Abstract
Many aspects of plant development are regulated by antagonistic interactions between the plant hormones auxin and cytokinin, but the molecular mechanisms of this interaction are not understood. To test whether cytokinin controls plant development through inhibiting an early step in the auxin response pathway, we compared the effects of cytokinin with those of the dgt (diageotropica) mutation, which is known to block rapid auxin reactions of tomato (Lycopersicon esculentum) hypocotyls. Long-term cytokinin treatment of wild-type seedlings phenocopied morphological traits of dgt plants such as stunting of root and shoot growth, reduced elongation of internodes, reduced apical dominance, and reduced leaf size and complexity. Cytokinin treatment also inhibited rapid auxin responses in hypocotyl segments: auxin-stimulated elongation, H+ secretion, and ethylene synthesis were all inhibited by cytokinin in wild-type hypocotyl segments, and thus mimicked the impaired auxin responsiveness found in dgt hypocotyls. However, cytokinin failed to inhibit auxin-induced LeSAUR gene expression, an auxin response that is affected by the dgt mutation. In addition, cytokinin treatment inhibited the auxin induction of only one of two 1-aminocyclopropane-1-carboxylic acid synthase genes that exhibited impaired auxin inducibility in dgt hypocotyls. Thus, cytokinin inhibited a subset of the auxin responses impaired in dgt hypocotyls, suggesting that cytokinin blocks at least one branch of the DGT-dependent auxin response pathway.
The balance between auxin and cytokinin controls a wide range of processes in plant development, including the formation of roots, shoots, and callus tissue in vitro (Skoog and Miller, 1957), the outgrowth of shoot axillary buds (Sachs and Thimann, 1967), and the formation of lateral roots (Wightman et al., 1980; Hinchee and Rost, 1986). Mutual control of active auxin and cytokinin pools, interactive control of gene expression, and posttranslational effects have been described as possible mechanisms underlying such physiological interactions (Coenen and Lomax, 1997). However, the relationship between classical hormone interactions at the physiological level and molecular auxin-cytokinin interactions is presently not well defined.
Auxin-Cytokinin Interactions during Hypocotyl Elongation
Auxin-cytokinin interactions can be observed in the elongation response of dicot hypocotyl segments. Auxin-induced elongation of sunflower (Helianthus annuus; DeRopp, 1956) and soybean (Glycine max; Vanderhoef et al., 1973; Vanderhoef and Stahl, 1975) hypocotyl segments is inhibited in the presence of cytokinins. This inhibition is detectable within 10 min of cytokinin addition and is not mediated by changes in ethylene synthesis (Vanderhoef and Stahl, 1975). Based on the time course of auxin-induced elongation growth in the presence and absence of cytokinin, Vanderhoef and Stahl (1975) proposed that cytokinin selectively inhibits the later phase of auxin-induced elongation and may not influence rapid growth processes mediated by H+ secretion. This hypothesis has, however, not been tested directly thus far.
Among the molecular target reactions at which auxin and cytokinin could conceivably interact to control cellular elongation is the expression of rapidly auxin-inducible genes, such as members of the SAUR (small auxin up-regulated RNAs) gene family. Auxin addition to the incubation medium activates SAUR expression in soybean epicotyl segments within 2 to 5 min (McClure and Guilfoyle, 1987), and the kinetics and location of expression from the SAUR promoter show a strong correlation with auxin-induced elongation processes (Gee et al., 1991; Li et al., 1991). Although the biochemical function of SAUR RNAs in elongation growth is unknown, their rapid induction renders them valuable for defining inhibitory effects on early events in auxin signaling. For example, the reduced auxin inducibility of SAUR genes in the axr2 and axr1 mutants of Arabidopsis (Timpte et al., 1994, 1995) provides evidence that AXR1 and AXR2 proteins are required for an early step in the auxin response.
Treatment with the cytokinin isopentenyladenine does not inhibit the auxin induction of SAUR genes in isolated nuclei from soybean plumules (Guilfoyle and Hagen, 1986). However, cytokinin reduces auxin-induced accumulation of SAUR mRNAs in soybean by up to 50% (McClure and Guilfoyle, 1987), and also eliminates ectopic expression from the SAUR promoter in roots of the axr3 mutant of Arabidopsis (Leyser et al., 1996). In contrast, cytokinin alone increases SAUR expression slightly in Arabidopsis rosette leaves (Timpte et al., 1995). The influence of cytokinin on auxin-induced SAUR gene expression and its relation to elongation growth, therefore, is unresolved.
Interactions during Ethylene Synthesis
In addition to stimulating elongation growth, auxin rapidly induces ethylene synthesis in many tissues (Abeles, 1966), and cytokinin enhances this auxin effect in hypocotyl segments of mung bean (Vigna radiata; Lau and Yang, 1973; Imaseki et al., 1975) and pea (Pisum sativum; Fuchs and Lieberman, 1968). Lau and Yang (1973) proposed that this interaction might be based on cytokinin-induced increases in free indole-3-acetic acid (IAA) levels. However, in a later study, increases in free IAA could not account for the cytokinin effect on ethylene synthesis in this tissue (Imaseki et al., 1975). Auxin stimulates ethylene synthesis by increasing the expression of genes encoding 1-aminocyclopropane-1-carboxylic acid (ACC) synthase, the key enzyme in ethylene synthesis, in a tissue-specific manner (Zarembinski and Theologis, 1994). In hypocotyl segments, transcripts for several specific members of the tomato (Lycopersicon esculentum; Yip et al., 1992), mung bean (Kim et al., 1992), and Arabidopsis (Abel et al., 1995; Vogel et al., 1998) ACS gene families show strong auxin inducibility. In contrast, cytokinin-induced increases in the expression of ACS genes are small; therefore, cytokinin has been proposed to stimulate ethylene synthesis by a posttranscriptional mechanism (Vogel et al., 1998). Synergistic effects of cytokinins on auxin-induced ACS-mRNA expression have, however, been described for a mung bean ACS gene (Yoon et al., 1999).
Mechanism of Antagonistic Signaling
Antagonistic interactions between auxins and cytokinins in many aspects of development have been confirmed through studies of transgenic plants expressing genes that affect auxin and cytokinin metabolism: Transgenic cytokinin-overproducing plants (Medford et al., 1989) generally exhibit morphological aberrations similar to auxin-degrading plants (Romano et al., 1991; Spena et al., 1991) and decreased auxin responses (Li et al., 1994), whereas transgenic cytokinin-degrading plants (Werner et al., 2001) morphologically resemble auxin-overproducing plants (Klee et al., 1987; Sitbon et al., 1992) and have increased auxin responses (Martin et al., 1997).
One possible explanation for antagonisms between auxin and cytokinin is a mutual regulation of hormone levels. Applied or internally produced cytokinin decreases levels of free IAA (Eklöf et al., 1997), and applied or internally produced auxin reduces levels of major cytokinins (Zhang et al., 1995; Eklöf et al., 1997), in part through stimulating cytokinin conjugation (Martin et al., 1997). However, it is not clear whether the changes in hormone pools at the organ or tissue level adequately reflect cellular or subcellular changes in active hormone concentrations.
Additional interactions between auxin and cytokinin likely are mediated by intersections between their respective signaling pathways. Emerging response pathways for auxin and cytokinin signaling are surprisingly parallel, potentially reflecting a case of convergent evolution (Hutchison and Kieber, 2002). Both auxin- and cytokinin-responsive genes are activated by constitutively expressed transcription factors (type B Arabidopsis response regulators for cytokinin and auxin response factors (ARFs) for auxin) that form inactive complexes with negative regulator proteins (type A Arabidopsis response regulators for cytokinins and AUX/IAA proteins for auxin). The negative regulators are themselves encoded by primary hormone-responsive genes, so that the response of primary hormone-inducible genes is rapidly terminated by increased synthesis of the negative regulator proteins (Hutchison and Kieber, 2002; Kepinski and Leyser, 2002).
