Abstract
Phosphoenolpyruvate carboxykinase (PEPCK) catalyzes the conversion of oxaloacetate to phosphoenolpyruvate in the gluconeogenic production of sugars from storage oil in germinating oilseeds. Here, we present the results of analysis on PEPCK antisense Arabidopsis plants with a range of enzyme activities from 20% to 80% of wild-type levels. There is a direct correlation between enzyme activity and seedling establishment during early post-germinative growth, thus demonstrating the absolute requirement of PEPCK and gluconeogenesis in this process. Soluble sugar levels in the 35S-PCK1 antisense seedlings are reduced and seedling establishment can be rescued with an exogenous supply of sucrose. We observed an increase in the respiration of acetyl coenzyme A units released from fatty acid β-oxidation and a corresponding decrease in the production of sugars with decreasing enzyme activity in 2-d-old antisense seedlings. The 35S-PCK1 antisense lines have a more extreme phenotype when compared with Arabidopsis mutants disrupted in the glyoxylate cycle. We conclude that the 35S-PCK1 antisense seedlings are compromised in the ability to use both storage lipid and storage protein through gluconeogenesis to produce soluble sugars.
In oilseed plants, ATP-dependent phosphoenolpyruvate carboxykinase (PEPCK; EC 4.1.1.49) is proposed to have a fundamental role in the provision of Suc during germination and early post-germinative seedling growth in oilseed species. Fatty acids from seed storage lipids are catabolized to acetyl CoA to produce sugars for seedling growth and development by β-oxidation, the glyoxylate cycle, and gluconeogenesis (Kornberg and Beevers, 1957; Canvin and Beevers, 1961; Beevers, 1980).
There is strong evidence that PEPCK is a key enzyme in the regulation of flux through the gluconeogenic pathway in early post-germinative seedling growth. First, studies in marrow (Cucurbita pepo) have demonstrated that the maximum catalytic activity of PEPCK is higher than and changes simultaneously with the flux through the gluconeogenic pathway during post-germinative growth (Leegood and ap Rees, 1978a). Second, PEPCK and Fru 1,6-bisphosphatase (FBPase) are the only enzymes in the conversion of oxaloacetate to Glc 6-phosphate during gluconeogenesis that are substantially displaced from equilibrium in vivo (Leegood and ap Rees, 1978b). Third, studies using the PEPCK inhibitor 3-mercaptopicolinic acid demonstrated that PEPCK has a regulatory role in gluconeogenesis (Leegood and ap Rees, 1978a), with a high flux control coefficient of between 0.7 to 1.0 reported in gluconeogenic marrow cotyledons (Trevanion et al., 1995). More recently, however, Runquist and Kruger (1999) calculated the flux control coefficient for the glyoxylate cycle enzyme isocitrate lyase (ICL) in germinating castor bean (Ricinus communis) seedlings to be 0.66 ± 0.09, suggesting that the flux control coefficient for PEPCK is unlikely to be as high as previously reported. The flux through the gluconeogenic pathway is also regulated by FBPase (Leegood and ap Rees, 1978b), but the relative contributions of PEPCK and FBPase in the regulation of gluconeogenesis have yet to be determined.
Besides a role in gluconeogenesis, PEPCK fulfils additional functions in plants. The photosynthetic carbon dioxide-concentrating mechanisms found in plants with Crassulacean acid metabolism and both PEPCK-type and some NADP-malic enzyme-type C4 plants contain PEPCK activity that plays a role in decarboxylating C4 acids (Leegood et al., 1996; Walker and Leegood, 1996; Wingler et al., 1999). A broad range of C3 plant tissues also contain PEPCK activity, including cauliflower (Brassica oleracea) florets (Mazelis and Vennesland, 1957), turnip taproot (Cooper and Benedict, 1968), grape (Vitis vinifera; Ruffner and Kliewer, 1975), apple (Malus domestica), kiwifruit (Actinidia deliciosa), and aubergine (Solanum melongena; Blanke et al., 1988). Within plants, PEPCK has been located in developing seeds (Walker et al., 1999), phloem and trichome tissues, oil and resin ducts (Leegood and Walker, 1999), and ripening tomato (Lycopersicon esculentum) fruit (Bahrami et al., 2001), but the role of PEPCK in these tissues remains speculative. There is support for the involvement of PEPCK in pH stability and nitrogen and amino acid metabolism (Walker et al., 1999, 2001; Lea et al., 2001) and more recently, evidence that PEPCK plays a role in decarboxylating C4 acids via a partial C4 cycle in the vascular system of C3 plants (Hibberd and Quick, 2002).
Two PCK genes have been identified in the Arabidopsis genome, PCK1 on chromosome 4 (Munich Information Center for Protein Sequences [MIPS; http://mips.gsf.de]; MIPS code At4g37870) and PCK2 on chromosome 5 (MIPS code At5g65690). Both are predicted to encode cytosolic enzymes, with PCK1 the predominant gene expressed during early post-germinative growth (Rylott et al., 2001).
The PCK1 and PCK2 genes exhibit 80% identity at the cDNA level. The PCK1 gene shares 91% identity with a 1,509-bp cold-inducible canola (Brassica napus) PEPCK cDNA (GenBank accession no. U21745; Sáez-Vásquez et al., 1995) and 81% identity with a 2,408-bp PEPCK cDNA isolated from senescing cucumber (Cucumis sativus) cotyledons (Kim and Smith, 1994; GenBank accession no. L31899). The predicted open reading frames of PCK1 and PCK2 encode for proteins of 671 and 628 amino acids, respectively, with 71% identity. During purification, PEPCK has been shown to undergo a rapid proteolytic cleavage, with the native protein for cucumber of 74 kD cleaved to 64 kD (Walker et al., 1995). The predicted molecular masses of PCK1 and PCK2 are 73.4 and 68.7 kD, respectively.
