Abstract
The monoclonal antibody, CCRC-M1, which recognizes a fucose (Fuc)-containing epitope found principally in the cell wall polysaccharide xyloglucan, was used to determine the distribution of this epitope throughout the mur1 mutant of Arabidopsis. Immunofluorescent labeling of whole seedlings revealed that mur1 root hairs are stained heavily by CCRC-M1, whereas the body of the root remains unstained or only lightly stained. Immunogold labeling showed that CCRC-M1 labeling within the mur1 root is specific to particular cell walls and cell types. CCRC-M1 labels all cell walls at the apex of primary roots 2 d and older and the apices of mature lateral roots, but does not bind to cell walls in lateral root initials. Labeling with CCRC-M1 decreases in mur1 root cells that are undergoing rapid elongation growth such that, in the mature portions of primary and lateral roots, only the walls of pericycle cells and the outer walls of epidermal cells are labeled. Growth of the mutant on Fuc-containing media restores wild-type labeling, where all cell walls are labeled by the CCRC-M1 antibody. No labeling was observed in mur1 hypocotyls, shoots, or leaves; stipules are labeled. CCRC-M1 does label pollen grains within anthers and pollen tube walls. These results suggest the Fuc destined for incorporation into xyloglucan is synthesized using one or the other or both isoforms of GDP-d-mannose 4,6-dehydratase, depending on the cell type and/or developmental state of the cell.
All plant cells are encased by walls; primary walls predominate in young, dividing, and growing cells, whereas secondary walls are characteristic of the thickened walls of woody tissues. Primary walls consist of several inter-digitated and interconnected matrices of polysaccharides and (glyco)proteins (McNeil et al., 1984; Bacic et al., 1988; McCann and Roberts, 1991; Carpita and Gibeaut, 1993; Rose et al., 2000). Examples of such matrices include one consisting of cellulose and associated hemicelluloses (e.g. xyloglucan) and another made up of pectic polysaccharides (e.g. homogalacturonan, rhamnogalacturonan I, and rhamnogalacturonan II). The precise structures of these matrices and how they interact with each other within the wall remain largely unknown.
Walls give shape and structure to plant cells and, ultimately, organs, while at the same time maintaining strength, flexibility, and plasticity to accommodate growth and respond to biotic and abiotic changes in the plant's environment. It has also become increasingly clear that cell walls play important roles in the biology of plant cells, particularly with respect to their development and differentiation (McCabe et al., 1997; Fleming et al., 1999; Lally et al., 2001; O'Neill et al., 2001). Thus, it is important to gain a better understanding of the structure and function of the macromolecular components of plant cell walls, how their synthesis is coordinated and regulated, and how these components interact to form a functional wall.
Models of plant cell wall structure have remained relatively unchanged in their essential elements since their earliest form (Albersheim, 1975; McCann and Roberts, 1991; Carpita and Gibeaut, 1993), and provide an overall framework for the macromolecular organization of the wall. However, research over the past several years (Carpita et al., 2001) demonstrates that these models are insufficient to capture the full complexities of cell wall structure, composition, and organization necessary to fulfill the physiological role(s) increasingly ascribed to the cell walls of higher plants.
A small but growing number of monoclonal antibodies against plant cell wall polysaccharides and glycoproteins have been used to determine the localizations of these macromolecules within plant cells and tissues (Knox, 1997). These studies have documented a wide variety of labeling patterns demonstrating that walls can differ among different cell types (Knox et al., 1989, 1990, 1991; Dolan and Roberts, 1995; Dolan et al., 1995; Freshour et al., 1996; Casero et al., 1998; Vicré et al., 1998; Willats et al., 1999; Majewska-Sawka et al., 2002; McCartney and Knox, 2002), and even among the walls surrounding a single cell (Freshour et al., 1996; Šamaj et al., 1999; Majewska-Sawka et al., 2002). Antibodies have also provided evidence for the existence of subdomains within a single wall that contain different glycoconjugates (Knox et al., 1990; Freshour et al., 1996; Bush and McCann, 1999; Serpe et al., 2002). Moreover, monoclonal antibodies have been used to demonstrate developmental regulation of carbohydrate epitopes on glycoproteins (Pennell and Roberts, 1990; Pennell et al., 1991; Van Aelst and Van Went, 1992; Stacey et al., 1995; McCabe et al., 1997; Casero et al., 1998; Butowt et al., 1999) and polysaccharides (Stacey et al., 1995; Freshour et al., 1996; Willats et al., 1999).
There are only a few examples where the available antibodies have been used to examine the effects of mutations on the structures of plant cell wall components (Barry et al., 1991; Benfey et al., 1993; Di Laurenzio et al., 1996; Rhee and Somerville, 1998; Nickle and Meinke, 1998; Sinha and Lynch, 1998; Shevell et al., 2000; His et al., 2001; Orfila et al., 2001). Examination of plants carrying mutations affecting wall components may reveal wall-related structural patterns that are obscured or do not exist in the walls of wild-type plants.
One mutant having altered cell walls is mur1, which was isolated by screening the leaves of a mutagenized population of Arabidopsis for changes in monosaccharide composition (Reiter et al., 1993). Initial chemical analyses of the mur1 walls detected only trace amounts of Fuc in the aboveground tissues of the plant, whereas Fuc levels in the roots were reduced by 40% compared with wild-type plants (Reiter et al., 1993). The gene associated with the mur1 phenotype, GMD2, has been isolated and shown to encode a GDP-Man 4,6-dehydratase (Bonin et al., 1997), the first enzyme in the specific pathway for biosynthesis of GDP-Fuc, the sugar nucleotide substrate required by the fucosyltransferases responsible for incorporation of Fuc into cell wall polysaccharides and other glycoconjugates.
We have generated a monoclonal antibody, CCRC-M1, that recognizes terminal fucosyl residues linked α-(1→2) to a galactosyl residue, an epitope commonly found in the side chains of xyloglucan and, to a lesser extent, of rhamnogalacturonan I (Puhlmann et al., 1994). In a previous study, we demonstrated that this epitope is present in almost all cell walls of wild-type Arabidopsis seedlings (Freshour et al., 1996). We have now used CCRC-M1 to localize this Fuc-containing epitope throughout mur1 plants and show that its insertion into the walls of this Arabidopsis mutant is cell and tissue specific. Our results also provide insights into how plant cells regulate the biosynthesis of their walls during plant growth and development.
