Abstract
In Chlamydomonas reinhardtii, the presence of a defective STA11 locus results in significantly reduced granular starch deposition displaying major modifications in shape and structure. This defect simultaneously leads to the accumulation of linear malto-oligosaccharides (MOS). The mutants of STA11 were showed to lack d-enzyme, a plant α-1,4 glucanotransferase analogous to the Escherichia coli amylomaltase. We have cloned and characterized both the cDNA and gDNA corresponding to the C. reinhardtii d-enzyme. We now report allele-specific modifications of the d-enzyme gene in the mutants of STA11. These allele-specific modifications cosegregate with the corresponding sta11 mutations, thereby demonstrating that STA11 encodes d-enzyme. MOS production and starch accumulation were investigated during day and night cycles in wild-type and mutant C. reinhardtii cells. We demonstrate that in the algae MOS are produced during starch biosynthesis and degraded during the phases of net polysaccharide catabolism.
Until recently plant starch was believed to be synthesized from ADP-Glc solely through a combination of starch synthases and branching enzymes. However the finding of low-starch or starchless mutants defective for a particular form of debranching enzyme in four different plant systems established that other enzymes of starch metabolism are equally important in ensuring normal starch granule biogenesis (James et al., 1995; Mouille et al., 1996; Nakamura et al., 1996; Zeeman et al., 1998). This came as a surprise because debranching enzymes were initially thought to be enzymes involved solely in starch breakdown. Mutants of the corresponding activities in yeast (Teste et al., 2000) or Escherichia coli (I. Kinderf, Z. Li, M.S. Samuel, B. Koshar-Hashezmi, S. Ball, L. Rampling, and M. Morell, unpublished data) are clearly glycogen over-producers, confirming the initial suspicion. Although the detailed interpretation of the results obtained with the plant mutants vary somewhat, there is now a general agreement that isoamylases, the particular form of debranching enzyme affected in these studies, are enzymes required during starch biosynthesis exclusively. It has been comforting to realize that all mutants affected in starch metabolism behaved in a similar fashion in plants as different as Chlamydomonas reinhardtii, Arabidopsis, pea (Pisum sativum), maize (Zea mays), or rice (Oryza sativa). Some discrepancies in expressivity of mutant phenotypes could be easily explained most of the time by subtle differences in the pathways. For instance, the presence of extraplastidial and plastidial ADP-Glc pyrophosphorylases in cereals easily explains why mutants of cereals lacking the major enzyme form displayed reduced expressivity in their starch accumulation phenotype (for review, see Kossmann and Lloyd, 2000). The starch accumulation phenotypes of the Arabidopsis or C. reinhardtii mutants defective for the catalytic subunit of their sole plastidial ADP-Glc pyrophosphorylase were much more severe (Lin et al., 1988; Zabawinski et al., 2001).
At variance with animals and fungi, plants including C. reinhardtii have retained the bacterial pathway of malto-oligosaccharide (MOS) assimilation in the plastid stroma. Mutants of the plant d-enzyme (the equivalent of the E. coli MalQ encoded amylomaltase) display a strong phenotype on starch metabolism (Critchley et al., 2001). d-enzyme is an α-1,4 glucanotransferase that transfers mostly maltosyl residues from a donor oligosaccharide to the nonreducing end of an acceptor. The donor and acceptor molecules can be glucans of identical length. d-enzyme thus disproportionates a homogenous population into a series of oligosaccharides of increasing length (Peat et al., 1956; Jones and Whelan, 1969). The first mutants defective for d-enzyme were reported in C. reinhardtii and synthesized 10-fold less starch while displaying modifications in starch structure and amylose content (Colleoni et al., 1999a, 1999b). This correlated with an accumulation of unbranched soluble MOS in the plastid stroma. An equivalent mutant of Arabidopsis also accumulated MOS and high amylose starch but did not display any consistent decrease in starch accumulation (Critchley et al., 2001). On the contrary, the Arabidopsis leaf contained more starch at the end of the night than the wild-type reference, suggesting a function of d-enzyme in polysaccharide breakdown (Critchley et al., 2001). In addition, accumulation of MOS seemed confined to the dark phase. This is the first time that such conflicting behaviors between mutants of starch metabolism are reported. The results obtained in C. reinhardtii suggest an important function of d-enzyme in starch biosynthesis whereas those of Arabidopsis point to a role in starch breakdown only. The C. reinhardtii mutant was obtained during a classical screen for low-starch phenotypes after UV mutagenesis (Colleoni et al., 1999a), whereas the Arabidopsis mutants were obtained as gene disruptions after screening a T-DNA library of Arabidopsis for an insertion in a particular d-enzyme sequence (Critchley et al., 2001). Several issues require clarification before any further speculation is made on the functions of d-enzyme in plants. It is still unclear whether the C. reinhardtii STA11 locus defines the structural gene encoding the same type of d-enzyme activity as in Arabidopsis. If so, it remains possible that the UV-generated sta11-1 did not generate a true null mutant allele and that the phenotype would be different with a gene disruption at the STA11 locus. Finally, the low-starch phenotype in C. reinhardtii was recorded under nitrogen starvation, which defines culture conditions that mimic the constant accumulation of starch occurring in storage organs. On the other hand, the Arabidopsis phenotype was investigated in leaf cells undergoing recurrent cycles of synthesis and degradation. Therefore additional documentation of the defects occurring in the sta11 mutants of C. reinhardtii was needed before any further speculation could be made on the function of α-1,4 glucanotransferases in starch metabolism. We now report the molecular characterization of the STA11 locus and mutants. We prove that STA11 defines the structural gene of a form d-enzyme analogous to that analyzed in Arabidopsis. We report the isolation of a gene disruption at the STA11 locus and have investigated the behavior of the d-enzyme mutants following recurrent cycles of synthesis and degradation. We show that the MOS are produced mainly during the phases of active starch biosynthesis and are degraded during polysaccharide catabolism. These results confirm an important function of α-1,4 glucanotransferases during the normal process of starch biosynthesis in green algae. The possible reasons underlying the conflicting results obtained in C. reinhardtii and Arabidopsis are discussed.
