Abstract
N-Acylethanolamines (NAEs) are fatty acid derivatives found as minor constituents of animal and plant tissues, and their levels increase 10- to 50-fold in tobacco (Nicotiana tabacum) leaves treated with fungal elicitors. Infiltration of tobacco leaves with submicromolar to micromolar concentrations of N-myristoylethanolamine (NAE 14:0) resulted in an increase in relative phenylalanine ammonia-lyase (PAL) transcript abundance within 8 h after infiltration, and this PAL activation was reduced after co-infiltration with cannabinoid receptor antagonists (AM 281 and SR 144528). A saturable, high-affinity specific binding activity for [3H]NAE 14:0 was identified in suspension-cultured tobacco cells and in microsomes from tobacco leaves (apparent Kd of 74 and 35 nm, respectively); cannabinoid receptor antagonists reduced or eliminated specific [3H]NAE 14:0 binding, consistent with the physiological response. N-Oleoylethanolamine activated PAL2 expression in leaves and diminished [3H]NAE 14:0 binding in microsomes, whereas N-linoleoylethanolamine did not activate PAL2 expression in leaves, and did not affect [3H]NAE 14:0 binding in microsomes. The nonionic detergent dodecylmaltoside solubilized functional [3H]NAE 14:0-binding activity from tobacco microsomal membranes. The dodecylmaltoside-solubilized NAE-binding activity retained similar, but not identical, binding properties to the NAE-binding protein(s) in intact tobacco microsomes. Additionally, high-affinity saturable NAE-binding proteins were identified in microsomes isolated from Arabidopsis and Medicago truncatula tissues, indicating the general prevalence of these binding proteins in plant membranes. We propose that plants possess an NAE-signaling pathway with functional similarities to the “endocannabinoid” pathway of animal systems and that this pathway, in part, participates in xylanase elicitor perception in tobacco.
N-acylethanolamines (NAEs) constitute a class of lipid compounds naturally present in both animal and plant membranes as constituents of the membrane-bound phospholipid, N-acylphosphatidylethanolamine (NAPE). NAPE is composed of a third fatty acid moiety linked to the amino head group of the commonly occurring membrane phospholipid, phosphatidylethanolamine (Schmid et al., 1990; Chapman and Moore, 1993). NAEs are released from NAPE by phospholipase d-type hydrolases in response to a variety of stimuli (Di Marzo et al., 1994; Schmid et al., 1996; Chapman et al., 1998; Hansen et al., 1998). During the past decade, transient NAE release and accumulation has been attributed a variety of biological activities, including neurotransmission (Schmid et al., 1996; Di Marzo, 1998a, 1998b), membrane protection (Hansen et al., 2000), immunomodulation in animals (Klein et al., 1998), and defense signaling in plants (Tripathy et al., 1999; Chapman, 2000).
In animals, anandamide (NAE 20:4) was the first NAE type to be identified as an endogenous signaling ligand for cannabinoid (CB) receptors (Devane et al., 1992), and the diverse physiological functions of CBs are mediated in part through the CB receptors CB1 (Matsuda et al., 1990) and CB2 (Munro et al., 1993). These CB receptors are G-protein coupled and mostly localized to the central nervous and immune systems (Pertwee, 1997, 1999; Martin et al., 1999), respectively. Identification of these receptors and their endogenous ligands has led to the development of several CB analogs (WIN 55, 212-2, AM 281, SR 144528, etc.) that interact more specifically and potently with CB receptors (Reggio, 1999). Together, the NAEs and their receptors have emerged as active signaling components of an “endocannabinoid” system affecting both neuronal and immune functions in animal systems (Salzet et al., 2000) and have become targets of potential therapeutic applications (De Petrocellis et al., 2000; Straus, 2000).
In plants, NAPE-NAE metabolism is widespread (Chapman and Moore, 1993) and appears to be involved in several physiological processes (Chapman, 2000). For example, a phospholipase D-mediated accumulation of extracellular NAE 14:0 was triggered in tobacco (Nicotiana tabacum) cell suspensions (Chapman et al., 1998) and leaves (Tripathy et al., 1999) within minutes after elicitor perception. Nanomolar concentrations of NAE 14:0 activated Phe ammonia-lyase (PAL) gene expression in both cell suspensions and leaves of intact plants in a manner similar to, but independent of, pathogen elicitor treatment. In addition, exogenously supplied NAE 14:0 (and other NAE types) could reduce the characteristic alkalinization response induced by various pathogen elicitors. In fact, micromolar concentrations of NAE 14:0 essentially eliminated the alkalinization response in tobacco suspension cells, reminiscent of the CB-receptor-mediated ion flux modulation by NAE 20:4 in N18 neuroblastoma cells and murine AtT-20 tumor cells (Mackie et al., 1993, 1995). We proposed that the elicitor-induced release of nanomolar to micromolar amounts of NAE 14:0 in vivo acted both to attenuate the short-term alkalinization response and to activate downstream PAL2 gene expression in tobacco (Tripathy et al., 1999).
At present little is known about the detailed sequence of events or the components of NAE-mediated signaling in plants. Here, we provide evidence that NAE action in plant cells is mediated via NAE-binding protein(s). Further, we demonstrate saturable, high-affinity [3H]NAE 14:0-specific binding to a protein in tobacco membranes with biochemical properties appropriate for the physiological responses. In addition, antagonists of mammalian CB receptors blocked both of the biological activities previously attributed to NAE 14:0, an endogenous NAE that accumulated in tobacco cell suspensions and leaves after pathogen elicitor perception (Chapman et al., 1998; Tripathy et al., 1999). The membrane-associated tobacco NAE-binding protein was solubilized from tobacco membranes in the nonionic detergent dodecylmaltoside (DDM), and a similar membrane-associated NAE-binding activity was characterized in leaf and root tissues of Arabidopsis and Medicago truncatula. Consequently, we propose that NAE signaling in plant cells may operate through ligand interaction with a membrane-associated protein similar to the endocannabinoid-signaling pathway found in other multicellular eukaryotes.
RESULTS
CB Receptor Antagonists Reverse NAE Inhibition of Elicitor-Induced Alkalinization Response
The fungal elicitor, xylanase, induces well-characterized defense responses in tobacco cell suspensions and leaves including Ca2+ influx, K+/H+ exchange, ethylene biosynthesis, and accumulation of defense gene transcripts (pathogenesis-related proteins) and defense-related compounds (phytoalexins; Anderson et al., 1990; Lotan and Fluhr, 1990; Felix et al., 1993; Moreau et al., 1994). A high-affinity binding protein (66 kD) for xylanase was found in tobacco membranes (Hanania and Avni, 1997). The elicitor activity of xylanase is independent of its enzymatic activity, as deduced from site-directed mutagenesis in the catalytic domain of the xylanase II gene of Trichderma reesei (Enkerli et al., 1999) and amino acid substitutions in the active site of the EIX (ethylene-inducing xylanase) gene of Trichderma viride (Furman-Matarasso et al., 1999).