The coordinate induction of β-oxidation and glyoxylate cycle genes during storage lipid mobilization has been well documented. The acyl-CoA oxidase (ACX3; Eastmond et al., 2000; Froman et al., 2000) and keto-acyl-CoA thiolase (PED1/KAT2; Hayashi et al., 1998; Germain et al., 2001) genes of β-oxidation are both induced transcriptionally during early post-germinative seedling growth. Transcription also plays a major role in regulating the abundance of the glyoxylate cycle enzymes. Genes encoding the glyoxylate cycle enzymes ICL and malate synthase (MS) are controlled at the level of transcription (Comai et al., 1989; Graham et al., 1990; Sarah et al., 1996) and ICL and MS are both coordinately expressed during early post-germinative growth and senescence (Gut and Matile, 1988; Comai et al., 1989). However, relatively little is known about the regulation of plant PEPCKs. In cucumber, PCK1 expression parallels MS and ICL (Kim and Smith, 1994), and more recently, coordinate expression of genes of the β-oxidation pathway, glyoxylate cycle, and gluconeogenesis have all been demonstrated during germination in Arabidopsis (Rylott et al., 2001).
Two allelic mutants in the β-oxidation keto-acyl-thiolase gene, ped1 (Hayashi et al., 1998) and kat2 (Germain et al., 2001) and the acyl-CoA oxidase gene, acx3 (Eastmond et al., 2000) have recently been characterized. Germinating seedlings of the ped1 and kat2 mutants are unable to catabolize storage lipid into acetyl CoA, and in the absence of an exogenous supply of Suc, do not establish. This failure to establish is characterized by an arrest in seedling growth, with failure of the cotyledons to expand or roots to elongate. However, icl seedlings are able to use storage lipid and establish without an exogenous supply of Suc under optimal growth conditions. Arabidopsis seeds contain approximately 25% storage protein (Baud et al., 2002), and it is likely that this contributes gluconeogenic substrate for establishment of icl seedlings.
Following the observations and conclusions drawn from both β-oxidation and glyoxylate cycle mutants, we hypothesize that gluconeogenesis would be essential for oilseed germination and seedling establishment. In this study, we have used the PCK1 gene, which is the predominant PCK gene expressed during early seedling growth, to produce 35S-PCK1 antisense Arabidopsis plants with a range of PEPCK activities to functionally characterize the role of PEPCK.
RESULTS
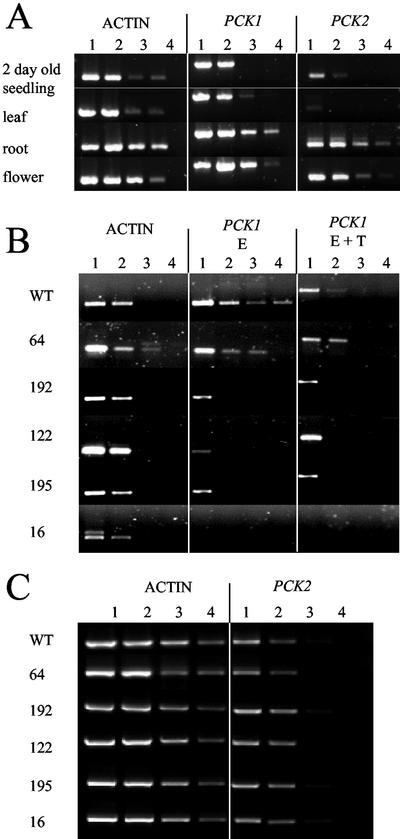
To determine the level of expression of the PCK genes in Arabidopsis, reverse transcriptase (RT)-PCR was used. Figure 1A shows that PCK1 is the predominant PCK gene expressed in 2-d-old seedlings. The PCK1 gene is also highly expressed in mature leaf, root, and flower tissue whereas PCK2 is expressed principally in root and flower.
Figure 1.
A, RT-PCR on cDNA from 2-d-old seedlings, leaf, root, and flower of untransformed wild-type Col0. Actin cDNA was amplified using primers ACT2S and ACT2A, PCK1 using PCK1-1001 and PCK1-1709, and PCK2 using PCK2-1032 and PCK2-1536. B, RT-PCR on cDNA from 2-d-old seedlings from untransformed wild-type Col0 and five 35S-PCK1 antisense lines: 64, 192, 122, 195, and 16. Endogenous (E) PCK1 cDNA only, was amplified using PCK1-72 and PCK1-573. Endogenous (E) and transgene (T) PCK1 cDNA was amplified using primers PCK1-1001 and PCK1-1709. C, PCK2 cDNA from 2-d-old seedlings was amplified using primers PCK2-1032 and PCK2-1536. PCR was carried out on undiluted (lanes 1), one-tenth (lanes 2), one-hundredth (lanes 3), and one-thousandth (lanes 4) dilutions of cDNA.
One hundred and ninety-six 35S-PCK1 antisense transformants were identified after selection on hygromycin. Of these, 60% showed a significant reduction in the level of seedling establishment in the T2 generation (data not shown). After initial characterization, five homozygous lines with T-DNA insertions at one or two loci, based on segregation analysis in the T2 and T3 generations, and representing a range of PEPCK activities were selected for further analysis (lines 16, 64, 122, 192, and 195). Analysis using RT-PCR on cDNA from 2-d-old seedlings indicated that transcript levels of PCK1 were decreased in all five lines, with line 16 showing the lowest steady-state levels of PCK1 mRNA (Fig. 1B). Transcript levels of PCK2 in 2-d-old seedlings were much lower than PCK1 (Fig. 1A), and PCK2 levels were unaltered in the 35S-PCK1 antisense lines (Fig. 1C). Primers were made to the putative cytosolic FBPase (MIPS code At1g43670) based on similarity with a confirmed cytosolic FBPase from canola reported by Strand et al. (2000). Analysis using RT-PCR showed that the transcript levels of this FBPase were unaltered in the 35S-PCK1 antisense lines (data not shown).