RESULTS
Isolation of sta11-2 Allele
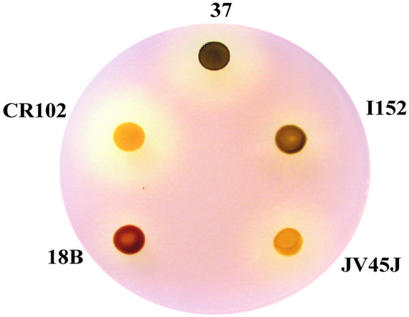
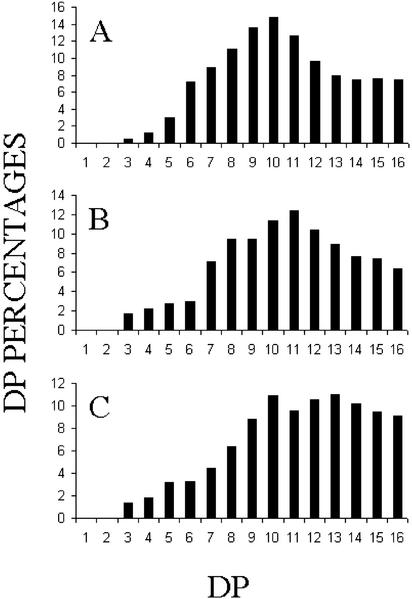
During the first of our two large-scale campaigns of insertional mutagenesis (see “Materials and Methods”), we isolated one particular low-starch mutant that accumulated unbranched MOS (Fig. 1). Because of this phenotype, we immediately set out to assay d-enzyme activity and to perform complementation tests with the previously defined sta11-1 UV-generated mutant (Colleoni et al., 1999a, 1999b). As expected, the mutant contained no trace of d-enzyme activity and did not complement the previously characterized sta11-1 mutants in diploids. A set of meiotic recombinants carrying the new sta11-2::ARG7 mutation were obtained upon crossing with a wild-type reference strain. A set of three sta11-1 and three sta11-2::ARG7 segregants were compared with respect to starch and soluble glucans accumulation. In addition, a more detailed investigation of the structure of both starch and glucans was performed on strain CR102 and compared with the reference JV45J sta11-1 mutant strain. The results summarized in Table I suggest that the sta11-2::ARG7 mutation displays a phenotype very similar to that of the previously characterized sta11-1 carrying mutants with a somewhat increased effect. During crosses (n = 8), the sta11-2 mutants always accumulated less than 5% of the wild-type starch amount under nitrogen starvation, whereas the sta11-1 mutants could accumulate up to 12% of that amount. This correlated with a significant increase in MOS production in the sta11-2::ARG7 mutants. The chain-length distribution of this material was highly variable but always consisted in both sta11-1 and sta11-2::ARG7 of unbranched chains typically inferior to degree of polymerization 16 with a significant amount of maltose. In addition, the chain-length distributions of the amylopectin from both the sta11-1 and sta11-2::ARG7 were significantly modified. Using fluorophore-assisted capillary electrophoresis, we had previously documented a small but significant difference in the chain-length distribution of the wild-type and mutant (sta11-1) amylopectin (Colleoni et al., 1999a). Using high-performance anion exchange chromatography with pulsed amperometric detection (Fig. 2), we confirm these differences and demonstrate that the presence of sta11-2::ARG7 leads to an exacerbation of the phenotype. The strains carrying the gene disruption display a more dramatic relative increase of chains of DP 12 to 16 and similar modification of the very small glucans (DP 3–6). Here again, the expressivity of the sta11-2::ARG7 mutant phenotype is significantly increased. In nitrogen-supplied medium in the light and in the presence of acetate, the relative decrease in starch amount in the mutants compared with the wild type is far less spectacular. However, even in these conditions, the sta11-2::ARG7 mutants displayed a higher expressivity than the sta11-1 carrying strains because they contained higher amylose and MOS amounts.
Figure 1.
