Abstract
Previous work with model transgenic plants has demonstrated that cellular accumulation of mannitol can alleviate abiotic stress. Here, we show that ectopic expression of the mtlD gene for the biosynthesis of mannitol in wheat improves tolerance to water stress and salinity. Wheat (Triticum aestivum L. cv Bobwhite) was transformed with the mtlD gene of Escherichia coli. Tolerance to water stress and salinity was evaluated using calli and T2 plants transformed with (+mtlD) or without (−mtlD) mtlD. Calli were exposed to −1.0 MPa of polyethylene glycol 8,000 or 100 mm NaCl. T2 plants were stressed by withholding water or by adding 150 mm NaCl to the nutrient medium. Fresh weight of −mtlD calli was reduced by 40% in the presence of polyethylene glycol and 37% under NaCl stress. Growth of +mtlD calli was not affected by stress. In −mtlD plants, fresh weight, dry weight, plant height, and flag leaf length were reduced by 70%, 56%, 40%, and 45% compared with 40%, 8%, 18%, and 29%, respectively, in +mtlD plants. Salt stress reduced shoot fresh weight, dry weight, plant height, and flag leaf length by 77%, 73%, 25%, and 36% in −mtlD plants, respectively, compared with 50%, 30%, 12%, and 20% in +mtlD plants. However, the amount of mannitol accumulated in the callus and mature fifth leaf (1.7–3.7 μmol g−1 fresh weight in the callus and 0.6–2.0 μmol g−1 fresh weight in the leaf) was too small to protect against stress through osmotic adjustment. We conclude that the improved growth performance of mannitol-accumulating calli and mature leaves was due to other stress-protective functions of mannitol, although this study cannot rule out possible osmotic effects in growing regions of the plant.
Water stress and salinity are major abiotic factors that limit crop productivity in drought-prone areas. One way of increasing productivity in stressful environments is to breed crops that are more tolerant to stress. However, success in breeding for tolerance has been limited because (a) tolerance to stress is controlled by many genes, and their simultaneous selection is difficult (Richards, 1996; Yeo, 1998; Flowers et al., 2000); (b) tremendous effort is required to eliminate undesirable genes that are also incorporated during breeding (Richards, 1996); and (c) there is a lack of efficient selection procedures particularly under field conditions (Ribaut et al., 1997). Genetic engineering offers an alternative approach for developing tolerant crops. Unlike classical breeding, genetic engineering is a faster and more precise means of achieving improved tolerance (Cushman and Bohnert, 2000) because it avoids the transfer of unwanted chromosomal regions. Moreover, through genetic engineering, multiple genes can be assembled and simultaneously introduced to the crop of interest. There are many functional targets for engineering tolerance to water stress and salinity, one of them being accumulation of osmoprotectants (Rathinasabapathi, 2000).
The osmolyte mannitol is normally synthesized in numerous plant species, but not in wheat (Triticum aestivum). In celery (Apium graveolens), mannitol is synthesized in equal proportion to that of Suc. It also constitutes as much as 50% of the translocated photoassimilate (Loester et al., 1992). Mannitol accumulation increases when plants are exposed to low water potential (Ψw; Patonnier et al., 1999), and accumulation is regulated by inhibition of competing pathways and decreased mannitol consumption and catabolism (Pharr et al., 1995; Stoop et al., 1996). In celery, salt stress inhibits Suc synthesis but does not affect the enzymes for mannitol biosynthesis. Moreover, the rate of mannitol use in sink tissues decreases during salt stress mainly because of the suppression of the NAD+-dependent mannitol dehydrogenase, which oxidizes mannitol to Man (Pharr et al., 1995; Stoop and Pharr, 1996). Studies using transgenic tobacco (Nicotiana tabacum) and Arabidopsis also showed improved growth of mannitol-accumulating plants under stress (Tarczynski et al., 1992, 1993; Thomas et al., 1995). However, these studies lack actual measurements of Ψw and osmotic potential (Ψs) in assessing the role of mannitol in stress tolerance. Subsequent work suggested that mannitol accounted for only 30% to 40% of Ψs changes observed in transgenic tobacco (Karakas et al., 1997). The function of mannitol in stress tolerance has not been evaluated in plants of agronomic importance. Here, we introduced the mtlD gene of Escherichia coli (Davis et al., 1988) into wheat to evaluate its role in improving tolerance to water stress and salinity. MtlD encodes for mannitol-1-phosphate dehydrogenase that catalyzes the reversible conversion of Fru-6-phosphate to mannitol-1-phosphate. In transgenic plants, mannitol-1-phosphate is converted to mannitol via nonspecific phosphatases (Thomas et al., 1995).