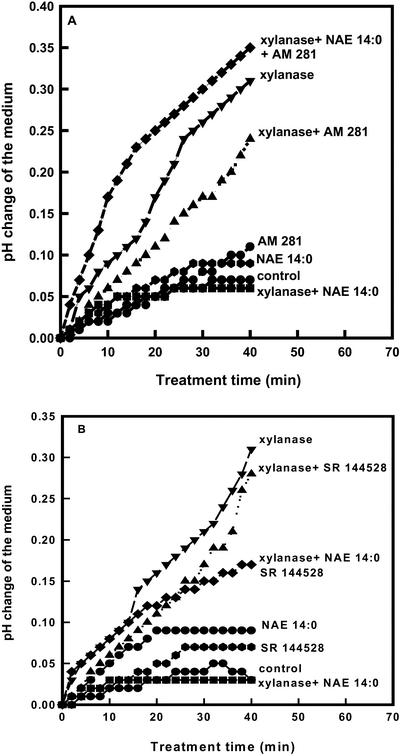
Medium alkalinization is among the best documented, early plant defense responses induced by pathogen elicitors (Atkinson et al., 1990; Baker et al., 1991) or wounding (Meindl et al., 2000). Xylanase-induced alkalinization of tobacco cell suspension culture medium (Bailey et al., 1992; Felix et al., 1993) and its inhibition by NAEs (Tripathy et al., 1999) provided an easily measurable biological response for preliminary pharmacological experiments. The involvement of a NAE 14:0-binding protein in this response was explored using two CB receptor antagonists, AM 281 and SR 144528 (Rinaldi-Carmona et al., 1998). Tobacco cell cultures treated with xylanase (1.0 μg mL−1) responded with a characteristic rapid approximately 0.3-unit pH change within 40 min, which was reduced to approximately control levels in the presence of 10 μm NAE 14:0 (Fig. 1, A and B). NAE 14:0 alone did not appreciably affect the pH of the culture medium (Tripathy et al., 1999; also shown in Fig. 1). When the mammalian CB receptor antagonists AM 281 and SR 144528 (at 10 μm, equimolar to NAE 14:0) were included with NAE 14:0 and xylanase, the NAE inhibitory effect was reversed, although the effect by SR144528 was less pronounced. The antagonists by themselves did not seem to influence appreciably the xylanase-induced alkalinization response (Fig. 1, A and B, respectively), and had no substantial influence on pH of the media in the absence of xylanase. Thus, antagonists of mammalian NAE (NAE 20:4) receptors effectively reversed this NAE 14:0-mediated response in tobacco cells, predicting the occurrence of NAE receptors in plant cells.
Figure 1.
A and B, Effect of mammalian CB receptor antagonists AM 281 and SR 144528 on NAE 14:0-mediated inhibition of xylanase-induced alkalinization of tobacco cell culture medium. Medium alkalinization was recorded in 15 mL of cultured cells that were pre-equilibrated to a constant pH for 20 to 30 min at room temperature. Xylanase (1.0 μg mL−1), NAE 14:0 (10.0 μm), AM 281 (10.0 μm), and/or SR 144528 (10.0 μm) were added alone or in combinations as preparations in water, spent culture medium, and 0.1% (v/v) DMSO, respectively, as described in “Materials and Methods.” Results shown are from a typical experiment using a single population of cells for all treatments in A or B. Replicate experiments with different batches of cell suspensions showed similar results, although the magnitude of the pH change varied between culture batches.
CB Receptor Antagonists Inhibit Elicitor and NAE 14:0-Induced Increases in PAL2 Transcript Abundance
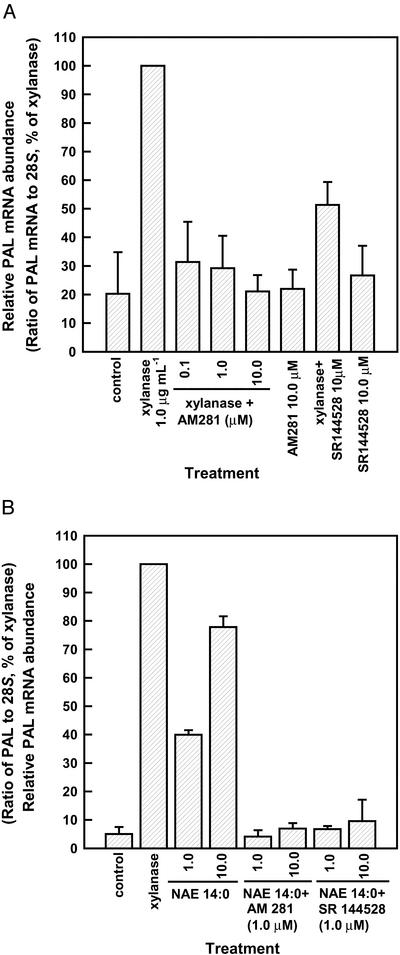
Xylanase treatment triggered the accumulation of NAE 14:0 in tobacco cell suspensions (Chapman et al., 1998) and leaf tissues (Tripathy et al., 1999) within minutes, and this in turn was associated with an increase in relative PAL2 transcript abundance. The kinetics of PAL transcript induction in tobacco leaves by xylanase or by NAE 14:0 is similar, both peaking at about 8 h after treatment. When tested with the CB receptor antagonists AM 281 and SR 144528, the xylanase-induced increase in PAL2 transcript abundance after 8 h was reduced (Fig. 2A). Quantification of PAL transcript abundance from multiple northern blots confirmed these results. Moreover, when NAE 14:0-treated tobacco leaves were co-infiltrated with 1.0 μm of either AM 281 or SR 144528 for 8 h, the accumulation of PAL transcripts reduced to near control levels compared with NAE 14:0 alone and water-only controls (Fig. 2B). Taken together, these results corroborated our results with cell suspensions (alkalinization response above) by supporting the existence of a CB receptor-like NAE 14:0-binding protein in tobacco cells that mediates its biological activities and suggests that NAE signaling may participate in xylanase perception.
Figure 2.
Reduction of xylanase- or NAE-induced PAL mRNA transcript accumulation by mammalian CB receptor antagonists AM 281 and SR 144528. A, PAL2 transcript abundance was evaluated by northern blotting in experiments wherein tobacco leaves were infiltrated with water (control), xylanase (1.0 μg mL−1) alone, or xylanase in combination with different concentrations of AM 281 or SR 144528 for 8 h. B, PAL2 transcript abundance was evaluated by northern blotting in experiments wherein tobacco leaves infiltrated with water (control, including 0.1% [v/v] DMSO), different concentrations of NAE 14:0 (as aqueous preparations diluted from DMSO stock, up to 0.1% [v/v] DMSO final) alone, or in combination with AM 281 (1.0 μm in 0.1% [v/v] DMSO) or SR 144528 (1.0 μm in 0.1% [v/v] DMSO). In both A and B, results are a quantitative representation of relative abundance of PAL mRNA transcripts (normalized to 28S rRNA by scanning densitometry and NIH imaging software). These results are presented as the percent of xylanase-induced levels of PAL, which was arbitrarily set to 100%. The data plotted correspond to the means ± sd of three independent experiments analyzed under identical conditions of electrophoresis, hybridization, and film exposure.
Other NAEs Increase PAL2 Transcript Accumulation
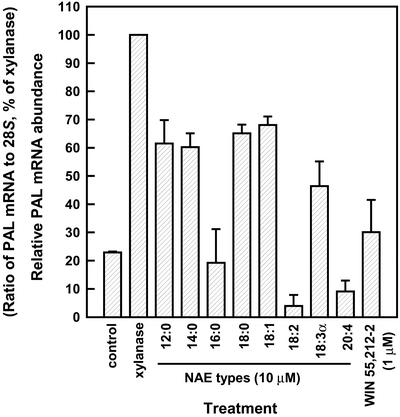
In addition to NAE 12:0 and NAE 14:0 identified in tobacco leaves and cell suspensions, a variety of NAE types with different chain lengths and degree of unsaturation were identified in seeds of higher plants (Chapman et al., 1999). Consequently, we tested several synthetic NAE types for their ability to modulate PAL transcript levels. Most of the NAE species (added at 10.0 μm) except NAE 18:2 and NAE 20:4 (and to a lesser degree NAE 16:0) induced PAL transcript accumulation in tobacco leaves (Fig. 3). NAE 18:2 and NAΕ 16:0 were the most abundant NAE types identified in seeds from several higher plant species (Chapman et al., 1999), approaching 1,000 and 400 ng g−1 fresh weight, respectively in cotton (Gossypium hirsutum) seeds, and yet they were mostly inactive with respect to PAL expression in leaves of tobacco plants. Also interesting, NAE 20:4 (not identified in plants, but the endogenous ligand for mammalian CB1 receptor; Devane et al., 1992) and a synthetic CB receptor agonist, WIN 55,212-2, did not appear to induce PAL transcripts appreciably in these tissues. Curiously NAE 18:0, NAE 18:1, and NAE 18:3 were active, whereas NAE 18:2 was not. The reason for this difference in PAL induction is unclear at this point, but may reflect a different fate for NAE 18:2, because this NAE was shown to be metabolized by lipo-xygenase-13 pathway in germinating seeds (Shrestha et al., 2002). Thus, although NAE modulation of short and long-term defense responses in plants shares some similarities with the endocannabinoid-signaling pathways in animals, there are some distinct differences with respect to NAE types. Clearly, abrogation of NAE effects by CB receptor antagonists suggests the existence of an analogous receptor for NAEs in tobacco. However, such a putative receptor must be somewhat different from the mammalian receptor because the endogenous ligand in tobacco leaf tissues is likely NAE 14:0, a medium chain, saturated species, and anandamide, the endogenous polyunsaturated. long chain mammalian NAE, is inactive in modulating tobacco PAL transcript accumulation.
