Abstract
Transgenic potato (Solanum tuberosum cv Désirée) plants overexpressing a soybean (Glycine max) type 1 sterol methyltransferase (GmSMT1) cDNA were generated and used to study sterol biosynthesis in relation to the production of toxic glycoalkaloids. Transgenic plants displayed an increased total sterol level in both leaves and tubers, mainly due to increased levels of the 24-ethyl sterols isofucosterol and sitosterol. The higher total sterol level was due to increases in both free and esterified sterols. However, the level of free cholesterol, a nonalkylated sterol, was decreased. Associated with this was a decreased glycoalkaloid level in leaves and tubers, down to 41% and 63% of wild-type levels, respectively. The results show that glycoalkaloid biosynthesis can be down-regulated in transgenic potato plants by reducing the content of free nonalkylated sterols, and they support the view of cholesterol as a precursor in glycoalkaloid biosynthesis.
Glycoalkaloids are a family of steroidal toxic secondary metabolites present in plants of the Solanaceae family. In cultivated potato (Solanum tuberosum) the main glycoalkaloids, α-chaconine and α-solanine, are triglycosylated products of the same aglycone, solanidine, but they differ in their sugar moieties (Friedman and McDonald, 1997). The highest glycoalkaloid level in potato plants is found in flowers and sprouts, followed by the leaves, and the lowest amounts are detected in stems and tubers. The amount of glycoalkaloids increases upon wounding and light exposure, something that may render tubers unsuitable for human consumption. Mild clinical symptoms of glycoalkaloid poisoning include abdominal pain, vomiting, and diarrhea, and an upper safe limit in tubers of 200 mg total glycoalkaloids (TGA) kg−1 fresh weight has been recommended by leading authorities. However, this upper limit is close to levels found in tubers destined for human consumption, and efforts should be made to keep TGA levels low when introducing new varieties on the market (see Valkonen et al., 1996).
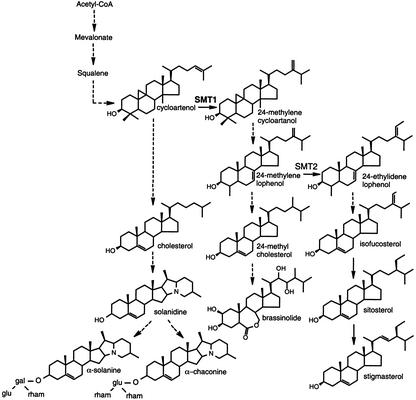
The biosynthesis of glycoalkaloids in potato is currently not fully understood. Solanidine has been proposed to be synthesized from the key precursor in plant sterol synthesis, cycloartenol, in a biosynthetic route including cholesterol, a sterol lacking alkylations at the C-24 position in the side chain (Heftmann, 1983; Bergenstråhle et al., 1996; Friedman and McDonald, 1997; Fig. 1). Cholesterol is in most plant species only a minor sterol, but is present at relatively high levels, approximately 15% to 20% of total sterols, in Solanaceous plants such as potato and tobacco (Nicotiana tabacum). One of the final reactions in the synthesis of glycoalkaloids is the glucosylation or galactosylation of solanidine to yield γ-chaconine or γ-solanine, respectively. A cDNA encoding the solanidine glucosyltransferase (SGT) enzyme has been cloned (Moehs et al., 1997). The SGT mRNA increased after wounding, in line with previous measurements of wound-induced SGT activity and glycoalkaloid levels. However, the galactosylation of solanidine is likely catalyzed by a separate enzyme (Bergenstråhle et al., 1992; Zimowski, 1998). The final glycosylation steps leading to α-chaconine and α-solanine have not been characterized.
Figure 1.
Schematic presentation of proposed sterol and glycoalkaloid biosynthesis pathways in potato plants (adapted from Bergenstråhle et al. [1996] and Choe et al. [1999]). Dashed arrows indicate more than one enzymatic step. The methylation steps catalyzed by SMT1 and SMT2 are indicated.
Cycloartenol metabolism leads also to the synthesis of other plant sterols. Plant plasma membranes commonly contain a mixture of sterols, the main ones being the 24-ethyl sterols sitosterol and stigmasterol, which together often constitute more than 70% of total sterols. The alkylations of the sterol side chain are performed by the sequential action of two distinct S-adenosyl-l-Met:sterol C24-methyltransferases, SMT type 1 (SMT1) and type 2 (SMT2). In the first step, cycloartenol is methylated to 24-methylene cycloartanol by the enzymatic action of SMT1, whereas in the second alkylation step, 24-methylene lophenol is methylated to 24-ethylidene lophenol by action of SMT2 (Bouvier-Navé et al., 1998; Fig. 1). Several lines of evidence suggest a key role of the SMTs in the synthesis of sterols and brassinosteroids, sterol-derived plant growth hormones. Hartmann and Benveniste (1974) reported a build-up of cycloartenol in ageing potato discs and suggested that the activity of cycloartenol-C24-methyltransferase (SMT1) was limiting in sterol synthesis. In line with this, transgenic plants overexpressing 3-hydroxy-3-methylglutaryl CoA reductase, an early-acting enzyme in sterol synthesis, displayed up to 60-fold higher levels of cycloartenol but much lower increases of 24-methylene cycloartanol and its further metabolites (Schaller et al., 1995). Furthermore, overexpression of SMT1 in transgenic tobacco plants increased 24-methylated sterols at the expense of cholesterol, whereas overexpression of SMT2 mainly increased the 24-ethyl sterols, this also at the expense of cholesterol (Schaeffer et al., 2000; Sitbon and Jonsson, 2001). In the SMT2 transformants, growth was reduced, presumably due to a reduction of sterols needed in brassinosteroid synthesis. On the basis of the analysis of transgenic Arabidopsis plants over- or underexpressing SMT2, Schaeffer et al. (2001) proposed a crucial role of SMT2 in balancing the ratio of campesterol to sitosterol to fit both growth requirements and membrane integrity.
