Abstract
Potato (Solanum tuberosum) homeobox 1 (POTH1) is a class I homeobox gene isolated from an early-stage tuber cDNA library. The RNA expression pattern of POTH1, unlike that of most other class I knotted-like homeobox genes, is widespread in the cells of both indeterminate and differentiated tissues. Using in situ hybridization, POTH1 transcripts were detected in meristematic cells, leaf primordia, and the vascular procambium of the young stem. Overexpression of POTH1 produced dwarf plants with altered leaf morphology. Leaves were reduced in size and displayed a “mouse-ear” phenotype. The mid-vein was less prominent, resulting in a palmate venation pattern. The overall plant height of overexpression lines was reduced due to a decrease in internode length. Levels of intermediates in the gibberellin (GA) biosynthetic pathway were altered, and the bioactive GA, GA1, was reduced by one-half in sense mutants. Accumulation of mRNA for GA 20-oxidase1, a key biosynthetic enzyme, decreased in overexpression lines. In vitro tuberization was enhanced under both short- and long-day photoperiods in several POTH1 overexpression lines. Sense lines produced more tubers at a faster rate than controls. These results imply that POTH1 mediates the development of potato by acting as a negative regulator of GA biosynthesis.
Homeobox genes, a family of transcription factors highly conserved in animals, plants, and yeast (Chan et al., 1998), are implicated in the control of cell fate. The Antennapedia homeobox gene in fruitfly (Drosophila melanogaster), for example, specifies leg identity while inhibiting the formation of an antenna (Mann and Chan, 1996). Ectopic expression of the eyeless gene in the wing imaginal disc tissue of fruitfly embryos causes a normal eye to form on the wings (Halder et al., 1995). The first plant homeobox gene to be discovered was knotted1 (kn1) from maize (Zea mays; Vollbrecht et al., 1991). Dominant gain-of-function mutations of Kn1 formed knot-like structures along lateral veins. These knots were composed of cells that continued to divide rather than differentiate normally (Vollbrecht et al., 1991; Smith et al., 1992), indicating that kn1 is involved in regulating cell fate (Clark et al., 1996; Kerstetter et al., 1997; Chan et al., 1998).
Knotted-like homeobox (knox) genes have been isolated from several plant species (for review, see Reiser et al., 2000) and can be divided into two classes based on expression patterns and sequence similarity (Kerstetter et al., 1994). Class I knox genes have high similarity to the kn1 homeodomain and generally have a meristem-specific mRNA expression pattern. Class II knox genes usually have a more widespread expression pattern. Knox genes are members of the three amino acid loop extension (TALE) superclass of homeobox genes (Bürglin, 1997). The TALE superclass includes members from plants, animals, and fungi and is characterized by the addition of three amino acids, Pro-Tyr-Pro (PYP), between helices 1 and 2 of the homeodomain. Knox genes share conserved regions outside of the homeodomain including the KNOX and ELK domains. The ELK domain has been postulated to be involved in nuclear localization, protein-protein interactions, or suppression of gene activation (Meisel and Lam, 1996; Nagasaki et al., 2001). The KNOX domain is composed of two α-helices separated by a flexible linker and is conserved with the animal Meis and PBC domains (Bürglin, 1997). By overexpressing chimeric proteins of the tobacco (Nicotiana tabacum) knox family, Sakamoto et al. (1999) showed that the second half of the KNOX domain was most important for determining severity of the mutant phenotypes. Recent studies indicate that the KNOX domain is essential for the formation of homo- and heterodimers (Bellaoui et al., 2001; Müller et al., 2001; Nagasaki et al., 2001).
Genetic analysis has demonstrated the role of knox class I genes in the formation and maintenance of the shoot apical meristem (SAM). Knock-out mutants of the Arabidopsis knox gene, shoot meristemless, are characterized by deficiencies in the development of the SAM (Barton and Poethig, 1993; Clark et al., 1996; Endrizzi et al., 1996). Loss-of-function mutants of kn1 of maize resulted in fewer lateral meristems but more lateral organs, such as leaves and carpels (Kerstetter et al., 1997), whereas gain-of-function mutants resulted in the formation of ectopic meristems (Smith et al., 1992). Gain-of-function mutations in class I knox genes are generally a result of ectopic expression that results in changes in cell fate and disruption of differentiation (Smith et al., 1992; Muehlbauer et al., 1999; Reiser et al., 2000). The dominant mutants of tomato (Lycopersicon esculentum), Mouse-ear and Curl, lead to distinct phenotypes with altered architecture and morphology of leaves. Both mutations are the result of ectopic expression of the same tomato knox gene, TKn2/LeT6 (Chen et al., 1997; Parnis et al., 1997). Overexpression of kn1, NTH15, and OSH1 (maize, tobacco, and rice [Oryza sativa] knox class I genes, respectively) in transgenic tobacco or LeT6/TKn2 (Janssen et al., 1998) and kn1 (Hareven et al., 1996) in transgenic tomato resulted in plants displaying altered leaf morphology, dwarfism, and loss of apical dominance (Sinha et al., 1993; Sato et al., 1996; Tamaoki et al., 1997). In tomato, overexpression resulted in a severalfold increase in the complexity of the compound structure of the leaves (Janssen et al., 1998; Hareven et al., 1996). Hay et al. (2002) show that SHOOT MERISTEMLESS, a KNOX protein, inhibits gibberellin (GA) synthesis in the meristem and that other KNOX genes of tomato are implicated in the regulation of leaf architecture.
Alterations in morphology caused by ectopic expression of knox genes were accompanied by changes in hormone levels. Expression of the maize homeobox gene kn1 has been linked to the accumulation of the hormone cytokinin. Leaf explants of tobacco that overexpress kn1 were capable of cytokinin-independent growth and exhibited elevated cytokinin levels (Hewelt et al., 2000). Ori et al. (1999) showed that kn1 ectopic expression driven by a senescence-associated gene (SAG12) promoter delayed senescence and increased cytokinin levels. The phenotype produced by transgenic activation of KNAT2 expression suggested that KNAT2 acts synergistically with cytokinins and antagonistically with ethylene (Hamant et al., 2002). The specific accumulation of cytokinins in leaves and altered leaf morphology were observed when KNAT1 was overexpressed in lettuce (Frugis et al., 2001). Although elevated cytokinin levels were also observed in transgenic tobacco overexpressing NTH15 and OSH1, levels of GAs decreased (Tamaoki et al., 1997; Kusaba et al., 1998b). Northern analysis revealed that expression of the gene encoding an important GA biosynthesis enzyme, GA 20-oxidase, decreased in transgenic plants resulting in the reduction of GA levels (Kusaba et al., 1998a; Tanaka-Ueguchi et al., 1998). The NTH15 protein of tobacco binds to specific elements in regulatory regions of the GA 20-oxidase gene of tobacco to suppress its activity (Sakamoto et al., 2001).
The results of this study demonstrate that a potato (Solanum tuberosum) homeobox gene, POTH1, is involved in vegetative pattern formation, accompanied by a decrease in GA levels. On the basis of sequence similarity, POTH1 is classified as a class I knox gene, however, its expression pattern is more widespread than most of the class I knox genes. The phenotype of POTH1 overexpression mutants includes altered leaf morphology, dwarfism, decreased GA1 levels, and enhanced tuber formation in vitro.
RESULTS
Isolation and Characterization of POTH1
An early-stage tuber cDNA library (Kang and Hannapel, 1995) from potato cv Superior was screened for members of the homeobox gene family. PCR primers were designed from the consensus sequence of the homeoboxes of the class I genes kn1 from maize (Vollbrecht et al., 1991), KNAT1 and KNAT2 from Arabidopsis (Lincoln et al., 1994), OSH1 from rice (Matsuoka et al., 1993), and SBH1 from soybean (Glycine max; Ma et al., 1994). A mass excision of the tuber cDNA library was performed, and this DNA was used as the PCR template. A band corresponding to the expected size of 158 nucleotides was purified, cloned, and sequenced. This potato homeobox fragment was 87% identical to the conserved positions of the consensus sequence created from the five class I genes (data not shown), and was used as a probe to screen the cDNA library. Library screening resulted in the isolation of a truncated, 1,053-nucleotide homeobox cDNA from the library, which was used as a probe to screen the library again. Three clones were isolated, and the full-length 1,383-nucleotide potato homeobox cDNA, POTH1, was selected for further study. The cDNA (GenBank accession no. U65648) includes an open reading frame of 1,035 nucleotides coding for a 345-residue protein estimated to have a mass of 37.95 kD. It contains a 134-nucleotide 5′-untranslated region, and a 216-nucleotide 3′-untranslated region, including the poly(A) tail. The coding sequence of the protein includes the 97-amino acid KNOX domain, the 22-amino acid ELK domain, and the 64-amino acid homeodomain.
