Abstract
It is demonstrated that, in etiolated pea (Pisum sativum) epicotyls, ethylene affects the activation of both monomeric GTP-binding proteins (monomeric G-proteins) and protein kinases. For monomeric G-proteins, the effect may be a rapid (2 min) and bimodal up-regulation, a transiently unimodal activation, or a transient down-regulation. Pretreatment with 1-methylcyclopropene abolishes the response to ethylene overall. Immunoprecipitation studies indicate that some of the monomeric G-proteins affected may be of the Rab class. Protein kinase activity is rapidly up-regulated by ethylene, the effect is inhibited by 1-methylcyclopropene, and the activation is bimodal. Immunoprecipitation indicates that the kinase(s) are of the MAP kinase ERK1 group. It is proposed that the data support the hypothesis that a transduction chain exists that is separate and antagonistic to that currently revealed by studies on Arabidopsis mutants.
Great progress has been made in the elucidation of components of the ethylene signal transduction pathway (for a review, see Hall et al., 2001), mainly through studies on Arabidopsis mutants. Such components include five partially functionally redundant receptors (Chang et al., 1993; Hua et al., 1995; Sakai et al., 1998), a protein (CTR1) having homology with mitogen-activated protein kinase kinase kinases (MAPKKK; Kieber et al., 1993), further downstream components such as the EIN series (Johnson and Ecker, 1998; Alonso et al., 1999), and ethylene response element-binding proteins (Solano et al., 1998). A significant feature of the system is that the receptors appear to be negative regulators, that is, they are active in the absence of ligand and inactive in its presence (Hua and Meyerowitz, 1998).
Implicit in the presence of a putative MAPKKK in the transduction sequence is that protein phosphorylation via a mitogen-activated protein kinase (MAPK) cascade(s) is involved in mediating responses to ethylene. It has been shown in tobacco (Nicotiana tabacum; Raz and Fluhr, 1993), pea (Pisum sativum; Berry et al., 1996), and Arabidopsis (Novikova et al., 1999) that ethylene up-regulates protein phosphorylation overall and that in the dominant receptor mutant etr1-1, the process is down-regulated relative to wild type (Novikova et al., 1999). Equally, there is evidence that ethylene affects protein kinase activity. Thus, in tobacco, ethylene activates PK12, a protein kinase of the LAMMER type and increases levels of its transcript (Sessa et al., 1996). Equally, in Arabidopsis, ethylene increases protein kinase activity, which immunoprecipitation studies with anti-phospho-Tyr antibodies and antibodies to the canonical MAPK ERK1 indicate is of the MAPK family (Novikova et al., 2000). Moreover, the same work showed that ethylene-treated tissue had higher concentrations of phosphorylated MAPK than controls, suggesting that, at least in part, the activation evoked by ethylene is of pre-existing enzyme. Recently, Kumar and Klessig (2000) have demonstrated that, in tobacco cells, treatment with the ethylene precursor aminocyclopropane carboxylic acid leads to increased MAPK activity. In the ctr1-1 mutant, MAPK activity is much higher than in wild type, but clearly this activity and the increased activity observed in wild type in response to ethylene cannot be part of the MAPK cascade initiated by CTR1 (Novikova et al., 2000).
Other candidates that have emerged as possible components of the ethylene signal transduction pathway are monomeric GTP-binding proteins (monomeric G-proteins). Thus, both in pea (Novikova et al., 1997) and in Arabidopsis (Novikova et al., 1999), it has been shown that ethylene at physiological concentrations activates monomeric G-proteins. The activation in Arabidopsis leaves is antagonized by cytokinin, and in the etr1-1 mutant, activity is constitutively down-regulated. Other work by Zegzouti et al. (1999) has shown that, in tomato (Lycopersicon esculentum) fruit, the transcription of a gene, which showed high homology to a monomeric G-protein from pea, is rapidly but transiently up-regulated by ethylene. Monomeric G-proteins are key components of many signal transduction pathways in both animals (Bos, 2000) and yeast (Schmidt and Hall, 1998), and there is increasing evidence for a role for them in plants (Schiene et al., 2000; Valster et al., 2000; Zhang et al., 2000; Li et al., 2001; Lu et al., 2001; Ono et al., 2001). Like MAPKs, monomeric G-proteins can themselves form cascades (Feig et al., 1996; Van Aelst and D'Souza-Schorey, 1997; Bar-Sagi and Hall, 2000)—that is, they can interact both synergistically or antagonistically—and have a number of different effectors including MAPKKKs (Leevers et al., 1994; Morrison and Cutler, 1997; Lewis et al., 1998).
We have argued that the data on the activation of monomeric G-proteins and MAPK suggest a role for these components in ethylene signal transduction but that they are somehow antagonistic to the linear transduction chain revealed by studies on mutants (Chang and Shockey, 1999; Larsen and Chang, 2001). Clearly, however, it is necessary to explore further these effects. Thus, the objectives of the work described here were to characterize the effect of ethylene on these two signaling components in more detail in terms of their pattern, kinetics, and specificity. The results presented here indicate the involvement of several monomeric G-proteins in the transduction of ethylene effects and that the patterns and duration of the activation of these and of MAPK activity closely parallel those observed in some animal systems (Meloche et al., 1992; Lenormand et al., 1993; Foschi et al., 1997).
RESULTS
Ethylene Induces Changes in Activation of Monomeric G-Proteins
As shown previously (Novikova et al., 1997, 1999), ethylene promoted GTP binding to proteins in the appropriate range of molecular masses for monomeric G-proteins (20–30 kD); other bands detected at 30 to 40 kD are the result of nonspecific binding, as indicated by the inability of unlabeled GTP to compete. Activation of monomeric G-proteins by ethylene was abolished by the receptor-directed inhibitor 1-methylcyclopropene (MCP; Sisler and Serek, 1997) and markedly reduced by the receptor-directed inhibitor 2,5-norbornadiene (NBD).
MCP antagonizes the triple response in pea seedlings (Table I). Thus, a 2-h pretreatment of epicotyls with 100 nL L−1 MCP followed by treatment with 1 μL L−1ethylene significantly reduced the ethylene response; MCP alone had little effect other than that attributable to suppression of responses to endogenous ethylene. NBD showed similar effects.
Table I.
The effects of MCP and NBD on the ethylene-induced triple response in pea seedlings
| Treatment | Hook Angle | Epicotyl Width | Epicotyl Length |
|---|---|---|---|
| % | |||
| Control | 100 | 100 | 100 |
| Ethylene (1 μL L−1) | 198 ± 5 | 136 ± 1 | 70 ± 1 |
| MCP (100 nL L−1) | 91 ± 11 | 94 ± 2 | 111 ± 4 |
| MCP + ethylene | 108 ± 5 | 102 ± 2 | 104 ± 2 |
| NBD (2,000 μL L−1) | 88 ± 5 | 93 ± 1 | 115 ± 4 |
| NBD + ethylene | 131 ± 7 | 95 ± 2 | 99 ± 3 |
Four-day-old seedlings were incubated for 48 h at 23°C in the dark. Where ethylene was used together with inhibitors, the latter were added at zero time, and ethylene was added after 1 h. Data are presented as a percentage of the control. Values indicate the means ± se of 45 measurements.