RESULTS
Distribution of the Fuc-Containing CCRC-M1 Epitope in Primary Roots of mur1 Plants
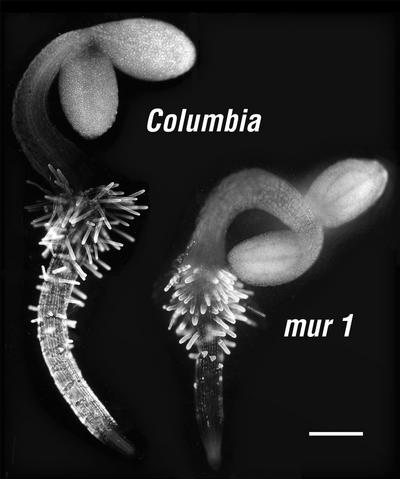
Immunofluorescent labeling of the surface of intact, unfixed roots of mur1 seedlings showed that CCRC-M1 labels the walls of root hairs strongly, whereas the body of the root is labeled weakly, if at all (Fig. 1). In contrast, CCRC-M1 labels both root hairs and the body of the root in wild-type Arabidopsis seedlings (Fig. 1). CCRC-M1 does not label any aboveground tissues in intact seedlings of both wild type and mur1, nor does the antibody label cells at the root apex.
Figure 1.
Immunofluorescent labeling with CCRC-M1 of intact, unfixed 4-d-old wild-type and mur1 seedlings. Measurement bar is 0.2 mm.
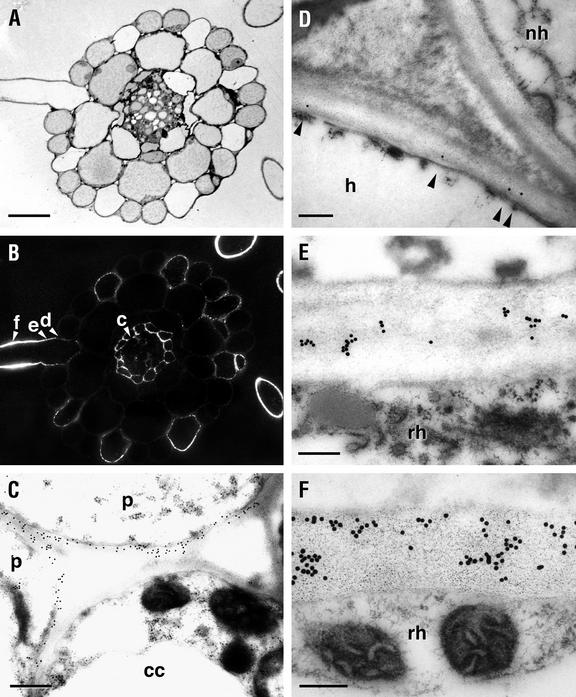
Labeling of thin sections taken from mur1 seedlings confirmed and extended the observations made with intact seedlings. Immunofluorescent labeling of transverse sections taken from the upper part (5 mm from the root apex, a point at which all cells are fully differentiated; Dolan et al., 1993) of the mur1 root results in intense labeling of root hair cell walls (Fig. 2B). The intensity of immunogold labeling increases along the root hair wall with increasing distance from the body of the root-hair forming cell (Fig. 2, D–F). The walls of the body of the root hair-forming cell are weakly labeled (Figs. 2, B and D), whereas those of the non-root hair-forming epidermal cells do not label (Fig. 2D). Pericycle cell walls are the only other cell walls that are labeled with CCRC-M1 in transverse sections taken from the upper part of mur1 roots (Fig. 2, B and C). Immunofluorescent labeling with CCRC-M1 of equivalent sections from wild-type plants yields uniform labeling of all cell walls (Freshour et al., 1996).
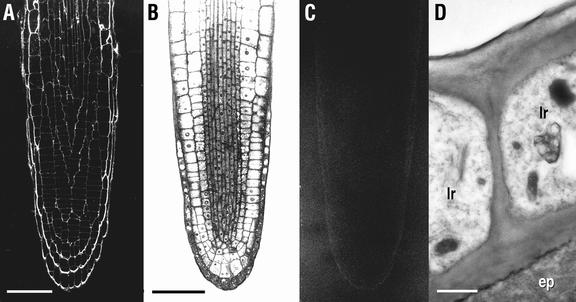
Figure 2.
CCRC-M1 labeling of serial transverse sections of mur1 roots taken about 5 mm from the root apex, where all cells are fully differentiated (Dolan et al., 1993). A, Section stained with toluidine blue. B, Immunofluorescent labeling with CCRC-M1. Lettered arrowheads in B indicate approximate positions of sections taken for immunogold labeling shown in C through F. C, Close-up showing immunogold labeling of pericycle (p), but not central cylinder cell (cc) walls. D, Junction between hair-forming (h) and non-hair-forming (nh) cells showing sparse labeling (arrowheads) of the wall of the hair-forming cell, but absence of label in the wall of the non-hair forming cell. E and F, Root hair (rh) cell walls at increasing distances from the body of the hair-forming cell. Immunofluorescent labeling with CCRC-M1 of equivalent sections from wild-type plants yields uniform labeling of all cell walls (Freshour et al., 1996). Measurement bars = 20 μm in A and B, 0.3 μm in C and D, and 0.2 μm in E and F.
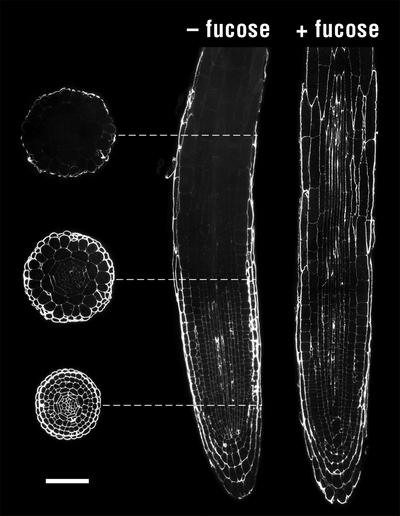
Examination of longitudinal sections taken from the root apex of 4-d-old mur1 seedlings demonstrated that CCRC-M1 labels the walls of all cells located within the meristematic zone (Dolan et al., 1993), approximately 200 μm from the apex (Fig. 3), in contrast to the absence of labeling in this region observed in intact seedlings (Fig. 1). The absence of surface labeling at the tip of intact seedlings could be due to the presence on the root tip surface of material (e.g. root slime) that blocks access of the CCRC-M1 antibody to the epidermal and root cap cell walls. CCRC-M1 labeling diminishes rapidly at distances greater than 200 μm from the root apex, where cell elongation occurs (Dolan et al., 1993), such that labeling is restricted to the outer wall of epidermal cells by approximately 350 μm from the root apex. Immunolabeling of transverse sections taken at various distances from the root apex confirm these observations (Fig. 3). Growing mur1 seedlings on a medium supplemented with Fuc, which restores Fuc content of walls to the levels observed in wild-type plants (Reiter et al., 1993), results in labeling of all walls throughout the root (Fig. 3), the pattern observed in wild-type plants (Freshour et al., 1996).
