Abstract
The phytochromes are one of the means via which plants obtain information about their immediate environment and the changing seasons. Phytochromes have important roles in developmental events such as the switch to flowering, the timing of which can be crucial for the reproductive success of the plant. Analysis of phyB mutants has revealed that phyB plays a major role in this process. We have recently shown, however, that the flowering phenotype of the phyB monogenic mutant is temperature dependent. A modest reduction in temperature to 16°C was sufficient to abolish the phyB mutant early-flowering phenotype present at 22°C. Using mutants null for one or more phytochrome species, we have now shown that phyA, phyD, and phyE, play greater roles with respect to phyB in the control of flowering under cooler conditions. This change in the relative contributions of individual phytochromes appears to be important for maintaining control of flowering in response to modest alterations in ambient temperature. We demonstrate that changes in ambient temperature or photoperiod can alter the hierarchy and/or the functional relationships between phytochrome species. These experiments reveal new roles for phyD and phyE and provide valuable insights into how the phytochromes help to maintain development in the natural environment.
Plant growth and development is intimately linked to external cues that signal changes in the environment. Alterations in light quality, quantity, and duration provide the plant with information that accurately reflects changes in both local environment and the changing seasons. To detect and respond to these different light signals, plants have evolved a series of highly specialized photoreceptors. This photoreceptor system includes the red (R) and far-red (FR) light-absorbing phytochromes and the blue/UV-A light-absorbing cryptochromes and phototropins (Whitelam et al., 1998).
The Arabidopsis phytochromes comprise the products of a family of five closely related genes, designated PHYA through PHYE (Mathews and Sharrock, 1997). The photosensory activity of the phytochromes resides in their unique capacity for reversible light-induced interconversion between a R light-absorbing Pr form and a FR light-absorbing Pfr form. Light-triggered Pfr formation also induces cytosolic to nuclear translocation and the activation of signaling via molecular interaction (Kircher et al., 2002; Quail, 2002). In the nucleus, phyA and phyB interact directly with PIF3 and phyB interacts with PIF4 to regulate transcription (Ni et al., 1998, 1999; Martinez-Garcia et al., 2000; Huq and Quail, 2002). Direct interaction with ZTL/ADO1, ELF3, and COP1 provides a means for phyB to connect with the circadian clock and activate the de-etiolation switch (Jarillo et al., 2001; Liu et al., 2001; Yang et al., 2001). phyA and phyB interact with PKS1 and phyA with NDPK2 in the cytosol (Choi et al., 1999; Fankhauser et al., 1999). Furthermore, interactions have been demonstrated between phyA and phyB with cry1 and cry2, respectively (Ahmad et al., 1998; Mas et al., 2000). This may be the means via which at least some of the reported physiological interactions between phyA/phyB and cry1/cry2 occur (Casal and Mazzella, 1998; Neff and Chory, 1998; Mockler et al., 1999).
It is now well established that individual photoreceptors do not act in isolation, but as an interconnected network (Casal, 2002; Nagy and Schafer, 2002). Analysis of mutants null for one or more photoreceptors grown under specific conditions has provided valuable insights into how the photoreceptor network operates within the natural environment. Complex interactions that involve phyA, phyB, phyD, cry1, and cry2 have been described for de-etiolation (Casal, 1995; Casal and Boccalandro, 1995; Casal and Mazzella, 1998; Neff and Chory, 1998; Hennig et al., 1999, 2001; Mazzella et al., 2001). The impact of the cry1 and cry2 mutations on Lhcb*2 promoter-gusA expression was shown to be markedly affected by the absence of phyA and phyB (Mazzella et al., 2001). Furthermore, functional interaction between phyA, phyB and cry1 was shown for accumulation of chlorophyll and anthocyanin (Neff and Chory, 1998; Hennig et al., 2001). Flowering is also subject to strong regulatory control by the photoreceptors. We now have evidence that phyA, phyB, phyD, phyE, cry1, and cry2 regulate flowering through an interconnected network (Devlin et al., 1998, 1999; Mockler et al., 1999).
Temperature is also an important environmental cue in the regulation of flowering. Many plants have adopted a reproductive strategy that requires long periods of cold (1°C–10°C) to promote flowering. This strategy ensures that flowering does not occur in winter months but instead in the more favorable spring climate (Simpson and Dean, 2002). We have recently demonstrated that ambient temperature is a significant modulator of photoreceptor action in the control of flowering (Halliday et al., 2002). A modest reduction in growth temperature, from 22°C to 16°C, completely abolished the phyB mutant early-flowering phenotype frequently observed at higher temperatures. Thus, small changes in ambient temperature can have a large impact on photoreceptor action. These light- and temperature-controlled flowering pathways appear to regulate expression of FT, a known convergence point for the photoperiod and vernalization pathways (Halliday et al., 2002; Hepworth et al., 2002; Simpson and Dean, 2002; Yanovsky and Kay, 2002; Izawa et al., 2002). Therefore, FT (together with LFY and SOC1/AGL20) is an important integration point for multiple flowering pathways.