Wild-type and mutant iodine-staining phenotypes. Strain 37 corresponds to the wild-type reference use in this work. Strains 18B (sta2-1) and I152 (sta3-1) are defective for GBSSI and SSIII-like soluble starch synthase, respectively (Delrue et al., 1992; Fontaine et al., 1993). Both JV45J (sta11-1) and CR102 (sta11-2) are defective for d-enzyme activity. Mutations at STA11 locus in JV45J and CR102 have been generated after UV and insertional mutagenesis, respectively. Cells patches were incubated 5 d on solid nitrogen-deprived medium and were subsequently stained by iodine vapors.
Table I.
Phenotypes of 137C (wild-type), JV45J (sta11-1), and CR102 (sta11-2) strains during storage (−N) and growth (+N) starch synthesis
| Starcha
|
MOSb
|
λmaxc
of
Amylopectin
|
Amylopectind
|
Amylosed
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Culture conditions | −N | +N | −N | +N | −N | +N | −N | +N | −N | +N |
| μg million cells−1 | nm | % | ||||||||
| 137C (wt) | 20.1 | 1.60 | 0.012 | 0.08 | 552 | 570 | 82 | 90 | 18 | 10 |
| JV45J (sta11hyphen]1) | 2.3 | 0.15 | 0.46 | 0.33 | 562 | 577 | 79 | 78 | 21 | 22 |
| CR102 (sta11-2) | 0.4 | 0.30 | 1.15 | 0.60 | 568 | 591 | 72 | 67 | 28 | 33 |
Amounts of insoluble polysaccharide purified through sedimentation and b amounts of soluble MOS as measured by the standard amyloglucosidase assay.
Wavelength at the maximal absorbance of the iodine-polysaccharide complex of amylopectin purified by gel filtration chromatography on Sepharose CL-2B column.
The percentage of each fraction of starch was calculated after gel filtration chromatography on Sepharose CL-2B column of the dispersed polysaccharides.
Figure 2.
Chain-length distributions of the two sta11 allelic mutants and the wild-type reference strain. Isoamylase-debranched chains were analyzed by HPAED-PAD. Percentages of chains ranging between DP 1 to 16 (chains containing 1–16 Glc residues) are scaled on the y axis. A, Debranched chains of purified amylopectin from our “waxy” reference strain BAFR1 (sta2-29::ARG7). B, Debranched chains of gel permeation chromatography-purified amylopectin from the mutant JV45J (sta11-1). C, Debranched chains of gel permeation chromatography purified amylopectin from the mutant CR102 (sta11-2).
Cloning and Characterization of a C. reinhardtii cDNA Homologous to Vascular Plants d-Enzymes
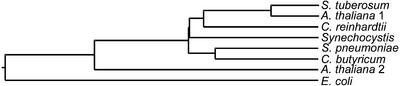
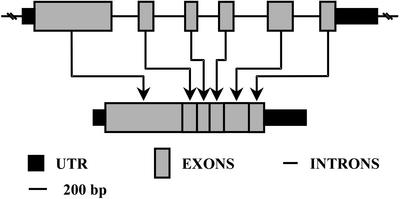
Alignment of known d-enzyme protein sequences revealed three blocks (Fig. 3, regions I–III) of highly conserved amino acid sequences between enzymes as distantly related as those of S. pneumoniae, C. butyricum, potato, and B. burgdorferi. We used degenerated oligonucleotide primers to amplify by PCR from genomic DNA, a 1,564-bp sequence with homology to vascular plant sequences. We then used this as a probe to fish out a 2,071-bp cDNA lacking the 133 bp of 5′ sequence that were subsequently obtained by RACE-PCR to yield the full cDNA sequence. The complete protein sequence is displayed in Figure 3 and was compared with the E. coli MalQ amylomaltase, the Synechocystis sp. α-1,4 glucanotransferase, the potato tuber d-enzyme, and the leaf-expressed d-enzyme sequence of Arabidopsis. A phylogenetic tree of selected α-1,4 glucanotransferases was built (Fig. 4), showing once again that the C. reinhardtii enzyme sequence could be placed as an intermediate between cyanobacteria and vascular plants. In addition to this, we selected a set of two distinct cosmids covering the entire gDNA corresponding to the STA11 gene (see “Materials and Methods”). The organization of the STA11 gene is displayed in Figure 5.
Figure 3.
Sequences comparison of α-1,4 glucanotransferases from different organisms. C. reinhardtii d-enzyme sequence (AAG29840) was compared with those from Streptococcus pneumoniae (NP_346528), Clostridium butyricum (Q59266), potato (Solanum tuberosum; QO6801), and Borrelia burgdorferi (NP_212300). Highlighted boxes display conserved regions used for oligonucleotide primers design. Alignment was constructed using the ClustalW method with the PAM250 residue weight table. The amino acid (G) located on the right side within region I of C. reinhardtii d-enzyme sequence is modified to a Cys residue in the sta11-1 mutant allele.
Figure 4.
Phylogenetic tree of selected α-1,4 glucanotransferases. Accession numbers (from top to bottom) are as follow: Q06801, NP_201291, AAG29839, NP_440120, NP_346526, Q59266, AAL91204, and NP_417875. This phylogenetic tree was built using the ClustalW method with the PAM250 residue weight table.
Figure 5.
Intron/exon organization of C. reinhardtii α-1,4 glucanotransferase gene (GenBank accession no. AF307843).
