Abstract
Previous studies implicated the involvement of a heterotrimeric G protein in red (R) and far-red (FR) light signal transduction, but these studies utilized pharmacological or gain-of-function approaches and, therefore, are indirect tests. Here, we reexamine the role of the single canonical heterotrimeric G protein in R and FR control of hypocotyl growth using a loss-of-function approach. Single- and double-null mutants for the GPA1, AGB1 genes encoding the alpha and beta subunit of the heterotrimeric G protein, respectively, have wild-type sensitivity to R and FR. Ectopic overexpression of wild type and a constitutive active form of the alpha subunit and of the wild-type beta subunit had no effect that can be unequivocally attributed to altered R and FR responsiveness. These results preclude a direct role for the heterotrimeric G complex in R and FR transduction in Arabidopsis leading to growth control in the hypocotyl.
The classic example of the molecular coupling of signals by a heterotrimeric G protein to a downstream effector is vision in animals where the alpha subunit of the cognate heterotrimeric complex, transducin, couples the activated heptahelical membrane receptor rhodopsin to its cGMP phosphodiesterase effector in rod photoreceptor cells (Baylor, 1996). Plant cells are also light sensitive, especially in the red (R)/far-red (FR) light spectral region due to its highly light-sensitive family of photoreceptors called phytochrome. Therefore, an obvious question has been whether phytochrome light perception is similarly coupled by a heterotrimeric G protein to an unidentified downstream effector. Two influential papers of the early 1990s suggested that it is (Bowler et al., 1994; Neuhaus et al., 1993). In these elegant studies, some phenotypes of a tomato (Lycopersicon esculentum) phytochrome mutant could be rescued to wild type by pertussis and cholera toxins, agents that stabilize the activated form of the G protein subunit by different means. Furthermore, microinjection of cGMP induced some phytochrome-mediated events in the dark. These observations led these authors to conclude that a heterotrimeric G protein was positioned downstream of phytochrome in the light signal transduction pathway and upstream of a cGMP-mediated step, in analogy to light perception in animals. Several other labs used pharmacological approaches in different systems and came to the same conclusion. Electroporation of GDPβS blocked R-induced protoplast swelling, whereas GTPγS induced swelling in darkness (Bosson et al., 1990). Cholera toxin was shown to increase the steady-state mRNA levels of the light-regulated gene, CAB (Romero and Lam, 1993).
More recently, Okamota and colleagues took a gain-of-function approach to test this hypothesis and concluded with all previous authors that a heterotrimeric G protein is involved in phytochrome-mediated signal transduction (Okamota et al., 2001). The authors reported that Arabidopsis ectopically overexpressing the alpha subunit of the heterotrimeric G protein, regardless of the Gα activation state, was hypersensitive to R and FR.
Because the previous pharmacological and gain-of-function studies are indirect tests for the role of a heterotrimeric G protein in light signaling in plants, we chose to examine the light sensitivities of G protein null mutants in Arabidopsis. Arabidopsis has a single gene encoding a canonical alpha subunit of a heterotrimeric G protein (GPA1; Ma, 1994), a single beta subunit (AGB1) and possibly two gamma subunits (Mason and Botella, 2000, 2001). The modeled structure of the Arabidopsis heterotrimeric complex is robustly supported by fold recognition and compactability tests (Ullah et al., 2003).
The etiolated and light-grown phenotypes of these mutants have been described extensively (Ullah et al., 2001; 2003). The main defect can be summarized as reduced control of cell division throughout development that manifests as fewer cells in many organs, altered lateral root formation, and altered apical dominance. The gpa1 and agb1 null mutants do not share phytochrome loss-of-function mutant phenotypes.
RESULTS AND DISCUSSION
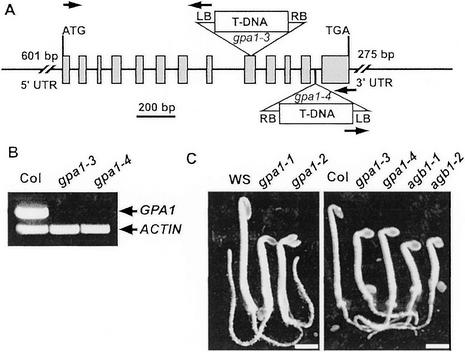
This study introduces two new alleles of gpa1. As shown in Figure 1, gpa1-3 and gpa1-4 are T-DNA insertion alleles that disrupt GPA1 expression. Both of these Col alleles represent transcript null mutants (Fig. 1B). The etiolated phenotype of gpa1-3 and gpa1-4 is identical to the previously reported gpa1-1 and gpa1-2 Ws mutant phenotype (Fig. 1C).