RESULTS
Response of Calli to Water and Salt Stress
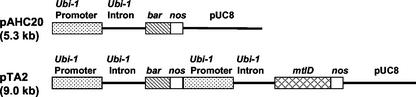
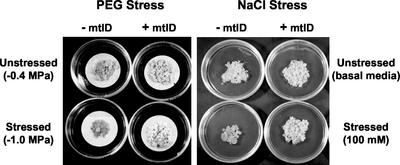
Recombinant constructs with (pTA2) or without (pAHC20) the mannitol-1-phosphate dehydrogenase (mtlD) gene of E. coli (Davis et al., 1988) were introduced into wheat calli and were subsequently regenerated into plants (Fig. 1). Calli transformed with pAHC20 and expressing the bar gene alone (−mtlD) exhibited a 40% reduction in growth in the presence of −1.0 MPa of polyethylene glycol (PEG) and a 37% growth reduction under 100 mm NaCl stress. Calli transformed with pTA2 expressed mtlD (+mtlD), and PEG and NaCl stresses had no effect on their growth (Table I; Fig. 2). PEG and NaCl increased accumulation of soluble carbohydrates in both −mtlD and +mtlD calli (Table II). The +mtlD calli accumulated 81% and 118% more mannitol in the presence of PEG and NaCl, respectively, relative to their unstressed counterparts. Depending on treatment type, mannitol accounted for 8% to 14% of the total soluble carbohydrates in +mtlD calli (Table II). Stress also increased Glc, Fru, and Suc in both types of calli. Accumulation of mannitol in +mtlD calli coincided with reduced Suc content regardless of whether the calli were stressed (Table II). PEG and NaCl reduced the Ψw and Ψs of both −mtlD and +mtlD calli. However, there were no significant differences in Ψw or Ψs between the two callus types under either PEG or NaCl stress (Table III). Calli exposed to stress showed osmotic adjustment of −0.34 to −0.29 MPa.
Figure 1.
Plasmids used for wheat transformation. Plasmid pAHC20 contains only the selectable marker bar. Plasmid pTA2 contains bar and the E. coli mtlD gene for biosynthesis of mannitol-1-phosphate. Both genes were under the control of the maize (Zea mays) ubi-1 promoter. Calli and plants transformed with pTA2 were used as mannitol-accumulating lines (+mtlD), and those transformed with pAHC20 served as negative controls (−mtlD).
Table I.
Fresh weight of transgenic wheat calli grown under PEG and NaCl stresses
| Stress Level | Callus Type | Stress Typea
|
|
|---|---|---|---|
| PEG | NaCl | ||
| g fresh wt | |||
| Unstressed | −mtlD | 4.0b | 3.8b |
| +mtlD | 3.6b | 4.5b | |
| Stressed | −mtlD | 2.4a | 2.4a |
| +mtlD | 3.7b | 4.3b | |
| lsd0.05 | 0.7 | 0.5 | |
Stress was applied to 0.25 g of 6-month-old calli (C1–11, −mtlD; and C2–20, +mtlD) by supplementing the Murashige and Skoog medium with −1.0 MPa of PEG or 100 mm NaCl. Measurements were taken 60 d after stress. Data are means of three replications.
Means followed by the same letter in a column are not significantly different at P < 0.05 as determined by Fisher's protected lsd test.
Figure 2.
Effect of osmotic stress on the growth of transgenic wheat calli. The mannitol-accumulating callus line C2-20 (+mtlD) and the nonaccumulating line C1-11 (−mtlD) were grown in Murashige and Skoog medium containing PEG 8,000 (−1.0 MPa) or 100 mm NaCl for 60 d.
Table II.
Mannitol and soluble sugar content of transgenic wheat grown under stress
| Growth stage | Stress Level | Callus/Plant Type | Mannitol | Glc | Fru | Suc |
|---|---|---|---|---|---|---|
| μmol g−1 fresh wt | ||||||
| Callus | ||||||
| PEG stress | Unstressed | −mtlD | 0.0a | 5.7a | 4.4a | 7.7b |
| +mtlD | 2.1b | 5.3a | 3.9a | 5.1a | ||
| −1.0 MPa | −mtlD | 0.0a | 9.4b | 8.1b | 12.0c | |
| +mtlD | 3.8c | 8.2ab | 6.5b | 9.1b | ||
| lsd0.50 | 1.2 | 3.4 | 2.0 | 1.5 | ||
| NaCl stress | Unstressed | −mtlD | 0.0a | 8.2a | 5.4b | 10.8ab |
| +mtlD | 1.7b | 6.6a | 4.2a | 9.0a | ||
| 150 mm | −mtlD | 0.0a | 14.2b | 8.1d | 13.6c | |
| +mtlD | 3.7c | 12.2b | 6.8c | 11.3bc | ||
| lsd0.05 | 0.7 | 3.5 | 1.1 | 2.0 | ||
| Whole plant | ||||||
| Water stress | Unstressed | −mtlD | 0.0a | 5.6a | 4.9ab | 7.1b |
| +mtlD | 0.6b | 4.9a | 3.5a | 5.3a | ||
| Stressed | −mtlD | 0.0a | 15.2b | 8.7c | 19.5c | |
| +mtlD | 1.5c | 12.9b | 6.7bc | 14.6c | ||
| lsd0.05 | 0.16 | 4.5 | 3.0 | 1.7 | ||
| NaCl stress | Unstressed | −mtlD | 0.0a | 8.7a | 6.9a | 9.7ab |
| +mtlD | 0.9b | 7.0a | 4.1a | 6.3a | ||
| Stressed | −mtlD | 0.0a | 13.1b | 10.1b | 25.8c | |
| +mtlD | 2.0c | 10.3ab | 6.4a | 16.7b | ||
| lsd0.05 | 0.51 | 3.4 | 2.8 | 8.8 | ||
Six-month-old calli C1–11 (−mtlD) and C2–20 (+mtlD) were grown in Murashige and Skoog media containing −1.0 MPa of PEG or 100 mm NaCl for 60 d. Wheat plants P1-13-1 (−mtlD) and P2-19-1 (+mtlD) were first grown in soil or hydroponically. Then stress was imposed for 30 d by withholding water from plants grown in soil or by adding 150 mm NaCl to plants grown hydroponically. In plants, carbohydrate content was measured on the fifth leaf. Data are means of three replications.a
Means followed by the same letter in a column are not significantly different at P < 0.05 as determined by Fisher's protected lsd test.
Table III.