Molecular Characterization of sta11-2::ARG7
Because insertional mutagenesis in C. reinhardtii is known to lead to local rearrangements and deletions of genomic DNA that are easy to detect, we used the d-enzyme cDNA as a probe in Southern blots of wild-type and mutant recombinants of sta11-2::ARG7. The results shown in Figure 6A show cosegregation of a RFLP evidenced with the d-enzyme probe and the sta11-2::ARG7 mutation. The d-enzyme transcript levels, although low in the wild-type strain, could be detected both in northern-blot analysis (data not shown) and in reverse transcriptase (RT-PCR) experiments (Fig. 6B). On the contrary, the sta11-2::ARG7 mutants contained no detectable transcripts corresponding to d-enzyme, whereas mRNAs encoding d-enzyme could be routinely found in sta11-1 mutants with levels similar to those found in wild-type strains (Fig. 6B). These results confirmed sta11-2::ARG7 as a bona fide null mutant.
Figure 6.
A, RFLP analysis from sta11-2::ARG7 (indicated as sta11-2) mutant and wild-type strains. Molecular hybridization has been carried out using as a probe a 1.5 kb corresponding to a internal region of the α-1,4 glucanotransferase gene. B, A 0.8% (w/v) agarose gel loaded with RT-PCR products from a reaction performed with specific primers corresponding to the 3′ end of the d-enzyme mature transcript (for more details, see “Materials and Methods”).
Molecular Characterization of sta11-1
When we set out to amplify the sta11-1 cDNA by using the same set of degenerated primers as those that enabled us to clone our wild-type probe, we systematically met with failure. However the presence of mRNA was suggested by the use of other primers corresponding to the C. reinhardtii sequence. We then sequenced the whole-mutant sta11-1 cDNA and found only one consistent change in the middle of the conserved region I, thereby explaining our failure to amplify cDNAs by using oligonucleotide primers corresponding to this region of the gene. We then sequenced this region in a total of five wild-type and five sta11-1 mutants and found the modified sequence to cosegregate with the mutant allele. This cosegregation analysis was completed by an additional round of PCR performing directly on gDNA, using the degenerated primers corresponding to region I and II on eight wild-type and six mutant progeny. The sequence modification consisted of change of a GGC codon specifying Gly to a TGC codon specifying Cys at the level of the last Gly of region I (highlighted in Fig. 3)
Analysis of Enzyme Activity and Corresponding mRNA Levels during Recurrent Starch Synthesis and Degradation
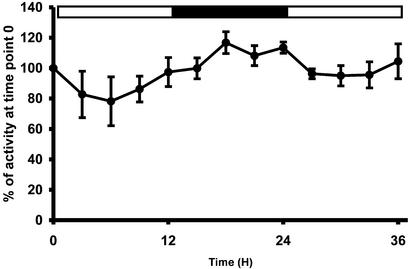
To approach the conditions used during the analysis of the Arabidopsis mutants, we subjected C. reinhardtii to a 12-h day/12-h night cycle of growth in nitrogen-supplied and CO2-enriched or acetate-supplied environment. These optimal conditions ensure that C. reinhardtii will not be restricted for growth. The enzyme activity levels were assayed while the relative mRNA abundance was estimated by semiquantitative RT-PCR experiments (data not shown). No major oscillations of mRNA abundance were observed at the experiment's level of detection (2-fold) that could be compared with those that we previously showed for the small subunit of the C. reinhardtii ADP-Glc pyrophosphorylase (data not shown; Zabawinski et al., 2001). The enzyme assays afforded for more precise measurements that revealed a small but significant increase of d-enzyme activity in darkness (Fig. 7).
Figure 7.
d-Enzyme activity (●) measured on wild-type cells grown under 12-h d/12-h night cycle with 4% (v/v) CO2 constant bubbling. The results displayed are the mean ± sds of four distinct and independent experiments. The results were compared by taking T = 0 measure as an arbitrary standard (assigned value of 100%).
Analysis of Starch and MOS Contents
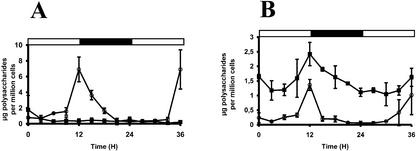
We followed starch and MOS accumulation in two sta11-1 mutants and two wild-type recombinants for 36 h as detailed in “Materials and Methods.” According to the preculture used and the particular strain under study, the time of minimal starch and MOS content varied somewhat (±3 h). However, the results were all essentially the same with the minimal starch content being obtained most often in the middle of the light phase as was previously reported (Mérida et al., 1999) and the maximal starch content being always reached at the light to dark transition (Fig. 8, A and B). Most importantly, all sta11-1 mutants MOS behaved similarly and followed closely the starch accumulation curves being degraded at the time of starch breakdown and produced at the time of polysaccharide synthesis.
Figure 8.
Starch (○) and WSP (▪) accumulations in wild-type and sta11-1 mutant strains. Cells were grown under 12-h day (white horizontal bars)/12-h night (black horizontal bars) cycle with 4% (v/v) CO2 constant bubbling. Results displayed in A are the mean ± sd of two distinct and independent experiments performed on wild-type strain. Results displayed in B are the mean ± sd of two distinct and independent experiments carried out on sta11-1 mutant strain.
