Abstract
An analysis was made of progressive changes in patterns of cavitation in the sapwood of three species of conifer (Larix kaempferi, Abies sachalinensis, and Picea jezoensis) that were growing in a sub-frigid zone. In all three conifers, all tracheids of the newly forming outermost annual ring were filled with water or cytoplasm during the period from May to August. However, many tracheids in the transition zone from earlywood to latewood lost water in September, presumably through drought-induced cavitation. Cavitated tracheids tended to be continuously distributed in a tangential direction. Subsequently, some earlywood tracheids of the outermost annual ring lost water during the period from January to March. This was associated with freeze-thaw cycles. In the second and third annual rings from the cambium of all three conifers, the lumina of most tracheids in the transition zone from earlywood to latewood contained no water. In contrast, some latewood tracheids near the annual ring boundary and many earlywood tracheids retained water in their lumina. The third annual ring had more cavitated tracheids than the second annual ring. Our observations indicated that cavitation progressed gradually in the tracheids of the conifers and that they were never refilled once cavitation had occurred. The region involved in water transport in conifers did not include the entire sapwood and differed among annual rings.
In trees, water is transported from roots to leaves through the xylem conduit of the stem. However, not all conduits in the stem do function in the transport of water. The water that is in a metastable state in conduits of transpiring plants renders these conduits vulnerable to the formation of cavities by a process known as cavitation. A cavitated conduit is filled primarily with vapor and is eventually filled with air. A conduit in such an air-filled state is considered to be embolized and no longer available for water transport (Zimmermann, 1983; Sperry et al., 1988a). In general, cavitation is induced by water stress, which is caused by excessive transpiration from leaves or by a decrease in soil water potential, or by freezing of xylem sap, when the ambient temperature falls in winter (Tyree and Sperry, 1989; Sperry, 1993; Utsumi et al., 1999).
In dicotyledonous trees, water is transported mainly through vessels that are composed of vessel elements. Vessel elements are connected axially via perforations, and they make contact laterally with walls of adjacent vessel elements via bordered pits. In diffuse-porous dicotyledonous trees, in which vessels are scattered in annual rings, several outer annual rings function within water transport (Greenidge, 1958). In contrast, in ring-porous trees, which have large earlywood vessels near the annual ring boundary, a great deal of water is transported exclusively via the earlywood vessels of the outermost annual ring. In ring-porous trees of northern temperate zones, the earlywood vessels of the outermost annual ring lose their ability to transport water during the period from autumn to winter as a result of freezing of the xylem sap (Sperry et al., 1994; Utsumi et al., 1996, 1999). In ring-porous trees, the refilling of earlywood vessels does not occur in the following spring. In some diffuse-porous trees, vessels that have gradually become cavitated in winter are at least partially refilled in spring, before the onset of transpiration, in several outer annual rings (Utsumi et al., 1996, 1998). Therefore, in dicotyledonous trees, the region that functions in water transport depends on the anatomical features of the vessels.
In conifers, water transport in the stem occurs in the sapwood, which includes living parenchyma and is located in the outer part of the xylem (Zimmermann and Brown, 1971; Zimmermann, 1983). Conifers have tracheids, which have no perforations but make contact with neighboring tracheids via pairs of intertracheary bordered pits for the transport of water from the soil to the leaves. Water in one tracheid is transferred to the next tracheid via pairs of bordered pits. An annual ring consists of earlywood, in which tracheids have large diameters and thin cell walls, and latewood, in which tracheids have narrow diameters and thick cell walls. Dye injection studies of pathways of water transport in conifers suggest that the entire sapwood might not be involved in water transport. In these studies, some of the outer annual rings (Rudinsky and Vitè, 1959; Kozlowski and Winget, 1963; Kozlowski et al., 1967) and the earlywood tracheids in each annual ring (Harris, 1961; Kozlowski et al., 1965, 1966) were generally stained by a solution of dye. However, it remains uncertain whether or not all earlywood tracheids of the outer annual rings function in water transport. In addition, the timing of cavitation in earlywood and latewood tracheids has not yet been determined. Moreover, it has not yet been ascertained at the cellular level whether cavitated tracheids are refilled with water, although it has been shown that moisture content changes seasonally (Clark and Gibbs, 1957; Gibbs, 1958; Grace, 1993), and hydraulic conductivity recovers in spring from their lower level in winter (Sperry and Sullivan, 1992; Sperry, 1993; Sperry et al., 1994).
To examine these issues, we monitored the distribution of water in the sapwood of two evergreen conifers and one deciduous conifer in situ. We analyzed the distribution of water in large areas of the xylem of sapwood by soft x-ray photography. Soft x-ray photography has been used by others to determine the distribution of water in sapwood and heartwood at the tissue level (Sano et al., 1995; Nakada et al., 1999a, 1999b; Sakamoto and Sano, 2000). In each annual ring, the distribution of water was determined precisely by cryo-scanning electron microscopy (SEM), which allows visualization of water at the cellular level (Ohtani and Fujikawa, 1990; Huang et al., 1994; Sano et al., 1995; Utsumi et al., 1996; Nijsse and Aelst, 1999; Canny et al., 2001). In this study, we attempted to clarify the seasonal and perennial changes in the water transport regions of the sapwood of these conifers at the cellular level.