A phylogenetic analysis of the sequences of KNOX proteins most similar to POTH1 was performed (Fig. 1). POTH1 shares 86% similarity with the homeodomain of KN1, classifying it as a class I homeobox protein (Kerstetter et al., 1994). However, over the entire protein sequence, POTH1 shares only 51% similarity with KN1. The five proteins with the most similarity to POTH1 include TKN3 from tomato (U76408), NTH22 of tobacco (Nishimura et al., 1999), PKN2 of Ipomoea nil (AB016000), and KNAT6 and KNAT2 of Arabidopsis (Lincoln et al., 1994) with 94%, 88%, 73%, 65%, and 63% similarity overall, respectively. As expected, relatively high levels of conservation were observed in the homeodomains (97%–83%) and in the KNOX domains (95%–63%) of this group.
Figure 1.
Phylogenetic tree of KNOX proteins similar to the amino acid sequence of the POTH1 protein of potato. These data were organized into a phylogenetic tree with the ME-Boot program of the MEGA package v1.0 and the neighbor-joining program (Saitou and Nei, 1987). The numbers listed at the branching points are boot-strapping values that indicate the level of significance (percentage) for the separation of two branches. The length of the branch line indicates the extent of difference according to the scale at the lower left-hand side. Databank accession numbers are listed on the dendrogram, and nomenclature for the protein and the common name of the species are listed in the right column.
Accumulation of POTH1 mRNA
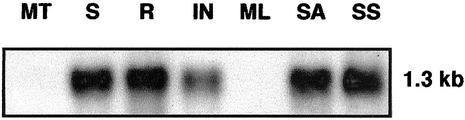
Northern-blot analysis was used to determine the pattern of POTH1 mRNA accumulation in various organs of potato (Fig. 2). Poly(A+)-enriched RNA samples were hybridized with a digoxygenin-UTP-labeled 780-nucleotide RNA antisense probe with the conserved ELK region, homeobox region, and poly(A) tail deleted. A single band, approximately 1.3 kb in length, representing POTH1 mRNA, was present in RNA extracted from stem, root, inflorescence, shoot apex, and swollen stolon apex (Fig. 2, lanes S, R, IN, SA, and SS, respectively). POTH1 transcripts were not detected in either mature tuber or mature leaf RNA (Fig. 2, lanes MT and ML). Equal loading and the quality of the RNA loaded were ascertained via ethidium bromide staining (data not shown). This autoradiograph was representative of several replicate hybridization blots.
Figure 2.
POTH1 mRNA accumulation in various organs of the potato plants. Poly(A)-enriched RNA (5 μg in each lane) was hybridized to a digoxygenin-rUTP-labeled POTH1 RNA antisense probe with the ELK and homeodomain deleted. MT, Mature tuber; S, stem; R, root; IN, inflorescence; ML, mature leaf; SA, shoot apex; SS, swollen stolon apex. Equal loading of intact poly(A+) RNA in each lane was confirmed by ethidium bromide staining (not shown). The hybridizing bands are approximately 1.3 kb in length.
To determine more precisely the location of POTH1 mRNA accumulation, in situ hybridization was performed on vegetative meristems of potato (Fig. 3). The potato SAM comprises two tunica layers, which divide anticlinally to produce the epidermis and contribute to lateral organs such as leaves, and three corpus layers, which divide both periclinally and anticlinally to contribute to lateral organ and stem development (Sussex, 1955; Esau, 1977). POTH1 mRNA accumulates in the two tunica and three corpus layers of the SAM, the leaf primordia, the procambium, and the lamina of young leaves (Fig. 3A). Lower levels of POTH1 transcript can also be detected in the developing leaflets of an older leaf (Fig. 3A, OL). A slightly lower level of POTH1 transcript can be detected in the central zone of the SAM, where initials divide less rapidly than adjacent cells.
Figure 3.
Localization of POTH1 mRNA in potato plants is revealed by in situ hybridization. Presence of POTH1 mRNA is indicated by an orange/brown stain under dark-field microscopy. All micrographs are the same magnification. Bar = 300 μm. A, Longitudinal section through a vegetative shoot apex, probed with antisense POTH1. AP, Apical meristem; L, leaf lamina; OL, older leaf lamina. Asterisks indicate leaf primordia (beneath AP) and procambium (to left of AP). B, Unswollen stolon apex, antisense POTH1. AP, Apical meristem; P, procambium; asterisk, lamina of young leaf; V, perimedullary parenchyma associated with vascular tissue; X, xylem element. C, Unswollen stolon apex, sense POTH1. D, Swollen stolon apex, antisense POTH1. AP, Apical meristem; P, procambium; V, perimedullary parenchyma and vascular tissue; L, lamina of young leaf. E, Swollen stolon, subapical longitudinal section, basal to section in 5D, antisense POTH1. IC, Inner cortex; V, perimedullary parenchyma and vascular tissue; PI, pith. F, Swollen stolon, subapical section, sense POTH1.
Potato plants produce underground stems called stolons that grow horizontally (Jackson, 1999). Under optimum conditions, the subapical region of the stolon tip will begin to swell and eventually develop into a tuber. A non-tuberizing stolon will elongate with most of its growth occurring in the tunica and corpus layers. The greatest concentration of POTH1 signal can be detected in the apical meristem of the elongating stolon (Fig. 3B). Expression levels are also high in the lamina of the youngest leaf, the procambium, and the perimedullary parenchyma associated with the vascular tissue (Fig. 3B). Differentiation of the procambium into mature vascular tissue is marked by the appearance of xylem elements (Esau, 1977), and POTH1 transcript accumulates in this differentiated tissue as well (Fig. 3B). No signal is detected in an elongating stolon tip hybridized with a sense POTH1 probe (Fig. 3C).
The apex of a tuberizing, visibly swollen stolon (Fig. 3D) continues to accumulate POTH1 mRNA in the apical meristem, the procambium, the lamina of new leaves, and the perimedullary parenchyma, but the signal is less intense than in the elongating stolon apical meristem (Fig. 3B). In the subapical portion of the swollen stolon tip (Fig. 3E), where rapid radial expansion is occurring (Xu et al., 1998b), POTH1 signal is detected in the vascular tissue, especially in the perimedullary parenchyma. There is some signal as well in the pith and inner cortex (Fig. 3E). Figure 3F is the sense probe control corresponding to the section in Figure 3E. Similar results were observed with sense probe controls in each section examined (data not shown). The data presented in Figure 3 are representative of several independent replications. Because Figure 3, A through D, shows longitudinal sections through various apices at the same magnification, the locations of labeled tissues appear similar from one apex to the next one.
The Overexpression of POTH1 in Transgenic Potato Plants
To determine the effect of POTH1 overexpression on the development of potato, the full-length POTH1 sequence was placed under the control of the cauliflower mosaic virus 35S promoter in the binary vector, pCB201 (Xiang et al., 1999). To examine the role of POTH1 in tuberization, two potato cultivars (potato cv FL-1607 and potato subsp. andigena) were selected for transformation. Potato subsp. andigena plants are photoperiod sensitive and tuberize only under short-day conditions (Carrera et al., 2000), whereas potato cv FL-1607 plants tuberize under both long- and short-day photoperiods. A total of 30 independent transgenic lines from potato subsp. andigena and 20 independent transgenic lines from potato cv FL-1607 were generated and screened for increased POTH1 mRNA expression. Among 10 sense lines of potato subsp. andigena and 15 lines of potato cv FL-1607 that showed high levels of POTH1 mRNA accumulation, five independent transgenic lines of potato subsp. andigena and four lines of potato cv FL-1607 were chosen for further analysis. An aberrant phenotype was observed only in those lines with detectable levels of POTH1 mRNA from total RNA samples. Two transgenic lines, potato subsp. andigena lines 15 and 18 had the highest levels of POTH1 mRNA accumulation (Fig. 4A), whereas potato subsp. andigena lines 11, 20, and 29 had intermediate levels of POTH1 mRNA (Fig. 4A). Similar high levels of POTH1 accumulation were observed in potato cv FL-1607 overexpression lines that exhibited mutant phenotypes (data not shown). Equivalent loading of RNA samples was verified by using an 18S rRNA probe from wheat (Triticum aestivum; Fig. 4B).
Figure 4.