DISCUSSION
α-1,4 Glucanotransferases were recently shown to define important components of starch metabolism in Arabidopsis and C. reinhardtii (Colleoni et al., 1999a, 1999b; Critchley et al., 2001). Both mutants accumulated MOS and high amylose starch when defective for d-enzyme activity. However, the levels of starch in the Arabidopsis mutant were either equivalent or higher than those displayed by the wild-type reference, whereas in the C. reinhardtii mutants, starch levels were as severely reduced as in strains defective for the large subunit of ADP-Glc pyrophosphorylase (Van den Koornhuyse et al., 1996). These vastly different phenotypes argued that in C. reinhardtii, d-enzyme is required in the normal process of starch biosynthesis, whereas in Arabidopsis, it appears that the same enzyme activity is required for starch degradation only.
In an attempt to resolve these contradictions, we undertook a detailed molecular characterization of the sta11 mutants of C. reinhardtii. The multiplicity of enzyme forms in plants is such that it remained possible that the enzyme defective in both systems belonged to different families in a fashion reminiscent of the debranching enzymes of the isoamylase or pullulanase type. Both forms of debranching enzyme catalyze the same biochemical reaction, but isoamylase was shown to be selectively involved in amylopectin synthesis, whereas pullulanase is suspected to be active in starch degradation. Because two different kinds of α-1,4 glucanotransferases are known to occur in Arabidopsis, it was of paramount importance to better define the nature of the missing enzyme activity in C. reinhardtii. It is now clear that the d-enzyme of C. reinhardtii is more related to the Arabidopsis chromosome V form of α-1,4 glucanotransferase and to the previously characterized potato d-enzyme than to that of the Arabidopsis chromosome II. Yet it is the chromosome V form of α-1,4 glucanotransferase that is missing in the Arabidopsis mutant. Because only one kind of α-1,4 glucanotransferase is presently documented in the extensive expressed sequence tag data gathered for C. reinhardtii (more than 110,000 expressed sequence tags are currently available for C. reinhardtii), it still remains possible that α-1,4 glucanotransferases sequences have been duplicated during evolution of vascular plants and that different forms of enzymes have acquired specialized anabolic or catabolic functions.
Another possible explanation for the conflicting results obtained in Arabidopsis and C. reinhardtii could come from the different molecular nature of the mutations under investigation. The Arabidopsis mutant carries a T-DNA insertion essentially inactivating the gene, whereas the C. reinhardtii UV-generated mutant is now shown to consist of a single-amino acid change in an otherwise intact protein. It is thus possible that the very nature of these different mutations could explain the observed phenotypic contradictions. However, the report we now make of a gene disruption of the C. reinhardtii STA11 gene disproves this. the absence of d-enzyme mRNA and protein in C. reinhardtii correlates with a further decrease in starch amount to less than 3% of the wild-type amount during nitrogen starvation, an observation that is very hard to reconcile with an absence of direct or indirect function of d-enzyme in the normal process of starch biosynthesis. The increased phenotypic expressivity of the sta11-2::ARG7 gene disruption was also evidenced on the chain-length distribution of amylopectin. This modification can be explained through a direct function of α-1,4 glucanotransferases in amylopectin synthesis as suggested by Colleoni et al. (1999b). In that case, it was proposed that the function of the α-1,4 glucanotransferase would be to transfer most of the glucans produced by isoamylases during biosynthesis back to the external pre-amylopectin chains. The modification in chain-length distribution of the mutant amylopectin can also be explained by an indirect and more likely effect consisting of the lowering of the ADP-Glc concentration. Such a substrate concentration decrease is expected if the function of d-enzyme is to harvest efficiently the energy contained in the glucans released by isoamylase by assisting the release of G1P through starch phosphorylase. The modified chain-length distribution witnessed here is very similar to that evidenced on the amylopectin of the low-starch mutants defective for either phosphoglucomutase or the large subunit of ADP-Glc pyrophosphorylase (Van den Koornhuyse et al., 1996). Future characterization of the phenotypes witnessed in both Arabidopsis and C. reinhardtii should therefore focus on metabolic profiling of the mutant and wild-type strains.
In a final attempt to resolve the contradictions observed between the Arabidopsis and C. reinhardtii mutants, we have investigated the phenotype of the algal mutants in physiological conditions that are closer to those of the Arabidopsis leaf, which defines a typical plant “source” tissue. Our previous report concerned nitrogen-starved culture where starch accumulates to very high levels and adopts a structure similar to that of storage starch, which is synthesized in the plant “sink” reserve tissues (the kernel endosperm, the tuber, the seed embryo, etc.). During nitrogen starvation, the C. reinhardtii cell becomes progressively non-photosynthetic. It was therefore possible that if starch turnover occurs in these conditions, the energy losses occasioned by the presence of futile cycles would not be compensated by photophosphorylation. The fact that preamylopectin processing during biosynthesis is expected to release MOS through the action of isoamylases, may explain why inefficient MOS metabolism may trigger a collapse of polysaccharide synthesis under nitrogen starvation. We therefore have investigated the behavior of the sta11 mutants in nitrogen-supplied medium and growing in log phase in the presence of a 12-h-light and 12-h-dark cycles. The experiments detailed in this work prove that the ATP supplied through photosynthesis does not bypass the requirement for d-enzyme activity during biosynthesis. Most importantly, the MOS accumulated at the time of starch biosynthesis and were degraded at the time of starch breakdown, a result that is in clear contradiction with the situation documented in Arabidopsis. It must be stressed that the C. reinhardtii system presents important differences with respect to the mature Arabidopsis leaf cell. Among these differences, the cell cycle remains active in the algae. Together with the ticking of the circadian clock, this may be responsible for the very different timing of starch biosynthesis and degradation observed in C. reinhardtii. This may however not be a unique property of algae because other vascular plant leaf tissues are also known to anticipate the arrival of darkness and trigger starch degradation in the light (Li et al., 1992; Mérida et al., 1999).