Figure 3.
Transverse sections of mur1 roots were taken at the approximate position shown (dashed lines) from a seedling grown on normal medium. The longitudinal section on the right was taken from a mur1 seedling grown on medium supplemented with 20 mm l-Fuc. Immunofluorescent labeling with CCRC-M1 of equivalent sections from wild-type plants yields essentially uniform labeling of all cell walls (Freshour et al., 1996). Measurement bar = 50 μm.
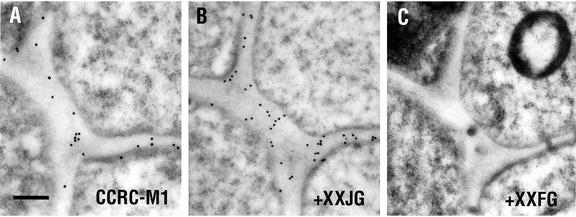
The specificity of mur1 labeling with CCRC-M1 was checked by pre-incubation of the antibody with different xyloglucan-derived oligosaccharides (Fig. 4). Xyloglucan isolated from the mur1 mutant contains l-Gal in place of l-Fuc (Zablackis et al., 1996). Pre-incubation of CCRC-M1 with XXJG, the xyloglucan nonasaccharide containing a terminal l-galactosyl residue, had no apparent affect on binding of the antibody to cell walls in the meristematic zone of mur1 roots. On the other hand, pre-incubation of CCRC-M1 with XXFG, the xyloglucan nonasaccharide containing a terminal l-fucosyl residue, completely abolishes binding of the antibody to cell walls of mur1 plants. Thus, CCRC-M1 is specific for the fucosylated form of xyloglucan.
Figure 4.
Pre-adsorbtion controls for immunogold labeling of mur1 cell walls with the CCRC-M1 antibody. A, Labeling with CCRC-M1. B, Labeling with CCRC-M1 that had been pre-incubated with XXJG (100 μg mL−1). C, Labeling with CCRC-M1 that had been pre-incubated with XXFG (100 μg mL−1). Sections were taken from roots of 5-d-old mur1 seedlings and show the walls of columella cells. Measurement bar is 0.3 μm.
We also examined whether the labeling observed in mur1 roots could be attributed to localization of fucosylated rhamnogalacturonan I, because CCRC-M1 will bind to both xyloglucan and rhamnogalacturonan I when assayed by in vitro immunoassays (Puhlmann et al., 1994). CCRC-M1 does not label any walls in the fut1 mutant (Fig. 5), in which fucosylation of xyloglucan is specifically abolished without affecting fucosylation of other macromolecules (Perrin et al., 2003). Labeling of the wild-type background for fut1 (ecotype Wassilewskija) with CCRC-M1 is ubiquitous (Fig. 5A) and is identical to that observed previously for wild-type Arabidopsis ecotype Columbia (Freshour et al., 1996). Thus, CCRC-M1 binding to fucosylated rhamnogalacturonan I cannot be detected under the conditions used for our immunolabeling studies, indicating that the labeling observed in mur1 is due exclusively to binding to fucosylated xyloglucan.
Figure 5.
Immunolabeling with CCRC-M1 of thin sections taken from root apices of 5-d-old wild-type (ecotype Wassilewskija; A) and fut1 (B–D) seedlings. A and C, Immunofluorescent labeling with CCRC-M1. B, Section adjacent to the one shown in C stained with toluidine blue. D, Immunogold labeling of epidermal (ep) and lateral root cap (lr) cell walls about 125 μm from the root apex. Measurement bars are 50 μm in A through C and 0.5 μm in D.
Developmental Regulation of the CCRC-M1 Epitope in mur1 Roots
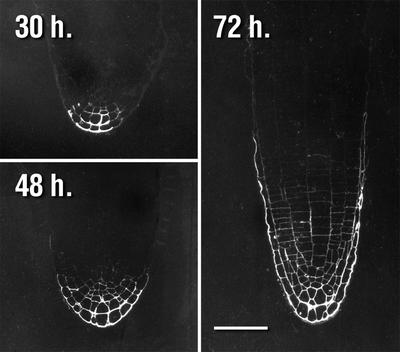
Labeling of mur1 seedlings with CCRC-M1 24 h after imbibition shows no labeling of any cell walls in the seedling, whereas all cell walls label in wild-type seedlings at this time (Fig. 6). Occasionally, the walls of a small number of epidermal cells located at a hypocotyl-cotyledon junction were observed to label with CCRC-M1 (Fig. 7). Beginning at 30 h postimbibition, a small number of cells at the apex of mur1 roots are labeled with CCRC-M1. The zone of antibody labeling at the root apex expands with time (Fig. 8), such that by 96 h all walls below the elongation zone of the mur1 root are labeled (Fig. 3).
Figure 6.
Longitudinal sections of germinating seeds of wild-type (A and B) and mur1 (C and D) 24 h postimbibition. A and C, Sections stained with toluidine blue. B and D, Immunofluorescent labeling of adjacent sections with CCRC-M1. The different appearance of the mur1 seed is due to loss of the seed coat during the fixation process, leading to partial unfolding of the germling. Measurement bars = 50 μm.
Figure 7.
Longitudinal sections of mur1 germling 24 h postimbibition showing labeling with CCRC-M1 of cells at the junction between a cotyledon and the hypocotyl. A, Section stained with toluidine blue; arrow indicates region of labeling shown in B. B, Immunofluorescent labeling with CCRC-M1; arrow points to region examined by immunogold labeling shown in C. C, Immunogold labeling showing the presence of gold particles in the walls of two cells, but no labeling in the walls of two other adjacent cells (arrowheads). Measurement bars = 50 μm in A, 25 μm in B, and 0.5 μm in C.
Figure 8.
Immunofluorescent labeling with CCRC-M1 of the mur1 radicle apex with CCRC-M1 at 30, 48, and 72 h postimbibition. Measurement bar = 50 μm.