RESULTS
Soft X-Ray Photography
The seasonal and perennial changes in the distribution of water in the outer six annual rings of Larix kaempferi, Abies sachalinensis, and Picea jezoensis were visualized at the tissue level by soft x-ray photography. In soft x-ray photographs, dark zones indicate relatively large amounts of water because water absorbs the X-rays. In the sapwood of the three species examined, amounts of water differed among annual rings. Outer annual rings contained large amounts of water, and inner annual rings contained less water than outer annual rings over the course of the experiment (Fig. 1).
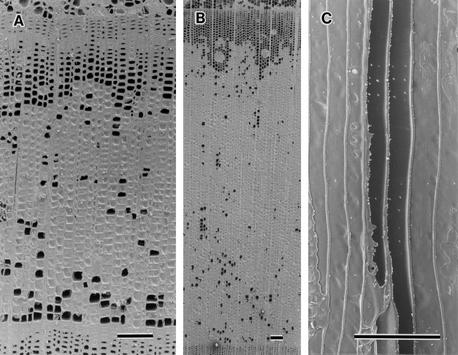
Figure 1.
Soft x-ray photographs of stems of P. jezoensis. Each number indicates the number of the annual ring, counted from the bark. The large arrow indicates the liner region with reduced amounts of water. Small arrows indicate the similar punctate regions. A, Transverse section of five outer annual rings and phloem in August. B, Transverse section of five outer annual rings and phloem in February. ph, Phloem. Bars = 2 mm.
From May to August, all tracheids of the newly forming outermost annual ring contained water in the three species examined. In the second to sixth annual rings counted from the bark, liner region with less water were seen in the outer layer of each annual ring (Fig. 1A, large arrow). In P. jezoensis, punctate regions with less water were also found in the respective middle to outer layer of the second to the sixth annual ring (Fig. 1A, small arrow).
From September to April, there was a liner region with less water in the respective outer layers of the outer six annual rings in the three species examined (Fig. 1B, large arrow). In A. sachalinensis, samples from one tree in September and in November exceptionally revealed the presence of water in all tracheids of the outermost annual ring. In P. jezoensis, punctate regions with less water were visible in the middle to outer layer of each annual ring (Fig. 1B, small arrow).
Analysis by Cryo-SEM
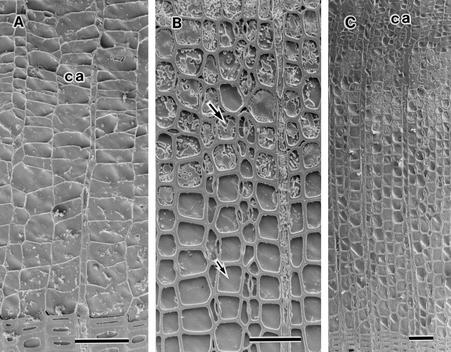
The seasonal and perennial changes in the distribution of water in the outer three annual rings of the three conifers were visualized at the cellular level by cryo-SEM. In all three conifers, all tracheids of the newly forming outermost annual ring were filled with water or cytoplasm from May to August. In May, formation of new xylem had already started. The differentiating earlywood tracheids of the outermost annual ring and cambial cells were filled with cytoplasm (Fig. 2A). The formation of the outermost annual ring progressed during June and July. The cytoplasm and water in tracheid lumina could be distinguished by features of cut surfaces. Figure 2B shows a portion of the outermost annual ring of A. sachalinensis in July. The cut surface of cytoplasm was not flat (Fig. 2B, large arrow) because the freeze-etching treatment before observations generated differences in levels between water and other materials as a result of sublimation. In contrast, the cut surface of water was relatively even (Fig. 2B, small arrow). All tracheids of the outermost annual ring were also filled with water or cytoplasm in August (Fig. 2C).
Figure 2.
Cryo-SEM photographs of A. sachalinensis, P. jezoensis, and L. kaempferi. A, Transverse surface of the outermost annual ring of P. jezoensis in May. The lumina of all tracheids are filled with water or cytoplasm. B, Transverse surface of the earlywood tracheids of the outermost annual ring of A. sachalinensis in July. The large arrow indicates cytoplasm in the lumen of a tracheid. The small arrow indicates water in the tracheid lumen. C, Transverse surface of the outermost annual ring of L. kaempferi in August. The lumina of all tracheids are filled with water and cytoplasm. ca, Cambial zone. Bars = 50 μm.