It is now apparent that different species of plants as well as different tissues of a same plant harbor very different levels of enzyme activities of starch and MOS metabolism. This remains true when one compares those tissues actively engaged in starch synthesis. The Arabidopsis mature leaf, the C. reinhardtii nitrogen-supplied cell growing in the light, the C. reinhardtii nitrogen-starved cell, the potato tuber, the maize endosperm, and the pea embryo all contain vastly different types and amounts of enzyme activities concerned with starch metabolism. Although the “core” synthetic pathway composed of ADP-Glc pyrophosphorylases, starch synthases, branching enzymes, and isoamylases are present in all cases (for review, see Kossmann and Lloyd, 2000; Myers et al., 2000), the systems contain very different amounts of those other enzymes whose functions in starch metabolism remain unclear. d-Enzyme activity is easy to detect in C. reinhardtii, in the Arabidopsis leaf, and in the potato tuber. However, the activity could not be detected at comparable levels in the maize endosperm. When maize and C. reinhardtii pullulanase are assayed and compared, the algae proved to contain one to two orders of magnitude less of this enzyme activity, whereas plastidial phosphorylase defines one of the most abundant enzymes of the maize endosperm amyloplast. The MOS produced by isoamylase during starch biosynthesis could be metabolized through many distinct pathways with different consequences. They can be very efficiently recovered by providing primers to the soluble or granule-bound starch synthases. They can be equally efficiently recovered by being transferred on pre-amylopectin by d-enzyme. They can be degraded with moderate energy loss by a combination of d-enzyme and phosphorylase digestion, or they can feed a futile cycle by degradation through endo or exo type of amylases or by glucosidases. The phenotypic consequences will depend on the limited or unlimited availability of substrate and ATP, which in turn will depend on the highly variable physiological conditions prevailing in the tissue under consideration. d-Enzyme as a major enzyme of MOS metabolism is likely to be of use in both polysaccharide synthesis and degradation. The consequences of mutations affecting d-enzyme activity on starch metabolism will largely depend on the enzymatic make-up and physiological status of the cell under consideration.
MATERIALS AND METHODS
Materials
[α-32P]dCTP was purchased from Amersham Biosciences UK Ltd. (Little Chalfont, Buckinghamshire, UK). CL-2B Sepharose column and Percoll were obtained from Amersham Biosciences AB (Uppsala). Starch assay kit was obtained from Roche Diagnostics (Mannheim, Germany).
Chlamydomonas reinhardtii Strains, Growth Conditions, and Media
The reference strains of C. reinhardtii used in this study are 137C (mt− nit1 nit2), 37 (mt+ pab2 ac14), and BAFR1 (mt+ nit1 nit2 sta2-29::ARG7). The strain used for nuclear transformation is TerBD20 (mt− sta2-1 nit1 nit2 cw15 arg7-7). JV45J (mt− nit1 nit2 sta11-1) and all “CO” strains were previously described (Colleoni et al., 1999a). RC21 (mt− sta11-2::ARG7 sta2-1 nit1 nit2 cw15 arg7-7) was obtained by nuclear transformation of TerBD20 using plasmid pASL and strain CR102 (sta11-2::ARG7) represents a progeny from a cross involving both RC21 and 37 strains. Standard media are fully detailed by Harris (1989), whereas growth conditions and nitrogen-starved media are described by Ball et al. (1990, 1991), Delrue et al. (1992), and Libessart et al. (1995). For the studies concerning enzyme activity, mRNA level, starch, and MOS measurement in 12-h-day/12-h-night cycle, experiments were performed under high CO2 level (4%) bubbling in Sueoka medium (Sueoka, 1960).
Insertional Mutagenesis
Transformations of strain TerBD20 with plasmid pASL (Adam and Loppez, 1998) were performed following method described by Kindle (1990).
d-Enzyme Assays
Protocols used for crude extracts preparations and enzyme assays were previously described by Colleoni et al. (1999a, 1999b).
Determination of Starch Levels, Starch Purification, and Spectral Properties of the Iodine-Starch Complex
A full account of amyloglucosidase assays, starch purification on Percoll gradient, and λmax (maximal absorbance wavelength of the iodine polysaccharide complex) measures can be found in Delrue et al. (1992).