Figure 3.
Analysis of PAL mRNA transcript accumulation after treatment of tobacco leaves with different NAE types and the mammalian CB analog WIN 55,212-2. All NAEs (NAEs 12:0, 14:0, 16:0, 18:0, 18:1, 18:2, 18:3α, and 20:4) were infiltrated into leaves at 10.0 μm, and WIN 55, 212-2 was infiltrated at 1.0 μm. PAL2 transcript abundance was evaluated by northern blotting. Results are a quantitative representation of relative abundance of PAL mRNA transcripts (means ± sd) in tobacco leaves subjected to the above treatments for 8 h (normalized to 28S rRNA and percent of xylanase-induced levels of PAL) in three independent experiments analyzed under identical conditions of electrophoresis, hybridization, and film exposure.
NAE-Binding Proteins in Membranes of Tobacco Cell Suspensions, Tobacco Leaves, and Other Plant Tissues
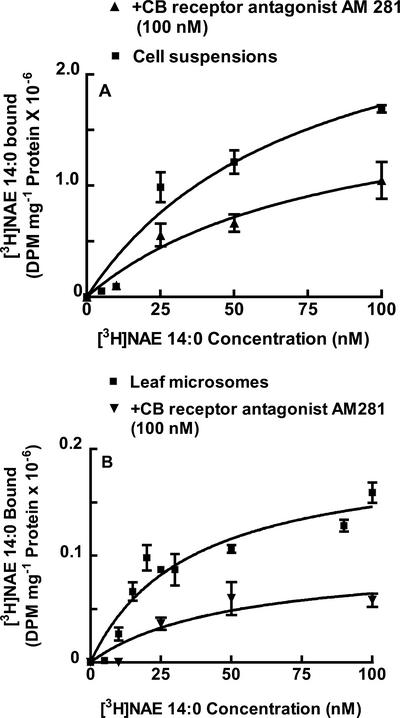
Because the CB receptor antagonists effectively suppressed NAE 14:0-mediated biological activities as described above, a mammalian CB receptor-binding assay (Hillard et al., 1995) was modified for tobacco cells/plant extracts. Binding of [3H]NAE 14:0 (49.0 Ci mmol−1) to intact tobacco suspension cells was examined. Preliminary binding experiments were conducted to standardize pH and bovine serum albumin (BSA) concentration in binding assays. Also, protein amount (cell leaf−1 microsomal proteins) and incubation time were optimized for reproducible estimates of specific binding. For tobacco suspension-cultured cells, specific binding of [3H]NAE 14:0 was determined at pH 5.6 with increasing concentration of radioligand (1–100 nm) in the absence (total binding) or presence (nonspecific binding) of excess unlabeled NAE 14:0. Saturable, specific binding of [3H]NAE 14:0 as a function of ligand concentration (Fig. 4A) indicated the existence of a high-affinity tobacco NAE 14:0-binding protein. The binding affinity of NAE 14:0 for tobacco cells was estimated by fitting the experimental data to nonlinear regression analysis for a one-site binding equation (Prism 3.0, GraphPad Software, San Diego) and showed an apparent dissociation constant (Kd) of 74 nm (Table I). The binding affinity of NAE 14:0 was less than the range estimated for some elicitins for their receptors in tobacco membrane preparations (Kd of 5.8–13.5 nm; Bourque et al., 1998, 1999) but was higher than that of harpin (Kd of 425 nm; Lee et al., 2001), an elicitor of Erwinia amylovora and Pseudomonas syringae pv syringae origin (Baker et al., 1993; He et al., 1993). Moreover, the apparent Kd was comparable with that determined for CB receptor ligands in many mammalian cell types (for review, see Khanolkar et al., 2000). The Kd values for CB receptors in mammalian systems vary greatly depending on tissue type, temperature, the amount of protein, and other incubation factors (for review, see Pertwee, 1999). Under the conditions for optimal NAE 14:0 binding to tobacco cells, the mammalian endocannabinoid, [3H]NAE 20:4, did not bind (data not shown).
Figure 4.
Specific binding of [3H]NAE 14:0 to tobacco suspension-cultured cells and microsomes isolated from tobacco leaves. A, Analysis of specific binding activity of [3H]NAE 14:0 to tobacco suspension cells (20 μg of protein) with concentrations ranging from 5 to 100 nm in the absence and presence of the mammalian CB receptor antagonist, AM 281 (100 nm). B, Analysis of specific binding activity of [3H]NAE 14:0 to tobacco leaf microsomes (50 μg of protein) with concentrations ranging from 5 to 100 nm in the presence and absence of AM 281. Specific binding was determined by subtracting nonspecific binding (binding in the presence of approximately 500× nonradioactive NAE 14:0) from total radioligand binding. Data shown are means and range of duplicate samples within a given experiment and are representative of results obtained in six replicate experiments.
Table I.
Binding properties of NAE-binding proteins identified in tobacco cell suspensions and in microsomes isolated from tobacco leaves
| Protein Source | Radioligand Only
|
+ AM 281
|
+ SR 144528
|
|||
|---|---|---|---|---|---|---|
| Kd | Bmax | Kd | Bmax | Kd | Bmax | |
| nm | DPM mg−1 protein | nm | DPM mg−1 protein | nm | DPM mg−1 protein | |
| Tobacco cell suspensions | 74 | 2,991,000 | 82 | 1,888,000 | ND | ND |
| Tobacco leaf microsomes | 35 | 196,994 | 49 | 96,554 | 65 | 139,177 |
| Tobacco leaf solubilized microsomes | 11 | 66,720 | 16 | 59,888 | 15 | 53,774 |
Results shown are Kd and Bmax values estimated from the specific binding data like that shown in Fig. 4 using a single-site binding equation (GraphPad Prism 3.0 software). Binding assays were conducted in the absence (radioligands only) or presence of CB antagonists, AM 281 or SR 144528 (each at 100 nm for cell suspension or microsomes and at 10 nm for DDM solubilized microsomes). ND, not determined; DPM, disintegrations per minute.
Specific binding of [3H]NAE 14:0 was reduced substantially in the presence of 100 nm AM 281 (Fig. 4), although the binding affinity (Kd, approximately 82 nm) remained essentially unchanged (Table I). At higher concentrations (1.0 μm) both the antagonists, AM 281 and SR 144528, completely eliminated [3H]NAE 14:0 binding (data not shown).
Similar binding experiments with tobacco leaf microsomes were conducted to develop optimal, reproducible [3H]NAE 14:0-specific binding conditions. [3H]NAE 14:0-specific binding was saturable in leaf microsomes (Fig. 4B) with an equilibrium Kd of 35 nm (Table I), which is comparable but somewhat lower than that estimated for tobacco cells (Kd, 74.0 nm). Consistent with results of suspension cells, the CB antagonist AM 281 (100 nm) reduced NAE 14:0-specific binding without substantially affecting the apparent binding affinity (Kd, 50 nm with AM 281). In addition, SR 144528 (100 nm) also reduced NAE 14:0-specific binding in tobacco microsomes (Table I). Under no circumstances did plant membranes bind [3H]NAE 20:4, again reflecting a consistent difference between tobacco NAE-binding protein(s) and mammalian CB receptors.