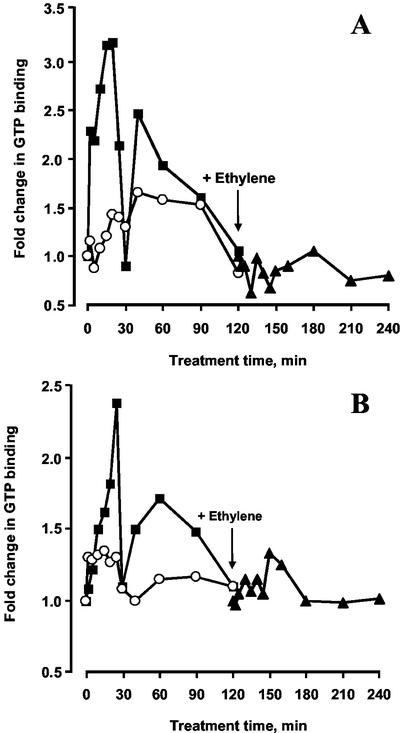
Time courses were carried out on the effect of ethylene on both monomeric G-proteins released by treatment with 750 mm KCl and those subsequently solubilized from membranes by 1% (w/v) Triton X-100 (representing extrinsic and integral membrane proteins, respectively); samples were initially examined by one-dimensional electrophoresis. In five separate experiments, the same pattern was observed in all cases. Thus, in the KCl-solubilized fraction, activity doubled within 2 min of ethylene application reaching a peak at about 3-fold activation at 20 min and falling thereafter to control levels at about 30 min (Fig. 1A). There was then a further increase, representing a more than 2-fold activation after 40 min and a subsequent decrease to control levels at 2 h. Within the five experiments, the activation at 20 min ranged between 2- and 4-fold and that at 40 min between 1.5- and 3-fold. The proteins extracted with Triton X-100 exhibited a similar pattern to the KCl-extracted proteins except that the response was slower and the overall activation was lower with the first peak occurring at 25 min and the second at 60 min (Fig. 1B; but see below). Again, by 2 h, activity had returned to basal levels. The activation at 25 min ranged from 2- to 4-fold and in the second peak from 1.5- to 3-fold. It should be noted, however, that more than 75% of the total activated monomeric G-proteins is present in the Triton X-100 fraction (Novikova et al., 1997).
Figure 1.
Time course of GTP binding to monomeric G-proteins extracted with 750 mm KCl (A) and 1% (w/v) Triton X-100 (B) as affected by ethylene (1 μL L−1, ●), MCP (100 nL L−1, ○) and when ethylene was applied after pretreatment with MCP (▾). The arrow indicates time point of ethylene application. Experimental points are derived from scans of one-dimensional autoradiographs of SDS-PAGE separations of labeled proteins.
Treatment with MCP had a small but consistent promotory effect in both fractions, reminiscent of the effects of ethylene. When ethylene was added immediately after the treatment with MCP, no significant response was observed in either fraction.
Two-Dimensional Separation of Monomeric G-Proteins Reveals Great Complexity
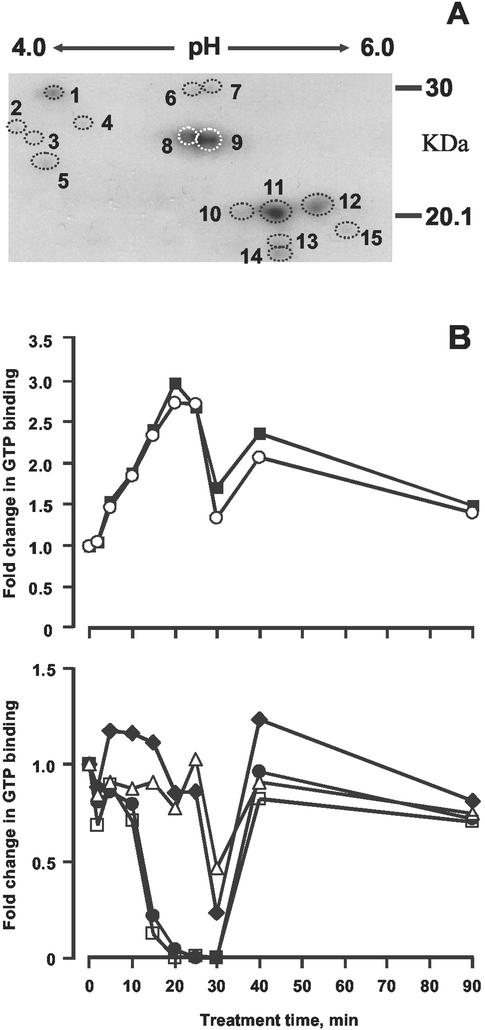
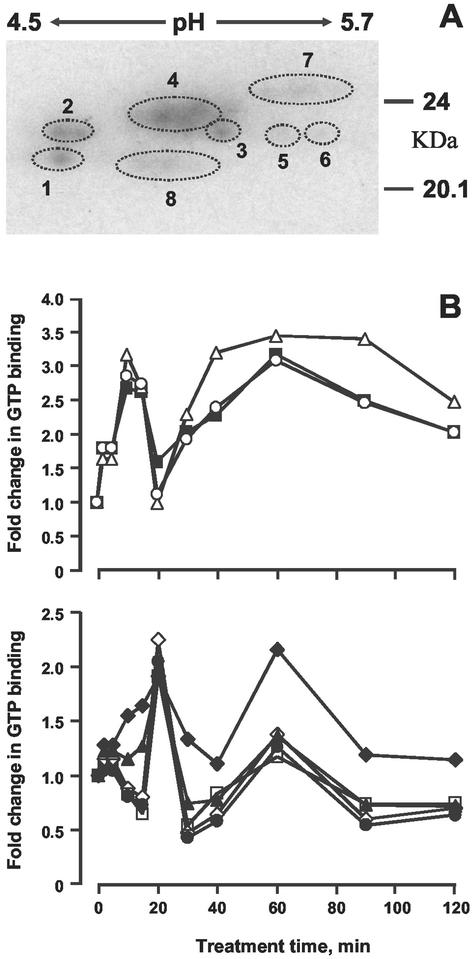
Autoradiographs of two-dimensional separations of monomeric G-proteins in both fractions are shown in Figures 2A and 3A. At least two gels were run for each of the five biological replicates and representative autoradiographs are presented. In the KCl fraction, 15 (at least) distinct spots are observable, of which 14 could be rigorously quantified by densitometry, and the patterns of activation are shown in Figures 2B. In the Triton fraction, the components could not be resolved rigorously, a common problem with hydrophobic proteins; nevertheless, eight distinct groupings could be identified (Fig. 3B).