Studies to date have demonstrated roles for phyD and phyE in a range of developmental processes including germination, seedling establishment, elongation, and flowering responses to end-of-day-FR and low R/FR ratio light (Aukerman et al., 1997; Devlin et al., 1998, 1999; Hennig et al., 1999, 2002). For many of these responses, phyD and phyE have been shown to have redundant roles. However, our earlier studies suggest that in some instances, redundancy of action for an individual phytochrome may simply reflect suboptimal conditions for the particular phytochrome-mediated response. We have conducted a series of experiments that illustrate that changes in the photoperiod and temperature, important environmental cues, change the hierarchy of phytochrome action, revealing prominent roles for phyD and phyE in the natural environment. These experiments also highlight important changes in the functional relationships between the phytochromes that underlie developmental plasticity.
RESULTS
In SDs the phyE Monogenic Mutant Is Early Flowering
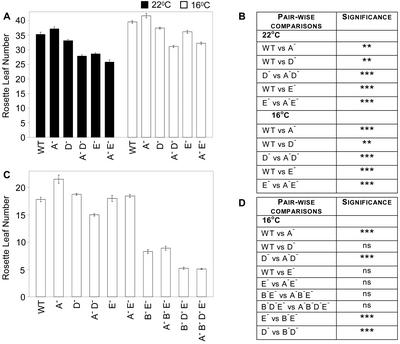
Consistent with earlier studies, when grown under 8-h photoperiods (SDs), the vegetative morphology of the monogenic phyE mutant was similar to that of the wild type (Devlin et al., 1998). However, under our growth conditions (photon irradiance 180 μmol m−2 s−1), the phyE mutant flowered consistently earlier than the wild type, both in terms of rosette leaf number and time to bolting (Fig. 1A; data not shown). The statistical significances for the wild type versus phyE and all other pairwise genotype comparisons were calculated using the Bonferroni multiple comparisons test (Fig. 1, B and D). As previously reported, the phyA mutant flowered slightly later than the wild type (Johnson et al., 1994; Neff and Chory, 1998). However, in SDs, plants null for both phyA and phyE flowered earlier than the monogenic phyE mutant (Fig. 1, A and B). This suggests an interaction of phyA- and phyE-mediated signaling in the control of flowering under SDs. The phyD mutant produced very slightly fewer rosette leaves than the wild type at bolting, whereas the phyAphyD double mutant flowered earlier than the phyD monogenic mutant. As for phyE, this suggests an interaction between the phyA and phyD mutations under SDs.
Figure 1.
Flowering time in SDs and LDs. A, Plants were grown in SDs at either 22°C or 16°C. C, Plants were grown in LDs at 16°C. Rosette leaf number was determined at bolting (photon irradiance, 400–700 nm, 180 μmol m−2 s−1). Bars represent the se. WT, Laer wild type; A−, phyA; D−, phyD; and E−, phyE null mutations. B and D, Statistical significance of differences in flowering time. Pairwise comparisons for genotypes were undertaken using the Bonferroni multiple comparisons test.
At 16°C phyB Acts Redundantly to Control Flowering
When grown at 16°C, the growth of wild-type plants is slower compared with plants maintained at 22°C (Halliday et al., 2002). However, the vegetative developmental phase is only slightly extended because these plants consistently produce only about four more leaves under these cooler conditions (Fig. 1A). Under SDs, the early-flowering phenotype of phyE was maintained at both 22°C and 16°C, although its severity was slightly reduced at the cooler temperature (Fig. 1, A and B). Likewise, the flowering responses of phyA, phyD, phyA phyD, and phyA phyE mutants relative to the wild type were similar under both temperature regimes. Thus, the changes in flowering time imposed by phyE, phyA, and phyD mutations were not markedly altered in the 16°C to 22°C temperature range. We have recently demonstrated that the phyB mutant flowers at the same time as the wild type under 16°C (Halliday et al., 2002). Collectively, these results suggest more prominent roles for phyE, phyA, and phyD in the regulation of flowering under cooler conditions. Although the monogenic phyB mutant is not early flowering when grown at 16°C, the phyB null allele does lead to accelerated flowering in the phyE or phyD backgrounds in both 16-h photoperiods (LDs) and SDs (Figs. 1, C and D, and 3). These data demonstrate synergistic interactions between phyD and phyE with phyB. This suggests that although phyB has a more minor role in repressing flowering at 16°C than it does at 22°C, it still exerts a degree of control on flowering at the cooler temperatures via synergistic interactions with other phytochromes.
Under LDs at 16°C, the monogenic phyE and phyD mutants flowered with a similar number of rosette leaves to the wild type (Fig. 1, C and D). In contrast, the late-flowering phenotype of the phyA mutant was retained under these conditions. This suggests that under cool LDs, the hierarchy changes such that phyA has a more prominent role, with respect to phyE and phyD in the control of flowering.