POTH1 mRNA accumulation in transgenic potato plants and the evaluation of leaf and stem traits in POTH1 overexpression lines. A, Total RNA (5 μg) from shoot tips of wild-type (WT) and independent transgenic lines, potato subsp. andigena 15, 18, 20, 29, and 11 were hybridized to a 32P-labeled POTH1 probe with the ELK and homeodomain deleted. B, Membranes were stripped and hybridized with 32P-labeled 1.2-kb wheat 18S rRNA to ascertain equal loading and transfer. C through F, Three plants each of wild-type and overexpression lines, potato subsp. andigena 15, 18, 20, 29, and 11 were examined. se is indicated for each mean. Plant height (C) and internode length (D) were examined for 75-d-old plants. Petiole length (E) and (F) the terminal leaflet length were measured for the sixth expanded leaf of 84-d-old plants.
Phenotype of POTH1 Overexpression Lines
Overexpression of POTH1 resulted in a phenotype characterized by a reduction in plant height and leaf size (Fig. 4, C–F). Lines with the most abundant POTH1 RNA levels had the greatest reduction in overall height. The height of potato subsp. andigena lines 15 and 18 was reduced by at least 64% compared with wild-type plants (Fig. 4C). Transgenic lines with an intermediate phenotype (potato subsp. andigena lines 20, 29, and 11) showed a 20% to 25% reduction in plant height (Fig. 4C). The decrease in plant height was due to a corresponding decrease in internode elongation (Fig. 4D). The average internode length of the severe mutant, potato subsp. andigena line 15, was 4.0 mm compared with 16 mm for wild-type potato subsp. andigena plants. The same pattern was observed for petiole and leaflet length (Fig. 4, E and F) with the severe phenotypes displaying the greatest reduction in size. Among the five sense lines, petiole length was reduced by 70% to 96%, whereas leaflet length was reduced by 29% to 87% compared with wild-type plants. The sixth expanded leaf from the shoot apex was used to measure petiole and terminal leaflet length. Similar results were seen for potato cv FL-1607 overexpression lines (data not shown).
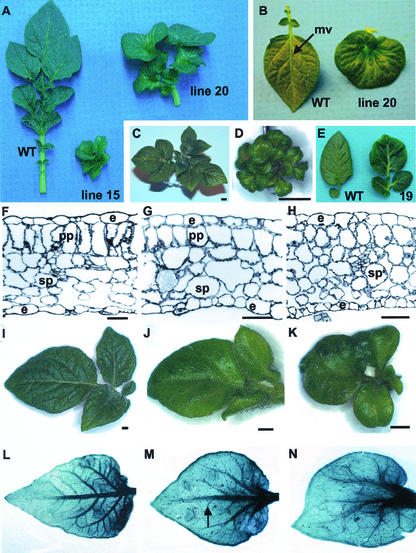
Transgenic plants that overexpressed POTH1 also exhibited malformed leaves. The overall size of the leaflets was greatly reduced, and they were rounded, curved, and wrinkled (Fig. 5, A and B). Wild-type leaflets have an ovate form and display pinnate venation with a prominent mid-vein (Fig. 5B, left). In the overexpression mutants, the mid-vein is less prominent, and the most severe phenotypes exhibited a mouse-ear leaf phenotype (Fig. 5, B–D). The leaflets are heart-shaped with a shortened mid-vein. In addition, there has been a switch from pinnate to palmate venation (Fig. 5B). The phyllotaxy is not altered in overexpression lines, although, compared with wild-type plants (Fig. 5C), the leaves are clustered closer to the stem due to shortened petioles (Fig. 5D). In tomato, the dominant mutations Mouse-ear and Curl were caused by a change in the spatial and temporal expression of the tomato knox gene TKn2/LeT6 (Chen et al., 1997; Parnis et al., 1997). Overexpression of kn1 (Hareven et al., 1996) in tomato caused up to a 6-fold increase in the level of leaf compoundness resulting in a leaf bearing 700 to 2,000 leaflets. Such a marked increase in the level of compoundness was not observed in POTH1 overexpression lines. Increased proliferation of leaflets from sense lines, however, was common (compare wild-type and line 19 leaflets in Fig. 5E).
Figure 5.
Phenotype of the leaves of POTH1 overexpression lines. A, Overall size and shape of leaves from the potato subsp. andigena intermediate and severe overexpression lines, line 20 and line 15, respectively, have been altered compared with wild-type leaves (WT). B, Wild-type leaflets (WT) have a prominent mid-vein (mv) and pinnate venation pattern. The potato subsp. andigena intermediate overexpression mutant (line 20) has a mouse-ear shape, a shortened mid-vein, and palmate venation pattern. C, Shoot tip of WT potato subsp. andigena line. D, Shoot tip of the severe mutant, potato subsp. andigena line 15, showing a mouse-ear leaf phenotype and shortened petioles causing leaves to cluster closely to the stem. The bars in C and D = 5 mm. E, The rachis and associated leaflets were detached from the petiole of a wild-type plant (WT) and a representative sense line (19), to show a slight increase in the lobing of leaflets. F, Cross section through a wild-type leaf showing the arrangement of cell layers: e, epidermis; sp, spongy parenchyma; pp, palisade parenchyma. Bar = 50 μm. G, Cross section through potato subsp. andigena line 15 leaf after treatment with GA3 showing an intermediate level of cell organization. Bar = 50 μm. H, Cross section through potato subsp. andigena line 15 leaf showing that the cell layers lack a palisade parenchyma layer. Bar = 50 μm. I, Wild-type leaf from potato subsp. andigena showing the morphology of a compound leaf. J and K, The compound leaf structure is shown for the overexpression mutant, potato subsp. andigena line 15. Shoot tips were treated with either 10 μm GA3 in 0.002% (v/v) ethanol (J) or 0.002% (v/v) ethanol alone (K). Terminal leaflets from compound leaves of wild-type plants (L), GA3-treated line 15 (M), and untreated line 15 were cleared to show the venation pattern. M, The mid-vein is marked with an arrow. Note that the morphology and venation of the GA3-treated leaf (J and M) is more similar to the wild-type leaf (I and L) than to the potato subsp. andigena line15 untreated leaf (K and N). Bars in I through K = 1.0 mm. The second expanded leaf was used for the leaf samples in F through N.
To determine whether POTH1 overexpression affected the leaf at the cellular level, leaf cross sections of the severe mutant, potato subsp. andigena line 15, were examined. Wild-type leaves consist of a palisade parenchyma layer on the adaxial side and a spongy parenchyma layer on the abaxial side (Fig. 5F). The cells of the palisade layer are aligned in a vertical orientation and are tightly packed, whereas the spongy parenchyma are more loosely arranged (Fig. 5F). In leaves of potato subsp. andigena line 15, the palisade parenchyma layer is absent, and the spongy parenchyma cells are more closely packed (Fig. 5H). Overall cell size in the leaves of potato subsp. andigena line 15 is reduced by about one-half.
Many of the traits of the phenotypes observed in POTH1 overexpression lines are similar to GA-deficient mutants. These similarities include decreased plant height, decreased internode length, and darker green coloration of the leaves (van den Berg et al., 1995b). Because of these differences, exogenous GA3 was applied to determine whether the overexpression lines were responsive to GA treatment. The shoot apex of overexpression lines was sprayed to runoff with 10 μm GA3 in 0.002% (v/v) ethanol or with 0.002% (v/v) ethanol alone. Application of GA3 not only caused plants with a severe phenotype to increase in height (data not shown), but it also partially rescued the leaf morphology of both severe and intermediate phenotypes. Palisade and spongy parenchyma organization is partially rescued in leaves from line 15 treated with GA3 (Fig. 5G). The compound leaf structure of the potato subsp. andigena wild-type leaf is shown in Figure 5I. The GA3-treated leaf (Fig. 5J) of the severe mutant, line 15, is more similar in morphology to the wild-type leaf (Fig. 5I) than to the mutant untreated leaf (Fig. 5K). Leaflets are more ovate in form rather than the typical mouse-ear shape. Wild-type leaves have a prominent mid-vein (Fig. 5L), whereas the mid-vein (Fig. 5M, arrow) is more prominent in the mutant GA3-treated leaf than in the mutant untreated leaf (Fig. 5N).