Figure 2.
Two-dimensional separations of G-proteins. Proteins were extracted with 750 mm KCl from untreated or ethylene-treated (1 μL L−1) epicotyls and separated by two-dimensional gel electrophoresis. A, Designation of spots; B, quantification of GTP binding during time course: 2 (●), 6 (♦), 8 (▪), 9 (○), 12 (▵), 14 (□).
Figure 3.
Two-dimensional separations of G-proteins. Proteins were extracted with 1% (w/v) Triton X-100 from untreated or ethylene-treated (1 μL L−1) epicotyls and separated by two-dimensional gel electrophoresis. A, Designation of spots; B, quantification of GTP binding during time course: 1 (⋄), 2 (●), 3 (♦), 4 (▴), 5 (▪), 6 (▵), 7 (○), 8 (□).
In the KCl fraction, two components were rapidly activated reaching a maximum of 3-fold after 20 min (Fig. 2B). Activity fell back significantly by 30 min but rose again at 40 min, thereafter decreasing to a constant level by 60 min. Four further components showed a deactivation with two reaching a minimum at about 15 to 20 min and the others at 30 min. Both groups returned to the baseline by 40 min, remaining more or less constant thereafter.
In the Triton fraction, three components show a bimodal pattern of activation as in the KCl fraction, with a significant increase after 2 min and peaks at 10 and 40 min (Fig. 3B). A further component also shows a bimodal pattern, but the peaks appear at 20 and 60 min. Three further components show a distinct transient activation with a peak at 20 min; changes after the first peak are too small to be significant, and the overall pattern can be seen as unimodal. It should be noted that not all of the components are necessarily separate entities because it is well established that procedures before electrophoresis may modify proteins such that a single component can give rise to more than one spot (Celis and Gromov, 1999), although the several different patterns we have observed here indicate that a number of different monomeric G-proteins are involved. However, unlike the situation with heterotrimeric G-proteins, activation and inactivation of monomeric G-proteins requires the participation of several accessory proteins (Buday and Downward, 1993; Aronheim et al., 1994; Joneson and Bar-Sagi, 1997), and hence the differences in the timing of activation between the two fractions may reflect the kinetics of assembly of such accessory proteins into signaling complexes. An alternative but not mutually exclusive possibility is that because monomeric G-proteins can themselves form cascades (Ridley et al., 1992; Chang et al., 1994; Chant and Stowers, 1995; Nobes and Hall, 1995; Bender et al., 1996), then the pattern may in part reflect this. It is also relevant to observe that not all the monomeric G-proteins present in the one-dimensional gels from which the data in Figures 2 and 3 were obtained are present in the two-dimensional gels because several monomeric G-proteins have predicted pIs >6.5 (Huber et al., 1994).
Immunoprecipitation with Rab8 Antibodies Shows Differential Effects
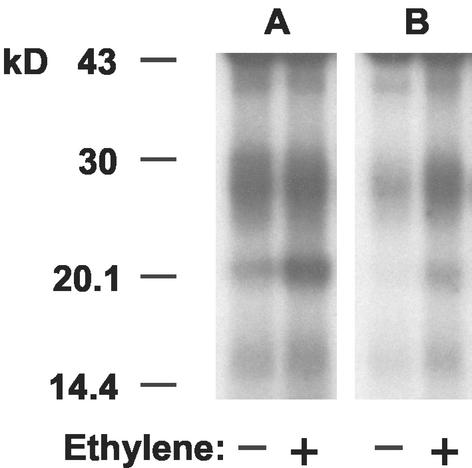
We have observed rapid activation of transcription of the Ara3 and Rab8 genes in Arabidopsis (Moshkov et al., 2003) and therefore undertook immunoprecipitation studies with antibodies to the latter protein; the results are shown in Figure 4. Two diffuse bands were revealed between 20 and 30 kD, but with much higher abundance of antigens in the KCl-solubilized fraction. Activation by ethylene was observed in the lower molecular mass band in both fractions. Although this was also the case for the higher molecular mass band in the Triton fraction, it was not observable in the KCl fraction.
Figure 4.
Immunoprecipitation of [α-32P]GTP-labeled monomeric G-proteins with anti-Rab 8 antibodies. A, KCl (750 mm)-extracted membrane proteins; B, 1% (w/v) Triton X-100-solubilized membrane proteins. Fractions were derived from epicotyls treated with ethylene (1 μL L−1, 20 min).
Protein Kinase Activity Increases Are Bimodal in Response to Ethylene
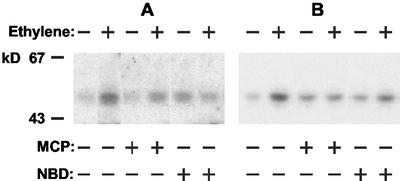
Pea epicotyls were incubated for 1 h in the presence of 1 μL L−1 ethylene, and extracts were subjected to immunoprecipitation with antibodies to either the mammalian MAPK ERK1 or phospho-Tyr. The immunoprecipitates were used in in-gel assays using myelin basic protein (MBP) as a substrate. In both cases, ethylene treatment led to a marked increase in a band at 48 ± 2 kD (Fig. 5, A and B). The results from five separate experiments indicated an activation of at least 2-fold and up to 5-fold. MCP reduced the ethylene-induced increase by more than 50%. Interestingly, MCP alone consistently increased MBP phosphorylation by up to 3-fold, albeit always much less than that shown by ethylene in the same experiments. Similar but less marked effects were obtained with NBD; again, the antagonist alone increased MBP phosphorylation. Experiments using in vitro assays of extracts gave similar results as did the use of Histone H1 as a substrate (albeit in the latter case with lower overall activity).
Figure 5.
MAP kinase activity in pea epicotyls as affected by ethylene, MCP, and NBD. Pea seedlings were treated with ethylene (1 μL L−1, 20 min), MCP (100 nL L−1, 2 h), NBD (2,000 μL L−1, 2 h), or inhibitors of ethylene binding (as indicated above) followed by ethylene treatment. MAP kinase was assayed after precipitation with either anti-ERK1 (A) or anti-phospho-Tyr (B) antibodies by means of in-gel assays.