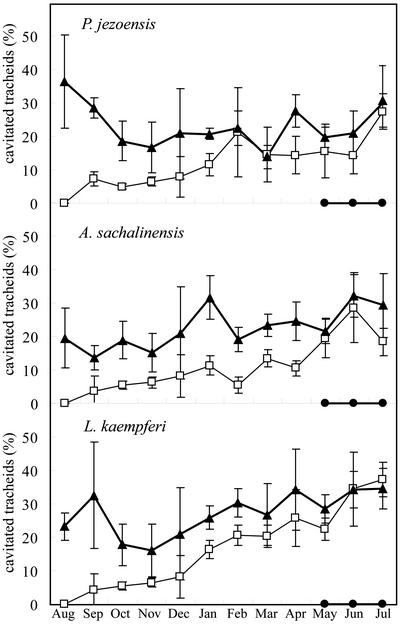
In September in all three conifers, many tracheids in the transition zone from earlywood to latewood of the outermost annual ring had lost water, whereas the lumina of tracheids in the latewood near the cambial zone and earlywood tracheids retained water. All earlywood tracheids were filled with water until December in A. sachalinensis and P. jezoensis, and until February in L. kaempferi. Figure 3A shows the distribution of water in the outer zone of the outermost annual ring of P. jezoensis in September. Tracheids of latewood near the cambial zone had thick cell walls (Fig. 3A, large arrow), and their lumina contained water. Many tracheids in the transition zone from earlywood to latewood contained no water (Fig. 3A, small arrow). In one unusual specimen of A. sachalinensis, sampled in September and in November, water remained in the lumina of all tracheids of the outermost annual ring. The percentage of cavitated tracheids of the outermost annual ring tended to increase gradually from September to January in all three conifers (Fig. 4).
Figure 3.
Cryo-SEM photographs of P. jezoensis and L. kaempferi. A, Transverse surface of the outermost annual ring of P. jezoensis in September. The latewood tracheids have thick cell walls (large arrow). Some tracheids in the transition zone from earlywood to latewood (small arrow) contain no water. B, Transverse surface of the outermost annual ring of L. kaempferi in January. Some tracheids in the transition zone from earlywood to latewood (arrow) contain no water. C, Transverse surface of the outermost annual ring of L. kaempferi in January. All earlywood tracheids are filled with water. Arrows indicate materials in the tracheid lumina. D, Transverse surface of the outermost annual ring of P. jezoensis in January. The arrow indicates a resin canal. E, Transverse surface of the outermost annual ring of P. jezoensis in January. The tracheids adjacent to the resin canal (re) contain no water. The small arrow indicates an epithelial cell. The large arrow indicates a water-filled tracheid. F, Transverse surface of the earlywood tracheids of the outermost annual ring of P. jezoensis in January. Some earlywood tracheids have no water. ca, Cambial zone. Bars = 50 μm.
Figure 4.
Seasonal changes in percentage of cavitated tracheids in P. jezoensis, A. sachalinensis, and L. kaempferi from August 1999 to July 2000. Mean for four areas of radial files in the annual rings that were formed in 1998 (solid triangles), 1999 (white squares), and 2000 (solid circles) are shown with 95% confidence intervals. The formation of new tracheids started until May 2000.
In January, the distribution of water in the outermost annual rings in A. sachalinensis and P. jezoensis was a similar to the distribution of water in the second annual ring during the growing season. In L. kaempferi, a similar change in the distribution of water in the outermost annual ring was observed in March. Figure 3, B and C, show the outermost annual ring of L. kaempferi in January. The lumina of latewood tracheids near the cambial zone and of earlywood tracheids retained water. In contrast, some tracheids in the transition zone from earlywood to latewood contained no water (Fig. 3B, arrow). Figure 3C shows that some materials (arrows) were present in the tracheid lumina of the earlywood that was adjacent to ray parenchyma. In January, the lumina of some earlywood tracheids of the outermost annual ring of A. sachalinensis and P. jezoensis no longer contained water. In L. kaempferi, some tracheid lumina of earlywood tracheids contained no water in March. Figures 3, D to F, show the outermost annual ring of P. jezoensis in January. Some earlywood tracheids of the outermost annual ring contained no water (Fig. 3F).
In January, we noted an additional pronounced change in P. jezoensis: Water was lost from the lumina of tracheids around axial resin canals (Fig. 3, D and E). Each resin canal was surrounded by epithelial cells (Fig. 3E, small arrow) and contained resin (Fig. 3E, re). The cut surface of the resin was flat and appeared to have undergone less sublimation than the cut surface of the water that filled the tracheid lumina (Fig. 3E, large arrow). This phenomenon was not detected in L. kaempferi even though axial resin canals were present in the xylem just as they were in P. jezoensis. The discrepancy might be attributable to the difference in terms of location between the axial resin canals of the two species. In L. kaempferi, the location of axial resin canals corresponded to the transition zone from earlywood to latewood, in which many tracheids had already undergone cavitation in early autumn.