Auxin-resistant mutants of Arabidopsis define a number of genes in the auxin-signaling pathway: Lesions of the recessive axr1 and tir1 mutants are localized in component proteins of the ubiquitin-dependent protein degradation machinery, whereas several semidominant mutations affect AUX/IAA or ARF proteins (Kepinski and Leyser, 2002). Most of these mutants show cross resistance to cytokinin, and the cytokinin-resistant rpn12 mutant, which encodes a component of the 26S proteasome, in turn is resistant to auxin (Smalle et al., 2002), supporting the idea that both auxin- and cytokinin-signaling pathways may share some components.
We have used the dgt (diageotropica) mutant of tomato to investigate the mechanism of interaction between auxin and cytokinin. Hypocotyl segments of the dgt mutant are auxin resistant with respect to ethylene synthesis, elongation growth (Kelly and Bradford, 1986), and auxin-induced H+ secretion (Coenen et al., 2002). The single-gene, recessive dgt mutant exhibits pleiotropic phenotypic effects including reduced apical dominance; stunting of root and shoot growth; dark-green, hyponastic leaves; thin, rigid stems; and primary and adventitious roots that lack lateral root primordia, unless the root apex has been severely damaged (Zobel, 1972, 1973). Similar to the auxin-resistant Arabidopsis mutants axr2 and axr3 (Hobbie and Estelle, 1994), roots of dgt seedlings are resistant to auxin (Muday et al., 1995; Coenen et al., 2002) and also show reduced sensitivity to cytokinin (Coenen and Lomax, 1998). However, cytokinin inhibition of hypocotyl growth in intact dgt seedlings is nearly indistinguishable from the wild type (Coenen and Lomax, 1998).
We have tested the possibility that cytokinin interferes with an early step in the auxin response pathway by comparing the effects of cytokinin with those of the dgt mutation. In addition to assessing cytokinin effects on morphogenesis, we examined auxin-stimulated elongation, H+ secretion, and ethylene synthesis in hypocotyl segments, as well as the expression of primary auxin-responsive genes that are associated with these physiological responses. Our results support a model in which cytokinin inhibits one branch of the DGT-dependent auxin signal transduction pathway.
RESULTS
Phenocopy of dgt Morphology by Cytokinin Application
After 7 weeks of continuous exposure to benzyladenine (BA), the effects of cytokinin treatment on the development of wild-type tomato seedlings were similar to the phenotypic effects of the dgt mutation (Fig. 1A). Compared with untreated wild-type plants (Fig. 1A, left), cytokinin-treated wild-type tomato plants showed stunted roots and shoots, reduced internode length, reduced leaf complexity, increased pigmentation, and reduced senescence of the cotyledons (Fig. 1A, center). For each of these traits, the altered morphology of cytokinin-treated wild-type plants closely resembled that of untreated dgt plants (Fig. 1A, right). Leaf size, the number of leaflets per leaf, and the ornateness of the leaf margins were strongly reduced in dgt plants (Fig. 1B, bottom row) as compared with untreated wild-type leaves (Fig. 1B, top row), and cytokinin treatment of wild-type plants phenocopied each of these leaf characteristics (Fig. 1B, center row).
Figure 1.
Morphology of mature tomato plants. A, Seven-week-old plants: untreated wild type (left), wild type watered with 10 μm BA (center), and untreated dgt (right). B, Leaves from 7-week-old plants, arranged from cotyledon (left) to youngest leaf (right); untreated wild-type (top row), wild type treated with 10 μm BA (middle row), and untreated dgt (bottom row). C, Leaves emerging from first internode of untreated dgt plant (left) and wild-type plant treated with 10 μm BA (right). D, Lateral branches emerging from first internode of a 3-month-old dgt plant.
Both dgt plants and cytokinin-treated wild-type plants exhibited reduced apical dominance. In tomato, lateral shoots normally emerge from the axils of true leaves. However, in 7-week-old dgt and cytokinin-treated wild-type plants, lateral branches also originated from the first internodes, directly above the cotyledonary leaf axils (Fig. 1, C and D). These lateral branches appeared before the onset of lateral branch outgrowth from the other leaf axils, and they showed an unusual, downward angle of insertion (Fig. 1C). Their placement suggested that they were produced by normally quiescent buds in the cotyledonary axils. A similar displacement of axillary buds up the internode during internode elongation is typical of flowering branches of potato (Solanum tuberosum; Troll, 1969), a species closely related to tomato. Unlike regular lateral branches, the outgrowths initially consisted of a single leaf with no visible apical meristem. However, after these leaves had fully developed, a shoot meristem became visible near the basal end of the petiole of each leaf (Fig. 1C, left plant). These meristems later produced complete lateral branches (Fig. 1D).
Cytokinin Inhibition of Auxin-Induced Elongation, H+ Secretion, and LeSAUR Expression
We compared the effects of cytokinin treatment and the dgt mutation on the concentration dependence of IAA-induced growth by measuring hypocotyl segment elongation in the presence and absence of 100 μm BA (Fig. 2). In the absence of BA, auxin-induced elongation of wild-type segments was observed at 1, 10, and 100 μm IAA and did not show saturation within the range of auxin concentrations tested. Mutant hypocotyl segments did not elongate in response to auxin. When the auxin treatments were performed in the light, all three auxin concentrations still stimulated growth in the presence of 100 μm BA (Fig. 2A). However, the BA reduced the magnitude of the wild-type auxin response, and even high auxin concentrations did not overcome this inhibitory effect (Fig. 2A). Therefore, the complete inhibitory effect of the dgt mutation on auxin-induced hypocotyl segment elongation was partially mimicked by short-term cytokinin treatment of wild-type hypocotyl segments in the light. When the auxin treatment was conducted in darkness, cytokinin inhibited auxin-induced elongation of wild-type segments only for the highest auxin concentration, and the maximum inhibition was reduced to approximately 20% of the auxin response (Fig. 2B). Mutant hypocotyl segments failed to respond to auxin in either presence or absence of BA (Fig. 2B). Effects of zeatin on segment elongation were similar to those of BA (data not shown). At concentrations of 10 μm or less, neither zeatin nor BA had reproducible effects on segment elongation (data not shown).
Figure 2.
Dose response curve for auxin-induced elongation of tomato hypocotyl segments in the presence and absence of 100 μm BA. Segment growth was determined after 14 h of incubation in the light (A) or in the dark (B). In addition to receiving BA during the auxin treatment, segments were pre-incubated for 2 h in the presence or absence of the indicated BA concentration. Error bars show ses from at least three independent experiments.
To test whether the relatively high cytokinin concentration of 100 μm had auxin-unrelated, toxic effects on tomato hypocotyl segments, we measured elongation responses to the fungal toxin fusicoccin (FC) in the absence and presence of 100 μm BA. Cytokinin-treated dgt and wild-type segments showed normal elongation responses to various concentrations of FC (data not shown). Thus, both the dgt mutation and cytokinin treatment appeared to inhibit auxin-specific aspects of elongation growth that are not required for FC-induced elongation.
Because cytokinin inhibits only sustained growth of soybean hypocotyls but does not affect early auxin-induced growth (Vanderhoef and Stahl, 1975), we investigated the timing of auxin-induced tomato hypocotyl growth in the presence and absence of cytokinin (Fig. 3). Measurements of light-incubated wild-type tomato hypocotyls in hourly intervals suggested that IAA stimulated the rate of tomato hypocotyl elongation for the first 3 to 4 h, and that BA inhibited IAA effects over this entire period (Fig. 3). Treatment with 100 μm BA did not inhibit the elongation of control segments receiving no IAA (Fig. 3).
Figure 3.
