Abstract
The aroma of roses (Rosa hybrida) is due to more than 400 volatile compounds including terpenes, esters, and phenolic derivatives. 2-Phenylethyl acetate, cis-3-hexenyl acetate, geranyl acetate, and citronellyl acetate were identified as the main volatile esters emitted by the flowers of the scented rose var. “Fragrant Cloud.” Cell-free extracts of petals acetylated several alcohols, utilizing acetyl-coenzyme A, to produce the corresponding acetate esters. Screening for genes similar to known plant alcohol acetyltransferases in a rose expressed sequence tag database yielded a cDNA (RhAAT1) encoding a protein with high similarity to several members of the BAHD family of acyltransferases. This cDNA was functionally expressed in Escherichia coli, and its gene product displayed acetyl-coenzyme A:geraniol acetyltransferase enzymatic activity in vitro. The RhAAT1 protein accepted other alcohols such as citronellol and 1-octanol as substrates, but 2-phenylethyl alcohol and cis-3-hexen-1-ol were poor substrates, suggesting that additional acetyltransferases are present in rose petals. The RhAAT1 protein is a polypeptide of 458 amino acids, with a calculated molecular mass of 51.8 kD, pI of 5.45, and is active as a monomer. The RhAAT1 gene was expressed exclusively in floral tissue with maximum transcript levels occurring at stage 4 of flower development, where scent emission is at its peak.
Roses (Rosa hybrida) are grown as garden plants, for the cut-flower industry, and as a source of natural fragrances. Many modern cut-flower rose cultivars were selected for long vase life, flower shape, and color. Intensive breeding has also generated garden cultivars that have an intense “rose” scent. More than 400 different volatile compounds have been identified in rose scent, and these compounds have been classified into several chemical groups including hydrocarbons, alcohols, esters, aromatic ethers, and “others” (aldehydes such as geranial and nonanal, rose oxide, and norisoprenes such as β-ionone; Flament et al., 1993; Weiss, 1997).
Volatile esters such as geranyl acetate and 2-phenylethyl acetate are important contributors to the aroma of roses and many other flowers (Knudsen and Tollsten, 1993). Volatile esters also contribute to the unique aroma of fruits such as banana (Musa acuminata), apple (Malus domestica), melon (Cucumis melo), strawberry (Fragaria ananassa), and spice plants such as lavender (Lavandula officinalis; Croteau and Karp, 1991; Ueda et al., 1992). Despite the knowledge of the contribution of volatile acetate esters to the aroma of roses, including the demonstration of the circadian emission of some major volatile acetate esters by flowers of the rose var. “Honesty” (Helsper et al., 1998), little is known about the mechanisms by which these compounds are formed in roses.
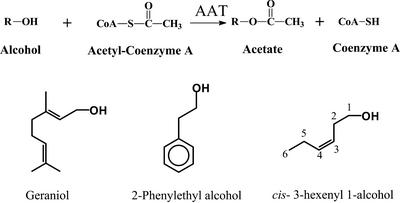
Acetate esters in plants are normally generated as a result of the action of alcohol acetyltransferase (AAT) enzymes that transfer the acetyl moiety from acetyl-CoA to an alcoholic substrate (Fig. 1; Harada et al., 1985; Fellman and Mattheis, 1995; Perez et al., 1996; Aharoni et al., 2000; Shalit et al., 2001). To date, only two genes that code for AAT enzymes involved in volatile esters formation in flowers have been identified (Dudareva et al., 1998; D'Auria et al., 2002). They include the gene BEAT, which codes for a benzyl AAT, and the gene BEBT, which codes for a benzyl alcohol benzoyl transferase. The gene products generate benzyl acetate and benzylbenzoate, respectively, two important volatiles emitted from the flowers of Clarkia breweri. BEAT and BEBT both belong to the BAHD family of acyltransferases, a group of monomeric enzymes of roughly 450 amino acids whose two main hallmarks are the HXXXD motif believed to constitute the active site and an additional motif, DFGWG, of unknown function (St-Pierre and De Luca, 2000).
Figure 1.
Formation of volatile esters by AAT enzymatic activity. AAT enzyme catalyzes the formation of esters using acetyl-CoA and alcohols as the substrates. A few of the main substrates of these enzymes are shown.
The floral scent of “Fragrant Cloud,” a rose garden variety that is profoundly scented, consists largely of esters (mainly acetate derivatives), aromatic alcohols (mainly 2-phenylethyl alcohol), monoterpene alcohols (such as citronellol and geraniol), and other monoterpenes and sesquiterpenes (primarily germacrene D; Flament et al., 1993; Guterman et al., 2002). To study volatile ester formation in roses, we have followed an integrated approach, combining data obtained from the analysis of volatile composition in the headspace of flowers, together with enzymatic activity data from petal cell-free extracts, bioinformatic analysis of expressed sequence tag (EST) databases, and functional expression of candidate genes in Escherichia coli. This approach has already led to the isolation and characterization of several genes involved in biosynthesis of terpenoid and methylether volatile components of the rose floral scent (Channeliere et al., 2002; Guterman et al., 2002; Lavid et al., 2002). Here, we describe the determination of the main volatile acetate esters present in “Fragrant Cloud” roses headspace, the identification of AAT activities involved in volatile acetate formation, and the isolation and characterization of a new gene, highly expressed in developing rose petals, which encodes a protein that catalyzes the formation geranyl acetate in vitro.