As might be expected, microsomal membranes isolated from tobacco leaves were enriched in NAE-binding activity relative to other cell fractions derived by differential centrifugation (data not shown). NAE-binding activity was not released from microsomal membranes by salt solutions, but two nonionic detergents (Triton X-100 and DDM) effectively solubilized active NAE-binding activity (S. Tripathy, K. Kleppinger-Sparace, R.A. Dixon, and K.D. Chapman, unpublished data), indicating that the NAE-binding protein was an integral membrane protein according to classical biochemical criteria. DDM-solubilized NAE-binding proteins retained saturable, specific binding toward [3H]NAE 14:0 with apparent affinities that were similar to, although somewhat higher than, the proteins in intact microsomes (Table I). The apparent Bmax values were somewhat lower in DDM-solubilized protein preparations compared with intact microsomes, perhaps reflecting incomplete recovery of appropriately folded native proteins, especially because little NAE-binding activity was detected in detergent-insoluble fractions. Nonetheless recovery of functional DDM-solubilized NAE-binding activity from tobacco microsomal membranes supports our conclusion that NAE-binding activity is tightly associated with plant membranes (like that in animal tissues) and should facilitate future protein purification and characterization strategies.
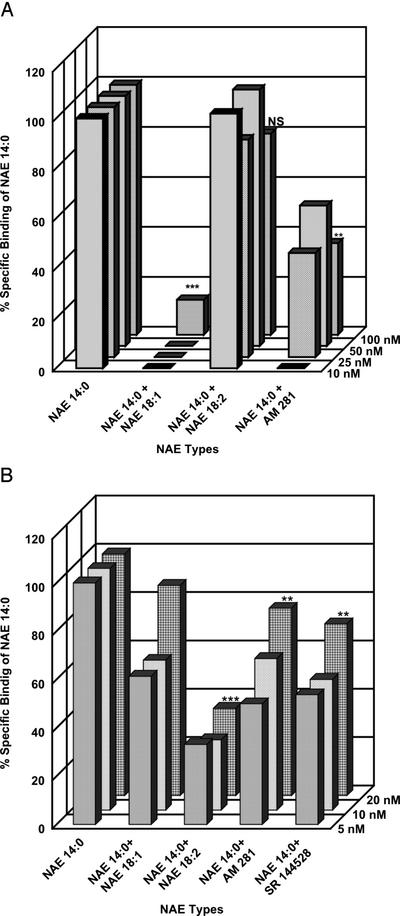
Interference of other NAEs with specific binding of [3H]NAE 14:0 to its membrane-associated binding protein was analyzed (Fig. 5) because several other NAE types were potent activators of PAL expression (e.g. NAE 18:1) or were altogether inactive (e.g. NAE 18:2). In addition, a comparison was made between [3H]NAE 14:0-specific binding activity in intact microsomes (Fig. 5A) and in DDM-solubilized proteins (Fig. 5B). When binding studies for microsomes (Fig. 5A) with [3H]NAE 14:0 were carried out in the presence NAE 18:1 (100 nm), specific binding was completely eliminated at lower NAE 14:0 concentrations and was reduced significantly at equimolar concentrations of NAE 14:0 (P < 0.001, n = 3). By contrast, NAE 18:2 did not seem to affect [3H]NAE 14:0-specific binding (not significant) in microsomes at any concentration tested. When tested with xylanase at 1.0 μg mL−1, [3H]NAE 14:0-specific binding to tobacco leaf microsomes was not affected (data not shown). Taken together, these binding studies for microsomal membranes are consistent with the notion that a CB receptor-like NAE-binding protein(s) exists in tobacco and that it mediates the biological activity(ies) of NAEs.
Figure 5.
Comparison of [3H]NAE 14:0-specific binding to microsomal membranes (A) or to DDM-solubilized microsomal proteins (B) of tobacco leaves in the presence of other NAE types and CB receptor antagonist. NAE 18:1, which induced PAL transcript accumulation; NAE 18:2, which did not induce PAL in tobacco leaves; and AM281, which blocked NAE and xylanase-induced PAL activation, were included at 100 nm in the [3H]NAE 14:0-binding assays for microsomes, and the radioligand concentration was varied from 10 to 100 nm. These same competitors as well as SR144528 were included at 10 nm in the [3H]NAE 14:0-binding assays for DDM-solubilized microsomal proteins, and the radioligand concentration was varied from 10 to 100 nm. A, Values represent the means (triplicate assays) of an individual experiment reproduced two times using different preparations of microsomes (50 μg of protein). One-way ANOVA was used (GraphPad InStat v3.0) to compare (for 100 nm of each treatment) the data for statistically significant differences (***, P < 0.001; **, P < 0.01; NS, non-significant) in specific NAE 14:0 binding (control) in the presence of different NAE types or the antagonist, AM 281. For NAE 14:0 versus NAE 14:0 + NAE 18:1, P < 0.001(***); for NAE 14:0 versus NAE 14:0 + NAE 18:2, P > 0.05 (NS); for NAE 14:0 versus + AM 281, P < 0.01 (**); for NAE 14:0 + NAE 18:1 versus NAE 14:0 + NAE 18:2, P < 0.01 (**); for NAE 14:0 + NAE 18:1 versus NAE 14:0 + AM 281, P > 0.10 (NS); and for NAE 14:0 + NAE 18:2 versus NAE 14:0 + AM 281, P < 0.01 (**). B, Values represent the means (triplicate assays) of an individual experiment reproduced four times using different preparations of DDM-solubilized microsomes (5–10 μg of solubilized protein). One-way ANOVA was used (GraphPad InStat v3.0) as above to compare (for 10 nm of each treatment) the data for statistically significant differences (***, P < 0.001; **, P < 0.01; NS, non-significant) for specific binding for NAE 14:0 alone (control, set at 100%) compared with NAE 14:0 in the presence of different NAE types or the antagonists, AM 281 and SR 144528. For NAE 14:0 versus NAE 14:0 + NAE 18:1, P < 0.05; NAE 14:0 versus NAE 14:0 + NAE 18:2, P < 0.001 (***); for NAE 14:0 versus NAE 14:0+ AM 281, P < 0.01 (**); for NAE 14:0 versus NAE 14:0+ SR 144528, P < 0.01 (**); for NAE 14:0 + NAE 18:1 versus NAE 14:0 + NAE 18:2, P < 0.01 (**); for NAE 14:0 + AM 281 versus NAE 14:0 + SR 144528, P > 0.05 (NS); NAE 14:0 + AM 281 versus NAE 14:0 + NAE 18:1, P > 0.05 (NS); NAE 14:0 + AM 281 versus NAE 14:0 + NAE 18:2, P > 0.05 (NS); NAE 14:0 + SR 144528 versus NAE 14:0 + NAE 18:1, P > 0.05 (NS); NAE 14:0 + SR 144528 versus NAE 14:0 + NAE 18:2, P > 0.05 (NS). In both A and B, error bars are omitted for clarity; sd was generally less than 15%.
In DDM-solubilized fractions, NAE 14:0-specific binding was diminished in the presence of 10 nm each of NAE 18:1 (particularly at equimolar and lower radioligand concentrations), NAE 18:2, AM 281, or SR 144528 (Fig. 5B). Higher concentrations of these putative competitors (30, 50, or 100 nm) completely inhibited/prevented specific [3H]NAE 14:0 binding at 5 to 20 nm radioligand (data not shown). Inhibition of [3H]NAE 14:0 binding was reduced as the concentration of radioligand was increased, consistent with the likelihood that these competitors are reversibly bound to DDM-solubilized protein. Somewhat surprising was the fact that NAE 18:2 appeared to quite effectively inhibit [3H]NAE 14:0-specific binding in DDM-solubilized preparations, opposite from the results with intact microsomes (Fig. 5A), suggesting that NAE 18:2 does bind to the putative NAE receptor. As mentioned above, NAE 18:2 is readily converted by a microsomal 13-LOX to novel NAE oxylipins (Shrestha et al., 2002) that may not interact with the NAE-binding protein. This functional NAE-LOX activity, although present in microsomes, may not be present in our DDM-solubilized preparation, and consequently, unmetabolized NAE18:2 has access to the NAE-binding protein. Of course other explanations are possible as well, and this apparent discrepancy can be addressed with purified or recombinant NAE-binding proteins in the future.
