Abstract
To assess antioxidative protection by carnosic acid (CA) in combination with that of other low-molecular weight (Mr) antioxidants (α-tocopherol [α-T] and ascorbate [Asc]) in chloroplasts, we measured endogenous concentrations of these antioxidants, their redox states, and other indicators of oxidative stress in chloroplasts of three Labiatae species, differing in their CA contents, exposed to drought stress in the field. Damage to the photosynthetic apparatus was observed neither in CA-containing species (rosemary [Rosmarinus officinalis]) and sage [Salvia officinalis]) nor in CA-free species (lemon balm [Melissa officinalis]) at relative leaf water contents between 86% and 58%, as indicated by constant maximum efficiency of photosystem II photochemistry ratios and malondialdehyde levels in chloroplasts. The three species showed significant increases in α-T, a shift of the redox state of α-T toward its reduced state, and increased Asc levels in chloroplasts under stress. Lemon balm showed the highest increases in α-T and Asc in chloroplasts under stress, which might compensate for the lack of CA. Besides, whereas in rosemary and sage, the redox state of CA was shifted toward its oxidized state and the redox state of Asc was kept constant, lemon balm displayed a shift of the redox state of Asc toward its oxidized state under stress. In vitro experiments showed that both CA and Asc protect α-T and photosynthetic membranes against oxidative damage. These results are consistent with the contention that CA, in combination with other low-Mr antioxidants, helps to prevent oxidative damage in chloroplasts of water-stressed plants, and they show functional interdependence among different low-Mr antioxidants in chloroplasts.
Mediterranean plants are exposed to a combination of environmental stress conditions, including low water availability, high irradiance, temperature fluctuations, and nutrient deprivation. Such stresses may lead to an imbalance between antioxidant defenses and the amount of activated oxygen species (AOS), resulting in oxidative stress (Smirnoff, 1993; Pastori and Foyer, 2002; Xiong et al., 2002). AOS are necessary for inter- and intracellular signaling (Doke, 1997; Foyer and Noctor, 1999), but at high concentrations they can cause damage at various levels of organization, including chloroplasts (Halliwell and Gutteridge, 1989; Asada, 1999). Apart from the xanthophyll cycle, photorespiration and other changes in metabolic activity, which may protect the chloroplasts from oxidative damage (Demmig-Adams and Adams, 1996; Kozaki and Takeba, 1996; Eskling et al., 1997; Osmond et al., 1997), a number of enzymatic and nonenzymatic antioxidants are present in chloroplasts that control oxygen toxicity (Smirnoff, 1993; Foyer et al., 1994; Asada, 1999). Carotenoids, α-tocopherol (α-T, vitamin E), ascorbate (Asc, vitamin C), and glutathione help to maintain the integrity of the photosynthetic membranes under oxidative stress (Havaux, 1998; Noctor and Foyer, 1998; Asada, 1999; Smirnoff and Wheeler, 2000; Munné-Bosch and Alegre, 2002a). Besides, some Labiatae plants, including rosemary (Rosmarinus officinalis) and sage (Salvia officinalis), contain the diterpene carnosic acid (CA), which displays high antioxidant properties in vitro (Aruoma et al., 1992; Schwarz et al., 1992). We have recently found that CA is present in chloroplasts, where it is oxidized to rosmanol (ROM) and isorosmanol (ISO; Munné-Bosch and Alegre, 2001). However, data supporting a protective effect of CA to photosynthetic membranes against oxidative damage has not been provided so far.
Estimations of the redox state of low-Mr antioxidants may allow us to better understand the relationship between drought and oxidative stress in plants. Although levels of antioxidants indicate the potential extent of antioxidative protection and the balance between their synthesis, oxidation, and regeneration, their redox state indicates an oxidative load toward these compounds and provides us with a reliable estimation of the extent of oxidative stress in the cell. Changes in the redox state of Asc and glutathione (Boo et al., 2000; Robinson and Bunce, 2000; Tausz et al., 2001; Mittler et al., 2001; Herbinger et al., 2002) and in that of CA (Munné-Bosch and Alegre, 2000) have been studied in drought-stressed plants. By contrast, to our knowledge, drought-induced changes in oxidation products of α-T or carotenoids and therefore in their redox states have not been reported so far in plants.
It is thought that low-Mr antioxidants cooperate to provide protection against oxidative damage to plants. However, evidence of this occurring in chloroplasts of water-stressed (WS) plants is still limited. To date, it has been shown that Asc recycles α-T from its α-tocopheroxyl radical in vitro (Packer et al., 1979; Smirnoff and Wheeler, 2000) and that a deficiency in Asc may affect antioxidative protection by α-T under stress in Arabidopsis (Munné-Bosch and Alegre, 2002b). Cooperation between α-T and β-carotene (β-C) has also been shown in the scavenging of singlet oxygen in lipid membranes (Burton and Ingold, 1984) and in the protection of photosystem II structure and function in Chlamydomonas reinhardtii (Trebst et al., 2002). In addition, it has been suggested that CA cooperates with α-T to inhibit lipid peroxidation in vitro (Hopia et al., 1996). Both CA and α-T are membrane-associated scavengers of 1O2 and lipid peroxyl radicals (Aruoma et al., 1992; Haraguchi et al., 1995; Munné-Bosch and Alegre, 2002a), and the leaves of rosemary and sage contain similar amounts of α-T as in other species, which suggests that CA may cooperate with α-T in chloroplasts rather than replace its activity.
This study seeks to identify the role of CA, in combination with that of α-T and Asc, in protecting the chloroplasts from drought-induced oxidative stress in Labiatae plants. We measured endogenous concentrations of these antioxidants, their redox states, and other indicators of oxidative stress in chloroplasts of two CA-containing species (rosemary and sage) and a CA-free species (lemon balm [Melissa officinalis]) exposed to drought stress in the field. In addition, in vitro experiments using lemon balm chloroplasts were performed to test the protective effect of CA to photosynthetic membranes against oxidative damage.
RESULTS
In Vivo Experiments
The response of two CA-containing species, i.e. rosemary and sage, and a CA-free species, i.e. lemon balm, to drought stress under Mediterranean field conditions was evaluated. To attain similar relative leaf water contents (RWC) of approximately 58%, lemon balm and sage were exposed to 30 and 49 d of water deficit, respectively. By contrast, the RWC in rosemary decreased only from approximately 85% to 66% after 67 d of water deficit (Table I). This was associated with higher LMA in rosemary, compared with sage and lemon balm, the latter showing the lowest LMA both in irrigated and WS conditions. No damage to the photosynthetic apparatus was observed in any of the species at RWCs between 86% and 58%, as indicated by constant maximum efficiency of photosystem II photochemistry (Fv/Fm) ratios and MDA levels in chloroplasts. MDA levels in leaves were also kept constant in the three species throughout the study (Table I).
Table I.