RESULTS
Volatile Acetate Esters Are Major Constituents of “Fragrant Cloud” Floral Scent
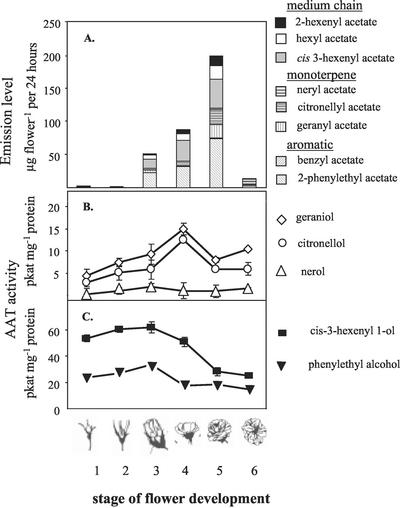
The levels of volatile acetate esters emitted from flower buds (stages 1 and 2) of “Fragrant Cloud” roses are very low (0.4 μg per flower per 24 h and 0.2 μg per flower per 24 h, respectively) and consist almost exclusively of cis-3-hexenyl acetate (Fig. 2A). During flower opening, the level of emission of acetate esters increased to 50 μg per flower per 24 h at stage 3 and 90 μg per flower per 24 h at stage 4, reaching maximal levels of 200 μg per flower per 24 h at fully open flowers (stage 5) and decreasing to only 15 μg per flower per 24 h in fully open flowers (stage 6; Fig. 2A). 2-Phenylethyl acetate and cis-3-hexenyl acetate were the major acetates emitted. Geranyl acetate, citronellyl acetate, hexyl acetate, and 2-hexenyl-acetate were also abundant, whereas benzyl acetate and neryl acetate were emitted only at low levels. Although the total levels of emission of acetate esters changed during development, the ratios between the different acetate esters in each flowering stage (except when the flowers are still closed, stages 1 and 2) were generally constant throughout flower opening. At stage 6, only 2-phenylethyl, cis-3-hexenyl, and hexyl acetate were noted.
Figure 2.
Emission of acetate esters from intact flowers (A) and alcohol:acetyl-CoA enzymatic activity levels in cell-free extracts of rose petals (B and C). Emission of acetate esters from “Fragrant Cloud” flowers was assessed by the headspace technique (A). Means of five to 10 determinations per flower stage are given. The levels of AAT activity in cell-free extracts utilizing the monoterpenoids geraniol, citronellol or nerol as substrates (B), and the aromatic 2-phenylethyl alcohol or the aliphatic cis-3-hexenyl-1-alcohol as substrates (C). Means and se of two replicates, each representing an individual flower. The experiment was replicated three times with similar results.
AAT Activities in Cell-Free Extracts Derived from Petals
Cell-free extracts from open flowers were prepared to evaluate the possible involvement of AATs in the formation of volatile acetate esters. Alcoholic substrates putatively involved in the formation of the major acetate esters emitted in “Fragrant Cloud” open flowers (stages 4 and 5) were tested (Table I). The highest levels of AAT enzymatic activity were detected with medium-chain aliphatic alcohols such as cis-3-hexene 1-alcohol and 1-hexanol (237% and 204%, respectively, as compared with the rates obtained with geraniol). High activity (80%–150% as compared with the rate obtained for geraniol) was also detected when medium-chain aliphatic 1-octanol and the aromatic alcohol 2-phenylethyl alcohol, and the aliphatic isoamyl alcohol and butanol and the monoterpene citronellol were used as substrates. Moderate levels of activity (30%–50% of the rate of geraniol) were observed when the monoterpene alcohol nerol, the medium-chain aliphatic1-decanol, and the aromatic benzyl alcohol were used as substrates. Relatively low levels of activity (15%–20% of the rate of geraniol) were detected with ethanol, and with linalool, a tertiary monoterpene alcohol.
Table I.
Relative alchohol acetyltransferase activity of crude + 3 + 95 extracts derived from “Fragrant Cloud” rose petals (stages 4 and 5 combined) and of partially purified recombinant RhAAT gene product with selected alcohol substrates. Activity with geraniol was set as 100%. Means and ses of two replicates. The experiments were repeated three times with similar results.
| Substrate Testeda | Crude Extract | RhAAT1 |
|---|---|---|
| Monoterpene alcohols | ||
| Geraniol | 100 ± 15 | 100 ± 7.7 |
| Citronellol | 87 ± 8.8 | 60 ± 3.1 |
| Nerol | 35 ± 2.8 | 15.6 ± 1.6 |
| Linalool | 22 ± 0.3 | 3.6 ± 0.6 |
| Aromatic alcohols | ||
| 2-Phenylethyl alcohol | 144 ± 5.4 | 7.9 ± 0.5 |
| Benzyl alcohol | 35 ± 0.7 | 8.9 ± 0.5 |
| Aliphatic alcohols | ||
| cis-3-Hexene 1-ol | 237 ± 11 | 10.6 ± 1.2 |
| 1-Hexanol | 204 ± 21 | 13.9 ± 1 |
| 1-Octanol | 147 ± 7.7 | 34.8 ± 7 |
| 1-Butanol | 101 ± 11 | 4.2 ± 0.1 |
| Isoamyl alcohol | 97.4 ± 14 | 4.0 ± 2.0 |
| 1-Decanol | 50 ± 0.1 | 5.1 ± 0.2 |
| 3-Hexanol | 28.7 ± 1.2 | 4.5 ± 0.2 |
| Ethanol | 16.5 ± 1.2 | 2.4 ± 0.3 |
All substrates were tested at a conentration of 10 mm.