In LDs phyE Is Epistatic to phyA in the Control of Flowering Time
When grown under LDs, the phyAphyD double mutant flowered significantly earlier than the wild type (Fig. 1C). Monogenic phyD flowered at the same time, and monogenic phyA flowered later than the wild type, suggesting a functional interaction between phyA and phyD in the control of flowering. A similar relationship for phyA and phyD and for phyA and phyE was observed under SDs (Fig. 1A; see above). In contrast, under LDs, impact of the phyA mutation in a phyE background was negligible at both 22°C (data not shown) and 16°C (Fig. 1, C and D). Under LDs, plants carrying the phyA and phyE mutations flowered at the same time as the phyE mutant. Furthermore, the phyAphyBphyE and phyAphyBphyDphyE mutants flowered at the same times as phyBphyE and phyBphyDphyE, respectively. These data suggest that under LDs phyE is required for the phyA mutant phenotype.
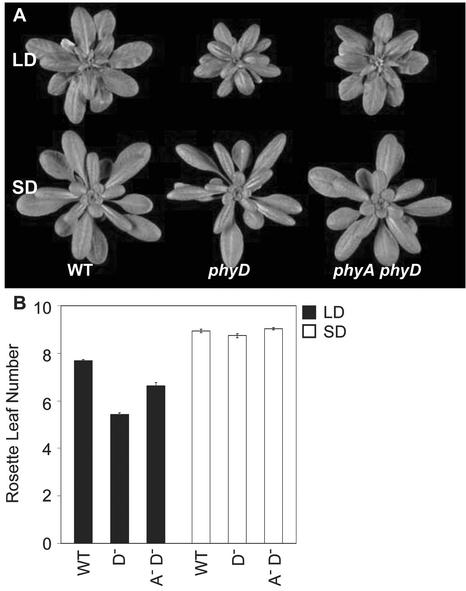
The Monogenic phyD Mutant Has Reduced Leaf Size in LDs
Like phyE, phyD has been shown to act redundantly with phyB to control leaf shape (Devlin et al., 1998, 1999). However, we have shown that small adjustments in temperature reveal a striking leaf phenotype in the monogenic phyD mutant. When grown in LDs under cooler conditions (16°C), phyD produced markedly smaller leaves than the wild type, revealing a new role for phyD in the promotion of leaf expansion (Fig. 2, A and B). Removal of phyA in addition to phyD restored much of the wild-type phenotype, suggesting that phyA was required for the monogenic phyD mutant phenotype. This phenotype is not only temperature conditional, it is also photoperiod dependent. When grown under SDs, the rosette diameter of phyD was very similar to the wild type. Under these conditions, phyD leaf area was slightly smaller than the wild type; however, the removal of phyA in addition to phyD completely restored the wild-type phenotype (Fig. 2, A and B). Taken together, these data suggest that the role of phyD in controlling leaf development is photoperiod and temperature conditional. Furthermore, phyA appears to have a role in moderating phyD action in this response.
Figure 2.
Basal rosette diameter in LDs and SDs. A, Laer WT, and phyD, phyAphyD mutants grown in LDs or SDs for 28 d. B, Basal rosette leaf diameter (centimeters) was determined for 28-d-old plants grown in LDs or SDs (photon irradiance, 400–700 nm, 180 μmol m−2 s−1) at 16°C. Bars represent the se. WT, Laer wild type; A−, phyA; and D−, phyD.
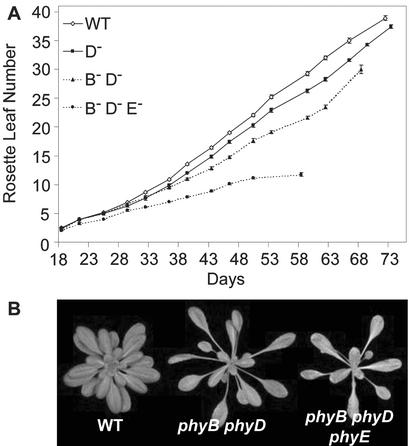
phyD Slows Rosette Leaf Formation Rate
Earlier work established a prominent role for phyB in controlling the rate of rosette leaf production (Mazzella et al., 2001; Halliday et al., 2002). Analysis of the monogenic phyD mutant revealed a role for phyD in the control of rosette leaf production rate throughout vegetative development, but one that gains prominence in the second half of the vegetative phase. When grown in SDs at 16°C, the first seven to eight leaves were produced at a similar rate in the phyD mutant and the wild type, thereafter in phyD, leaf production slowed (Fig. 3A). A further slowing of leaf production was observed during the final third of the developmental phase. These data are consistent with our recent analysis of the phyAphyBphyD mutant that suggested this role for phyD in the second half of the vegetative developmental phase (Halliday et al., 2002). As for the phyB mutant, the phyD phenotype was seen at both 22°C and 16°C (Halliday et al., 2002; data not shown). This phenotype contrasts with that of phyA and phyE, both of which produce leaves at a wild-type rate (Halliday et al., 2002; data not shown). Removal of phyB in addition to phyD slowed leaf production further (Fig. 3, A and B). Leaf production of mutants null for phyB, phyD, and phyE was very severely retarded. On occasion, growth was more severely disrupted in phyBphyDphyE mutants, these plants appeared pale and sickly and developed necrotic lesions (data not shown). We have not observed these phenotypic traits in our phyAphyBphyD or phyAphyBphyE triple mutants, which may reflect the relative importance of phyB, phyD and phyE for normal vegetative development.
