. 4). Six-day-old seedlings were incubated as indicated in Figure 4, before phloem exudate was collected to analyze: A, ATP; B, ADP; C, AMP; D, ATP/ADP; E, energy charge; F, total AdN; G, total UdN; H, UDP-Glc; I, UTP; J, UDP; K, UTP/UDP; L, PPi; M, Pi; N, ATP/(ADP × Pi); O, UTP/(UDP × Pi); and P, PPi/(Pi × Pi). Metabolite ratios were calculated using the molar concentration of the respective metabolites. Results are the mean ± se (n = 3).
Uridine nucleotides serve as cofactors in the pathway of Suc degradation via Suc synthase (SuSy) and UDP-Glc pyrophosphorylase (Geigenberger and Stitt, 1993; Loef et al., 1999). Overall uridine nucleotide concentrations (Fig. 5G) declined in parallel with the adenine nucleotides. UDP-Glc accounted for the majority of the uridine nucleotide pool in the phloem and declined progressively as the oxygen tension was decreased (Fig. 5H). UTP concentration remained unaltered as oxygen was decreased from 21% to 3% (v/v), and declined sharply in 0.5% (v/v) oxygen (Fig. 3I). UDP rose progressively as the oxygen tension was decreased (Fig. 5J). UDP is the substrate of SuSy, which is responsible for Suc mobilization in the phloem complex. The UTP to UDP ratio fell progressively from approximately 8 in 21% (v/v) oxygen to 1.7 in 0.5% (v/v) oxygen (Fig. 5K), similar to the ATP to ADP ratio (for comparison, see Fig. 5D). GTP, GDP, and the overall guanine nucleotide concentrations were approximately 90% lower than the corresponding adenine nucleotide concentrations and showed a similar response to oxygen (data not shown).
The sieve tube concentration of PPi was high in 21% (v/v) oxygen, decreased only gradually at 6% and 3% (v/v) oxygen, and fell to very low levels at 0.5% (v/v) oxygen (Fig. 5L). Pi increased gradually as the oxygen concentration decreased (Fig. 5M). Because phloem sap is pure cytosolic, values for Pi can be used to calculate the phosphorylation potential of ATP, UTP, and PPi directly. The ATP phosphorylation potential (ATP × ADP−1 × Pi−1) was approximately 1,200 m−1 at 21% (v/v) oxygen, decreased more than 50% at 6% (v/v) oxygen, and showed a further marked decrease at 3% and 0.5% (v/v) oxygen (Fig. 5N). The phosphorylation potential of UTP (Fig. 5O) was similar to that of ATP (Fig. 5N) and showed the same response when oxygen was decreased. The phosphorylation potential of PPi (Fig. 5P) was much lower than that of ATP and UTP, decreased only slightly at 6% (v/v) oxygen, and dropped markedly at 3% and 0.5% (v/v) oxygen.
Influence of the Oxygen Tension on the Concentrations of Glycolytic Metabolites, Organic Acids, and Ethanol in the Phloem Sap of Seedlings
Figure 6 summarizes the effect of low oxygen tensions on glycolytic metabolites and organic acids in the phloem. The concentrations of Glucose-1-Phosphate (Glc1P), Glucose-6-Phosphate (Glc6P), and Fructose-6-Phosphate (Fru6P) increased 1.3-fold when oxygen was decreased to 6% (v/v), remained high at 3% (v/v) oxygen, and decreased by approximately 50% when oxygen was further reduced to 0.5% (v/v; Fig. 6, A–C). Fructose-1,6-bisPhosphate (Fru1,6bP) (Fig. 6D) and dihydroxyacetone phosphate (Fig. 6E) increased only slightly at 6% (v/v) oxygen, rose 3-fold and 1.5-fold, respectively, in 3% (v/v) oxygen, and stayed high at 0.5% (v/v) oxygen. Glycerate-3-phosphate decreased gradually (by 40%) from 21% to 3% (v/v) oxygen and declined by a further 50% at 0.5% (v/v) oxygen (Fig. 6F). Pyruvate concentration showed a biphasic response increasing nearly 2-fold when oxygen was decreased from 21% to 6% (v/v), remaining high when oxygen was decreased to 3% (v/v), and decreasing markedly when oxygen was further decreased down to 0.5% (v/v; Fig. 6G). The concentration of lactate was approximately 2 mm in 21% (v/v) oxygen, increased slightly in 6% and 3% (v/v) oxygen, and increased more dramatically in 0.5% (v/v) oxygen (Fig. 6H). Malate was high (12 mm) in 21% (v/v) oxygen and decreased progressively by 45%, 80%, and 63% in 6%, 3%, and 0.5% (v/v) oxygen, respectively (Fig. 6I). Succinate concentration was approximately 3 mm in 21% (v/v) oxygen, did not change in 6% (v/v) oxygen, but increased up to 5 and 7 mm in 3% and 0.5% (v/v) oxygen, respectively (Fig. 6J). Ethanol concentration was low (approximately 1 mm), except in 0.5% (v/v) oxygen where it rose approximately 4-fold (Fig. 6K).
Figure 6.