RWC, leaf mass area (LMA), malondialdehyde (MDA) levels in leaves (MDAleaf) and chloroplasts (MDAchlor), and maximum efficiency of photosystem II photochemistry (Fv/Fm) in leaves of well-watered (WW) and WS rosemary, sage, and lemon balm plants
| Rosemary
|
Sage
|
Lemon Balm
|
||||
|---|---|---|---|---|---|---|
| WW | WS | WW | WS | WW | WS | |
| RWC (%) | 84.4 ± 1.8 | 66.0 ± 3.9a | 85.9 ± 2.2 | 58.9 ± 2.1a | 86.0 ± 2.6 | 58.1 ± 3.3a |
| LMA(g dm−2) | 1.17 ± 0.04 | 2.23 ± 0.15a | 0.75 ± 0.04 | 1.17 ± 0.05a | 0.35 ± 0.01 | 0.57 ± 0.02a |
| MDAleaf (nmol g−1 dry wt) | 10.80 ± 2.21 | 12.75 ± 2.82 | 21.12 ± 3.9 | 24.81 ± 2.96 | 100.03 ± 4.55 | 95.82 ± 4.02 |
| MDAchlor (nmol g−1 dry wt) | 2.95 ± 0.30 | 3.11 ± 0.19 | 4.78 ± 0.55 | 5.38 ± 0.77 | 35.08 ± 1.98 | 39.90 ± 5.12 |
| Fv/Fm | 0.79 ± 0.04 | 0.80 ± 0.03 | 0.80 ± 0.04 | 0.77 ± 0.02 | 0.78 ± 0.02 | 0.77 ± 0.03 |
Data correspond to the mean ± se of four to six measurements made on leaves or chloroplasts isolated from leaves collected at predawn (1 h before sunrise).
Statistical difference (t test, P ≤ 0.05) between WS and WW plants.
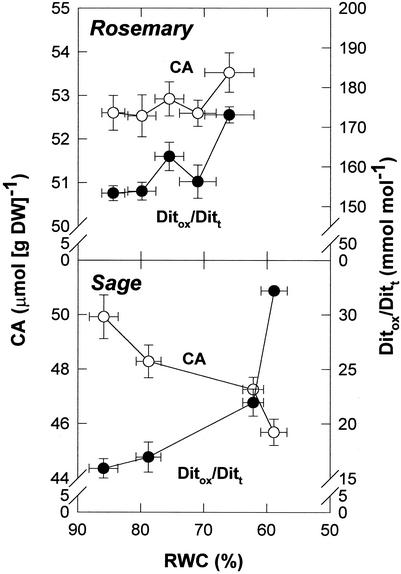
Both rosemary and sage showed a significant oxidation of CA under stress, as indicated by the redox state of CA, given as the ratio of oxidized diterpenes (Ditox = ROM + ISO) to total diterpenes (Ditt = CA + ROM + ISO), which increased by approximately 13% in rosemary and 2-fold in sage (Fig. 1). Though such differences could be partly attributed to the higher water loss in sage, both species differed in CA synthesis. Whereas rosemary could compensate CA oxidation under stress (CA levels increased by approximately 0.9 μmol g−1 dry weight), the highest oxidation of CA in WS sage plants resulted in CA decreases by approximately 4.2 μmol g−1 dry weight.
Figure 1.
Relationship between the RWC and CA contents and the redox state of CA (estimated as Ditox/Ditt, where Ditox = ROM + ISO and Ditt = CA + Ditox) in leaves of rosemary and sage exposed to water deficit in the field. Data correspond to the mean ± se of four independent measurements made on leaves collected at predawn (1 h before sunrise).
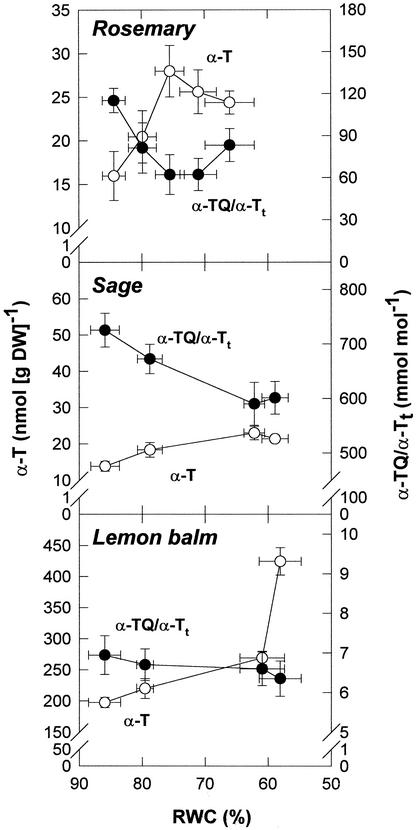
α-T levels increased under stress in the three species studied (Fig. 2). However, α-T levels in lemon balm were approximately 15-fold higher than those in rosemary and sage throughout the experiment, and the highest drought-induced increases of α-T were observed in CA-free lemon balm leaves. In this species, α-T increased by approximately 230 nmol g−1 dry weight under stress, compared with increases of approximately 10 and 8 nmol g−1 dry weight in rosemary and sage, respectively. The highest α-T increases in lemon balm leaves served to maintain the redox state of α-T (given as the α-TQ/[α-TQ + α-T]) toward a reduced state. The slight increases in α-T decreased this ratio significantly in rosemary and sage, because α-TQ was kept constant throughout the study in these species. Thus, CA-free lemon balm leaves showed a approximately 25-fold higher increase of α-T than CA-containing species to keep the redox state of α-T constant under stress. This α-T increase in WS lemon balm leaves (approximately 230 nmol g−1 dry weight) corresponds to approximately 34% of the CA oxidized to ROM + ISO in WS sage leaves, where ROM + ISO increased by approximately 677 nmol g−1 dry weight.
Figure 2.
Relationship between the RWC and α-T contents and the redox state of α-T (estimated as α-TQ/α-Tt, where α-Tt = α-T + α-TQ) in leaves of rosemary, sage, and lemon balm exposed to water deficit in the field. Data correspond to the mean ± se of four independent measurements made on leaves collected at predawn (1 h before sunrise).