Time course of elongation for wild-type hypocotyl segments incubated in the light. Segments were treated with or without 100 μm IAA in the presence of 100 μm BA after a 2-h pre-incubation with 100 μm BA and measured at the indicated times after auxin addition. Error bars show SEs from three independent experiments.
To resolve initial elongation response kinetics in greater detail, we measured changes in the elongation rate of tomato hypocotyl segments with a CCD camera-auxanometer (Fig. 4). The measurements confirmed that cytokinin inhibited auxin-induced elongation of tomato hypocotyl segments in the light from the onset of the auxin response (Fig. 4A). As had been suspected from end point measurements for dark-incubated hypocotyl segments shown in Figure 2B, 100 μm BA failed to inhibit the elongation response elicited by 10 μm IAA in low light (Fig. 4B). BA concentrations of 10 μm or less failed to inhibit growth in abraded or nonabraded segments (data not shown).
Figure 4.
Elongation kinetics of abraded wild-type tomato hypocotyl segments. Growth rates were monitored with a CCD camera. Arrows indicate addition of 10 μm IAA to the incubation medium at 120 min. A, Elongation rates in bright white light. B, Elongation rates in dim light. Experiments were repeated three or more times, and representative results for each treatment are shown.
To explain the lack of cytokinin effects on early phases of auxin-induced growth in soybean hypocotyls, Vanderhoef and Stahl (1975) proposed that cytokinin does not affect auxin-induced H+ secretion. We tested this hypothesis directly by continuously monitoring the equilibrium-pH of a solution containing abraded tomato hypocotyl segments (Fig. 5A). Abraded tomato hypocotyl segments equilibrate the pH of an unbuffered incubation medium to a stable pH within 2 h, and this equilibrium pH is maintained for several hours in the absence of added hormones (Coenen et al., 2002). After the equilibration period, the addition of auxin to the incubation medium induced the segments to acidify the medium to approximately 0.2 pH units below the equilibrium pH (Fig. 5, A and B). This auxin-induced H+ secretion by tomato hypocotyl segments was severely inhibited in the presence of growth-inhibiting concentrations of cytokinin (Fig. 5A). The inhibitory effect of 100 μm BA could not be explained as a buffering effect, because 100 μm adenine did not affect auxin-induced H+ secretion (Fig. 5A). Furthermore, the effect of BA on H+ secretion was auxin specific, because FC-induced H+ secretion was not inhibited in the presence of 100 μm BA (Fig. 5A). Ten micromolar BA was insufficient to inhibit auxin-induced H+ secretion (Fig. 5B), thus correlating with the lack of effect of this cytokinin treatment on elongation responses (data not shown).
Figure 5.
H+ secretion in abraded wild-type tomato hypocotyl segments. The pH of the unbuffered incubation medium (10 mm KCl and 1 mm CaCl2) was monitored continuously. A, Continuous pH measurements. After equilibration of the incubation medium to a stable pH, a pH-adjusted aqueous IAA solution (white triangles), or ethanolic FC solution (black triangles, final ethanol concentration 0.1% [v/v]) were added at the indicated times. Additions to the medium indicated in the left column were made from aqueous stocks and were present during the entire experiment. One representative experiment for each treatment is shown. Equilibrium pH before IAA or FC addition is given to the left of each graph. B, Quantitative comparison of IAA-induced changes in the pH of the medium at 0.5 and 2 h after IAA addition. Bars represent averages from two or three experiments. Error bars show SEs.
Auxin-induced elongation of hypocotyl segments is preceded not only by increased H+ secretion but also by the rapid induction of SAUR genes (Cleland, 1995). Therefore, the accumulation of SAUR mRNAs has become a molecular assay for rapid auxin action. In agreement with previous reports (Zurek et al., 1994; Mito and Bennett, 1995), our RNA gel-blot analysis demonstrated that the auxin-induced accumulation of LeSAUR transcripts was reduced in dgt versus wild-type hypocotyl segments (Fig. 6). The effect of the dgt mutation on the accumulation of LeSAUR transcripts was auxin specific, because cycloheximide induced LeSAUR expression to the same extent in dgt and wild-type hypocotyl segments (data not shown). In both wild-type and dgt segments, treatment with BA failed to inhibit IAA-induced LeSAUR accumulation and, therefore, did not mimic the effects of the dgt mutation (Fig. 6).
Figure 6.
Influence of auxin, cytokinin, and the dgt mutation on the expression of the LeSAUR gene as determined by RNA gel blots. Hypocotyl segments were harvested and treated as described for elongation assays. The final incubation was for 2 h in Suc/MES (SM) buffer containing the indicated hormones at 100 μm. A representative autoradiograph is shown above the quantification of signals from three independent experiments. Values from densitometer scans of films were expressed as percent of the highest signal in each respective experiment and subsequently averaged. Error bars indicate the se from three independent experiments. The ethidium bromide-stained gel is shown at the bottom.
Cytokinin Inhibition of Auxin-Induced Ethylene Synthesis
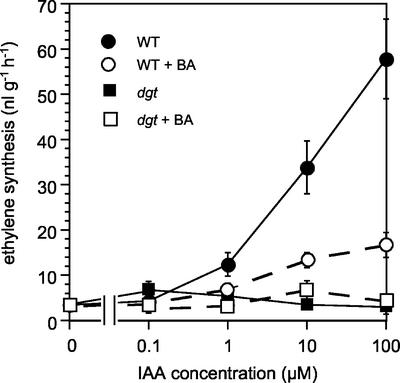
In addition to elongation and H+ secretion, cytokinin treatment also inhibited auxin-induced ethylene synthesis in tomato hypocotyl segments (Fig. 7), another auxin response that is impaired by the dgt mutation (Kelly and Bradford, 1986). Treatment with auxin strongly stimulated ethylene synthesis in wild-type hypocotyl segments (approximately 15-fold), whereas ethylene synthesis in dgt segments was not stimulated by increasing auxin concentrations, either alone or in the presence of cytokinin (Fig. 7). Treatment with 100 μm BA reduced the magnitude of auxin-induced ethylene synthesis in wild-type hypocotyl segments, and thus produced a partial phenocopy of the dgt effect. As seen for segment elongation, effects of BA on auxin-induced ethylene synthesis were similar to those of zeatin, and 100 μm BA or zeatin were required for reproducible inhibition of auxin-induced responses in wild-type segments (data not shown). Interestingly, cytokinin alone did not stimulate ethylene synthesis in excised hypocotyl segments (Fig. 7), although it does increase ethylene synthesis by intact seedlings approximately 7-fold (Coenen and Lomax, 1998).
Figure 7.
Influence of cytokinin on auxin-induced ethylene synthesis in wild-type and dgt tomato hypocotyl segments. Accumulated ethylene was measured after a 3-h treatment with the indicated auxin concentrations in the presence and absence of 100 μm BA, after a 2-h pre-incubation with or without BA. Segments were harvested as described for elongation experiments and incubated in the dark. Error bars represent the se from independent experiments (n = 4 for wild type, n = 2 for dgt).