Ψw and Ψs of transgenic wheat
| Growth Stage | Stress Level | Callus/Plant Type | Ψw | Ψs
|
|
|---|---|---|---|---|---|
| Fresh Tissue | Fully Turgid Tissue | ||||
| MPa | |||||
| Callus | |||||
| PEG stress | Unstressed | −mtlD | −0.44a | −0.57a | −0.42a |
| +mtlD | −0.43a | −0.60a | −0.48a | ||
| −1.0 MPa | −mtlD | −1.03b | −1.23b | −0.71b | |
| +mtlD | −1.04b | −1.31b | −0.82b | ||
| lsd0.05 | 0.20 | 0.28 | 0.18 | ||
| NaCl stress | Unstressed | −mtlD | −0.48a | −0.63a | −0.50a |
| +mtlD | −0.43a | −0.65a | −0.47a | ||
| 150 mm | −mtlD | −0.84b | −1.20b | −0.80b | |
| +mtlD | −0.82b | −1.17b | −0.76b | ||
| lsd0.05 | 0.17 | 0.31 | 0.22 | ||
| Whole plant | |||||
| Water stress | Unstressed | −mtlD | −1.02a | −1.67a | −1.18a |
| +mtlD | −0.97a | −1.66a | −1.16a | ||
| Stressed | −mtlD | −2.29b | −2.22b | −1.41b | |
| +mtlD | −1.43c | −2.20b | −1.53b | ||
| lsd0.05 | 0.25 | 0.30 | 0.21 | ||
| NaCl stress | Unstressed | −mtlD | −0.89a | −1.19a | −1.14a |
| +mtlD | −0.84a | −1.21a | −1.10a | ||
| 150 mm | −mtlD | −2.00b | −2.71b | −1.70b | |
| +mtlD | −1.74c | −2.14c | −1.63b | ||
| lsd0.05 | 0.23 | 0.50 | 0.17 | ||
For calli, water stress was recorded after exposing lines C1–11 (−mtlD) and C2–20 (+mtlD) to −1.0 MPa of PEG and 100 mm NaCl for 60 d. For plants, lines P1-13-1 (−mtlD) and P2-19-1 (+mtlD) were subjected to water stress and 150 mm NaCl for 30 d. In plants, water stress was measured on the fifth leaf. Data are means of three replications.a
Means followed by the same letter in a column are not significantly different at P < 0.05 as determined by Fisher's protected lsd test.
Response to Water Stress and Salinity at the Whole-Plant Level
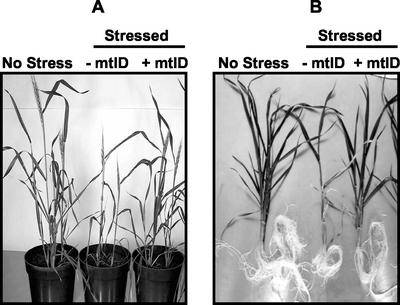
Water stress reduced growth of both −mtlD and +mtlD plants. However, the effect of stress was more severe on −mtlD than on +mtlD plants (Table IV; Fig. 3). In −mtlD plants, shoot fresh weight was reduced by 70%, dry weight by 56%, plant height by 40%, flag leaf length by 45%, and number of tillers by 75%. In +mtlD plants, fresh weight was reduced by 40%, dry weight by 8%, plant height by 18%, and flag leaf length by 29%. Water stress did not affect the number of tillers produced in +mtlD plants (Table IV; Fig. 3).
Table IV.
Shoot fresh weight, dry weight, plant height, and number of tillers of transgenic wheat plants exposed to water stress
| Stress Level | Plant Type | Shoot weight
|
Plant Height | Flag Leaf Length | No. of Tillers | |
|---|---|---|---|---|---|---|
| Fresh Wt | Dry Wt | |||||
| g | cm | |||||
| Unstressed | −mtlD | 11.7c | 2.5b | 47.0c | 28.3b | 4.0b |
| +mtlD | 11.9c | 2.6b | 45.0c | 26.3b | 3.0b | |
| Stressed | −mtlD | 3.5a | 1.1a | 28.0a | 15.6a | 1.0a |
| +mtlD | 7.1b | 2.4b | 36.7b | 18.7a | 3.0b | |
| lsd0.05 | 2.9 | 0.8 | 7.3 | 7.0 | 1.6 | |
T2 plants (P1-13-1, −mtlD; and P2-19-1, +mtlD) were grown in soil and exposed to stress by withholding water. Measurements were taken after 30 d of the imposition of stress. Data are means of three replications.a
Means followed by the same letter in a column are not significantly different at P < 0.05 as determined by Fisher's protected lsd test.
Figure 3.
Effect of water stress and salinity on the growth of +mtlD and −mtlD plants. The mannitol-accumulating transgenic wheat line P2-19-1 (+mtlD) and the nonaccumulating P1-13-1 (−mtlD) were stressed by withholding water (A) or by supplementing the nutrient solution with 150 mm NaCl (B) for 30 d. Pictures were taken 30 d after the imposition of water stress and 20 d after NaCl stress. In the absence of stress, −mtlD and +mtlD plants were similar in size; thus, for unstressed controls, only the −mtlD plants are shown.
Water stress increased the concentration of soluble carbohydrates in both −mtlD and +mtlD plants (Table II). In +mtlD plants, the concentration of mannitol was increased by 150%. In the absence of water stress, the +mtlD plants had lower Suc content than the −mtlD plants. There was no difference in the concentration of other soluble sugars between the two plants whether the plants were stressed or not.