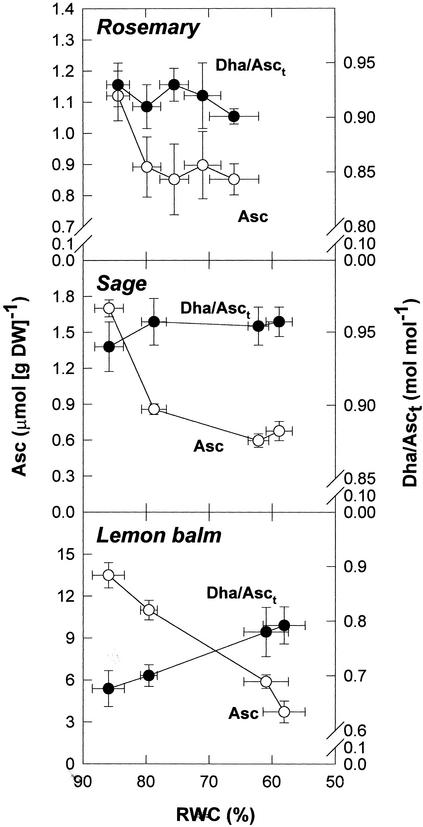
Asc levels were 10-fold higher in lemon balm leaves than in those of rosemary and sage under irrigated conditions. Asc levels in leaves decreased under stress in the three species studied. Asc in leaves decreased by approximately 9.76 μmol g−1 dry weight in lemon balm, whereas it decreased by approximately 0.27 and 1.03 μmol g−1 dry weight in rosemary and sage, respectively (Fig. 3). Despite Asc levels decreased in leaves, the amounts of Asc in chloroplasts increased under stress in the three species (Table II). Asc in chloroplasts increased by approximately 11.02 μmol g−1 dry weight in lemon balm, and it increased by approximately 0.06 and 0.27 μmol g−1 dry weight in rosemary and sage, respectively. Thus Asc levels increased at least 10 μmol g−1 dry weight more in lemon balm chloroplasts than in those of rosemary or sage. This amount doubles the decrease of CA in WS sage leaves. The highest increases of Asc in chloroplasts (given as a percentage of that found in leaves) were observed in WS lemon balm plants. In this species, Asc in chloroplasts increased from approximately 2% to 33% of that found in leaves. Besides, the ratio of DHA to total Asc (DHA/Asct) in chloroplasts, which changed in parallel with that of the leaf, increased significantly (P ≤ 0.05) only in WS lemon balm plants (Fig. 3; Table II).
Figure 3.
Relationship between the RWC and Asc contents and the redox state of Asc (estimated as DHA/Asct, where Asct = Asc + DHA) in leaves of rosemary, sage, and lemon balm exposed to water deficit in the field. Data correspond to the mean ± se of four independent measurements made on leaves collected at predawn (1 h before sunrise).
Table II.
Asc levels, given per dry wt and as a percentage of that found in leaves, and ratio of dehydroascorbate (DHA) to total Asc (DHA/Asct) in chloroplasts of rosemary, sage and lemon balm at the onset of the experiment (well-watered [WW] plants) and after exposure to water deficit in the field (WS plants)
| Rosemary
|
Sage
|
Lemon Balm
|
||||
|---|---|---|---|---|---|---|
| WW | WS | WW | WS | WW | WS | |
| Asc (μmol g−1 dry wt) | 0.21 ± 0.02 | 0.27 ± 0.01a | 0.20 ± 0.01 | 0.47 ± 0.02a | 2.46 ± 0.05 | 13.58 ± 0.42a |
| Asc (%) | 1.29 | 2.61 | 1.43 | 7.13 | 1.82 | 33.60 |
| DHA/Asct (mol mol−1) | 0.92 ± 0.03 | 0.91 ± 0.02 | 0.95 ± 0.02 | 0.96 ± 0.03 | 0.72 ± 0.03 | 0.86 ± 0.04a |
Data correspond to the mean ± se of four measurements made on chloroplasts isolated from leaves collected at predawn (1 h before sunrise). Chloroplasts were obtained from leaves as described in “Materials and Methods.” Asc, Reduced Asc in chloroplasts given per unit of dry wt of chloroplast and as a percentage of that found in leaves. This percentage was calculated as Asc (%) = 100 × A/B, where A is the Asc measured in chloroplasts and B is that found in leaves; both A and B are given as nmol Asc mol−1 Chl.
Statistical difference (t test, P ≤ 0.05) between WS and WW plants.
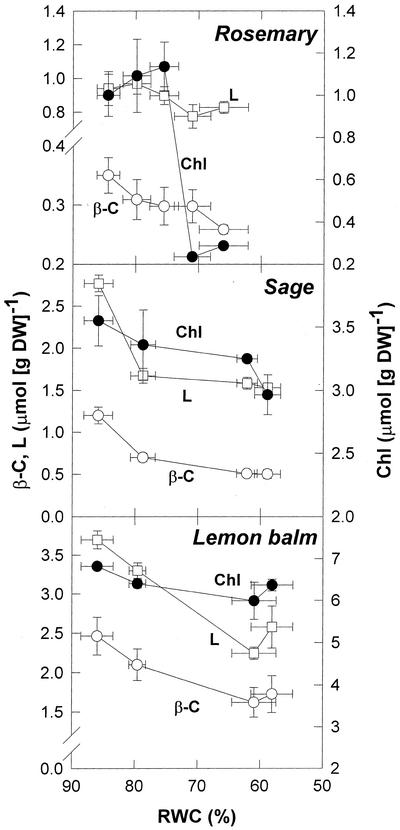
β-C and lutein (L) decreased progressively under stress in the three species studied (Fig. 4). The highest amounts of β-C and L were observed in sage and lemon balm which, in turn, showed the highest stress-induced decreases in carotenoids. Violaxanthin and neoxanthin decreased in parallel with β-C and L, and the de-epoxidation state of the xanthophyll cycle remained at approximately 0.1 throughout the experiment in the three species (data not shown). Rosemary and sage were the species showing the most pronounced decrease in chlorophyll a+b (Chl) under stress (by approximately 0.8 μmol g−1 dry weight). This was especially relevant in rosemary, because this change corresponded to a 80% decrease in Chl under stress. Because carotenoid levels decreased only slightly in this species, rosemary was the only species showing higher carotenoid to Chl ratios under stress (Table III). The Chl ratios were nearly unaffected by drought in either species; and ranged between 2.86 and 2.92 in rosemary, between 2.29 and 2.33 in sage, and between 2.47 and 2.68 in lemon balm, throughout the experiment (data not shown).
Figure 4.
Relationship between the RWC and β-C, and L and Chl contents in leaves of rosemary, sage, and lemon balm exposed to water deficit in the field. Data correspond to the mean ± se of four independent measurements made on leaves collected at predawn (1 h before sunrise).
Table III.