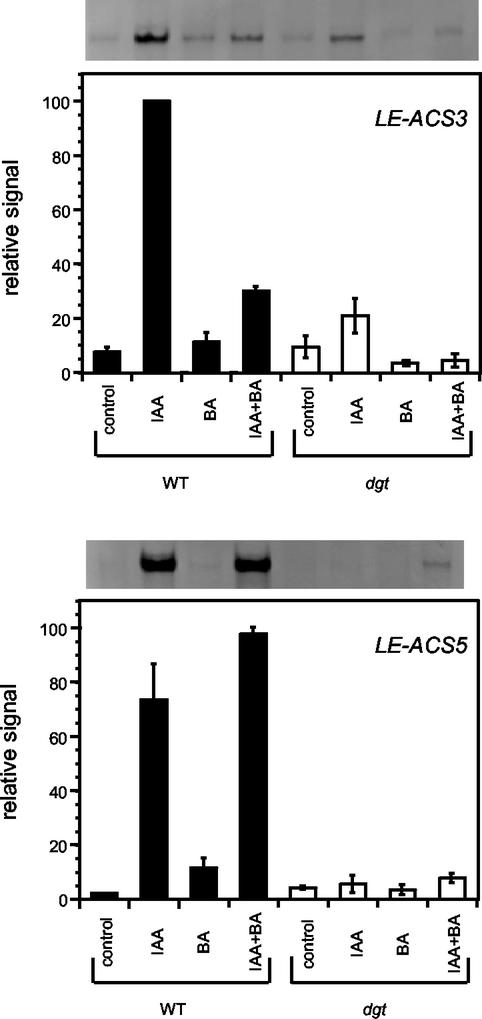
To pinpoint a possible molecular target for the interaction of auxin and cytokinin in the regulation of ethylene synthesis, we investigated the effects of cytokinin on the auxin induction of two genes encoding ACC synthase, LE-ACS3 and LE-ACS5, that are specifically auxin-inducible in tomato hypocotyl segments (Yip et al., 1992). Because the level of expression of these genes is significantly lower than that of the LeSAUR gene, we used RNAse protection assays to quantify relative LE-ACS3 and LE-ACS5 transcript levels. Consistent with the results obtained by Yip et al. (1992), mRNA levels for LE-ACS3 and LE-ACS5 increased in response to IAA in wild-type hypocotyl segments (Fig. 8). In the presence of 100 μm BA, the auxin inducibility of LE-ACS3 transcripts was strongly inhibited, whereas BA did not inhibit the auxin effect on LE-ACS5 transcript levels. In dgt hypocotyl segments, auxin inducibility of LE-ACS3 transcripts was markedly reduced and induction of LE-ACS5 transcripts by auxin was absent (Fig. 8). Thus, cytokinin treatment partially mimicked the effects of the dgt mutation on the expression of LE-ACS3 but not on LE-ACS5 expression.
Figure 8.
Influence of auxin, cytokinin, and the dgt mutation on the expression of two ACC synthase genes (LE-ACS3 and LE-ACS5) as determined by RNAse protection assays. Hypocotyl segments were harvested and treated as described for ethylene biosynthesis assays. The final incubation was for 2 h in SM buffer containing the indicated hormones at 100 μm. A representative fluorograph (LE-ACS3 and LE-ACS5) is shown above the quantification of signals from three independent experiments. Values from densitometer scans of films were expressed as percent of the highest signal in each respective experiment and subsequently averaged. Error bars indicate the se from three independent experiments.
DISCUSSION
Cytokinin Treatment Produces a Phenocopy of the Auxin-Resistant dgt Mutant
Auxins and cytokinins control many morphogenetic processes through antagonistic interactions (DeRopp, 1956; Skoog and Miller, 1957; Sachs and Thimann, 1967; Klee, 1994; Miyazawa et al., 1999), suggesting that these two hormone classes target a common set of molecular response pathways. If antagonistic auxin-cytokinin interactions are mediated through common signaling mechanisms, a defect in an auxin response pathway should produce a similar spectrum of morphological changes as treatment with cytokinin.
The DGT protein of tomato is required for a specific set of rapid, primary auxin responses, indicating that the dgt mutation disrupts an early step in an auxin-signaling pathway (Mito and Bennett, 1995; Nebenführ et al., 2000; Coenen et al., 2002). The DGT-dependent auxin response pathway likely plays a role throughout plant development, because the dgt mutation pleiotropically affects plant morphogenesis (Zobel, 1972, 1973; Kelly and Bradford, 1986; Muday et al., 1995; Coenen and Lomax, 1998). Our finding that cytokinin-treatment of wild-type plants resulted in a phenocopy of dgt morphology (Fig. 1) indicates that cytokinins affect a similar set of developmental and morphogenic responses as the dgt mutation and, thus, supports the idea that cytokinin and auxin effects on plant development may be mediated through shared signaling pathways. The close resemblance between the auxin-resistant dgt mutant and cytokinin-treated wild-type plants cannot be interpreted as cytokinin hypersensitivity of the dgt mutant because dgt tissues do not show increased cytokinin sensitivity with respect to any of a wide range of developmental processes (Coenen and Lomax, 1998). The dgt phenotype also cannot be explained by increased cytokinin levels in the mutant because dgt seedlings do not show measurable increases in major endogenous cytokinins (C. Coenen and T.L. Lomax, unpublished data). To test whether cytokinins antagonize auxin action by inhibiting a DGT-dependent auxin response pathway, we investigated whether cytokinins inhibit the same early auxin responses that are impaired in hypocotyls of dgt seedlings: elongation, ethylene synthesis, and the induction of auxin-inducible genes.
Cytokinins Inhibit Auxin-Induced Elongation and H+ Secretion, But Not SAUR Gene Expression
Although cytokinin inhibition of auxin-stimulated hypocotyl elongation was demonstrated in the classic experiments by DeRopp (1956) in sunflower and by Vanderhoef and Stahl (1975) in soybean, the mechanism of this auxin-cytokinin interaction has not been investigated. Based on the observation that cytokinins do not influence the early phase of auxin- or acid-induced growth in soybean hypocotyls, whereas they strongly inhibit sustained auxin-induced elongation, Vanderhoef and Stahl (1975) proposed that auxin-induced H+ secretion is not affected by cytokinin treatment. However, to our knowledge, cytokinin effects on auxin-induced H+ secretion in soybean hypocotyl segments have not been measured directly. Our measurements of growth kinetics (Fig. 4) and H+ secretion (Fig. 5) in tomato hypocotyls demonstrate that cytokinins inhibit rapid auxin effects on both elongation growth and H+ secretion and that this cytokinin effect is likely enhanced by light. Our results differed from those obtained for soybean hypocotyls by Vanderhoef et al. (1975) in that cytokinin inhibition of auxin-induced elongation in tomato was only partial (up to approximately 75% of the auxin effect, as compared with complete inhibition reported for soybean). Furthermore, as little as 4.2 μm cytokinin is sufficient to completely inhibit auxin-induced elongation in soybean (Vanderhoef et al., 1973), whereas 100 μm cytokinin was required for a reproducible inhibition of auxin-induced elongation of tomato segments (10 and 50 μm BA had no effect; data not shown). In addition, zeatin is more effective than BA in soybean (Vanderhoef et al., 1973), whereas zeatin and BA effects in tomato were similar (data not shown). The discrepancy in growth kinetics and concentration requirements between tomato and soybean may relate to species-specific differences in physiology, such as the much larger amount of storage proteins, lipids, and carbohydrates available for the sustained growth of soybean hypocotyls. Irrespective of the reasons for the observed differences, however, our results clearly negate a general applicability of Vanderhoef and Stahl's proposal that H+ secretion is unaffected by growth-inhibiting concentrations of cytokinin. Although the signal transduction mechanism for auxin-induced H+ secretion is still uncertain, our results indicate that at least one element of this pathway in tomato hypocotyls may be controlled by cytokinin. The H+-ATPase enzyme itself is likely not the direct target of cytokinin inhibition, because FC-induced H+ secretion was unaffected by BA (Fig. 5A).