To determine whether GA biosynthesis was disrupted in POTH1 overexpression lines, levels of intermediates in the GA biosynthesis pathway in potato (van den Berg et al., 1995a) were measured. Levels of the intermediates GA53 and GA19 increased in POTH1 overexpression lines, whereas GA1 and GA8 levels decreased (Fig. 6). In potato subsp. andigena lines 29 and 20, GA53 and GA19 levels increased approximately 2-fold compared with wild-type lines (Fig. 6). The levels of GA1 and GA8 present in potato subsp. andigena overexpression lines were approximately one-half that of wild-type levels (Fig. 6). Accumulation of GA53 and GA19 with a concomitant decrease in GA1 and GA8 indicates that the GA biosynthetic pathway is blocked at the oxidation of GA19 to GA20, leading to a decrease in the levels of bioactive GA1. Similar patterns of accumulation for GA intermediates were also observed for potato subsp. andigena sense line 15 (data not shown).
Figure 6.
Levels of intermediates in the GA biosynthetic pathway. GAs were extracted from shoot tips down to the sixth expanded leaf from wild-type plants and potato subsp. andigena POTH1 overexpression lines 29, 20, and 11. GAs were separated by HPLC and levels were measured by gas chromatography-mass spectrometry. GA53, GA19, and GA20 are precursors to GA1, the physiologically active GA, whereas GA8 is the inactive metabolite. GA53 and GA19 levels increased, whereas GA20, GA1, and GA8 levels decreased in POTH1 overexpression lines. Measurements are the average of three replications. se is indicated for each mean. Concentrations of GA53, GA19, GA20, GA1, and GA8 were determined by calculating the area of the peaks at the correct Kovats retention indices (KRI) at 448/450 (KRI = 2,497), 434/436 (2,596), 418/420 (2,482), 506/508 (2,669), and 594/596 (2,818), respectively.
Overexpression lines were deficient in bioactive GAs but were responsive to the exogenous application of GA3. This suggests that GA biosynthesis is inhibited in the overexpression lines. In addition, accumulation of GA53 and GA19, with a decrease in GA20, GA1, and GA8 (Fig. 6), indicates that the activity of the biosynthetic gene, GA 20-oxidase, may be suppressed. GA 20-oxidase catalyzes the oxidation of carbon 20 of GA53 to GA44 to GA19 to GA20. The enzyme GA 3-oxidase then converts GA20 to the active GA1 (Hedden and Kamiya, 1997). To determine whether POTH1 overexpression causes a change in GA 20-oxidase mRNA levels, RNA-blot analysis was performed using one of the potato genes encoding GA 20-oxidase, StGA20ox1, as a probe (Carrera et al., 1999). In the overexpression lines, StGA20ox1 mRNA levels were reduced substantially compared with levels in wild-type lines (Fig. 7).
Figure 7.
The accumulation of mRNA for GA 20-oxidase1 in transgenic plants that overexpress the potato knox gene, POTH1. A, Five micrograms of total RNA from the shoot tips of wild-type lines (designated 2, 9, and 10) and the overexpression lines, potato subsp. andigena 11, 15, and 18 were hybridized with a 1.2-kb fragment of the potato GA 20-oxidase1 cDNA, StGA20ox1 (Carrera et al., 1999). B, The membrane was stripped and reprobed with 18S wheat rRNA to ascertain equal loading and efficient transfer.
GA is involved in regulating cell growth in a tuberizing stolon (Xu et al., 1998a) and in contributing to the control of the photoperiodic response of tuber formation (Martínez-García et al., 2001). Because levels of active GAs were reduced in transgenic plants, an in vitro tuberization assay (Konstantinova et al., 1999) was used to determine the effect of POTH1 overexpression on tuberization. After 2 weeks under a 16-h light/8-h dark photoperiod to induce rooting, plants were cultured on 6% (w/v) Suc under either an 8-h light/16-h dark (inductive) or 16-h light/8-h dark (noninductive) photoperiod. After 14 d, overexpression lines produced an average of 0.7 to 1.5 tubers per plant under short days, whereas wild-type plants produced an average of 0.08 tubers per plant (Table I). Similar results were observed under long days and after 21 d in culture (Table I). Overall, the POTH1 overexpression lines could produce more tubers in less time than controls. The potato cv FL-1607 overexpression lines also exhibited increased tuber activity under both photoperiods (data not shown).
Table I.
In vitro tuberization of POTH1 overexpression lines
| Transgenic Line | No. of Tubers per Plant
|
|||
|---|---|---|---|---|
| Short Day | Long Day | |||
| 14 d | 21 d | 14 d | 21 d | |
| Control | 0.08 | 0.66 | 0.06 | 0.43 |
| 1200-29 | 1.40 | 1.70 | 0.88 | 1.25 |
| 1200-11 | 0.72 | 0.88 | 1.16 | 1.30 |
| 1200-15 | 1.50 | 2.30 | 0.12 | 0.38 |
| 1200-18 | 0.86 | 1.50 | 0.5 | 0.86 |
Potato ssp. andigena transgenic plants were placed on Murashige-Skoog medium supplemented with 6% (w/v) Suc under either short- or long-day conditions. At least 12 plants per line were monitored for total number of tubers that formed. The average number of tubers produced per plant is shown.
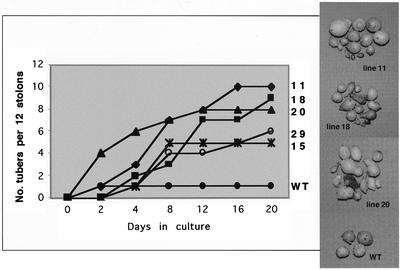
Low levels of GA in the stolon tip are correlated with tuber induction (Xu et al., 1998a). To determine whether the effect of overexpressing POTH1 can activate tuber development locally in the stolon tip without a leaf-mediated signal, we cultured stolon tips excised from in vitro plantlets overexpressing POTH1 that were not tuberizing. After a 20-d incubation in the dark on 8% (w/v) Suc, stolons from all five POTH1 sense lines produced more tubers than wild-type stolons (Fig. 8). Line 11 exhibited almost a 10-fold increase in tuber yield (262 mg stolon tip−1) after 35 d in culture compared with wild-type plants (27 mg stolon tip−1).
Figure 8.
Rate of tuberization for stolons from overexpression lines 11, 18, 20, 29, and 15 of POTH1 and from wild-type plants (WT). Excised stolon tips (approximately 1.5 cm in length) from plants grown under long-day conditions were grown in vitro in the dark in media supplemented with 8% (w/v) Suc and monitored for 20 d. Tubers shown on the right were harvested at 35 d. Twelve stolon tips per independent line were evaluated for tuber production.
DISCUSSION
Isolated from an early-stage tuber cDNA library, POTH1 is a homeobox gene belonging to the knox gene family. It contains the conserved homeodomain, ELK, and KNOX domains. The homeodomain contains the invariant residues, PYP, between helices 1 and 2, making it a member of the TALE superclass. Because of its close sequence match with the KN1 homeodomain, POTH1 is classified as a knox class I homeobox gene.
POTH1 Has a Widespread mRNA Expression Pattern
Even though POTH1 is classified as a class I knox gene, it has a more widespread mRNA expression pattern than other class I genes. Unlike the mRNA expression pattern of kn1, which is limited to corpus cells of the apical meristem (Jackson et al., 1994), in situ hybridization showed that POTH1 mRNA accumulates in the meristematic and indeterminate cells of the SAM, determinate leaf primordia, the expanding lamina of new leaves, and developing leaflets of older leaves. In an unswollen stolon tip, the accumulation of POTH1 mRNA was highest in undetermined, meristematic cells, but was also detected in the lamina of young leaves and the vascular tissue of the stem. Once tuberization has been initiated, the signal becomes less intense at the stolon apex but is present in the vascular tissue in the subapical portion of the stolon. At this stage of tuberization, elongation of the stolon has stopped, and rapid, radial expansion occurs in the subapical region (Reeve et al., 1969).
Most class I knox genes have a more limited pattern of mRNA expression, restricted to undifferentiated cells of the meristem (Reiser et al., 2000). Members of the tobacco knox family have distinct expression patterns within the SAM. NTH15 and NTH1 are expressed throughout the corpus, NTH20 is expressed in the periphery zone, and NTH9 is expressed in the rib zone of the SAM (Nishimura et al., 1999). The tomato knox class I genes TKn1 and TKn2/LeT6 have an expression pattern similar to POTH1 with transcripts detectable in meristematic and differentiated cells. Expression of TKn2/LeT6 was detected in the corpus of the meristem, developing leaf primordia, leaflet primordia and margins, and the vascular cells of the leaf (Chen et al., 1997; Janssen et al., 1998). This expanded expression pattern in tomato has been attributed to the differences in compound leaf development compared with simple leaf development and the expansion of undifferentiated tissues to include developing leaflets.