Because overexpression of either SMT1 or SMT2 in transgenic tobacco plants leads to reduced levels of cholesterol, presumably due to an increased channeling of cycloartenol into alkylated sterols, we reasoned that this might enable the precursor role of cholesterol in TGA synthesis to be experimentally tested in transgenic potato plants. Considering the negative effects of SMT2 overexpression on plant growth, we chose a SMT1 for this purpose. We here report on an altered sterol composition and a reduced TGA level in such SMT1-overexpressing potato plants.
RESULTS
Generation and Analysis of Transgenic Potato Plants Overexpressing a Soybean (Glycine max) SMT1 cDNA
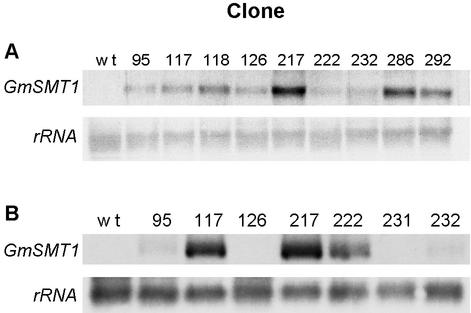
Potato cv Désirée was transformed with the pTET1:GmSMT1.kana construct, and a total number of 44 kanamycin-resistant transformants were regenerated. Compared with wild-type control plants, the transformants did not display any visible difference in their general growth and development, nor was their dry weight to fresh weight ratio in leaves and tubers significantly altered (results not shown). Northern analysis of RNA extracted from leaves and tubers revealed in the transgenic plants a single 1.5-kb GmSMT1 transcript, the level of which varied considerably among the different clones (Fig. 2). Two of the transgenic clones with a strong GmSMT1 expression in the leaves (118 and 217) were selected for analysis of SMT activity in vitro. Enzyme assays were carried out using microsomal preparations incubated with the SMT1 substrate cycloartenol and the SMT2 substrate 24-methylene lophenol (Table I). With cycloartenol as the substrate, the level of SMT activity was increased about 10-fold in the transgenic clones as compared with the wild-type plants, but with 24-methylene lophenol as substrate, the activity was similar between the different genotypes. This demonstrates that the GmSMT1 cDNA is expressed in the plants as an active enzyme and that cycloartenol is the preferred substrate. Three additional transgenic clones (95, 286, and 292) were analyzed and displayed a 10-fold increase in SMT activity as compared with wild-type plants (not shown). Due to the limited available amount of 24-methylene lophenol, these clones were however only analyzed with cycloartenol as the substrate. For all genotypes, the microsomes exhibited much higher enzyme activities with 24-methylene lophenol than with cycloartenol, approximately 100-fold in wild-type plants and 10-fold in the transformants.
Figure 2.
Northern-blot analysis of GmSMT1 expression in leaves (A) and tubers (B) from wild-type and transgenic GmSMT1 potato plants. Total RNA in young leaves and tubers was extracted from plants grown in a climate chamber or a greenhouse, respectively. Twenty micrograms of RNA was separated on formaldehyde gels, blotted onto a nylon filter, and hybridized with labeled DNA probes for GmSMT1 and rRNA (loading control). B, In tubers, a weak signal was detected for the clones 126 and 231 after prolonged exposure of the film.
Table I.
SMT activity in wild-type and transgenic GmSMT1 potato plants
| Clone | Cycloartenol | 24-Methylene Lophenol |
|---|---|---|
| fkat mg−1 protein | ||
| wt (1) | 0.02 | 2.56 |
| wt (2) | 0.07 | 3.48 |
| 118 | 0.39 | 3.26 |
| 217 | 0.41 | 3.47 |
Microsomes were prepared from young leaves of mature greenhouse-grown plants, and SMT activity was measured with cycloartenol and 24-methylene lophenol, the preferred substrates of SMT1 and SMT2, respectively. Values are corrected for the SMT activity with endogenous substrate.
GmSMT1 Transformants Display Increased Total Sterol Levels in Leaves and Tubers
Thirteen transgenic clones representing the range of GmSMT1 expression were analyzed for their total sterol content in the leaves. This revealed a general increase of the total sterol content in the transformants, ranging from a moderate increase of about 50%, up to 360% higher than in wild-type plants (Table II). Transgenic clones with a strong GmSMT1 expression in leaves, as measured by the northern analysis, generally also displayed a high total sterol level, demonstrating that the increased sterol level in the transformants was due to expression of GmSMT1.
Table II.