Separation of Starch Polysaccharides by Gel Permeation Chromatography
Starch (1.5 mg) dissolved in 500 μL of 10 mm NaOH was applied to a column (0.5-cm i.d. × 65 cm) of Sepharose CL-2B, which was equilibrated and eluted with 10 mm NaOH. Fractions of 300 to 320 μL were collected at a rate of one fraction per 1.5 min. Glucans in the fractions were detected by their reaction with iodine, and the levels of amylopectin and amylose were determined by amyloglucosidase assays (Roche Diagnostics).
Chain Length Distribution Analysis
Fractions from CL-2B column containing amylopectin were pooled and dialyzed 12 h against water. After lyophilization, the samples were digested by isoamylase (2 units in 55 mm sodium acetate buffer, pH 3.5; Megazyme International, Bray, County Wicklow, Ireland) before HPAED-PAD analysis. For a complete description of this technique, refer to Fontaine et al. (1993).
Cloning of the Full-Length d-Enzyme gDNA
A complete description of algal DNA extraction can be found in Rochaix et al. (1991). Degenerated primers corresponding to the two first of the three highly conserved regions of d-enzyme protein sequence (see Fig. 2) were designed as following: primer I, 5′-RTTRTCRTGNGTACCNGTRTA; and primer II, 5′-RAARCCVGCRAAATGRTCRATVCG. PCR amplification performed on wild-type genomic DNA with both primers I and II gave a specific fragment of 1,564 bp whose sequence was strongly similar to that of d-enzymes already cloned from higher plants. To isolate the full genomic copy of the structural gene of C. reinhardtii d-enzyme, 11,280 Escherichia coli clones from a cosmid library (Zhang et al., 1994) were screened using the previously obtained 1,564-bp fragment as a radiolabeled probe. This genomic library is indexed in 120 microtitration plates, and the corresponding E. coli clones were transferred onto nylon filters and consequently treated as described by Sambrook et al. (1989) before hybridization with the specific nucleotide probe. Out of a total of four positives clones, two were selected for further analysis because of their strong hybridizations with the probe. This prompted us to use these cosmids for complete sequencing of the d-enzyme gene subsequently submitted to GenBank (accession no. AF307843).
Cloning of the Full-Length d-Enzyme cDNA
A partial cDNA clone corresponding to algal d-enzyme was isolated as follows. Approximately 500,000 lysis plaques of a C. reinhardtii λZAP II cDNA library were screened with the 1,564-bp genomic probe previously described. A cDNA clone with an insert of 2,071 bp was isolated and fully sequenced on both strand. The 5′ end of the d-enzyme cDNA was obtained by RACE-PCR (Invitrogen, Carlsbad, CA) following the supplier's instructions. In brief, a total fraction of RNA from the wild-type strain was reverse transcribed using the following specific primer 5′-CTCCAGCAGTCCGTCCTTG. A first PCR amplification of the subsequently produced cDNA was done using the specific primer 5′-GCTCCTCAATGCTCACCACAA while the nested PCR amplification was carried out with the next specific primer 5′-CGGGGGCACCAGCGGCAGCAG. The complete cDNA obtained was submitted to GenBank (accession no. AF307842). Total RNA was extracted from the wild-type strain 330 with the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the supplier's instructions.
RFLP Analysis
Standard protocols for molecular biology as described by Sambrook et al. (1989) were used for RFLP analysis, including gDNA restriction and subsequent electrophoresis on agarose gel, transfer onto nylon membranes, and hybridization with a specific probe. Approximately 10 μg of gDNA was digested with 50 units of SmaI. Restriction fragments were then separated on 0.8% (w/v) agarose gel and transferred onto a nylon membrane (Porablot, NY Amp, Macherey-Nagel, Germany). Hybridization was performed overnight at 65°C in the following hybridization buffer: 5× SSC, 5× Denhardt's, 0.1% (w/v) SDS, and 0.1 g mL−1 denatured salmon sperm DNA, where 1× SSC is 0.15 m NaCl, 0.015 m sodium citrate and 1× Denhardt's is 0.2 g L−1 Ficoll 400, 0.2 g L−1 PVP40, and 0.2 g L−1 bovine serum albumin. Probes were radiolabeled by random priming method as described by supplier's instruction (Amersham Biosciences). Membranes were typically washed twice in 2× SSC and 0.1% (w/v) SDS at 65°C for 10 min and twice in 0.5× SSC and 0.1% (w/v) SDS at 65°C for 10 min before exposure to x-ray film.
RT-PCR Experiments
Total RNA was extracted using the RNeasy plant mini kit as described by the manufacturer (Qiagen). Total RNA was then quantified by agarose gel analysis and spectrophotometry. Reverse transcription was performed with SuperScript II kit (Invitrogen). The product of the reaction was then purified on mini-column (PCR purification kit, BIO 101, La Jolla, CA), and the subsequent PCR amplification was performed using the “Jump start ready mix” (Sigma-Aldrich, St Louis). Primers corresponding to the d-enzyme cDNA were the following: RTD3, 5′-TGATGCGGCTGGACAACACG; and RTD4, 5′-TCCCCACAGAAACGCACCCCTACA.