Figure 1.
Col (Columbia) null alleles of gpa1. A, Two new gpa1 alleles in the Col background have T-DNA insertions in the ninth exon and 12th intron as shown. Positions of primers used to identify the mutants are indicated by arrows. B, Reverse transcribed-PCR of cDNA prepared from Col and the two mutants is described in “Materials and Methods.” C, Phenotype of wild-type (Wassilewskija [Ws] and Col) and G protein mutants. The genotypes of gpa1-1, gpa1-2, agb1-1, and agb1-2 are described elsewhere. RB, T-DNA right border; LB, T-DNA left border.
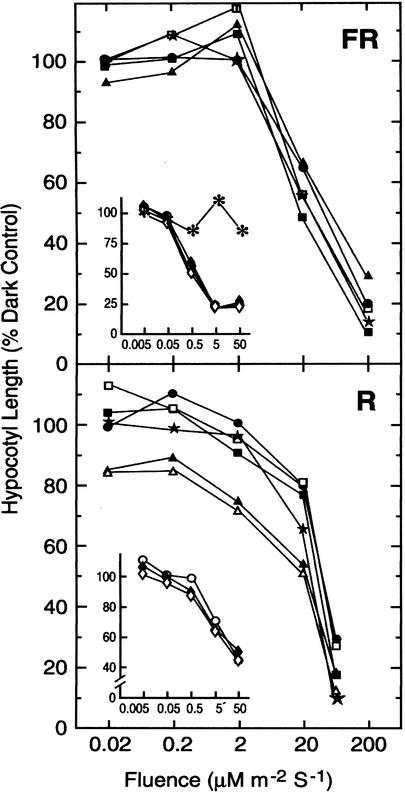
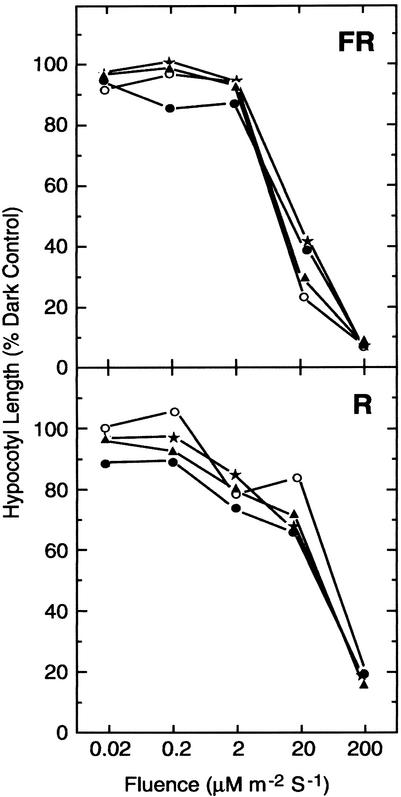
The R and FR fluence responsiveness of gpa1 and agb1 mutants were determined exactly as described by Reed et al. (1998). All mutants responded to the full extent of the wild-type response across a broad fluence range of R and FR light (Fig. 2). Null alleles of gpa1 displayed wild-type R and FR sensitivity in both Col (Fig. 2) and Ws (Fig. 2, insets) backgrounds. To determine if there is compensating effects of the two subunit genes, the double mutant was analyzed and found to share identical R and FR fluence sensitivity (Fig. 3). These results indicate that the canonical Arabidopsis heterotrimeric G protein complex does not directly couple phytochrome signaling.
Figure 2.
Fluence response for G protein mutants. The FR (top) and R (bottom) fluence responsiveness for wild types: Col (black circle) and Ws (white circle); for G protein mutants: gpa1-1 (solid diamond), gpa1-2 (white diamond), gpa1-3 (solid square), gpa1-4 (white square), agb1-1 (solid triangle), and agb1-2 (white triangle); for a line constituently expressing an active form (Q222L mutant) of GPA1 (designated GPA1*, star symbol), and for a phytochrome mutant (phyA-211, asterisk) is described in “Materials and Methods.” Insets, Responsiveness of the Ws gpa1 alleles is similar to the Col. gpa1 alleles. A second line expressing the Q222L mutant had the same light-responsiveness phenotype (not shown).
Figure 3.