To test for changes in AAT enzymatic activities in rose petals during flower development, cell-free extracts of petals of the six flowering stages were prepared and tested for potential enzymatic acetylation activity with five putative alcohol substrates (Fig. 2, B and C). Enzymatic activity leading to the synthesis of geranyl acetate and citronellyl acetate from the monoterpene alcohols geraniol and citronellol, respectively, moderately increased during flower development, reaching a maximum level of about 15 pkat mg−1 protein for geranyl acetate formation at stages 3 and 4 and a maximum of 12 pkat mg−1 protein at stage 4 for citronellyl acetate formation (Fig. 2B). In contrast, the potential for neryl acetate formation was low during all flower developmental stages. In addition, AAT activities with 2-phenylethyl alcohol and cis-3-hexene 1-alcohol, which led to the formation of 2-phenylethyl acetate and cis-3-hexenyl acetate, respectively, were already high at stage 1 (20 pkat mg−1 protein and 55 pkat mg−1 protein, respectively; Fig. 2C), reached maximal levels at stages 2 to 3 (35 pkat mg−1 protein for 2-phenylethyl alcohol and 60 pkat mg−1 protein for cis-3-hexene 1-alcohol), and subsequently declined at stages 4 to 6 to lower levels than those seen in the first stages (15 pkat mg−1 protein for 2-phenylethyl alcohol and 25 pkat mg−1 protein for cis-3-hexene 1-alcohol).
Isolation and Characterization of RhAAT1, an AAT Gene, from “Fragrant Cloud” Petals
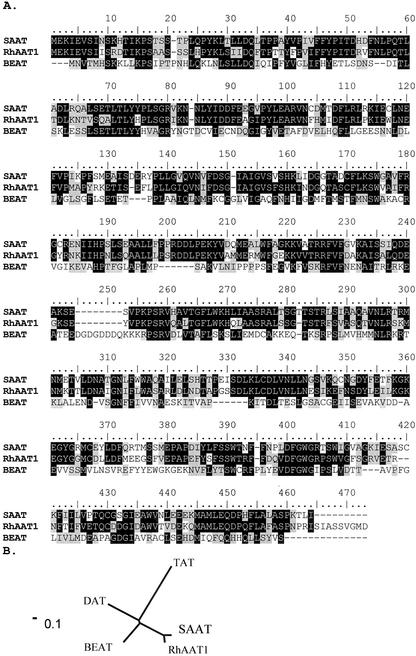
An EST database of “Fragrant Cloud” petals that contains more than 1,834 unigenes has been established (Guterman et al., 2002). A search in this database for genes encoding potential AAT enzymes highlighted three ESTs displaying sequence similarity with known BAHD AAT genes from other sources. One such cDNA, which we designated as RhAAT1, encodes a polypeptide of 458 amino acid residues (Fig. 3) with a calculated molecular mass of 51.8 kD and a pI of 5.45. The protein contains the two highly conserved motifs HXXXD and DFGWG present in the enzymes of the plant BAHD O-acyltransferases family.
Figure 3.
Comparison of the amino acid sequence of the RhAAT1 enzyme with closely related sequences (A) and positioning of RhAAT1 gene on the phylogenetic map (B). A, RhAAT1 (accession no. BQ106456), BEAT (accession no. AF043464), and SAAT (accession no. AF193789) amino acid sequences are compared. Amino acid shaded in black represent identical matches; gray-shaded boxes represent conservative changes. B, Non-rooted phylogenetic tree. Alignments and phylogenetic tree program used was ClustalX (Thompson et al., 1997).
The protein sequence shows the highest identity, 69%, to a strawberry protein designated SAAT, which was isolated from ripening strawberry fruits and shown to catalyze the formation of medium-chain aliphatic acetate esters (Aharoni et al., 2000). RhAAT1 is also 31% identical to the protein encoded by the benzyl AAT gene (BEAT), which catalyzes the formation of benzyl acetate in flowers of C. breweri (Dudareva et al., 1998).
RhAAT1 was highly similar (E value = 10−49) to the pfam transferase (PF02458) domain and is also relatively closely related to two other enzymes of the BAHD plant acetyltransferases. It is 31% identical to DAT, an acetyltransferase involved in vindoline formation in Catharanthus roseus (St-Pierre et al., 1998; Laflamme et al., 2001) and 27% identical to TAT, an acetyltransferase involved in taxol formation in Taxus cuspidata (Walker et al., 2000; Fig. 3B).
Substrate Preference of the Recombinant RhAAT1 Enzyme
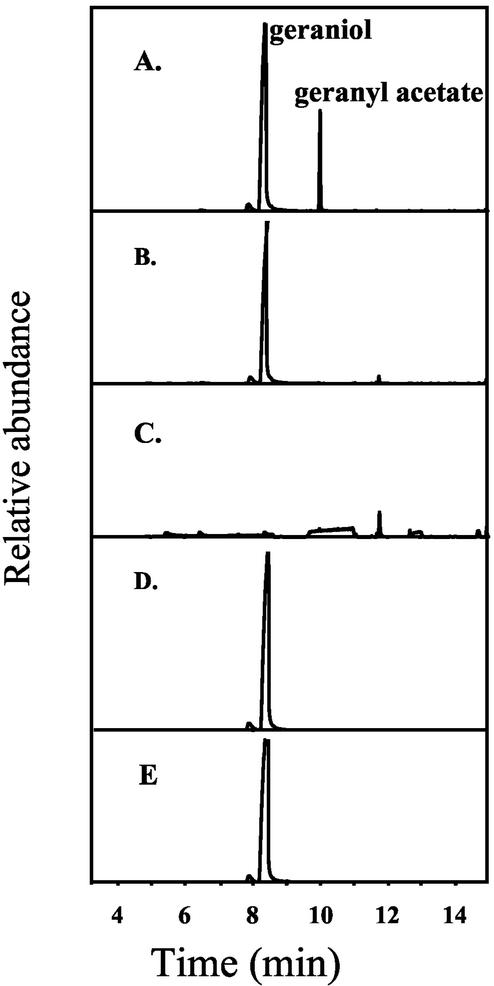
The coding region of RhAAT1 was subcloned into a pET (11a) expression vector (Studier et al., 1990; Lavid et al., 2002) for functional expression in E. coli. To test for the substrate specificity of the RhAAT1 gene product for potential alcoholic substrates and to determine its general catalytic and kinetic parameters, we used a simple and sensitive radio-assay as well as gas chromatography-mass spectrometry (GC-MS) analysis for the identification of products (Dudareva et al., 1998; Shalit et al., 2001). The RhAAT1 protein was partially purified (see “Materials and Methods”), and a number of alcohols were tested as substrates (Table I). RhAAT1 was most active with geraniol, catalyzing the production of geranyl acetate from geraniol and acetyl-CoA (Fig. 4A). The reaction depended on the presence of enzyme (Fig. 4B), on the presence of the alcoholic substrate geraniol (Fig. 4C), and acetyl-CoA (Fig. 4D). Controls of the same cells lacking a recombinant RhAAT1 gene did not possess AAT activity (Fig. 4E). The 2-saturated derivative citronellol was accepted as a substrate at an intermediate rate (60% as compared with geraniol). Only low levels of activity were observed with nerol (16%), a cis isomer of geraniol, and almost no activity (3.6%) was attained with the tertiary monoterpene alcohol linalool. A moderate level of activity was also observed with 1-octanol (35%), lesser activity with 1-hexanol (14%), and cis-3-hexen 1-alcohol (10%). Only a slight level of activity was observed when benzyl alcohol, n-butanol, ethanol, and isoamyl alcohol (9%–2%) were offered as substrates. Interestingly, RhAAT1 had low activity (8%) with 2-phenylethyl alcohol compared with geraniol, even though 2-phenylethyl alcohol is the apparent substrate for the formation of 2-phenylethyl acetate, the major volatile emitted from “Fragrant Cloud” flowers, and AAT enzymatic activity catalyzing the formation 2-phenylethyl acetate is higher than geranyl acetate-forming AAT activity in cell-free extracts derived from petals (Table I).