Phenotype of POTH1 Overexpression Transgenic Lines
Overexpression of POTH1 resulted in altered leaf morphology, dwarfism, and increased rates of in vitro tuberization. Leaves were small, wrinkled, and curved. Both severe and intermediate phenotypes were characterized by a mouse-ear leaf phenotype. Leaves were heart-shaped with a decreased mid-vein and palmate venation. The petioles were reduced in length resulting in leaves clustered closer to the stems. Overexpression lines exhibited dwarfism as a result of reduced internode length. The severity of the phenotype was correlated with the greatest levels of POTH1 sense transcript accumulation. Cross sections of leaves revealed that the mesophyll organization was disrupted with the palisade parenchyma layer missing in POTH1 overexpression lines. The tightly packed cells were about one-half the size of the wild-type cells. A similar disruption in leaf parenchyma layers was observed in sense mutants of KNAT1 and KNAT2 (Chuck et al., 1996; Frugis et al., 2001; Pautot et al., 2001). Because class I knox genes are implicated in maintaining the undifferentiated state of cells (Chan et al., 1998), disruption in leaf architecture is likely a result of a defect in the normal differentiation program.
Similar to the knox genes NTH15 of tobacco and OSH1 of rice, our results indicate that POTH1 is a negative regulator of GA biosynthesis. POTH1 overexpression transgenic lines share many phenotypes with GA-deficient mutants including dwarfism, decreased internode elongation, and darker leaf coloration (van den Berg et al., 1995b). Exogenous application of GA3 partially rescued the aberrant leaf phenotype, indicating that overexpression lines were responsive to GA. Levels of the bioactive GA, GA1, were reduced in overexpression lines, and the further product of GA1 metabolism GA8 even more noticeably, whereas intermediates before GA20 in the pathway accumulated. Additionally, the mRNA levels of a key GA biosynthetic enzyme, GA 20-oxidase1, were reduced in overexpression lines. When NTH15 and OSH1 were overexpressed in tobacco, the levels of the hormones, auxin, cytokinin, abscisic acid, and GA were altered. GA1 levels were reduced and cytokinin levels increased (Tamaoki et al., 1997; Kusaba et al., 1998b). In tobacco, NTH15 affects plant growth by negatively regulating GA levels by suppressing the transcription of the tobacco GA 20-oxidase gene through a direct interaction with regulatory elements (Sakamoto et al., 2001). POTH1 sense lines described here also exhibited a 2- to 4-fold increase in bioactive cytokinins measured in SAMs (data not shown).
POTH1 overexpression lines exhibited an increase in both the rate of tuberization and the total number of tubers formed under both short- and long-day photoperiods. Enhanced tuberization can be partially attributed to the decrease in GA1 levels caused by POTH1 suppression of GA 20-oxidase1. Phytochrome B (PHYB) and GAs are involved in inhibiting tuberization under long-day photoperiods. GA levels in the leaf decreased under short-day photoperiods and increased under long-day conditions (Railton and Wareing, 1973). High levels of GA in the stolon tip favor elongation of stolon meristems, whereas decreasing levels are required for initiation of tuberization (Xu et al., 1998a). GA 20-oxidase is a key enzyme in the GA biosynthetic pathway. In potato, the GA 20-oxidase genes are regulated by GA1 feedback inhibition, blue light, and PHYB (Jackson et al., 2000). Whereas PHYB antisense plants were able to form tubers under both long- and short-day photoperiods (Jackson et al., 1996), transgenic antisense potato subsp. andigena plants with suppressed levels of GA 20-oxidase1 (StGA20ox1) were not able to overcome the negative effects of photoperiod on tuberization in soil-grown plants (Carrera et al., 2000). In this study, the POTH1 sense lines exhibited an increase in the rate of tuberization under both short- and long-day photoperiod conditions.
Regulation of POTH1 Activity during Development
Overexpression of POTH1 potentially regulates development in the SAM and in underground stolons through a reduction in bioactive GA levels in vegetative meristems. Whereas GA levels are high in the elongating unswollen stolon and decrease in swollen stolons (Xu et al., 1998a), POTH1 mRNA accumulates in both unswollen and swollen stolons. If POTH1 is a negative regulator of GA synthesis, how can its expression mediate a decrease in GA levels in the swollen stolon leading to tuberization, but not in the elongating unswollen stolon tip? With other transcription factors, an interaction with a partner protein can regulate development by affecting the binding of the homeodomain(s) to the DNA of a target gene. In Antirrhinum spp., for example, formation of a ternary complex consisting of the MADS box proteins SQUA, DEF, and GLO greatly increases DNA binding compared with SQUA homodimers or DEF/GLO heterodimers alone (Egea-Cortines et al., 1999). The interaction of HOX proteins with PBC proteins in animals modulates the affinity of the HOX proteins for specific DNA-binding sites (Chang et al., 1997). HOX homodimers have different DNA-binding sites than HOX-PBC heterodimers (Mann and Chan, 1996), indicating that the target gene (and function) is dependent on protein-protein interactions. Additionally, HOX-PBC complexes can be activators or repressors of transcription depending on the cell type and the presence of a third interacting partner (Saleh et al., 2000). With the formation of different combinations of heterodimers and ternary complexes, the potential to regulate growth by interacting with different target genes is greatly increased.
It is clear that the interaction of KNOX proteins with other proteins is an important mechanism for regulating development. Protein-protein interactions between BEL1-type transcription factors and KNOX proteins have been reported in barley (Hordeum vulgare; Müller et al., 2001), Arabidopsis (Bellaoui et al., 2001), and maize (Smith et al., 2002). Homodimerization of KNOX proteins of barley (Müller et al., 2001) and rice (Nagasaki et al., 2001) has also been demonstrated. Sakamoto et al. (1999) showed by expressing chimeric proteins in transgenic tobacco plants that the region of the KNOX domain (designated KNOX2) involved in protein interaction was more important than the homeodomain in determining the severity of the mutant phenotype. By using a yeast two-hybrid library screen, seven unique proteins were isolated from potato stolons that interact with POTH1 (Chen et al., 2003). These seven proteins are homeobox genes of the BEL1-like family and are members of the TALE superclass. Whereas POTH1 has a widespread mRNA expression pattern, the seven potato BELs have a differential pattern of expression. It is possible that POTH1 interacts with one BEL protein to negatively regulate GA levels in the tuberizing stolon but interacts with a different BEL partner in the elongating stolon or SAM. Overexpression of one of the POTH1-interacting proteins, StBEL5, enhances tuberization under both long- and short-day photoperiods. However, unlike POTH1 overexpression, leaf morphology is not drastically altered (data not shown). In a tandem complex with a specific BEL partner, POTH1 could activate transcription of a set of target genes in one organ or set of cells and with another partner suppress those same genes in a different organ.
MATERIALS AND METHODS
Amplification of Potato (Solanum tuberosum) Homeobox Fragment for Use as Probe
Two primers, primer 1 (5′-AAGAAGAAGAAGAAAGGGAA) and primer 2 (5′-ATGAACCAGTTGTTGAT) were designed based on comparison of the homeobox regions of five class I homeobox genes (kn1, KNAT1, KNAT2, OSH1, and SBH1) to correspond to the most highly conserved portions of the homeobox and were synthesized at the DNA Synthesis Facility at Iowa State University. Template DNA was prepared from a mass in vivo excision of a 4-d axillary bud tuber λZAPII cDNA library (Stratagene, La Jolla, CA) from potato cv Superior. The potato homeobox fragment was amplified using an annealing temperature of 45°C and cloned into the pCR2.1 vector of the TA Cloning kit (Invitrogen, Carlsbad, CA).
Library Screening and Sequence Analysis
The early tuberization stage library was constructed as previously described (Kang and Hannapel, 1996). Screening of 400,000 plaque-forming units was accomplished using 100 ng of 32P-labeled PCR-generated probe in 50% (w/v) formamide, 6× SSC, 3.4× Denhardt's solution, 25 mm sodium phosphate buffer, pH 7.0, 120 μg mL−1 denatured salmon sperm DNA, and 0.4% [w/v] SDS at 42°C for 48 h. Membranes were washed with 2× SSC/0.1% (w/v) SDS at 25°C for 5 min and then twice with 2× SSPE/0.1% (w/v) SDS at 65°C for 20 min. POTH1 was sequenced at the Nucleic Acid Sequencing Facility at Iowa State University. Sequence analyses performed included BLAST (Altschul et al., 1990) and GAP (Genetics Computer Group, Madison, WI).