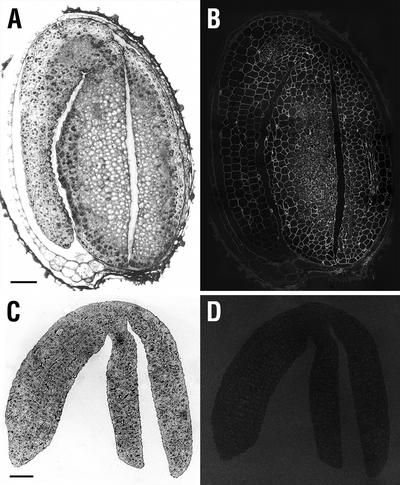
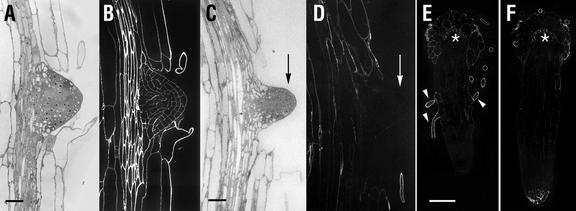
The developmental pattern exhibited by CCRC-M1 labeling at the primary root apex is recapitulated in developing lateral roots. CCRC-M1 labels all cell walls in wild-type lateral root primordia (Fig. 9B), just as it does at the primary root apex (Freshour et al., 1996). In contrast, lateral root primordia of mur1 seedlings do not label with the CCRC-M1 antibody (Fig. 9D). Indeed, no labeling of mur1 lateral root tips occurs until lateral roots reach a length >0.25 mm (Fig. 9F), though labeling of root hairs was observed in shorter lateral roots (Fig. 9E). The tips of longer mur1 lateral roots are labeled with CCRC-M1 (Fig. 9F) in a pattern that is indistinguishable from that observed in the primary root (Fig. 3).
Figure 9.
Longitudinal sections of lateral roots of wild-type (A and B) and mur1 (C–F) seedlings. Lateral root initials are shown in A and C (sections stained with toluidine blue) and B and D (immunofluorescent labeling of adjacent sections with CCRC-M1). Longer lateral roots are shown in E and F; the primary root is seen in cross section at the tops of these plates (asterisk). E, Immunofluorescent labeling of a lateral root approximately 0.25 mm in length; arrowheads point to labeled root hairs. F, Immunofluorescent labeling of a lateral root approximately 0.3 mm in length; root hairs are not observed due to the plane of section taken. Measurement bars = 20 μm for A and B, 25 μm for C and D, and 50 μm for E and F.
Distribution of the CCRC-M1 Epitope in Aboveground Tissues of mur1 Plants
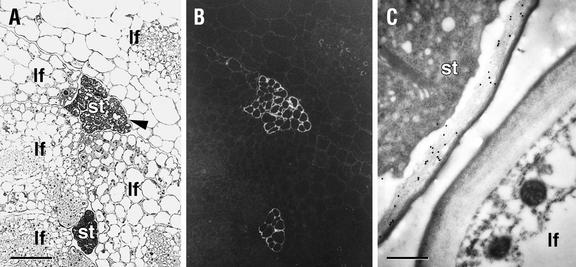
Stipules are the only aboveground vegetative tissues of mur1 plants whose walls stain with CCRC-M1 (Fig. 10) in mur1 seedlings. No labeling with this antibody of other leaf and hypocotyl cell walls was observed in tissue sections taken at various stages of mur1 growth and development. In wild-type plants, all cell walls of the hypocotyl, leaves, and stipules were labeled by CCRC-M1 when examined in sectioned tissue (data not shown). The absence of labeling of aboveground tissues in intact wild-type seedlings (Fig. 1) probably reflects blocking of antibody access to the cell walls of these tissues by cutin.
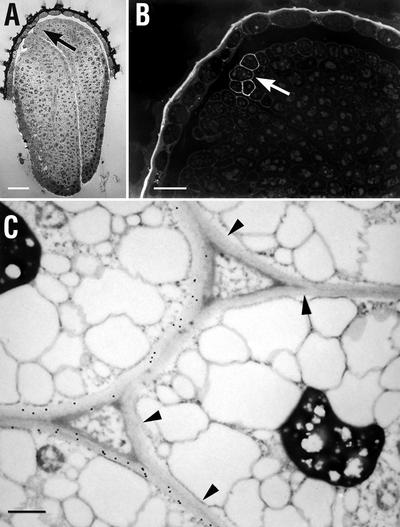
Figure 10.
Immunofluorescent and immunogold labeling with CCRC-M1 of transverse sections through the rosette of a 21-d-old mur1 plant. A, Stipule (st) and portions of adjacent leaves (lf); arrowhead points to region examined by immunogold labeling shown in C. B, Adjacent section with immunofluorescent labeling with CCRC-M1. C, Immunogold labeling demonstrating the presence of the CCRC-M1 epitope in the wall of a stipule cell (st) and its absence from the wall of a nearby leaf cell (lf). Measurement bar = 50 μm in A and B, and 0.5 μm in C.
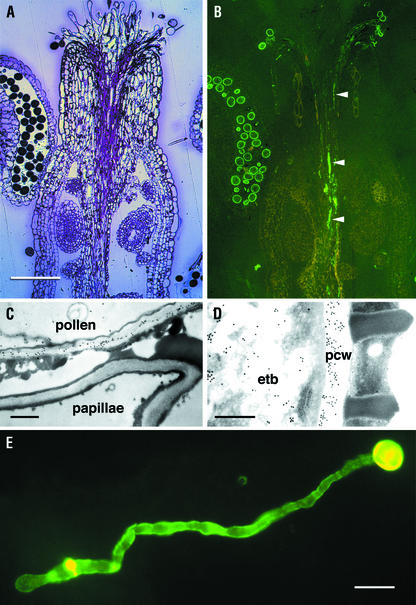
Labeling of mur1 floral tissues with CCRC-M1 was observed, but was restricted to pollen grains and to pollen tubes growing within the style (Fig. 11). Immunogold labeling of intact pollen revealed that CCRC-M1 labeling is confined to the inner, electron-translucent layer (intine) of the walls in both wild-type (data not shown) and mur1 (Fig. 11D) pollen grains. In addition, CCRC-M1 labeling was observed in electron-translucent bodies within mur1 pollen (Fig. 11D), a pattern also observed in wild-type pollen (data not shown). CCRC-M1 labels both the grain and the growing tube of pollen that had been germinated in vitro (Fig. 11E).
Figure 11.
Immunofluorescent and immunogold labeling with CCRC-M1 of mur1 flowers, pollen grains, and tubes. A and B, Longitudinal sections taken from stage 13 (defined as described by Bowman, 1994b) flowers of mur1 plants either stained with toluidine blue (A) or labeled with CCRC-M1 (B); arrowheads indicate pollen tubes in the style. C, Immunogold labeling of a pollen wall adjacent to an unlabeled papillar wall in the pollinated flower. D, Immunogold labeling of the wall (pcw) and electron translucent bodies (etb) in a mur1 pollen grain. E, Immunofluorescent labeling of mur1 pollen germinated in vitro. Measurement bars = 80 μm in A and B, 0.5 μm in C, 0.4 μm in D, and 30 μm in E.