Carotenoid contents per unit of Chl and de-epoxidation state (DPS) of the xanthophyll cycle in leaves of well-watered (WW) and WS rosemary, sage, and lemon balm plants
| Rosemary
|
Sage
|
Lemon Balm
|
||||
|---|---|---|---|---|---|---|
| WW | WS | WW | WS | WW | WS | |
| mol mol−1 Chl | ||||||
| β-C | 0.35 ± 0.03 | 0.90 ± 0.03a | 0.34 ± 0.04 | 0.17 ± 0.01a | 0.36 ± 0.03 | 0.27 ± 0.03a |
| L | 0.94 ± 0.09 | 2.88 ± 0.12a | 0.78 ± 0.03 | 0.52 ± 0.02a | 0.54 ± 0.02 | 0.40 ± 0.04a |
| N | 0.31 ± 0.03 | 0.80 ± 0.05a | 0.19 ± 0.01 | 0.16 ± 0.01a | 0.11 ± 0.01 | 0.10 ± 0.01 |
| VZA | 0.32 ± 0.02 | 1.40 ± 0.12a | 0.35 ± 0.02 | 0.27 ± 0.02a | 0.41 ± 0.01 | 0.20 ± 0.01a |
| DPS | 0.13 ± 0.02 | 0.10 ± 0.03 | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.12 ± 0.02 | 0.09 ± 0.02 |
Data correspond to the mean ± se of four measurements made on leaves collected at predawn (1 h before sunrise). β-C, β-Carotene; L, lutein; N, neoxanthin; VZA, violaxanthin + zeaxanthin + antheraxanthin. DPS was calculated as (Z + A)/VZA.
Statistical difference (t test, P ≤ 0.05) between WS and WW plants.
In Vitro Experiments
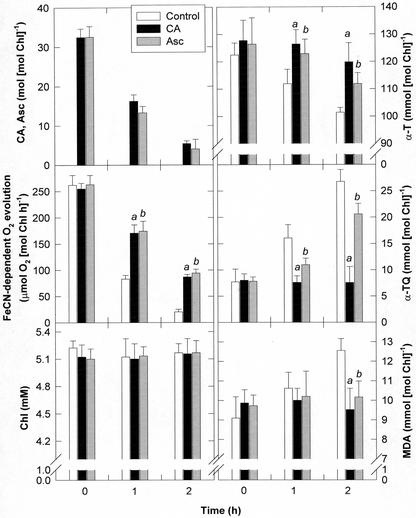
CA-free lemon balm chloroplasts were used to evaluate the protective effects of CA to photosynthetic membranes against oxidative damage. Ferricyanide-dependent O2 evolution, photosynthetic pigments, MDA, and low-Mr antioxidants were measured in osmotically shocked chloroplasts (a) devoid of exogenous antioxidants (controls), (b) treated with CA at concentrations between 5 and 50 mol mol−1 Chl, which is within the range of concentrations found in rosemary and sage chloroplasts, or (c) treated with Asc at the same concentrations and exposed to a light intensity of 300 μmol m−2 s−1 at 22°C for 2 h (Fig. 5). Ruptured lemon balm chloroplasts showed progressive decreases in FeCN-dependent oxygen evolution when exposed to light. After 2 h of light exposure, CA-treated chloroplasts maintained approximately 34% of the initial oxygen evolving capacity, whereas similar values were attained in CA-free chloroplasts after 1 h of light exposure. Whereas photosynthetic pigments were kept constant, α-T decreased progressively up to 18% after 2 h of light exposure in CA-free chloroplasts. In these chloroplasts, decreases in α-T were correlated with increases in α-TQ at equimolar concentrations. By contrast, a complete inhibition of α-T oxidation to α-TQ was observed in CA-treated chloroplasts. The same extent of α-T protection was observed at all CA concentrations tested (5–50 mol mol−1 Chl). In addition, whereas MDA remained unchanged in CA-treated chloroplasts, MDA accumulated in CA-free chloroplasts after 2 h of light exposure, once α-T was partially oxidized. Asc inhibited lipid peroxidation in light-exposed ruptured chloroplasts and also protected α-T, though to a lesser extent than CA.
Figure 5.
Protective effects of CA and Asc at a concentration of 32 mol mol−1 Chl on α-T oxidation to α-TQ and lipid peroxidation (estimated as MDA accumulation) in CA-free osmotically shocked lemon balm chloroplasts exposed to a light intensity of 300 μmol m−2 s−1. Chloroplast intactness before osmotic shock was approximately 85%. Asc concentrations in intact lemon balm chloroplasts were approximately 20 mmol mol−1 Chl. Data correspond to the mean ± se of three independent measurements. a and b indicate statistical difference (t test, P ≤ 0.05) between chloroplasts treated with CA or chloroplasts treated with Asc, respectively, and controls. For details, see “Materials and Methods.” Protection against oxidative damage was observed at all CA and Asc concentrations tested (between 5–50 mol mol−1 Chl).
DISCUSSION
Studying Mediterranean plants within their natural habitat may reveal novel mechanisms of resistance to environmental stresses. The response of rosemary and sage, two CA-containing species, to drought stress was compared with that of lemon balm, which belongs to the same family (Labiatae) but does not contain CA in leaves. CA is a diterpene with potent antioxidant properties that has received considerable attention in food science (Schwarz et al., 1992; Richheimer et al., 1999) and biomedicine (Singletary and Nelshoppen, 1991; Haraguchi et al., 1995). We have recently shown that CA is oxidized to ROM and ISO in chloroplasts (Munné-Bosch and Alegre, 2001) and that both rosemary and sage increase ROM and ISO formation in response to drought stress (Munné-Bosch et al., 1999, 2001; Munné-Bosch and Alegre, 2000), which supports an antioxidative function of CA in WS plants. In the present study, we provide further evidence for the antioxidative function of CA in chloroplasts and present results showing functional interdependence between low-Mr antioxidants in chloroplasts of WS Labiatae plants.
Osmotically shocked lemon balm chloroplasts exposed to light showed α-T oxidation to α-TQ at equimolar concentrations. Neither α-T nor α-TQ levels were affected in dark-exposed chloroplasts (data not shown), which indicates that α-T oxidation to α-TQ was caused by photogenerated AOS. At the same FeCN-dependent oxygen evolution, CA-treated chloroplasts showed lower α-T oxidation than controls (CA-free chloroplasts), which indicates that CA may play a role as an antioxidant in photosynthetic membranes. Field experiments showed that CA was oxidized before α-T and before damage to the photosynthetic apparatus occurred in WS rosemary and sage plants. Because both CA and α-T are present in chloroplastic membranes (Havaux, 1998; Munné-Bosch and Alegre, 2001, 2002a) and scavenge singlet oxygen and lipid peroxyl radicals (Luis, 1991; Aruoma et al., 1992; Munné-Bosch and Alegre, 2002a), our results are consistent with cooperation between CA and α-T in the prevention of oxidative damage to photosynthetic membranes under stress. CA is found at higher concentrations than α-T in chloroplasts (Munné-Bosch and Alegre, 2001), and it is oxidized more readily than α-T (Aruoma et al., 1992), thus CA may indirectly protect α-T by scavenging singlet oxygen and lipid peroxyl radicals. It has also been suggested that CA recycles α-tocopheroxyl radicals to α-T in vitro (Hopia et al., 1996), but this remains to be demonstrated in vivo.