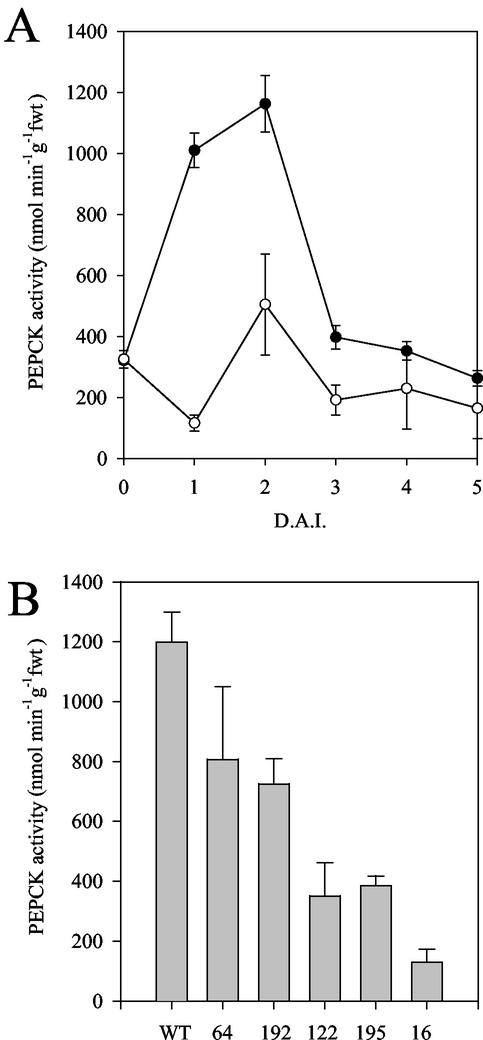
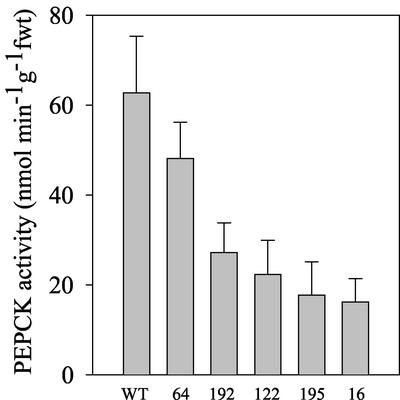
Figure 2A illustrates the peak in PEPCK activity observed in 2-d-old, untransformed, wild-type Arabidopsis seedlings. This corresponds with the peak in activity in β-oxidation and glyoxylate cycle enzymes (Germain et al., 2001; Rylott et al., 2001) and the rapid decline in storage lipid levels seen in Arabidopsis seedlings grown under these conditions (Germain et al., 2001). This peak was significantly reduced in the 35S-PCK1 antisense line 122. The decreased PEPCK activity also mirrored the pattern of transcript expression seen in the antisense lines; line 16 exhibited the lowest level of activity, <11% of untransformed, wild-type Arabidopsis in 2-d-old seedlings (Fig. 2B). These results demonstrate that the PCK1 gene is responsible for the peak in activity seen in 2-d-old seedlings.
Figure 2.
A, PEPCK activity in 0- to 5-d-old untransformed wild-type Col0 (●) and 35S-PCK1 antisense line 122 (○) seedlings. B, PEPCK activity in 2-d-old untransformed wild-type Col0 and 35S-PCK1 antisense seedlings. Seedlings were grown on one-half strength Murashige and Skoog medium supplemented with 20 mm Suc. Values are the mean ± se of three measurements made on three separate batches.
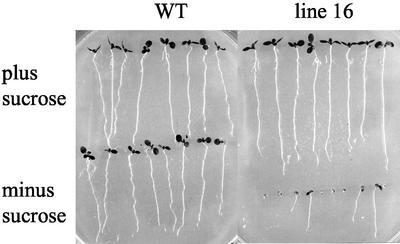
In the absence of an exogenous supply of Suc, the ability of the 35S-PCK1 antisense seedlings to establish was compromised. This was characterized by an arrest in seedling growth, with failure of the cotyledons to expand or roots to elongate. No true leaves were produced in these seedlings (Fig. 3). Seedlings of the 35S-PCK1 antisense lines that had failed to establish could be rescued by transferring to plates containing 20 mm Suc; these seedlings then grew and established normally.
Figure 3.
Untransformed wild-type Col0 and 35S-PCK1 antisense seedlings grown on one-half-strength Murashige and Skoog media with or without 20 mm Suc for 10 d at 160 μm m−2 s−1.