Total sterol content in leaves from wild-type and transgenic GmSMT1 potato plants
| Clone | Series 1
|
Series 2
|
||||
|---|---|---|---|---|---|---|
| n | TS | Increase | n | TS | Increase | |
| mg kg−1 | % | mg kg−1 | % | |||
| w t | 8 | 82 ± 13 | 0 | 8 | 65 ± 10 | 0 |
| 95 | 3 | 185 ± 49 | 126 | 3 | 117 ± 11 | 81 |
| 103 | 3 | 187 ± 15 | 129 | n.a. | ||
| 117 | n.a. | 3 | 299 ± 4 | 361 | ||
| 118 | 3 | 230 ± 35 | 181 | 3 | 284 ± 39 | 337 |
| 126 | 3 | 140 ± 31 | 71 | 3 | 109 ± 21 | 67 |
| 217 | n.a. | 3 | 178 ± 55 | 174 | ||
| 218 | 3 | 218 ± 28 | 168 | n.a. | ||
| 222 | 3 | 147 ± 50 | 81 | 3 | 160 ± 34 | 146 |
| 231 | 3 | 123 ± 11 | 51 | n.a | . | |
| 232 | n.a. | 3 | 116 ± 29 | 78 | ||
| 286 | 3 | 197 ± 56 | 142 | 3 | 235 ± 27 | 262 |
| 292 | 3 | 210 ± 60 | 158 | 3 | 178 ± 74 | 174 |
| 304 | 3 | 193 ± 70 | 137 | n.a. | ||
Sterols (free sterols and steryl-esters) were extracted from leaf tissue from shoots grown in a climate chamber, and analyzed in duplicate by gas liquid chromatography (GC) confirmed by mass spectroscopy (MS) using 5α-cholestane as an added internal standard. Two independent experiments were carried out. Total sterols (TS) were calculated as a sum of the main sterols in potato (cholesterol, 24-methyl cholesterol, isofucosterol, sitosterol, and stigmasterol). Mean value ± sd; n, number of plants analyzed; n.a., not analyzed.
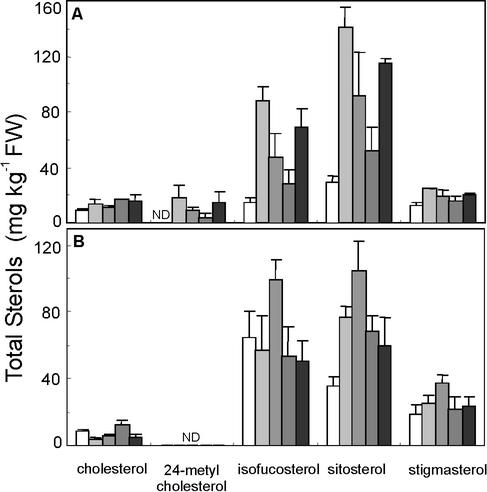
When the concentration of the main sterols in potato was analyzed, it was found that in leaves the higher total sterol level in transgenic plants was mainly due to increased levels of the 24-ethyl sterols isofucosterol and sitosterol (Fig. 3). The 24-methyl sterol 24-methyl cholesterol also increased but to a lesser extent. The level of cycloartenol, the preferred substrate of SMT1, was 39 ± 19 mg kg−1 in the wild-type plants (means ± sd, n = 8 plants), but it was not significantly different from this in the transgenic plants (Student's t test, results not shown). In tubers, the main increase in total sterols was due to higher levels of sitosterol. The total level of cholesterol, a 24-desmethyl sterol, was not significantly altered in leaves, but was reduced in tubers from three of the four analyzed transformed clones.
Figure 3.
Sterol composition in leaves (A) and tubers (B) from wild-type and transgenic GmSMT1 potato plants. Sterols were extracted from leaves and tubers and analyzed in duplicate by gas chromatography confirmed with mass spectrometry. White staples, Wild-type plants (A, n = 8 plants; B, n = 3); black staples, transgenic plants (n = 3), from left to right clones 118, 217, 232, and 286. Mean values + sd. ND, Not detected.
GmSMT1 Transformants Display Altered Levels of Free and Esterified Sterols in Leaves and Tubers
To investigate whether the increase in total sterol levels was due to an increase in free sterols or steryl-esters, these sterol classes were analyzed in wild-type plants and two transgenic clones (118 and 217), both of which contained an increased total sterol level compared with the wild type (Table II). The results showed that in leaves, there was an increase both in free and esterified forms of isofucosterol and sitosterol and in tubers, an increase of free and esterified sitosterol (Fig. 4). For both leaves and tubers, levels of free and esterified stigmasterol and esterified cholesterol were similar to those in wild-type plants. However, the level of free cholesterol in both of these transgenic clones was reduced to about 50% of that in the wild type.
Figure 4.
Free and esterified sterols in leaves (A and B) and tubers (C and D) from wild-type and transgenic GmSMT1 potato plants. Sterols were extracted from leaves and tubers and analyzed in duplicate by gas chromatography confirmed with mass spectrometry. White staples, Wild type; black staples, transgenic clones, from left to right clones 118 and 217. Mean values + sd, n = 3 plants. ND, Not detected.