Asc added to osmotically shocked chloroplasts partly prevented α-T oxidation in the light. In addition, α-T and Asc in chloroplasts increased, whereas no photoinhibitory damage to the photosynthetic apparatus occurred at RWCs between 86% to 58% in any of the three species studied. In agreement with previous studies (Packer et al., 1979; Niki et al., 1982; Asada and Takahashi, 1987; Munné-Bosch and Alegre, 2002b), which suggest a positive interplay between Asc and α-T, Asc may indirectly protect α-T by scavenging AOS, and it may participate in the recycling of α-tocopheroxyl radicals to α-T. Thus, both CA and Asc seem to play a major role in the protection of α-T and photosynthetic membranes against oxidative damage.
Neither of the species studied showed a redox shift of α-T toward α-TQ accumulation in leaves. By contrast, differences in the redox states of CA and Asc and in the extent of α-T and Asc accumulation in chloroplasts were observed in CA-containing (rosemary and sage) and CA-free species (lemon balm) exposed to drought. Whereas CA oxidation products (ROM and ISO) increased and the redox state of Asc was kept constant in drought-stressed rosemary and sage plants, lemon balm showed a significant shift of the redox state of Asc toward its oxidized state in stressed chloroplasts. Lemon balm showed the highest increases in α-T and Asc in stressed chloroplasts. Lemon balm leaves showed approximately 25-fold higher increases of α-T than the CA-containing species, and this increase corresponded to approximately 34% of the CA oxidized to ROM + ISO in WS sage leaves. In addition, Asc levels increased at least 10 μmol g−1 dry weight more in lemon balm chloroplasts than in those of rosemary or sage, which doubles the decrease of CA in WS sage leaves. Because no photoinhibitory damage to the photosynthetic apparatus was observed in either species, α-T and Asc accumulation in chloroplasts might, therefore, compensate for the lack of CA in WS lemon balm plants. α-T increases could partly compensate for the lack of CA by performing a similar antioxidative function. In addition, enhanced Asc levels might favor recycling of α-tocopheroxyl radicals (whose formation might increase in the absence of CA), thus resulting in DHA accumulation under stress.
Asc in chloroplasts increased in WS plants of the three species, especially lemon balm. Although intracellular Asc transport in stressed plants is still not fully understood (Rautenkranz et al., 1994; Horemans et al., 2000), our results support increased transport of Asc from the cytosol to chloroplasts in stressed plants. The DHA/Asct ratios measured in the three species were considerably higher than those of other species. It has been shown that the Asc pool decreases and is oxidized during the lignifying process in leaves (Otter and Polle, 1994). Thus, it is likely that the high DHA/Asct ratios measured in the present study might be associated, among other factors, with the xeric characteristics of such leaves (especially in rosemary and sage). DHA may additionally have important functions beyond the Asc-glutathione cycle (Deutsch, 2000; for review, see Smirnoff and Wheeler, 2000). DHA appears to have antioxidant properties on its own, beyond that of Asc. DHA could also play a role in controlling cell expansion and probably as a precursor of oxalate, which may control concentrations of ionic calcium in the wall by formation of calcium oxalate crystals.
The oxidation products of CA (ROM and ISO) and α-T (α-TQ) may also have important functions in plants. All CA derivatives bearing two hydroxyl groups in ortho-position at C11 and C12 of the molecule (e.g. ROM and ISO) display antioxidant properties (Aruoma et al., 1992). Thus, CA may function as a “cascading” antioxidant, in which oxidation products are further oxidized, thus enhancing antioxidative protection by CA. In addition, CA, ROM, and ISO can be methoxylated, and the resulting derivatives accumulate in the plasma membrane, where they may affect membrane fluidity (Munné-Bosch and Alegre, 2001). In turn, α-TQ, the location of which is similar to that of α-T (both compounds are found in the envelope, thylakoids, and plastoglobuli of chloroplasts), has been suggested to play a role in cyclic electron transport around photosystem II, thus conferring photoprotection to the photosynthetic apparatus (Kruk and Strzalka, 1995, 2001). The increase in α-TQ levels in lemon balm may therefore contribute, in combination with enhanced α-T and Asc levels, to the prevention of oxidative damage in drought. Further research is needed to have a more complete understanding of the plethora of CA and α-T oxidation products that accumulate in different species of the Labiatae family under stress.
Chl levels decreased in WS rosemary, sage, and lemon balm plants, a phenomenon which has already been described for several Mediterranean plants (Kyparissis et al., 1995; Havaux et al., 1998; Munné-Bosch and Alegre, 2000). In agreement with these studies, our results suggest that Chl loss is not necessarily a symptom of unsuccessful adaptation to stress, because none of the three species showed damage to the photosynthetic apparatus at RWCs between 86% to 58%. A reduction in chlorophyll levels might damper the potentially damaging effects of high solar radiation in drought-stressed plants, because it decreases leaf light absorption and therefore increases the photoprotective and antioxidative capacity of leaves per amounts of photons absorbed. Rosemary was the only species showing increases of carotenoids per unit of Chl, which may contribute to the high resistance of this species to severe water loss (Munné-Bosch and Alegre, 2000).
In conclusion, the results are consistent with the contention that CA, in combination with other low-Mr antioxidants, helps to prevent oxidative damage to photosynthetic membranes in WS rosemary and sage plants. In addition, the results show functional interdependence between low-Mr antioxidants in chloroplasts of WS Labiatae plants, in which the absence of CA in lemon balm leaves is compensated by enhanced α-T and Asc accumulation and increased Asc oxidation in stressed chloroplasts.
MATERIALS AND METHODS
Plant Material
Thirty-five plants of rosemary (Rosmarinus officinalis) obtained from cuttings, and 35 plants of sage (Salvia officinalis L. sub. officinalis) and lemon balm (Melissa officinalis) obtained from seeds were grown in a greenhouse under controlled conditions as described (Munné-Bosch and Alegre, 2000). Eighteen-month-old plants were transplanted to the experimental fields at the University of Barcelona during October of 1999.
In Vivo Experiments
Experiments performed on plants growing under Mediterranean field conditions started on April 23, 2001. Thirty days before the experiment started, plants received approximately 100 mm of water. During the experiment, plants did not receive water at all and were WS by covering the plants with a clear polyvinyl chloride sheet only when rainfall was expected. Plant water status, LMA, chlorophyll fluorescence, MDA and pigment contents, and levels of reduced and oxidized low-Mr antioxidants (α-T, Asc, and CA) in fully developed young leaves collected at predawn (1 h before sunrise) were measured. MDA contents and levels of reduced and oxidized Asc were also measured in chloroplasts obtained from leaves collected at predawn. For measurements in leaves, samples were collected, frozen in liquid nitrogen, and stored at −80°C until analysis. For measurements in chloroplasts, leaves were immediately subjected to cell fractionation as described below and stored at −80°C until analysis.