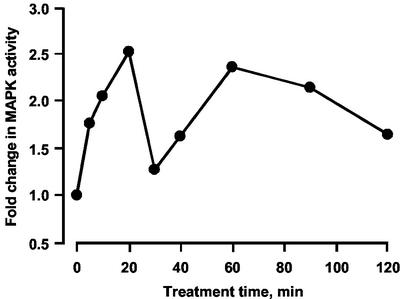
Time-course studies on in vitro MBP phosphorylation, representative of five separate experiments, are shown in Figure 6. Activity increases within 5 min of ethylene treatment and peaks at more than 2-fold after 20 min. Activity falls back almost to control levels by 30 min, but there is then a further rise to the level seen at 20 min between 40 and 60 min followed by a slow decrease over the next hour. The ranges of activity at 20 min varied between 2- and 5-fold and for the second peak at a similar or slightly lower level.
Figure 6.
Time course of ethylene-modulated MAP kinase activity. Pea seedlings were treated with 1 μL L−1 ethylene for different time periods followed by isolation of cytosolic proteins. MAP kinase activity was assayed in vitro. Experimental points were derived from scans of autoradiographs of SDS-PAGE separations phosphorylated MBP.
DISCUSSION
The data presented here clearly demonstrate that ethylene affects the activation of both monomeric G-proteins and protein kinase(s) in pea epicotyls, reflecting our previous findings in Arabidopsis (Novikova et al., 1999, 2000); the immunoprecipitation data suggest that the protein kinase(s) is of the MAPK ERK1 type and that at least some of the monomeric G-proteins affected are of the Rab type. Some of the effects on activation are, moreover, very rapid and show a distinct bimodal pattern. This latter phenomenon is well established in animal systems for both monomeric G-proteins and for MAPKs in response to a continuous signal (Meloche et al., 1992; Lenormand et al., 1993; Foschi et al., 1997) but has not, to our knowledge, been demonstrated in plants. The magnitudes of the activations observed are comparable with those seen in animals (Denhardt, 1996).
Transient activation of MAPKs has now been observed in response to both abiotic and biotic stresses (Droillard et al., 2000; Grant et al., 2000; Ichimura et al., 2000; Kovtun et al., 2000; Kumar and Klessig, 2000; Mikolajczyk et al., 2000; Samuel et al., 2000; Zhang et al., 2000; Desikan et al., 2001) and to growth regulators other than ethylene (Huttly and Phillips, 1995; Knetsch et al., 1996; Kovtun et al., 1998; Burnett et al., 2000; Mockaitis and Howell, 2000). The work of Kumar and Klessig (2000) on tobacco cells detected such a transient increase in response to 1-aminocyclopropane-1-carboxylic acid, but bimodality was not observed. This may indicate a fundamental difference in response in their system or possibly reflects the choice and number of time points at which activity was recorded.
It is notable that in animal systems, it is now clear that the kinetics of the response elicited in terms of MAPK activation may be as important as the identity of the MAPK itself and the magnitude of the response (Marshall, 1995; York et al., 1998). It may be significant that there is a correlation between the patterns for the two signaling components investigated here because of the widespread involvement of monomeric G-proteins in the activation of MAPKs (Tan and Kim, 1999).
At first sight, the apparent multiplicity of monomeric G-proteins modulated by ethylene appears surprising. However, animal paradigms indicate that several G-proteins may be involved in transduction for a single signal (Mira et al., 2000; Zondag et al., 2000; Sahai et al., 2001), and signaling between individual monomeric G-proteins is a feature of many systems (Feig et al., 1996; Van Aelst and D'Souza-Schorey, 1997; Bar-Sagi and Hall, 2000). It should be noted here, however, that the two fractions used represent extrinsic or integral membrane proteins and that some of the changes—including the down-regulation of some components—may reflect a transformation of extrinsic proteins into the integral state, something that is a characteristic of monomeric G-proteins (Zhang and Casey, 1996; Choy et al., 1999; Rodriguez-Concepcion et al., 1999). On the other hand, we have observed that in Arabidopsis, ethylene treatment may result in either up-regulation or down-regulation of transcription of genes for monomeric G-proteins (Moshkov et al., 2003).
Apart from any role in the induction of MAPK activity, Rab class monomeric G-proteins are known to be involved in membrane trafficking (Olkkonen and Stenmark, 1997; Chavrier and Goud, 1999; Waters and Pfeffer, 1999; Batoko et al., 2000) and transgenic tomato plants containing antisense Rab11 constructs exhibit abnormal phenotypes and reduced fruit softening (Li et al., 2001). Similarly, we have demonstrated rapid up-regulation of transcription of two Rab class genes (Rab8 and Ara3) in Arabidopsis leaves in response to ethylene (Moshkov et al., 2003).
It is not clear how the present findings, together with those for MAPKs and monomeric G-proteins in Arabidopsis (Novikova et al., 1999, 2000; Moshkov et al., 2003), fit in with the pathway established for ethylene signal transduction as derived from work on Arabidopsis mutants and which also appears to be the case with tomatoes (Tieman et al., 2000). If this pathway operates in pea, then ethylene perception will lead to down-regulation of a CTR1 homolog, which will, in turn, presumably down-regulate the MAPK cascade dependent upon it (and there is evidence that such a cascade exists, at least in maize [Zea mays; Kovtun et al., 1998]). Clearly, an increase in MAPK activation in response to ethylene cannot be directly attributable to CTR1; we have already shown that in the ctr1-1 mutant itself, MAPK activity is much higher than in wild type (Novikova et al., 2000). Equally, no Arabidopsis mutants have been produced where the lesion has been shown to be in a gene for a monomeric G-protein. Conversely, the rapidity of activation of some monomeric G-proteins demonstrated here suggests direct receptor activation; we know of no other such rapid effect of ethylene on a biochemical process in an intact plant. Moreover, in animals and yeast, activation of monomeric G-proteins is almost invariably directly via a receptor.
We have argued elsewhere (Hall et al., 2001) that the explanation of these results lies in the existence of a transduction chain(s) whose role is antagonistic to that of the chain whose existence is established. There is now a not dissimilar paradigm in plants in relation to auxin signaling. Thus, Mizoguchi et al. (1994) showed that auxin treatment of tobacco cv Bright Yellow-2 cells led to the activation of MAPK. More recently, Mockaitis and Howell (2000) have shown in Arabidopsis roots that auxin treatment leads to a rapid but transient activation of MAPK. On the other hand, transient expression of the MAPKKK NPK1 in maize mesophyll protoplasts led to a suppression of auxin-induced gene expression, an effect reversed by expression of the protein phosphatase MP2C (Kovtun et al., 1998). This indicates the presence of antagonistic signaling chains.