SMT1 Overexpression Is Associated with Reduced Glycoalkaloid Levels in Potato Leaves and Tubers
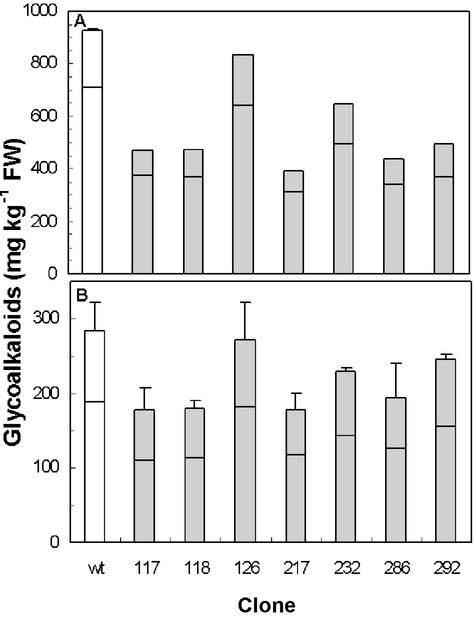
In the light of cholesterol as a proposed intermediate in glycoalkaloid biosynthesis, the lower level of free cholesterol in transgenic plants prompted an analysis of their TGA content. In wild-type plants, TGA levels were higher in leaves than in tubers, about 3.3- or 7.1-fold higher on a fresh weight or dry weight basis, respectively (Fig. 5). The α-solanine: α-chaconine ratio was 1:3 in leaves and 1:2 in tubers. Transgenic plants displayed a reduced TGA level both in leaves and tubers, down to 41% and 63%, respectively, as compared with the wild type (Fig. 5). Both α-solanine and α-chaconine levels were decreased, but the α-solanine:α-chaconine ratio was not altered. The relative reduction of TGA levels in leaves corresponded to a similar reduction in tubers of the different clones. For instance, clone 217, which displayed the lowest level of TGA in leaves also had the lowest level in tubers, whereas clone 126 had negligible TGA reductions in both of these tissues.
Figure 5.
TGA levels in leaves (A) and tubers (B) from wild-type and transgenic GmSMT1 potato plants. Levels of α-chaconine (lower part of staple) and α-solanine (upper part of staple) were analyzed in duplicate by HPLC. The average difference from the mean in duplicate analyses was 1.9%. White staples, Wild-type plants (A, n = 3; B, n = 6 plants); black staples, transgenic plants (A, n = 1; B, n = 2, for clones 118 and 217, n = 3). Mean values + sd. sd was calculated from the sum of α-chaconine and α-solanine in the samples analyzed. The analysis of tubers was carried out in two independent experiments, giving similar results.
DISCUSSION
Transgenic potato plants overexpressing a soybean SMT1 cDNA from a strong constitutive promoter were generated. The growth and general phenotype of the transformants was similar to that of wild-type plants, in line with what has been shown for equivalent transformations of tobacco (Schaeffer et al., 2000; Sitbon and Jonsson, 2001). In contrast, overexpression of SMT2 cDNAs in tobacco and Arabidopsis has been associated with decreased growth (Schaller et al., 1998; Schaeffer et al., 2001; Sitbon and Jonsson, 2001), presumably due to a concomitant negative effect on the production of brassinosteroids (Schaeffer et al., 2001). Our results demonstrate that SMT1, rather than SMT2, is a useful tool for transformation of potato to alter the metabolic channeling between sterols and glycoalkaloids without affecting growth or viability.
The SMT activity with cycloartenol as the substrate was 10-fold higher in transgenic plants as compared with the wild type, whereas the activity with 24-methylene lophenol was similar between the two genotypes (Table I). This demonstrates in a plant background that the GmSMT1 exhibits no, or only a slight, activity with 24-methylene lophenol as the substrate. This agrees with a study on tobacco NtSMT1-1 expressed in the yeast erg6 sterol mutant, showing 1.5% enzymatic NtSMT1 activity with 24-methylene lophenol as substrate (type 2), as compared with activity with cycloartenol (type 1; Bouvier-Navé et al., 1998) and with results presented by Nes (2000) showing a corresponding 13% activity for GmSMT1 expressed in Escherichia coli with 24-methylene lophenol as substrate as compared with activity with cycloartenol. The higher SMT1 activity in transgenic potato plants was associated with an up to 360% higher total sterol content compared with wild-type plants (Table II), mainly due to increased levels of the 24-ethyl sterols isofucosterol and sitosterol (Fig. 3). An increase was found both in free sterol and steryl-ester fractions, with the highest relative increase in the latter (Fig. 4). This is in accordance with earlier findings from a sterol-overproducing tobacco mutant (Maillot-Vernier et al., 1991; Gondet et al., 1994) and transgenic plants overexpressing a 3-hydroxy-3-methylglutaryl CoA reductase cDNA (Schaller et al., 1995), although in these studies, the increase was more clearly confined to the steryl-ester fraction. The increased total sterol level with an increased level of 24-ethyl sterols is therefore explained by an increased flow of sterol precursors into the 24-alkylated pathway due to a increased rate of cycloartenol methylation carried out by GmSMT1. The results are however in some contrast with a previous investigation of ours on similarly transformed GmSMT1-overexpressing tobacco plants, where neither total sterol nor sitosterol levels were markedly altered, but where the levels of 24-methyl cholesterol were increased in relation to other sterols (Sitbon and Jonsson, 2001).
One explanation of these species differences in sterol metabolism is that the endogenous SMT2 activity is higher in potato than in tobacco and that increased amounts of 24-methylene lophenol in potato are therefore more efficiently converted into 24-ethylidene lophenol and its further metabolites. Our enzyme activity measurements support this suggestion inasmuch as the activity in microsomes from wild-type potato plants was 100-fold higher with 24-methylene lophenol (SMT2 activity) than with cycloartenol (SMT1 activity; Table I). In wild-type tobacco plants, the corresponding difference in SMT activities with the two substrates was 5-fold (Schaeffer et al., 2000). It should however be noted that the enzymatic measurements from tobacco and potato are not readily comparable, i.e. due to different assay conditions, and that the species differences under identical assay conditions may be less. Interestingly, despite a higher level of sitosterol, the level of stigmasterol, the C-22 desaturated product of sitosterol, was not increased in our potato transformants (Figs. 3 and 4). A similar situation has been noted also in studies of transgenic SMT2-overexpressing tobacco and Arabidopsis plants (Schaller et al., 1998; Schaeffer et al., 2001; Sitbon and Jonsson, 2001) and indicates that the conversion of sitosterol to stigmasterol is a rate-limiting step in sterol biosynthesis.
