Abstract
In flowering plants, maternal seed integument encloses the embryo and the endosperm, which are both derived from double fertilization. Although the development of these three components must be coordinated, we have limited knowledge of mechanisms involved in such coordination. The endosperm may play a central role in these mechanisms as epigenetic modifications of endosperm development, via imbalance of dosage between maternal and paternal genomes, affecting both the embryo and the integument. To identify targets of such epigenetic controls, we designed a genetic screen in Arabidopsis for mutants that phenocopy the effects of dosage imbalance in the endosperm. The two mutants haiku 1 and haiku 2 produce seed of reduced size that resemble seed with maternal excess in the maternal/paternal dosage. Homozygous haiku seed develop into plants indistinguishable from wild type. Each mutation is sporophytic recessive, and double-mutant analysis suggests that both mutations affect the same genetic pathway. The endosperm of haiku mutants shows a premature arrest of increase in size that causes precocious cellularization of the syncytial endosperm. Reduction of seed size in haiku results from coordinated reduction of endosperm size, embryo proliferation, and cell elongation of the maternally derived integument. We present further evidence for a control of integument development mediated by endosperm-derived signals.
In flowering plants, the two female gametes, the egg cell and the central cell, are fertilized by one of the two male gametes delivered by the pollen tube. The zygotic product of the fusion of one male gamete with the egg cell develops into the embryo of the daughter plant. The fertilized central cell develops as the endosperm that nurtures embryo development. In most species, endosperm development is initiated by a proliferative syncytial phase accompanied by cell growth that generates a large multinucleate cell (Olsen, 2001; Berger, 2003). This syncytium is partitioned into individual cells by a specific type of cytokinesis called cellularization. In cereal species, the cellular endosperm stores the reserves of the seed during a phase marked by endoreduplication. Although the endosperm does not store the reserves of the seed in Arabidopsis, it most probably controls the flux of nutrients delivered by the vascular tissue of the mother to the embryo and protects the embryo from physical and osmotic stresses.
Because the embryo is surrounded by the endosperm, which, in turn, is enclosed within the ovule integument, these three structures must coordinate their development to produce a mature seed of the appropriate size. The endosperm plays a central role in the control of seed size as indicated by a series of experiments in Arabidopsis and maize (Zea mays), where the dosage balance between maternal and paternal genomes was perturbed (Lin, 1984; Kermicle and Allemand, 1990; Scott et al., 1998). In most flowering plants, the endosperm contains two maternal copies and one paternal copy of the genome (2m/1p). In Arabidopsis, increased paternal dosage in endosperm causes an increase of seed size (Scott et al., 1998), whereas increased maternal dosage has the opposite effect. Dosage imbalance has been reported to affect primarily the timing of cellularization of the endosperm and its degree of proliferation. In turn, the amount of endosperm produced would affect proliferation of the embryo and the size of the mature seed. These studies suggest that the endosperm is a key player in the control of seed size through epigenetic controls.
Mutants that phenocopy the effects of m/p dosage imbalance might allow identification of genes, the expression of which is affected by m/p dosage imbalance in endosperm. Paternal excess in the endosperm is at least partially phenocopied by mutations in the Polycomb Group genes of the FIS class (Grossniklaus et al., 1998; Luo et al., 1999; Ohad et al., 1999). Until now, despite phenotypical similarities, no molecular link has been made between imbalances in the m/p dosage and the FIS genes. In contrast to paternal excess, no mutation at single loci that phenocopy maternal excess in the m/p dosage has been isolated. However, DNA methylation is likely involved because pollination of wild type (WT) with transgenic pollen carrying a maintenance DNA methyltransferase 1 antisense construct (MET1 a/s line; Finnegan et al., 1996) causes precocious endosperm cellularization and seed size reduction similar to maternal excess (Adams et al., 2000; Luo et al., 2000). We have screened for such a phenotype and report the isolation of mutants at two loci, haiku 1 and haiku 2. These mutants are sporophytic recessive and cause premature arrest of endosperm growth, which triggers precocious cellularization, restricts cell proliferation in the embryo, and limits cell elongation of the maternally derived seed integument. Our results provide new evidence for feedback communication from the endosperm to the mother plant and identify single loci potentially involved in parental dosage compensation.
RESULTS
Screens for Endosperm Mutants with Viable Seeds and Genetic Characterization of the haiku Mutants
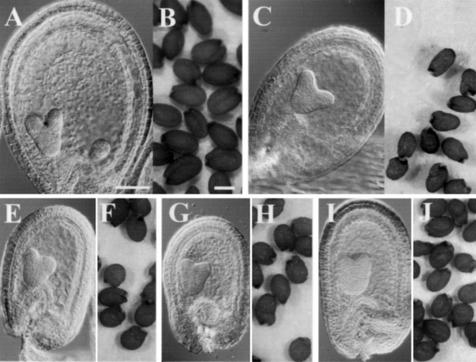
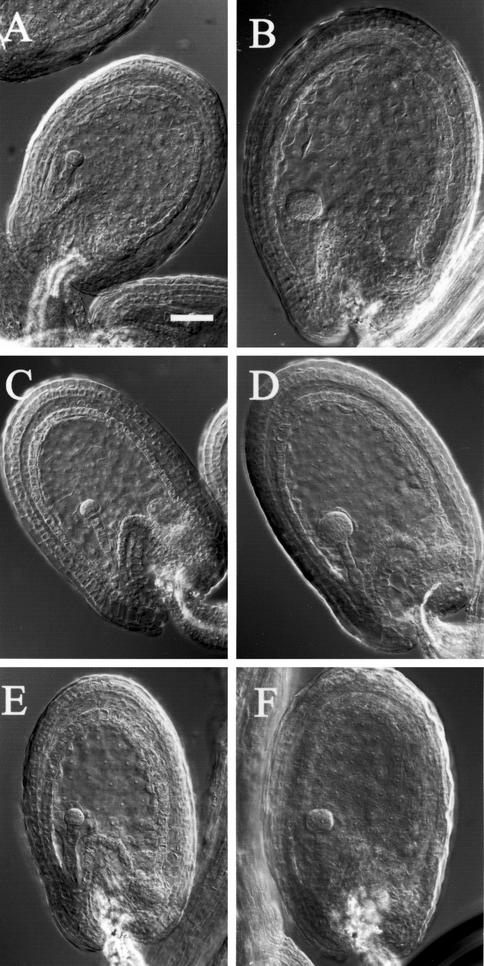