Figure 4.
Identification by GC-MS of the acetate esters product formed in vitro by the product of RhAAT1. Bacterial lysates overexpressing RhAAT1 were incubated with geraniol and acetyl-CoA in assay buffer as described in “Materials and Methods.” The identification of the geranyl acetate product was done by matching with the retention time and the mass spectrum of authentic geranyl acetate, and by comparison with the computerized Wiley library. The Kovac index of geranyl acetate also corresponded with that of authentic standard. A, Lysates (derived from cells overexpressing RhAAT1) + geraniol + acetyl-CoA. B, Reaction buffer + geraniol + acetyl-CoA. C, Lysates derived from cells overexpressing RhAAT1 + acetyl-CoA. D, Lysates derived from cells overexpressing RhAAT1 + geraniol (no acetyl-CoA). E, Control lysates (derived from control E. coli BL21 [DE3] pLysS cells, not overexpressing RhAAT1) + geraniol + acetyl-CoA.
Characterization of Kinetic Parameters of the RhAAT1 Enzymatic Activity
The determination of the general catalytic properties and kinetic parameters of the recombinant partially purified RhAAT1 enzyme was done utilizing geraniol, citronellol, 1-hexanol, and 1-octanol as alcoholic substrates and acetyl-CoA as acyl donor. The apparent Km values were: geraniol, 0.16 mm; citronellol, 0.20 mm; 1-hexanol, 0.5 mm; and 1-octanol, 0.46 mm. The Km value for acetyl-CoA was 65 μm regardless of the alcoholic substrate used. The size of the purified native enzyme, determined by gel filtration chromatography, was 53 kD, similar to the predicted size of the subunit (51.8 kD), indicating that the enzyme is active as a monomer, as noted earlier for other plant AATs of the BAHD family (St-Pierre and De Luca, 2000). The optimum temperature for catalysis was in the range of 25°C to 35°C. The pH optimum was between pH 7.0 and 8.0. The addition of EDTA to the reaction buffer did not affect the activity, indicating a divalent metal-independent catalysis.
RhAAT1 Expression Is Regulated during Flower Development
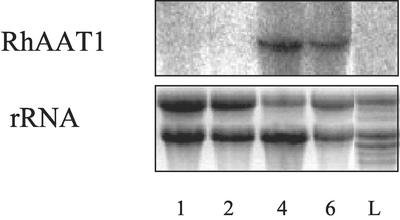
We monitored the level of RhAAT1 transcript levels in petals from different developmental stages (1, 2, 4, and 6) as well as from leaves (Fig. 5). No RhAAT1 transcripts were detected in leaves and in the early stages of flower development (stage 1). RhAAT1 transcript reached maximum level at open flowers (stage 4), and lower transcript was detected at full bloom (stage 6).
Figure 5.
RNA gel-blot analysis of RNA samples derived from “Fragrant Cloud” roses. RNA gel-blot analyses of RhAAT1. RNA was extracted from rose cv “Fragrant Cloud” leaves (L) and from petals at different developmental stages (1, 2, 4, and 6) and analyzed for RhAAT1 expression. Ethidium bromide staining of rRNA is presented (bottom).
DISCUSSION
Petals of “Fragrant Cloud” Roses Display AATs Capable of Synthesizing Many Volatile Acetyl Esters
In previous work, the major constituents of the floral scent of the rose var. “Fragrant Cloud” were determined and found to be mostly acetate esters, alcohols, monoterpenes, and sesquiterpenes (Guterman et al., 2002; Lavid et al., 2002). The emission of high levels of volatile acetate esters suggested that AAT enzymatic activities might be involved in flower scent production as noted before for C. breweri (Dudareva et al., 1998). We tested this hypothesis by measuring AAT activity in crude extracts derived from petals with acetyl-CoA and various alcoholic substrates. The results (Fig. 2; Table I) show a significant correlation between the presence of specific acetate esters in the headspace and the ability of petal cell-free extracts to produce these acetate esters in vitro. Nonetheless, the cell-free extracts have the potential to acetylate alcohols whose acetate esters are not detected in the headspace of “Fragrant Cloud” flowers. Thus, the production of acetate esters in rose petals depends not only on the specificity of the AATs present, but also on other factors that may include substrate availability and cell and tissue compartmentalization of substrates and enzymes.