It is generally accepted that the known ethylene receptors are negative regulators, that is, that they are “signaling active” in the absence of ethylene but that they become “inactivated” on binding the ligand hence resulting in the down-regulation of CTR1 and leading to an ethylene effect. If an antagonistic chain(s) does exist, then the corollary would be that the “active” receptor suppresses this, directly or indirectly. The fact that in the dominant negative mutant etr1-1 (where the receptor appears to be in the active state), both constitutive MAPK activity (Novikova et al., 2000) and monomeric G-protein activity (Novikova et al., 1999) are down-regulated lends some support to this proposition. Equally, we have shown that in ctr1-1, not only constitutive MAPK activity is up-regulated, but monomeric G-protein activity is also (Moshkov et al., 2003). The parallel effects of these mutations and of ethylene itself on gene transcription are outlined elsewhere (Moshkov et al., 2003). It is interesting that some of the monomeric G-proteins show transient down-regulation in response to ethylene, suggesting that in some cases the active receptor may be responsible for the up-regulation of some components; however, as noted above, there are other explanations for this phenomenon. Proof of the involvement of the components reported here must await their precise identification and the production of appropriate transgenics, work which is ongoing in our laboratory.
MATERIALS AND METHODS
Plant Material and Treatments
Pea (Pisum sativum L. cv Sugar Snap) seeds were soaked overnight in tap water at room temperature, and after removal of the testas, the seeds were sown in trays containing vermiculite. The seedlings were grown for 4 d in the dark at 23°C and watered daily. The intact seedlings were transferred into 1-L Kilner jars (15 seedlings in each) for treatments. Treatments were carried out at 23°C in the dark and performed as follows: with 1 μL L−1 ethylene for different time intervals as indicated, with 100 nL L−1 MCP for different time intervals, with 2,000 μL L−1 NBD gas phase applied as a liquid at appropriate volume onto a filter paper strip, or in the case of pretreatment with MCP or NBD, with 100 nL L−1 or 2,000 μL L−1, respectively, for 2 h followed by 1 μL L−1 ethylene for different time intervals as indicated. The apical 1.5 to 2 cm of the epicotyls was then excised, immediately frozen in liquid nitrogen, and stored at −70°C.
Bioassays
Four-day-old seedlings were placed into 1-L Kilner jars, which were then airtight sealed with injection ports. Appropriate compounds were injected at the following concentrations: ethylene at 1 μL L−1, MCP ranging from 0.5 to 200 nL L−1, and NBD ranging from 250 to 10,000 μL L−1 (gas phase). Treatments were incubated in the dark at 23°C for 48 h, and then epicotyl length and width and plumular hook angle were measured. For each treatment, 45 seedlings were measured and means and ses were calculated. The data are expressed as percentage of control.
Isolation of Membrane-Enriched Fractions
All procedures were carried out at 4°C. The epicotyl tips were homogenized in a buffer A (1:2, w/v) that contained 50 mm Tris-HCl (pH 7.6), 10 mm MgCl2, 1 mm MnCl2, 1 mm EDTA, 1 mm EGTA, 2 mm Na3VO4, 10 mm β-glycerophosphate, 1 mm benzamidine, 1 mm dithiothreitol (DTT), 1 mm phenylmethylsulfonyl fluoride (PMSF), 1 mm diethyldithiocarbamic acid sodium salt, and 250 mm Suc. Polyvinylpolypyrrolidone was added to the buffer in a ratio of 1:20 (w/w) of plant tissue. The homogenate was filtered through 200-μm nylon mesh, and the filtrate was centrifuged at 12,000g for 20 min. The pellet was discarded, and the supernatant was centrifuged at 130,000g for 4 h. The pellet was resuspended in the same buffer supplemented with 20% (w/v) glycerol to a protein concentration of about 10 mg mL−1 and was used for isolation of membrane proteins. The supernatant was used for measuring protein kinase activity. Where appropriate, the extracts were divided into aliquots, frozen in liquid nitrogen, and stored at −70°C.
Solubilization of Membrane Proteins
Resuspended membrane-enriched fractions were mixed (1:5, v/v) with a buffer B containing 25 mm sodium-HEPES (pH 7.5), 5 mm MgCl2, 1 mm EDTA, 0.5 mm DTT, and 0.1 mm PMSF supplemented with KCl to give a final concentration of 100 mm and stirred for 30 min. The suspension was centrifuged at 130,000g for 2 h, and the supernatant was discarded because previously we have demonstrated that there was no specific ethylene-regulated GTP binding in this fraction (Novikova et al., 1997). The pellet was resuspended in buffer B but containing 750 mm KCl. After stirring for 30 min, the suspension was centrifuged at 130,000g for 1 h. The supernatant was collected and dialyzed overnight against 50 to 100 volumes of a buffer containing 25 mm sodium-HEPES (pH 7.5), 10 mm MgCl2, 150 mm NaCl, and 2 mm EDTA. The pellet was resuspended in buffer B but containing 1% (w/v) Triton X-100. After stirring for 30 min the suspension was centrifuged at 130,000g for 1 h, and the detergent-solubilized fraction was retained and dialyzed overnight against 50 to 100 volumes of the buffer 25 mm sodium-HEPES (pH 7.5), 10 mm MgCl2, 150 mm NaCl, 2 mm EDTA, and 0.05% (w/v) Triton X-100. The final pellet was then discarded. Protein content was measured with BCA Protein Assay Reagent (Pierce, Rockford, IL) according to the manufacturer's instructions.
Affinity Labeling with [α-32P]GTP
Affinity labeling of G-proteins was carried out according to the method of Löw et al. (1992), using [α-32P]GTP (specific activity 110 TBq mmol−1; Amersham Biosciences AB, Uppsala). Reaction mixtures (25–50 μL) that included 25 to 50 μg of membrane protein extracted with either 750 mm KCl or 1% (w/v) Triton X-100 and 74–148 kBq [α-32P]GTP were incubated at 37°C for 10 min. NaIO4 was then added to a final concentration of 4 mm and oxidation allowed to proceed for 1 min at 37°C. This was followed by reduction using NaCNBH3 at a final concentration of 80 mm for 1 min at 37°C. Further reduction was then accomplished by the addition of NaBH4 to a final concentration of 100 mm and incubation for 1.5 h at 0°C. Oxidizing and reducing agents were freshly prepared and kept at 0°C before use. The specificity of binding was assessed by using a 100-fold excess of unlabeled GTP. After labeling, the proteins were precipitated with 80% (v/v) acetone at −20°C and pelleted by centrifugation. The pellets were washed twice with 80% (v/v) acetone. For electrophoretic separation, proteins were dissolved either in sample buffer for SDS-PAGE (Laemmli, 1970) or sample buffer for two-dimensional electrophoresis (7.5 m urea, 2 m thiourea, 1% [w/v] Triton X-100, 4% [w/v] CHAPS, 20 mm DTT, and 0.2% [v/v] Pharmalyte 3–10 [Amersham Biosciences AB]) to achieve a protein concentration of 2 mg mL−1. The distribution of [α-32P]GTP binding was analyzed by SDS-PAGE or two-dimensional electrophoresis followed by autoradiography. For immunoprecipitation, the pellets were dissolved in a buffer consisting of 50 mm Tris-HCl (pH 7.6), 150 mm NaCl, 1 mm DTT, 0.2 mm PMSF, 1% (w/v) Lubrol (ICN Pharmaceuticals, Costa Mesa, CA), 1% (w/v) sodium-deoxycholate, and 0.5% (w/v) SDS.