In addition to its rapid effects on H+ secretion, auxin also stimulates elongation through altering gene expression (Cleland, 1995), but the relationship between altered gene expression and H+ secretion remains unclear. Based on their expression patterns and kinetics, SAUR transcripts have been proposed to be involved in auxin-induced elongation (Li et al., 1991), and dgt hypocotyl segments, which do not elongate in response to auxin (Kelly and Bradford, 1986), are impaired in auxin-induced SAUR accumulation (Mito and Bennett, 1995). In contrast to the effects of the dgt mutation, cytokinin application partially inhibits auxin-induced growth in wild-type hypocotyls (Figs. 2 and 4) without inhibiting the auxin-induced accumulation of LeSAUR transcripts (Fig. 6). There are several conceivable explanations for the failure of cytokinin to inhibit this rapid auxin response while still affecting hypocotyl growth. First, because SAURs are a gene family, we cannot exclude that inhibition of the auxin-induced expression of another SAUR family member may be inhibited by cytokinin. Second, transient decreases in SAUR expression levels would not have been detected in our experiments. Third, cytokinins may inhibit hypocotyl elongation downstream from the accumulation of LeSAUR mRNAs, for example by inhibiting the action of SAURs via posttranscriptional effects. Fourth, if elongation requires auxin-induced H+ secretion in addition to SAUR induction, the inhibition of H+ secretion (Fig. 5) alone may suffice to inhibit auxin-induced elongation. However, regardless of the reason for the failure of cytokinin treatment to mimic dgt effects on SAUR expression, this result demonstrates that cytokinin treatment does not mimic dgt effects on all rapid auxin responses. To assess whether cytokinin effects on DGT-dependent auxin responses may be limited to membrane events, and thus not apply to reactions involving changes in gene expression, we compared dgt and cytokinin effects on ethylene production, which is mediated through auxin-stimulated transcriptional activation of ACC synthase genes.
Cytokinins Inhibit Auxin-Induced Ethylene Synthesis and LE-ACS3 Expression, But Not LE-ACS5 Expression
In addition to auxin-induced elongation, the induction of ethylene synthesis by auxin is also blocked in dgt hypocotyl segments (Kelly and Bradford, 1986). Cytokinin application inhibited auxin-stimulated ethylene production in wild-type tomato hypocotyl segments (Fig. 7) and, thus, partially mimicked the auxin insensitivity of dgt hypocotyl segments in this response. This cytokinin effect appears to be species specific, because cytokinin and auxin were found to stimulate ethylene synthesis synergistically in mung bean (Lau and Yang, 1973; Imaseki et al., 1975) and in pea (Fuchs and Lieberman, 1968).
Because the auxin induction of ethylene synthesis in tomato hypocotyl segments is accompanied by a large increase in transcripts for at least two genes encoding ACC synthase (Yip et al., 1992), we tested whether cytokinin or the dgt mutation affected the expression of these genes. Whereas the dgt mutation impaired the auxin induction of both genes, cytokinin application inhibited IAA-induced accumulation of transcripts for only one of the ACC synthase genes, LE-ACS3 (Fig. 8). The strong cytokinin inhibition of both ethylene production (Fig. 7) and of LE-ACS3 mRNA accumulation (Fig. 8) suggests that the LE-ACS3-encoded isoenzyme may produce the bulk of auxin-induced ethylene. The generally accepted paradigm that auxin increases ethylene synthesis through transcriptional stimulation of ACC synthase genes (Zarembinski and Theologis, 1994) makes this interpretation plausible, although our results do not preclude cytokinin effects on other auxin-inducible ACC synthase isoforms or posttranscriptional effects on the activity of ACC synthase or ACC oxidase enzymes. Synergistic cytokinin enhancement of the auxin-induced expression of a mung bean ACC synthase gene, VR-ACS6, is at least in part transcriptionally mediated (Yoon et al., 1999).
The lack of cytokinin effects on the auxin response of LE-ACS5 demonstrates that auxin-signaling pathways for LE-ACS3 and LE-ACS5 induction are different from each other, although both depend on the presence of a functional DGT gene product. Differences in the regulation of LE-ACS3 and LE-ACS-5 expression were also discovered in response to other stimuli: LE-ACS3 is induced early after root flooding, whereas LE-ACS5 does not respond to this stimulus (Shiu et al., 1998), and only LE-ACS3 is expressed in petals (Llop-Tous et al., 2000) and in ripening fruit (Nakatsuka et al., 1998). Our data provide additional evidence for differential regulation of LE-ACS3 and LE-ACS5 expression: The LE-ACS3-activating pathway must contain at least one cytokinin-regulated step, either before or after the action of the DGT gene product. The promoters of these two genes have not been isolated, but the auxin-inducible promoter of the Arabidopsis ACS4 gene contains two auxin response elements (Aux-REs) that are also present in SAUR and AUX/IAA promoters (Abel et al., 1996). Thus, DGT is likely required for the activation of one or both of these elements.
Cytokinin Regulates a Subset of DGT-Dependent Auxin Response Pathways
The fact that cytokinin treatment phenocopied dgt effects on plant morphology and on various physiological target reactions (hypocotyl segment elongation, H+ secretion, and ethylene synthesis) implies that cytokinin and DGT may control a shared set of auxin responses. Furthermore, the cytokinin inhibition of auxin-induced LE-ACS3 expression suggests that at least some of the interactions between cytokinin and auxin response pathways occur during the control of gene expression.
The dgt mutant resembles several different auxin-resistant mutants of Arabidopsis in its morphology, auxin physiology, and effects on auxin-inducible genes, although there is no single Arabidopsis mutant that has all of the characteristics of dgt. Recessive auxin-resistant mutants of Arabidopsis have been shown to affect the degradation of AUX/IAA proteins that act as repressors of Aux-REs by complexing with Aux-RE-binding proteins called ARFs (Kepinski and Leyser, 2002). The intact dgt response to cycloheximide makes it unlikely that DGT is an ARF: Once repressor proteins are removed by turning off their synthesis with the help of cycloheximide, gene expression in dgt tissues proceeds unhampered, demonstrating that the Aux-RE-activating proteins are intact in mutant cells. DGT may, however, function as a negative regulator of specific AUX/IAA proteins (Nebenführ et al., 2000; Balbi and Lomax, 2002). The differential effects of cytokinin on LE-ACS3 and LE-ACS5 mRNA induction by auxin suggests that their promoters may bind different AUX/IAA or ARF proteins. Our data predict that Aux-REs in both promoters directly or indirectly depend on DGT for their activation and that LE-ACS3 Aux-REs are repressed in the presence of cytokinins.
Our results support a model in which cytokinin effects on auxin responses are mediated through interactions between specific auxin- and cytokinin-signaling pathways rather than through a global effect of cytokinins on active auxin levels or all auxin responses. For example, selective inhibition of LE-ACS3 expression by cytokinin cannot be explained by cytokinin effects on active auxin pools, because decreased auxin concentrations should affect all auxin-inducible genes, including LE-ACS5 and SAURs. Other researchers have also reported an inhibitory effect of cytokinin on the expression of a subset of auxin-inducible genes (van der Zaal et al., 1987; Young et al., 1994), supporting the idea that cytokinins affect the transcription or stability of a subset of auxin-inducible mRNAs. The most parsimonious model for the observed interactions suggests that cytokinin inhibits a branch of the DGT-dependent auxin-signaling pathway (Fig. 9).
Figure 9.
Hypothetical model for the action of cytokinin and the DGT gene product on auxin responses in tomato hypocotyl segments. Through a DGT-dependent process, auxin stimulates the expression of auxin-inducible genes and H+ secretion, leading to auxin-induced ethylene synthesis and elongation. Cytokinin antagonizes a subset of these DGT-dependent auxin responses by inhibiting one branch of the auxin signal transduction pathway.
The model minimizes interaction points between auxin- and cytokinin-signaling pathways by predicting that cytokinin acts upon a signaling step that is required for both plasma membrane-localized and nuclear auxin effects, namely the activation of H+ pumping and LE-ACS3 expression (Fig. 9). Recent analyses of a rice (Oryza sativa) auxin-binding protein have suggested that auxin controls H+ pumping through binding to an intracellular protein that directly activates the H+-ATPase (Kim et al., 2000, 2001). Such an extremely short auxin signal transduction chain is potentially difficult to reconcile with our finding that the DGT gene product and cytokinin both affect events in the nucleus and at the plasma membrane. Any single auxin-binding protein mediating all of these interactions would then have to be controlled not only by DGT but also by the cytokinin-signaling pathway, and it would have to interact not only with the H+-ATPase but also with proteins able to relay the auxin signal to the nucleus. The auxin-binding protein-H+-ATPase complex potentially contains further, yet unidentified, proteins that could mediate such interactions.