Metabolite concentrations and metabolite ratios in the sieve tube sap of R. communis seedlings after incubation at different oxygen tensions (see legend to Fig. 4). Six-day-old seedlings were incubated as indicated in Figure 4, before phloem exudate was collected to analyze: A, Glucose-1-Phosphate; B, Glucose-6-Phosphate; C, Fructose-6-Phosphate; D, Fructose-1,6-bisPhosphate; E, dihydroxyacetone phosphate; F, glycerate-3-phosphate; G, pyruvate; H, lactate; I, malate; J, succinate; K, ethanol; and L, NADH to NAD ratio. Results are the mean ± se (n = 3).
Influence of the Oxygen Tension on the NAD+ Reduction State in the Phloem of Seedlings
The NAD+ reduction state in the cytosol is an important parameter of cellular metabolism (Stryer, 1990), reflecting the balance between NAD+ reduction via glycolysis and NADH reoxidation via respiratory reactions. However, cytosolic concentrations of NADH cannot be measured accurately because the reduction state of NAD+ in the cytosol is very low and most of the NADH will be bound to enzymes (Heineke et al., 1991). Alternatively, NADH to NAD+ ratios can be estimated from the concentrations of metabolites supposed to be in near equilibrium with the NADH/NAD+ couple in the cytosol (Heineke et al., 1991). Assuming the reaction catalyzed by lactate dehydrogenase is close to equilibrium in vivo, the NADH to NAD+ ratio can be calculated according to the following equation:
 |
The literature value of K is 2.3 × 10−12 m and represents the equilibrium constant of lactate dehydrogenase (Bergmeyer, 1987). The NADH to NAD+ ratio in the phloem at different oxygen concentrations was calculated using the molar concentrations of lactate and pyruvate displayed in Figure 6, H and G, and assuming a pH of 8 in the phloem symplast ([H+] = 1 × 10−8 m). In 21% (v/v) oxygen, the phloem NADH to NAD+ ratio was very low (approximately 1.4 × 10−3; Fig. 6L), which is similar to the values previously found in the cytosol of spinach (Spinacia oleracea) leaves or in animal tissues (0.5–1.7 × 10−3; see Heineke et al., 1991). The phloem NADH to NAD+ ratio remained at this constant low level in 6% (v/v) oxygen, increased slightly in 3% (v/v) oxygen, and increased dramatically in 0.5% (v/v) oxygen (Fig. 6L). The data indicate that there is no substantial increase in the reduction state of NAD+ unless oxygen is decreased down to 0.5% (v/v).
Influence of the Oxygen Tension on the Concentrations of Amino Acids and the C to N Ratio in the Sieve Tube Sap of Seedlings
Amino acids are transported in significant concentrations in the phloem symplast of R. communis plants (see Komor et al., 1989; Schobert and Komor, 1989). In 21% (v/v) oxygen, the total amino acid concentration in the sieve tube sap of the seedlings was approximately 19 mm (Fig. 7A). Glu (Fig. 7B) and Gln (Fig. 7C) were the main amino acids transported (contributing to 32% of total amino acids).
Figure 7.
Concentrations of amino acids in the sieve tube sap of R. communis seedlings after incubation at different oxygen tensions (see legend to Fig. 4). Six-day-old seedlings were incubated as indicated in Figure 4, before phloem exudate was collected to analyze the concentrations of various amino acids: A, total amino acids; B, Glu; C, Gln; D, Ser; E, Ala; F, γ-aminobutyric acid (GABA); G, Ile; H, Leu; I, Val; J, Met; K, Phe; L, tryptophane; M, Tyr; N, Asp; O, Asn; P, Thr; Q, Lys; R, His; S, Arg; T, Gly; and U, citrulline. Results are the mean ± se (n = 3).
When external oxygen was decreased, the total amino acid concentration in the phloem rose up to 45, 52, and 71 mm in 6%, 3%, and 0.5% (v/v) oxygen, respectively. There were also major changes in the pattern of individual amino acids in response to low oxygen. A decrease in the oxygen tension to 6%, 3%, and 0.5% (v/v) led to a gradual decrease in the concentrations of Glu, Gln, and Ser (Fig. 7, B–D). The contribution of Glu, Gln, and Ser to the total amino acid pool in the phloem decreased markedly from 16.0%, 16.0%, and 17.4% of the total pool in 21% (v/v) oxygen to 2.5%, 2.1%, and 2.6% of the total pool in 0.5% (v/v) oxygen, respectively (calculated from the data in Fig. 7, A–D). The concentrations of the other amino acids either decreased only slightly (Arg and Gly; Fig. 7, S and T) or increased in response to low oxygen (Fig. 7, E–R and U). There was a massive increase in the concentrations of Ala (7-fold) and GABA (5.5-fold) when oxygen was decreased from 21% to 6% (v/v; Fig. 7, E and F). This trend was further accentuated in 3% and 0.5% (v/v) oxygen, where Ala increased 12- and 14-fold and GABA 5.8- and 6.4-fold, respectively, compared with 21% (v/v) oxygen. Interestingly, under low oxygen, Ala and GABA were the main amino acids in the phloem, counting for 42% and 15% of the total amino acids and reaching concentrations of 22 and 8 mm at 3% (v/v) oxygen, respectively. There were also major increases in the concentrations of Ile (Fig. 7G), Leu (Fig. 7H), Val (Fig. 7I), Met (Fig. 7J), and Asn (Fig. 7O), whereas Phe (Fig. 7K), Tyr (Fig. 7M), Asp (Fig. 7N), Thr (Fig. 7P), Lys (Fig. 7Q), His (Fig. 7R), and citrulline (Fig. 7U) increased only moderately.