Fluence response for gpa1 and agb1 single and double mutants. The FR (top) and R (bottom) fluence responsiveness for Col (white circle), gpa1-4 (solid triangle), agb1-2 (solid circle), and the gpa1-4,agb1-2 double mutant (star symbol) is described in “Materials and Methods.”
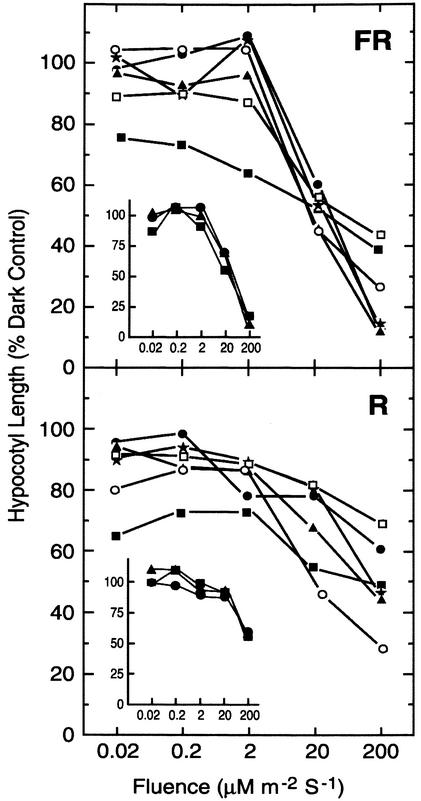
We replicated the design of published gain-of-function experiments using independently engineered inducible expression of native GPA1 (for description, see Ullah et al., 2003). In addition, we generated plants that constitutively express a constitutively active (Q222L mutant) GPA1 and plants that inducibly express AGB1. Induction of GPA1 and AGB1 was obtained using the dexamethasone promoter, and we show no effect of dexamethasone on light responsiveness in wild-type or vector-only plants as indicated by similar light fluence/response slopes. The amount of dexamethasone used in this study induced gene expression of GPA1 and AGB1 in the range of 6- to 10-fold (Ullah et al., 2003). Our study included two independent lines for each construct. With one exception, overexpression of G protein subunits had no effect on the response extent and fluence range of R and FR perception, again precluding a direct role for this G protein in R and FR perception (Fig. 3). In one of two lines (Fig. 4, H2), inducible expression of the wild-type GPA1 decreased the slope, suggesting a decrease in light sensitivity. These results are at variance to the results of Okamota et al. (2001), who reported an increase in light sensitivity upon expression of either the wild-type GPA1 or the Q222L mutant.
Figure 4.
Fluence response for Arabidopsis lines expressing G protein subunits (Col). The FR (top) and R (bottom) fluence responsiveness for wild type (asterisk), a vector-only control (black circle), GPA1 overexpression lines (solid square, line H2; and white square, line C3), and AGB1 overexpression lines (white circle, line 6-4; and solid triangle, line 8-3). Insets, Indicated fluence response curves in the absence of induction (i.e. no dexamethasone).
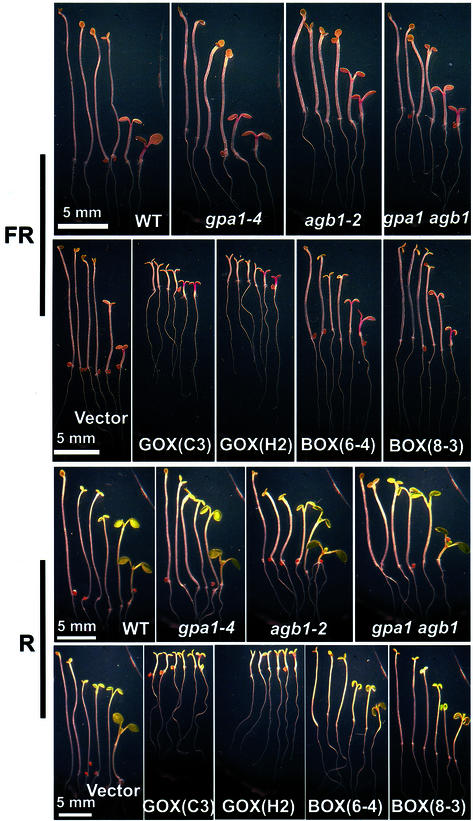
The phenotypes of the mutants and the overexpressing lines in R and FR are shown in Figure 5. Note that overexpression of GPA1 results in dramatic shortening of the hypocotyl at all fluences and opening but not expansion of the cotyledons in darkness. R and FR induces hook opening, R induces cotyledon expansion, and FR induces anthocyanin production in G protein mutants to an apparent wild-type degree. Thus, with regard to other photomorphogenic responses controlled by phytochrome, the G protein mutants are R and FR responsive.