A perhaps surprising symmetry in the model is that ethylene synthesis and elongation in hypocotyl segments each rely on one pathway that is inhibited by cytokinin and one that is not. Although the exogenous cytokinin concentrations required for the inhibition of these auxin responses appear high and may not reflect the endogenous cytokinin concentrations required for these responses, a similar interaction pattern between the hormones produced in planta might permit plants to fine-tune auxin responses via cytokinin without allowing cytokinins to shut off auxin responses completely. Alternatively, the network may allow individual cells and tissues to modulate the cytokinin dependence of their auxin responses by favoring one of the two response pathways.
MATERIALS AND METHODS
Plant Materials
For experiments testing the effect of long-term cytokinin application on the development of wild type and dgt tomato (Lycopersicon esculentum Mill.) plants in the light, we used dgt and its isogenic parent VFN8, which were originally a gift from Dr. Kent Bradford (University of California, Davis). The dgt mutant extensively backcrossed into the more fertile Ailsa Craig background (originally obtained from Dr. Charles M. Rick, University of California, Davis) was used for studies on hypocotyl segments because of the large amounts of mutant seed required in these experiments. The morphological traits of dgt are the same in the Ailsa Craig and VFN8 backgrounds (data not shown). All seeds used in this study came from field plants propagated by selfing at the Oregon State University Botany Farm. Before sowing, seeds were treated with 20% (v/v) household bleach for 10 min and rinsed in tap water.
Long-Term Cytokinin Treatment
Seeds were sown in Magenta boxes (7.5 × 7.5 × 10 cm, Sigma, St. Louis) on absorbent paper (Kimtowels, Kimberly-Clark, Roswell, GA) wetted with aqueous solutions of BA. After 2 d in the dark at 28°C, the boxes were transferred to an incubator equipped with wide-spectrum fluorescent lights (General Electric Plant and Aquarium, General Electric, Fairfield, CT). Seedlings were grown for 7 d at 28°C under a cycle of 16 h of light (50 μE of photosynthetically active radiation m−2 s−1) and 8 h of dark. Nine days after sowing, seedlings were transplanted into 5- × 6- × 6-cm plastic pots containing a soil-free potting mix wetted with BA solutions. The soil-free mix consisted of 3 L of vermiculite:1 L of expanded clay:50 g of Osmocote (14:14:14 [w/w] N:P:K):3 g of Micromax Micronutrients (Osmocote and micronutrients from Grace-Sierra Horticultural Products Company, Milpitas, CA). After 2 more d in the incubator, transplanted seedlings were grown in a greenhouse under natural light conditions at temperatures of 24°C (day) and 18°C (night). During this period the plants were watered with BA solutions and fertilized as needed with Osmocote and micronutrients. Plants were photographed 7 weeks after sowing.
Auxin-Induced Elongation and H+ Secretion
For discontinuous elongation measurements, seeds were sown in plastic boxes (32 × 26 × 10 cm) onto two layers of filter paper number 3 (Whatman, Clifton, NJ) moistened with distilled water, and incubated for 3 to 5 d at 28°C in the dark. Segments for elongation experiments were harvested and handled under dim-green light (<0.05 μmol m−2 s−1). Hypocotyl segments 6 mm in length were cut from immediately below the hook of seedlings that were 1 to 2 cm tall. For each treatment, 15 segments were floated on SM buffer (1% [w/v] Suc and 5 mm MES/KOH [pH 6.0]) for a 2-h pre-incubation period in darkness at 28°C. At the end of the pre-incubation, segments were measured with a dissecting microscope equipped with an ocular micrometer and transferred to SM buffer containing the appropriate growth regulators. Segments receiving BA during the incubation period also received BA during the pre-incubation. After specified incubation times in white light (145 μE m−2 s−1) or in darkness, the segments were remeasured, and the mean length increase was calculated. Mean segment length increases from three independent experiments were pooled to calculate final mean and se.
For continuous elongation measurements and for H+ secretion assays, seeds were spread on moist filter paper in Magenta boxes (Sigma) and incubated at 26°C in the dark. Four to 5 d after sowing, 1.5- to 2.5-cm-tall seedlings (measured from the hook to the root shoot node) were selected, and 1-cm hypocotyl segments were cut from immediately below the hook. Continuous growth measurements were performed with a custom-made CCD camera auxanometer as previously described (Christian and Lüthen, 2000) under either regular white light or under low light (6 μE m−2 s−1).
H+ secretion was monitored as described by Coenen et al. (2002). The cuticles of excised hypocotyl segments were abraded according to the method of Lüthen et al. (1990) by vortexing 0.2 to 0.3 g of hypocotyl segments in 5 mL of a 0.2 g mL−1 aqueous suspension of SiC-powder (1,200 mesh; K. Schriever, Hamburg, Germany) in a 50-mL conical polypropylene tube for 20 s at top speed on a Vortex Genie 2 model vortexer (Scientific Industries, Bohemia, NY). This abrasion time was optimized for accessibility of the apoplast, demonstrated by staining with Neutral Red, and lack of damage to epidermal cells, demonstrated by absence of staining with Evans blue (data not shown). Abraded segments (0.2–0.3 g) were rinsed extensively with distilled water and immediately transferred to 1 mL of well-aerated, unbuffered incubation medium (1 mm CaCl2 and 10 mm KCl) in a glass vial. The pH of the medium was continuously monitored with a pH electrode (InLab 423, Mettler Toledo, Steinbach, Germany) connected to a pH meter (Wissenschaftlich-Technische Werkstätten, Weilheim, Germany) and a chart recorder. IAA (Merck, Darmstadt, Germany) was added to 10 μL of a pH-adjusted aqueous solution, and FC was added in 1 μL of ethanol.
Auxin-Induced Ethylene Synthesis
Measurements of ethylene synthesis were performed essentially as described by Kelly and Bradford (1986). Hypocotyl segments were harvested and pre-incubated as for discontinuous elongation assays. Subsequently, 15 segments 1 cm in length were selected for each treatment and floated on 1 mL of SM buffer containing the appropriate growth regulators in a 10-mL vial. Vials were incubated uncapped for 2 h, then sealed and incubated for an additional 3 h to allow ethylene to accumulate. All incubations were under agitation at 28°C in the dark. One milliliter of the gas phase of each sample was analyzed on a gas chromatograph (Shimadzu, Columbia, MD) equipped with a 4-foot Poropak Q column and a flame ionization detector. Afterward, the segments were gently blotted on Kimtowels and weighed to normalize ethylene production for tissue fresh weight.
Gene Expression
Hypocotyls were excised from 5-d-old etiolated seedlings and cut into pieces approximately 1 cm in length. The segments were pre-incubated for 2 h in SM buffer and then incubated for an additional 2 h in SM buffer containing 100 μm IAA, 100 μm BA, or a combination thereof. Segments treated with BA during the final incubation period also received 100 μm BA during pre-incubation. All incubations were done at 28°C in the dark under gentle agitation.