Immunoprecipitation
For immunoprecipitation procedures, 500 to 1,000 μg of [α-32P]GTP-labeled membrane proteins were mixed with 5 to 10 μg of polyclonal anti-Rab 8 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and 25 to 50 μL of a 50% (v/v) suspension of prewashed Protein G-Sepharose CL-6B (Amersham Biosciences AB). Incubations were carried out overnight at 4°C with continuous shaking. Protein G-Sepharose was pelleted at 12,000g for 5 min. The supernatants were carefully removed and discarded. The pellets were washed twice with a buffer (0.5 mL) containing 50 mm Tris-HCl (pH 7.6), 600 mm NaCl, 1% (w/v) Lubrol, and 0.5% (w/v) SDS and once further with a buffer containing 100 mm Tris-HCl (pH 7.6), 10 mm EDTA, and 300 mm NaCl. Antibody-antigen complexes bound to Protein G-Sepharose were eluted with SDS sample buffer, boiled for 5 min, and precipitated with 80% (v/v) acetone at −20°C. The precipitated proteins were centrifuged, washed twice with 80% (v/v) acetone, and dissolved in SDS sample buffer. Samples were subjected to SDS-PAGE, and [α-32P]GTP binding was detected by autoradiography.
For immunoprecipitation of MAP kinase(s), 130,000g supernatants were transferred in a buffer containing 50 mm Tris-HCl (pH 7.6), 100 mm NaCl, 1 mm PMSF, 1 mm EGTA, 2 mm Na3VO4, 10 mm β-glycerophosphate, 1 mm benzamidine, 1 mm DTT, and 0.1% (w/v) Tween 40 by gel filtration on PD-10 columns (Amersham Biosciences AB). The extracts (1 mg protein) were mixed with 10 μg of anti-ERK1 rabbit polyclonal or 5 μg of anti-phospho-Tyr mouse monoclonal antibodies (both from Santa Cruz Biotechnology) and 25 μL of 50% (v/v) prewashed Protein G-Sepharose and incubated with continuous shaking at 4°C overnight. The mixtures were centrifuged for 5 min at 12,000g, and the pellets were washed twice with 0.5 mL of the incubation buffer followed by two further washes with a buffer consisted of 40 mm sodium-HEPES (pH 8.0), 2 mm DTT, 10 mm MgCl2, 1 mm MnCl2, and 0.1 mm EGTA.
In Vitro MAP Kinase Assay
MAP kinase activity was assayed using a modified method of Sontag et al. (1993). Supernatant fractions after 130,000g centrifugation (5–15 μg mL−1 protein) were incubated with MBP (0.25 mg mL−1; Invitrogen, Carlsbad, CA) for 20 min at 30°C in a final volume of 50 μL of buffer containing 20 mm Tris-HCl (pH 7.6), 10 mm MgCl2, 1 mm MnCl2, 1 mm EGTA, 1 mm dithiothreitol, 1 mm PMSF, 2 mm Na3VO4, 10 mm β-glycerophosphate, 1 mm benzamidine, 10 μm ATP, and 74 kBq of [γ-32P]ATP (specific activity 110 TBq mmol−1; Amersham Biosciences AB). The reaction was terminated by adding an equal volume of double concentration Laemmli SDS sample buffer (Laemmli, 1970), and samples were boiled for 3 min. Phosphorylated MBP was resolved in 15% (w/v) PAG under denaturing conditions and visualized by autoradiography. Analysis of autoradiographs was carried out by scanning on a GS-690 Imaging Densitometer (Bio-Rad, Hercules, CA) and quantified using Phoretix (Nonlinear Dynamics, Newcastle-upon-Tyne, UK) software.
When MAP kinase activity was assayed after immunoprecipitation, the pelleted Protein G-Sepharose with bound antibody-antigen complexes was mixed with reaction mixture containing 40 mm sodium-HEPES (pH 8.0), 2 mm DTT, 10 mm MgCl2, 1 mm MnCl2, 0.1 mm EGTA, 0.25 mg mL−1 MBP, 40 μm ATP, and 185 kBq [γ-32P]ATP. The reaction was allowed to proceed for 30 min at room temperature and was terminated by the addition of equal volume of double SDS sample buffer and boiling for 5 min. After pelleting, the Protein G-Sepharose supernatant was electrophoresed in 15% (w/v) PAG under denaturing conditions, and thereafter gels were exposed to x-ray film. Autoradiographs were analyzed as above.
In-Gel MAP Kinase Assay
Supernatant fractions after 130,000g centrifugation were mixed 1:1 with double SDS sample buffer containing 10 mm EDTA and 200 mm DTT and boiled for 3 min before electrophoresis. Electrophoresis was performed in 10% (w/v) SDS-PAG into which 0.5 mg mL−1 MBP was added before gel polymerization. All following incubations were performed according to Lee et al. (1993). Gels were washed twice with 20% (v/v) 2-propanol in 50 mm Tris-HCl (pH 8.0) and further for 1 h in several gel volumes of 50 mm Tris-HCl (pH 8.0) containing 5 mm 2-mercaptoethanol. Proteins were denatured by incubation in the same buffer but containing 6 m guanidine-HCl for 1 h and then renatured by five washes each of 10 min in several gel volumes of 50 mm Tris-HCl (pH 8.0) containing 0.04% (w/v) Tween 40 and 5 mm 2-mercaptoethanol. The gels were pre-incubated for 1 h with 40 mm HEPES (pH 8.0) containing 2 mm DTT, 10 mm MgCl2, 1 mm MnCl2, and 0.1 mm EGTA. Phosphorylation of MBP within the gel was carried out by incubation for 1 h in the same buffer but supplemented with 74 kBq mL−1 of [γ-32P]ATP and 40 μm ATP. The reaction was terminated by washing the gels several times in the trichloroacetic acid-sodium pyrophosphate stop solution (5% [w/v] and 1% [w/v], respectively) until the radioactivity in the washings reached background levels. Finally, the gels were fixed for 1 h in ethanol-acetic acid (20% [v/v] and 7.5% [v/v], respectively), dried, and autoradiographed.