DISCUSSION
The immunolocalization studies reported here demonstrate that the fucosylated epitope recognized by the monoclonal antibody CCRC-M1 is present in the primary walls of specific root cells, in contrast to wild-type plants where the epitope is found in the primary walls of almost all root cells (Freshour et al., 1996). In particular, this epitope is detectable in the meristematic zones of all primary and lateral roots of this mutant, but only after the cells in these zones have reached a specific developmental stage (Figs. 8 and 9). Outside of the meristematic zone of roots, the epitope is present in root hair-forming cells and especially in the root hairs themselves (Figs. 1 and 2).
More surprising was the discovery that the fucosylated epitope recognized by CCRC-M1 is also present in aboveground tissues of the mur1 plant, specifically in stipules (Fig. 10), and in pollen and pollen tubes (Fig. 11). Previous chemical analyses had only found trace amounts of Fuc in aboveground tissues (Reiter et al., 1993; Zablackis et al., 1995). These chemical analyses lacked the sensitivity to determine whether or not the trace amounts of Fuc were confined to specific aboveground cells or organs.
The fucosylated epitope recognized by CCRC-M1 is present on the cell wall polysaccharides, xyloglucan, and rhamnogalacturonan I, as assayed by in vitro immunoassays (Puhlmann et al., 1994). Other fucosylated macromolecules (e.g. rhamnogalacturonan II, arabinogalactans, and glycoproteins) present in Arabidopsis cell walls are not recognized by this antibody. CCRC-M1 does not label any walls in the fut1 mutant (Fig. 5), in which fucosylation of xyloglucan is selectively abolished by an insertional knockout of the gene encoding the xyloglucan-specific fucosyltransferase, FUT1 (Perrin et al., 2003). The fucosylation of other glycoconjugates, including rhamnogalacturonan I, remains unaffected in the fut1 mutant (Perrin et al., 2003). The extent of fucosylation of rhamnogalacturonan I in Arabidopsis is low (approximately 1 mol %; Malcolm O'Neill, personal communication). Thus, the absence of CCRC-M1 labeling in the fut1 mutant leads us to conclude that CCRC-M1 cannot detect fucosylated rhamnogalacturonan I in tissue sections under our immunolabeling conditions, at least in the tissues of Arabidopsis. More detailed chemical analyses of mur1 walls demonstrated that wall polysaccharides of the mutant carried l-galactosyl residues in place of the l-fucosyl residues found in wild-type polysaccharides (Zablackis et al., 1996). CCRC-M1 does not recognize the l-galactosylated xyloglucan present in mur1 walls (Fig. 4). Taken together, these data suggest that the CCRC-M1 labeling of the mur1 mutant exclusively reflects the localization of fucosylated xyloglucan.
The pattern of CCRC-M1 labeling observed in mur1 plants clearly reflects genetic redundancy in the de novo synthesis of l-Fuc. mur1 plants carry a missense mutation in the GMD2 gene, which results in the expression of a nonfunctional form of GDP-d-Man 4,6-dehydratase, an enzyme required for the biosynthesis of Fuc (Bonin et al., 1997). A second gene, GMD1, with significant sequence similarity to GMD2, is present in the Arabidopsis genome and has been shown recently to encode a second isoform of GDP-d-Man 4,6-dehydratase (C.P. Bonin, G. Freshour, M.G. Hahn, G.F. Vanzin, W.-D. Reiter, submitted for publication). Thus, the pattern of Fuc incorporation into xyloglucan in mur1 plants as recognized by the CCRC-M1 antibody likely results from the expression of GMD1 activity in specific cells and at specific times during the plant's growth and development.
Fucosylated xyloglucans are first detected in the columellar root cap and root meristems, and are eventually present throughout the cell division zone of the root (Figs. 8 and 9). Our data suggest that GMD1 expression is activated around 24 to 30 h postimbibition in the primary root (Fig. 8A) and is delayed in lateral roots until these reach a length > 0.25 mm (Fig. 9). In the elongation zone, the amount of fucosylated xyloglucan present in cell walls drops off dramatically (Fig. 3), suggesting that GMD1 expression is switched off and the protein degraded, or that GMD1 activity is down-regulated by posttranslational modification. The incorporation of fucosylated xyloglucan into the walls of growing root hairs (Figs. 1 and 2) and pericycle cells (Fig. 2) suggests renewed expression of GMD1 or reactivation of existing enzymes in these cell types. These results suggest that the mutant phenotype is cell autonomous and that UDP-l-Fuc does not move from cell to cell within the root.
The observation that fucosylated xyloglucans are incorporated in mur1 into the walls of root hair-forming cells during the course of root hair development is consistent with other reports suggesting that root hair walls differ from those of the body of the root hair-forming cell and also differ from those of neighboring epidermal cells that do not form root hairs (Freshour et al., 1996; Šamaj et al., 1999). Our data further suggest that GMD1 expression may be regulated at least in root epidermal cells by genes such as TRANSPARENT TESTA GLABRA1, GLABRA2, CAPRICE, and WEREWOLF known to control root hair development (Galway et al., 1994; Masucci et al., 1996; Wada et al., 1997; Lee and Schiefelbein, 1999).
The presence of fucosylated xyloglucan in aboveground tissues is restricted to stipules (Fig. 10) and to pollen grains and tubes (Fig. 11), suggesting a corresponding restricted pattern of GMD1 expression. The presence within pollen grains of internal deposits labeled by CCRC-M1 (Fig. 11D) raises the possibility that fucosylated xyloglucan is synthesized and sequestered in the pollen grain during maturation. The stored xyloglucan could then later be mobilized during pollen germination, at which time its glycosyl components could be used for the biosynthesis of the fucosylated xyloglucan being incorporated into the growing pollen tube wall (Fig. 11E), thereby avoiding the need for expression of GDP-d-Man 4,6-dehydratase activity during pollen tube growth.
The localization data reported here suggest that in wild-type Arabidopsis, the GMD2 gene is expressed in all of those tissues not labeled by CCRC-M1 in the mur1 mutant. Whether or not GMD2 is also expressed in the wild type together with GMD1 in those tissues showing CCRC-M1 labeling in mur1 plants cannot be ascertained from our data. It is possible that the two genes alternate in their expression patterns in the different parts of wild-type Arabidopsis during the course of plant growth and development. Detailed gene expression studies for both GMD1 and GMD2 using gene-specific probes and immunolocalizations using isoform-specific antibodies would likely answer these questions.