Under water stress, Ψw and Ψs were significantly lower in both −mtlD and +mtlD plants (Table III). In −mtlD plants, Ψw was reduced to −2.29 MPa compared with −1.43 MPa in +mtlD plants. This difference was not related to changes in Ψs because Ψs in both types of plants was similar. Decreased Ψw also resulted in wilting and leaf rolling of the −mtlD plants in the 2nd and 3rd d of withholding water. In +mtlD plants, these symptoms were delayed until the 4th d. The −mtlD and +mtlD plants showed osmotic adjustment by −0.23 and −0.37 MPa, respectively.
Plants grown hydroponically had twice the shoot fresh weight and dry weight of those grown in soil. Besides, hydroponically grown plants had more tillers than plants grown in soil. Apart from these differences, +mtlD plants grew better in 150 mm NaCl than −mtlD plants, as did +mtlD plants in the water stress experiment. In −mtlD plants, salt stress reduced shoot fresh weight by 77%, dry weight by 73%, plant height by 25%, flag leaf length by 36%, and number of tillers by 67%. In +mtlD plants, shoot fresh weight was reduced by 50%, dry weight by 30%, plant height by 12%, length of the flag leaf by 20%, and number of tillers by 57% (Table V; Fig. 3). A similar pattern was observed in root growth. In −mtlD plants, salt stress reduced root fresh weight by 80%, dry weight by 82%, and root length by 32%. In +mtlD plants, root fresh weight was reduced only by 50%, dry weight by 55%, and root length by 23% (Table V; Fig. 3).
Table V.
Shoot weight, root weight, plant height, length of the flag leaf, length of root, and number of tillers of transgenic wheat plants exposed to NaCl
| Stress Level | Plant Type | Shoot
|
Root
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Fresh Wt | Dry Wt | Plant Height | Flag Leaf Length | No. of Tillers | Fresh Wt | Dry Wt | Length | ||
| g | cm | g | cm | ||||||
| Unstressed | −mtlD | 28.3c | 4.8c | 50.0b | 34.7c | 6.0c | 17.8c | 1.1c | 63b |
| +mtlD | 24.2c | 3.7c | 51.0b | 33.7c | 7.0d | 16.9c | 1.1c | 61b | |
| Stressed | −mtlD | 6.4a | 1.3a | 37.7a | 22.7a | 2.0a | 3.5a | 0.2a | 43a |
| +mtlD | 12.2b | 2.6b | 45.0b | 27.0b | 3.0b | 8.5b | 0.5b | 47a | |
| lsd0.05 | 5.7 | 1.1 | 6.6 | 2.3 | 0.5 | 4.2 | 0.2 | 12.1 | |
T2 plants (P1-13-1, −mtlD; and P2-19-1, +mtlD) were grown hydroponically, and stress was imposed by raising the salt concentration of the nutrient medium to 150 mm NaCl. Measurements were taken after 30 d of stress. Data are means of three replications.a
Means followed by the same letter in a column are not significantly different at P < 0.05 as determined by Fisher's protected lsd test.
Exposure to 150 mm NaCl increased soluble carbohydrate content in both −mtlD and +mtlD plants (Table II). Suc showed the largest increase. In −mtlD plants, Suc content rose by 166% and in +mtlD plants by 165%. In +mtlD plants, salt stress increased the mannitol content by 122%. The increase in mannitol content coincided with reduced Suc content in +mtlD plants.
Salt stress significantly reduced Ψw and Ψs in both types of plants (Table III). The −mtlD plants had lower Ψw and Ψs than the +mtlD plants. However, there was no difference in Ψs at full turgor, and both plant types osmotically adjusted by the same magnitude: −mtlD plants by −0.56 MPa and +mtlD plants by −0.53 MPa. Lower leaves of −mtlD plants showed greater wilting and more chlorotic tips than the +mtlD plants, although the young fifth leaf was turgid, as revealed by the difference between Ψw and Ψs (Table III).
DISCUSSION
Our results demonstrate that mannitol improves growth of transgenic wheat under water stress and salinity both at the callus and whole-plant level (Tables I, IV, and V; Figs. 2 and 3). These findings are in agreement with earlier studies that used the same mtlD gene in tobacco (Tarczynski et al., 1992, 1993; Karakas et al., 1997; Shen et al., 1997a) and Arabidopsis (Thomas et al., 1995). The amount of mannitol accumulated in transgenic wheat was in the low end of the range reported for tobacco and Arabidopsis. In tobacco, mannitol accumulated to between 1 and 7 μmol g−1 fresh weight (Tarczynski et al., 1992, 1993; Shen et al., 1997a). Transgenic Arabidopsis accumulated between 0.05 and 12 μmol g−1 fresh weight mannitol (Thomas et al., 1995). In our experiment, depending on the severity of stress, wheat accumulated 1.7 to 3.7 μmol g−1 fresh weight in the callus and 0.6 to 2.0 μmol g−1 fresh weight in the mature fifth leaf. Earlier studies concluded that the amount of mannitol accumulated was inadequate to account for osmotic effects (Tarczynski et al., 1992, 1993; Thomas et al., 1995). However, no direct measurement of plant water status was made. In a subsequent study, Karakas et al. (1997) estimated that in salt-stressed transgenic tobacco mannitol contributes only 3 × 10−3 to 4 × 10−3 MPa to osmotic adjustment.