In the second and third annual rings, counted from the bark, there were no obvious seasonal changes in the pattern of distribution of water in the three species examined. Percentage of cavitated tracheids of the second annual ring was nearly larger than that of the outermost annual ring over the course of the experiment in all three conifers (Fig. 4). In the second and third annual rings, most tracheid lumina in the transition zone from earlywood to latewood were not filled with water. In contrast, the lumina of some latewood tracheids near the annual ring boundary and many earlywood tracheids retained water. Figure 5A shows the third annual ring from the bark of P. jezoensis in August. Many tracheids in the transition zone from earlywood to latewood contained no water, but some latewood tracheids and many earlywood tracheids were filled with water. The earlywood tracheids near the annual ring boundary contained less water than other parts of earlywood tracheids. A similar distribution of water in annual ring was observed during the growing season (Fig. 5A) and the dormant season (Fig. 5B). Figure 5C shows the radial surface of the third annual ring of P. jezoensis in February. Some tracheids were completely filled with water, whereas other neighboring tracheids contained none at all.
Figure 5.
Cryo-SEM photographs of P. jezoensis. A, Transverse surface of the second annual ring, counted from the bark, in August. B, Transverse surface of the second annual ring, counted from the bark, in February. C, Radial surface of earlywood tracheids of the third annual ring in February. Bars = 100 μm.
The seasonal changes in the distribution of water in the three annual rings of P. jezoensis are shown schematically in Figure 6.
Figure 6.
A schematic representation of the seasonal changes in the distribution of water in the outer three annual rings in P. jezoensis. The double-headed arrow indicates the outermost annual ring.
DISCUSSION
The present study demonstrated that the loss of water from the newly formed annual rings occurs in two phases. The first phase occurs in the transition zone from earlywood to latewood soon after the formation of the xylem in late summer or early autumn. As a result of this process, cavitated tracheids tend to be distributed continuously in a tangential direction. The second phase occurs in the earlywood in winter. During this phase, cavitated tracheids appear sporadically in the earlywood of all the three species examined. In P. jezoensis, cavitation also occurs in the tracheids that surround the resin canals that are located in the middle to outer zone of the annual ring. To our knowledge, these patterns of the temporal progression of cavitation in coniferous trees have not been reported previously.
The cavitation that occurred in the newly formed annual ring before late winter seems likely to be irreversible. The distribution of cavitated tracheids in the second and third annual rings was similar to that in the outermost annual ring in late winter even though the percentage of the cavitated tracheids was larger in the inner annual rings than in the outermost annual ring (Figs. 1 and 4). There were no apparent changes in the pattern of distribution of cavitated tracheids in the second and third annual rings during the course of the present study. It is unlikely that cavitated tracheids are refilled with water in the sapwood.
In September, many tracheids in the transition zone from earlywood to latewood in the outermost annual ring lost water (Figs. 1A and 3A). This disappearance of water might have been due to cavitation induced by water stress. During the growing season, mature tracheids of the outermost annual ring were filled with water and were exposed to the water stress that causes cavitation as a result of leaf transpiration. However, even if cavitation occurs in one tracheid, the functional tracheids adjacent to the embolized tracheid can avoid the entry of gas: The water-conducting tracheids can be sealed off from the cavitated tracheids by pit aspiration (Zimmermann, 1983). In conifers, the intertracheary pit membrane consists of a centrally thickened torus and a porous region that surrounds the torus, known as the margo. The pit membrane is displaced to one side of the pit border and the pit aperture is occluded by the torus if the pressure between the two tracheids becomes significantly different, and the pit membrane has sufficiently flexibility. If the bordered pits of all tracheids have thin and flexible pit membranes, the progression of cavitation can be avoided during the growing season. However, the structures of intertracheary pit membranes in earlywood and latewood are very different (Ishida and Fujikawa, 1970; Fujikawa and Ishida, 1972; Butterfield and Meylan, 1980). In Pinus sylvestris, the fibrillar texture of the margo of latewood is much denser than that of earlywood (Bauch et al., 1972). Furthermore, the frequency of the bridge between the torus and the periphery of the pit membrane, which is defined as an extended torus, increases from earlywood to latewood in A. sachalinensis (Sano et al., 1999). Therefore, the bordered pit membranes of tracheids in the transition zone from earlywood to latewood might be too rigid to allow closure of the aperture. Most of the bordered pits of tracheids in the transition zone from earlywood to latewood are located in the radial wall. Large-scale cavitation would be facilitated in the tangential direction when such tracheids are exposed to water stress (Fig. 1).
Some latewood tracheids retained water for several years (Fig. 5). In A. sachalinensis, the bordered pit membrane of the last few latewood tracheids have no or little visible openings (Sano et al., 1999). Low permeability of such bordered pit membranes would contribute to prevent gas entry from the once cavitated tracheid to the adjacent water-filled tracheid by air seeding. Moreover, the diameter of the lumina of latewood tracheids is smaller than that of tracheids in the transition zone from earlywood to latewood. It is likely that strong capillary pressure in the lumina of latewood tracheids allows retention of water in these lumina for several years. In addition, latewood tracheids at the annual ring boundary are connected to the initial earlywood tracheids of the next annual ring by pit pairs in their tangential walls. Water might move from earlywood to latewood through the annual ring boundary as a result of differences in capillary pressure between the lumina of earlywood and latewood tracheids.