Plant M2 families were screened on a cytological basis for abnormal endosperm development. Cleared seeds were observed at stages ranging from late-heart to mid-torpedo embryo stage, when the endosperm has passed the initial syncytial proliferation stage and is cellularized (Fig. 1A). At these stages, seeds from WT × MET1 a/s crosses show a remarkable reduction of size of the endosperm and a slight delay in embryo development (Fig. 1C), and as reported previously, mature dried seeds are of reduced size in comparison with WT (Adams et al., 2000; Luo et al., 2000; Fig. 1, B and D). We isolated two mutant lines that produce seeds with a phenotype similar to seeds from WT × MET1 a/s crosses (Fig. 1, E–H). The two lines were named haiku 1 (iku1) and haiku 2 (iku2), reminiscent of the aphoristic literary form of Japanese poetry. In both iku1/iku1 and iku2/iku2, the size of the seed is reduced by 25% in length and 14% in width (Table I). As a consequence, iku seeds are more spherical than oblong, as compared with the WT (Fig. 1, B, F, and H). Parallel to the reduction in seed size, the mass of the seed is reduced by 32% in iku mutants (Table I). In comparison with the WT seed, the growth of the embryo and the size of the endosperm are reduced in iku seeds (Fig. 1, A, E, and G). A variable small proportion of iku seeds (less than 10%) of very reduced size collapse at maturation and die. In most iku seeds, the embryo reaches the bent-cotyledon stage, and seed maturation (seed browning and drying) occurs as in WT (Fig. 1, F and H). These small seeds are viable and germinate like WT. Seedlings homozygous for iku develop into morphologically normal plants producing small seeds. The number of seed produced per silique in selfed homozygous iku plants is similar to WT. Except for seed size, we did not detect any morphological difference between iku and WT plants. Therefore, we conclude that iku1 and iku2 reduce seed volume but do not affect plant morphogenesis.
Figure 1.
Morphology of seeds from a cross of WT × METI a/s compared with iku mutants. Nomarski micrographs of cleared seeds at the embryo torpedo stage of WT (A), crosses of WT × METI a/s (C), iku1 (E), iku2 (G), and iku1/iku1;iku2/iku2 (I) show reduced size of the seed in WT × METI a/s and iku mutant. The endosperm is of reduced size, and the embryo does not appear affected. Mature seeds from WT plants (B), crosses of WT × METI a/s (D), and homozygous mutants iku1 (F), iku2 (H), and iku1;iku2 (J). Scale bars = 85 μm in A, C, E, G, and I, and scale bars = 250 μm in B, D, F, H, and J.
Table I.
Morphometric measurements of dimensions and mass of mature seeds
| Wild Type | iku1/iku1 | iku2/iku2 | iku1/iku1, iku2/iku2 | |
|---|---|---|---|---|
| Seed length (μm) | 440 | 328 | 328 | 312 |
| n, se | 220, 40 | 228, 32 | 199, 32 | 110, 30 |
| Seed width (μm) | 270 | 232 | 232 | 225 |
| n, se | 218, 16 | 225, 24 | 197, 24 | 110, 30 |
| Seed weight (μg) | 13.6 | 9.1 | 9.3 | 9.3 |
| n, se | 1,019, 0.1 | 1,640, 0.1 | 1,440, 0.1 | 970, 0.2 |
Selfed iku1 and iku2 heterozygous mutants produce 24.5% (n = 800; se = 0.2) and 25.8% (n = 750; se = 0.3) small seeds, respectively. The progeny of backcrosses for each mutant segregate 50% of plants heterozygous for the mutation (n = 240 plants). This shows that iku1 and iku2 are sporophytic recessive mutations and, thus, affect the development of the embryo, the endosperm, or both. Crosses to test for genetic complementation between homozygous iku1 and iku2 plants produce 100% WT seeds (n = 700 seeds). Hence, iku1 and iku2 are mutated in different loci. This is confirmed by mapping analyses that place iku1 1.6 cM south of the marker Cop1a on chromosome 2 and iku2 4.4 cM north of the marker ArLIM15 on chromosome 3. In subsequent screens of gamma ray-mutagenized populations, two alleles of iku1 and one allele of iku2 were isolated. All iku alleles show identical phenotypes. Double-mutant plants homozygous for iku1 and iku2 mutations produce seed that by morphology and size are not significantly different from those of single mutants (Fig. 1, I and J; Table I). Thus, both haiku mutations are likely to be loss-of-function mutations affecting two genes active in the same genetic pathway.
Development of the Seed in haiku Mutants
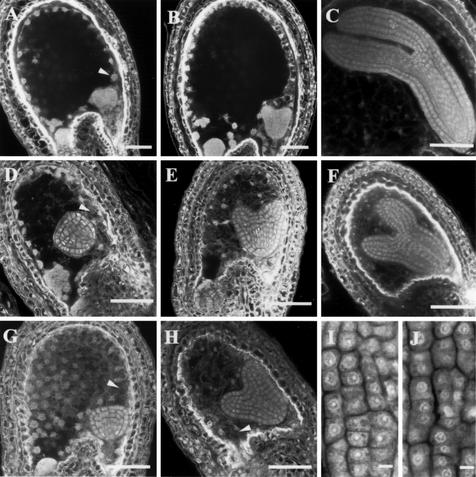
Because the earliest defect reported for seed development in WT × MET1 a/s crosses is a precocious cellularization of the endosperm (Adams et al., 2000), we characterized in detail the development of the endosperm in seeds of selfed heterozygous iku plants (Fig. 2, n = 120 seeds). Identical results were obtained when selfed homozygous iku plants were compared with WT (n = 200). Multiple aspects of the development of the endosperm are affected in iku mutants. In the WT endosperm, at the beginning of the embryo triangular stage, the syncytial endosperm that has undergone a series of nuclear division is partitioned into individual cells, a process referred to as cellularization (Boisnard-Lorig et al., 2001). This process is initiated in the micropylar endosperm that surrounds the embryo at the anterior pole (Fig. 2A). In the peripheral endosperm, which comprises the central large vacuole, cellularization occurs after the eighth mitotic cycle (stage IX; Sørensen et al., 2002; Fig. 2B). In contrast to the WT, the iku endosperm undergoes cellularization in a single step during stage VIII in the anterior and in the peripheral endosperm (Fig. 2, D and G). Additional endosperm cell layers are produced by conventional cell division in iku as in the WT. However, the number of such divisions is reduced by one-half in iku mutant endosperm (Fig. 2, C and F). In less than 10% of the iku seeds the endosperm is cellularized at endosperm stage VII (not shown). In these seeds, the embryo reaches the globular embryo stage, does not develop further, and dies at seed maturation.