Mannitol has been proposed to enhance tolerance to water deficit stress primarily through osmotic adjustment (Loester et al., 1992). Our data show that there was no difference in the Ψs of −mtlD and +mtlD transformants at the callus level or in mature fifth leaves and that both adjusted osmotically to an equal extent when exposed to water and osmotic stresses (Table III). The amount of mannitol accumulated in response to stress was small (Table II), and its effect on osmotic adjustment was less than that of other carbohydrates. We estimated the contribution of mannitol to osmotic adjustment using the van't Hoff's equation, Ψs = −cRT, where c is the solute concentration in mol L−1, R is a constant (8.2 × 10−3 L MPa mol−1 K−1) and T is temperature in Kelvins (Table VI). Accordingly, at 85% relative water content (stressed), 3.8 μmol g−1 fresh weight of mannitol in PEG-stressed calli and 3.7 μmol g−1 fresh weight in NaCl-stressed calli would contribute only −1.1 × 10−2 MPa to Ψs of fresh calli. At 95% relative water content (unstressed), the contribution of mannitol to Ψs of turgid calli would be −9.9 × 10−3 (PEG stress) and −9.5 × 10−3 MPa (NaCl stress). This represents only 1.2% of the Ψs at full turgor or 2% to 3% of the osmotic adjustment of stressed calli. The fate of mannitol in transgenic plants is unknown. Because wheat does not naturally synthesize mannitol, it may not have the mechanism to transport or metabolize this sugar alcohol, and mannitol is most likely stored in the cytosol as a dead-end product. Assuming that mannitol is accumulated in the cytosol and that the cytosol represents 5% of the total water content of fully turgid tissue, mannitol would contribute −2.0 × 10−1 and −1.9 × 10−1 MPa to Ψs of calli exposed to PEG and NaCl, respectively (Table VI). If Ψs and osmotic adjustment of the cytosol are similar to the values measured on the tissue basis, this will represent 25% of the Ψs at full turgor of PEG- and NaCl-stressed calli.
Table VI.
Contribution of mannitol to osmotic potential in transgenic wheat (+mtlD)
| Growth Stage | Stress Type | Stress Level | Mannitol Content | Ψsc
|
Contribution to Cytosolic Ψsf | ||
|---|---|---|---|---|---|---|---|
| Fresh Tissued | Fully Turgid Tissuee | ||||||
| μmol g−1fresh wta | Mol L−1b | MPa | |||||
| Calli | PEG | Unstressed | 2.1 | 2.5 × 10−3 | −6.1 × 10−3 | −5.8 × 10−3 | −1.1 × 10−1 |
| −1.0 MPa | 3.8 | 4.8 × 10−3 | −1.1 × 10−2 | −9.9 × 10−3 | −2.0 × 10−1 | ||
| NaCl | Unstressed | 1.7 | 2.0 × 10−3 | −4.8 × 10−3 | −4.6 × 10−3 | −9.2 × 10−2 | |
| 150 mm | 3.7 | 4.6 × 10−3 | −1.1 × 10−2 | −9.5 × 10−3 | −1.9 × 10−1 | ||
| Whole plant | Water | Unstressed | 0.6 | 0.8 × 10−3 | −2.0 × 10−3 | −1.9 × 10−3 | −3.8 × 10−2 |
| Stressed | 1.5 | 2.0 × 10−3 | −4.9 × 10−3 | −4.3 × 10−3 | −8.6 × 10−2 | ||
| NaCl | Unstressed | 0.9 | 1.1 × 10−3 | −2.7 × 10−3 | −2.6 × 10−3 | −5.2 × 10−2 | |
| 150 mm | 2.0 | 2.6 × 10−3 | −6.3 × 10−3 | −5.6 × 10−3 | −1.1 × 10−1 | ||
Osmotic potentials were calculated from mannitol contents in Table II using van't Hoff's equation, Ψs = −cRT, where c is solute concentration in mol L−1, R is a constant (8.2 × 10−3 L MPa mol−1 K−1), and T is temperature in K (at room temperature T = 298 K).
Mannitol content for stressed tissues are from Table II.
Mol L−1 = [(μmol mannitol g−1fresh weight × 100/water content per fresh weight)/1,000]. Water accounted for 85% and 80% of the fresh weight of unstressed and stressed calli, respectively. For plants in water stress experiment, 76% (unstressed leaf) and 73% (stressed leaf) of fresh weight was water. For plants in salt stress, 80% (unstressed leaf) and 78% (stressed leaf) of fresh weight was water.
Relative water content (a) Unstressed calli: fresh calli, 95%; fully turgid calli, 98%; stressed calli: fresh calli; 85%; fully turgid calli; 95%. (b) Unstressed fifth leaf: fresh leaf, 95%, fully turgid leaf, 97%; stressed fifth leaf: fresh leaf, 88%; fully turgid leaf, 96%.
Ψs of fresh tissue under stress = −cRT.
Ψs of (a) fully turgid calli: unstressed calli, −cRT × (95/100); stressed calli, −cRT × (85/100); and (b) fully turgid leaf: unstressed leaf, −cRT × (96/100); stressed leaf, −cRT × (88/100).
Osmotic contribution of mannitol to the cytosol (assuming all the mannitol is in the cytosol, which accounts for 5% of cell volume) = Ψs of turgid tissue × (100/5).