We have previously shown that cholesterol levels can be reduced in transgenic tobacco plants by GmSMT1 overexpression, and a similar reduction was expected in the present study. However, when the total sterol content was analyzed, a reduced cholesterol content in the transgenic clones was not always resolved (Fig. 3). Only when free sterols and steryl-esters were analyzed separately was a reduction of free cholesterol levels consistently seen in transgenic leaves and tubers (Fig. 4). Our results suggest that an increased metabolic flux of cycloartenol into alkylated sterols by GmSMT1 overexpression leads to a reduction of free nonalkylated sterols (e.g. cholesterol), presumably due to a competition between the alkylated and nonalkylated sterol-biosynthetic routes for their common substrate cycloartenol. This is in keeping with the opposite effect in an Arabidopsis smt1 mutant, deficient for the Arabidopsis single SMT1 gene, where total cholesterol levels increased 5-fold compared with wild-type plants (Diener et al., 2000). These findings imply an important role of SMT1 in plant sterol synthesis.
Concurrent with a reduced level of free cholesterol, our GmSMT1-overexpressing transgenic potato plants displayed a reduced TGA level in leaves and tubers (Fig. 5). This is in good agreement with previous metabolic studies leading to the proposal of cholesterol as an intermediate in the biosynthesis of glycoalkaloids (Heftmann, 1983; Bergenstråhle et al., 1996; Friedman and McDonald, 1997). We currently do not know to what extent SMT1 expression is regulating TGA levels in potato plants under natural circumstances, but several studies indicate that SMT activity may be a regulatory point in the biosynthesis of sterols and glycoalkaloids in this species. In wounded potato tubers (tuber discs), sterol synthesis is induced. The SMT activity increased in the discs, but nevertheless, the relative amount of cholesterol and TGA accumulated (Bergenstråhle et al., 1993, 1996). However, when SMT activity was further increased by ethephon treatment, neither cholesterol nor TGA levels were induced, and the total sterol synthesis was not affected. This suggests that elevated SMT activity can inhibit the accumulation of TGA that occurs as an effect of the increased sterol synthesis in wounded potato tubers. Similarly, the light-induced accumulation of TGA may be related to an up-regulation of sterol synthesis in combination with low levels of SMT activity, a suggestion supported by the finding in Arabidopsis that SMT mRNA levels are down-regulated by light (Ma et al., 2001). Down-regulation of SMT1 mRNA levels has in soybean also been demonstrated as an effect of fungal elicitors (Shi et al., 1996). However, a regulatory role for SMT in suppressing TGA synthesis in potato exposed to microbial pathogens appears less likely, because it has been shown that treatment of potato with fungal elicitors affect sterol synthesis by down-regulation of squalene synthase (Brindle et al., 1988; Zook and Kúc, 1991).
We have here demonstrated that overexpression of a SMT1 cDNA can be used to down-regulate cholesterol and TGA levels in potato. To the best of our knowledge, this is the first report on a modification of TGA levels in transgenic plants, and it demonstrates the applicability of genetic engineering for studies of TGA metabolism and the regulation of its pool size in potato. Using more specific promoters, it might be possible to specifically direct the increased SMT1 activity, and hence reduced TGA level, to the pith of tubers. This might enable the use of potato pulp wastes from starch industry as animal fodder or increase the genetic pool available to breeding program, because today, many interesting genotypes are overlooked due to their high TGA levels.
MATERIALS AND METHODS
Generation of Transgenic Potato (Solanum tuberosum cv Désirée) Plants
To obtain a kanamycin-selectable GmSMT1 vector construct, the entire promoter cassette of plasmid pTET1:GmSMT1.hyg (Sitbon and Jonsson, 2001) containing the soybean (Glycine max) SMT1 cDNA (Shi et al., 1996) expressed in sense orientation from the cauliflower mosaic virus 35S-derived pTET1 promoter, was excised as a HindIII + EcoRI fragment and cloned into the corresponding sites of the binary Ti-plasmid pPCV702.kana (Koncz and Schell, 1986). The resulting plasmid pTET1:GmSMT1.kana was electroporated into the Agrobacterium tumefaciens 3101 pMP90RK strain. Leaves and internodes of potato were transformed in two separate experiments following both a leaf disc (Knapp et al., 1988) and an internode (Beaujean et al., 1998) transformation protocol. Regenerated shoots were rooted on a selective medium containing 1× Murashige and Skoog salts (Duchefa Biochemie bv, Haarlem, The Netherlands), pH 5.6, supplemented with 3% (w/v) Suc, 0.25% (w/v) gellan, and 75 μg mL−1 kanamycin. Forty-four transgenic plantlets with well-developed roots on selective medium were further cultured on the same medium without antibiotics and were routinely kept under axenic conditions in a growth room maintained at 22°C for a 16-h day and an 8-h night. When needed, primary transformants were multiplied vegetatively from axillary shoots. Plantlets were transferred to black plastic pots (1 L) with fertilized peat and grown in a climate chamber at 16-h day (24°C) and 8-h night (17°C) at 65% relative humidity. After 5 weeks, plants were transferred to black pots (5 L) and grown during the summer season in a greenhouse with natural daylight. Successful transformation was confirmed by PCR analysis on genomic DNA preparations, as well as by northern analysis of total RNA. Transgenic plants were also initially screened for an altered sterol composition by extraction and analysis of total sterols on a gas chromatograph essentially as described (Bergenstråhle et al., 1996) but with an initial extraction of total lipids from leaf discs with chloroform:methanol (2:1, v/v) for 1 h at room temperature, and sterols were extracted twice with n-hexane after saponification.