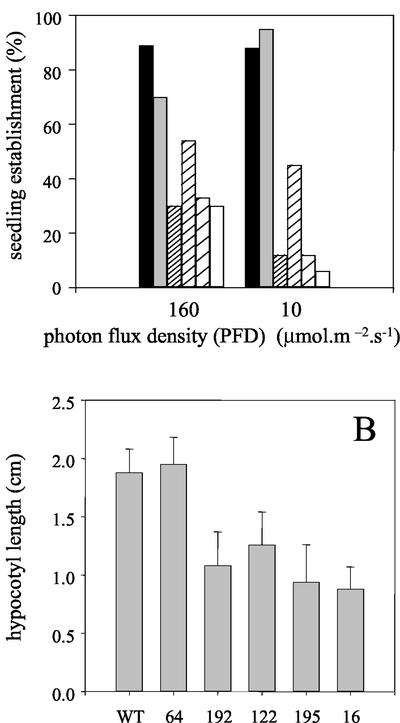
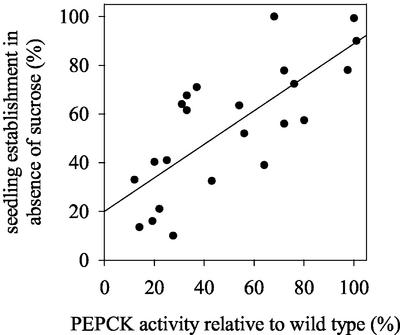
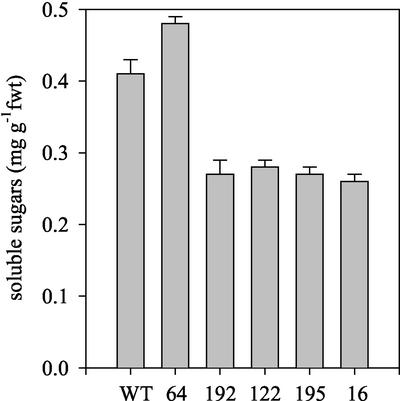
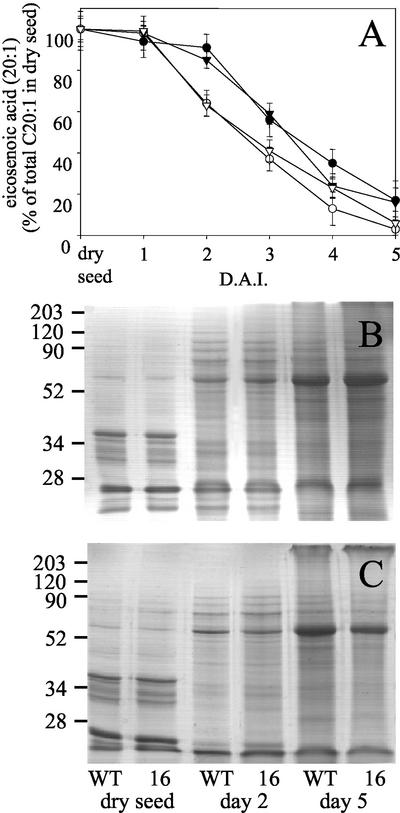
Seedling establishment, without exogenous Suc, was further compromised by a reduction in light intensity (Fig. 4A) and in the dark, hypocotyl lengths in 5-d-old 35S-PCK1 antisense seedlings were also reduced, by up to 68% in line 16 (Fig. 4B). To test the relationship between PEPCK activity and seedling establishment, measurements were made on 17 additional homozygous antisense lines to provide a more complete range of PEPCK activities. There was a statistically significant correlation between PEPCK activity and seedling establishment (r = 0.750, P < 0.01; Fig. 5). To investigate whether a decreased level of endogenous Suc in the seedlings was linked to the reduction in seedling establishment, we measured the soluble sugar levels (Glc, Fru, and Suc) in seedlings grown without an exogenous supply of Suc. As seedling growth is compromised in the antisense lines, we measured the levels of Suc in 2-d-old seedlings, when the wild-type and antisense lines are at similar developmental stages. We found a significant reduction in the levels of soluble sugars in the four lines with the lowest PEPCK enzyme activities (Fig. 6). To examine whether storage lipid catabolism was compromised in these 35S-PCK1 antisense seedlings, total fatty acid levels were analyzed using GC-based fatty acid analysis (Browse et al., 1986). All five of the 35S-PCK1 antisense lines revealed no significant alterations in the rate of storage lipid catabolism from that of untransformed Arabidopsis seedlings. Figure 7A shows that the levels of eicosenoic acid (20:1), which is specific to storage triacylglycerol (Lemieux et al., 1990), in seedlings from line 16 decreased at the same rate as wild-type seedlings both with and without Suc. The observed delay in storage lipid breakdown in the presence of Suc is in agreement with previous reports (Eastmond et al., 2000; Martin et al., 2002). In germinating castor bean seedlings, storage protein is converted to Suc via gluconeogenesis (Stewart and Beevers, 1967). To investigate whether storage protein breakdown was reduced in the antisense lines, protein extracts from germinating seedlings from wild-type and 35S-PCK1 antisense line 16 were visualized on SDS-PAGE gels (Fig. 7, B and C). The legumin-type globulins (also referred to as 12S globulin or cruciferin in Arabidopsis; Sjodahl et al., 1991) are present between approximately 45 and 16 kD. Dry seeds of both wild type and line 16 have comparable levels of storage proteins. The storage proteins are catabolized at similar rates in wild-type and line 16 seedlings grown on media with (Fig. 7B) or without 20 mm Suc (Fig. 7C).
Figure 4.
A, Seedling establishment in high (160 μmol m−2 s−1) and low (10 μmol m−2 s−1) photon flux densities. Black box, untransformed wild-type Col0; gray box, line 64; narrow hatched box, line 122; medium hatched box, line 192; wide hatched box, line 195; white box, line 16. Suc was not included in the medium; values are the mean ± se of measurements on three separate batches of 30 seedlings. B, Hypocotyl length of 35S-PCK1 antisense and wild-type etiolated seedlings. Seedlings were grown in the dark on medium without Suc for 5 d. Values are the mean ± se of measurements on three separate batches of 30 seedlings.
Figure 5.
The relationship between seedling establishment and PEPCK activity in 2-d-old seedlings from independent 35S-PCK1 antisense lines grown on one-half-strength Murashige and Skoog medium without Suc.
Figure 6.
Soluble sugar levels (Glc, Fru, and Suc) in 2-d-old untransformed wild-type Col0 and 35S-PCK1 antisense seedlings; mean ± se of measurements made on five replicates. All values are the mean ± se of measurements made on three separate batches of seedlings.
Figure 7.
A, Levels of eicosenoic acid (20:1), which is specific to storage triacylglycerol in wild-type and 35S-PCK1 antisense line 16 seedlings 0 to 5 d after imbibition (D.A.I.). Wild type (●) and line 16 (▾) grown on one-half-strength Murashige and Skoog media with 20 mm Suc, wild type (○) and line 16 (▿) grown without Suc. SDS-PAGE gel of protein extracts from seedlings of wild-type and 35S-PCK1 antisense line 16 grown on one-half-strength Murashige and Skoog medium containing 20 mm Suc (B) and on one-half-strength Murashige and Skoog medium without Suc (C). Protein extract equivalent to eight seedlings was loaded onto each lane. Molecular weight markers are in kilodaltons.