In addition to tobacco, high-affinity, saturable [3H]NAE 14:0-specific binding activity was measured in microsomes from Arabidopsis and M. truncatula plant parts (Table II), which indicates the general prevalence of these NAE-binding proteins in plant membranes, and suggests that NAE-signaling pathways may operate in other tissues of a variety of plant species.
Table II.
Binding properties of NAE-binding proteins identified in microsomes (150,000g supernatant) isolated from tobacco leaves, Arabidopsis leaves or roots, and M. truncatula leaves and roots
| Protein Source | Kd | Bmax |
|---|---|---|
| nm | DPM mg−1 protein | |
| Tobacco leaves | 35 | 196,994 |
| Arabidopsis leaves | 9 | 10,302 |
| Arabidopsis roots | 51 | 208,862 |
| M. truncatula leaves | 38 | 38,758 |
| M. truncatula roots | 50 | 120,941 |
Results shown are Kd and Bmax values calculated by fitting experimental data to a single-site binding equation (GraphPad Prism 3.0). Approximately 50 μg of protein was used in each assay, and specific [3H]NAE 14:0 binding was determined over a range of 5 to 80 nm radioligand (similar to plots shown in Fig. 4). DPM, disintegrations per minute.
DISCUSSION
Induction of defense-signaling cascades in plants is the consequence of elicitor recognition and perception by plant cells (Boller, 1995) through specific receptors. This interaction leads to well-known transient modulation of several components of the signaling pathways, such as lipases, [Ca2′2b], G-proteins, kinases and phosphatases, reactive oxygen intermediates, nitric oxide, salicylic acid, and various metabolites in an orderly and timely fashion to activate defense gene expression (for review, see Trewavas, 2000). Our previous studies have indicated that elicitor-induced endogenous levels of NAE 14:0 release were sufficient to activate PAL2 gene expression (Tripathy et al., 1999). In mammals, endogenously released NAE 20:4 (Devane et al., 1992; Das et al., 1995) and sn-2-arachidonylglycerol (Mechoulam et al., 1995; Sugiura et al., 1995) activate signaling pathways mostly by binding to CB1 (Matsuda et al., 1990; Gerard et al., 1991; Chakrabarti et al., 1995) and CB2 (Munro et al., 1993) CB receptors, respectively. With this in mind, we have used the mammalian CB receptor antagonists AM 281 and SR 144528 as pharmacological tools coupled to NAE 14:0-induced biological activities as indexes for a systematic approach to identify an NAE receptor/binding protein in plants.
Two physiological responses, inhibition of elicitor-induced alkalinization response (Fig. 1) and induction of PAL2 transcript accumulation (Fig. 2), attributed to NAE 14:0 were reversed by mammalian CB receptor antagonists. Moreover data are presented supporting the occurrence of an NAE 14:0-binding protein(s) in tobacco with binding characteristics closely matching the modulation of these physiological responses. Although this represents only the beginning of the characterization of NAE signaling in plants, these results indicate substantial similarities with endocannabinoid signaling in animal cells. For example, in Chinese hamster ovary cells, SR 144528 is a selective antagonist of the CB2-mediated induction of the immediate-early response gene krox24 and inhibition of adenylyl cyclase activity (Portier et al., 1999). In mammalian neuronal cells, CB modulation of N- and P/Q-type ion channels is altered by SR 141716A (Twitchell et al., 1997), an analog of AM 281 (Gatley et al., 1998). Thus, the reversal/inhibition by CB receptor antagonists of NAE 14:0-induced responses in tobacco cell suspensions and leaves suggests the parallel existence of a mammalian-like endocannabinoid system where NAE 14:0 mediates its function by binding to a CB receptor-like binding protein.
Our working hypothesis is that, in tobacco (cells and leaves), NAE 14:0 is released within minutes of fungal xylanase perception (Chapman et al., 1998; Tripathy et al., 1999), and this endogenous rise in NAE concentration is perceived by a membrane-associated NAE-binding protein that transduces this signal to attenuate the alkalinization response in the short term (time course of minutes) and to activate PAL2 expression in the long term (time course of hours). Here, the complete inhibition of the elicitor-induced alkalinization response by NAE 14:0 is likely a manifestation of higher levels of exogenous NAE 14:0 (10 μm) added at time of elicitor treatment. Endogenous, activated levels (induced by elicitor) of NAE 14:0 are in the low- to mid-nanomolar range (Tripathy et al., 1999), and this rising concentration of NAE 14:0 may be responsible for limiting (not completely inhibiting) the extent of elicitor-induced alkalinization that occurs some 40 to 60 min after elicitor treatment. Likewise, the endogenous, activated NAE 14:0 levels in the nanomolar range were sufficient to activate PAL2 expression (Tripathy et al., 1999). Hence, we propose that NAE release could participate in both the attenuation of the primary signal (xylananse) and the propagation of this signal to activate defense gene expression (e.g. PAL2). Clearly, NAE release represents only part of a complex scheme of signaling circuits that provides plant cells the flexibility to respond to multiple abiotic and biotic stresses, and the relationship of this NAE-signaling pathway to other defense-related signal transduction cascades (Boller, 1995; Zhu et al., 1996; Zhang and Klessig, 2001) remains to be elucidated.
Work by the Boller group, based on experiments with the kinase inhibitor K-252a, indicated that xylanase activation of PAL activity was dependent upon kinase-mediated activation of the alkalinization response (Felix et al., 1993, 1994); our results suggest that the NAE pathway may, in part, bypass this requirement in terms of PAL2 activation, because NAE treatment alone does not induce the alkalinization response but is sufficient to activate PAL2 expression (Figs. 1 and 2B; Tripathy et al., 1999). One reasonable scenario to explain this apparent discrepancy is that more than one kinase likely is activated in response to xylanase (and other elicitors), one of which activates the alkalinization response independent of NAE action, another of which participates in the activation of PAL2 transcription and is dependent upon NAE action, and both of which would be inhibited by K-252a treatment. CB receptor-dependent modulation of mitogen-activated protein kinase activity occurs in several vertebrate cell types (Di Marzo, 1998a), although NAE modulation of kinase activity in plants has not yet been demonstrated. It should be noted that in tobacco, the xylanase-induced alkalinization-response was reported not to operate in leaf discs, despite activation of several downstream characteristic defense responses (Bailey et al., 1992), suggesting that the alkalinization response may be in a pathway separate from some plant defense responses. Future work to delineate the signaling components that interact with NAE metabolism will help to more clearly define the role of NAE signaling in plant defense responses. In any case, NAE-mediated signaling may represent one of several pathways that converge to lead to PAL2 gene activation (and/or other stress-induced genes).
Although a more complete characterization of NAE-binding protein(s) in plants must await purification and molecular cloning of candidate protein(s), the binding assay developed here for the endogenous plant NAE 14:0 will no doubt aid in the functional characterization of such proteins. Some clear differences with mammalian CB receptor-ligand interactions are evident from our results, such as the reduction in [3H]NAE 14:0-specific binding by the longer-chain, monounsaturated NAE 18:1, suggesting binding affinity of the plant protein to both saturated and unsaturated NAE types. Several medium to long chain NAE types induced PAL transcript accumulation in tobacco leaves. Interestingly, the mammalian natural and synthetic CB receptor agonists NAE 20:4 and WIN 55212-2, respectively, did not induce this activity. NAE 20:4 is reported to have low affinity for the CB2 receptor in some mammalian cell types (Schowalter et al., 1996; Felder and Glass, 1998). NAE 16:0 also did not substantially induce PAL activity and in mammalian tissues does not bind with high affinity to any of the known CB receptors (Lambert and Di Marzo, 1999). Perhaps the most striking difference from mammalian systems is the nature of the NAE itself. In tobacco leaves, NAE 14:0, a saturated, medium chain acylethanolamide, appears to function as the primary endogenous ligand, whereas the endogenous mammalian ligand, NAE 20:4, cannot activate PAL expression (Fig. 3) or bind to tobacco membranes. Similarly, NAE 18:2, a diunsaturated NAE type, neither induced PAL expression (Fig. 3) nor affected [3H]NAE 14:0-specific binding in microsomal membranes (Fig. 5A), although this may be a consequence of its rapid peroxidation by microsomal membranes (Shrestha et al., 2002), because DDM-solubilized NAE-binding activity appears to interact with NAE18:2 (Fig. 5B).