Isolation of Chloroplasts
Chloroplasts were isolated from leaves as described (Munné-Bosch and Alegre, 2001). In short, after grinding the samples in isolation buffer (0.5–1.5 m sorbitol [depending on the plant water status], 50 mm Tricine, 1 mm MgCl2, 0.1% [w/v] bovine serum albumin, 1 mm butylated hydroxytoluene [BHT], and 1 mm citric acid), the homogenate was filtered through four layers of cheesecloth and centrifuged at 3°C and 2,500 g for 4 min. The pellet was resuspended in isolation buffer and then centrifuged at 3°C and 200g for 1 min. The chloroplasts in the supernatant were sedimented by centrifugation at 3°C and 2,500g for 4 min. Chloroplasts were purified by resuspending the pellets in isolation buffer, layering onto 10 mL of 25% (v/v) of Percoll (in isolation buffer), and centrifuging at 3°C and 15,800g for 20 min. The chloroplast pellets were resuspended in isolation buffer, centrifuged at 3°C and 2,500g for 4 min, and used immediately for analyses. The identity and purity of chloroplasts were determined by assaying amounts and activities of appropriate markers, and confirmed further by microscopic observation as described (Munné-Bosch and Alegre, 2001). Chloroplasts-enriched fractions did not show detectable activities of NADPH-cytochrome c reductase, latent IDPase, vanadate-sensitive ATPase, and cytochrome c oxidase, which are markers of the endoplasmic reticulum, Golgi apparatus, plasma membrane, and mitochondrion, respectively. The chloroplast intactness was determined by the ferricyanide reducing assay (Lilley et al., 1975). The intactness of chloroplasts exceeded 80% in preparations used both for in vitro and in vivo experiments.
Climatologic Measurements
Environmental conditions were monitored at 5-min intervals throughout the experiment with a weather station (Delta-T Devices, Newmarket, UK). The photosynthetically active photon flux density was measured with a Quantum sensor (Li-Cor, Lincoln, NE). Air temperature and relative humidity were measured with a thermohygrometer (Vaisala, Helsinki). Vapor pressure deficit was calculated from air temperature and relative humidity data according to Nobel (1991). During the in vivo experiments (from April 23 to June 29, 2001), maximum diurnal photosynthetically active photon flux density, air temperature, and vapor pressure deficit ranged between 1,830 and 1,910 μmol m−2 s−1, 20.0°C and 23.2°C, and 0.98 and 1.67 KPa, respectively.
Water Status and LMA
Leaves were weighted and leaf area was immediately measured using a flatbed scanner (model GT-5000, Epson, Nagano, Japan) and an image-processing program. Then, leaves were rehydrated for 24 h at 4°C in darkness and subsequently oven-dried for 24 h at 80°C. The RWC was determined as 100 × (FW − DW)/(TW − DW), where FW is the fresh matter, TW is the turgid matter after re-hydrating the leaves, and DW is the dry matter after oven-drying the leaves. The LMA was determined as DW/leaf area.
Chlorophyll Fluorescence
Chlorophyll fluorescence measurements were made on leaves at predawn with a portable fluorimeter mini-PAM (Walz, Effeltrich, Germany) according to Munné-Bosch and Alegre (2000). The Fv/Fm was calculated from chlorophyll fluorescence data by using the equations described by Genty et al. (1989).
Estimation of Lipid Peroxidation
The extent of lipid peroxidation in leaves and chloroplasts was estimated by measuring the amount of MDA by HPLC as described (Munné-Bosch and Alegre, 2002b). In short, samples were repeatedly extracted with 80:20 (v/v) ethanol:water containing 1 μL L−1 BHT using ultrasonication (Vibra-Cell ultrasonic processor, Sonics and Materials Inc., Danbury, CT). After centrifugation, supernatants were pooled, and an aliquot of appropriately diluted sample was added to a test tube with an equal volume of thiobarbituric acid (TBA) solution containing 20% (w/v) trichloroacetic acid, 0.01% (w/v) BHT, and 0.65% (w/v) TBA. A blank was prepared by replacing the sample with extraction medium, and controls for each sample were prepared by replacing TBA with 50 mm NaOH. Samples were heated at 95°C for 25 min, and after cooling, the (TBA)2-MDA adduct was isocratically separated on a Hypersyl ODS-5 μm (250 × 4.6 mm, Teknokroma, St. Cugat, Spain) by using 5 mm potassium phosphate buffer (pH 7.0) containing 15% (v/v) acetronitrile and 0.6% (v/v) tetrahydrofuran as an eluant at a flow rate of 0.9 mL min−1. The (TBA)2-MDA adduct was quantified through its A537 (diode array detector 1000S, Applied Biosystems, Foster City, CA) and was identified by its characteristic spectrum and by coelution with an authentic standard. 1,1,3,3-Tetraethoxypropane (Sigma, Steinheim, Germany), which was used as a standard, is stoichiometrically converted into MDA during the acid-heating step of the assay.
Pigment Determination
The extraction and HPLC analysis of pigments was carried out as described (Munné-Bosch and Alegre, 2000). In short, leaves were ground in liquid nitrogen and repeatedly extracted with ice-cold acetone using ultrasonication (Vibra-Cell ultrasonic processor). Pigments were separated on a nonendcapped Zorbax ODS-5 μm column (250 × 4.6 mm; DuPont, Wilmington, DE; 20% C, Teknokroma) at 30°C at a flow rate of 1 mL min−1. The solvents consisted of (A) acetonitrile:methanol (85: 15, v/v) and (B) methanol:ethyl acetate (68: 32, v/v). The gradient used was: 0 to 14 min, 100% A; 14 to 16 min, decreasing to 0% A; 16 to 28 min, 0% A; 28 to 30 min, increasing to 100% A; and 30 to 38 min, 100% A. Detection was carried out at 445 nm (diode array detector 1000S, Applied Biosystems). Compounds were identified by their characteristic spectra and by coelution with chlorophyll and carotenoid standards, which were obtained from Fluka (Buchs, Switzerland) and Hoffman-La Roche (Basel).
Analyses of Reduced and Oxidized Low-Mr Antioxidants
For measurement of α-T and its oxidation product, α-TQ, leaves were repeatedly extracted with ice-cold n-hexane containing 1 μL L−1 BHT using ultrasonication (Vibra-Cell ultrasonic processor) as described (Munné-Bosch and Alegre, 2000). α-T and α-TQ were analyzed by HPLC essentially as described (Hogg et al., 1996). α-T and α-TQ were separated on a Partisil 10 ODS-3 column (250 × 4.6 mm, Scharlau, Barcelona) at a flow rate of 1 mL min−1. The solvents consisted of (A) methanol:water (95: 5, v/v) and (B) methanol. The gradient used was: 0 to 10 min, 100% A; 10 to 15 min, decreasing to 0% A; 15 to 20 min, 0% A; 20 to 23 min, increasing to 100% A; and 23 to 28 min, 100% A. α-T and α-TQ were quantified through their A283 and A256, respectively (diode array detector 1000S, Applied Biosystems). Both compounds were identified by their characteristic spectra and by coelution with authentic standards provided by Sigma and Prof. Strzalka (Jagiellonian University, Krakov, Poland).