Figure 3.
Rosette leaf production rate in 16°C SDs. A, Rosette leaf number was counted at time intervals (days) until flowering time in plants grown at 16°C in SDs (photon irradiance, 400–700 nm, 180 μmol m−2 s−1). Bars represent the se. WT, Laer wild type; B−, phyB; D−, phyD; and E−, phyE null mutations. B, Laer WT, and phyBphyD, phyBphyDphyE mutants grown in SDs for 46 d.
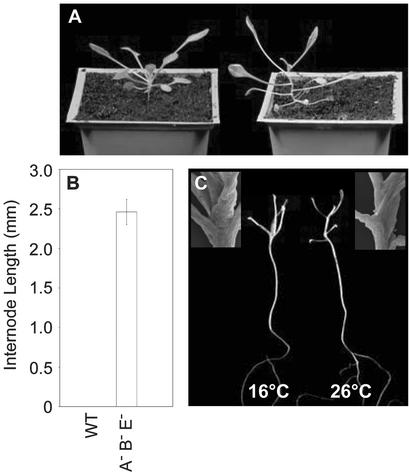
The Elongated Internode Phenotype of phyAphyBphyE Is Temperature Dependent
The identification of phytochrome controlled internode elongation, and flowering responses in the phyAphyB double mutant provided the basis of a screen that identified the phyE null mutation (Devlin et al., 1996, 1998). The constitutively early flowering and elongated internode phenotype of the phyAphyBphyE mutant provided evidence for the role of phyE in these aspects of photomorphogenesis. However, in a similar fashion to mutants lacking phyA, phyB, and cry1, the elongated internode phenotype of the phyAphyBphyE mutant is only evident if plants are grown at an inductive temperature (Mazzella et al., 2000). When phyAphyBphyE plants were grown under SDs at 16°C, the mutant exhibited a normal rosette habit, whereas growth at or above 22°C resulted in the phyAphyBphyE mutant producing distinct internodes (Fig. 4, A and B; Devlin et al., 1998). The elongated internode phenotype was not observed in double mutant combinations of phyA, phyB, or phyE under these conditions. We therefore reasoned that under warmer growth conditions, internode elongation may be the default situation and that phyE (together with phyA and phyB) inhibit this elongation to maintain the rosette growth habit. To test this, wild-type seedlings were grown in darkness on vertically oriented Suc-containing plates at either 16°C or 26°C. Suc availability in the aerial part of the plant is known to promote seedling development in the absence of photoreceptor action (Roldan et al., 1999). Although the seedlings exhibited an elongated growth habit under both temperature regimes, internodes were only elongated in seedlings grown at the warmer temperature (Fig. 4C). This suggests that at permissive temperature, internode elongation is the default position and that phyE, phyB and phyA are important for maintaining the compact rosette habit under these conditions.
Figure 4.
Temperature-dependent internode elongation. A, The phyAphyBphyE triple mutant, grown at 16°C (left) and 21°C (right) at photon irradiance, 400 to 700 nm, 100 μmol m−2 s−1. B, Rosette internode length (millimeters) of 21°C-grown wild type and phyAphyBphyE. Plants were grown in SDs for these experiments; bars represent the se. C, Wild-type seedlings grown in the dark at 16°C and 26°C. Fresh and electron microgram images were taken of seedlings grown on 3% (w/v) Suc for 3 weeks.
DISCUSSION
We set out to gain further insights into how the phytochrome network controls development in the natural environment. By growing plants deficient in one or more phytochrome species under different photoperiods and temperatures, we have been able to establish new roles for phyD and phyE. We have also demonstrated that changes in photoperiod and temperature dramatically alter functional relationships between phytochrome species. A growing body of evidence suggests that this is the means via which the photoreceptor system manipulates development in response to changed environmental conditions. We show this also provides a mechanism for photoreceptors to maintain developmental stability under different ambient temperatures.