Typical seven-transmembrane G-protein-coupled receptors are not prevalent in plant databases, which may indicate a structurally diverged NAE-binding protein that recognizes different NAE types. Alternatively, our results could be explained by the co-existence (and functional activity) of more than one protein, although we have modeled binding at one site (Fig. 4). Also, we cannot rule out at this point a cell surface-binding protein similar to vertebrate vanilloid receptors, which have been shown to bind NAEs (Szallasi and Di Marzo, 2000), even though the NAE-binding site for mammalian vanilloid receptors is intracellular, and in tobacco cell suspensions, NAE 14:0 accumulated extracellularly. At this point, we favor a model where an NAE 14:0-binding protein in tobacco, more similar to mammalian CB receptors, mediates its biological activity, and this is strongly supported by the pharmacological results. Nonetheless, future work is aimed at the isolation and structural characterization of the functional NAE-binding protein(s) from plants to better understand their role in mediating an NAE-signaling cascade. This should be facilitated by the development of our radioligand-binding assay and the detergent solubilization of functional NAE-binding proteins from plant membranes.
The Kd value for [3H]NAE 14:0-specific binding activity was comparable with the range of Kd values reported for CB receptor ligands in animals, and binding activity was reduced by CB receptor antagonists (see Figs. 4 and 5). Somewhat surprising was the fact that both mammalian CB receptor antagonists, usually used to distinguish between CB1 and CB2 in animal cells, abrogated NAE 14:0 binding and function in tobacco. The affinities of plant membrane proteins for NAE 14:0 (Tables I and II) were in the range of those reported for elicitors such as cryptogein (10.3 nm) and other elicitins (5.8–13.5 nm; Bourque et al., 1998, 1999) in tobacco plants. However, NAE 14:0 binding and activation of defense responses occurred at lower agonist concentrations than the activation of responses by the endogenous defense signal, salicylic acid, which is increased upon pathogen/elicitor treatment and may induce defense gene (pathogenesis related) expression in tobacco by binding specifically to salicylic acid-binding proteins with varying affinities (Kd, 90 nm–14 μm; Chen and Klessig, 1991; Du and Klessig, 1997). Suramin, an inhibitor of cytokine and growth factor receptor interactions in animal cells, was recently reported to inhibit binding of systemin to its receptor in plant cells (Stratmann et al., 2000), providing a precedence for potentially conserved signaling pathways for activation of immune and defense responses in plants and animals.
In summary, the present findings support the existence of a membrane-associated CB receptor-like plant NAE-binding protein(s) for NAE 14:0 that mediates NAE biological activities, suggesting the existence of an endocannabinoid-like signaling system that is conserved from primitive organisms to vertebrate mammals (for review, see Salzet et al., 2000) and now to plants.
MATERIALS AND METHODS
Plant Materials and Experimental Treatments
Tobacco (Nicotiana tabacum cv Xanthi) cell suspensions were periodically initiated from callus cultures and maintained as described previously (Chapman et al., 1995). Suspension cells from exponential log phase (72 h post-subculture) were used for elicitor treatment (xylanase 1.0 μg mL−1; Sigma-Aldrich, St. Louis) and binding assays. Fully expanded tobacco leaves from 3- to 6-month-old plants, grown as previously described (Tripathy et al., 1999), were used to evaluate PAL2 expression. NAE-specific binding activities were assayed in microsomes isolated from Arabidopsis and M. truncatula plants tissues. Arabidopsis (ecotype Columbia) plants were grown under a 12-h photoperiod (120 μE m−2) and were harvested at approximately 6 weeks old. M. truncatula (cv A17) plants were grown under a 16-h photoperiod (daylength extended with supplemental high-intensity Na vapor lamps), and vegetative tissues were harvested from 3-month-old plants.
Xylanase was added either directly to suspension cells or infiltrated as aqueous solutions into tobacco leaves. NAEs were synthesized chemically and added as aqueous solutions (prepared in either spent culture medium for culture treatment or diluted into water for leaf infiltration) essentially as described previously (Tripathy et al., 1999).
Stock solutions of CB receptor antagonists, AM 281 and SR 144528 (kindly provided by Dr. Guenter Gross, Department of Biological Sciences, University of North Texas, Denton) in dimethyl sulfoxide (DMSO) were infiltrated alone or with elicitor and/or NAEs as DMSO-aqueous preparations (final concentration of DMSO, <0.1% [v/v]). Control experiments were carried out with DMSO (0.1%, v/v) only.
Alkalinization Response and Defense Gene Expression
Suspension cell cultures were equilibrated to steady pH values before treatment with elicitor, NAEs, and/or CB receptor antagonists. The change in medium pH was recorded as described previously (Tripathy et al., 1999) at 2-min intervals for approximately 40 min. 0
Relative PAL transcript abundance was evaluated by RNA gel-blot analysis of total RNA preparations as described previously (Tripathy et al., 1999). In brief, tobacco leaves were infiltrated with either xylanase (1.0 μg mL−1) or NAE 14:0 alone or in combination with CB receptor antagonists, and total RNAs were isolated after 8 h according to Chomczynski and Sacchi (1987). RNA samples (10 μg) were separated in agarose-formaldehyde gels and transferred to nylon membranes (Hybond N+, Amersham Biosciences AB, Uppsala). Evaluation of RNA loading was by methylene blue-staining of blots (Herrin and Schmidt, 1988). Blots were probed with tobacco PAL2 and visualized by chemiluminescence as described previously (Tripathy et al., 1999). Relative PAL mRNA levels were estimated by normalizing to 28S RNA using scanning densitometry (NIH Imaging software developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Blots from three independent RNA extractions were evaluated under identical conditions of hybridization and film exposure and were averaged for quantitative estimates of relative transcript abundance. All experiments included water-only and xylanase-only controls for comparison. Relative PAL transcript levels induced by xylanase treatment were arbitrarily set to 100%, and experimental treatments were calculated proportionally.
Radioligands for Binding Assays
[3H]NAE 14:0 was synthesized from [9,10-3H(N)]myristic acid (49 Ci mmol−1, PerkinElmer Life Sciences, Boston) following the method of Hillard and coworkers (1995) with some modifications. In brief, acylchloride was prepared using 10 to 25 μCi of [3H]14:0, 800 μL of dichloromethane, and 1.2 equivalents of oxalyly chloride in the presence of 1.0 equivalent of dimethylformamide on ice for 1 h (Devane et al., 1992). The acylchloride formed was added to a 10-fold excess of ethanolamine, and incubation was carried out for another 2 h on ice. The reaction was stopped by the addition of 1 mL of water, and the organic layer was washed three times with water. [3H]NAE 14:0 was collected in the organic layer, dried under N2, and stored in anhydrous methanol. For further purification, [3H]NAE 14:0 was separated by thin-layer chromatography (K6 Silica Gel 60A, Whatman, Clifton, NJ) in hexane:ethyl acetate:methanol (60:40:5, v/v), quantified by radiometric scanning (Chapman et al., 1998), and eluted from silica gel with chloroform:methanol (1:2, v/v). Purity was 98% or greater as determined by thin-layer chromatography. [3H]NAE 20:4 (223 Ci mmol−1) was purchased from PerkinElmer Life Sciences.