RNA was extracted in the presence of guanidinium thiocyanate (Ausubel et al., 1998). Hypocotyl segments were weighed, frozen in liquid N2, and stored at −80°C. Tissue was thawed at 37°C in 1.5 mL g−1 extraction buffer (4 m guanidinium thiocyanate, 25 mm sodium citrate [pH 7.0], 0.5% [w/v] sarcosyl, and 0.76% [v/v] β-mercaptoethanol), and ground using a Tekmar homogenizer fitted with a small probe. After grinding, 150 μL of 2 m sodium acetate (pH 4.0), 1.5 mL of water-saturated phenol, and 300 μL of chloroform:isoamyl alcohol (49:1 [v/v]) were added for each gram fresh weight, and the extracts were centrifuged for 20 min at 3,600 rpm (3,000g) in a Beckman GPR (Beckman Instruments, Fullerton, CA) tabletop centrifuge. The upper aqueous phase was re-extracted with an equal volume of chloroform:isoamyl alcohol (49:1 [v/v]) and then precipitated with an equal volume of isopropanol. The RNA was further purified by subsequent reprecipitations with lithium chloride and sodium chloride.
RNA gel blots (Sambrook et al., 1989) were performed on 10 μg of total RNA using Hybond N+ nylon membranes (Amersham, Arlington Heights, IL) according to the manufacturer's instructions. Membranes were hybridized with [32P]-labeled probes generated by random primer labeling using the Decaprime kit (Ambion, Austin, TX). The LeSAUR probe was synthesized by labeling a 100-bp XhoI-BamHI fragment of the plasmid described by Mito and Bennett (1995). Probe (1.5 × 105 cpm) were added to 5 mL of hybridization solution. Filters were washed twice at room temperature in 2× SSC and 0.1% (w/v) SDS, and then for 1 h at 55°C in 1× SSC and 0.1% (w/v) SDS.
RNAse protection assays were carried out using the Ambion RPAII kit. The [35S]-labeled probes for partial sequence of the tomato ACC synthase genes LE-ACS3 (GenBank accession no. M83320) and LE-ACS5 (GenBank accession no. M83322) were generated by linearizing plasmids pBTAS2 and pBTAS3 (Yip et al., 1992) with BamHI and transcribing those templates from their T7 promoters using the Maxiscript kit (Ambion). The probes (2 × 105 cpm) were hybridized with 20 μg of total RNA at 42°C overnight in a hybridizing oven (VWR 1540, VWR, Seattle), and unhybridized RNA was digested with a mixture of ribonucleases A and T1. Radiolabeled fragments were analyzed by native PAGE and fluorography.
The signals on films from northern blots and RNAse protection assays were quantified by scanning the films in a densitometer (Molecular Dynamics, Sunnyvale, CA). The relative signal intensities were expressed as percentage of the maximum signal obtained in each experiment. Relative signal intensities were averaged for three independently grown and treated batches of hypocotyl tissue. Variation was expressed as the se from the three experiments.
Distribution of Materials
All chemicals were purchased from Sigma unless stated otherwise. Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We are grateful to Dr. Shang Fa Yang and his collaborators for providing the pBTAS clones and to Drs. Alan Bennett and Nobuaki Mito for the LeSAUR clone. We thank Drs. David L. Rayle and Mary J. Ellard-Ivey for valuable discussions and practical advice and Chris Lundberg for critical reading of the manuscript.
Footnotes
This work was supported by the National Science Foundation (grants to T.L.L.) and by the Deutsche Forschungsgemeinschaft (grant no. BO 537/16–2 to H.L. and M.C. and postdoctoral fellowship no. CO 235/1–1 to C.C.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.016196.
LITERATURE CITED
- Abel S, Ballas N, Wong LM, Theologis A. DNA elements responsive to auxin. BioEssays. 1996;18:647–654. doi: 10.1002/bies.950180808. [DOI] [PubMed] [Google Scholar]
- Abel S, Nguyen MD, Chow W, Theologis A. ASC4, a primary indole acetic acid-responsive gene encoding 1-aminocyclopropane 1-carboxylate synthases in Arabidopsis. J Biol Chem. 1995;270:19093–19099. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- Abeles FB. Auxin stimulation of ethylene production. Plant Physiol. 1966;41:585–588. doi: 10.1104/pp.41.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley and Sons, Inc.; 1998. [Google Scholar]
- Balbi V, Lomax TL. Regulation of early tomato fruit development by the Diageotropica gene. Plant Physiol. 2003;131:186–197. doi: 10.1104/pp.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Lüthen H. New methods to analyse auxin-induced growth I: classical auxinology goes Arabidopsis. Plant Growth Regul. 2000;32:107–114. [Google Scholar]
- Cleland RE. Auxin and cell elongation. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 214–227. [Google Scholar]
- Coenen C, Bierfreund N, Lüthen H, Neuhaus G. Developmental regulation of proton ATPase-dependent auxin responses in the diageotropica mutant of tomato (Lycopersicon esculentum Mill.) Physiol Plant. 2002;114:461–471. doi: 10.1034/j.1399-3054.2002.1140316.x. [DOI] [PubMed] [Google Scholar]
- Coenen C, Lomax TL. Auxin-cytokinin interactions in higher plants: old problems and new tools. Trends Plant Sci. 1997;2:351–356. doi: 10.1016/S1360-1385(97)84623-7. [DOI] [PubMed] [Google Scholar]
- Coenen C, Lomax TL. The DIAGEOTROPICA gene differentially affects auxin and cytokinin responses throughout development in tomato (Lycopersicon esculentum Mill.) Plant Physiol. 1998;117:63–72. doi: 10.1104/pp.117.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRopp R. Kinetin and auxin activity. Plant Physiol. 1956;31:253–254. doi: 10.1104/pp.31.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklöf S, Åstot C, Blackwell J, Moritz T, Olsson O, Sandberg G. Auxin-cytokinin interactions in wild-type and transgenic tobacco. Plant Cell Physiol. 1997;38:225–235. [Google Scholar]
- Fuchs Y, Lieberman M. Effect of kinetin, IAA, and gibberellin on ethylene production, and their interactions in growth of seedlings. Plant Physiol. 1968;43:2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee MA, Hagen G, Guilfoyle TJ. Tissue-specific and organ-specific expression of soybean auxin-responsive transcripts GH3 and SAURs. Plant Cell. 1991;3:419–430. doi: 10.1105/tpc.3.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle TJ, Hagen G. Transcriptional regulation of auxin responsive genes. In: Fox JE, Jacobs M, editors. Molecular Biology of Plant Growth Control. New York: Alan Liss Inc.; 1986. pp. 85–89. [Google Scholar]
- Hinchee MAW, Rost TL. The control of lateral root development in cultured pea seedlings. Bot Gaz. 1986;147:137–147. [Google Scholar]
- Hobbie L, Estelle MA. Genetic approaches to auxin action. Plant Cell Environ. 1994;17:525–540. doi: 10.1111/j.1365-3040.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Hutchison CE, Kieber JJ. Cytokinin signaling in Arabidopsis. Plant Cell Suppl. 2002;14:S47–S59. doi: 10.1105/tpc.010444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaseki H, Kondo K, Watanabe A. Mechanism of cytokinin action on auxin-induced ethylene production. Plant Cell Physiol. 1975;16:777–787. [Google Scholar]
- Kelly M, Bradford KJ. Sensitivity of the diageotropica tomato mutant to auxin. Plant Physiol. 1986;82:713–717. doi: 10.1104/pp.82.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser HMO. Ubiquitination and auxin signaling: a degrading story. Plant Cell Suppl. 2002;14:S81–S95. doi: 10.1105/tpc.010447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WT, Silverstone A, Yip WK, Dong JG, Yang SF. Induction of 1-aminocyclopropane-1-carboxylate synthase mRNA by auxin in mung bean hypocotyls and cultured apple shoots. Plant Physiol. 1992;98:465–471. doi: 10.1104/pp.98.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kim D, Jung J. Two isoforms of soluble auxin receptor in rice (Oryza sativa L.) plants: binding property for auxin and interaction with plasma membrane H+-ATPase. Plant Growth Regul. 2000;32:143–150. [Google Scholar]