RhAAT1 Is an Acetyltransferase with Limited Substrate Specificity
Screening a rose petal EST database for sequences with similarity to known AATs of the BAHD acyltransferase family of plants (St-Pierre and De Luca, 2000) yielded three cDNAs. One of them, RhAAT1, was analyzed in detail in this study and shown to encode an enzyme with high affinity for geraniol that catalyzes the formation of geranyl acetate. The enzyme also readily accepts citronellol, a 2-saturated derivative of geraniol (approximately 60% the rate obtained with geraniol), but is relatively inefficient (approximately 16% the rate obtained with geraniol) in acetylating nerol, the cis isomer of geraniol. The enzyme had also lower levels of activity with cis-3-hexene 1-alcohol and 2-phenylethyl alcohol (Table I). Therefore, it is likely that cis-3-hexenyl acetate and 2-phenylethyl acetate, the two main esters emitted from “Fragrant Cloud” roses (Fig. 2A), are synthesized by another enzyme or enzymes. This hypothesis is also supported by the observation that the patterns of the changes in cis-3-hexenyl-1-acetate and 2-phenylethyl acetate-forming AAT activity levels over the developmental stages of the flower, although similar to each other (Fig. 2C), are different from the corresponding pattern of geranyl acetate-forming AAT activity levels (Fig. 2B). The maximum for the former occurs at stage 3, whereas the maximum for the latter occurs at stage 4.
RhAAT1 Is Similar to SAAT and Other Acetyltransferases in the BAHD Family
The phylogenetic analysis (Fig. 3B) indicates that the enzyme encoded by RhAAT1 is most similar to SAAT, an enzyme involved in the production of volatile acetate esters in strawberry fruits. Both rose and strawberry belong to the Rosaceae family, and the high sequence similarity (69%) may indicate that the two sequences are descendants from a common gene, diverging after recent gene duplications (Pichersky and Gang, 2000). Although SAAT has marked AAT activity with medium-chain alcohol substrates and is less active with short-chain alcohols, the highest activity of RhAAT1 was obtained when the monoterpene alcohol geraniol was used as a substrate. However, RhAAT1 also has marked activity with medium-chain alcohols and some but lesser activity with short-chain alcohols. The potential acetylating activity of SAAT toward the monoterpene alcohols geraniol and citronellol has not been reported. It is also interesting that the Km value of RhAAT1 for geraniol, the preferred substrate, is 0.16 mm, whereas it is 0.20, 0.45, and 0.5 mm for citronellol, 1-octanol, and 1-hexanol, respectively. In contrast, the calculated Km value of SAAT with 1-octanol and 1-hexanol, the best alcoholic substrates, are much higher (5.7 and 8.9 mm, respectively).
Regulation of Volatile Emission from Rose Petals at the Enzymatic and Transcriptional Level
Emission of acetate volatiles is very low at stages 1 and 2, it became apparent at stage 3 of flower development, and then rapidly increased to a maximum at stage 5. The major acetates emitted are 2-phenyl ethylacetate and cis-3-hexenyl acetate. Nevertheless, acetyltransferase activity able to utilize 2-phenylethyl alcohol and cis-3-hexenyl alcohol (the corresponding precursors) is already prominent at stages 1 and 2. It could be possible that substrate availability is limiting the formation of the corresponding acetates. The levels of the emission of the corresponding alcohol substrates are very low at stages 1 and 2 and increase at stage 3 (M. Shalit and E. Lewinsohn, unpublished data), paralleling the increase in acetate emissions. It is also possible that the alcohols and/or the acetates might be formed in the petals at stages 1 and 2 but not emitted due to anatomical, developmental, or other physiological constrains. This possibility is unlikely, as indicated by studies in which rose petals were solvent (methyl-tert-butyl ether), extracted, and found to be devoid of these alcohols and their acetates (data not shown). Nevertheless, other processes, such as tissue or subcellular compartmentization of the enzymes and substrates, can explain these observations. Moreover, maximal emission of these compounds occurs up to stage 5, whereas the maximal recorded enzyme activity is at stage 3. Therefore, it seems that the nonoptimal levels of enzyme activity can account for the emissions observed. It can also be possible that due to low turnover rates of the ester products, they are maximally emitted at a stage in which enzyme activities are already declining. Concerning the emission of geranyl and citronellyl acetates, the maximal levels of the measured AAT enzyme activities also do not correlate exactly with the highest levels of volatile emission. It could be that the high geraniol- and citronellol-specific AAT activities observed in cell-free extracts in stages 1 and 2 are due to other AAT enzyme activities compartmentized from their substrates. This is corroborated by the RhAAT1 expression analyses, which indicate an apparent lack of RhAAT1 transcript at stages 1 and 2. Therefore, we assume that other acetyltransferases that could use geraniol and citronellol as potential substrates might yield substantial activity measurements in stages 1 and 2. Moreover, two additional cDNAs with sequences similar to genes of the BAHD family have been identified in the “Fragrant Cloud” rose EST database, but have not yet been fully characterized. In accordance, it seems that additional AAT genes could be involved in formation of volatile esters emitted from the flowers.
CONCLUSION
Volatile acetate esters are very important contributors to the aroma of many plants, including commercially important crops. Only a few genes encoding enzymes involved in volatile ester formation have been identified and characterized. We have described the identification and characterization of the gene that codes for RhAAT1, an enzyme able to catalyze the formation of volatile esters in a major ornamental crop. The rose RhAAT1 is currently the only known AAT that can utilize the monoterpene geraniol as substrate to generate geranyl acetate, a compound with a fruity rose note reminiscent of pear (Pyrus communis) and slightly of lavender that occurs in the scent of many plants (Bauer et al., 2001). The availability of RhAAT1 and similar AAT genes that encode enzymes for the formation of fragrances will allow a better understanding of the factors that influence and limit the biogeneration of scent compounds. This information can be used to generate crops with improved or modified fragrance using the modern biotechnological tools available (Galili et al., 2002).