The Early-Flowering Phenotype of phyE Is Specific to SDs
PhyB has been shown to be an important regulator of flowering time in response to light quality and photoperiod (Whitelam et al., 1998; Salome et al., 2002). We have shown that in SDs, like phyB, the monogenic phyE mutant also flowers early. Furthermore, we did not observe this phenotype under LDs, which suggests the early-flowering phenotype of phyE is specific to SDs. Thus it appears that under SDs, phyB and phyE play major roles in regulating flowering time. Devlin and co-workers (1998) previously described the phyE phenotype as wild type, however, this apparent contradiction may simply reflect the comparatively high-light levels used in our experiments. The apparent specificity of phyE action to SDs may occur as an indirect consequence of the short photoperiod. Alternatively, this may represent a mechanism via which light interacts with the circadian system to delay flowering under SDs.
The phyD mutant flowered slightly earlier than the wild type under SDs. Again like phyE, this effect was not observed under LDs. The enhanced effect of the phyE and to a lesser extent the monogenic phyD mutations under SDs may reflect more influential roles for phyD and phyE under shorter photoperiods in the inhibition of flowering. Although the effects of the monogenic phyD and phyE mutations were not severe, in a phyA background, they had a larger impact. In SDs, we have shown the genetic interactions of phyD and phyE with phyA are synergistic. This genetic relationship of phytochrome genes enables specific modification of flowering when phyA signaling is perturbed in addition to phyD or phyE. Because phyA, phyD, and phyE are differentially regulated by light and exhibit different action kinetics, this may be a means for the plant to distinguish and respond to a simultaneous change in two or more parameters in the light environment (Eichenberg et al., 2000; Kircher et al., 2002). This type of mechanism may facilitate acceleration of flowering in response to neighboring vegetation. Under these circumstances, fluence rates are high (degrading phyA), but the light reflected from the potential competitors is FR-enhanced, lowering the proportion of active phyD or phyE. This type of signaling provides plants with a means to interpret and process complex changes in the light environment.
Photoperiod Affects the Functional Relationship of phyA and phyE in the Control of Flowering
The synergistic relationship of phyA and phyE in the control of flowering observed in SDs was not observed under LDs. In LDs, we have shown that phyE is epistatic to phyA in this response. These data suggest that the length of the photoperiod has a significant impact on how the phyA and phyE pathways interact. Under SDs, the phyA- and phyE-signaling pathways are functionally distinct, whereas under LDs phyE is necessary for phyA action. One could speculate that altering the functional relationships of phyA and phyE in this way provides one route via which flowering can be adjusted in response to the prevailing photoperiod. For example, in LDs, the absence of both phyA and phyE had practically no effect on flowering time, whereas in SDs, phyAphyE was early flowering. Thus, the combined action of the phyA and phyE appears to be inhibitory under SDs, conditions that delay flowering in the wild type. Conditional synergism has previously been demonstrated for cry1 and phyB in the control of hypocotyl length (Casal and Mazzella, 1998). They demonstrated that in saturating light conditions, phyB and cry1 acted independently, but under conditions that were non-saturating for either phyB or cry1 action, they acted synergistically. These types of experiments illustrate how changes in the light environment can dramatically change the functional relationship between photoreceptors. Our data suggest that photoperiod-mediated changes in the functional relationship between phyA and phyE may contribute to the changes in flowering time observed in different photoperiods.
At 16°C, phyE and phyD Have More Prominent Roles in the Control of Flowering
The early-flowering phenotype of the monogenic phyB mutant is well known (Whitelam et al., 1998). However, we have recently shown that this phenotype is abolished when plants are grown at 16°C, a typical summertime temperature in a range of northern latitudes (Halliday et al., 2002). The data in this paper demonstrate that phyB does have a role in the control of flowering under cooler conditions, but its role is redundant in the presence of phyD and phyE. Under LDs at 16°C, the monogenic phyD and phyE mutations had no impact on flowering time, however, loss of phyB in addition to phyD or phyE accelerated flowering. This suggests that under LDs at 16°C, phyD, phyE, and phyB have largely redundant roles in the control of flowering, however, the interaction between phyB and phyD or phyE was synergistic. Under SDs at 16°C, a redundant role was still observed for phyB, however, the phyE and, to a lesser extent, the monogenic phyD mutations accelerated flowering. Thus, at lower temperatures, phyE and phyD have more prominent roles in the control of flowering under SDs.
We observed that the late-flowering phenotype of the monogenic phyA mutant was retained at 16°C under both SDs and LDs. Thus, phyA appears to have a more prominent role in LDs, but shares prominence with phyE and phyD in SDs at cooler temperatures. Our recent work demonstrated that wild-type plants display a normal early-flowering response to low R/FR ratio at 16°C (Halliday et al., 2002). This, together with our current findings suggests that phyB takes the principle role under warmer conditions, however, the action of phyA, phyE, and phyD gain importance under cooler conditions. This change in the hierarchy of phytochrome action at 16°C maintains phytochrome control of flowering under these conditions. This type of accommodative action or “developmental canalization” has been proposed for phyA, phyB, cry1, and cry2 in the control of seedling de-etiolation (Mazzella et al., 2001). This type of complex, highly connected, and yet plastic network is thought to be essential for normal development as it buffers both environmental change and genetic variation (Stearns, 2002). Our observations are interesting in context with recent findings that a drop in temperature from 23°C to 16°C enhanced the late flowering phenotype of cry2 considerably (Blazquez et al., 2003). Therefore, like phyB, the cry2 phenotype is also very sensitive to changes in temperature. However, in contrast to phyB, cry2 action appears to be enhanced under cooler conditions.