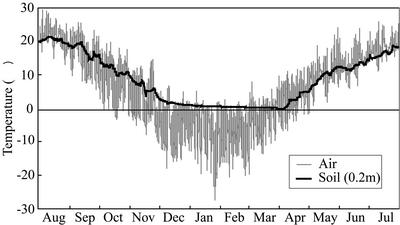
In all three conifers examined, cavitation occurred in some of the earlywood tracheids of the outermost annual ring during the winter (Fig. 3F). In dicotyledonous trees, a cycle of freezing and thawing induces loss of hydraulic conductivity in the xylem (LoGullo and Salleo, 1993; Langan et al., 1997; Pockman and Sperry, 1997) and disappearance of water from vessel lumina (Utsumi et al., 1999). When the xylem sap in conduits freezes, air bubbles appear as a result of the difference between the solubility of air in water and in ice. After the ice has melted, the retained air bubbles expand in conduits if tension forces are generated in the vascular system (Tyree and Sperry, 1989; Sperry, 1993). In our study area, the air temperature fell below −10°C and rose above 0°C on several occasions from December to March (Fig. 7). In addition, the large-diameter conduit is more susceptible to cavitation by freeze-thaw stress than small one (Davis et al., 1999). Therefore, freezing and thawing of water during the winter probably resulted in cavitation in some earlywood tracheids, which have larger diameter than latewood tracheids, of the outermost annual rings of these conifers that we studied.
Figure 7.
Changes in air and soil temperatures from August 1999 to July 2000. Soil temperatures were measured 20 cm beneath the surface of the soil.
The seasonal changes in water distribution in the second and third annual rings were not clearly discernable. However, the proportion of the cavitated tracheids was larger in the fourth annual ring than in the third annual ring during the period of our experiment. Thus, cavitation induced by freezing and thawing might have occurred in the second and third annual rings during the winter. Dicotyledonous trees experience considerable decreases in hydraulic conductivity during the winter (Sperry et al., 1988b, 1994; Tognetti and Borghetti, 1994; Magnani and Borghetti, 1995), and the loss of hydraulic conductivity in conifers is less than that in dicotyledonous trees in wintertime (Sperry and Sullivan, 1992; Sperry, 1993). However, earlywood tracheids were cavitated by the stress of freezing and thawing in our experiment. The difference in the extent of the loss of hydraulic conductivity between conifers and dicotyledonous trees during the winter might result from the lower vulnerability of tracheids than vessels to freeze-thaw-induced cavitation, even if all tracheids and vessels are at risk of cavitation when expose to freeze-thaw stress.
The timing of cavitation during the winter differed among the three species examined. In both P. jezoensis and A. sachalinensis, there was no water in some earlywood tracheids of the outermost annual ring in January, whereas earlywood tracheids of L. kaempferi lost water from their lumina in March. L. kaempferi is a deciduous tree that loses its leaves in November, with leaf expansion in May, at our study site. Xylem water pressure in winter might be less negative in L. kaempferi than in the other two species.
In P. jezoensis, tracheids adjacent to resin canals contained no water (Fig. 3E). It was reported that the injection of hydrophobic materials into stems of a conifer (Pinus thunbergii Parl.) promoted the development of tracheid embolisms (Kuroda, 1991). Therefore, hydrophobic materials in resin canals or epithelial cells might facilitate cavitation.
In all three conifers examined, some materials were present in the lumina of tracheids adjacent to ray parenchyma (Fig. 3C). This observation indicates that a pathway for the transport of water and certain materials exists between ray parenchyma and adjacent tracheids. The radial transport of water via tracheids would be interrupted by the transition zone from earlywood to latewood, where there is no water in most tracheid lumina (Fig. 5). However, water would be transported axially through the tracheids of several outer rings of the stem and, finally, to the outermost annual ring, which is connected to leaves. Ray cells might play an important role in radial transport of water across several annual rings. In P. jezoensis and L. kaempferi, a ray consists of ray parenchyma cells and ray tracheids, which have bordered pits. Such ray tracheids in P. jezoensis and L. kaempferi also might be a pathway for the radial transport of water.
The cross-sectional area of sapwood is a simple biometric parameter that is widely used for extrapolating the transpiration data from trees to forest stands (Èermák and Nadezhdina, 1998). However, our results show that regions involved in water transport are not equal to the cross-sectional area of sapwood in conifers. In the outermost annual ring, water transport occurs mainly in mature water-filled earlywood tracheids. In the second and the third annual rings, many, but not all, earlywood tracheids function in water transport. The tracheids in the transition zone from earlywood to latewood cannot transport water because their lumina contain little water. Latewood tracheids near the annual ring boundary also play a minimal role in water transport because these small diameter tracheids retain water for its capillary pressure. Thus, water transport occurs mainly in earlywood tracheids, whereas latewood tracheids and tracheids in the transition zone from earlywood to latewood play no role or only a minor role in water transport. Previous studies have demonstrated radial variations in sap velocity in tree trunks (Èermák et al., 1992; Èermák and Nadezhdina, 1998, Nadezhdina et al., 2002). To characterize water transport in whole trees more accurately, further precise studies of water transport regions and radial variations in sap velocity at cellular level are now required.