MATERIALS AND METHODS
Plant Material
Flowers of rose (Rosa hybrida) cv “Fragrant Cloud” were harvested from plants grown in a greenhouse in the Newe-Ya'ar Research Center, Israel, under controlled conditions (Lavid et al., 2002).
Chemicals and Radiochemicals
All chemicals and radiochemicals were purchased from Sigma (St. Louis) unless otherwise noted.
Headspace Volatile Collection
Intact individual rose flowers, still attached to the bush, were enclosed in a 1-L glass container with the appropriate openings, and headspace was trapped for 24 h at 25°C using a method modified from Raguso and Pichersky (1995), utilizing a Porapak Q 80/100 (Waters Corp., Milford, MA) polydivinylbenzene filter. Photon fluence was 22 ± 2 μE m−2 s−1 as determined by an LI-188B integrating quantum radiometer/photometer (LI-COR, Lincoln, NE). The photoperiod was 15 h, and the starting of the sampling was initiated 4 h after the lights were turned on. Volatiles were eluted utilizing 10 mL of HPLC-grade hexane containing 100 μg of ethylmyristate as an internal standard and evaporated to 0.5 mL. One microliter of each sample was analyzed by GC-MS (Lavid et al., 2002).
GC-MS Analysis
The volatile compounds collected from the headspace were analyzed on an Hewlett-Packard-GCD apparatus equipped with an HP-5 (30 m × 0.25 mm) fused-silica capillary column. Helium (1 mL min−1) was used as a carrier gas. The injector temperature was 250°C, set for splitless injection. The oven was set to 50°C for 1 min, and then the temperature was increased to 200°C at a rate of 4°C min−1. The detector temperature was 280°C. Mass range was recorded from 45 to 450 mass-to-charge ratio, with electron energy of 70 eV. Identification of the main components was done by comparison of mass spectra and retention time data with those of authentic samples and supplemented with a Wiley GC-MS library (Lewinsohn et al., 2001; Shalit et al., 2001). Quantification of the compounds was performed by utilizing the total mass ions detected and compared with the internal standard.
Cloning of Rose AAT Gene
RhAAT1 was identified in the “Fragrant Cloud” petal EST database (Guterman et al., 2002) by homology search (BLAST) with other AATs. RhAAT1 was subcloned into a T7-dependent expression vector pET (11a) by PCR with the appropriate oligonucleotides according to the manufacturer's instructions as previously described (Wang and Pichersky, 1999).
RNA Extraction and Analysis
Total RNA was extracted from petals and leaves as previously described (Manning, 1991). RNA samples (10 μg) were fractionated in a 1% (w/v) agarose gel containing formaldehyde and blotted onto Hybond N+ membranes (Amersham, Buckinghamshire, UK). The blots were hybridized in a solution containing 0.26 m Na2PO4, 7% (w/v) SDS, 1 mm EDTA, and 1% (w/v) bovine serum albumin at 60°C with 32P-labeled RhAAT1 cDNA probe (Redprime, Amersham). The membranes were washed twice in 2× SSC and 0.1% (w/v) SDS at 60°C for 20 min each and exposed to x-ray film (Fuji, Tokyo; Lavid et al., 2002).
Preparation of Cell-Free Extracts Derived from Rose Petals
Cell-free soluble protein extracts were prepared from petals of “Fragrant Cloud” using a protocol modified from Lavid et al. (2002). Petals were chosen from several stages of flower development: from a green closed bud (stage1), beginning of anthocyanin accumulation (stage 2), full red closed flower (stage 3), flowers start to open (stage 4), fully open flower (stage 5), and a wilting faded flower (stage 6). Fresh flowers were weighed and frozen in liquid nitrogen in a chilled mortar. Tissue was ground with a pestle in the presence of 1% (w/w) sand and 1% (w/w) polyvinylpolypyrrolidone until a uniform powder was obtained. Ice-cold extraction buffer (50 mm bis-Tris [pH 6.9] containing 10% [v/v] glycerol, 5 mm Na2S2O5, 10 mm dithiothretiol [DTT], 1% [w/w] polyvinylpyrrolidone 40) was added at a 10:1 ratio (w/v), and grinding was continued at 4°C until reaching an homogenous texture. The slurry was centrifuged at 20,000g for 10 min at 4°C. The supernatant (crude extract) was either used fresh or kept for up to 2 weeks at −40°C until its use for enzymatic assays as described below.
Expression of RhAAT1 in Escherichia coli
Recombinant E. coli BL21 (DE3) Gold (Stratagene, La Jolla, CA) bacteria were plated in Luria-Bertani broth (LB)-agar containing 50 μg mL−1 ampicillin and 34 μg mL−1 chroramphenicol. Individual colonies were grown in 2 mL of LB liquid medium containing 50 μg mL−1 ampicillin overnight to be used as starter cultures. Five hundred microliters of bacterial cell suspensions was transferred into 50 mL of LB liquid medium containing ampicillin and grown at 37°C with shaking (200 rpm) until the OD600 reached 0.6. Isopropylthio-β-galactoside was then added to a final concentration of 0.3 mm, and the cultures were grown for another 4 to 5 h at room temperature and aliquoted in 2-mL polypropylene tubes to 1.5-mL aliquots. Cells were harvested by centrifugation at 20,000g for 10 min at 4°C and frozen at −20°C until use (Lavid et al., 2002).