Figure 5.
Fluence response of G protein mutants and lines overexpressing G protein subunits. The FR (top two panels) and R (bottom two panels) fluence responsiveness is described in “Materials and Methods.” A representative seedling for each fluence is shown. Arrow indicates from left to right: dark, 0.02, 0.2, 2, 20, and 200 μm m−2 s−1. Magnification is the same and comparable for each panel. GOX and BOX indicate GPA1 and AGB1 overexpressing plants, respectively, with the transgenic line designation indicated in parentheses.
Phytochrome controls transcriptional activity (Schäfer and Bowle, 2002); therefore, we cannot exclude the possibility that a canonical G protein couples light perception by phytochrome to changes in transcription. However, as shown in Figure 5, photomorphogenetic changes in seedling development mediated by R and FR appear to be normal in the G protein mutants; thus, if phytochrome control of transcription is coupled by a canonical G protein, then one must argue that these particular transcriptional changes are not directly involved in controlling hypocotyl growth, hook opening, cotyledon expansion, and anthocyanin production. Published evidence does not support this argument (Schäfer and Bowle, 2002), thus favoring the conclusion that G proteins also do not couple light perception to transcriptional control during photomorphogenesis, at least not directly.
At a superficial level, gpa1 and agb1 (Fig. 1C) might appear to be photomorphogenic mutants because their hypocotyls are transiently shorter than wild type, and the hooks are partially open in the dark. However, this can be ascribed entirely to a defect in cell division, rather than cell elongation, which is the case for the constitutive photomorphogenic (COP) mutants (Schwechheimer and Deng, 2000). The reduction in cell number in G protein mutants is compensated in many cases by cell elongation to achieve nearly normal morphology. Nonetheless, G protein mutants have altered sensitivities to several hormones (Ashikari et al., 1999; Fujisawa et al., 1999; Ueguchi-Tanaka et al., 2000; Ullah et al., 2001, 2002, 2003; Wang et al., 2001). The reduced perception of some hormones and the complete loss in others in the G protein mutants must impact cellular responsiveness to many signals. We offer this as an explanation of why G protein mutants share some COP phenotypes but are otherwise wild type in their sensitivity to R and FR.
Although Arabidopsis has a single canonical Gα-subunit gene, there are three other genes that share some deduced amino acid sequence identity to GPA1, and one of these has been shown to bind GTP. These are described as extra-large G proteins because they are approximately twice the size of classical Gα (Assmann, 2002). Could one or more of these subunits be the primary coupler of light perception in Arabidopsis? We think not, at least not by the classical mechanism. The extra-large Gα proteins have N-terminal extensions that are incompatible with the conserved heterotrimeric G protein complex. Modeling and structural studies of canonical Gα indicate that modifications of the N terminus disrupt interaction with Gβγ; thus, if the extra-large Gα proteins do interact with a β-propeller protein, it probably is not a classical Gβ-subunit. Furthermore, a functional interaction with the single Gβ-subunit in Arabidopsis is inconsistent with these extra-large G alpha mutants operating in the light pathway because we show here that agb1 mutants have wild-type R and FR sensitivity.
In conclusion, because loss-of-function in the single-copy genes encoding canonical Gα and Gβ subunits does not result in altered R and FR sensitivity, the predominant theory of the last decade that phytochrome control of seedling photomorphogenesis involves a heterotrimeric G protein is not supported.
MATERIALS AND METHODS
Plant Material
gpa1-1, gpa1-2, and agb1-2 are described by Ullah et al. (2003). agb1-1 is described by Lease et al. (2001). gpa1-3 and gpa1-4 were obtained from the Salk Institute sequence-indexed insertion mutant collection (J.R. Ecker, unpublished data). Plants homozygous for gpa1-3 and gpa1-4, respectively, were isolated, and the insertion was confirmed by sequencing at the University of North Carolina (Chapel Hill). The GPA1 transcript levels in gpa1-3 and gpa1-4 mutants were checked by reverse transcribed-PCR. Total RNAs were isolated from seedlings grown in light for 10 d. Arabidopsis GPA1 primers (5′-ATGGGCTTACTCTGCAGTA-3′ and 5′-TCATAAAAGGCCAGCCTCCAGT-3′) and Arabidopsis actin primers (5′-GTTGGGATGAACCAGAAGGA-3′ and 5′- GAACCACCGATCCAGACACT-3′) were added together in each PCR reaction.