RNA Isolation and Northern-Blot Analysis
For Figure 2, total RNA was isolated (Dix and Rawson, 1983) from potato plants grown in the greenhouse at 20°C to 25°C under 16 h of light. Total RNA was enriched for poly (A+) RNA by separation over an oligo(dT) column, and northern gel electrophoresis was performed using methyl mercury as a denaturant. Ethidium bromide staining under UV light was used to ascertain equal gel loading and efficient transfer to nylon membranes. The Genius non-radioactive nucleic acid labeling and detection system (Roche Diagnostics, Indianapolis) was used. Fifteen nanograms per milliliter of digoxygenin-UTP-labeled antisense RNA probe in 50% (w/v) formamide was hybridized to filters at 55°C overnight. Membranes were washed twice for 5 min in 2× SSC/0.1% (w/v) SDS at 25°C and then washed twice for 15 min in 0.1× SSC/0.1% (w/v) SDS at 68°C. The membranes were then incubated 30 min in blocking solution:maleic acid buffer, pH 7.5 (1:10), 30 min in anti-digoxygenin-alkaline-phosphatase conjugate:maleic acid buffer (1:10,000), washed twice for 15 min in maleic acid buffer, and equilibrated 5 min in detection buffer before the addition of disodium 3-[4-metho xyspiro {1,2-dioxetane-3,2′-[5′-chloro]tricyclo [3.3.1.13,7]decan}-4-yl] phenyl phosphate substrate solution. Membranes were exposed to film for 30 to 45 min at 25°C.
For Figure 7, total RNA was isolated with TriPure Isolation Reagent (Roche Diagnostics) and gel electrophoresis was performed using 10 mm methyl mercury (II) hydroxide as a denaturant. For hybridization with STGA20ox1, shoot tip samples were collected at the same time of day to avoid variations due to diurnal variation. Probes were labeled with [α-32P]dCTP (RadPrime DNA Labeling System, Invitrogen). The 1.5-kb EcoRI-XhoI fragment of StGA20ox1 cDNA (Carrera et al., 1999) was provided by Salomé Prat (Madrid). All membranes were hybridized at 42°C for 70 h in 50% (w/v) formamide. The membranes were rinsed in 2× SSC/0.1% (w/v) SDS at 25°C, followed by 1× SSC/0.1% (w/v) SDS for 20 min at 65°C, then 0.1× SSC/0.1% (w/v) SDS for 20 to 30 min at 65°C. Film was exposed for 4 to 7 d.
In Situ Hybridization Analysis
Preparation of tissue samples and in situ hybridizations were performed as previously described (Cañas et al., 1994). Digoxygenin-UTP-labeled RNA probes, both sense and antisense, were transcribed with RNA polymerases according to instructions (Roche Diagnostics) and were hydrolyzed using 0.2 m sodium carbonate and 0.2 m sodium bicarbonate at 65°C for 51 min. Unincorporated nucleotides were removed over a Sephadex G-50 column.
For immunological detection, the slides were incubated in buffer 1 (1% [w/v] blocking solution, 100 mm Tris, pH 7.5, and 150 mm NaCl) for 1 h and then equilibrated with buffer 2 (100 mm Tris pH 7.5, 150 mm NaCl, 0.5% [w/v] bovine serum albumin, and 0.3% [w/v] Triton X-100). Tissue sections were then incubated with anti-digoxygenin-alkaline-phosphatase conjugate diluted 1:1,000 in buffer 2 in a humidified box for 2 h and then washed three times for 20 min in 100 mm, Tris pH 7.5, and 150 mm NaCl. The tissue sections were equilibrated in buffer 3 (100 mm Tris, pH 9.5, 100 mm NaCl, and 50 mm MgCl2) for 10 min and then incubated in 3.2 μg mL−1 5-bromo-4-chloro-3-indolyl-phosphate:6.6 μg mL−1 nitroblue tetrazolium salt in buffer 3 in a humidified box for 13 h (aboveground tissues) or 7 h (underground tissues). Accumulation of POTH1 mRNA is visualized as an orange/brown stain under dark-field illumination. Sections were viewed and captured with a 35 mm camera (Leitz, Midland, Ontario) using the dark-field mode on a Leitz Orthoplan light microscope.
35S-POTH1 Transformation of Potato Plants
The full-length POTH1 cDNA was cloned into the binary vector pCB201 (Xiang et al., 1999) between the cauliflower mosaic virus 35S promoter and the nos terminator. Two potato cultivars, subsp. andigena and cv FL-1607, were transformed by the Agrobacterium tumefaciens (strain GV2260)-mediated leaf-disc transformation method (Liu et al., 1995). A total of 30 independent transgenic lines from potato subsp. andigena and 20 independent transgenic lines from potato cv FL-1607 were screened for insertion of the transgene and accumulation of POTH1 mRNA. Five independent transgenic lines of potato subsp. andigena and four lines of potato cv FL-1607 that showed high levels of POTH1 mRNA accumulation were selected for further analysis. Untransformed tissue culture plants were used as wild-type controls.
Light Microscopy
Leaf tissue was fixed in 2% (v/v) glutaraldehyde and 2% (v/v) paraformaldehyde in 0.1 m cacodylate buffer, pH 7.2, at 4°C for 72 h, dehydrated in a graded ethanol series, and embedded in LR White resin (Electron Microscopy Sciences, Ft. Washington, PA). One-micrometer-thick sections were cut on an ultramicrotome (Reichert/Leica, Deerfield, IL) and stained with 1% (w/v) toludine blue O. For leaf clearing, leaves were soaked in 95% ethanol until all chlorophyll was removed and then in bleach until all cellular material was removed. The samples were dehydrated in increasing amounts of ethanol up to 95%, stained for 5 min in 0.5% (w/v) chlorozol black E in 95% ethanol, washed three times in 100% ethanol, two times in xylene, and mounted with Permount. Sections were viewed and captured by using an Axiocam HRc digital camera (Zeiss, Welwyn Garden City, UK) and bright-field microscopy with a Zeiss Axioplan 2 microscope. Leaf samples were captured by using an identical digital camera with a stereomicroscope (SZH10, Olympus, Tokyo).
GA Analysis
Three replicates of shoot tips down to the sixth expanded leaf (10 g each), were harvested in liquid nitrogen and frozen at −80°C. The tissue was ground with 80% methanol (MeOH) and incubated at 4°C overnight. 2H2-GA internal standards (L. Mander, Canberra, Australia) were added in the following amounts: 1 ng g−1 fresh weight GA1, 10 ng g−1 fresh weight GA8, 10 ng g−1 fresh weight GA19, 20 ng g−1 fresh weight GA20, and 5 ng g−1 fresh weight GA53. The extract was filtered through Highflo Supercel and washed with 80% MeOH. After evaporation of the MeOH in vacuo, 0.5 m Na2HPO4 was added to bring the pH to about 8.5, followed by addition of 20 mL of hexane. The processed extract was mixed well, and the hexanes were evaporated off in vacuo. The solution was than acidified to pH 3 to 3.5 with glacial acetic acid and incubated for 15 min. The sample was than filtered through polyvinylpolypyrrolidone (PVPP) and washed with 0.2% (v/v) acetic acid. The eluate was loaded onto a prepared Baker SPE (C18) cartridge and washed with 0.2% (v/v) acetic acid. The sample was eluted off the column with 7 mL of 80% MeOH, evaporated to dryness, and dissolved in 1 mL of 100% MeOH. The MeOH-insoluble precipitate was removed by centrifugation, and the supernatant was evaporated to dryness, redissolved in 0.8 mL of 0.2% (v/v) acetic acid, and filtered through a 45-μm filter. A 1-mL loop was used to load the sample onto the C18 HPLC column (Econosphere: Phenomenex, Torrance, CA) run with the following 0.2% (v/v) acetic acid to acetonitrile gradient: 5% to 20% over 2 min, 20% to 35% over 15 min, and 35% to 75% over 15 min. Fractions for the following GAs were taken as follows: 10 to 14.3 min for GA8, 15.3 to 17.45 min for GA1, 23 to 27 min for GA19 and GA20, and 27 to 29.3 min for GA53. Fractions were collected separately and methylated with diazomethane in ether. Samples were dried, redissolved in 1 mL of ethyl acetate, and partitioned against water. The aqueous phase was partitioned against another 1 mL of ethyl acetate, and the ethyl acetate fractions were combined. The samples were dried and placed under high vacuum over P2O5. The samples were dissolved in 2 μL of dry pyridine and 10 μL of bis(trimethylsilyl) trifluoro-acetamide with 1% (v/v) trimethylchlorosilane (Sylon BFT, Pierce, Rockford, IL) and heated at 80°C for 20 min. Samples were analyzed by gas chromatography-selected ion monitoring on a gas chromatograph-mass spectrometer (5890 GC + 5970B MS, Hewlett Packard, Palo Alto, CA) with a 15-m Zebron ZB1 column (Phenomenex, Torrance, CA). The carrier gas, He, was set at a flow rate of approximately 35 cm s−1. The initial column temperature was 60°C for 1 min and then increased at a rate of 30°C min−1 to 240°C and then to 290°C at a rate of 4°C min−1. The injector temperature was 225°C, and the temperature of the detector was 300°C. Concentrations of GA53, GA19, GA20, GA1, and GA8 were determined by calculating the area of the peaks, 448/450, 434/436, 418/420, 506/508, and 594/596, respectively, at the correct Kovats retention indices. Reference spectra were obtained from Gaskin and MacMillan (1991). Cross-ion corrections were calculated according to the following formula:
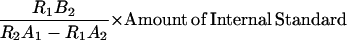 |
where R1 is the measured endogenous ion in sample, R2 is the measured heavy ion in sample, A1 is the percentage of endogenous ion in natural unlabeled sample (= 100%), A2 is the percentage of heavy ion in natural unlabeled sample (= 10%), and B2 is the percentage of heavy ion in labeled internal standard (= 100%).