Preparation of Bacterial Lysates
Individual bacterial pellets were suspended in reaction buffer. Ten micrograms per milliliter chicken egg white lysosyme (grade VI, Sigma, 60,000 units mg−1 protein) was added. The samples were vigorously mixed and incubated in ice water (4°C) for 15 min. After the cells lysed, the suspensions were centrifuged (20,000g for 10 min at 4°C). The supernatants were used fresh for characterization of the enzymatic activity of the gene products.
Partial purification of RhAAT1 Recombinant Enzyme
Three milliliters of crude cell-free extracts was purified on a 10-mL P6 column (15 × 120 mm; Bio-Rad, Munich), the active fractions were taken for further purification on a Sepharose Q HiTrap 1-mL column (0.7 × 2.5 cm; Pharmacia Biotech, Piscataway, NJ), and eluted with 25 mm bis-Tris (pH 6.9) buffer containing 5% (v/v) glycerol, 5 mm Na2S2O5, and 10 mm DTT in a gradient of 0 to 1 m NaCl. Fractions containing the highest AAT activity (eluted at 200 mm NaCl) were pooled and utilized for characterization experiments.
AAT Enzymatic Activity from Cell-Free Extracts Derived from Rose Petals and Recombinant E. coli
Radioactive Assay
Small-scale assays were performed by mixing10 μL of crude extract, 10 mm alcohol substrate, and 23 μm (7.8 μCi μmol−1 [14C]acetyl-CoA, Amersham) into a final volume of 50 μL of assay buffer (50 mm bis-Tris [pH 6.9], 10% [v/v] glycerol, 5 mm Na2S2O5, and 10 mm DTT). The assays were incubated for 1 h at 30°C. One milliliter of hexane was added to each tube, which was then vigorously vortexed and spun for 30 s at 5,000g to separate phases. The upper hexane layer, containing the newly formed radiolabeled alcohol acetate esters, was transferred to 5-mL scintillation tubes containing 3 mL of scintillation liquid (4 g L−1 2,5 phenyloxazol, 0.05 g L−1 2,2-ρ-phenylene-bis-5-phenyloxazol, and 10% [v/v] Triton X-100 in toluene). The radioactivity was quantified using a liquid scintillation counter (model 810, Kontron Instruments, Watford, Herts, UK). Enzyme activity in picokatals was calculated based on the specific activity of the substrate and using appropriate correction factors for the counting efficiency of the scintillation machine (Shalit et al., 2001). The reaction velocities were linear for all substrates tested.
GC-MS Assay
Enzymatic assays were performed by mixing 10 mm of the appropriate alcohol substrate, 0.2 mm acetyl-CoA, and 200 μL of crude extract in a total volume of 2 mL in assay buffer incubated for 8 h at 30°C. Two milliliters of hexane was added to each tube, which was then vigorously vortexed and spun for 30 s at 2,000g to separate phases. The upper hexane layers were dried with sodium sulfate and concentrated by a Turbo Vap II (Zymark, Hopkinton, MA) to a final volume of 400 μL. One microliter was injected to the GC-MS for the identification of volatiles (Shalit et al., 2001).
Protein Determination
The Bradford assay (Bradford, 1976) utilizing the Bio-Rad Protein assay reagent was used. A595 was determined using a spectrophotometer (810, Uvikon, Rotkreuz, Switzerland). Bovine serum albumin (Sigma) served as a standard.
Sequence Selection, Alignment, and Phylogenetic Analysis
The RhAAT1 clone was used to conduct BLASTX (Altschul et al., 1990) analysis for related proteins in Viridiplantae using the GenBank nonredundant database of July 2002. The sequences with an E value cutoff of 1 × 10−10 were retrieved. From these sequences, those with confirmed in vitro acetyl-CoA acetyltransferase activity were compiled, aligned, and analyzed by using ClustalX (Thompson et al., 1997). The weighing matrix used was PAM250. The aligned sequences were analyzed with the Neighbor Joining method of Saitou and Nei (1987).
ACKNOWLEDGMENTS
We thank Dr. Vitaly Portnoy for his help with cloning procedures and Dr. Micha Raviv, Shlomit Medina, and Arkady Krasnovsky for helpful discussions and for growing the plants.
Footnotes
This work was supported by the Israeli Ministry of Sciences, Culture, and Sport (grant no. 1410–2–00 to E.L., D.Z., Z.A., A.V., and D.W.), and by a BARD scholarship (to E.P.). This is publication no. 143/2002 of the Agricultural Research Organization (Bet Dagan, Israel).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018572.