Northern Analysis of GmSMT1 Expression
The GmSMT1 transcript level was analyzed by northern gel blot in two independent experiments in a total of 23 kanamycin-resistant transformants, using 20 μg of total RNA extracted from young leaves with a RNeasy Plant Mini Kit (Operon, Qiagen, Valencia, CA) and from tubers of seven transformants extracted according to Chang et al. (1993). RNA was separated on formaldehyde agarose gels and transferred onto HybondN membranes (Amersham Biosciences AB, Uppsala). A 1.2-kb PCR fragment spanning the entire GmSMT1-coding sequence was labeled with [32P]dCTP and used as a hybridization probe. Blots were washed under stringent conditions, and exposed to autoradiographic film (MP-film, Amersham Biosciences AB) at −70°C. No cross-hybridization to the endogenous potato SMT transcripts was observed under these conditions. Equal RNA loading was confirmed by control hybridization with a rDNA probe. For 12 clones, the analysis (plant growth, leaf RNA extraction, and hybridization) was repeated either two or three times, with good reproducibility between the experiments (not shown).
SMT1 Enzymatic Assay
Microsomal fractions were isolated at 4°C from 5 g of freshly isolated young leaves from mature greenhouse-grown plants as described (Sitbon and Jonsson, 2001). Proteins were quantified by the Bradford assay using bovine serum albumin as the reference. The SMT1 assay volume of 0.1 mL contained 60 μL of microsomal suspension (approximately 0.20 mg of protein) in sample buffer (pH 7.5), 1 mg mL−1 Tween 80, 10 μm S-adenosyl-l-Met, and 1.3 μm (740 kBq mL−1) S-adenosyl-l-(methyl-14C) Met (Amersham Biosciences UK Ltd, Little Chalfont, Buckinghamshire, UK). Cycloartenol (a gift from Dr. W. David Nes, Department of Chemistry and Biochemistry, Texas Tech University, Lubbock) and 24-methylene lophenol (a gift from Dr. P. Bouvier-Navé, Départemente de Biologie Cellulaire et Moléculaire, Institut de Botanique, Strasbourg, France) were used as the substrate at a concentration of 25 μm. Incubations with or without substrate were carried out at 35°C for 1 and 2 h. Sterol products were separated by thin-layer chromatography (TLC) with dichloromethane as developing solvent, and the radioactivity in 4-desmethyl sterols, 4-monomethyl sterols, and 4,4-dimethyl sterols was determined separately as described (Sitbon and Jonsson, 2001). The main assay products with cycloartenol and 24-methylene lophenol as the substrates were recovered in the 4-dimethyl sterol and 4-monomethyl fractions, respectively.
Extraction of Total Sterols from Leaves and Tubers in GmSMT1 Potato Plants
Discs (200 mg fresh weight) from young leaves were harvested from plants grown in a climate chamber at two separate occasions (series 1 and 2), frozen in liquid nitrogen, and stored at −70°C until analysis. Average plant height was 14 and 18 cm in series 1 and 2, respectively. Total sterol fractions were isolated from crushed leaf discs using a slightly modified direct saponification (Dionisi et al., 1998) of samples with 3 mL of 2 m KOH in 95% (v/v) ethanol for 30 min at 85°C, before sterols were extracted with n-hexane essentially as described (Dutta and Normén, 1998). Four micrograms of 5α-cholestane was added to samples before the extraction and was used as an internal standard.
Tubers (10 g fresh weight) were harvested from greenhouse-grown plants and stored at 4°C in the dark for 2 months before analysis. Total sterols from lyophilized and frozen potato tuber powder were extracted and analyzed essentially as described (Bergenstråhle et al., 1996), but the initial extraction of total lipids with chloroform:methanol (2:1, v/v) was performed for 90 min at 70°C, and sterols were extracted twice with n-hexane after saponification. Ten micrograms of 5α-cholestane was added to samples as standard.
Isolation of Free Sterols and Esterified Sterols from Leaves and Tubers
The levels of free and esterified sterols in GmSMT1 potato transformants were analyzed using leaf and tuber samples (described above) from two transgenic clones and the wild type. Total lipids were extracted with chloroform:methanol (2:1, v/v) for 1 h (leaves) or 1.5 h (tubers) at 70°C. Free sterols and steryl-esters were then separated by two runs of TLC with dichloromethane as developing solvent. Steryl-ester fractions (RF = 0.9) were extracted from the plate, saponified, and extracted with n-hexane. Free sterol fractions (RF = 0.3) were extracted from the plate. Sterol moieties from steryl-ester from leaves and tubers and free sterol from tubers were then separated again by TLC with dichloromethane as developing solvent. The desmethylsterol fraction was analyzed by GC, as described below. Five or 10 μg of 5α-cholestane was added to samples as standard.