From the data in Figure 7, the transport of total N can be calculated (Fig. 8). In low oxygen, there was a marked increase in the amount of total N in the sieve tube sap (sum of all the nitrogen in amino acids; Fig. 8A). Because this was largely paralleled by a decrease in the exudation rate, there were no substantial changes in the rate of total N transport (Fig. 8B; calculated by multiplying the total N in Fig. 8A by the exudation rates in Fig. 4A), except for 6% (v/v) oxygen, where the total N transport rate was nearly 2-fold increased. The Suc to amino acid ratio was approximately 15 in 21% (v/v) oxygen and decreased continuously to 7, 5, and 4 in 6%, 3%, and 0.5% (v/v) oxygen, respectively (calculated from Figs. 4B and 7A), indicating that low oxygen affects the C/N balance in the phloem. The C to N ratio in the phloem was calculated directly by dividing the amount of total C (sum of the carbons in amino acids, calculated from Fig. 7, plus the carbons in Suc, calculated from Fig. 4A) by the amount of total N (Fig. 8A) in the tube sap. When oxygen was decreased, there was a continuous decrease in the phloem C to N ratio from 150 to 25 (Fig. 8C).
Figure 8.
Transport of total N and C to N ratios in the sieve tube sap of R. communis seedling after incubation at different oxygen tensions (see legend to Fig. 4). Six-day-old seedlings were incubated as indicated in Figure 4, before phloem exudate was collected to analyze: A, total N; B, the rate of total N translocation; and C, the C to N ratio. Results are the mean ± se (n = 3).
DISCUSSION
Oxygen Concentrations Are Low inside the Phloem in Planta
Our results indicate that transport phloem is hypoxic. Direct measurements of oxygen profiles across stems of adult R. communis plants using microsensors showed that oxygen was decreased down to approximately 7% (v/v) in the vascular regions (Fig. 1), although plants were growing in the light at 21% (v/v) external oxygen. Oxygen levels down to 15% (v/v) were measured in the phloem sap exuding from small incisions in the bark. The tissues in which the transport phloem is embedded in may form a serious oxygen diffusion barrier. In respect to this, it is interesting that the hollow center of the stem acts as a buffer to counteract changes in oxygen availability (Fig. 1). In many stems, the central part has prominent intercellular spaces or is destroyed during growth (Esau, 1977). Such gas-filled spaces (aerenchyma) are known to facilitate internal oxygen transport (for review, see Drew, 1997), and as shown in the present study, the inner gas space prevented total anoxia in the stem tissue when external oxygen was deprived (see above).
Oxygen concentrations also fall to low levels within R. communis seedlings germinating in 21% (v/v) oxygen. Within the endosperm embedding the cotyledons where Suc is loaded into the phloem, oxygen decreased from approximately 14% (v/v) in 6-d-old seedlings down to 6% (v/v) in 10-d-old seedlings. This decrease during seedling development is reflected in the transport phloem in the hypocotyl (Fig. 3).
The presence of hypoxic conditions within the phloem provides an explanation why specific SuSy genes like Sh1 from maize (Zea mays; Yang and Russell, 1990), Asus1 from Arabidopsis (Martin et al., 1993), and potato StSus3 (Fu and Park, 1995) that are known to be up-regulated by hypoxia are preferentially expressed in the phloem.
Phloem Energy State and Suc Transport Rate Are Decreased at the Low Oxygen Levels Found in the Phloem of Intact Plants
This study shows that the oxygen concentrations occurring in the phloem of normoxic plants (approximately 5%–7% [v/v]) are in a range where they limit energy metabolism (Fig. 5) and where they start to become limiting for phloem transport (Fig. 4). When oxygen levels within the phloem are decreased further, phloem function is severely inhibited (Fig. 2). This is probably due to decreased Suc import or reloading (retrieval) into the phloem, resulting from energy deprival in the tissue.
Energy levels in phloem tissue of normoxic plants are fairly low already. Under full aerobic conditions, the ATP to ADP ratios measured in the cytosol of plant leaves or in animal tissues are in the range of 9 to 10 and the adenylate energy charge is over 0.9 (Lilley et al., 1982; Stitt et al., 1982; Stryer, 1990). In contrast, the values determined for the phloem of stems of intact normoxic plants are 4.1 and 0.78, respectively (Fig. 2). In seedlings, the phloem energy state (ATP to ADP ratio = 6.1; adenylate energy charge = 0.88) seems to be higher than in adult stems, but it must be noted that severing the hypocotyl will increase the access of oxygen from the atmosphere (21% [v/v]) to the phloem tissue in the residual hypocotyl stump. This could have led to a partial recovery of the adenylate energy state measured in the exudate.