- Kim YS, Min JK, Kim D, Jung J. A soluble auxin-binding protein, ABP(57): purification with anti-bovine serum albumin antibody and characterization of its mechanistic role in the auxin effect on plant plasma membrane H+-ATPase. J Biol Chem. 2001;276:10730–10736. doi: 10.1074/jbc.M009416200. [DOI] [PubMed] [Google Scholar]
- Klee HJ. Transgenic plants and cytokinin biology. In: Mok DW, Mok MC, editors. Cytokinins: Chemistry, Activity and Function. Boca Raton, FL: CRC Press; 1994. pp. 289–293. [Google Scholar]
- Klee HJ, Horsch RB, Hinchee MAW, Hein MB, Hoffmann NL. The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev. 1987;1:86–96. [Google Scholar]
- Lau O, Yang SF. Mechanism of a synergistic effect of kinetin on auxin-induced ethylene production. Plant Physiol. 1973;51:1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HMO, Pickett FB, Dharmasiri S, Estelle MA. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. An auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell. 1991;3:1167–1175. doi: 10.1105/tpc.3.11.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shi X, Strabala T, Hagen G, Guilfoyle TJ. Transgenic tobacco plants that overproduce cytokinins show increased tolerance to exogenous auxin and auxin transport inhibitors. Plant Sci. 1994;100:9–14. [Google Scholar]
- Llop-Tous I, Barry CS, Grierson D. Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol. 2000;123:971–978. doi: 10.1104/pp.123.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthen H, Bigdon M, Böttger M. Reexamination of the acid growth theory of auxin action. Plant Physiol. 1990;93:931–939. doi: 10.1104/pp.93.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Mok MC, Mok DW. Protein processing and auxin response in transgenic tobacco harboring a putative cDNA of zeatin O-xylosyltransferase from Phaseolus vulgaris. Plant J. 1997;12:305–312. doi: 10.1046/j.1365-313x.1997.12020305.x. [DOI] [PubMed] [Google Scholar]
- McClure BA, Guilfoyle TJ. Characterization of a class of small auxin-inducible soybean polyadenylated RNAs. Plant Mol Biol. 1987;6:611–623. doi: 10.1007/BF00020537. [DOI] [PubMed] [Google Scholar]
- Medford JI, Horgan R, El-Sawi Z, Klee HJ. Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell. 1989;1:403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito N, Bennett AB. The diageotropica mutation and synthetic auxins differentially affect the expression of auxin-regulated genes in tomato. Plant Physiol. 1995;109:293–297. doi: 10.1104/pp.109.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa Y, Sakai A, Miyagishima S, Takano H, Kawamo S, Kuroiwa T. Auxin and cytokinin have opposite effects on amyloplast development and the expression of starch synthesis genes in cultured bright yellow-2 tobacco cells. Plant Physiol. 1999;121:461–469. doi: 10.1104/pp.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta. 1995;195:548–553. doi: 10.1007/BF00195714. [DOI] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, White T, Lomax TL. The diageotropica mutation alters auxin induction of a subset of the Aux/IAA gene family of tomato. Plant Mol Biol. 2000;44:73–84. doi: 10.1023/a:1006437205596. [DOI] [PubMed] [Google Scholar]
- Romano CP, Hein MB, Klee HJ. Inactivation of auxin in tobacco transformed with the indole acetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev. 1991;5:438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Sachs T, Thimann KV. The role of auxins and cytokinins in the release of buds from dominance. Am J Bot. 1967;54:136–144. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shiu OY, Oetiker JH, Yip WK, Yang SF. The promoter of LE-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transcript. Proc Natl Acad Sci USA. 1998;95:10334–10339. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon F, Hennion S, Sundberg B, Little CHA, Olsson O, Sandberg G. Transgenic tobacco plants coexpressing the Agrobacterium tumefaciens iaaM and iaaH gene display altered growth and indole acetic acid metabolism. Plant Physiol. 1992;99:1062–1069. doi: 10.1104/pp.99.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–131. [PubMed] [Google Scholar]
- Smalle J, Kurepa J, Yang P, Babiychuk E, Kushnir S, Durski A, Vierstra RD. Cytokinin growth responses in Arabidopsis involve the 26S proteasome subunit RPN12. Plant Cell. 2002;14:17–32. doi: 10.1105/tpc.010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spena A, Prinsen E, Fladung M, Schulze SC, Van Onckelen H. The indole acetic acid-lysine synthetase gene of Pseudomonas syringae subsp. savastanoi induces developmental alterations in transgenic tobacco and potato plants. Mol Gen Genet. 1991;227:205–212. doi: 10.1007/BF00259672. [DOI] [PubMed] [Google Scholar]
- Timpte C, Lincoln CA, Pickett FB, Turner JC, Estelle MA. The AXR1 and AUX1 genes of Arabidopsis function in separate auxin response pathways. Plant J. 1995;8:561–569. doi: 10.1046/j.1365-313x.1995.8040561.x. [DOI] [PubMed] [Google Scholar]
- Timpte C, Wilson AK, Estelle MA. Effects of the axr2 mutation on Arabidopsis on cell shape in hypocotyl and inflorescence. Planta. 1994;188:271–278. doi: 10.1007/BF00216824. [DOI] [PubMed] [Google Scholar]
- Troll W. Die Infloreszenzen, Typologie und Stellung im Aufbau des Vegetationskörpers. Stuttgart: Fischer; 1969. [Google Scholar]
- Vanderhoef LN, Stahl CA. Separation of two responses to auxin by means of cytokinin inhibition. Proc Natl Acad Sci USA. 1975;72:1822–1825. doi: 10.1073/pnas.72.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef LN, Stahl C, Siegel N, Zeigler R. The inhibition by cytokinin of auxin-promoted elongation in excised soybean hypocotyl. Physiol Plant. 1973;29:22–27. doi: 10.1104/pp.52.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zaal EJ, Memelink J, Mennes AM, Quint A, Libbenga KR. Auxin-induced mRNA species in tobacco cell cultures. Plant Mol Biol. 1987;10:145–157. doi: 10.1007/BF00016152. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Woesk KE, Theologis A, Kieber J. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA. 1998;95:4766–4771. doi: 10.1073/pnas.95.8.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F, Schneider EA, Thimann KV. Hormonal factors controlling the initiation and development of lateral roots: II. Effects of exogenous growth factors on lateral root formation in pea roots. Physiol Plant. 1980;49:304–314. [Google Scholar]
- Yip WK, Moore T, Yang SF. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci USA. 1992;89:2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IS, Park DH, Mori H, Imaseki H, Kang BG. Characterization of an auxin-inducible 1-aminocyclopropane-1-carboxylate synthase gene, VR-ACS6, of mungbean (Vigna radiata (L.) Wilczek) and hormonal interactions on the promoter activity in transgenic tobacco. Plant Cell Physiol. 1999;40:431–438. doi: 10.1093/oxfordjournals.pcp.a029559. [DOI] [PubMed] [Google Scholar]
- Young RJ, Scheuring CF, Harris-Haller L, Taylor BH. An auxin-inducible proteinase inhibitor gene from tomato. Plant Physiol. 1994;104:811–812. doi: 10.1104/pp.104.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang X, Wang J, Letham DS, McKinney SA, Higgins TJV. The effect of auxin on cytokinin levels and metabolism in transgenic tobacco tissue expressing an ipt gene. Planta. 1995;196:84–94. [Google Scholar]
- Zobel RW. Genetics of the diageotropica mutant of the tomato. J Hered. 1972;63:91–97. [Google Scholar]
- Zobel RW. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol. 1973;52:385–389. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek DM, Rayle DL, McMorris TC, Clouse SD. Investigation of gene expression, growth kinetics, and wall extensibility during brassinosteroid-regulated stem elongation. Plant Physiol. 1994;104:505–513. doi: 10.1104/pp.104.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]