Similarly, at 88% relative water content (stressed), mannitol levels of 1.5 μmol g−1 fresh weight in water-stressed plants and 2.0 μmol g−1 fresh weight in salt-stressed plants would contribute −4.9 × 10−3 MPa and −6.3 × 10−3 MPa to Ψs of the fresh leaf, respectively. At 96% relative water content (unstressed), the contribution of mannitol to Ψs of turgid leaves would be −4.3 × 10−3 (water stress) and −5.6 × 10−3 MPa (NaCl stress). This represents 0.4% and 0.3% of the Ψs at full turgor of water- and NaCl-stressed leaves, respectively. With the same assumptions made above, the contribution of mannitol to Ψs in the cytosol of water- and salt-stressed plants would be −8.6 × 10−2 and −1.1 × 10−1 MPa at full turgor, respectively (Table VI). This corresponds to 5.6% and 6.7% of the Ψs of leaves at full turgor. This is rather insignificant relative to the total osmotic adjustment. Besides, there was no significant difference in the osmotic adjustment between the −mtlD and +mtlD wheat either at the callus or whole-plant level (Table III), suggesting that the beneficial effect of mannitol resulted from protective mechanisms other than osmotic adjustment. Although very unlikely, the possibility that mannitol may have an osmotic effect in growing regions of +mtlD wheat plants remains to be determined. As mentioned above, previous studies in tobacco and Arabidopsis have shown accumulation of two to three times higher levels of mannitol than we found in wheat, yet they were not considered meaningful in terms of osmotic adjustment (Tarczynski et al., 1992, 1993; Thomas et al., 1995; Karakas et al., 1997; Shen et al., 1997a). Studies with other osmolytes also showed that marginal accumulation of fructan, Pro, and trehalose improves growth of transgenic tobacco under stress without being involved in osmotic adjustment (Kavi Kishor et al., 1995; Pilon-Smits et al., 1995; Holmström et al., 1996).
How could a small amount of mannitol enhance the tolerance of transgenic wheat to water stress and salinity? Besides its function in osmotic adjustment, mannitol improves tolerance to stress through scavenging of hydroxyl radicals (OH•) and stabilization of macromolecular structures (Smirnoff and Cumbes, 1989; Crowe et al., 1992; Shen et al., 1997a, 1997b). Reactive oxygen species in general react aggressively with biological molecules and can cause lipid peroxidation, breakdown of macromolecules and damage to nucleic acids (Smirnoff, 1998). Oxidative stress is common in plants during water stress (Smirnoff, 1993, 1998). The importance of mannitol as a scavenger of the hydroxyl radical (OH•) has been demonstrated in vitro (Smirnoff and Cumbes, 1989) and in vivo using transgenic tobacco (Shen et al., 1997a). In tobacco, mannitol protects the thiol-regulated enzyme phosphoribulokinase, thioredoxin, ferredoxin, and glutathione from OH• (Shen et al., 1997b). The mechanism by which mannitol interacts with OH• remains to be explained. Stabilization of macromolecular structure involves formation of hydrogen bonds. Under limited water availability, osmolytes can form hydrogen bonds with macromolecules and thus prevent formation of intramolecular H-bonds that would otherwise irreversibly change their three-dimensional structure (Crowe et al., 1992). Unlike osmotic adjustment, OH• scavenging and other protective functions require only small amounts of mannitol, and it is likely that the improved performance of transgenic wheat observed in this study was the result of OH• scavenging and/or improved stability of macromolecular structures.
We have shown that similar to previous studies in model plants, the improved performance of mannitol-accumulating wheat under stress was not likely to involve osmotic adjustment in calli and mature leaves. For osmotic adjustment to be important, a higher concentration of mannitol is needed. Theoretically, increased accumulation of mannitol can be achieved by diverting more carbon to mannitol biosynthesis. However, accumulation of too much mannitol may have adverse effects. First, diverting carbon that is normally destined to Suc synthesis will reduce or deplete the Suc pool with a deleterious effect on growth especially in plants where Suc is the major carbohydrate translocated. Second, even though mannitol is a compatible solute, the target plant may not tolerate high levels of mannitol. The appropriateness of the term compatible solute for osmolytes is questionable because marginal accumulations can induce pleiotropic effects (Hare et al., 1998). The plant line P2-19-1 (+mtlD) used in our study accumulated only 0.7 μmol g−1 fresh weight mannitol in the flag leaf under unstressed conditions. This was the highest amount of mannitol accumulated without causing any noticeable side effects in transgenic wheat. Plants that accumulated higher mannitol had severe abnormalities including sterility, stunted growth, twisted heads, and curled leaves (Fig. 4). For instance, the sterile plant line P2-16-1 (Fig. 4) accumulated 1.6 μmol g−1 fresh weight mannitol in the flag leaf. The Suc content in this line was exceptionally low; only 1.9 μmol g−1 fresh weight compared with 3.5 μmol g−1 fresh weight in the fertile +mtlD line P2-19-1 and 4.5 μmol g−1 fresh weight in the −mtlD line P1-13-1. In a related study, transgenic tobacco plants expressing mtlD were 20% to 25% smaller in size and had reduced Suc compared with the wild type (Karakas et al., 1997). Sheveleva et al. (2000) found that tobacco plants expressing mtlD and IMT1 (myo-inositol-O-methyltransferase) had abnormal flower development and reduced sugar content. Exogenous application of Gly betaine to a nonaccumulating plant was found to destabilize membranes and to inhibit protein synthesis and osmotic-induced accumulation of Pro (Gibon et al., 1997). Stress-induced accumulation of Pro also results in reduced growth (Hare and Cress, 1998). These results point to the need to carefully optimize the use of existing osmoprotectant-based mechanisms and to explore the development of alternative engineering strategies, such as the use of stress-inducible expression systems for stress tolerance determinants, which lack potential detrimental effects on growth.
Figure 4.