Figure 2.
Cytology of iku seeds. Confocal sections of seeds of WT (A–C), iku1 (D–F), and iku2 (G and H) at successive embryo stages: triangular (A, D, and G), mid-heart (B, E, and H), and bent cotyledon (C and F). At triangular embryo stage, the endosperm is completely cellularized in iku1 and iku2 seed (D and G, arrowheads), in contrast to the WT (A, arrowhead). Reduction of the size of the posterior cyst in iku seeds is observed after the triangular stage. Embryo morphology of WT and iku mutant is similar (B, E, and H), although embryo growth is reduced after early torpedo stage (C and F). Embryo cotyledon cells in WT (I) and in iku1 (J) at late torpedo stage show similar size. Scale bars = 50 μm in A through H, and scale bars = 7.5 μm in I and J.
In contrast to endosperm development, embryo development shows no obvious deviation from WT until the late-heart embryo stage in most iku seeds, implying that the regular cell divisions associated with the establishment of the apical-basal axis, the tissue layers, and the bilateral symmetry are normal (Fig. 2, B, E, and H). After early torpedo embryo stage, unlike WT embryos (Fig. 2C), iku embryos do not undergo increased cell proliferation in cotyledon primordia and in the hypocotyl (Fig. 2F). Cell size is similar between iku and WT embryos (Fig. 2, I and J), which indicates that reduction of the embryo size in iku results from reduction in the total number of cells.
In conclusion, the iku mutations cause a precocious onset of endosperm cellularization, reduce proliferation of the cellularized endosperm, and cause a reduction of the embryo proliferation after the early torpedo stage.
Impact of Precocious Cellularization of Endosperm in haiku Mutants on Seed Size
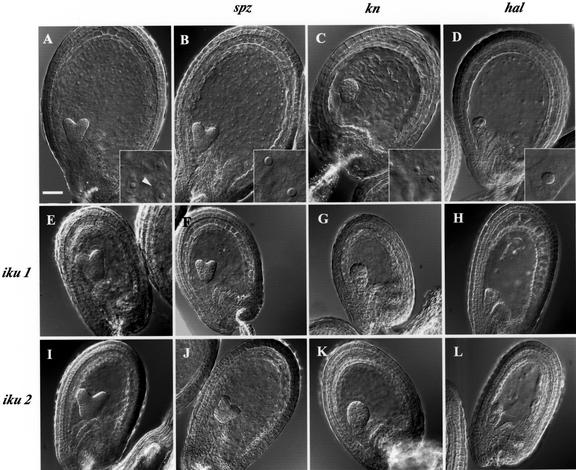
We hypothesized that, in iku seeds, precocious endosperm cellularization contributed to the reduction of endosperm and embryo proliferation. To test this hypothesis, the iku mutants were crossed with mutants where endosperm cellularization does not occur. If precocious cellularization was a major cause in the reduction of seed size in iku, the double mutant without cellularized endosperm should show restoration of a larger seed size. Alternatively, the double-mutant seed would be of the size of iku seed but with non-cellularized endosperm. In iku/iku backgrounds, we introduced sporophytic recessive mutations that cause defects of cellularization: kn (knolle), spz (spätzle), and hallimasch, respectively (Sørensen et al., 2002). The mutant affects both cytokinesis in the embryo and cellularization of the endosperm as a result of the loss of function of the syntaxin KNOLLE, targeted to the cell plate (Fig. 3C; Lukowitz et al., 1996; Lauber et al., 1997). Double-mutant plants iku1/iku1;kn/+ produce one-quarter of seeds bearing the typical knolle phenotype with enlarged multinucleate cells in the embryo and a partially syncytial endosperm (Fig. 3G). Irrespective of the presence or absence of the kn phenotype, all the seeds produced by iku1/iku1;kn/+ plants are of a comparable size to seeds of the single mutant iku1/iku1. The mutant spz is characterized by the absence of cellularization in the endosperm, but in contrast to knolle, it does not affect cytokinesis in the embryo (Fig. 3B; Sørensen et al., 2002). spz/spz seeds are viable and produce homozygous plants indistinguishable from the WT. Similarly, iku1/iku1;spz/spz double-mutant plants produce seeds of reduced size as does the single-mutant iku1/iku1, but with non-cellularized endosperm (Fig. 3F). In kn and spz, partial cellularization of the endosperm is observed in a few seeds. To examine the effect of a complete loss of cellularization, we used the mutant hallimasch that belongs to the pilz class, characterized by the complete absence of microtubule in the embryo and in the endosperm (Mayer et al., 1999). The pilz mutant seed is completely unable to perform endosperm cellularization, and mitosis is severely prevented, leading to the generation of large nuclei in the endosperm (Fig. 3D). The pilz embryo development is reduced to a few multinucleate cells. Double-mutant iku1/iku1;hal/+ plants produce one-quarter of seeds showing additive iku and hal phenotypes (Fig. 3H). Identical results are obtained in double mutants with iku2 (Fig. 3, I–L). All combinations of iku mutations with cellularization-defective mutants result in additive phenotypes, without increasing seed size in comparison with iku seeds. Hence, reduction of seed size in iku mutants does not depend on endosperm cellularization.
Figure 3.