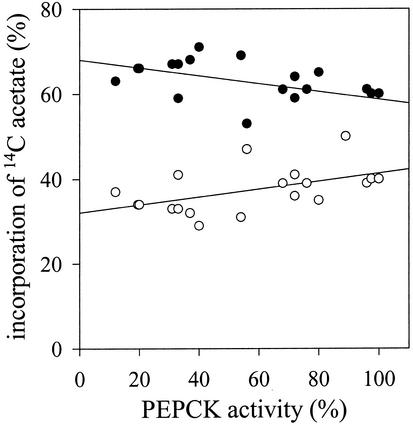
Because lipid and protein catabolism appeared unaltered, but soluble sugar levels were reduced, presumably as a consequence of compromised gluconeogenesis, we investigated whether more of the storage carbon was being respired in the 35S-PCK1 antisense lines than in untransformed Arabidopsis by feeding [14C]acetate to 2-d-old seedlings. To test the relationship between PEPCK activity and 14C incorporation into carbon dioxide and sugars, measurements were made on the same additional set of 17 lines used in Figure 5 (Fig. 8). There was a negative correlation between PEPCK activity and the incorporation of 14C from acetate into carbon dioxide and a positive correlation for the accumulation of 14C from acetate into sugars. Although the r values for these correlations were not significant (r = −0.420 and r = 0.370 respectively), similar trends were seen in two separate experiments.
Figure 8.
Incorporation of [14C]acetate into sugars (○) and carbon dioxide (●) in wild-type Col0 and 35S-PCK1 antisense 2-d-old seedlings.
The reduction of PEPCK activity seen for 2-d-old seedlings in Figure 2B was mirrored in the rosette leaves, with line 16 showing the greatest reduction (Fig. 9). However, the level of PEPCK activity in rosette leaves was approximately 20-fold lower than in 2-d-old seedlings. Once seedlings had become established and transplanted to soil, there was no observable difference in the growth habit throughout the remainder of the life cycle.
Figure 9.
PEPCK activity in rosette leaves of untransformed wild-type Col0 and 35S-PCK1 antisense lines. Values are the mean ± se of measurements made on three separate batches of tissue.
DISCUSSION
The direct correlation between seedling establishment and PEPCK activity (Fig. 5) in the different antisense lines demonstrates the essential role of PCK1 in this process. However the [14C]acetate feeding experiments presented in Figure 8 imply that PEPCK does not exert a high level of control over gluconeogenic flux. Previous studies have suggested higher levels of flux control for PEPCK. Leegood and ap Rees (1978a) noted a 3-fold increase in incorporation of [2-14C]acetate into carbon dioxide in the presence of the PEPCK inhibitor 3-mercaptopicolinic acid. Trevanion et al. (1995), also using 3-mercaptopicolinic acid reported the flux control coefficient of PEPCK as 0.7 to 1.0. However, our results and those of Leegood and ap Rees (1978a) and Trevanion et al. (1995) were all obtained by feeding exogenous [14C]acetate, which gives only an indirect measurement of the fate of β-oxidation-derived acetyl CoA units. More recently, the flux control coefficient for another enzymatic step in the conversion of acetyl CoA to Suc, namely ICL, was reported to be 0.66 ± 0.09 (Runquist and Kruger, 1999) thus questioning a major role for PEPCK in this process. Irrespective of the data obtained through the [14C]acetate feeding experiments, the fact remains that decreasing PEPCK activity has a profound effect on the ability of seedlings to establish and therefore must play a significant role in regulating flux through the pathway.
The linear curve fitted to the data in Figure 5 suggests that in the total absence of PEPCK activity, 20% of the seedlings would still establish. To determine whether this is the case would require the removal of PCK2 activity in addition to PCK1 because the PCK2 gene is also expressed in young seedlings albeit at lower levels (Fig. 1A).
In the 35S-PCK1 antisense lines, storage lipid is still catabolized during early post-germinative growth, even without a supply of exogenous sugars. Our results suggest that there is an increase in the respiration of acetyl CoA units released from fatty acid β-oxidation and a corresponding decrease in the production of sugars in the 35S-PCK1 antisense lines with decreasing PEPCK activity (Fig. 8). These results indicate that when the capacity for the conversion of storage lipid to Suc is reduced, there is an increased flux of carbon derived from fatty acid β-oxidation into respiration. A similar conclusion was reported for an Arabidopsis mutant of the glyoxylate cycle gene, ICL (Eastmond et al., 2000). In 2-d-old icl seedlings, the glyoxylate cycle is blocked; consequently, there is no net flux of carbon from lipid into gluconeogenesis, and lipid is instead respired. However, seedling establishment is not compromised in the icl mutant when grown under optimal conditions, demonstrating that the glyoxylate cycle is not essential for seedling establishment under these conditions.
In addition to lipid reserves, Arabidopsis seeds contain reserves of storage protein (Baud et al., 2002). Stewart and Beevers (1967) demonstrated that amino acids from storage protein in the endosperm of castor bean are converted to Suc by gluconeogenesis and translocated to the germinating seedling. In the icl mutant, gluconeogenic substrate can still be provided by the breakdown of this storage protein, which is presumably in sufficient quantities for seedling establishment under optimal conditions. Under the suboptimal conditions of reduced light, or photoperiod, the frequency of seedling establishment in the icl mutant decreases as these additional reserves are depleted before photosynthetic competency is attained (Eastmond et al., 2000; Eastmond and Graham, 2001). Decreasing the amount of light available for photosynthesis also further reduces the percentage of seedling establishment in the 35S-PCK1 antisense lines. In these lines, the combination of suboptimal growth conditions and reduction in available Suc from the restricted gluconeogenic pathway further compromise seedling establishment. The decrease in soluble sugars in the 35S-PCK1 antisense seedlings at d 2 is already 30% (Fig. 6), which is similar to that reported for the icl1 mutant (Pritchard et al., 2002). We conclude that the levels of soluble sugars are close to the threshold required for seedling establishment at this time, and even a relatively small drop can have a profound effect.