In the case of seedlings, phloem ATP to ADP ratio and adenylate energy state increased when their endosperm was removed and cotyledons were exposed to a continuous stream of 21% (v/v) oxygen. This confirms that the endosperm is acting as a diffusion barrier and that removal of this barrier allows better access of oxygen to the phloem tissues within the cotyledons, leading to a relief of energy metabolism within the phloem. R. communis cotyledons are only 100 μm thick, and the diffusion path of oxygen from the incubation medium to the sieve elements is very short (in the 10-μm range; Kriedemann and Beevers, 1969). When external oxygen around the cotyledons was decreased from 21% to 6% (v/v), which is comparable with the in planta oxygen concentration in the vicinity of the phloem in stems, the adenylate energy state decreased markedly inside the phloem (Fig. 5). Interestingly, the relation between oxygen tension and ATP to ADP ratio within the phloem (Fig. 9) is similar to that previously described for potato tuber discs (Geigenberger et al., 2000).
Figure 9.
Correlation between phloem oxygen tension and energy state. In case of adult plants, ATP/ADP data were taken from Figure 2D, where oxygen tensions in the phloem were determined to be 7% (v/v; normoxic stems) and 2% (v/v; N2-treated stems) using microsensors (see Fig. 1 and data given in the text). In the case of intact seedlings (6 DAG), ATP/ADP data were taken from the text and oxygen data from Figure 3. In the case of seedlings (6 DAG) incubated at different oxygen tensions after removing their endosperm, ATP/ADP and oxygen data were both taken from Figure 5.
All together, this study shows by using three independent parameters, namely ATP to ADP ratio, adenylate energy charge, and ATP phosphorylation potential, that a stepwise decrease in oxygen leads to a progressive reduction in the phloem energy state. Similar parameters of the UTP and PPi energy systems showed a similar response. Because phloem sap derives purely from the cytosol, all these parameters could be measured without complication due to subcellular compartmentation. This allows a direct comparison of ATP to ADP and UTP to UDP ratios. Interestingly, these ratios matched very well (compare Fig. 5, K with D), indicating that the UTP and ATP systems are equilibrated via nucleoside diphosphate kinase in the phloem symplast. This apparently contrasts with analyses of whole tissues like leaves (Dancer et al., 1990) or tubers (Loef et al., 2001), where the overall values for the ATP to ADP ratios are always smaller than those for the UTP to UDP ratios. The reason for this difference lies in the differential compartmentation of adenine and uridine nucleotides in plant cells. In leaves (Stitt et al., 1982; Dancer et al., 1990) and tubers (Farré et al., 2001), uridine nucleotides are predominantly located in the cytosol, whereas adenine nucleotides are present in the cytosol and the plastid. Subcellular analysis of leaves (Stitt et al., 1982) and tubers (Tiessen et al., 2002) demonstrated lower ATP to ADP ratios in the plastid compared with the cytosol.
Low Oxygen Leads to a Sequential Induction of Fermentative Pathways and to Large Changes in the Composition of the Phloem Sap
The decrease in oxygen has complex consequences on the composition of the phloem sap (Figs. 6 and 7). Remarkable are the increased levels of total N in the phloem under low oxygen (Fig. 8). Two factors appear to be responsible for this: (a) the massive increase in the concentrations of Ala and GABA, especially at 6% (v/v) oxygen, and (b) the decreased loading of Suc, especially at 3% and 0.5% (v/v) oxygen, leading to a lower phloem flux that will result in a rise in amino acid concentrations even when the entry of amino acids into the phloem remains unaltered. Obviously, the transport of N is less sensitive to low oxygen than the transport of C. The more than proportional increase in the concentrations of Ala and GABA could be due to a selective increase in the import of these individual amino acids into the phloem or to changes in phloem metabolism in response to low oxygen.
The latter alternative is more likely because accumulation of Ala and GABA has been reported frequently as an early response to hypoxia and often precedes the accumulation of succinate (Davies, 1980). Ala and GABA are synthesized by Ala aminotransferase and Glu decarboxylase, respectively, both enzymes being induced by low oxygen (Klok et al., 2002) and using Glu as a common substrate. Accumulation of Ala and GABA in the phloem was paralleled by a decrease in Glu (Fig. 7). Ala aminotransferase also leads to the production of oxoglutarate, which is one of the substrates of a fermentative pathway that leads to accumulation of succinate. This pathway was initially discovered in facultative anaerobic mollusks (Hochachka et al., 1973) and is also supposed to be active in plants (Davies, 1980).