GMD1 could also be functionally redundant with GMD2 to ensure fucosylation of cell wall polymers to preserve wall properties critical to specific cell types within the plant. In that regard, it is interesting to note the tissues in which GMD1 expression is apparent in mur1 plants. These include the two meristematic tissues present in roots, i.e. the apical meristems and the pericycle, and the only two cell types in plants known to expand via tip growth, root hair cells and pollen tubes. Perhaps the unique characteristics of these tissues and cells require the fucosylation of one or more of the macromolecules that make up their walls.
The need for fucosylation of wall polymers is less apparent in the other cells whose walls are labeled by CCRC-M1, namely stipules (Fig. 10) and epidermal cells located at the junctions of cotyledons and hypocotyls (Fig. 7). The function of stipules is not known, though the densely stained cytoplasm (Fig. 10A) and the extensive endomembrane system of these tissues (Bowman, 1994a) suggest intense metabolic activity. The cells at the cotyledon/hypocotyls junction may play an important role in the proper unfolding of the embryo as the seed germinates, and cell wall properties may have an important role in that process.
The Fuc deficiency in the mur1 mutant has an impact on plant growth and cell wall properties. The mutant plants have been reported to be stunted in their aboveground parts, where almost no Fuc is produced (Reiter et al., 1993; O'Neill et al., 2001). We also observed stunting in the roots of mur1, both in terms of overall organ size (Fig. 1) and the sizes of individual cells (Fig. 3), an observation that was also reported recently by others (Van Hengel and Roberts, 2002). In addition, the walls of mur1 cells are more brittle than wild-type walls (Reiter et al., 1993). Recent evidence suggests that the growth defects exhibited in aboveground mur1 tissues are the result of the absence of Fuc in rhamnogalacturonan II, leading to a reduced ability of this polysaccharide to form essential pectin cross-links within the wall (O'Neill et al., 2001).
The Fuc synthesized via GDP-d-Man 4,6-dehydratase is incorporated into various cell wall glycoconjugates, including xyloglucan, rhamnogalacturonan I, rhamnogalacturonan II, and arabinogalactan glycoproteins. Fucosylation of all of these cell wall polymers is affected in the mur1 mutant (Zablackis et al., 1996; Rayon et al., 1999; O'Neill et al., 2001; Van Hengel and Roberts, 2002). The data reported here depict only the localization of the fucosylated forms of xyloglucan that are recognized by CCRC-M1. Recently, Van Hengel and Roberts (2002) reported that a lectin from the eel Anguilla anguilla specifically recognizes fucosylated arabinogalactan glycoproteins but does not recognize fucosylated xyloglucans. Thus, it is now possible to compare the fucosylation of different polymers within individual cells of mur1 plants. Such analyses, especially if specific probes for other fucosylated polymers become available, are likely to yield interesting information as to whether activated sugar nucleotide precursors are available to the biosynthetic machinery within the Golgi for all fucosylated polymers, or are selectively directed to specific synthases, which appear to be specific for each polymer (Faik et al., 2000; Vanzin et al., 2002). Our results reported here add to the growing body of information that suggests that the composition of cell wall polysaccharides, and, hence, the composition and properties of the resulting cell wall, are controlled both at the level of the synthases/transferases responsible for the biosynthesis of the polymers and at the level of the activated sugar nucleotide precursors that supply those synthases.
MATERIALS AND METHODS
Plant Culture Conditions
Seeds of wild-type (Columbia or Wassilewskija ecotype) Arabidopsis were from laboratory stocks. The fut1 mutant of Arabidopsis, which contains a T-DNA insert in the gene encoding the xyloglucan-specific fucosyltransferase (Perrin et al., 2003), was a gift from Kenneth Keegstra (Michigan State University, East Lansing). Generation and characterization of the mur1 mutant was described previously (Reiter et al., 1993). Seeds were surface sterilized, germinated, and grown aseptically as described previously (Freshour et al., 1996). In some cases, seedlings were grown on the same agar medium supplemented with 20 mm l-Fuc (Sigma Chemical Co., St. Louis).
Rosette tissues were collected from 3-week-old plants grown in 10-cm pots of Fafard number 2 soil mix at 23°C in a growth chamber under 16 h of fluorescent illumination (180 μE m−2 s−1) per day. Plants were watered as necessary and received one application of Peter's 20:10:20 peat-lite liquid fertilizer on d 8 after planting. Flowers were collected from mature plants grown in pots and maintained in a greenhouse. Pollen was collected from greenhouse-grown plants and germinated in vitro for 8 to 48 h as described (Pickert, 1988).
Antibodies
The generation of murine monoclonal antibody CCRC-M1 and the partial characterization of its epitope have been described (Puhlmann et al., 1994). Goat anti-mouse IgG (M-8642), goat anti-mouse IgG-gold conjugate (10 nm; G-7652), and goat anti-mouse IgG-FITC conjugate (F-0257) were purchased from Sigma Chemical Co.
Colloidal Gold Conjugation
Colloidal gold (approximately 15 nm) was prepared and conjugated to goat anti-mouse IgG as described previously (Freshour et al., 1996).
Tissue Fixation and Sectioning
Seedlings 4 d and older were initially fixed by flooding the petri plates at room temperature with fixative (50 mm potassium phosphate buffer [pH 6.9] containing 2.5% [w/v] glutaraldehyde). After 1 h, the root tissue was gently removed from the agar and transferred to 1-dram vials containing 2 mL of fresh fixative. Seedlings younger than 4 d postimbibition and vegetative and floral tissues from mature plants were collected and transferred directly to vials containing fixative at room temperature. Subsequent fixation, embedding, and sectioning of tissues was as described previously (Freshour et al., 1996).
Immunocytochemistry
Immunolocalizations (immunofluorescent and immunogold) on tissue sections were carried out as described (Freshour et al., 1996) with the following modifications. Polyethyleneglycol was omitted from all buffers, and the treatment of sections with 0.1 n HCl was skipped. For immunogold labeling, the gold-conjugated secondary antibody was typically used at dilutions of 1:10 or 1:20 (v/v). These changes did not alter immunolocalization patterns observed with the CCRC-M1 antibody in wild-type plants.
Immunofluorescent labeling of intact seedlings was carried out as described (Freshour et al., 1996), except that the unfixed tissue was immersed in reagents in 24-well tissue culture plates (Costar, Cambridge, MA).