Phenotypes observed in transgenic wheat plants. Lines P2-16-1 and P2-19-1 were transformed with plasmid pTA2 for accumulation of mannitol in the cytosol (+mtlD). Line P1-13-1 was transformed with pAHC20 (−mtlD) and did not accumulate mannitol. Most +mtlD plants were short and sterile and had twisted leaves and heads similar to P2-16-1. In addition, the sterile plants had high mannitol (more than 1.5 μmol g−1 fresh weight) and low Suc content (less than 2 μmol g−1 fresh weight). In the fertile +mtlD plants, mannitol content ranged from 0.4 to 0.7 μmol g−1 fresh weight.
MATERIALS AND METHODS
Gene Constructs and Biolistic Transformation
The open reading frame of mtlD (1.2 kb) was amplified by PCR from pCab-mtlD (a gift from Dr. Hans Bohnert) and ligated to the BamHI site of pAHC17 (Christensen and Quail, 1996). To the HindIII site of the resulting plasmid, the ubi-bar-nos region of pAHC20 (Christensen and Quail, 1996) was ligated to create pTA2 (Fig. 1). Both mtlD and the selectable marker bar in pTA2 were under the control of the maize (Zea mays) ubi-1 promoter (Christensen et al., 1992). For accumulation of mannitol in the cytosol, wheat (Triticum aestivum L. cv Bobwhite) was transformed with pTA2 using the He-driven PDS 1000 and regenerated as described by Weeks (1995). As a negative control, wheat was also transformed with pAHC20.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Plant Materials and Growth Conditions
Twenty T0 plants transformed with pTA2 (+mtlD) were obtained. Thirteen of these plants were sterile, were stunted in growth, and had twisted leaves and heads. In the remaining seven plants, mannitol concentration ranged from 0.4 to 0.7 μmol g−1 fresh weight. Mannitol concentration in calli ranged from 0.3 to 2.0 μmol g−1 fresh weight (data not shown). To be able to observe differences in the response of pTA2-transformed (+mtlD) and pAHC20-transformed (−mtlD) lines, a transformation event that resulted in progeny with a high mannitol content and phenotypically identical to the control for plant traits under non-stress conditions was selected for analyses of growth, water relations, and soluble carbohydrates in response to water or salinity stresses. The event represented by callus line C2-20 and plant line P2-19-1 was transformed with pTA2 (+mtlD) and accumulated 2.0 and 0.7 μmol g−1 fresh weight of mannitol, respectively; it was selected for characterization. Moreover, for experiments involving calli, tissues of the same age (6 months) were used to avoid variations due to differences in physiological states. Accordingly, the event represented by callus line C1-11 and plant line P1-13-1 transformed with pAHC20 (−mtlD) served as negative controls. Stress at the tissue level was imposed on 0.25 g of calli grown on Murashige and Skoog maintenance media (Weeks, 1995) containing PEG 8,000 (Ψs = −1.0 MPa) or 100 mm NaCl for 60 d. Calli were transferred to fresh media every 2 weeks.
Stress at the whole-plant level was imposed on T2 plants derived from immature embryos of +mtlD and −mtlD T1 kernels. Seedlings were first screened for the bar gene on media containing 3 mg L−1 bialaphos. After 2 weeks, healthy seedlings were transferred to 15- × 12-cm pots filled with 350 g of soil or to troughs filled with 3.5 L of aerated nutrient solution. Plants were maintained in a growth chamber at 200 μmol m−2 s−1 photosynthetically active radiation, 23°C/17°C day/night temperature, 70% relative humidity, and 16-h photoperiod. After 3 weeks, three uniform plants were randomly assigned to stress. Stress was imposed by watering plants with 50 mL of water at 3- to 4-d intervals compared with 150 mL for unstressed controls. For salt stress, plants were exposed to 150 mm NaCl by raising the NaCl concentration of the nutrient solution 30 mm per day over a 5-d period. The solution was changed every 3 to 4 d.
Measurement of Growth and Water Relations
Growth was measured at the end of the stress period. For calli, fresh weight was measured. For plants, fresh weight, dry weight, plant height, length of the flag leaf, and number of tillers were recorded. In addition, for salt-stressed plants, fresh weight, dry weight, and length of the root were measured. Ψw and Ψs were determined for calli and the fifth leaf. Ψw was measured with leaf cutter psychrometers (Merrill Specialty Equipment, Logan, UT) connected to an automatic Ψw measurement system (HP-115, Wescor, Logan, UT). Ψs was measured using a Vapro vapor pressure osmometer (Wescor). Osmotic adjustment was determined as the difference between Ψs at full turgor (after rehydration in distilled water for 2 h) between stressed and unstressed tissues.
Determination of Carbohydrate Content
Mannitol and other soluble carbohydrates were extracted from calli and leaves as described before (Adams et al., 1993) and separated using a high-performance anion-exchange chromatography system coupled to a pulsed amperometric detector. Fifty-microliter samples were injected into a 9- × 250-mm Carbopac PA1 column (Dionex, Sunnyvale, CA), and carbohydrates were separated isocratically in 150 mm degassed NaOH at a flow rate of 2.0 mL min−1. Peak areas were quantified, and retention times were determined using an integrator.
ACKNOWLEDGMENTS
We thank Dr. Peter Quail for the kind gift of pAHC17 and pAHC20, Dr. Hans J. Bohnert for the mtlD gene, Dr. Troy J. Weeks for help with wheat transformation, and Dr. Andrew Mort for help with analysis of carbohydrates. We thank Colleen Sweeney, David King, Dave Ferris, and Pamela Tauer for technical assistance.
Footnotes
This work was supported by the Oklahoma Agricultural Experiment Station, by the Oklahoma Wheat Research Foundation, by the National Science Foundation (grant no. EPS–9550478), by a scholarship from the Fulbright Foundation (to T.A.), and in part by the Nevada Agricultural Experiment Station (article no. 03031230).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.003616.