Our results indicate that a stepwise decrease in the oxygen concentration leads to a successive induction of biochemical pathways in the phloem, resulting in accumulation of Ala and GABA at 6% (v/v) external oxygen, succinate at 3% (v/v) external oxygen, and lactate and ethanol at 0.5% (v/v) external oxygen. The onset of lactic and ethanol fermentation was paralleled by a steep increase in the estimated cytosolic NADH to NAD+ ratio (Fig. 6L), which indicates that the phloem is entering anoxic metabolism. Under these conditions, cytochrome oxidase becomes oxygen limited (Km [O2] is 14 μm [Drew, 1997], corresponding to 0.01% [v/v] oxygen), ATP formation via oxidative phosphorylation is inhibited, and ATP has to be produced by fermentation (Drew, 1997). In contrast to this, induction of the pathways to Ala, GABA, and succinate occurred at oxygen concentrations that were much higher than the Km (O2) of cytochrome oxidase and were not accompanied by any substantial changes in the NAD+ reduction state. This indicates that these pathways can be induced well before anoxic conditions are reached. GABA (Shelp et al., 1999) and succinate (Menegus et al., 1989) synthesis have been proposed to play a role in counteracting cytoplasmatic acidification in low oxygen. Recent studies document that acidosis occurs rapidly and is due to proton-releasing hydrolysis of nucleotide-5-triphosphates when oxygen falls (Gout et al., 2001). This will require rapid synthesis of GABA, Ala, and succinate in response to relatively small decreases in oxygen tensions.
Low Oxygen Leads to Adaptive Changes in Phloem Metabolism
Based on studies in growing potato tubers, Geigenberger et al. (2000) concluded that falling internal oxygen leads to: (a) a restriction of glycolysis and respiration that decreases the adenylate energy status, (b) a widespread decrease in biosynthetic activity which decreases ATP consumption, and (c) a switch to pathways which consume less ATP. They proposed that this represents a metabolic acclimation to decrease oxygen consumption and prevent the tissue from driving itself into anoxia. It was clearly separated from the inhibition of cytochrome oxidase and the switch to lactic fermentation, which does not occur until much lower oxygen concentrations. Our results provide evidence for a similar adaptive response in the phloem.
First, there is a continuous decrease in phloem energy state while the NAD+ reduction state remains low, indicating a coordinated inhibition of glycolysis and respiration in response to low oxygen. This occurs at oxygen levels that are far higher than the Km (O2) of cytochrome oxidase. The increase in hexose phosphate levels under these conditions is consistent with an inhibition of glycolysis. Second, there is a decrease in total nucleotide levels with decreasing oxygen levels, indicating inhibition of nucleotide biosynthesis in the phloem. Inhibition of biosynthetic processes will save ATP and reduce respiration rates. Third, the need to conserve energy and oxygen provides an explanation why Suc is metabolized by SuSy and UGPase in the phloem (Geigenberger et al., 1993; Nolte and Koch, 1993), which costs less energy (only 1 mol PPi mol−1 Suc) compared with degradation via invertase and hexokinase (2 mol ATP mol−1 Suc; Huber and Akazawa, 1986). Interestingly, phloem transport is strongly impaired when PPi is removed by phloem-specific overexpression of pyrophosphatase, indicating an important role of PPi-dependent Suc degradation via SuSy in the phloem (Lerchl et al., 1995; Geigenberger et al., 1996). Low oxygen leads to increased expression of specific SuSy genes and to repression of invertase in maize roots (Zeng et al., 1999) and potato tubers (K.L. Bologa, A.R. Fernie, A. Leisse, M. Ehlers Loureiro, and P. Geigenberger, unpublished data).
These considerations imply that plant metabolism follows a similar defense strategy as initially described in hypoxia tolerant animals (Hochachka et al., 1997). This includes a reduction in energy turnover to reach an optimal hypometabolic steady state and an improved energy efficiency of the remaining metabolic processes to prevent internal anoxia when oxygen is falling. These metabolic acclimations occur at oxygen levels that are higher than the Km (O2) for cytochrome oxidase, indicating that oxygen is acting as a regulator in addition to its role as respiratory substrate. Oxygen sensing systems have been elaborated in bacteria and yeast (see Bunn and Poyton, 1996) and still have to find their counterparts in higher organisms (Wenger, 2000). Current models of oxygen sensing in mammals are based on a haem protein capable of reversibly binding oxygen and the production of reactive oxygen species by NAD(P)H oxidases and mitochondria (López-Barneo et al., 2001). In plants, non-symbiotic hemoglobins have been suggested to be involved in oxygen sensing (Appleby et al., 1988), but more studies are needed to identify the molecular sensor and the cellular mechanism(s) involved.
MATERIALS AND METHODS
Plant Material
Seeds of Ricinus communis cv Carmencita (Jelitto, Hamburg, Germany) were germinated in sterile conditions and grown under continuous aeration (21% [v/v]) in hydroculture in the dark as in Kallarackal et al. (1989). Unless stated otherwise in the text, 6-d-old seedlings were used for the experiments. Adult plants were grown on soil in a growth chamber at 300 μmol photons m−2 s−1 and 14 h of light/8 h of dark at 20°C and 50% relative humidity and were used after 4 weeks when they were approximately 1 m tall. Plants were used for experiments in the middle of the light period.
Experiments with Adult Plants
A stem segment of approximately 5 cm of an intact adult plant was exposed to a continuous stream of gaseous nitrogen (zero oxygen) using a plastic cuvette. After 90 min, phloem exudate was collected from small incisions with a sharp razor blade into the bark of the stem 3 to 10 cm above or below the treated segment (as described by Smith and Milburn, 1980) and frozen in liquid nitrogen.