Fluence Response Assays
Fluence response was determined as described by Reed et al. (1998). In brief, 12 to 20 sterilized seeds were place in a row on plates containing one-half-strength Murashige and Skoog plus 1% (w/v) Suc and vernalized for 2 d. Plates were stacked with neutral density filters between plates and held vertically before R or FR Q2200 light diode sources (Quantum Devices, Inc., Barneveld, WI) at 22°C for 96 h. Dark treatments were plates in the stack covered in foil. Hypocotyl lengths were visualized by microscopy and measured by a calibrated scale, and the lengths of a minimum of 12 hypocotyls were averaged. The measurements were done single blindly. Response is reported as the average length of the light-treated hypocotyl divided by the average length of the dark hypocotyl, times 100. Each experiment was repeated at least three times. For induction of the G protein transgenes, 500 nm dexamethasone was included in the plates.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We thank Ms. Khou Xiong and Hongwei Liu, who measured over 8,000 hypocotyls.
Footnotes
This work was supported by the National Institutes of Health (grant no. GM65989–01 to A.M.J.) and by the National Science Foundation (grant no. MCB–0209711 to A.M.J.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017624.
LITERATURE CITED
- Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A. Rice Gibberellin-insensitive Dwarf Gene Dwarf1 encodes the α subunit of GTP-binding Protein. Proc Natl Acad Sci USA. 1999;96:10284–10289. doi: 10.1073/pnas.96.18.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell. 2002;14:S355–S373. doi: 10.1105/tpc.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. How photons start vision. Proc Natl Acad Sci USA. 1996;93:560–565. doi: 10.1073/pnas.93.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosson ME, Kendrick RE, Vrendenberg WJ. The involvement of a G-protein in phytochrome-regulated, Ca2+-dependent swelling of etiolated wheat protoplasts. Physiol Plant. 1990;80:55–62. [Google Scholar]
- Bowler C, Neuhaus G, Yamagata H, Chua NH. Cyclic GMP and calcium mediated phytochrome phototransduction. Cell. 1994;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Takuji S, Asahi T, Yukimoto I. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism in rice. Proc Natl Acad Sci USA. 1999;96:7575–7580. doi: 10.1073/pnas.96.13.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC. A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell. 2001;13:2631–2641. doi: 10.1105/tpc.010315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. GTP-binding proteins in plants: new members of an old family. Plant Mol Biol. 1994;26:1611–1636. doi: 10.1007/BF00016493. [DOI] [PubMed] [Google Scholar]
- Mason MG, Botella JR. Completing the heterodimer: isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc Natl Acad Sci USA. 2000;97:14784–14788. doi: 10.1073/pnas.97.26.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR. Isolation of a novel G-protein gamma-subunit from Arabidopsis thaliana and its interaction with Gbeta. Biochim Biophys Acta. 2001;1520:147–153. doi: 10.1016/s0167-4781(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Neuhaus G, Bowler C, Kern R, Chua NH. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993;73:937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Okamota H, Matsui M, Deng XW. Overexpression of the heterotrimeric G-protein alpha subunit enhances phytochrome-mediated inhibition of hypocotyl elongation. Plant Cell. 2001;13:1639–1652. doi: 10.1105/TPC.010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Elumalai RP, Chory J. Suppressors of an Arabidopsis thaliana phyB mutation identify genes that control light signaling and hypocotyl elongation. Genetics. 1998;148:1295–1310. doi: 10.1093/genetics/148.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LC, Lam E. Guanine nucleotide binding protein involvement in early steps of phytochrome-regulated gene expression. Proc Natl Acad Sci USA. 1993;90:1465–1469. doi: 10.1073/pnas.90.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer E, Bowle C. Phytochrome-mediated photoperception and signal transduction in higher plants. EMBO Rep. 2002;3:1042–1048. doi: 10.1093/embo-reports/kvf222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Deng XW. The COP/DET/FUS proteins-regulator of eukaryotic growth and development. Semin Cell Dev Biol. 2000;11:495–503. doi: 10.1006/scdb.2000.0203. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M. Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Natl Acad Sci USA. 2000;97:11638–11643. doi: 10.1073/pnas.97.21.11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM. The beta subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell. 2003;15:393–409. doi: 10.1105/tpc.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Wang S, Jones AM. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 2002;129:897–907. doi: 10.1104/pp.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science. 2001;292:2066–2069. doi: 10.1126/science.1059040. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channels and abscissic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]