Unlike the icl and 35S-PCK1 antisense lines, the β-oxidation mutants ped1 and kat2 are unable to catabolize lipid reserves, even in the presence of an exogenous supply of Suc. Furthermore, without a supply of exogenous Suc, post-germinative growth does not occur in ped1 and kat2, even under optimal growth conditions (Hayashi et al., 1998; Germain et al., 2001). Thus in the icl mutant, the catabolism of storage protein, in combination with respiration of storage lipid, provides enough fuel to accomplish seedling establishment under optimal growth conditions. However, the ped1 and kat2 mutants demonstrate that the catabolism of protein reserves alone is insufficient to attain seedling establishment. Moreover, unlike the icl mutant, the β-oxidation cycle is essential under all environmental conditions. In the 35S-PCK1 antisense lines, although the phenotype is less acute than the ped1 and kat2 mutants, it is still more severe than the icl mutant. Even under optimal growth conditions, the frequency of seedling establishment is reduced compared with untransformed, wild-type seedlings. The 35S-PCK1 antisense seedlings are compromised in the ability to use both storage lipid and storage protein through gluconeogensis to produce soluble sugars. This demonstrates that whereas a disrupted glyoxylate cycle can be compensated for by optimal growth and early onset of photosynthetic capacity in young seedlings, gluconeogenesis is essential irrespective of growth conditions.
No alterations in mature plant phenotype in the 35S-PCK1 antisense lines were observed. Metabolite profiling analysis targeted at the detection of phenylpropanoids and amino acids also did not reveal any alterations in the antisense lines. In the rosette leaves, PEPCK activity is 20-fold less than in seedlings, consistent with leaves as photosynthetic organs, being capable of producing an autotrophic supply of Suc. Furthermore, the distribution of PEPCK activity in leaves is localized to particular cell types such as trichomes (Leegood and Walker, 1999). It is possible, therefore that alterations, particularly of metabolites, in specific cell types within whole organs in the 35S-PCK1 antisense lines may have been masked by an unaltered phenotype in the rest of the organ. Despite the large reduction in PEPCK activity (80% of wild type in line 16) achieved in the antisense lines, this may not be a sufficient reduction to produce an altered phenotype in the mature plant. In addition, PCK2 gene expression levels are unaltered in the 35S-PCK1 lines, and PCK2 may play a more dominant role in mature plant organs. Thus additional studies are required to further dissect the roles of PEPCK activity in whole plants.
CONCLUSIONS
In conclusion, we have used an antisense approach to functionally characterize the role of PCK1 during early post-germinative growth. In combination with the icl mutant studies, we have demonstrated that alternative storage reserves to lipid, most probably storage protein, can contribute substrate for gluconeogenesis, and thus gluconeogenesis is essential for seedling establishment in the oilseed Arabidopsis.
MATERIALS AND METHODS
35S-PCK1 Antisense Construct
The cDNA expressed sequence tag H36251 was identified in the GenBank DNA sequence database as a PEPCK (PCK1), and the clone was subsequently obtained from the Arabidopsis Biological Resource Center (DNA Stock Center, Ohio State University, Columbus). The cDNA clone was provided as an Escherichia coli bacterial colony containing the cloning vector λ-Ziplox (Invitrogen, Carlsbad, CA) into which the cDNA clone was directionally inserted 5′ to 3′. The sequence was confirmed by partial sequencing of the 3′ and 5′ ends. The 1,239-bp PEPCK fragment was excised from λ-Ziplox by restriction digest of the plasmid with XbaI and SmaI and ligated into pJO530 (a pBIN19 derivative; Bevan, 1984), which had been previously linearized by restriction digestion with XbaI and SmaI. Plant transformation using the binary vector system was conducted using Agrobacterium tumefaciens strain G3V101 containing a vir+ Ti-plasmid lacking the T-DNA region. Restriction digest and PCR analysis confirmed the insertion of the PEPCK cDNA fragment in pJO530 in the antisense orientation.
Plant Material and Growth Conditions
Arabidopsis ecotype Columbia (Col0) plants were transformed by vacuum infiltration (Bechtold and Pelletier, 1998). T1 seed was grown on hygromycin plates, and resistant seedlings were selected to soil. T2 and T3 progeny were screened for homozygous lines by segregation on hygromycin. Seeds were germinated in continuous light on 0.8% (w/v) agar plates containing one-half-strength Murashige and Skoog medium (Murashige and Skoog, 1962; plus 20 mm Suc where indicated) at 20°C after 3 d imbibition at 4°C in the dark. For experiments with etiolated seedlings, plates were transferred back to the dark at 20°C after 30-min exposure to white light.
RT-PCR Analysis
The cDNA was synthesized from 5 μg of DNase-treated RNA using a Prostar First-strand RT-PCR kit (Stratagene, La Jolla, CA). PCR was performed using serial dilutions of cDNA, the reactions were heated to 95°C for 2 min followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 1 min, and then a single 72°C for 10 min. As a marker for constitutive expression actin (ACT2; GenBank accession no. U41998), cDNA was amplified with the primers ACT2A (5′-CTT ACA ATT TCC CGC TCT GC-3′) and ACT2S (5′-GTT GGG ATG AAC CAG AAG GA-3′). Endogenous and transgene PCK1 cDNA was amplified using primers PCK1-1001 (5′-AGG GTC TTT TCA GTG TGA TGC-3′) and PCK1-1709 (5′-CCA TAA CTG CCA CCA GAC CA-3′). Only endogenous PCK1 cDNA was amplified using PCK1-72 (5′-GAA GAT AAC GAC CGG AGC AG-3′) and PCK1-573 (5′-GGG AGC ACG ACC AGT CTT AG-3′). PCK2 cDNA was amplified using PCK2-1032 (5′-CTG CAT TTT CTC AGC CAA CA-3′) and PCK2-1536 (5′-CCG GTA TTT GAT CGG AGA TG-3′).