Experiments with Seedlings
The endosperm of 6-d-old seedlings was carefully removed, and the cotyledons were placed in 100 mL of 2.5 mm KH2PO4 buffer (pH 5.5) containing 100 mm Suc and incubated for 3 h before the hypocotyl was severed and phloem sap was collected with graded micro-capillaries in an enclosed atmosphere at 95% to 100% relative humidity as described by Kallarackal et al. (1989). In all cases, sap was collected continuously for approximately 2 h, subsequent subsamples being immediately frozen in liquid nitrogen every 5 min (Geigenberger et al., 1993). A detailed time course revealed that fluctuations of the exudation rate of individual seedlings over this time interval were less than 30% of the mean value (data not shown). During the whole experiment, the cotyledons were incubated at different external oxygen concentrations (0.5%, 3%, 6%, or 21% [v/v]) by using premixed gases (Messer, Griesheim, Germany) streaming through the incubation solution. To avoid complication due to atmospheric oxygen (21% [v/v]) entering the phloem via the cut surface, the hypocotyl stump was exposed to the specific oxygen tensions, too. The oxygen concentration in the solution was measured by using an oxygen electrode (see below). During the 3-h pre-incubation period, the roots of the seedlings were immersed in a solution containing 0.5 mm CaCl2 at 21% (v/v) oxygen. The whole experimental setup was placed in a water bath to maintain a constant temperature of 27°C.
Metabolite Analysis
The frozen exudate was extracted with trichloroacetic acid, and phosphorylated intermediates (ATP, ADP, AMP, PPi, Pi, and pyruvate) were measured as by Geigenberger et al. (1993). Uridine and guanine nucleotides were analyzed by HPLC as by Geigenberger et al. (1997), amino acids by HPLC as by Geigenberger et al. (1996), organic acids and ethanol according to Bergmeyer (1987), and Suc as in Geigenberger et al. (1996).
Analysis of Oxygen Tensions
In situ oxygen tensions were measured using an oxygen microsensor with a tip diameter of approximately 30 μm connected to a fiber optic oxygen meter (MicroxTX2, Presens, Regensburg, Germany). This type of sensor enables oxygen measurements both in solution and in dry gas. It is very sensitive and reacts to changes in oxygen very fast. Furthermore, unlike conventional electrodes, the sensor does not consume oxygen. The microsensor was pierced through the tissue using a micromanipulator, and the location of the tip was derived from its scaling. At each position, the reading equilibrated for approximately 30 s before the oxygen tension was registered.
Stems of 4-week-old flowering plants were clamped with a laboratory stand to fix their position toward the micromanipulator, and oxygen tensions were measured throughout a transect perpendicular to the axis of the stem. The oxygen concentration of phloem sap was determined by placing the microsensor directly in a droplet of phloem exudate freshly appearing from an incision in the stem of adult plants. Oxygen tensions in the hypocotyl of seedlings 6 and 10 DAG were determined at the site were the hypocotyl makes a sharp hook. Seedlings were fixed on permanently kneadable sealant (Terostat-IX, Henkel Teroson GmbH, Heidelberg) to prevent moving during the measurement. Endosperm was impaled with the microsensor perpendicular to the surface of the cotyledons.
ACKNOWLEDGMENT
We thank Mark Stitt for stimulating discussions and helpful comments on the manuscript.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ge 878/1–3 to P.G.) and by the Max-Planck Society (to J.T.v.D.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017202.
LITERATURE CITED
- Appleby CA, Bogusz V, Dennis ES, Peacock WJ. A role for hemoglobin in all plant roots? Plant Cell Environ. 1988;11:359–367. [Google Scholar]
- Banks NH. Evaluation of methods for determining internal gases in banana fruit. J Exp Bot. 1983;34:871–879. [Google Scholar]
- Behnke H-D, Sjolund RD, editors. Sieve Elements. Comparative Structure, Induction and Development. Berlin: Springer-Verlag; 1990. [Google Scholar]
- Bergmeyer HU. Methods of Enzymatic Analysis. Weinheim, Germany: VCH; 1987. [Google Scholar]
- Bouché-Pillon S, Fleurat-Lessard P, Fromont JC, Serrano R, Bonnemain JL. Immunolocalisation of the plasma-membrane H+-ATPase in minor veins of Vicia faba L. in relation to phloem loading. Plant Physiol. 1994;105:691–697. doi: 10.1104/pp.105.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn HF, Poyton RO. Oxygen sensing and molecular adaptations to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- Dancer JE, Neuhaus HE, Stitt M. The subcellular compartmentation of uridine nucleotides and nucleoside-5′-diphosphate kinase in leaves. Plant Physiol. 1990;92:637–641. doi: 10.1104/pp.92.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DD. Anaerobic metabolism and the production of organic acids. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants. Vol. 2. New York: Academic Press; 1980. pp. 581–609. [Google Scholar]
- DeWitt ND, Sussman MR. Immunocytological localisation of an epitope-tagged plasma membrane proton pump (H+-ATPase) in phloem companion cells. Plant Cell. 1995;7:2053–2067. doi: 10.1105/tpc.7.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Esau K. Anatomy of Seed Plants. New York: John Wiley & Sons; 1977. [Google Scholar]