Role of endosperm cellularization in the phenotype of iku seeds. In WT seeds, the endosperm is entirely cellularized at heart embryo stage (arrowhead in inset; A). Cellularization does not occur in the mutants spz (B), kn (C), and hal (D). Inserts show details of endosperm (magnified 10 times). Absence of cellularization does alter reduction of endosperm size in double-mutant combinations with iku1 (E–H) or with iku2 (I–L). The severe reduction of embryo development in hal does not have any impact on the reduction of endosperm size by iku mutations (H and L). Scale bars = 50 μm for all Nomarski micrographs.
Polarity of Endosperm in haiku Mutants
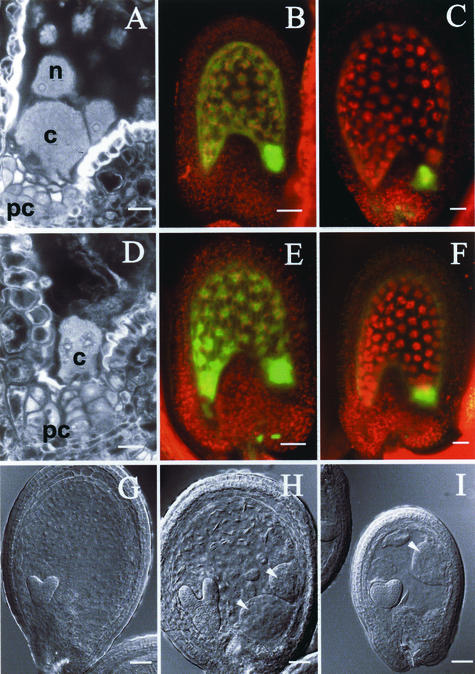
The posterior pole of the endosperm does not undergo cellularization and contains three structures (Fig. 4A): (a) single nuclei surrounded by a cytoplasmic unit that defines a nucleocytoplasmic domain (NCD; Brown et al., 1999), (b) the nodules that result from the fusion of NCDs, and (c) the posterior-most cyst, a multinucleate pool of cytoplasm that is formed by fusion of nodules (F. Berger, unpublished data). The cyst is located above the placentochalazal area of the seed integument, where vascular elements terminate. In iku seeds, the overall size of the posterior pole is reduced (Fig. 4D). The cyst of iku endosperm contains eight to 14 nuclei in comparison with 15 to 28 in the WT and is surrounded by zero to eight nodules and NCDs in comparison with 10 to 14 in the WT (n = 30 seeds for each genetic background). In a few iku seeds, cellularization reaches the posterior pole (Fig. 2H). These observations suggest that iku mutations cause a posterior displacement of the boundary between the peripheral endosperm and the posterior pole. To test this hypothesis, we introduced the polarity marker KS117 (Sørensen et al., 2001) into iku1 and iku2. In the WT endosperm, the expression of the marker KS117 is initially uniform (Fig. 4B) and becomes restricted to the posterior pole (Fig. 4C). In iku1 and iku2 endosperm, the expression of the marker KS117 follows a dynamic pattern similar to the WT (Fig. 4, E and F). However, the size of the posterior zone of expression of KS117 was much reduced in the iku endosperm, in agreement with the reduced size of the iku cyst. The posterior endosperm is of potential importance for transfer of maternal nutrients to the seed (Schultz and Jensen, 1971; Otegui et al., 2002). To test whether the reduction of the cyst in iku seeds is responsible for the reduction of the size of the endosperm we combined iku mutation to mutants of the fis class that are characterized by over-proliferation of posterior structures (Fig. 4H; Sørensen et al., 2001). The mutations fis are gametophytic maternal, and fis/+ plants generate 50% of seeds with enlarged, non-cellularized endosperm (Chaudhury et al., 1997; Ohad et al., 1996; Grossniklaus et al., 1998). The double mutant iku1/iku1;fis1/+ bears 50% of seeds of reduced size similar to that of iku seeds with an additive over-proliferation of the posterior endosperm (Fig. 4I). Similar observations were made with iku2 (not shown). Hence, over-proliferation of the posterior endosperm does not rescue the reduction of seed size caused by the iku mutations. In conclusion, the polarity of the endosperm does not appear to be perturbed by the iku mutations, and the reduction of the size of the posterior pole is probably a consequence and not a cause of the overall reduction of the size of the iku seed.
Figure 4.
Polarity in endosperm of iku mutants. In WT seeds, the endosperm is characterized by the absence of cellularization at the posterior pole, occupied by a syncytial cyst (c; A), which is extremely reduced in iku mutants (D). The WT expression of the green fluorescent protein (GFP) marker KS117 (green channel), initially uniform in the endosperm (B), becomes restricted to the posterior pole (C). In iku1 background, the posterior pole is reduced (D), but the restriction of expression of KS117 still occurs (E, F). In fis1, the relative size of the posterior pole increases (H, arrowheads) compared with WT (G). In double-mutant seeds iku1/iku1;fis1/fis1 (I), the ectopic cysts typical of fis 1 phenotype are present (arrowhead), but the size of seed remains as reduced as in iku1/iku1 (I). G to I, Nomarski micrographs; B, C, E, and F, projections of z series of confocal sections of GFP fluorescence and red autofluorescence. Confocal sections of posterior poles of WT and iku seed at heart embryo stage (A and D). Scale bars = 20 μm for A and D; scale bars = 35 μm for B, C, E, and F; scale bars = 50 μm for G to I.
Reduction of Endosperm Size by iku Mutations
WT seed volume increases markedly between the dermatogen and the mid-globular embryo stages after endosperm expansion (Fig. 5, A and B). During the same period, seed shape changes, becoming more oblong. At the dermatogen embryo stage, iku seeds cannot be distinguished from WT seeds (Fig. 5, A and C). The first difference in seed size clearly detected in iku seeds in comparison with WT seeds appears during globular embryo stage (Fig. 5, B and D), during which endosperm growth arrests (Fig. 5, A–D). The change of seed shape in WT does not take place in iku seeds that remain roundish as at the dermatogen embryo stage (Fig. 5, C and D). Identical defects are observed in seeds from crosses between WT ovules and MET1 a/s pollen (Fig. 5, E and F), which further supports similarities between iku phenotype and epigenetic changes that influence endosperm development.