Photoperiod and Temperature Affect the Role of phyD in the Control of Leaf Expansion
When grown in LDs at 16°C, the phyD mutant rosette leaves were notably smaller than those of the wild type. However, monogenic phyD mutant rosettes had a wild-type appearance under SDs and warmer LD conditions (Aukerman et al., 1997; Devlin et al., 1999). These data suggest that this rather striking phyD phenotype is dependent upon both photoperiod and temperature. Furthermore, while under permissive conditions, the phyD mutation inhibits leaf expansion; the removal of phyA in addition to phyD greatly attenuates this response. This suggests that phyA is required for the phyD small rosette phenotype. Recent work has demonstrated that phyD acts redundantly with phyB in the inhibition of leaf elongation when plants are grown in either LDs or SDs under warmer conditions (Aukerman et al., 1997; Devlin et al., 1999). In contrast, under cool LDs, phyD appears to be important for promotion of leaf blade expansion. The ecological significance of this finding is not clear, however, under these conditions, phyD appears to have an opposing action to phyB in the control of leaf shape.
phyD Controls the Rate of Rosette Leaf Formation
Recent reports have shown that the phyB mutation severely affects the rate of rosette leaf production (Mazzella et al., 2001; Halliday et al., 2002). Our recent analysis of the phyB, phyAphyB, and phyAphyBphyD suggested that phyD also regulated leaf production rate, but only in the second half of the vegetative phase (Halliday et al., 2002). Analysis of the monogenic phyD mutant revealed that phyD contributes to the control of rosette leaf production throughout development. However, its role was greatest in the final third of the vegetative phase. Thus, both phyB and phyD control the rate of rosette leaf formation, but their relative contributions are dependent on the developmental phase. These phytochrome-mediated effects are clearly a means of adjusting leaf production to suit the prevailing light environment. Such a strategy may be important when resources are limited, for example, under conditions of heavy vegetation shade.
The Elongated Phenotype of the phyAphyBphyE Mutant Is Temperature Dependent
Earlier work by Mazzella et al. (2000) demonstrated that the elongated internode phenotype of phyB, phyAphyB, phyBcry1, and phyAphyBcry1 mutants grown in continuous white light was a temperature-dependent phenomenon. Our experiments provide evidence that the elongated internode phenotype phyAphyBphyE is also temperature dependent. When grown under SDs at 16°C, phyAphyBphyE grew with a compact rosette, whereas at 22°C, internodes were clearly visible. Because double mutant combinations of phyA, phyB, and phyE did not produce internodes under our conditions, it appears that phyA, phyB, and phyE act redundantly to maintain the basal rosette during development. These data are consistent with previous data that demonstrate roles for phyA, phyB, and cry1 in this respect (Mazzella et al., 2001). Because multiple photoreceptors appear to suppress internode formation, we were interested to establish whether elongation was the default condition at warmer temperatures. To do this, we grew wild-type seedlings on Suc at 16°C and 26°C in darkness. These seedlings developed internodes at 26°C but not 16°C. These data are consistent with internode elongation being the default situation under warmer temperatures. When seedlings are grown in the light phyE, phyA, phyB, and cry1 act collectively to preserve the rosette growth habit.
Continually surveying their surroundings, the light receptors act as an integrated signaling network keeping development in tune with the environment. This complex task requires a flexible network that can both respond to and accommodate environmental change. The data presented in this paper provide a window into the complex light-signaling network that finely tunes development. Changes in the functional relationship between photoreceptors appear to be crucial for adjusting development in response to environmental cues such as photoperiod. However, they are also necessary for maintaining responses under varied environmental conditions. Changes in the hierarchy of phytochrome action under different ambient temperatures appear to be an important mechanism for maintaining control of flowering in the natural environment where temperatures fluctuate. Such accommodative behavior is an acknowledged characteristic of highly interconnected networks that act to buffer the effect of environmental or genetic perturbations (Casal, 2002; Stearns, 2002). Understanding the mechanisms that control both responsive and accommodative photoreceptor action will be one of our future challenges.
MATERIALS AND METHODS
Plant Material and Growth Conditions
In all of our experiments, we used Arabidopsis ecotype Landsberg erecta (Laer). Phytochrome mutant alleles were phyA-2 (Whitelam et al., 1993), phyB-1 (Koornneef et al., 1980), phyD-1 (Aukerman et al., 1997), and phyE-1 (Devlin et al., 1998). The phyD-1 mutation is a naturally occurring allele found in the Wassilewskija ecotype, therefore, near-isogenic Laer phyD-1 mutant lines were created by introgression of the phyD-1 mutation into the Laer ecotype (Aukerman et al., 1997).