- Farre EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L. Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, amino acids and sugar alcohols in potato tubers using a non-aqueous fractionation method. Plant Physiol. 2001;127:685–700. doi: 10.1104/pp.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Park W. Sink- and vascular-associated sucrose synthase functions are encoded by different gene classes in potato. Plant Cell. 1995;7:1369–1385. doi: 10.1105/tpc.7.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M. Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem. 2000;381:723–740. doi: 10.1515/BC.2000.093. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Langenberger S, Wilke I, Heineke D, Heldt HW, Stitt M. Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta. 1993;190:446–453. [Google Scholar]
- Geigenberger P, Lerchl J, Stitt M, Sonnewald U. Phloem-specific expression of pyrophosphatase inhibits long distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ. 1996;19:43–55. [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta. 1997;201:502–518. [Google Scholar]
- Geigenberger P, Stitt M. Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta. 1993;190:440–450. doi: 10.1007/BF00194429. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H. Response to oxygen deficiency in primary maize roots: I. Development of oxygen deficiency in the stele reduces radial ion solute transport to the xylem. Aust J Plant Physiol. 1998;25:745–758. [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M. Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J. 2002;30:221–235. doi: 10.1046/j.1365-313x.2001.01278.x. [DOI] [PubMed] [Google Scholar]
- Gout E, Boisson A-M, Aubert S, Douce R, Bligny R. Origin of the cytoplasmatic pH changes during anaerobic stress in higher plant cells: carbon-13 and phosphorous-31 nuclear magnetic resonance studies. Plant Physiol. 2001;125:912–925. doi: 10.1104/pp.125.2.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke D, Riens B, Grosse H, Hoferichter P, Peter U, Flügge U-I, Heldt HW. Redox transfer across the inner chloroplast membrane. Plant Physiol. 1991;95:1131–1137. doi: 10.1104/pp.95.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Felds J, Mustafa T. Animal life without oxygen: basic biochemical mechanisms. Am Zool. 1973;13:543–555. [Google Scholar]
- Hochachka PW, Land PC, Buck LT. Oxygen sensing and signal transduction in metabolic defence against hypoxia: lessons from vertebrate facultative anaerobes. Comput Biochem Physiol A Physiol. 1997;118:23–29. doi: 10.1016/s0300-9629(96)00372-6. [DOI] [PubMed] [Google Scholar]
- Huber SC, Akazawa T. A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 1986;81:1008–1013. doi: 10.1104/pp.81.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallarackal J, Orlich G, Schobert C, Komor E. Sucrose transport into the phloem of Ricinus communis L. seedlings as measured by the analysis of sieve-tube sap. Planta. 1989;177:327–335. doi: 10.1007/BF00403590. [DOI] [PubMed] [Google Scholar]
- Ke D, Yahia E, Hess B, Zhou L, Kader AA. Regulation of fermentative metabolism in avocado fruit under oxygen and carbon dioxide stresses. J Am Soc Hortic Sci. 1995;120:481–490. [Google Scholar]
- Kimmerer TW, Stringer MA. Alcohol dehydrogenase and ethanol in the stems of trees: evidence for anaerobic metabolism in the vascular cambium. Plant Physiol. 1988;87:693–697. doi: 10.1104/pp.87.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell. 2002;14:2481–2494. doi: 10.1105/tpc.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E. Sucrose uptake by cotyledons of Ricinus communis L.: characteristics, mechanism, and regulation. Planta. 1977;137:119–131. doi: 10.1007/BF00387548. [DOI] [PubMed] [Google Scholar]
- Komor E, Kallarackal J, Schobert C, Orlich G. Comparison of solute transport in the phloem of the Ricinus communis seedling and the adult plant. Plant Physiol Biochem. 1989;27:545–550. [Google Scholar]
- Kriedemann P, Beevers H. Sugar uptake and translocation in castor bean seedlings: I. Characteristics of transfer in intact and excised seedlings. Plant Physiol. 1969;42:161–173. doi: 10.1104/pp.42.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn C, Quick WP, Schulz A, Riesmeier JW, Sonnewald U, Frommer WB. Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ. 1996;19:1115–1123. [Google Scholar]
- Lerchl J, Geigenberger P, Stitt M, Sonnewald U. Impaired photoassimilate partitioning caused by phloem-specific removal of pyrophosphate can be complemented by a phloem-specific cytosolic yeast-derived invertase in transgenic plants. Plant Cell. 1995;7:259–270. doi: 10.1105/tpc.7.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley RMcC, Stitt M, Mader G, Heldt HW. Rapid fractionation of wheat leaf protoplasts using membrane filtration. Plant Physiol. 1982;70:965–970. doi: 10.1104/pp.70.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P. Feeding orotate leads to a specific increase in uridine nucleotide levels, resulting in a stimulation of sucrose degradation and starch synthesis in discs of growing potato tubers. Planta. 1999;209:314–323. doi: 10.1007/s004250050638. [DOI] [PubMed] [Google Scholar]