The specificity of labeling was tested by pre-incubation of the CCRC-M1 antibody with Fuc (2 m, Sigma Chemical Co.) or with the xyloglucan-derived oligosaccharides XXFG (100 μg mL−1, from sycamore maple [Acer pseudoplatanus] xyloglucan; William York, Complex Carbohydrate Research Center, Athens, GA) or XXJG (100 μg mL−1, from jojoba [Simmondsia chinensis] xyloglucan; W. York, CCRC; see Fry et al., (1993) for explanation of xyloglucan nomenclature). Pre-incubations were carried out for 1 to 2 h before application of the antibody to the tissue.
Distribution of Materials
Upon request, all novel material described in this article will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor. Requests for the CCRC-M1 monoclonal antibody should be directed to the corresponding author.
ACKNOWLEDGMENTS
The authors thank Kenneth Keegstra for the gift of seed of the fut1 mutant of Arabidopsis, Keith Roberts for communication of results before publication, and William Reeves for assistance with the preparation of the figures.
Footnotes
This work was supported by the U.S. Department of Energy (grant nos. DE–FG02–96ER20220 and DE–FG02–95ER20203) and in part by the U.S. Department of Energy-funded Center for Plant and Microbial Complex Carbohydrates (grant no. DE–FG05–93ER20097).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.016444.
LITERATURE CITED
- Bowman JL, editor. Arabidopsis: An Atlas of Morphology and Development. New York: Springer-Verlag; 1994a. pp. 34–37. [Google Scholar]
- Bowman JL, editor. Arabidopsis: An Atlas of Morphology and Development. New York: Springer-Verlag; 1994b. p. 140. [Google Scholar]
- Albersheim P. The walls of growing plant cells. Sci Am. 1975;232(4):80–95. doi: 10.1038/scientificamerican0475-80. [DOI] [PubMed] [Google Scholar]
- Bacic A, Harris PJ, Stone BA. Structure and function of plant cell walls. In: Preiss J, editor. Carbohydrates. Vol. 14. San Diego: Academic Press, Inc.; 1988. pp. 297–371. [Google Scholar]
- Barry P, Prensier G, Grenet E. Immunogold labelling of arabinoxylans in the plant cell walls of normal and bm3 mutant maize. Biol Cell. 1991;71:307–311. [Google Scholar]
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser M-T, Aeschbacher RA. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;119:57–70. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- Bonin CP, Potter I, Vanzin GF, Reiter W-D. The MUR1 gene of Arabidopsis thaliana encodes an isoform of GDP-d-mannose-4,6-dehydratase, catalyzing the first step in the de novo synthesis of GDP-l-fucose. Proc Natl Acad Sci USA. 1997;94:2085–2090. doi: 10.1073/pnas.94.5.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush MS, McCann MC. Pectic epitopes are differentially distributed in the cell walls of potato (Solanum tuberosum) tubers. Physiol Plant. 1999;107:201–213. [Google Scholar]
- Butowt R, Niklas A, Rodriguez-Garcia MI, Majewska-Sawka A. Involvement of JIM13- and JIM8-responsive carbohydrate epitopes in early stages of cell wall formation. J Plant Res. 1999;112:107–116. [Google Scholar]
- Carpita N, Tierney M, Campbell M. Molecular biology of the plant cell wall: searching for the genes that define structure, architecture and dynamics. Plant Mol Biol. 2001;47:1–5. [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Casero PJ, Casimiro I, Knox JP. Occurrence of cell surface arabinogalactan-protein and extensin epitopes in relation to pericycle and vascular tissue development in the root apex of four species. Planta. 1998;204:252–259. [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organisation of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Dolan L, Linstead P, Roberts K. An AGP epitope distinguishes a central metaxylem initial from other vascular initials in the Arabidopsis root. Protoplasma. 1995;189:149–155. [Google Scholar]
- Dolan L, Roberts K. Secondary thickening in roots of Arabidopsis thaliana: anatomy and cell surface changes. New Phytol. 1995;131:121–128. doi: 10.1111/j.1469-8137.1995.tb03061.x. [DOI] [PubMed] [Google Scholar]
- Faik A, Bar-Peled M, DeRocher AE, Zeng W, Perrin RM, Wilkerson C, Raikhel NV, Keegstra K. Biochemical characterization and molecular cloning of an α-1,2-fucosyltransferase that catalyzes the last step of cell wall xyloglucan biosynthesis in pea. J Biol Chem. 2000;275:15082–15089. doi: 10.1074/jbc.M000677200. [DOI] [PubMed] [Google Scholar]
- Fleming AJ, Caderas D, Wehrli E, McQueen-Mason S, Kuhlemeier C. Analysis of expansin-induced morphogenesis on the apical meristem of tomato. Planta. 1999;208:166–174. [Google Scholar]
- Freshour G, Clay RP, Fuller MS, Albersheim P, Darvill AG, Hahn MG. Developmental and tissue-specific structural alterations of the cell-wall polysaccharides of Arabidopsis thaliana roots. Plant Physiol. 1996;110:1413–1429. doi: 10.1104/pp.110.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, York WS, Albersheim P, Darvill A, Hayashi T, Joseleau J-P, Kato Y, Lorences EP, Maclachlan GA, McNeil M et al. An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiol Plant. 1993;89:1–3. [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol. 1994;166:740–754. doi: 10.1006/dbio.1994.1352. [DOI] [PubMed] [Google Scholar]
- His I, Driouich A, Nicol F, Jauneau A, Höfte H. Altered pectin composition in primary cell walls of korrigan, a dwarf mutant of Arabidopsis deficient in a membrane-bound endo-1,4-β-glucanase. Planta. 2001;212:348–358. doi: 10.1007/s004250000437. [DOI] [PubMed] [Google Scholar]
- Knox JP. The use of antibodies to study the architecture and developmental regulation of plant cell walls. Int Rev Cytol. 1997;171:79–120. doi: 10.1016/s0074-7696(08)62586-3. [DOI] [PubMed] [Google Scholar]
- Knox JP, Day S, Roberts K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development. 1989;106:47–56. [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta. 1990;181:512–521. doi: 10.1007/BF00193004. [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991;1:317–326. doi: 10.1046/j.1365-313X.1991.t01-9-00999.x. [DOI] [PubMed] [Google Scholar]
- Lally D, Ingmire P, Tong H-Y, He Z-H. Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell. 2001;13:1317–1331. doi: 10.1105/tpc.13.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J. WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell. 1999;99:473–483. doi: 10.1016/s0092-8674(00)81536-6. [DOI] [PubMed] [Google Scholar]