Figure 5.
Compared development of iku seeds and seeds from crosses of WT ovule × METI a/s pollen. In WT seeds, transition from dermatogen (A) to mid-globular (B) embryo stage coincides with increase of seed size. At dermatogen embryo stage, WT seeds (A), iku1 seeds (C), and seeds from METI a/s pollination of WT (E) have the same size. The transition from dermatogen to mid-globular embryo stage occurs with a normal timing in iku1- (D) and METI a/s-pollinated seeds (F), but the increase of seed size observed in WT seed is reduced. Scale bar = 50 μm for all Nomarski micrographs.
Developmental Effects of the iku Mutations on the Seed Integument
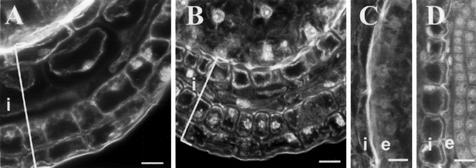
When homozygous iku plants are pollinated by WT pollen, seed development occurs as in the WT. Hence, the iku mutations do not have a maternal sporophytic effect on seed development. As a consequence, the integuments that are of maternal origin cannot be primarily affected by iku mutations. However, the integuments are likely to be affected indirectly to accommodate the overall changes in endosperm development resulting from the sporophytic recessive effect of iku mutations. The increase of the size of the integument takes place in two steps: an initial phase of cell proliferation after fertilization, followed by directional cell elongation (Western et al., 2000). Cell elongation is more pronounced along the axis defined by the apical-basal axis of the embryo. This leads to the characteristic oblong morphology of the WT seed. In contrast, the iku seeds remain nearly spherical (Fig. 1, F and H), and the average cell size in the integument does not increase (Fig. 6). We could not detect differences between WT seeds and iku seeds in the organization of the placentochalazal integument that might play a role in the maternal supply of nutrients to the endosperm (Fig. 4, A and D). In conclusion, the iku mutations specifically affect cell elongation in the seed integument. This ensures coordination of the development of the maternal integument with the reduced increase of endosperm size. Moreover, this suggests the existence of a signal from the endosperm that would normally trigger cell elongation in the integument.
Figure 6.
Effect of iku on the seed integument. In contrast to WT (A and C), the cells in the seed integument of iku1 (B) and iku2 (D) have not undergone elongation (embryo heart stage). This is most dramatic in the inner layers of the integument (i) that neighbor the endosperm (e). Scale bars represent 20 μm.
DISCUSSION
The haiku Mutations Affect Endosperm Growth and Might Identify Targets of Epigenetic Controls
Plants homozygous for iku produce seeds of reduced size and do not show any other vegetative or reproductive phenotype. Other mutants with reduced seed size have been reported and can be readily distinguished from iku because they are affected in other aspects of the plant life such as exs (Canales et al., 2002), which causes male sterility; ctr1 (Christensen et al., 2002; F. Berger, personal observations), which prevents cell elongation and ethylene signal transduction (Kieber et al., 1993); and ats (Léon-Kloosterziel et al., 1994), which causes reduction of layers in seed integument. Hence, iku mutations represent a new class of mutants specifically affected for seed size. Interestingly, the locus haiku2 colocalizes with one quantitative trait locus identified for seed size using natural variation between seed size of the ecotypes Landsberg erecta (Ler) and Cape Verde Islands (Alonso-Blanco et al., 1999). Once the HAIKU2 gene identified, a search for polymorphism and evaluation of its level of expression in Ler compared with Cape Verde Islands ecotypes will be valuable.
Endosperm development is affected by iku mutation before any defect is detected in the embryo. In the double mutant hal/+;iku/iku, a nearly complete absence of embryogenesis does not modify the effect of iku on the reduction of seed size. We conclude that the reduction of seed size by iku mutations is not directly mediated by the embryo but rather by the endosperm.
The iku mutations affect many features of endosperm development. The earliest phenotypic alteration in the iku seed is a premature arrest of growth of the endosperm, although proliferation of nuclei does not appear to be affected. This arrest becomes visible during the embryo globular stage. The iku endosperm undergoes a precocious complete cellularization at embryo triangular stage. We have shown recently that cellularization is coupled to the eighth mitotic wave in the peripheral endosperm (Sørensen et al., 2002). We hypothesize that, akin to cellularization of the syncytial Drosophila melanogaster embryo (Edgar and Lehner, 1996), cellularization of the Arabidopsis endosperm depends on the achievement of a critical threshold of the nucleocytoplasmic ratio. In iku endosperm, the mitotic activity is not affected, whereas the size is reduced. This would cause premature achievement of a threshold nucleocytoplasmic ratio and results in the precocious onset of cellularization. In conclusion, the iku mutations restrict initially the size of the endosperm, which, in turn, affects multiple aspects of endosperm development, such as cellularization, coordinated growth of the differentiated domains, and proliferation of the cellular endosperm.
We report that pollination of a WT plant with hypomethylated pollen causes arrest of endosperm growth during the globular embryo stage, and we observed precocious endosperm cellularization. Thus, WT × MET1 a/s crosses completely phenocopy the effects of iku mutations. A phenotype similar to iku is also produced by maternal excess in the endosperm (Scott et al., 1998). We hypothesize that the effects of maternal excess and hypomethylation of the paternal genome involve changes of the expression of many genes, some of which might be the IKU genes.