In Vitro Tuberization
Cuttings of transgenic and control plants were placed in Murashige-Skoog medium plus 6% (w/v) Suc (Konstantinova et al., 1999). After 2 weeks under long days (16 h of light, 8 h of dark) to promote rooting, plants were cultured separately under either long or short day (8 h of light, 16 h of dark) conditions. Plants were examined for tuber activity (percentage of plants that produced either swollen stolons or tubers), and the number of tubers was counted. For Figure 8, excised stolon tips (approximately 1.5 cm in length) from plants grown under long-day conditions were grown in vitro in the dark in media supplemented with 8% (w/v) Suc and monitored for 20 d. Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
ACKNOWLEDGMENTS
We thank Mary Tymeson for help with library screening and northern blots, Tracy Pepper and Bruce Wagner for help with tissue preparation and light microscopy for in situ hybridization, and Salomé Prat for assistance with plant transformation and screening.
Footnotes
This project was supported in part by the Iowa Agriculture and Home Economics Experiment Station (Ames) and an Iowa State University SPRIG. This is journal paper no. J–19520 of the Iowa Agriculture and Home Economics Experiment Station (project no. 3701).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.015560.
LITERATURE CITED
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Barton MK, Poethig RS. Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild type and in the shoot meristemless mutant. Development. 1993;119:823–831. [Google Scholar]
- Bellaoui M, Pidkowich MS, Samach A, Kushalappa K, Kohalmi SE, Modrusan Z, Crosby WL, Haughn GW. The Arabidopsis BELL1 and KNOX TALE homeodomain proteins interact through a domain conserved between plants and animals. Plant Cell. 2001;13:2455–2470. doi: 10.1105/tpc.010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25:4173–4180. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas LA, Busscher M, Angenent GC, Beltrán J-P, van Tunen AJ. Nuclear localization of the petunia MADS box protein FBP1. Plant J. 1994;6:597–604. [Google Scholar]
- Carrera E, Bou J, García-Martínez JL, Prat S. Changes in GA 20-oxidase gene expression strongly affect stem length, tuber induction and tuber yield of potato plants. Plant J. 2000;22:1–10. doi: 10.1046/j.1365-313x.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- Carrera E, Jackson SD, Prat S. Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato. Plant Physiol. 1999;119:765–773. doi: 10.1104/pp.119.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RL, Gago GM, Palena CM, Gonzalez DH. Homeoboxes in plant development. Biochim Biophys Acta. 1998;1442:1–19. doi: 10.1016/s0167-4781(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Chang C-P, Jacobs Y, Nakamura T, Jenkins NA, Copeland NG, Cleary ML. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol Cell Biol. 1997;17:5679–5687. doi: 10.1128/mcb.17.10.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-J, Janssen B-J, Williams A, Sinha N. A gene fusion at a homeobox locus: alterations in leaf shape and implications for morphological evolution. Plant Cell. 1997;9:1289–1304. doi: 10.1105/tpc.9.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Rosin FM, Prat S, Hannapel DJ (2003) Interacting transcription factors from the TALE superclass regulate tuber formation. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Chuck G, Lincoln C, Hake S. KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell. 1996;8:1277–1289. doi: 10.1105/tpc.8.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Jacobsen SE, Levin JZ, Meyerowitz EM. The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development. 1996;122:1567–1575. doi: 10.1242/dev.122.5.1567. [DOI] [PubMed] [Google Scholar]
- Dix KP, Rawson JRY. In vivo transcriptional products of the chloroplast DNA of Euglena gracilis. Curr Genet. 1983;7:265–273. doi: 10.1007/BF00376071. [DOI] [PubMed] [Google Scholar]
- Egea-Cortines M, Saedler H, Sommer H. Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus. EMBO J. 1999;18:5370–5379. doi: 10.1093/emboj/18.19.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 1996;10:967–979. doi: 10.1046/j.1365-313x.1996.10060967.x. [DOI] [PubMed] [Google Scholar]
- Esau K. Anatomy of Seed Plants. Ed 2. New York: John Wiley & Sons; 1977. The stem: primary state of growth; pp. 243–294. [Google Scholar]
- Frugis G, Giannino D, Nicolodi C, Chiappetta A, Bitonti MB, Innocenti AM, Dewitte W, Van Onckelen H, Mariotti D. Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot-like indeterminate growth associated with an accumulation of isopentenyl-type cytokinins. Plant Physiol. 2001;126:1370–1380. doi: 10.1104/pp.126.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin P, MacMillan J. GC-MS of the Gibberellins and Related Compounds: Methodology and a Library of Spectra. Bristol, UK: Cantock's Enterprises; 1991. [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Hamant O, Nogue F, Belles-Boix E, Jublot D, Grandjean O, Traas J, Pautot V. The KNAT2 homeodomain protein interacts with ethylene and cytokinin signaling. Plant Physiol. 2002;130:657–665. doi: 10.1104/pp.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E. The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell. 1996;84:735–744. doi: 10.1016/s0092-8674(00)81051-x. [DOI] [PubMed] [Google Scholar]
- Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M. The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol. 2002;12:1557–1565. doi: 10.1016/s0960-9822(02)01125-9. [DOI] [PubMed] [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Hewelt A, Prinsen E, Thomas M, Van Onckelen H, Meins FJ. Ectopic expression of maize knotted1 results in the cytokinin-autotrophic growth of cultured tobacco tissues. Planta. 2000;210:884–889. doi: 10.1007/s004250050693. [DOI] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. [Google Scholar]
- Jackson SD. Multiple signaling pathways control tuber induction in potato. Plant Physiol. 1999;119:1–8. doi: 10.1104/pp.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SD, Heyer A, Dietze J, Prat S. Phytochrome B mediates the photoperiodic control of tuber formation in potato. Plant J. 1996;9:159–166. [Google Scholar]
- Jackson SD, James PE, Carrera E, Prat S, Thomas B. Regulation of transcript levels of a potato gibberellin 20-oxidase gene by light and phytochrome B. Plant Physiol. 2000;124:423–430. doi: 10.1104/pp.124.1.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B-J, Lund L, Sinha N. Overexpression of a homeobox gene, LeT6, reveals indeterminate features in the tomato compound leaf. Plant Physiol. 1998;117:771–786. doi: 10.1104/pp.117.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S-G, Hannapel DJ. Nucleotide sequences of novel potato (Solanum tuberosum L.) MADS-box cDNAs and their expression in vegetative organs. Gene. 1995;166:329–330. doi: 10.1016/0378-1119(95)00593-5. [DOI] [PubMed] [Google Scholar]
- Kang S-G, Hannapel DJ. A novel MADS-box gene of potato (Solanum tuberosum L.) expressed during the early stages of tuberization. Plant Mol Biol. 1996;31:379–386. doi: 10.1007/BF00021798. [DOI] [PubMed] [Google Scholar]
- Kerstetter R, Vollbrecht E, Lowe B, Veit B, Yamaguchi J, Hake S. Sequence analysis and expression patterns divide the maize knotted1-like homeobox genes into two classes. Plant Cell. 1994;6:1877–1887. doi: 10.1105/tpc.6.12.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S. Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development. 1997;124:3045–3054. doi: 10.1242/dev.124.16.3045. [DOI] [PubMed] [Google Scholar]
- Konstantinova TN, Aksenova NP, Golyanovskaya S, Sergeeva LI. Photoperiodic control of tuber formation in potato Solanum tuberosum ssp. andigena in vivo and in vitro. Russ J Plant Physiol. 1999;46:763–766. [Google Scholar]