Analysis of Sterols by Capillary Column GC and GC-MS
Trimethylsilyl (TMS) ether derivatives of sterols was prepared as described (Dutta and Normén, 1998). A capillary column (30-m × 0.25-mm × 0.50-μm film thickness; DB-5MS, J&W Scientific, Folsom, CA) was used to quantify TMS ether derivatives of sterols. The column was connected to a gas chromatograph (Star 3400 CX, Varian, Walnut Creek, CA). Helium was used as a carrier gas at a velocity of 20 cm s−1, and nitrogen was used as makeup gas at the rate of 30 mL min−1. The detector temperature was 320°C. A falling needle injector was coupled with the GC for sample injection. A programmed oven was used at 290°C for 20 min, then raised to 310°C at a rate of 1°C min−1, and held for 1 min. An HP 3396A integrator (Hewlett-Packard, Avandole, PA) was used to integrate peak areas.
GC-MS analyses were performed on a gas chromatograph (GC 8000 Top Series, ThermoQuest Italia S.p.A., Rodano, Italy) coupled to a Voyager mass spectrometer with MassLab data system v1.4V (Finnigan, Manchester, UK). TMS ether derivatives of sterols were separated on the same column used for GC analysis. Helium was used as carrier gas at an inlet pressure of 80 kPa. The injector temperature was 250°C, the samples were injected in a splitless mode, and purge delay time was 0.6 min. A programmed oven temperature was used at 60°C for 1 min, then raised to 290°C at a rate of 50°C min−1, and then held at this temperature for 20 min before being finally raised to 300°C at 1°C min−1. The mass spectra were recorded at an electron energy of 70 eV, and the ion source temperature was 200°C. Campesterol, cholesterol, cycloartenol, sitosterol, and stigmasterol were identified by comparing the retention times and mass spectra with TMS ether derivatives of the corresponding standard samples. Other sterols were identified by comparing obtained mass spectra with published results on these sterols.
Glycoalkaloid Quantification
Leaf materials (20 g) from mature greenhouse-grown wild-type and transgenic plants was sampled after the summer season, frozen in liquid nitrogen, and stored at −70°C. Tubers (100 g) were harvested in parallel from the same plants. Before analysis, leaf samples were transported on dry ice, whereas intact tubers were packed to minimize mechanical stress and transported at ambient temperature in darkness. TGA levels (α-chaconine + α-solanine) in potato leaves and tubers were analyzed in duplicate by The Swedish Cereal Laboratory AB (Svalöv), using an HPLC-based assay modified from Hellenäs (1986) and according to the Swedish National Food Administration standards, Ref. NMKL 13.4. Genotypes and clone numbers were coded before analysis (blind test).
ACKNOWLEDGMENTS
We thank Dr. W. David Nes (Department of Chemistry and Biochemistry, Texas Tech University, Lubbock) and Dr. Pierette Bouvier-Navé (Départemente de Biologie Cellulaire et Moléculaire, Institut de Botanique, Strasbourg, France) for their generous gifts of cycloartenol and 24-methylene lophenol, respectively. We also thank Rita Svensson (The Swedish Cereal Laboratory, Svalöv) for performing the TGA analyses.
Footnotes
This work was supported by the Magnus Bergvall Foundation and by the C.F. Lundström Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018788.
LITERATURE CITED
- AOAC Official Method 997.13 (1997) Glycoalkoids (α-solanine and α-chaconine) in potato tubers. Liquid Chromatographic Method. First Action.
- Beaujean A, Sangwan RS, Lecardonnel A, Sangwan-Norreel BS. Agrobacterium-mediated transformation of three economically important potato cultivars using sliced internodal explants: an efficient protocol of transformation. J Exp Bot. 1998;49:1589–1595. [Google Scholar]
- Bergenstråhle A, Borgå P, Jonsson L. Sterol composition and synthesis in potato tuber discs in relation to glycoalkaloid synthesis. Phytochemistry. 1996;41:155–161. [Google Scholar]
- Bergenstråhle A, Tillberg E, Jonsson L. Characterization of UDP-glucose:solanidine glucosyltransferase and UDP-galactose:solanidine galactosyltransferase from potato tuber. Plant Sci. 1992;84:35–44. [Google Scholar]
- Bergenstråhle A, Tillberg E, Jonsson L. Effects of ethephon and norbornadiene on sterol and glycoalkaloid biosynthesis in potato tuber discs. Physiol Plant. 1993;89:301–308. [Google Scholar]
- Bouvier-Navé P, Husselstein T, Benveniste P. Two families of sterol methyltransferases are involved in the first and the second methylation steps of plant sterol biosynthesis. Eur J Biochem. 1998;256:88–96. doi: 10.1046/j.1432-1327.1998.2560088.x. [DOI] [PubMed] [Google Scholar]
- Brindle PA, Kuhn PJ, Threlfall DR. Biosynthesis and metabolism of sesquiterpenoid phytoalexins and triterpenoids in potato cell suspension cultures. Phytochemistry. 1988;27:133–150. [Google Scholar]
- Chang SJ, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE et al. The Arabidopsis dwf7/ste1 mutant is defective in the Δ7 sterol C-5 desaturation step leading to brassinosteroid synthesis. Plant Cell. 1999;11:207–221. [PMC free article] [PubMed] [Google Scholar]