LITERATURE CITED
- Adams P, Zegeer A, Bohnert HJ, Jensen RG. Anion exchange separation and amperometric detection of inositols from flower petals. Anal Biochem. 1993;214:321–324. doi: 10.1006/abio.1993.1495. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Hoekstra FA, Crowe LM. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- Cushman JC, Bohnert HJ. Genomic approaches to plant stress tolerance. Curr Opin Plant Biol. 2000;3:117–124. doi: 10.1016/s1369-5266(99)00052-7. [DOI] [PubMed] [Google Scholar]
- Davis T, Yamada M, Elgort MG, Saier MH. Nucleotide sequence of the mannitol (mtl) operon in Escherichia coli. Mol Microbiol. 1988;2:405–412. doi: 10.1111/j.1365-2958.1988.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Koyama ML, Flowers SA, Sudhakar C, Singh KP, Yeo AR. QTL: their place in engineering tolerance of rice to salinity. J Exp Bot. 2000;51:99–106. [PubMed] [Google Scholar]
- Gibon Y, Bessieres MA, Larher F. Is glycine betaine a non-compatible solute in higher plants that do not accumulate it? Plant Cell Environ. 1997;20:329–340. [Google Scholar]
- Hare PD, Cress WA. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1998;21:79–102. [Google Scholar]
- Hare PD, Cress WA, Van Staden J. Dissecting the role of osmolyte accumulation during stress. Plant Cell Environ. 1998;21:535–553. [Google Scholar]
- Holmström K-O, Mäntylä E, Welin B, Mandal A, Palva ET. Drought tolerance in tobacco. Nature. 1996;379:683–684. [Google Scholar]
- Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M, Rieger M. Salinity and drought tolerance in mannitol-accumulating transgenic tobacco. Plant Cell Environ. 1997;20:609–616. [Google Scholar]
- Kavi Kishor BP, Hong Z, Miao G-H, Hu C-AA, Verma DPS. Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108:1387–1394. doi: 10.1104/pp.108.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loester WH, Tyson RH, Everard JD, Redgwell RJ, Bieleski RL. Mannitol synthesis in higher plants: evidence for the role and characterization of a NADPH-dependent mannose-6-phosphate reductase. Plant Physiol. 1992;98:1396–1402. doi: 10.1104/pp.98.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patonnier MP, Peltier JP, Marigo G. Drought-induced increase in xylem malate and mannitol concentration and closure of Fraxinus excelsior L. stomata. J Exp Bot. 1999;50:1223–1229. [Google Scholar]
- Pharr DM, Stoop JMH, Williamson JD, Studer Feusi ME, Massel MO, Conkling MA. The dual role of mannitol as osmoprotectant and photoassimilate in celery. HortScience. 1995;30:1182–1188. [Google Scholar]
- Pilon-Smits EAH, Ebskamp MJM, Paul MJ, Jenken MJW, Weisbeek PJ, Smeekens SCM. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 1995;107:125–130. doi: 10.1104/pp.107.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinasabapathi B. Metabolic engineering for stress tolerance: installing osmoprotectant synthesis pathways. Ann Bot. 2000;86:709–716. [Google Scholar]
- Ribaut J-M, Jiang C, Gonzalez-de-Leon D, Edmeades GO, Hoisington DA. Identification of quantitative trait loci under drought conditions in tropical maize: 2. Yield components and marker-assisted selection strategies. Theor Appl Genet. 1997;94:887–896. [Google Scholar]
- Richards RA. Defining selection criteria to improve yield under drought. Plant Growth Regul. 1996;20:157–166. [Google Scholar]
- Shen B, Jensen RG, Bohnert HJ. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997a;113:1177–1183. doi: 10.1104/pp.113.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Jensen RG, Bohnert HJ. Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol. 1997b;115:527–532. doi: 10.1104/pp.115.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheveleva EV, Jensen RG, Bohnert HJ. Disturbance in the allocation of carbohydrates to regenerative organs in transgenic Nicotiana tabacum L. J Exp Bot. 2000;51:115–122. [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Plant resistance to environmental stress. Curr Opin Biotechnol. 1998;9:214–219. doi: 10.1016/s0958-1669(98)80118-3. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–1060. [Google Scholar]
- Stoop JMH, Pharr DM. Effect of different carbon sources on relative growth rate, internal carbohydrates, and mannitol-1-oxidoreductase activity in celery suspension cultures. Plant Physiol. 1996;103:1001–1008. doi: 10.1104/pp.103.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop JMH, Williamson JD, Pharr DM. Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci. 1996;1:139–144. [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ. Expression of a bacterial mtlD gene in transgenic tobacco leads to production and accumulation of mannitol. Proc Natl Acad Sci USA. 1992;89:2600–2604. doi: 10.1073/pnas.89.7.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ. Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science. 1993;259:508–510. doi: 10.1126/science.259.5094.508. [DOI] [PubMed] [Google Scholar]
- Thomas JC, Sepahi M, Arendall B, Bohnert HJ. Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana. Plant Cell Environ. 1995;18:801–806. [Google Scholar]
- Weeks JT. Stable transformation of wheat by microprojectile bombardment. In: Potrykus I, Spangenberg G, editors. Gene Transfer to Plants. Berlin: Springer-Verlag; 1995. pp. 157–161. [Google Scholar]
- Yeo A. Molecular biology of salt tolerance in context of whole-plant physiology. J Exp Bot. 1998;49:915–929. [Google Scholar]