When MAP kinase activity was assayed after immunoprecipitation, the pelleted Protein G-Sepharose with bound antibody-antigen complexes was mixed with double SDS sample buffer containing 10 mm EDTA and 200 mm DTT and boiled for 5 min. Then the beads were pelleted, and the supernatant was analyzed as above.
Electrophoresis
Labeled proteins were resolved using SDS-PAGE according to Laemmli (1970) or two-dimensional electrophoresis. Bio-Rad Mini-PROTEAN II and Mini 2-D electrophoresis cells were used. First dimension separation was carried out in 4% (w/v) polyacrylamide rods containing 9.2 m urea, 1% (w/v) Nonidet NP-40, and 2% (v/v) Pharmalyte, pH 4.0 to 6.5 (Amersham Biosciences AB). NaOH (20 mm) was used as catholyte and 10 mm H3PO4 as anolyte. On the top of the rods, 5 μL of sample buffer was laid. The rods were prefocused as follows: 10 min at 200 V, 15 min at 300 V, and 15 min at 400 V. Then the catholyte and anolyte solutions were discarded, and all of the liquid from the rods was removed and replaced with fresh catholyte. Then protein samples (20–50 μg) were loaded on the top of the rods and covered with overlay buffer containing 3.5 m urea, 0.5% (w/v) Triton X-100, and 0.5% (v/v) Pharmalyte 3–10. The running conditions were as follows: 15 min at 500 V and 4 h at 750 V. After isoelectrofocusing, the gels were carefully removed from glass capilliaries and equilibrated for 20 min in SDS-PAGE sample buffer. The rods were then placed on the top of 12.5% (w/v) PAG 1-mm thick and subjected to electrophoresis at 200 V. After electrophoresis, the gels were fixed, stained, dried, and subjected to autoradiography. The images were scanned as above.
ACKNOWLEDGMENT
We thank Prof. E.C. Sisler (North Carolina State University, Raleigh) for a sample of MCP.
Footnotes
This work was supported in part by INTAS (grant no. 99–01200) and by the Russian Foundation for Basic Research (grant no. 02–04–48414).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.015057.
LITERATURE CITED
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Aronheim A, Engelberg D, Li N, al-Alawi N, Schlessinger J, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–961. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D, Hall A. Ras and Rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Batoko H, Zheng HQ, Hawes C, Moore I. A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell. 2000;12:2201–2217. doi: 10.1105/tpc.12.11.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender L, Lo HS, Lee H, Kokojan V, Peterson V, Bender A. Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in yeast. J Cell Biol. 1996;133:879–894. doi: 10.1083/jcb.133.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AW, Cowan DSC, Harpham NVJ, Hemsley RJ, Novikova GV, Smith AR, Hall MA. Studies on the possible role of protein phosphorylation in the transduction of the ethylene signal. Plant Growth Regul. 1996;18:135–141. [Google Scholar]
- Bos JL. Ras. In: Hall A, editor. GTPases. Oxford: Oxford University Press; 2000. pp. 67–88. [Google Scholar]
- Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Burnett EC, Desikan R, Moser RC, Neill SJ. ABA activation of an MBP kinase in Pisum sativum epidermal peels correlates with stomatal responses to ABA. J Exp Bot. 2000;51:197–205. doi: 10.1093/jexbot/51.343.197. [DOI] [PubMed] [Google Scholar]
- Celis JE, Gromov P. 2D protein electrophoresis: Can it be perfected? Curr Opin Biotechnol. 1999;10:16–21. doi: 10.1016/s0958-1669(99)80004-4. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chang C, Shockey JA. The ethylene-response pathway: signal perception to gene regulation. Curr Opin Plant Biol. 1999;2:352–358. doi: 10.1016/s1369-5266(99)00004-7. [DOI] [PubMed] [Google Scholar]
- Chang EC, Barr M, Wang Y, Jung V, Xu HP, Wigler MH. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994;79:131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chant J, Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell. 1995;81:1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- Chavrier P, Goud B. The role of ARF and Rab GTPase in membrane transport. Curr Opin Cell Biol. 1999;11:466–475. doi: 10.1016/S0955-0674(99)80067-2. [DOI] [PubMed] [Google Scholar]
- Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of Ras: The CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- Denhardt DT. Signal transduction protein phosphorylation cascades mediated by Ras/Rho proteins in the mammalian cell: the potential for multiplex signalling. Biochem J. 1996;318:729–747. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Ichimura K, Shinozaki K, Neill SJ. Harpin induces activation of the Arabidopsis mitogen-activated protein kinases AtMPK4 and AtMPK6. Plant Physiol. 2001;126:1579–1587. doi: 10.1104/pp.126.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droillard M-J, Thibivilliers S, Cazalé A-C, Barbier-Brygoo H, Laurière C. Protein kinases induced by osmotic stresses and elicitor molecules in tobacco cell suspensions: two crossroad MAP kinases and one osmoregulation-specific protein kinase. FEBS Lett. 2000;474:217–222. doi: 10.1016/s0014-5793(00)01611-2. [DOI] [PubMed] [Google Scholar]
- Feig LA, Urano T, Cantor S. Evidence for a Ras/Ral signaling cascade. Trends Biochem Sci. 1996;21:438–441. doi: 10.1016/s0968-0004(96)10058-x. [DOI] [PubMed] [Google Scholar]
- Foschi M, Chari S, Dunn MJ, Sorokin A. Biphasic activation of p21ras by endothelin-1 sequentially activates the ERK cascade and phosphatidylinositol 3-kinase. EMBO J. 1997;16:6439–6451. doi: 10.1093/emboj/16.21.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JJ, Yun B-W, Loake GJ. Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J. 2000;24:569–582. doi: 10.1046/j.1365-313x.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Hall MA, Moshkov IE, Novikova GV, Mur LAJ, Smith AR. Ethylene signal perception and transduction: multiple paradigms? Biol Rev. 2001;76:103–128. doi: 10.1017/s1464793100005649. [DOI] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Huber LA, Ullrich O, Takai Y, Lütcke A, Dupree P, Olkkonen V, Virta H, de Hoop MJ, Alexandrov K, Peter M et al. Mapping of Ras-related GTP-binding proteins by GTP overlay following two-dimensional gel electrophoresis. Proc Natl Acad Sci USA. 1994;91:7874–7878. doi: 10.1073/pnas.91.17.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttly AK, Phillips AL. Gibberellin-regulated expression in oat aleurone cells of two kinases that show homology to MAP kinase and a ribosomal protein kinase. Plant Mol Biol. 1995;27:1043–1052. doi: 10.1007/BF00037031. [DOI] [PubMed] [Google Scholar]
- Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Knetsch MLW, Wang M, Snaar-Jagalska BE, Heimovaara-Dijkstra S. Abscisic acid induces mitogen-activated protein kinase activation in barley aleurone protoplasts. Plant Cell. 1996;8:1061–1067. doi: 10.1105/tpc.8.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu W-L, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu W-L, Zeng W, Sheen J. Suppression of auxin signal transduction by a MAPK cascade in higher plants. Nature. 1998;395:716–720. doi: 10.1038/27240. [DOI] [PubMed] [Google Scholar]