Enzyme and Sugar Assays
PEPCK assays were performed on plant tissue extracts according to the method of Cooper et al. (1968). Soluble sugars (Glc, Fru, and Suc) were measured according to the method of Stitt et al. (1989).
Metabolism of [14C]Acetate
The [14C]acetate feeding experiments were as described by Eastmond et al. (2000).
Amounts of label incorporated into ethanol-soluble (acidic, neutral, and basic) components, ethanol-insoluble material, and carbon dioxide were determined as described by Canvin and Beevers (1961). The neutral component contained the soluble sugar fraction.
Lipid Analysis
Fatty acids were extracted from dry seeds and 0- to 5 d-old-seedlings grown on one-half-strength Murashige and Skoog media with and without 20 mm Suc. Fatty acids were measured using the method of Browse et al. (1986).
Storage Protein Extraction and SDS-PAGE Analysis
Seedlings were homogenized in 50 mm HEPES, pH 7, and 5 mm dithiothreitol and 0.5% (w/v) SDS was added, and the samples were heated to 100°C for 1 h and centrifuged for 5 min at 8,000g. The supernatant was mixed with loading buffer (125 mm Tris, pH 6.8, 25% [v/v] glycerol, 2.5% [w/v] SDS, 0.05% [w/v] bromphenol blue, and 2.5% [v/v] β-mercaptoethanol) and the samples loaded onto a 12% (w/v) acrylamide gels. SDS-PAGE was as described by Laemmli (1970).
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019174.
LITERATURE CITED
- Bahrami AR, Chen Z-H, Walker RP, Leegood RC, Gray JE. Ripening-related occurrence of phosphoenolpyruvate carboxykinase in tomato fruit. Plant Mol Biol. 2001;47:449–506. doi: 10.1023/a:1011842828723. [DOI] [PubMed] [Google Scholar]
- Baud S, Boutin J-P, Miquel M, Lepiniec L, Rochat C. An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem. 2002;40:151–160. [Google Scholar]
- Bechtold N, Pelletier G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol. 1998;82:259–266. doi: 10.1385/0-89603-391-0:259. [DOI] [PubMed] [Google Scholar]
- Beevers H. The role of the glyoxylate cycle. In: Stumpf PK, editor. The Biochemistry of Plants. New York: Academic; 1980. pp. 117–130. [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke MM, Hucklesby DP, Notton BA. Phosphoenolpyruvate carboxykinase in aubergine, kiwi and apple fruit. Gartenbauwissenschaft. 1988;53:65–70. [Google Scholar]
- Browse J, Mc Court PJ, Somerville CR. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem. 1986;152:141–145. doi: 10.1016/0003-2697(86)90132-6. [DOI] [PubMed] [Google Scholar]
- Canvin DT, Beevers H. Sucrose synthesis from acetate in germinating castor bean: kinetics and pathway. J Biol Chem. 1961;236:988–995. [PubMed] [Google Scholar]
- Comai L, Dietrich RA, Maslyar DJ, Baden CS, Harada JJ. Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell. 1989;1:293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Benedict CR. PEP carboxykinase exchange reaction in photosynthesis bacteria. Plant Physiol. 1968;43:788–792. doi: 10.1104/pp.43.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Tchen TT, Wood HG, Benedict CR. The carboxylation of phosphoenolpyruvate and pyruvate. J Biol Chem. 1968;243:3857–3863. [PubMed] [Google Scholar]
- Eastmond PJ, Germain V, Lange PR, Bryce JH, Smith SM, Graham IA. Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc Natl Acad Sci USA. 2000;97:5669–5674. doi: 10.1073/pnas.97.10.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Graham IA. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001;6:72–77. doi: 10.1016/s1360-1385(00)01835-5. [DOI] [PubMed] [Google Scholar]
- Froman BE, Edwards PC, Bursch AG, Dehesh K. ACX3, a novel medium-chain acyl-coenzyme A oxidase from Arabidopsis. Plant Physiol. 2000;123:733–741. doi: 10.1104/pp.123.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde J-P, Bryce JH, Graham IA, Smith SM. Requirement for 3-ketoacyl CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 2001;28:1–12. doi: 10.1046/j.1365-313x.2001.01095.x. [DOI] [PubMed] [Google Scholar]
- Graham IA, Smith LM, Leaver CJ, Smith SM. Developmental regulation of expression of the malate synthase gene in transgenic plants. Plant Mol Biol. 1990;15:539–549. doi: 10.1007/BF00017829. [DOI] [PubMed] [Google Scholar]
- Gut H, Matile P. Apparent induction of key enzymes of the glyoxylic acid cycle in senescent barley. Planta. 1988;176:548–550. doi: 10.1007/BF00397663. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Toriyama K, Kondo M, Nishimura M. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid beta-oxidation. Plant Cell. 1998;10:183–195. doi: 10.1105/tpc.10.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd JM, Quick P. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature. 2002;415:451–454. doi: 10.1038/415451a. [DOI] [PubMed] [Google Scholar]
- Kim D-J, Smith SM. Molecular cloning of cucumber phosphoenolpyruvate carboxykinase and developmental regulation of gene expression. Plant Mol Biol. 1994;26:423–434. doi: 10.1007/BF00039551. [DOI] [PubMed] [Google Scholar]
- Kornberg HL, Beevers H. A mechanism of conversion of fat to carbohydrate in castor beans. Nature. 1957;180:35–36. doi: 10.1038/180035a0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lea PJ, Chen Z-H, Leegood RC, Walker RP. Does phosphoenolpyruvate carboxykinase have a role in both amino acid and carbohydrate metabolism? Amino Acids. 2001;20:225–241. doi: 10.1007/s007260170041. [DOI] [PubMed] [Google Scholar]