Regulation of Endosperm Size by IKU Might Control Embryo Size via Trophic Interactions
Reduction of seed size in iku mutants is accompanied by reduction of embryo size. This originates from a reduced cell proliferation after the embryo heart stage and likely results from defective development of the endosperm. This type of interaction between the respective sizes of the endosperm and of the embryo has been inferred from studies of other mutants in Arabidopsis, maize, and rice (Oryza sativa). The Arabidopsis mutants titan 3 (Liu and Meinke, 1998), fis1/medea, fis2 (Chaudhury et al., 1997; Sørensen et al., 2001), demeter (Choi et al., 2002), and spätzle (Sørensen et al., 2002), primarily affected in endosperm development, produce viable embryos with reduced growth that develop into normal-looking plants. In maize and rice, the endosperm stores reserves of the seed. Hence, endosperm developmental defects result in most cases in loss of seed viability (Neufer and Sheridan, 1980). In rice, a series of mutants show interdependence between the size of the embryo and the endosperm without variation of seed size (Hong et al., 1996). Although Arabidopsis endosperm does not store reserves, the reduced embryo growth as a consequence of reduced endosperm size suggests that nutrients are delivered from the endosperm to the embryo. In the WT, the endosperm acts as a sink for nutrient unloading from the phloem, which is essential for its storage function either directly or indirectly in the embryo cotyledons (Weber et al., 1997). IKU genes may encode housekeeping proteins and iku mutant endosperm may be a poor sink, causing reduced nutrient delivery and reserve storage. This may cause an initial reduction of the endosperm growth, and later in development would result in decreased proliferation in the iku embryo. According to such a hypothesis, the double mutant iku1/iku1;iku2/iku2 would be expected to show a cumulative effect on endosperm growth and seed size, which was not observed.
Seed Size Restriction in iku Results from Impaired Communication from the Endosperm to the Maternal Seed Integument
Reduction of seed size in iku mutants affects the integument that undergoes a precocious arrest of cell elongation. Because the mutations iku do not show maternal sporophytic effects, they cannot primarily affect the maternal seed integument. The precocious sporophytic recessive effect of iku mutation on endosperm is most likely the source of a signal toward the maternal tissues. Thus, the iku mutants demonstrate in Arabidopsis a feedback from the filial generation to the maternal generation that is involved in the coordination of seed development.
Developmental interactions between the integument and the endosperm have been demonstrated in cereals. Transfer of nutrients from the mother plant to the endosperm that stores reserves involves the specialized placentochalazal tissue of seed integument and the transfer layer in the endosperm (Thompson et al., 2001). Mutants affected for the development of the placentochalazal tissue show defects in seed growth (Felker et al., 1985; Cheng et al., 1996; Maitz et al., 2000). Most of these mutants have sporophytic maternal effects on endosperm and embryo development. In contrast, the maize mutant miniature1 is sporophytic recessive and produces small seeds with a reduction of endosperm size (Miller and Chourey, 1992; Cheng et al., 1996), similar to iku mutants in Arabidopsis. The reduction of size of the miniature1 endosperm results from a reduced proliferation of the cellular endosperm (Vilhar et al., 2002). Because earlier steps of endosperm development have not been studied in miniature1, it is difficult to conclude whether similarities with the iku phenotype extend to a reduction of growth of the syncytial endosperm. Miniature 1 encodes a cell wall invertase 2 (Carlson et al., 2000) that cleaves Suc in hexoses. The activity of the Miniature 1 cell wall invertase 2 is localized to the transfer layer of the endosperm that neighbors the placentochalazal tissue of the integument (Cheng et al., 1996). The abnormal development of the placentochalazal tissue in miniature1 seeds substantiates evidence for communications between the endosperm and the integument that would be involved in the coordination of maternal nutrients supply to the seed. The nature of such communications remains unknown.
In Arabidopsis, cytological organization of the posterior endosperm and of the integument suggests similarities with the transfer zone of cereals (Schultz and Jensen, 1971). Despite cytological similarities to cereals, the role of this zone in nutrients transport to the endosperm has not been demonstrated in Arabidopsis. However, unlike the miniature1 mutant, the placentochalazal region in the maternal integument is not affected in iku, suggesting that different functions are affected in both classes of mutants. As proposed above, the iku endosperm might be deficient in its normal function as a sink and would not provide enough turgor to drive cell elongation in seed integument. An alternative hypothesis to a mechanical signal could involve a molecular signal from the endosperm that triggers onset of cell elongation. The identification of the genes IKU might give some light on the nature of signals involved in this communication.
MATERIALS AND METHODS
Plant Lines
Arabidopsis WT ecotype Ler was used to generate populations of mutant lines. The WT ecotype Columbia was used for genetic mapping. The mutant allele ML159 of the pilz mutant hallimasch (Ler ecotype) was isolated during the same screen as the haiku mutants (Mayer et al., 1999). The mutant allele AP 6-16 (Ler ecotype) of KNOLLE has been described previously (Lukowitz et al., 1996). The mutant spätzle (allele DRU 42, WS ecotype) was isolated during a screen of collections provided by Loic Lepiniec (Institut National de la Recherche Agronomique, Versailles, France; Sørensen et al., 2002). The mutants fis1 and fis2 (Ler ecotype) were provided by A. Chaudhury (Canberra, ACT, Australia; Chaudhury et al., 1997). The marker line KS117 (C24 ecotype) originates from Jim Haseloff's enhancer trap line collection (Haseloff, 1999; http://www.plantsci.cam.ac.uk/Haseloff).
Growth Conditions
After vernalization for 3 d at 4°C in the dark, seeds were germinated on soil, and plants were cultured for 3 to 4 weeks in a growth chamber under short days (8 h of light at 20°C and 16 h of dark at 16°C, 60%–70% relative humidity). Flowering was induced by transfer to long days (20°C, 14 h of light/10 h of dark, 60%–70% relative humidity) where plants were cultured until seed harvest. Plants were grown under long days in the greenhouse for seed production and genetic mapping.
Mutagenesis and Isolation of the haiku Mutants
WT Ler seed were mutagenized with 0.3% (w/v) ethyl methanesulfonate (EMS) for 8 h or 20,000 rads x-rays, as previously described (Mayer et al., 1999). M2 families of seed were harvested from secondary branches after removal of the main shoot. For each M2 family, two plants were selected, and developing seeds were collected at the torpedo embryo stage from two siliques per plant. Seeds were cleared in chloral hydrate solution and observed microscopically with Normarski optics (Mayer et al., 1991). M2 families (1,600 and 800, respectively) were inspected in the EMS and x-ray populations. The percentage of embryo lethal mutations was 90% in EMS M2 families and 10% in x-ray families. During this screen, one to four alleles were found for the pilz mutants pfifferling, hallimasch, champignon, and porcino (Mayer et al., 1999).