MATERIALS AND METHODS
Plant Materials and Sampling
Trees of 8-year-old Abies sachalinensis (Fr. Schm.) Masters (height, 4 m; diameter at breast height, 4 cm), 12-year-old Picea jezoensis (Sieb. et Zucc.) Carr. (height, 7 m; diameter at breast height, 6 cm), and 9- to 13-year-old Larix kaempferi (Lamb.) Carr. (height, 6–7 m; diameter at breast height, 5–6 cm), grown in the Tomakomai Experimental Forest of Hokkaido University (Tomakomai, Hokkaido, Japan) were used in this study. Two trees of each species were felled monthly from August 1999 to July 2000.
Cylindrical samples of stems were collected from living trees at breast height after the xylem sap had been fixed by freezing of stems by a previously described procedure (Utsumi et al., 1996, 1998, 1999). In brief, watertight collars were made with plastic funnels to serve as containers for liquid nitrogen (LN2). The funnels were fitted to the sample stems, and then the collars were filled with LN2. The stems were allowed to freeze for approximately 10 min. Cochard et al. (2000) noted the possibility of artificial cavitation when freezing with LN2 is performed at a low xylem water potential. To avoid this possibility, samples were collected before sunrise, when the xylem water potential was high. Frozen stems were immediately removed from the sample trees and stored in a container with LN2.
Soft X-Ray Photography
Sample stems that had been stored in LN2 were transferred to a low-temperature room maintained at −20°C. Once the samples stems had equilibrated to −20°C, they were cut with a hand saw into transverse sections of about 2 mm in thickness in the low-temperature room. The sample discs were immersed in LN2 in the low-temperature room and transferred to an x-ray apparatus (Super Soft, Koizumi X-ray Co. Ltd., Tokyo). Frozen samples were placed on a film case in which an x-ray film (x-ray FR, Fuji Photo Film Co. Ltd., Tokyo) had been mounted and then irradiated at 20 kV and 5 mA for 120 s from a distance of 1.3 m (Sano et al., 1995). The negative films were enlarged for examination of the distribution of water.
Analysis by Cryo-SEM
The samples of frozen stems were transferred to a low-temperature room kept at −20°C and were equilibrated at −20°C. They were cut into small blocks (5 × 5 × 5 mm3) that included part of the xylem, cambial cells, and phloem. Between one and four blocks from each of stem sample were examined. Transverse, tangential, or radial surfaces of each block were cut cleanly with the steel blade of a sliding microtome (Yamato Koki, Tokyo) to expose cell lumina (Sano et al., 1993, 1995). Then, the blocks were attached to specimen holders with a drop of glycerol and fastened with a screw. The specimen holder with the sample, immersed in LN2, was transferred to a system for cryo-SEM (JSM840-a, JEOL, Tokyo) that was equipped with a freeze-etching unit. The holder and sample were fixed on the cold stage of the freeze-etching unit, which was maintained under a vacuum of approximately 1 × 10−4 Pa and equilibrated at −95°C. The specimen was freeze etched under these conditions for about 10 min to eliminate contamination by frost, and then it was rotary shadowed with a platinum-carbon pellet. The sample was transferred to the cold stage of the SEM, which was maintained at approximately −160°C, and secondary electron images were observed and recorded at an accelerating voltage of 5 kV (Fujikawa et al., 1988; Utsumi et al., 1996, 1998, 1999).
For quantitative evaluation of the occurrence of cavitation, two areas of radial files of the outer two to three annual rings were selected at random from the cryo-SEM photographs of each sample tree. One area consisted of four radial files and contained more than 100 tracheids in each annual ring. The number of cavitated tracheids was counted, and the percentage of cavitated tracheids was determined in every areas. In P. jezoensis and L. kaempferi, the radial files that were not adjacent to resin canal were selected.
Environmental Temperatures
To investigate the relationship between freezing stress and cavitation, we obtained daily air temperatures and the temperature of the soil 20 cm below the surface of the ground from data recorded in the Tomakomai Experimental Forest from August 1999 to July 2000.
ACKNOWLEDGMENTS
The authors thank Dr. Tsutomu Hiura for permission to collect sample trees and to access the weather data in Tomakomai Experimental Forest (Forest Research Station, Field Science Center for Northern Biosphere, Hokkaido University, Japan). The authors also thank Dr. Keita Arakawa for allowing use of the low-temperature room in Institute of Low Temperature Science (Hokkaido University).
Footnotes
This work was supported by the Japanese Society for the Promotion of Science Research Fellowships for Young Scientists.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.014795.