- Leegood RC, ap Rees T. Phosphoenolpyruvate carboxykinase and gluconeogenesis in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1978a;524:207–218. doi: 10.1016/0005-2744(78)90119-5. [DOI] [PubMed] [Google Scholar]
- Leegood RC, ap Rees T. Identification of the regulatory steps in gluconeogensis in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1978b;542:1–11. doi: 10.1016/0304-4165(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S, Osmond CB. Metabolite transport and photosynthetic regulation in C4 and CAM plants. In: Dennis DT, Turpin DH, Layzell DD, Lefebvre DK, editors. Plant Metabolism. London: Longman; 1996. pp. 341–369. [Google Scholar]
- Leegood RC, Walker RP. Phosphoenolpyruvate carboxykinase in plants: its role and regulation. In: Bryant JA, Burrell MM, Kruger NJ, editors. Plant Carbohydrate Biochemistry. Oxford: BIOS Scientific Publishers Ltd; 1999. pp. 202–213. [Google Scholar]
- Lemieux B, Miquel M, Somerville C, Browse J. Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor Appl Genet. 1990;80:234–240. doi: 10.1007/BF00224392. [DOI] [PubMed] [Google Scholar]
- Martin T, Oswald O, Graham IA. Arabidopsis seedling growth, storage lipid mobilization and photosynthetic gene expression are regulated by carbon: nitrogen availability. Plant Physiol. 2002;128:472–481. doi: 10.1104/pp.010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelis M, Vennesland B. Carbon dioxide fixation into oxaloacetate in higher plants. Plant Physiol. 1957;32:591–600. doi: 10.1104/pp.32.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–496. [Google Scholar]
- Pritchard SL, Charlton WL, Baker A, Graham IA. Germination and storage reserve mobilization are regulated independently in Arabidopsis. Plant J. 2002;31:639–647. doi: 10.1046/j.1365-313x.2002.01376.x. [DOI] [PubMed] [Google Scholar]
- Ruffner HP, Kliewer WM. Phosphoenolpyruvate carboxykinase activity in grape berries. Plant Physiol. 1975;56:67–71. doi: 10.1104/pp.56.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runquist M, Kruger NJ. Control of gluconeogenesis by isocitrate lyase in endosperm of germinating castor bean seedlings. Plant J. 1999;19:423–431. doi: 10.1046/j.1365-313x.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- Rylott EL, Hooks MA, Graham IA. Co-ordinate regulation of genes involved in storage lipid mobilization in Arabidopsis thaliana. Biochem Soc Trans. 2001;29:283–687. doi: 10.1042/0300-5127:0290283. [DOI] [PubMed] [Google Scholar]
- Sáez-Vásquez J, Raynal M, Delseny M. A rapeseed cold-inducible transcript encodes a phosphoenolpyruvate carboxykinase. Plant Physiol. 1995;109:611–618. doi: 10.1104/pp.109.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarah CJ, Graham IA, Reynolds SJ, Leaver CJ, Smith SM. Distinct cis-acting elements direct the germination and sugar responses of the cucumber malate synthase gene. Mol Gen Genet. 1996;250:153–161. doi: 10.1007/BF02174174. [DOI] [PubMed] [Google Scholar]
- Sjodahl S, Rodin J, Rask L. Characterization of the 12S globulin complex of Brassica napus: evolutionary relationship to other 11–12S storage globulins. Eur J Biochem. 1991;196:617–621. doi: 10.1111/j.1432-1033.1991.tb15857.x. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Beevers H. Glucoenogenesis from amino acids in germinating castor bean endosperm and its role in transport to the embryo. Plant Physiol. 1967;42:1587–1595. doi: 10.1104/pp.42.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, McLilley R, Gerhardt R, Heldt HW. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989;174:518–552. [Google Scholar]
- Strand A, Zrenner R, Trevanion S, Stitt M, Gustafsson P, Gardestrom P. Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J. 2000;23:759–70. doi: 10.1046/j.1365-313x.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- Trevanion SJ, Brooks AL, Leegood RC. Control of gluconeogenesis by phosphoenolpyruvate carboxykinase in cotyledons of Cucurbita pepo L. Planta. 1995;196:653–658. [Google Scholar]
- Walker RP, Chen Z-H, Johnson KE, Famiani LT, Tesci L, Leegood RC. Using immunohistochemistry to study plant metabolism: the examples of its use in the localization of amino acids in plant tissues, and of phosphoenolpyruvate carboxykinase and its possible role in pH regulation. J Exp Bot. 2001;52:565–576. [PubMed] [Google Scholar]
- Walker RP, Chen Z-H, Tecsi LI, Famiani F, Lea PJ, Leegood RC. Phosphoenolpyruvate carboxykinase plays a role in interactions of carbon and nitrogen metabolism during grape seed development. Planta. 1999;210:9–18. doi: 10.1007/s004250050648. [DOI] [PubMed] [Google Scholar]
- Walker RP, Leegood RC. Phosphorylation of phosphoenolpyruvate carboxykinase in plants: studies in plants with C4 photosynthesis and Crassulacean acid metabolism and in germinating seeds. Biochem J. 1996;317:653–658. doi: 10.1042/bj3170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RP, Trevanion SJ, Leegood RC. Phosphoenolpyruvate carboxykinase from higher plants: purification from cucumber and evidence of rapid proteolytic cleavage in extracts from a range of plant tissues. Planta. 1995;195:58–63. [Google Scholar]
- Wingler A, Walker RP, Chen Z-H, Leegood RC. Phosphoenolpyruvate carboxykinase is involved in the decarboxylation of aspartate in the bundle sheath of maize. Plant Physiol. 1999;120:539–545. doi: 10.1104/pp.120.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]