In each of the experiments, seeds were sown on 0.8% (w/v) Lehle medium (Lehle Seeds, Round Rok, TX), and stratified in darkness at 4°C for 5 d before transfer to SDs or LDs or at 16°C or 22°C. After a further 5 d, uniformly sized seedlings were transplanted to 5- × 5- × 5-cm pots containing a 3:1 compost:horticultural silver sand mix. Light was provided by L65/80W/30 warm-white fluorescent tubes (photon irradiance 400 to 700 nm, 180 μmol m−2 s−1; Osram Ltd., St. Helens, UK).
Seedlings in the dark internode elongation experiments were stratified and germinated as above, and then grown on 3% (w/v) Suc Murashige and Skoog medium in complete darkness for 3 weeks.
Fixation and Scanning of Tissue
A scanning electron microscope was used to obtain the close-up views of internodes. Samples were fixed in the fixing buffer 2% (w/v) gluteraldehyde in 30 mm sodium-cacodylate for 24 h. After three 10-min washes in fixing buffer, a secondary fix (1% [w/v] osmodium in fixing buffer) was applied for a further 24 h followed again by three 10-min washes. Samples were then dehydrated via 15-min soaks in each of the acetone series (v/v): 30%, 50%, 70%, 90%, and 100% × 3. After four 15-min exchanges through liquid CO2, the samples were dried using a Balzers Critical Point Drier CPD030. Samples were mounted on aluminum stabs and sputter coated with gold/palladium to an approximate thickness of 673A in a Polaron SC7640. Images were collected by a scanning electron microscope (S-3000H, Hitachi, Tokyo).
Plant Growth Assays
For plants grown under SDs, rosette leaf counts were carried out twice a week. Leaves were counted only when the petiole was visible to the naked eye. Flowering time was recorded as primary rosette leaf number at inflorescence production. Rosette leaves were distinguished from axillary leaves on the basis of morphological differences. Rosette diameter was measured at the widest point with a ruler. For quantification of internode length, images were taken with a digital camera, and measurements were made using Sigma Scan software (SPSS Science Software UK Ltd., Woking, Surrey, UK).
Statistical Analysis
Statistical analysis was performed using ANOVA and the Bonferroni multiple comparisons test. For each experiment, pairwise comparisons were made between all relevant genotypes, a subset of which is shown in Figure 1, B and D.
ACKNOWLEDGMENT
We thank Wendy Stoddart for technical assistance.
Footnotes
This work was supported by the Biotechnology and Biological Science Research Council (UK).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018135.
LITERATURE CITED
- Ahmad M, Jarillo JA, Smirnova O, Cashmore AR. The CRY1 blue light photoreceptor of Arabidopsisinteracts with phytochrome A in vitro. Mol Cell. 1998;1:939–948. doi: 10.1016/s1097-2765(00)80094-5. [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the ArabidopsisWassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez MA, Ahn JH, Weigel D. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat Genet. 2003;33:168–171. doi: 10.1038/ng1085. [DOI] [PubMed] [Google Scholar]
- Casal JJ. Coupling of phytochrome B to the control of hypocotyl growth in Arabidopsis. Planta. 1995;196:23–29. doi: 10.1007/BF00193213. [DOI] [PubMed] [Google Scholar]
- Casal JJ. Environmental cues affecting development. Curr Opin Plant Biol. 2002;5:37–42. doi: 10.1016/s1369-5266(01)00218-7. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G, Yi H, Lee J, Kwon YK, Soh MS, Shin B, Luka Z, Hahn TR, Song PS. Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature. 1999;401:610–613. doi: 10.1038/44176. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Halliday KJ, Harberd NP, Whitelam GC. The rosette habit of Arabidopsis thalianais dependent upon phytochrome action: novel phytochromes control internode elongation and flowering time. Plant J. 1996;10:1127–1134. doi: 10.1046/j.1365-313x.1996.10061127.x. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1487. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Robson PR, Patel SR, Goosey L, Sharrock RA, Whitelam GC. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsisby controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberg K, Baurle I, Paulo N, Sharrock RA, Rudiger W, Schafer E. Arabidopsisphytochromes C and E have different spectral characteristics from those of phytochromes A and B. FEBS Lett. 2000;470:107–112. doi: 10.1016/s0014-5793(00)01301-6. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science. 1999;284:1539–1541. doi: 10.1126/science.284.5419.1539. [DOI] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J. 2003;33:875–888. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- Hennig L, Funk M, Whitelam GC, Schafer E. Functional interaction of cryptochrome 1 and phytochrome D. Plant J. 1999;20:289–294. doi: 10.1046/j.1365-313x.1999.t01-1-00599.x. [DOI] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Sweere U, Martin A, Schafer E. Negative interference of endogenous phytochrome b with phytochrome a function in Arabidopsis. Plant Physiol. 2001;125:1036–1044. doi: 10.1104/pp.125.2.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig L, Stoddart WM, Dieterle M, Whitelam GC, Schafer E. Phytochrome E controls light-induced germination of Arabidopsis. Plant Physiol. 2002;128:194–200. [PMC free article] [PubMed] [Google Scholar]
- Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002;21:4327–4337. doi: 10.1093/emboj/cdf432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Quail PH. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 2002;21:2441–2450. doi: 10.1093/emboj/21.10.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. Phytochrome mediates the external light signal to repress FTorthologs in photoperiodic flowering of rice. Genes Dev. 2002;16:2006–2020. doi: 10.1101/gad.999202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo JA, Capel J, Tang RH, Yang HQ, Alonso JM, Ecker JR, Cashmore AR. An Arabidopsiscircadian clock component interacts with both CRY1 and phyB. Nature. 2001;410:487–490. doi: 10.1038/35068589. [DOI] [PubMed] [Google Scholar]
- Johnson E, Bradley M, Harberd NP, Whitelam GC. Photoresponses of light-grown phyA mutants of Arabidopsis: phytochrome A is required for the perception of daylength extensions. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Gil P, Kozma-Bognar L, Fejes E, Speth V, Husselstein-Muller T, Bauer D, Adam E, Schafer E, Nagy F. Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell. 2002;14:1541–1555. doi: 10.1105/tpc.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolf E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana(L.) Heynh. Z Planzenphysiol. 1980;100:147–160. [Google Scholar]
- Liu XL, Covington MF, Fankhauser C, Chory J, Wagner DR. ELF3 encodes a circadian clock-regulated nuclear protein that functions in an ArabidopsisPHYB signal transduction pathway. Plant Cell. 2001;13:1293–1304. doi: 10.1105/tpc.13.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia JF, Huq E, Quail PH. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 2000;288:859–863. doi: 10.1126/science.288.5467.859. [DOI] [PubMed] [Google Scholar]
- Mas P, Devlin PF, Panda S, Kay SA. Functional interaction of phytochrome B and cryptochrome 2. Nature. 2000;408:207–211. doi: 10.1038/35041583. [DOI] [PubMed] [Google Scholar]
- Mathews S, Sharrock RA. Phytochrome gene diversity. Plant Cell Environ. 1997;20:666–671. [Google Scholar]
- Mazzella MA, Bertero D, Casal JJ. Temperature-dependent internode elongation in vegetative plants of Arabidopsis thalianalacking phytochrome B and cryptochrome 1. Planta. 2000;210:497–501. doi: 10.1007/PL00008157. [DOI] [PubMed] [Google Scholar]
- Mazzella MA, Cerdan PD, Staneloni RJ, Casal JJ. Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsisdevelopment. Development. 2001;128:2291–2299. doi: 10.1242/dev.128.12.2291. [DOI] [PubMed] [Google Scholar]
- Mockler TC, Guo H, Yang H, Duong H, Lin C. Antagonistic actions of Arabidopsiscryptochromes and phytochrome B in the regulation of floral induction. Development. 1999;126:2073–2082. doi: 10.1242/dev.126.10.2073. [DOI] [PubMed] [Google Scholar]
- Nagy F, Schafer E. Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 2002;53:329–355. doi: 10.1146/annurev.arplant.53.100301.135302. [DOI] [PubMed] [Google Scholar]
- Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsisdevelopment. Plant Physiol. 1998;118:27–35. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell. 1998;95:657–667. doi: 10.1016/s0092-8674(00)81636-0. [DOI] [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature. 1999;400:781–784. doi: 10.1038/23500. [DOI] [PubMed] [Google Scholar]
- Quail PH. Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol. 2002;3:85–93. doi: 10.1038/nrm728. [DOI] [PubMed] [Google Scholar]
- Roldan M, Gomez-Mena C, Ruiz-Garcia L, Salinas J, Martinez-Zapater JM. Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsisin the dark. Plant J. 1999;20:581–590. doi: 10.1046/j.1365-313x.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Salome PA, Michael TP, Kearns EV, Fett-Neto AG, Sharrock RA, McClung CR. The out of phase 1 mutant defines a role for PHYB in circadian phase control in Arabidopsis. Plant Physiol. 2002;129:1674–1685. doi: 10.1104/pp.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Stearns SC. Progress on canalization. Proc Natl Acad Sci USA. 2002;99:10229–10230. doi: 10.1073/pnas.172388999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsisdisplay a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Patel S, Devlin PF. Phytochromes and photomorphogenesis in Arabidopsis. Philos Trans R Soc Lond B Biol Sci. 1998;353:1445–1453. doi: 10.1098/rstb.1998.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HQ, Tang RH, Cashmore AR. The signaling mechanism of ArabidopsisCRY1 involves direct interaction with COP1. Plant Cell. 2001;13:2573–2587. doi: 10.1105/tpc.010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]