- Loef I, Stitt M, Geigenberger P. Increased levels of adenine nucleotides modify the interaction between starch synthesis and respiration when adenine is supplied to discs from growing potato tubers. Planta. 2001;212:782–791. doi: 10.1007/s004250000461. [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Pardal R, Ortega-Sáenz P. Cellular mechanisms of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Lushuk JA, Salveit ME. Effects of rapid changes in oxygen concentration on respiration in carrot roots. Physiol Plant. 1991;82:559–568. [Google Scholar]
- Magness JR. Composition of gases in intercellular spaces of apples and potatoes. Bot Gaz. 1920;70:308–316. [Google Scholar]
- Martin T, Frommer WB, Salanoubat M, Willmitzer L. Expression of an Arabidopsis sucrose synthase gene indicates a role in metabolization of sucrose both during phloem loading and in sink organs. Plant J. 1993;4:367–377. doi: 10.1046/j.1365-313x.1993.04020367.x. [DOI] [PubMed] [Google Scholar]
- Menegus F, Cattaruzza L, Chersi A, Fronza G. Differences in the anaerobic lactate-succinate production and in the changes of cell sap pH for plants with high and low resistance to anoxia. Plant Physiol. 1989;90:29–32. doi: 10.1104/pp.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn JA, Kallarackal J. Physiological aspects of phloem translocation. In: Baker DA, Milburn JA, editors. Transport of Photoassimilates. Marlow, UK: Longman Scientific & Technical; 1989. pp. 264–305. [Google Scholar]
- Nolte KD, Koch KE. Companion-cell specific localization of sucrose synthase in zones of phloem loading and unloading. Plant Physiol. 1993;101:899–905. doi: 10.1104/pp.101.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober ES, Sharp RE. A microsensor for direct measurement of O2 partial pressure within plant tissues. J Exp Bot. 1996;47:447–457. [Google Scholar]
- Parthasarathy MV. Sieve-element structure. In: Zimmermann MH, Milburn JA, editors. Encyclopedia of Plant Physiology N.S. 1, Transport in Plants 1. Heidelberg: Springer; 1975. pp. 3–38. [Google Scholar]
- Porterfield DM, Kuang A, Smith PJS, Crispi ML, Musgrave ME. Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot. 1999;77:1439–1446. [PubMed] [Google Scholar]
- Rolletscheck H, Borisjuk L, Koschorreck M, Wobus U, Weber H. Legume embryos develop in a hypoxic environment. J Exp Bot. 2002;53:1099–1107. doi: 10.1093/jexbot/53.371.1099. [DOI] [PubMed] [Google Scholar]
- Schobert C, Komor E. The differential transport of amino acids into the phloem of Ricinus communis L. seedlings as shown by the analysis of sieve tube sap. Planta. 1989;177:342–349. doi: 10.1007/BF00403592. [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Bown AW, McLead MD. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999;4:446–451. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- Smith AC, Milburn JA. Osmoregulation and the control of phloem sap composition in Ricinus communis L. Planta. 1980;148:28–34. doi: 10.1007/BF00385438. [DOI] [PubMed] [Google Scholar]
- Stadler R, Brandner J, Schulz A, Gahrtz M, Sauer N. Phloem loading by the PmSuc2 sucrose carrier from Plantago major occurs into companion cells. Plant Cell. 1995;7:1545–1554. doi: 10.1105/tpc.7.10.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles W. The composition of the atmosphere (oxygen content of air, soil, intercellular spaces, diffusion, carbon dioxide and oxygen tension) In: Ruhland W, editor. Encyclopedia of Plant Physiology, Plant Respiration Inclusive Fermentations and Acid Metabolism. XII (Part 2) Heidelberg: Springer Verlag; 1960. pp. 114–148. [Google Scholar]
- Stitt M, Lilley RMcC, Heldt HW. Adenine nucleotide levels in the cytosol, chloroplasts and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982;70:971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Biochemistry. Heidelberg: Springer; 1990. [Google Scholar]
- Thomson CJ, Greenway H. Metabolic evidence for stelar anoxia in maize roots exposed to low O2 concentrations. Plant Physiol. 1991;96:1294–1301. doi: 10.1104/pp.96.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farré EM, Geigenberger P. Starch synthesis in potato tubers is regulated by post-translational redox-modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell. 2002;14:2191–2213. doi: 10.1105/tpc.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel AJE, Knoblauch M. Sieve element and companion cell: the story of the comatose patient and the hyperactive nurse. Aust J Plant Physiol. 2000;27:477–487. [Google Scholar]
- Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–1263. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]
- Willenbrink J. Über die Hemmung des Stofftransports in den Siebröhren durch lokale Inaktivierung verschiedener Atmungsenzyme. Planta. 1957;48:269–342. [Google Scholar]
- Yang NS, Russell D. Maize sucrose synthase-1 promoter directs phloem-cell specific expression of GUS gene in transgenic tobacco plants. Proc Natl Acad Sci USA. 1990;87:4144–4148. doi: 10.1073/pnas.87.11.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. Rapid repression of maize invertases by low oxygen: invertase/sucrose synthase balance, sugar signalling potential, and seedling survival. Plant Physiol. 1999;121:599–608. doi: 10.1104/pp.121.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]