- Kusaba S, Fukumoto M, Honda C, Yamaguchi I, Sakamoto T, Kano-Murakami Y. Decreased GA1 content caused by the overexpression of OSH1 is accompanied by suppression of GA 20-oxidase gene expression. Plant Physiol. 1998a;117:1179–1184. doi: 10.1104/pp.117.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba S, Kano-Murakami Y, Matsuoka M, Tamaoki M, Sakamoto T, Yamaguchi I, Fukumoto M. Alteration of hormone levels in transgenic tobacco plants overexpressing the rice homeobox gene OSH1. Plant Physiol. 1998b;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell. 1994;6:1859–1876. doi: 10.1105/tpc.6.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-HA, Stephens LC, Hannapel DJ. Transformation of Solanum brevidens using Agrobacterium tumefaciens. Plant Cell Rep. 1995;15:196–199. doi: 10.1007/BF00193719. [DOI] [PubMed] [Google Scholar]
- Ma H, McMullen MD, Finer JJ. Identification of a homeobox-containing gene with enhanced expression during soybean (Glycine max L.) somatic embryo development. Plant Mol Biol. 1994;24:465–473. doi: 10.1007/BF00024114. [DOI] [PubMed] [Google Scholar]
- Mann RS, Chan S-K. Extra specificity from extradenticle: the partnership between HOX and PBX/EXD homeodomain proteins. Trends Genet. 1996;12:258–262. doi: 10.1016/0168-9525(96)10026-3. [DOI] [PubMed] [Google Scholar]
- Martínez-García JF, García-Martínez JL, Bou J, Prat S. The interaction of gibberellins and photoperiod in the control of potato tuberization. J Plant Growth Regul. 2001;20:377–386. doi: 10.1007/s003440010036. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Ichikawa H, Saito A, Tada Y, Fujimura T, Kano-Murakami Y. Expression of a rice homeobox gene causes altered morphology of transgenic plants. Plant Cell. 1993;5:1039–1048. doi: 10.1105/tpc.5.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel L, Lam E. The conserved ELK-homeodomain of KNOTTED-1 contains two regions that signal nuclear localization. Plant Mol Biol. 1996;30:1–14. doi: 10.1007/BF00017799. [DOI] [PubMed] [Google Scholar]
- Muehlbauer GJ, Fowler JE, Girard L, Tyers R, Harper L, Freeling M. Ectopic expression of the maize homeobox gene Liguleless3 alters cell fates in the leaf. Plant Physiol. 1999;119:651–662. doi: 10.1104/pp.119.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Wang Y, Franzen R, Santi L, Salamini F, Rohde W. In vitro interactions between barley TALE homeodomain proteins suggest a role for protein-protein associations in the regulation of Knox gene function. Plant J. 2001;27:13–23. doi: 10.1046/j.1365-313x.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- Nagasaki H, Sakamoto T, Sato Y, Matsuoka M. Functional analysis of the conserved domains of a rice KNOX homeodomain protein, OSH15. Plant Cell. 2001;13:2085–2098. doi: 10.1105/TPC.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A, Tamaoki M, Sato Y, Matsuoka M. The expression of tobacco knotted1-type class1 homeobox genes correspond to regions predicted by the cytohistological zonation model. Plant J. 1999;18:337–347. doi: 10.1046/j.1365-313x.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Ori N, Juarez MT, Jackson D, Yamaguchi J, Banowetz GM, Hake S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under control of a senescence-activated promoter. Plant Cell. 1999;11:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Parnis A, Cohen O, Gutfinger T, Hareven D, Zamir MD, Lifschitz E. The dominant developmental mutants of tomato, Mouse-ear and Curl, are associated with distinct modes of abnormal transcriptional regulation of a Knotted gene. Plant Cell. 1997;9:2143–2158. doi: 10.1105/tpc.9.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautot V, Dockx J, Hamant O, Kronenberger J, Grandjean O, Jublot D, Traas J. KNAT2: evidence for a link between knotted-like genes and carpel development. Plant Cell. 2001;13:1719–1734. doi: 10.1105/TPC.010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Railton ID, Wareing PF. Effects of daylength on endogenous gibberellins in leaves of Solanum andigena: I. Changes in levels of free acidic gibberellin-like substances. Physiol Plant. 1973;28:88–94. doi: 10.1007/BF00386032. [DOI] [PubMed] [Google Scholar]
- Reeve RM, Hautala E, Weaver ML. Anatomy and compositional variation within potatoes: I. Developmental histology of the tuber. Am Pot J. 1969;46:361–373. [Google Scholar]
- Reiser L, Sánchez-Baracaldo P, Hake S. Knots in the family tree: evolutionary relationships and functions of knox homeobox genes. Plant Mol Biol. 2000;42:151–166. [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M. KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev. 2001;15:581–590. doi: 10.1101/gad.867901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Nishimura A, Tamaoki M, Kuba M, Tanaka H, Iwahori S, Matsuoka M. The conserved KNOX domain mediates specificity of tobacco KNOTTED1-type homeodomain proteins. Plant Cell. 1999;11:1419–1431. doi: 10.1105/tpc.11.8.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Rambaldi I, Yang X-H, Featherstone MS. Cell signaling switches HOX-PBX complexes from repressors to activators of transcription mediated by histone deacetylases and histone acetyltransferases. Mol Cell Biol. 2000;20:8623–8633. doi: 10.1128/mcb.20.22.8623-8633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Tamaoki M, Murakami T, Yamamoto N, Kano-Murakami Y, Matsuoka M. Abnormal cell divisions in leaf primordia caused by the expression of the rice homeobox gene OSH1 lead to altered morphology of leaves in transgenic tobacco. Mol Gen Genet. 1996;251:13–22. doi: 10.1007/BF02174339. [DOI] [PubMed] [Google Scholar]
- Sinha NR, Williams RE, Hake S. Overexpression of the maize homeo box gene, KNOTTED-1, causes a switch from determinate to indeterminate cell fates. Genes Dev. 1993;7:787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- Smith HM, Boschke I, Hake S. Selective interaction of plant homeodomain proteins mediates high DNA-binding affinity. Proc Natl Acad Sci USA. 2002;99:9579–9584. doi: 10.1073/pnas.092271599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Greene B, Veit B, Hake S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992;116:21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]
- Sussex IM. Morphogenesis in Solanum tuberosum L.: apical structure and developmental pattern of the juvenile shoot. Phytomorphology. 1955;5:253–273. [Google Scholar]
- Tamaoki M, Kusaba S, Kano-Murakami Y, Matsuoka M. Ectopic expression of a tobacco homeobox gene, NTH15, dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol. 1997;38:917–927. doi: 10.1093/oxfordjournals.pcp.a029252. [DOI] [PubMed] [Google Scholar]
- Tanaka-Ueguchi M, Itoh H, Oyama N, Koshioka M, Matsuoka M. Over-expression of a tobacco homeobox gene, NTH15, decreases the expression of a gibberellin biosynthetic gene encoding GA 20-oxidase. Plant J. 1998;15:391–400. doi: 10.1046/j.1365-313x.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- van den Berg JH, Davies PJ, Ewing EE, Halinska A. Metabolism of gibberellin A12 and A12-aldehyde and the identification of endogenous gibberellins in potato (Solanum tuberosum ssp. andigena) shoots. J Plant Physiol. 1995a;146:459–466. [Google Scholar]
- van den Berg JH, Simko I, Davies PJ, Ewing EE, Halinska A. Morphology and [14C]gibberellin A12 metabolism in wild-type and dwarf Solanum tuberosum ssp. andigena grown under long and short photoperiods. J Plant Physiol. 1995b;146:467–473. [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991;350:241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- Xiang C, Han P, Lutziger I, Wang K, Oliver D. A mini binary vector series for plant transformation. Plant Mol Biol. 1999;40:711–718. doi: 10.1023/a:1006201910593. [DOI] [PubMed] [Google Scholar]
- Xu X, van Lammeren AM, Vermeer E, Vreugdenhil D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998a;117:575–584. doi: 10.1104/pp.117.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Vreugdenhil D, van Lammeren AAM. Cell division and cell enlargement during potato tuber formation. J Exp Bot. 1998b;49:573–582. [Google Scholar]