LITERATURE CITED
- Bauch J, Liese W, Schultze R. The morphological variability of the bordered pit membranes in gymnosperms. Wood Sci Technol. 1972;6:165–184. [Google Scholar]
- Butterfield BG, Meylan BA. Three-Dimensional Structure of Wood. Ed 2. London: Chapman and Hall; 1980. [Google Scholar]
- Canny MJ, McCully ME, Huang CX. Cryo-scanning electron microscopy observations of vessel content during transpiration in walnut petioles: facts or artifacts? Plant Physiol Biochem. 2001;39:555–563. doi: 10.1104/pp.124.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Èermák J, Cienciala E, Kucer J, Hällgren J. Radial velocity profiles of water flow in trunks of Norway spruce and oak and the response of spruce to severing. Tree Physiol. 1992;10:367–380. doi: 10.1093/treephys/10.4.367. [DOI] [PubMed] [Google Scholar]
- Èermák J, Nadezhdina N. Sapwood as the scaling parameter: defining according to xylem water content or radial pattern of sap flow? Ann Sci For. 1998;55:509–521. [Google Scholar]
- Clark J, Gibbs RD. Studies in tree physiology: further investigations of seasonal changes in moisture content of certain Canadian forest trees. Can J Bot. 1957;35:219–253. [Google Scholar]
- Cochard H, Bodet C, Améglio T, Cruiziat P. Cryo-scanning electron microscopy observations of vessel content during transpiration in walnut petioles: fact or artifacts? Plant Physiol. 2000;124:1191–1202. doi: 10.1104/pp.124.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SD, Sperry JS, Hacke UG. The relationship between xylem conduit diameter and cavitation caused by freezing. Am J Bot. 1999;86:1367–1372. [PubMed] [Google Scholar]
- Fujikawa S, Ishida S. Study on the pit of wood cells using scanning electron microscopy: structural variation of bordered pit membrane on the radial wall between tracheids in Pinaceae species. Mokuzai Gakkaishi. 1972;18:477–483. [Google Scholar]
- Fujikawa S, Suzuki T, Ishikawa T, Sakurai S, Hasegawa Y. Continuous observation of frozen biological materials with cryo-scanning electron microscope and freeze-replica by a new cryo-system. J Electron Microsc. 1988;37:315–322. [PubMed] [Google Scholar]
- Gibbs RD. Patterns in the seasonal water content of trees. In: Thimann KV, editor. The Physiology of Forest Trees. New York: Ronald Press; 1958. pp. 43–69. [Google Scholar]
- Grace J. Consequences of xylem cavitation for plant water deficits. In: Smith JAC, Griffiths H, editors. Water Deficits. Oxford: BIOS Science Publishers; 1993. , pp109–145. [Google Scholar]
- Greenidge KNH. Rates and patterns of moisture movement in trees. In: Thimann KV, editor. The Physiology of Forest Trees. New York: Ronald Press; 1958. pp. 19–41. [Google Scholar]
- Harris JM. Water-conduction in the stems of certain conifers. Nature. 1961;25:678–679. [Google Scholar]
- Huang CH, Canny MJ, Oates K, McCully M. Planting frozen hydrated plant specimens for SEM observation and EDX microanalysis. Microsc Res Technol. 1994;28:67–74. doi: 10.1002/jemt.1070280108. [DOI] [PubMed] [Google Scholar]
- Ishida S, Fujikawa S. Study on the pit of wood cells using scanning electron microscopy. Report 2. Pit membrane of the tracheid bordered pit in a living tree trunk of Todomatsu, Abies sachalinensis Fr. Schm Res Bull Coll Exp Forest Coll Agric Hokkaido Univ. 1970;27:355–372. [Google Scholar]
- Kozlowski TT, Hughes JF, Leyton L. Patterns of water movement in dormant gymnosperm seedlings. Biorheology. 1966;3:77–85. [Google Scholar]
- Kozlowski TT, Hughes JF, Leyton L. Movement of injected dyes in gymnosperm stems in relation to tracheid alignment. Forestry. 1967;40:207–219. [Google Scholar]
- Kozlowski TT, Leyton L, Hughes JF. Pathways of water movement in young conifers. Science. 1965;205:830. [Google Scholar]
- Kozlowski TT, Winget CH. Patterns of water movement in forest trees. Bot Gaz. 1963;124:301–311. [Google Scholar]
- Kuroda K. Mechanism of cavitation development in the pine wilt disease. Eur J Forest Pathol. 1991;21:82–89. [Google Scholar]
- Langan SJ, Ewers FW, Davis SD. Xylem dysfunction caused by water stress and freezing in two species of co-occurring chaparral shrubs. Plant Cell Environ. 1997;20:425–437. [Google Scholar]
- LoGullo MA, Salleo S. Different vulnerabilities of Quercus ilex L. to freeze- and summer drought-induced xylem embolism: an ecological interpretation. Plant Cell Environ. 1993;16:511–519. [Google Scholar]