- Kumar D, Klessig DF. Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene, and jasmonic acid. Mol Plant-Microbe Interactions. 2000;13:347–351. doi: 10.1094/MPMI.2000.13.3.347. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Chang C. The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol. 2001;125:1061–1073. doi: 10.1104/pp.125.2.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ghose-Dastidar J, Winawer S, Friedman E. Signal transduction through extracellular signal-regulated kinase-like pp57 blocked in differentiated cells having low protein kinase Cβ activity. J Biol Chem. 1993;268:5255–5263. [PubMed] [Google Scholar]
- Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pagès G, L'allemain G, Brunet A, Pouysségur J. Growth factors indicate nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Can Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- Li H, Shen J-J, Zheng Z-L, Lin Y, Yang Z. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 2001;126:670–684. doi: 10.1104/pp.126.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw A, Faulhammer HG, Sprinzl M. Affinity labeling of GTP-binding proteins in cellular extracts. FEBS Lett. 1992;303:64–68. doi: 10.1016/0014-5793(92)80478-y. [DOI] [PubMed] [Google Scholar]
- Lu C, Zainal Z, Tucker GA, Lycett GW. Developmental abnormalities and reduced fruit softening in tomato plants expressing an antisense Rab11 GTPase gene. Plant Cell. 2001;13:1819–1833. doi: 10.1105/TPC.010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Meloche S, Seuwen K, Pagès G, Pouyssegur J. Biphasic and synergistic activation of p44 (mapk) (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992;6:845–854. doi: 10.1210/mend.6.5.1603090. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Awotunde OS, Muszyñeska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell. 2000;12:165–178. [PMC free article] [PubMed] [Google Scholar]
- Mira J-P, Benard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci USA. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Gotoh Y, Nishida E, Yamaguchi-Shinozaki K, Hayashida N, Iwasaki T, Kamada H, Shinozaki K. Characterization of two cDNAs that encode MAP kinase homologs in Arabidopsis thaliana and analysis of the possible role of auxin in activating such kinase activity in cultured cells. Plant J. 1994;5:111–122. doi: 10.1046/j.1365-313x.1994.5010111.x. [DOI] [PubMed] [Google Scholar]
- Mockaitis K, Howell SH. Auxin induces mitogenic activated protein kinase (MAPK) activation in roots of Arabidopsis seedlings. Plant J. 2000;24:785–796. doi: 10.1046/j.1365-313x.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Cutler RE. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- Moshkov IE, Mur LAJ, Novikova GV, Smith AR, Hall MA. Ethylene regulates monomeric GTP-binding protein gene expression and activity in Arabidopsis thaliana. Plant Physiol. 2003;131:1718–1726. doi: 10.1104/pp.014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Novikova GV, Moshkov IE, Smith AR, Hall MA. The effect of ethylene on GTP binding in extracts from pea seedlings. Planta. 1997;201:1–8. [Google Scholar]
- Novikova GV, Moshkov IE, Smith AR, Hall MA. The effect of ethylene on MAPKinase-like activity in Arabidopsis thaliana. FEBS Lett. 2000;474:29–32. doi: 10.1016/s0014-5793(00)01565-9. [DOI] [PubMed] [Google Scholar]
- Novikova GV, Moshkov IE, Smith AR, Kulaeva ON, Hall MA. The effect of ethylene and cytokinin on guanosine 5′-triphosphate binding and protein phosphorylation in leaves of Arabidopsis thaliana. Planta. 1999;208:239–246. doi: 10.1007/s004250050555. [DOI] [PubMed] [Google Scholar]
- Olkkonen V, Stenmark H. Role of Rab GTPases in membrane traffic. Int Rev Cytol. 1997;176:1–85. doi: 10.1016/s0074-7696(08)61608-3. [DOI] [PubMed] [Google Scholar]
- Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance in rice. Proc Natl Acad Sci USA. 2001;98:759–764. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V, Fluhr R. Ethylene signal is transduced via protein phosphorylation events in plants. Plant Cell. 1993;5:523–530. doi: 10.1105/tpc.5.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Yalovsky S, Gruissem W. Protein prenylation in plants: old friends and new targets. Plant Mol Biol. 1999;39:865–870. doi: 10.1023/a:1006170020836. [DOI] [PubMed] [Google Scholar]
- Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–766. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Miles GP, Ellis BE. Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 2000;22:367–376. doi: 10.1046/j.1365-313x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- Schiene K, Pühler A, Niehayus K. Transgenic tobacco plants that express an antisense construct derived from a Medicago sativa cDNA encoding a Rac-related small GTP-binding protein fail to develop necrotic lesions upon elicitor infiltration. Mol Gen Genet. 2000;263:761–770. doi: 10.1007/s004380000248. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall MN. Signaling to the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:305–338. doi: 10.1146/annurev.cellbio.14.1.305. [DOI] [PubMed] [Google Scholar]
- Sessa G, Raz V, Savaldi S, Fluhr R. PK12, a plant dual-specificity protein kinase of the LAMMER family, is regulated by the hormone ethylene. Plant Cell. 1996;8:2223–2234. doi: 10.1105/tpc.8.12.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant. 1997;100:577–582. [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE INSENSITIVE3 and ETHYLENE RESPONSE-FACTOR1. Genes Dev. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the MAP kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- Tan PBO, Kim SK. Signaling specificity: the RTK/RAS/MAP kinase pathway in metazoans. Trends Genet. 1999;15:145–149. doi: 10.1016/s0168-9525(99)01694-7. [DOI] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA. 2000;97:5663–5668. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valster AH, Hepler PK, Chernoff J. Plant GTPases: the Rhos in bloom. Trends Cell Biol. 2000;10:141–146. doi: 10.1016/s0962-8924(00)01728-1. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signalling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Waters MG, Pfeffer SR. Membrane tethering in intracellular transport. Curr Opin Cell Biol. 1999;11:453–459. doi: 10.1016/s0955-0674(99)80065-9. [DOI] [PubMed] [Google Scholar]
- York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJS. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- Zegzouti H, Jones B, Frasse P, Marty C, Maitre B, Latché A, Pech J-C, Bouzayen M. Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J. 1999;18:589–600. doi: 10.1046/j.1365-313x.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Klessig DF. Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J. 2000;23:339–347. doi: 10.1046/j.1365-313x.2000.00780.x. [DOI] [PubMed] [Google Scholar]
- Zondag GCM, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–782. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]