- Diener AC, Li H, Zhou W-X, Whoriskey WJ, Nes WD, Fink GR. STEROL METHYLTRANSFERASE 1 controls the level of cholesterol in plants. Plant Cell. 2000;12:853–870. doi: 10.1105/tpc.12.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisi F, Golay PA, Aeschlimann JM, Fay LB. Determination of cholesterol oxidation products in milk powders: methods comparison and validation. J Agric Food Chem. 1998;46:2227–2233. [Google Scholar]
- Dutta PC, Normén L. Capillary column gas-liquid chromatographic separation of Δ5-unsaturated and saturated phytosterols. J Chromatogr. 1998;816:177–184. [Google Scholar]
- Friedman M, McDonald GM. Potato glycoalkaloids: chemistry, analysis, safety and plant physiology. Crit Rev Plant Sci. 1997;16:55–132. [Google Scholar]
- Gondet L, Bronner R, Benveniste P. Regulation of sterol content in membranes by subcellular compartmentation of steryl-esters accumulating in a sterol-overproducing tobacco mutant. Plant Physiol. 1994;105:509–518. doi: 10.1104/pp.105.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann MA, Benveniste P. Effect of ageing on sterol metabolism in potato tuber slices. Phytochemistry. 1974;13:2667–2672. [Google Scholar]
- Heftmann E. Biogenesis of steroids in Solanaceae. Phytochemistry. 1983;22:1843–1860. [Google Scholar]
- Hellenäs K-E. A simplified procedure for quantification of potato glycoalkaloids in tuber extracts by HPLC: comparison with ELISA and a colorimetric method. J Sci Food Agric. 1986;37:776–782. [Google Scholar]
- Knapp S, Coupland G, Uhrig H, Starlinger P, Salamini F. Transposition of the maize transposable element Ac in Solanum tuberosum. Mol Gen Genet. 1988;213:285–290. [Google Scholar]
- Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimeric genes carried by a new type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- Ma L, Li J, Qu H, Hager J, Chen Z, Zhao H, Deng XW. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell. 2001;13:2589–2607. doi: 10.1105/tpc.010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillot-Vernier P, Gondet L, Schaller H, Benveniste P, Belliard G. Genetic study and further biochemical characterization of a tobacco mutant that overproduces sterols. Mol Gen Genet. 1991;231:33–40. doi: 10.1007/BF00293818. [DOI] [PubMed] [Google Scholar]
- Moehs CP, Allen PV, Friedman M, Belknap WR. Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J. 1997;11:227–236. doi: 10.1046/j.1365-313x.1997.11020227.x. [DOI] [PubMed] [Google Scholar]
- Nes WD. Sterol methyl transferase: enzymology and inhibition. Biochem Biophys Acta. 2000;1529:63–88. doi: 10.1016/s1388-1981(00)00138-4. [DOI] [PubMed] [Google Scholar]
- Schaeffer A, Bouvier-Navé P, Benveniste P, Schaller H. Plant sterol-C24-methyl transferases: different profiles of tobacco transformed with SMT1 or SMT2. Lipids. 2000;35:263–269. doi: 10.1007/s11745-000-0522-1. [DOI] [PubMed] [Google Scholar]
- Schaeffer A, Bronner R, Benveniste P, Schaller H. The ratio of campesterol to sitosterol that modulates growth in Arabidopsis is controlled by STEROL METHYLTRANSFERASE2;1. Plant J. 2001;25:605–615. doi: 10.1046/j.1365-313x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- Schaller H, Bouvier-Navé P, Benveniste P. Overexpression of an Arabidopsis cDNA encoding a sterol-C241-methyltransferase in tobacco modifies the ratio of 24-methyl cholesterol to sitosterol and is associated with growth reduction. Plant Physiol. 1998;118:461–469. doi: 10.1104/pp.118.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H, Grausem B, Benveniste P, Chye M-L, Tan C-T, Song Y-H, Chua N-H. Expression of the Hevea brasiliensis (H.B.K.) Müll. Arg. 3-hydroxy-3-methylglutaryl-coenzyme A reductase 1 in tobacco results in sterol overproduction. Plant Physiol. 1995;109:761–770. doi: 10.1104/pp.109.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Gonzales R, Bhattacharyya MK. Identification and charaterization of an S-adenosyl-l-methionine: Δ24-sterol-C-methyltransferase cDNA from soybean. J Biol Chem. 1996;271:9384–9389. doi: 10.1074/jbc.271.16.9384. [DOI] [PubMed] [Google Scholar]
- Sitbon F, Jonsson L. Sterol composition and growth of transgenic tobacco plants expressing type-1 and type-2 sterol methyltransferases. Planta. 2001;212:568–572. doi: 10.1007/s004250000417. [DOI] [PubMed] [Google Scholar]
- Valkonen JPT, Keskitalo M, Vasara T, Pietilä L. Potato glycoalkaloids: a burden or a blessing? Crit Rev Plant Sci. 1996;15:1–20. [Google Scholar]
- Zimowski J. Specificity and properties of UDP-galactose: tomatidine galactosyltransferase from tomato leaves. Plant Sci. 1998;136:139–148. [Google Scholar]
- Zook MN, Kúc JA. Induction of sesquiterpene cyclase and suppression of squalene synthethase activity in elicitor-treated or fungal-infected potato tuber tissue. Physiol Mol Plant Pathol. 1991;39:377–390. [Google Scholar]