- Magnani F, Borghetti M. Interpretation of seasonal changes of xylem embolism and plant hydraulic resistance in Fagus sylvatica. Plant Cell Environ. 1995;18:689–696. [Google Scholar]
- Nadezhdina N, Èermák F, Ceulemans R. Radial patterns of sap flow in woody stems of dominant and understory species: scaling errors associated with positioning of sensors. Tree Physiol. 2002;22:907–918. doi: 10.1093/treephys/22.13.907. [DOI] [PubMed] [Google Scholar]
- Nakada R, Fujisawa Y, Hirakawa Y. Soft X-ray observation of water distribution in the stem of Cryptomeria japonica D. Don: I. general description of water distribution. J Wood Sci. 1999a;45:188–193. [Google Scholar]
- Nakada R, Fujisawa Y, Hirakawa Y. Soft X-ray observation of water distribution in the stem of Cryptomeria japonica D. Don: II. Types found in wet-area distribution patterns in transverse sections of the stem. J Wood Sci. 1999b;45:194–199. [Google Scholar]
- Nijsse J, Aelst AC. Cryo-planting for cryo-scanning electron microscopy. Scanning. 1999;21:372–378. doi: 10.1002/sca.4950210603. [DOI] [PubMed] [Google Scholar]
- Ohtani J, Fujikawa S. Cryo-SEM observation on vessel lumina of a living tree: Ulmus davidiana var. japonica. Inter Assoc Wood Anatom Bull n s. 1990;11:183–194. [Google Scholar]
- Pockman WT, Sperry JS. Freezing-induced xylem cavitation and the northern limit of Larrea tridentata. Oecologia. 1997;109:19–27. doi: 10.1007/s004420050053. [DOI] [PubMed] [Google Scholar]
- Rudinsky JA, Vitè JP. Certain ecological and phylogenetic aspects of the pattern of water conduction in conifers. Forest Sci. 1959;5:259–266. [Google Scholar]
- Sakamoto Y, Sano Y. Inhibition of water conductivity caused by watermark disease in Salix sachalinensis. Inter Assoc Wood Anatom J. 2000;21:49–60. [Google Scholar]
- Sano Y, Fujikawa S, Fukazawa K. Studies on mechanisms of frost crack formation in tree trunks. Jpn J Freezing Drying. 1993;39:13–21. [Google Scholar]
- Sano Y, Fujikawa S, Fukazawa K. Detection and features of wetwood in Quercus mongolica var. grosseserrata. Trees. 1995;9:261–268. [Google Scholar]
- Sano Y, Kawakami Y, Ohtani J. Variation in the structure of intertracheary pit membranes in Abies sachalinensis, as observed by field emission scanning electron microscopy. Inter Assoc Wood Anatom J. 1999;20:375–388. [Google Scholar]
- Sperry JS. Winter xylem embolism and spring recovery in Betula cordifolia, Fagus grandifolia, Abies balsamea and Picea rubens. In: Borghetti M, Grace J, Raschi A, editors. Water Transport in Plants under Climatic Stress. Cambridge, UK: Cambridge University Press; 1993. pp. 86–98. [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. A method of measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 1988a;11:35–40. [Google Scholar]
- Sperry JS, Donnelly JR, Tyree MT. Seasonal occurrence of xylem embolism in sugar maple (Acer saccharum) Am J Bot. 1988b;75:1212–1218. [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE. Xylem embolism in ring-porous, diffuse-porous and coniferous trees of northern Utah and interior Alaska. Ecology. 1994;75:1736–1752. [Google Scholar]
- Sperry JS, Sullivan JEM. Xylem embolism in response to freeze-thaw cycles and water stress in ring-porous, diffuse-porous, and conifer species. Plant Physiol. 1992;100:605–613. doi: 10.1104/pp.100.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti R, Borghetti M. Formation and seasonal occurrence of xylem embolism in Alnus cordata. Tree Physiol. 1994;14:241–250. doi: 10.1093/treephys/14.3.241. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Phys Mol Bio. 1989;40:19–38. [Google Scholar]
- Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J. Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol. 1998;117:1463–1471. doi: 10.1104/pp.117.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y, Sano Y, Funada R, Fujikawa S, Ohtani J. The progression of cavitation in earlywood vessels of Fraxinus mandshurica var. japonica during freezing and thawing. Plant Physiol. 1999;121:897–904. doi: 10.1104/pp.121.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi Y, Sano Y, Ohtani J, Fujikawa S. Seasonal changes in the distribution of water in the outer growth rings of Fraxinus mandshurica var. japonica: a study by cryo-scanning electron microscopy. Inter Assoc Wood Anatom J. 1996;17:113–124. [Google Scholar]
- Zimmermann MH. Xylem Structure and the Ascent of Sap. Berlin: Springer-Verlag; 1983. [Google Scholar]
- Zimmermann MH, Brown CL. Trees. Structure and Function. Berlin: Spring-Verlag; 1971. [Google Scholar]