Seeds from 165 M3 plant lines with abnormal endosperm development were observed for confirmation of the phenotype. A subset of 20 lines was identified where embryo morphogenesis was not impaired, whereas endosperm development appeared abnormal. Three or four backcrosses were done with these lines. Mature dried seeds with phenotypic alterations were selected manually and planted on soil. In six lines, such seeds germinated and produced plants homozygous for the mutation. One line was characterized by a ratio of mutant seeds:WT close to 1:1 and showed gametophytic maternal reduced transmission of the mutation. Genetic mapping identified linkage with FIS2, and the line ML 319 was identified as an allele of the mutant fis2. Two other lines, UU3100 (EMS mutagenesis) and ML 590 (x-ray mutagenesis), showed seeds of very reduced size and were called haiku1 and haiku2, respectively. FD 726 and FD 1476, two alleles of haiku1, and GM 423, an allele of haiku2, were isolated in another screen of 2,000 gamma ray-mutagenized lines (300 grays; 40% of embryo lethal mutation).
Genetic Mapping
After two backcrosses of the original mutant lines (ecotype Ler), smaller seeds were selected from heterozygous plants and germinated. These gave rise to homozygous iku plants that were crossed with WT ecotype Columbia to produce a mapping population. Smaller F2 seeds were selected to produce a F2 mapping population enriched (90%) in iku/iku plants. DNA of iku/iku plants was extracted from single leaves, and polymorphic markers were PCR amplified. The following markers were used: chromosome 1, nga248; chromosome 2, GPA1, nga 1126, nga361, and Cop1a; chromosome 3, nga171, nga6, nga 126, nga 162, g4711a, GAPAB, athCHIB, and ArLIM15; and chromosome 4, AGa, nga1107, and nga12.
Generation of Double Mutants
Because both mutants, iku1 and iku2, shared nearly identical phenotypes, a series of crosses was used to obtain double-homozygous mutants iku1;iku2. Plants homozygous for each mutation were crossed. Double-heterozygous F1 iku1/+;iku2/+ plants were crossed to iku1/iku1, and small seeds (iku1/iku1;iku2/+) from the crosses were selected and germinated. After selfing, small seeds were selectively germinated, and complementation tests between the resulting F3 plants and homozygous iku2 mutants were performed to identify plants that were homozygous for both iku1 and iku2.
Cytological Characterization of haiku Mutants
Individual siliques were opened with two shallow longitudinal cuts on either side of the false septum. Siliques were stained with Schiff's reagent (Sigma, St. Louis) and embedded in LR White (Sigma) according to Braselton et al. (1996). All mutant lines were initially propagated as heterozygotes and produced siliques that contained both WT seeds and seeds with the mutant phenotype. Seeds that originated from individual siliques were isolated in each preparation to be able to compare mutant with WT development at corresponding stages. Confocal laser scanning microscopy was performed on an LSM-510 microscope (Zeiss, Jena, Germany) using the 488-nm excitation line of an argon laser and an emission filter long pass of 510 nm.
Fluorescence of the marker KS117 was observed directly with confocal laser scanning microscopy on fresh seeds mounted in 0.3% (w/v) agarose in 1% (w/v) Murashige and Skoog medium. For detection of GFP, the selective setting used was (excitation = 488 nm and emission 510–550 nm). Red autofluorescence was detected using the nonspecific setting (excitation = 543 nm and emission long-pass filter = 560 nm). Piles of 700- × 1,024-pixel sections were collected simultaneously for the green channel (GFP) and for the red channel (autofluorescence), and projections were realized using the Zeiss LSM 510 software.
Distribution of Materials
All novel materials described in this publication will be made available in a timely manner for noncommercial research purposes upon request to the corresponding author.
ACKNOWLEDGMENTS
We thank Prof. Christian Dumas for hosting our team in his laboratory, Jean-Christophe Geminard for his help with measuring seed weight, and Jean Finnegan for the gift of the MET1 a/s lines.
Footnotes
This work was supported by the Centre National de la Recherche Scientifique, Ecole Normale Supérieure de Lyon, and Institut National de la Recherche Agronomique (with a specific PhD fund to D.G.), by the Action Concertic Initiative Jeune (French Ministry of Research; to F.B.), by the Action Concertic Incitative Développement et Physiologic (to F.B.), by EMBO (short-term fellowship to F.B.), by the EMBO Young Investigator Program (to F.B.), and by the Deutsche Forschungsgemeinshaft (Leibniz award to G.J.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018762.