Binding Assays
Binding assays were performed as developed previously for mammalian CB receptors (Hillard et al., 1995). Several multiscreen filtration units from different companies were evaluated for NAE 14:0-binding studies before selecting the multiscreen Whatman filtration system with BC Durapore 1.2-μm filters (Millipore, Bedford, MA) with few modifications over mammalian CB receptor-binding assay. Preliminary binding studies with tobacco whole cells from suspension and leaf microsomes were carried out to standardize pH, BSA amount (milligrams per milliliter), protein concentration, and incubation time in assay buffer, and optimized conditions were followed for all subsequent binding assays. Whole cells with 20 to 30 μg of protein were incubated for 30 min in 200 μL of spent culture medium, pH 5.2 to 5.5, containing 1% (w/v) BSA and 5 mm dithiothreitol (DTT) on a platform rotary shaker set to 140 rpm. Microsomal fractions with 10 to 50 μg of protein were similarly incubated for 30 min in a total 200 μL of reaction volume containing final concentrations of 75 mm potassium-phosphate buffer, pH 7.2, 300 mm Suc, 7.5 mm KCl, 0.75 mm EDTA, 0.75 mm EGTA, 0.5 mm ascorbate, 1.5% (w/v) BSA, and 5 mm DTT. Radioactivity of the stock ligands was quantified before binding assays. To avoid organic solvent effects, measured amounts of radioligands were dried in the preparation vials under nitrogen, dissolved in the incubation buffer by repeated sonication, and dispensed into reaction wells. Nonspecific binding was measured by including 100 to 500× excess of unlabeled NAE 14:0 or NAE 20:4 in respective reactions. Binding assays were stopped upon filtration, and the wells were washed three times with 200 μL well−1 of ice-cold incubation buffer to remove unbound ligands. Filters were frozen to facilitate excision into scintillation vials (6.5 mL) for estimation of radioactivity (LS 3801 Beckman Coulter, Fullerton, CA). Raw counts were corrected for quenching and efficiency and were converted to disintegrations per minute. Specific [3H]NAE 14:0 binding was determined by subtracting nonspecific binding values from total binding values in duplicate assays.
Cell Fractionation, Isolation, and Detergent Solubilization of Microsomal Membranes
Cellular fractions were prepared according to Chapman and Sriparameswaran (1997). Usually, 25 to 50 g of tobacco cells or plant tissues were homogenized in 2 volumes of 100 mm potassium-phosphate buffer, pH 7.2, containing 400 mm Suc, 10 mm KCl, 1 mm EDTA, 1 mm EGTA, 10 mm ascorbate, and 5 mm DTT by 10- × 15-s bursts in a vortex blender. The crude homogenates were filtered through four layers of cheesecloth and centrifuged in a Sorvall SS-34 at 650g for 10 min at 4°C, and the resulting supernatant was centrifuged at 10,000g for 20 min at 4°C. The 10,000g supernatant was then centrifuged in a Beckman Coulter Ti 75 rotor at 150,000g for 60 min at 4°C. All pelleted fractions were resuspended in homogenization buffer to one-tenth of the original volume. Fractions either were used immediately for assays or detergent treatments or were stored frozen (−80°C) as aliquots. Protein content was estimated according to Bradford (1976) after precipitation in 10% (w/v) TCA and resuspension in 10 mm sodium or potassium-phosphate buffer (pH 7.2).
As an important step in the extraction of functional NAE-specific binding activity from microsomal membranes, this protein was effectively solubilized in nonionic detergent following the general procedure developed previously for cotton (Gossypium hirsutum) seed microsomal proteins (Chapman and Moore, 1994; Chapman et al., 1997). Microsomes (100 μg of protein) isolated from tobacco leaves were treated with increasing concentrations of DDM for 30 min on ice and centrifuged (150,000g, 60 min) to separate proteins into detergent-solubilized (supernatant) and insoluble (pellet) fractions. Specific [3H]NAE 14:0-binding activity was determined in these fractions, and 0.2 mm DDM was reproducibly found to result in the highest recovery of NAE 14:0-specific binding activity. Further experiments varying the detergent-to-protein ratio were conducted at 0.2 mm DDM, and a detergent-to-protein ratio of 0.4-to-1 (weight-to-weight) was selected as the most effective for the reproducible isolation of detergent-solubilized NAE-binding activity. Although NAE-binding activity was observed in Triton X-100-treated (up to 0.1% [v/v]) membranes, little or no activity remained soluble after ultracentrifugation. Treatments with the zwitterionic detergent CHAPS at various concentrations abolished NAE-specific binding activity.
Footnotes
This work was supported by the Texas Higher Education Coordinating Board (grant no. ARP 003594–028), by the U.S. Department of Agriculture National Research Initiative (grant no. 99–35304–8002), and by the Samuel R Noble Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.014936.
LITERATURE CITED
- Anderson JD, Bailey BA, Dean JFD, Taylor R. A fungal endoxylanase elicits ethylene biosynthesis in tobacco (Nicotiana tabacum L. cv. Xanthi) leaves. In: Flores HE, Arteca RN, Shannon JC, editors. Polyamines and Ethylene: Biochemistry, Physiology, and Interactions. Rockville, MD: American Society of Plant Physiologists; 1990. pp. 146–156. [Google Scholar]
- Atkinson MM, Keppler LD, Orlandi EW, Baker CJ, Mischke CF. Involvement of plasma membrane calcium influx in bacterial induction of the K+/H+ exchange mechanism. Plant Physiol. 1990;92:215–221. doi: 10.1104/pp.92.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, O'Neill NR, Keppler LD, Orlandi EW. Early responses during plant-bacterial interactions in tobacco cell suspensions. Phytopathology. 1991;81:1504–1507. [Google Scholar]
- Baker CJ, Orlandi EW, Mock NM. Harpin, an elicitor of the hypersensitive response in tobacco caused by Erwinia amylovora, elicits active oxygen production in suspension cells. Plant Physiol. 1993;102:1341–1344. doi: 10.1104/pp.102.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey BA, Korcak RF, Anderson JD. Alterations in Nicotiana tabacum L. cv Xanthi cell membrane function following treatment with an ethylene biosynthesis-inducing endoxylanase. Plant Physiol. 1992;100:749–755. doi: 10.1104/pp.100.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Mol Biol. 1995;46:189–214. [Google Scholar]
- Bourque S, Binet MN, Ponchet M, Pugin A, Lebrun-Garcia A. Characterization of the cryptogein binding sites on plant plasma membranes. J Biol Chem. 1999;274:34699–34705. doi: 10.1074/jbc.274.49.34699. [DOI] [PubMed] [Google Scholar]
- Bourque S, Ponchet M, Binet MN, Ricci P, Pugin A, Lebrun-Garcia A. Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 1998;118:1317–1326. doi: 10.1104/pp.118.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chakrabarti A, Onaivi ES, Chaudhuri G. Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq. 1995;5:385–388. doi: 10.3109/10425179509020870. [DOI] [PubMed] [Google Scholar]
- Chapman KD. Emerging physiological roles for N-acylphospha-tidylethanolamine metabolism in plants: signal transduction and membrane protection. Chem Phys Lipids. 2000;108:221–230. doi: 10.1016/s0009-3084(00)00198-5. [DOI] [PubMed] [Google Scholar]
- Chapman KD, Conyers-Jackson A, Moreau RA, Tripathy S. Increased N-acylphosphatidylethanolamine biosynthesis in elicitor-treated tobacco cells. Physiol Plant. 1995;95:120–126. [Google Scholar]
- Chapman KD, McAndrew RS, Huynh TT. Cottonseed calnexin: identification and isolation of a membrane-bound molecular chaperone. Plant Physiol Biochem. 1997;35:483–490. [Google Scholar]
- Chapman KD, Moore TS., Jr N-Acylphosphatidylethanolamine synthesis in plants: occurrence, molecular composition and phospholipid origin. Arch Biochem Biophys. 1993;301:21–33. doi: 10.1006/abbi.1993.1110. [DOI] [PubMed] [Google Scholar]
- Chapman KD, Moore TS., Jr Isozymes of cottonseed N-acylphospha-tidylethanolamine synthase: detergent solubilization and electrophoretic separation of active enzymes with different properties. Biochim Biophys Acta. 1994;1211:29–36. doi: 10.1016/0005-2760(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Chapman KD, Sriparameswaran A. Intracellular localization of N-acylphosphatidylethanolamine synthesis in cotyledons of cotton seedlings. Plant Cell Physiol. 1997;38:1359–1367. [Google Scholar]