- Majewska-Sawka A, Münster A, Rodríguez-García MI. Guard cell wall: immunocytochemical detection of polysaccharide components. J Exp Bot. 2002;53:1067–1079. doi: 10.1093/jexbot/53.371.1067. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development. 1996;122:1253–1260. doi: 10.1242/dev.122.4.1253. [DOI] [PubMed] [Google Scholar]
- McCabe PF, Valentine TA, Forsberg LS, Pennell RI. Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell. 1997;9:2225–2241. doi: 10.1105/tpc.9.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MC, Roberts K. Architecture of the primary cell wall. In: Lloyd CW, editor. Cytoskeletal Basis of Plant Growth and Form. London: Academic Press Ltd.; 1991. pp. 109–129. [Google Scholar]
- McCartney L, Knox JP. Regulation of pectic polysaccharide domains in relation to cell development and cell properties in the pea testa. J Exp Bot. 2002;53:707–713. doi: 10.1093/jexbot/53.369.707. [DOI] [PubMed] [Google Scholar]
- McNeil M, Darvill A, Fry SC, Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Nickle TC, Meinke DW. A cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality, incomplete cell walls, and excessive callose accumulation. Plant J. 1998;15:321–332. doi: 10.1046/j.1365-313x.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- Orfila C, Seymour GB, Willats WGT, Huxham IM, Jarvis MC, Dover CJ, Thompson AJ, Knox JP. Altered middle lamella homogalacturonan and disrupted deposition of (1→5)-α-l-arabinan in the pericarp of Cnr, a ripening mutant of tomato. Plant Physiol. 2001;126:210–221. doi: 10.1104/pp.126.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell. 1991;3:1317–1326. doi: 10.1105/tpc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell RI, Roberts K. Sexual development in the pea is presaged by altered expression of arabinogalactan protein. Nature. 1990;344:547–549. [Google Scholar]
- Perrin RM, Jia Z, Wagner TA, O'Neill MA, Sarria R, York WS, Raikhel NV, Keegstra K (2003) Analysis of xyloglucan fucosylation in Arabidopsis. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Pickert M. In vitro germination and storage of trinucleate Arabidopsis thaliana (L.) pollen grains. Arabidopsis Info Serv. 1988;26:39–42. [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AG, Hahn MG. Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1→2)-linked fucosyl-containing epitope. Plant Physiol. 1994;104:699–710. doi: 10.1104/pp.104.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayon C, Cabanes-Macheteau M, Loutelier-Bourhis C, Salliot-Maire I, Lemoine J, Reiter W-D, Lerouge P, Faye L. Characterization of N-glycans from Arabidopsis. Application to a fucose-deficient mutant. Plant Physiol. 1999;119:725–733. doi: 10.1104/pp.119.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W-D, Chapple CCS, Somerville CR. Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science. 1993;261:1032–1035. doi: 10.1126/science.261.5124.1032. [DOI] [PubMed] [Google Scholar]
- Rhee SY, Somerville CR. Tetrad pollen formation in quartet mutants of Arabidopsis thaliana is associated with persistence of pectic polysaccharides of the pollen mother cell wall. Plant J. 1998;15:79–88. doi: 10.1046/j.1365-313x.1998.00183.x. [DOI] [PubMed] [Google Scholar]
- Rose JKC, O'Neill MA, Albersheim P, Darvill A. The primary cell walls of higher plants. In: Ernst B, Sinaÿ P, Hart G, editors. Oligosaccharides in Chemistry and Biology: A Comprehensive Handbook. London: Wiley-VCH; 2000. pp. 783–808. [Google Scholar]
- Šamaj J, Braun M, Baluška F, Ensikat H-J, Tsumuraya Y, Volkmann D. Specific localization of arabinogalactan-protein epitopes at the surface of maize root hairs. Plant Cell Physiol. 1999;40:874–883. [Google Scholar]
- Serpe MD, Muir AJ, Driouich A. Immunolocalization of β-d-glucans, pectins, and arabinogalactan-proteins during intrusive growth and elongation of nonarticulated laticifers in Asclepias speciosa Torr. Planta. 2002;215:357–370. doi: 10.1007/s00425-002-0756-y. [DOI] [PubMed] [Google Scholar]
- Shevell DE, Kunkel T, Chua N-H. Cell wall alterations in the Arabidopsis emb30 mutant. Plant Cell. 2000;12:2047–2059. doi: 10.1105/tpc.12.11.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Lynch M. Fused organs in the adherent1 mutation in maize show altered epidermal walls with no perturbations in tissue identities. Planta. 1998;206:184–195. [Google Scholar]
- Stacey NJ, Roberts K, Carpita NC, Wells B, McCann MC. Dynamic changes in cell surface molecules are very early events in the differentiation of mesophyll cells from Zinnia elegans into tracheary elements. Plant J. 1995;8:891–906. [Google Scholar]
- Van Aelst AC, Van Went JL. Ultrastructural immuno-localization of pectins and glycoproteins in Arabidopsis thaliana pollen grains. Protoplasma. 1992;168:14–19. [Google Scholar]
- Van Hengel AJ, Roberts K. Fucosylated arabinogalactan-proteins are required for full root cell elongation in Arabidopsis. Plant J. 2002;32:105–113. doi: 10.1046/j.1365-313x.2002.01406.x. [DOI] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, Raikhel NV, Keegstra K, Reiter W-D. The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proc Natl Acad Sci USA. 2002;99:3340–3345. doi: 10.1073/pnas.052450699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicré M, Jauneau A, Knox JP, Driouich A. Immunolocalization of β-(1→4) and β-(1→6)-d-galactan epitopes in the cell wall and Golgi stacks of developing flax root tissues. Protoplasma. 1998;203:26–34. [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science. 1997;277:1113–1116. doi: 10.1126/science.277.5329.1113. [DOI] [PubMed] [Google Scholar]
- Willats WGT, Steele-King CG, Marcus SE, Knox JP. Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant J. 1999;20:619–628. doi: 10.1046/j.1365-313x.1999.00629.x. [DOI] [PubMed] [Google Scholar]
- Zablackis E, Huang J, Müller B, Darvill AG, Albersheim P. Characterization of the cell wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 1995;107:1129–1138. doi: 10.1104/pp.107.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablackis E, York WS, Pauly M, Hantus S, Reiter W-D, Chapple CCS, Albersheim P, Darvill A. Substitution of l-fucose by l-galactose in cell walls of Arabidopsis mur1. Science. 1996;272:1808–1810. doi: 10.1126/science.272.5269.1808. [DOI] [PubMed] [Google Scholar]