LITERATURE CITED
- Adams S, Vinkenoog R, Spielman M, Dickinson HG, Scott RJ. Parent-of-origin effects on seed development in Arabidopsis thaliana require DNA methylation. Development. 2000;127:2493–2502. doi: 10.1242/dev.127.11.2493. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:4710–4717. doi: 10.1073/pnas.96.8.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. Endosperm, the crossroad of seed development. Curr Opin Plant Biol. 2003;6:42–50. doi: 10.1016/s1369526602000043. [DOI] [PubMed] [Google Scholar]
- Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, Dumas C, Haseloff J, Berger F. Dynamic analyses of the expression of the histone:YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell. 2001;13:495–509. doi: 10.1105/tpc.13.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braselton JP, Wilkinson MJ, Clulow SA. Feulgen staining of intact plant tissues for confocal microscopy. Biotechnol Histochem. 1996;71:84–87. doi: 10.3109/10520299609117139. [DOI] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE, Nguyen H, Olsen OA. Development of endosperm in Arabidopsis thaliana. Sex Plant Reprod. 1999;12:32–42. [Google Scholar]
- Canales C, Bhatt AM, Scott R, Dickinson H. EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol. 2002;12:1718–1727. doi: 10.1016/s0960-9822(02)01151-x. [DOI] [PubMed] [Google Scholar]
- Carlson SJ, Shanker S, Chourey PS. A point mutation at the miniature1 seed locus reduces levels of the encoded protein, but not its mRNA, in maize. Mol Gen Genet. 2000;263:367–373. doi: 10.1007/s004380051180. [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ. Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:4223–4228. doi: 10.1073/pnas.94.8.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Taliercio EW, Chourey PS. The miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell. 1996;8:971–983. doi: 10.1105/tpc.8.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Gehring M, Johnson L, Hannon M, Harada JJ, Goldberg RB, Jacobsen SE, Fischer RL. DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell. 2002;110:33–42. doi: 10.1016/s0092-8674(02)00807-3. [DOI] [PubMed] [Google Scholar]
- Christensen CA, Gorsich SW, Brown RH, Jones LG, Brown J, Shaw JM, Drews GN. Mitochondrial GFA2 is required for synergid cell death in Arabidopsis. Plant Cell. 2002;14:2215–2232. doi: 10.1105/tpc.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA, Lehner CF. Developmental control of cell cycle regulators: a fly's perspective. Science. 1996;274:1646–1652. doi: 10.1126/science.274.5293.1646. [DOI] [PubMed] [Google Scholar]
- Felker FC, Peterson DM, Nelson OE. Anatomy of immature grains of eight maternal effect shrunken endosperm barley mutants. Am J Bot. 1985;72:248–256. [Google Scholar]
- Finnegan EJ, Peacock WJ, Dennis ES. Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proc Natl Acad Sci USA. 1996;93:8449–8454. doi: 10.1073/pnas.93.16.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- Haseloff J. GFP variants for multispectral imaging of living cells. Methods Cell Biol. 1999;58:139–151. doi: 10.1016/s0091-679x(08)61953-6. [DOI] [PubMed] [Google Scholar]
- Hong SK, Kitano H, Satoh H, Nagato Y. How is embryo size genetically regulated in rice? Development. 1996;122:2051–2058. doi: 10.1242/dev.122.7.2051. [DOI] [PubMed] [Google Scholar]
- Kermicle JL, Allemand M. Gametic imprinting in maize in relation to the angiosperm life cycle. Development Suppl. 1990;1:9–14. [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldman KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jürgens G. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol. 1997;139:1485–1493. doi: 10.1083/jcb.139.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Keijzer CJ, Koornneef M. A seed shape mutant in Arabidopsis that is affected in integument development. Plant Cell. 1994;6:385–392. doi: 10.1105/tpc.6.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B-Y. Ploidy barrier to endosperm development in maize. Genetics. 1984;107:103–115. doi: 10.1093/genetics/107.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Meinke DW. The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant J. 1998;16:21–31. doi: 10.1046/j.1365-313x.1998.00268.x. [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Mayer U, Jürgens G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell. 1996;84:61–71. doi: 10.1016/s0092-8674(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A. Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci USA. 2000;97:10637–10642. doi: 10.1073/pnas.170292997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitz M, Santandrea G, Zhang ZY, Lal S, Hannah LC, Salamini F, Thompson RD. Rgf1, a mutation reducing grain filling in maize through effects on basal endosperm and pedicel development. Plant Journal. 2000;23:29–42. doi: 10.1046/j.1365-313x.2000.00747.x. [DOI] [PubMed] [Google Scholar]
- Mayer U, Herzog U, Berger F, Inze D, Jürgens G. Mutations in the PILZ group genes disrupt the microtubule cytoskeleton and uncouple cell cycle progression from cell division in Arabidopsis embryo and endosperm. Eur J Cell Biol. 1999;78:100–108. doi: 10.1016/S0171-9335(99)80011-9. [DOI] [PubMed] [Google Scholar]
- Mayer U, Torres Ruiz RA, Berleth T, Misera S, Jürgens G. Mutations affecting body organisation in the Arabidopsis embryo. Nature. 1991;353:402–407. [Google Scholar]
- Miller ME, Chourey PS. The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell. 1992;4:297–305. doi: 10.1105/tpc.4.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufer MG, Sheridan WF. defective kernel mutants of maize: I. Genetic and lethality studies. Genetics. 1980;95:929–944. doi: 10.1093/genetics/95.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N, Margossian L, Hsu Y-C, Williams C, Repetti P, Fischer RL. A mutation that allows endosperm development without fertilization. Proc Natl Acad Sci USA. 1996;93:5319–5324. doi: 10.1073/pnas.93.11.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ, Goldberg RB, Fischer RL. Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell. 1999;11:407–416. doi: 10.1105/tpc.11.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OA. Endosperm development: cellularization and cell fate specification. Annu Rev Plant Phys Plant Mol Biol. 2001;52:233–267. doi: 10.1146/annurev.arplant.52.1.233. [DOI] [PubMed] [Google Scholar]
- Otegui MS, Capp R, Staehelin LA. Developing seeds of Arabidopsis store different minerals in two types of vacuoles and in the endoplasmic reticulum. Plant Cell. 2002;14:1311–1327. doi: 10.1105/tpc.010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz P, Jensen WA. Capsella embryogenesis: the chalazal proliferating tissue. J Cell Sci. 1971;8:201–227. doi: 10.1242/jcs.8.1.201. [DOI] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- Sørensen MB, Chaudhury AM, Robert H, Bancharel E, Berger F. Polycomb group genes control pattern formation in plant seed. Cur Biol. 2001;11:277–281. doi: 10.1016/s0960-9822(01)00072-0. [DOI] [PubMed] [Google Scholar]
- Sørensen MB, Mayer U, Lukowitz W, Robert H, Chambrier P, Jürgens G, Somerville C, Lepiniec L, Berger F. Cellularisation in the endosperm of Arabidopsis thaliana is coupled to mitosis and shares multiple components with cytokinesis. Development. 2002;129:5667–5676. doi: 10.1242/dev.00152. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Hueros G, Becker H, Maitz M. Development and functions of seed transfer cells. Plant Sci. 2001;160:775–783. doi: 10.1016/s0168-9452(01)00345-4. [DOI] [PubMed] [Google Scholar]
- Vilhar B, Kladnik A, Blejec A, Chourey PS, Dermastia M. Cytometrical evidence that the loss of seed weight in the miniature1 seed mutant of maize is associated with reduced mitotic activity in the developing endosperm. Plant Physiol. 2002;129:23–30. doi: 10.1104/pp.001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Sugar import and metabolism during seed development. Trends Plant Sci. 1997;22:169–174. [Google Scholar]
- Western TL, Skinner DJ, Haughn GW. Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol. 2000;122:345–355. doi: 10.1104/pp.122.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]