The extraction and HPLC analysis of reduced and oxidized Asc in leaves and chloroplasts was performed as described (Munné-Bosch and Alegre, 2002b). In short, leaves were ground in liquid nitrogen and repeatedly extracted with ice-cold extraction buffer (40% [v/v] methanol, 0.75% [w/v] m-phosphoric acid, 16.7 mm oxalic acid, and 0.127 mm diethylenetriaminepentaacetic acid) using ultrasonication (Vibra-Cell Ultrasonic Processor). After centrifugation, 0.1 mL of the supernatant was transferred to 0.9 mL of the mobile phase (24.25 mm sodium-acetate/acetic acid, pH 4.8, 0.1 mm diethylenetriaminepentaacetic acid, 0.015% [w/v] m-phosphoric acid, 0.04% [w/v] octylamine, and 15% [v/v] methanol) for determination of reduced Asc. For determination of total Asc (reduced plus oxidized; Asct), 0.1 mL of the supernatant was incubated for 10 min at room temperature in darkness with 0.25 mL of 2% (w/v) dithiothreitol and 0.5 mL of 200 mm NaHCO3. The reaction was stopped by adding 0.25 mL of 2% (v/v) sulfuric acid and 0.8 mL of the mobile phase. Asc was isocratically separated on a Spherisorb ODS C8 column (Teknokroma) at a flow rate of 0.8 mL min−1. Detection was carried out at 255 nm (diode array detector 1000S, Applied Biosystems). Asc was identified by its characteristic spectrum and by coelution with an authentic standard from Sigma.
Extraction and HPLC analysis of reduced diterpenes and Ditox were performed as described (Munné-Bosch and Alegre, 2001). In short, the samples were repeatedly extracted with methanol containing 0.005% (w/w) citric acid and 0.005% (w/w) isoascorbic acid using ultrasonication (Vibra-Cell ultrasonic processor). Diterpenes were separated on an ODS Hypersil-5 μm column (250 × 4 mm, Teknokroma) during 52 min at a flow rate of 0.6 mL min−1. The eluants consisted of: A, 51% (v/v) acetonitrile and 49% (v/v) water, containing 0.83% (v/v) 2 m citric acid; and B, 97% (v/v) acetonitrile and 3% (v/v) water, containing 0.5% (v/v) 2 m citric acid. The following gradient was used: 0 to 20 min, 100% A and 0% B; 20 to 34 min, decreasing to 50% A and 50% B; 34 to 40 min, decreasing to 0% A and 100% B; 40 to 48 min, increasing to 100% A and 0% B; and 48 to 52 min, 100% A and 0% B. Individual diterpenes were identified by their characteristic spectra. CA, which was provided by Prof. Schwarz (University of Kiel, Germany), was used for calibration. All diterpenes were quantified at 230 nm (diode array detector 1000S, Applied Biosystems).
In Vitro Experiments
In vitro experiments were performed with chloroplasts isolated from fully developed young leaves of well-watered lemon balm plants collected at predawn (1 h before sunrise) during November and December 2001. Lemon balm chloroplasts, which do not contain CA, were isolated as described above and were subjected to an osmotic shock by redissolving the chloroplast pellet in 2 mL of distilled water, after which 2 mL of 2× reaction buffer was added to yield a final concentration of 0.5 m sorbitol, 50 mm Tricine, 1 mm MgCl2, and 0.1% (w/v) bovine serum albumin. Oxidative stress in ruptured chloroplasts was induced by exposing the chloroplast solution (Chl concentration of approximately 5 mg mL−1) to a light intensity of 300 μmol m−2 s−1 at 22°C. The protective effect of CA on photosynthetic membranes was tested by adding CA to the chloroplast solution at concentrations between 5 and 50 mol mol−1 Chl, which is within the range of concentrations found in rosemary and sage chloroplasts. CA was dissolved in a minimum amount of methanol and then in water. The final methanol concentration in the chloroplast solution treated with CA was below 0.1% (v/v). The protective effect of CA was compared with that provided by Asc by adding Asc to the chloroplast solution at the same concentrations. Control experiments were performed by using ruptured chloroplasts devoid of exogenous antioxidants. Changes in ferricyanide-dependent O2 evolution, photosynthetic pigments, MDA, and low-Mr antioxidants (CA, α-T, and Asc) were simultaneously measured for 2 h of light exposure. Ferricyanide-dependent O2 evolution was determined as described (Lilley et al., 1975). O2 evolution was measured polarographically at 20°C in electrodes (Hansatech, King's Lynn, Norfolk, UK) illuminated by white light at 150 μmol m−2s−1. Analyses of photosynthetic pigments, MDA, and antioxidants were performed as described above.
Statistical Analyses
Statistical differences between measurements on different treatments or on different times were analyzed following the Student's t test using SPSS (Chicago). Differences were considered significant at a probability level of P < 0.05.
ACKNOWLEDGMENTS
We are very grateful to Prof. Kazimizierz Strzalka (Jagiellonian University, Krakov, Poland), Prof. Karin Schwarz (University of Kiel, Germany), and Hoffmann-La Roche for kindly providing us with α-TQ, CA, and carotenoid standards, respectively. We also thank the Serveis Científico-Tècnics and Serveis dels Camps Experimentals (University of Barcelona) for technical assistance.
Footnotes
This study was supported by the Ministerio de Ciencia y Tecnología (project no. MCYT BOS 2000–0560).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019265.