LITERATURE CITED
- Aharoni A, Keizer LCP, Bouwmeester HJ, Sun Z, Alvarez-Huerta M, Verhoeven HA, Blaas J, Van Houwelingen AMML, De Vos RCH, Van der Voet H et al. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell. 2000;12:647–661. doi: 10.1105/tpc.12.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bauer K, Garbe D, Surburg H. Common Fragrance and Flavor Materials. Wiley-VCH Velagsgesellschaft mbH, Germany: Weinheim; 2001. p. 44. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Channeliere S, Riviere S, Scalliet G, Jullien F, Szecsi J, Dolle C, Vergne P, Dumas C, Bendahmane M, Hugueney P et al. Analysis of gene expression in rose petals using expressed sequence tags. FEBS Lett. 2002;515:35–38. doi: 10.1016/s0014-5793(02)02413-4. [DOI] [PubMed] [Google Scholar]
- Croteau R, Karp F. Origin of natural odorants. In: Muller P, Lamparsky D, editors. Perfumes: Art, Science and Technology. New York: Elsevier Applied Science; 1991. pp. 101–126. [Google Scholar]
- D'Auria JC, Chen F, Pichersky E. Characterization of an acyltransferase capable of synthesizing benzylbenzoate and other volatile esters in flowers and damaged leaves of Clarkia breweri. Plant Physiol. 2002;130:466–476. doi: 10.1104/pp.006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, D'Auria JC, Hee Nam K, Raguso RA, Pichersky E. Acetyl-CoA:benzylalcohol acetyltransferase: an enzyme involved in floral scent production in Clarkia breweri. Plant J. 1998;14:297–304. doi: 10.1046/j.1365-313x.1998.00121.x. [DOI] [PubMed] [Google Scholar]
- Fellman JK, Mattheis JP. Ester biosynthesis in relation to harvest maturity and controlled-atmosphere storage of apples. In: Rousell RL, Leahy MM, editors. Fruit Flavors, Biogenesis, Characterization and Authentication. ACS Symposium Series 596. Washington, DC: American Chemical Society; 1995. pp. 149–162. [Google Scholar]
- Flament I, Debonneville C, Furrer A. Volatile constituents of roses. In: Teranishi R, Buttery RG, Sugisawa H, editors. Bioactive Volatile Compounds from Plants. Washington, DC: American Chemical Society; 1993. pp. 269–281. [Google Scholar]
- Galili G, Galili S, Lewinsohn E, Tadmor Y. Genetic, molecular, and genomic approaches to improve the value of plant foods and feeds. Crit Rev Plant Sci. 2002;21:167–204. [Google Scholar]
- Guterman I, Shalit M, Menda M, Piestun D, Dafny-Yelin M, Shalev G, Bar E, Davydov O, Ovadis M, Emanuel M et al. Rose scent: genomics approach to discovering novel floral fragrance-related genes. Plant Cell. 2002;14:2325–2338. doi: 10.1105/tpc.005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Ueda Y, Iwata T. Purification and some properties of alcohol acetyltransferase from banana fruit. Plant Cell Physiol. 1985;26:1067–1074. [Google Scholar]
- Helsper JPFG, Davis JA, Bouwmeester HJ, Krol AF, VanKampen MH. Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty Planta. 1998;207:88–95. [Google Scholar]
- Knudsen JT, Tollsten L. Trends in floral scent chemistry in pollination syndromes: floral scent composition in moth-pollinated taxa. Bot J Linn Soc. 1993;113:263–284. [Google Scholar]
- Laflamme P, St-Pierre B, De Luca V. Molecular and biochemical analysis of a Madagascar periwinkle root-specific minovincinine-19-hydroxy-O-acetyltransferase. Plant Physiol. 2001;125:189–198. doi: 10.1104/pp.125.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavid N, Wang J, Shalit M, Gutterman I, Bar E, Beuerle T, Weiss D, Menda N, Shafir S, Zamir D et al. O-methyltransferases involved in the biosynthesis of volatile phenolic derivatives in rose petals. Plant Physiol. 2002;129:1899–1907. doi: 10.1104/pp.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Kyoung-Hee N, Amar O, Lastochkin E, Larkov O, Ravid U et al. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 2001;127:1256–1265. [PMC free article] [PubMed] [Google Scholar]
- Manning K. Isolation of nucleic-acids from plants by differential solvent precipitation. Anal Biochem. 1991;195:45–50. doi: 10.1016/0003-2697(91)90292-2. [DOI] [PubMed] [Google Scholar]
- Perez AG, Sanz C, Olias R, Rios JJ, Olias JM. Evolution of strawberry alcohol acetyltransferase activity during fruit development and storage. J Agric Food Chem. 1996;44:3286–3290. [Google Scholar]
- Pichersky E, Gang DR. Genetics and biochemistry of specialized metabolites in plants: an evolutionary perspective. Trends Plant Sci. 2000;5:439–445. doi: 10.1016/s1360-1385(00)01741-6. [DOI] [PubMed] [Google Scholar]
- Raguso RA, Pichersky E. Floral volatiles from Clarkia breweri and Clarkia concinna (Onagraceae): recent evolution of floral scent and moth pollination. Plant Syst Evol. 1995;194:55–67. [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstruction of phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shalit M, Katzir N, Tadmor Y, Larkov O, Burger Y, Schalechet F, Lastochkin E, Ravid U, Amar O, Edelstein M et al. Acetyl-CoA: alcohol acetyl transferase activity and aroma formation in ripening melon fruits. J Agric Food Chem. 2001;49:794–799. doi: 10.1021/jf001075p. [DOI] [PubMed] [Google Scholar]
- St-Pierre B, De Luca V. Evolution of acyltransferase genes: origin and diversification of the BAHD superfamily of acyltransferases involved in secondary metabolism. In: Romeo T, Ibrahim R, Varin L, De Luca V, editors. Recent Advances in Phytochemistry Evolution of Metabolic Pathways. Vol. 34. Oxford, UK: Elsevier Science Ltd.; 2000. pp. 285–315. [Google Scholar]
- St-Pierre B, Laflamme P, Alarco AM, De Luca V. the terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J. 1998;14:703–713. doi: 10.1046/j.1365-313x.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, Dubendorff JW. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Tsuda A, Bai JH, Fujishita N, Chachin K. Characteristic pattern of aroma ester formation from banana, melon and strawberry with reference to the substrate specificity of ester synthethase and alcohol contents in pulp. J Jpn Soc Food Sci Technol. 1992;39:183–187. [Google Scholar]
- Walker K, Schoendorf A, Croteau R. Molecular cloning of a taxa-4(20), 11(12)-dien-5α-ol-D-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Arch Biochem Biophys. 2000;374:371–380. doi: 10.1006/abbi.1999.1609. [DOI] [PubMed] [Google Scholar]
- Wang J, Pichersky E. Identification of specific residues involved in substrate discrimination in two plant O-methyltransferases. Arch Biochem Biophys. 1999;368:172–180. doi: 10.1006/abbi.1999.1304. [DOI] [PubMed] [Google Scholar]
- Weiss EA. Rosaceae. CAB International, XXXX, XX. 1997. Essential oil crops; pp. 393–416. [Google Scholar]