- Chapman KD, Tripathy S, Venables B, Desouza A. N-Acylethanolamines: formation and molecular composition of a new class of plant lipids. Plant Physiol. 1998;116:1163–1168. doi: 10.1104/pp.116.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD, Venables B, Blair R, Jr, Bettinger C. N-Acylphospha-tidylethanolamines in seeds: quantification of molecular species and their degradation upon imbibition. Plant Physiol. 1999;120:1157–1164. doi: 10.1104/pp.120.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Klessig DF. Identification of a soluble salicylic acid-binding protein that may function in signal transduction in the plant disease-resistance response. Proc Natl Acad Sci USA. 1991;88:8179–8183. doi: 10.1073/pnas.88.18.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;102:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Das SK, Paria BC, Chakrabarty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proc Natl Acad Sci USA. 1995;92:4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Melck D, Bisogno T, Di Marzo V. Chem Phys Lipids . 2000;108:191–209. doi: 10.1016/s0009-3084(00)00196-1. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiological relevance. Biochim Biophys Acta. 1998a;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998b;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Du H, Klessig F. Identification of a soluble, high-affinity salicylic acid-binding protein in tobacco. Plant Physiol. 1997;113:1319–1327. doi: 10.1104/pp.113.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkerli J, Felix G, Boller T. The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol. 1999;121:391–397. doi: 10.1104/pp.121.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- Felix G, Regenass M, Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci USA. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman-Matarasso N, Cohen E, Quansheng Du, Chejanovsky N, Hanania U, Avni A. A point mutation in the ethylene-inducing xylanase elicitor inhibits the β-1–4-endoxylanase activity but not the elicitor activity. Plant Physiol. 1999;121:345–351. doi: 10.1104/pp.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Volkow ND, Pappas N, King P, Wong CT, Gifford N, Pyatt B, Dewey SL, Makriyannis A. Imaging the brain marijuana receptor: development of a radioligand that binds to cannabinoid CB1 receptors in vivo. J Neurochem. 1998;70:417–423. doi: 10.1046/j.1471-4159.1998.70010417.x. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau G, Vassart G, Parmentier M. Molecular-cloning of a human cannabinoid receptor which is also expressed in testies. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania U, Avni A. High-affinity binding site for ethylene-inducing xylanase elicitor on Nicotiana tabacum membranes. Plant J. 1997;12:113–120. [Google Scholar]
- Hansen HS, Lauritzen L, Moesgaard B, Strand AM, Hansen HH. Formation of N-acyl-phosphatidylethanolamines and N-acylethano-lamines-proposed role in neurotoxicity. Biochem Pharmacol. 1998;55:719–725. doi: 10.1016/s0006-2952(97)00396-1. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Moesgaard B, Hansen HH, Petersen G. N-Acylethano-lamines and precursor phospholipids: relation to cell injury. Chem Phys Lipids. 2000;108:135–150. doi: 10.1016/s0009-3084(00)00192-4. [DOI] [PubMed] [Google Scholar]
- He SY, Huang HC, Collmer A. Pseudomonas syringae pv syringae harpinpss a protein that is secreted via the hrp pathway and elicits the hypersensitive response in plants. Cell. 1993;73:1255–1266. doi: 10.1016/0092-8674(93)90354-s. [DOI] [PubMed] [Google Scholar]
- Herrin DL, Schmidt GW. Rapid, reversible staining of northern blots prior to hybridization. BioTechnique. 1988;6:196. [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Campbell WB. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem. 1995;64:677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- Khanolkar AD, Palmer SL, Makriyannis A. Molecular probes for the cannabinoid receptors. Chem Phys Lipids. 2000;108:37–52. doi: 10.1016/s0009-3084(00)00186-9. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Friedman H. Cannabinoid receptors and immunity. Immunol Today. 1998;19:373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: Are these two fatty acid amides cannabimimetic? Curr Med Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- Lee J, Klessig DF, Nurnberger T. A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell. 2001;13:1079–1093. doi: 10.1105/tpc.13.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T, Fluhr R. Xylanase, a novel elicitor of pathogenesis-related proteins in tobacco, use a non-ethylene pathway for induction. Plant Physiol. 1990;93:811–817. doi: 10.1104/pp.93.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Devane WA, Hill B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N 18 neuroblastoma cells. Mol Pharmacol. 1993;44:498–503. [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell RJ. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Mechoulam R, Razdan RK. Discovery and characterization of endogenous cannabinoids. Life Sci. 1999;65:573–595. doi: 10.1016/s0024-3205(99)00281-7. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Meindl T, Boller T, Felix G. The bacterial elicitor flagellin activates its receptor in tomato cells according to the address-message concept. Plant Cell. 2000;12:1783–1794. doi: 10.1105/tpc.12.9.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau RA, Powell MJ, Whitaker BD, Bailey BA, Anderson JD. Xylanase treatment of plant cells induces glycosylation and fatty acylation of phytosterols. Physiol Plant. 1994;91:575–580. [Google Scholar]
- Munro S, Thomas KL, Abu-Shar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Evidence for the presence of CB1 cannabinoid receptors on peripheral neurones and for the existence of neuronal non-CB1 cannabinoid receptors. Life Sci. 1999;65:597–605. doi: 10.1016/s0024-3205(99)00282-9. [DOI] [PubMed] [Google Scholar]
- Portier M, Rinaldi-Carmona M, Pecceu F, Combes T, Poinot-Chazel C, Calandra B, Barth F, Le Fur G, Casellas P. SR 144528, an antagonist for the peripheral cannabinoid receptor that behaves as an inverse agonist. J Phamacol Exp Ther. 1999;288:582–589. [PubMed] [Google Scholar]
- Reggio PH. Cannabinoid receptors. Tocris Rev. 1999;10:1–5. [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq J-M, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Salzet M, Christophe B, Bisogno T, Di Marzo V. Comparative biology of the endocannabinoid system. Eur J Biochem. 2000;267:4917–4927. doi: 10.1046/j.1432-1327.2000.01550.x. [DOI] [PubMed] [Google Scholar]
- Schmid HH, Schmid PC, Natarajan V. N-Acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Schmid HH, Schmid PC, Natarajan V. The N-acylation-phosphodiesterase pathway and cell signaling. Chem Phys Lipids. 1996;80:133–142. doi: 10.1016/0009-3084(96)02554-6. [DOI] [PubMed] [Google Scholar]
- Schowalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Shrestha R, Noordermeer MA, Van der Stelt M, Veldink GA, Chapman KD. N-Acylethanolamines are metabolized by lipoxygenase and amidohydrolase in competing pathways during cotton seed imbibition. Plant Physiol. 2002;130:391–401. doi: 10.1104/pp.004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann J, Scheer J, Ryan C. Suramin inhibits defense signaling by systemin, chitosan, and a B-glucan elicitor in suspension-cultured Lycopersicon peruvianum cells. Proc Natl Acad Sci. 2000;97:8862–8867. doi: 10.1073/pnas.97.16.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus SE. Immunoactive cannabinoids: therapeutic prospects for marijuana constituents. Proc Natl Acad Sci USA. 2000;97:9363–9364. doi: 10.1073/pnas.180314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Di Marzo V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491–497. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- Trewavas A. Signal perception and transduction. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 930–987. [Google Scholar]
- Tripathy S, Venables BJ, Chapman K. N-Acylethanolamines in signal transduction of elicitor perception: attenuation of alkalinization response and activation of defense gene expression. Plant Physiol. 1999;121:1299–1308. doi: 10.1104/pp.121.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–58. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Zhang S, Klessig D. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001;6:520–527. doi: 10.1016/s1360-1385(01)02103-3. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Droge-Laser W, Dixon RA, Lamb C. Transcriptional activation of plant defense genes. Curr Opin Genet Dev. 1996;6:624–630. doi: 10.1016/s0959-437x(96)80093-1. [DOI] [PubMed] [Google Scholar]