LITERATURE CITED
- Aruoma OI, Halliwell B, Aeschbach R, Löliger J. Antioxidant and prooxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Asada K, Takahashi M. Production and scavenging of active oxygens in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen DJ, editors. Photoinhibition. Amsterdam: Elsevier/North Holland Biomedical Press; 1987. pp. 227–280. [Google Scholar]
- Boo YC, Lee KP, Jung J. Rice plants with a high protochlorophyllide accumulation show oxidative stress in low light that mimics water stress. J Plant Physiol. 2000;157:405–411. [Google Scholar]
- Burton GW, Ingold KU. β-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Deutsch JC. Dehydroascorbic acid. J Chromatogr A. 2000;881:299–307. doi: 10.1016/s0021-9673(00)00166-7. [DOI] [PubMed] [Google Scholar]
- Doke N. The oxidative burst: role in signal transduction and plant stress. In: Pell EJ, Steffen KL, editors. Oxidative Stress and the Molecular Biology of Antioxidant Defences. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 785–813. [Google Scholar]
- Eskling M, Arvidsson PO, Akerlund HE. The xanthophyll cycle, its regulation and components. Physiol Plant. 1997;100:806–816. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Foyer CH, Noctor G. Leaves in the dark see the light. Science. 1999;284:599–601. doi: 10.1126/science.284.5414.599. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 1989. [Google Scholar]
- Haraguchi H, Saito T, Okamura N, Yagi A. Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med. 1995;61:333–336. doi: 10.1055/s-2006-958094. [DOI] [PubMed] [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998;3:147–151. [Google Scholar]
- Havaux M, Tardy F, Lemoine Y. Photosynthetic light-harvesting function of carotenoids in higher-plant leaves exposed to high light irradiances. Planta. 1998;205:242–250. [Google Scholar]
- Herbinger K, Tausz M, Wonisch A, Soja G, Sorger A, Grill D. Complex interactive effects of drought and ozone stress on the antioxidant defence systems of two wheat cultivars. Plant Physiol Biochem. 2002;40:691–696. [Google Scholar]
- Hogg N, Jit Singh R, Goss SPA, Kalyanaraman B. The reaction between nitric oxide and α-tocopherol: a reappraisal. Biochem Biophys Res Commun. 1996;224:696–702. doi: 10.1006/bbrc.1996.1086. [DOI] [PubMed] [Google Scholar]
- Hopia AI, Huang S, Schwarz K, German JB, Frankel EN. Effect of different lipid systems on antioxidant activity of rosemary constituents carnosol and carnosic acid with and without α-tocopherol. J Agric Food Chem. 1996;44:2030–2036. [Google Scholar]
- Horemans N, Foyer CH, Potters G, Asard H. Ascorbate function and associated transport systems in plants. Plant Physiol Biochem. 2000;38:531–540. [Google Scholar]
- Kozaki A, Takeba G. Photorespiration protects C3 plants from photooxidation. Nature. 1996;384:557–560. [Google Scholar]
- Kruk J, Strzalka K. Occurrence and function of alpha-tocopherol quinone in plants. J Plant Physiol. 1995;145:405–409. [Google Scholar]
- Kruk J, Strzalka K. Redox changes of cytochrome b559 in the presence of plastoquinones. J Biol Chem. 2001;276:86–91. doi: 10.1074/jbc.M003602200. [DOI] [PubMed] [Google Scholar]
- Kyparissis A, Petropoulou Y, Manetas Y. Summer survival of leaves in a soft-leaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: avoidance of photoinhibitory damage through decreased chlorophyll contents. J Exp Bot. 1995;46:1825–1831. [Google Scholar]
- Lilley RMC, Fitzgerald MP, Rienits KG, Walker DA. Criteria of intactness and the photosynthetic activity of spinach chloroplasts preparations. New Phytol. 1975;75:1–10. [Google Scholar]
- Luis JG. Chemistry, biogenesis, and chemotaxonomy of the diterpenoids of Salvia. In: Harborne JB, Tomas-Barberan FA, editors. Ecological Chemistry and Biochemistry of Plant Terpenoids. Oxford: Clarendon Press; 1991. pp. 63–82. [Google Scholar]
- Mittler R, Merquiol E, Hallk HE, Rachmilevitch S, Kaplan A, Cohen M. Living under a “dormant” canopy: a molecular acclimation mechanism of the desert plant Retama raetam. Plant J. 2001;25:407–416. doi: 10.1046/j.1365-313x.2001.00975.x. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta. 2000;210:925–931. doi: 10.1007/s004250050699. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol. 2001;125:1094–1102. doi: 10.1104/pp.125.2.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci. 2002a;21:31–57. [Google Scholar]
- Munné-Bosch S, Alegre L. Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett. 2002b;524:145–148. doi: 10.1016/s0014-5793(02)03041-7. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Müller M, Schwarz K, Alegre L. Diterpenes and antioxidative protection in drought-stressed Salvia officinalis plants. J Plant Physiol. 2001;158:1431–1437. [Google Scholar]
- Munné-Bosch S, Schwarz K, Alegre L. Enhanced formation of α-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary plants. Plant Physiol. 1999;121:1047–1052. doi: 10.1104/pp.121.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E, Tsuchiya J, Tanimura R, Kamiya Y. Regeneration of vitamin E from α-chromanoxyl radical by glutathione and vitamin C. Chem Lett. 1982;6:789–792. [Google Scholar]
- Nobel PS. Physicochemical and Environmental Plant Physiology. San Diego: Academic Press; 1991. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Osmond B, Badger M, Maxwell K, Björkman O, Leegod R. Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci. 1997;2:119–120. [Google Scholar]
- Otter T, Polle A. The influence of apoplastic ascorbate on the activities of cell wall-associated peroxidase and NADH oxidase in needles of Norway spruce (Picea abies L.) Plant Cell Physiol. 1994;35:1231–1238. [Google Scholar]
- Packer JE, Slater TF, Willison RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- Pastori G, Foyer CH. Common components, networks, and pathways of cross-tolerance to stress: the central role of “redox” and abscissic acid-mediated controls. Plant Physiol. 2002;129:460–468. doi: 10.1104/pp.011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenkranz AAF, Li L, Machler F, Martinoia E, Oertli JJ. Transport of ascorbic and dehydroascorbic acids across protoplast and vacuole membranes isolated from barley (Hordeum vulgare L. cv Gerbel) leaves. Plant Physiol. 1994;106:187–193. doi: 10.1104/pp.106.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richheimer SL, Bailey DT, Bernart MW, Kent M, Vininski JV, Anderson LD. Antioxidant activity and oxidative degradation of phenolic compounds isolated from rosemary. Recent Res Dev Oil Chem. 1999;3:45–58. [Google Scholar]
- Robinson JM, Bunce JA. Influence of drought-induced water stress on soybean and spinach leaf ascorbate-dehydroascorbate level and redox status. Int J Plant Sci. 2000;161:271–279. doi: 10.1086/314257. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Ternes W, Schmauderer E. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis: III. Stability of phenolic diterpenes of rosemary extracts under thermal stress as required for technological processes. Z Lebensm-Unters -Forsch. 1992;195:104–107. doi: 10.1007/BF01201767. [DOI] [PubMed] [Google Scholar]
- Singletary KW, Nelshoppen JM. Inhibition of 7,12-dimethylbenzanthracene (DMBA)-induced mammary tumorgenesis and of in vivo formation of mammary DMBA-DNA adducts by rosemary extract. Cancer Lett. 1991;60:169–175. doi: 10.1016/0304-3835(91)90224-6. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Plant Sci. 2000;19:267–290. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- Tausz M, Bytnerowicz A, Arbaugh MJ, Wonisch A, Grill D. Multivariate patterns of biochemical responses of Pinus ponderosa trees at field plots in the San Bernardino Mountains, southern California. Tree Physiol. 2001;21:329–336. doi: 10.1093/treephys/21.5.329. [DOI] [PubMed] [Google Scholar]
- Trebst A, Depka B, Holländer-Czytko H. A specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett. 2002;516:156–160. doi: 10.1016/s0014-5793(02)02526-7. [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu J. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]