Footnotes
This work was supported by the Université des Sciences et Technologies de Lille, by the Ministère de l'Education Nationale et de la Recherche, by the Centre National de la Recherche Scientifique (Unité Mixte de Recherche 8576 du Centre National de la Recherche Scientifique), and by the Région Nord-Pas de Calais (fellowship no. 99060060 to F.W.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.016527.
LITERATURE CITED
- Adam M, Loppez R. Use of the ARG7 gene as an insertional mutagen to clone PHON24, a gene required for derepressible neutral phosphatase activity in Chlamydomonas reinhardtii. Mol Gen Genet. 1998;258:123–132. doi: 10.1007/s004380050714. [DOI] [PubMed] [Google Scholar]
- Ball S, Marianne T, Dirick L, Fresnoy M, Delrue B, Decq A. A Chlamydomonas reinhardtiilow-starch mutant is defective for 3-phosphoglycerate activation and orthophosphate inhibition of ADP-glucose pyrophosphorylase. Planta. 1991;185:17–26. doi: 10.1007/BF00194509. [DOI] [PubMed] [Google Scholar]
- Ball SG, Dirick L, Decq A, Martiat JC, Matagne RF. Physiology of starch storage in the monocellular alga Chlamydomonas reinhardtii. Plant Sci. 1990;66:1–9. [Google Scholar]
- Colleoni C, Dauvillée D, Mouille G, Buléon A, Gallant D, Bouchet B, Morell M, Samuel M, Delrue B, D'Hulst C et al. Genetic and biochemical evidence for the involvement of α-1,4 glucanotransferases in amylopectin synthesis. Plant Physiol. 1999a;120:993–1003. doi: 10.1104/pp.120.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleoni C, Dauvillée D, Mouille G, Morell M, Samuel M, Slomiany M-C, Liénard L, Wattebled F, D'Hulst C, Ball S. Biochemical characterization of the Chlamydomonas reinhardtiiα-1,4 glucanotransferase supports a direct function in amylopectin biosynthesis. Plant Physiol. 1999b;120:1005–1014. doi: 10.1104/pp.120.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JM, Zeeman SC, Takaha T, Smith AM, Smith SM. A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J. 2001;26:89–100. doi: 10.1046/j.1365-313x.2001.01012.x. [DOI] [PubMed] [Google Scholar]
- Delrue B, Fontaine T, Routier F, Decq A, Wieruszeski JM, Van Den Koornhuyse N, Maddelein ML, Fournet B, Ball S. Waxy Chlamydomonas reinhardtii: monocellular algal mutants defective in amylose biosynthesis and granule-bound starch synthase activity accumulate a structurally modified amylopectin. J Bacteriol. 1992;174:3612–3620. doi: 10.1128/jb.174.11.3612-3620.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine T, D'Hulst C, Maddelein ML, Routier F, Marianne-Pépin T, Decq A, Wieruszeski JM, Delrue B, Van den Koornhuyse N, Bossu JP et al. Toward an understanding of the biogenesis of the starch granule: evidence that Chlamydomonassoluble starch starch synthase II controls the synthesis of intermediate size glucans of amylopectin. J Biol Chem. 1993;268:16223–16230. [PubMed] [Google Scholar]
- Harris EH, editor. The Chlamydomonas Sourcebook. A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- James MG, Robertson DS, Myers AM. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell. 1995;7:417–429. doi: 10.1105/tpc.7.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Whelan WJ. The action pattern of D-enzyme, a transmaltodextrinylase from potato. Carbohydr Res. 1969;9:483–490. [Google Scholar]
- Kindle K. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Genetics. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann J, Lloyd J. Understanding and influencing starch biochemistry. Crit Rev Biochem Mol Biol. 2000;35:141–196. [PubMed] [Google Scholar]
- Li B, Geiger DR, Shieh WJ. Evidence for circadian regulation of starch and sucrose synthesis in sugar beet leaves. Plant Physiol. 1992;99:1393–1399. doi: 10.1104/pp.99.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libessart N, Maddelein ML, Van den Koornhuyse N, Decq A, Delrue B, Ball SG. Storage, photosynthesis and growth: the conditional nature of mutations affecting starch synthesis and structure in Chlamydomonas reinhardtii. Plant Cell. 1995;7:1117–1127. doi: 10.1105/tpc.7.8.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J. Isolation and characterization of a starchless mutant of Arabidopsis thaliana(L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol. 1988;86:1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mérida A, Rodriguez-Galan JM, Vincent C, Romero JM. Expression of the granule-bound starch synthase I (Waxy) gene from snapdragon is developmentally and circadian clock regulated. Plant Physiol. 1999;120:401–410. doi: 10.1104/pp.120.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouille G, Maddelein M-L, Libessart N, Talaga P, Decq A, Delrue B, Ball S. Phytoglycogen processing: a mandatory step for starch biosynthesis in plants. Plant Cell. 1996;8:1353–1366. doi: 10.1105/tpc.8.8.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AM, Morell MK, James MG, Ball SG. Recent progress in understanding biosynthesis of the amylopectin crystal. Plant Physiol. 2000;122:989–998. doi: 10.1104/pp.122.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Umemoto T, Takahata Y, Komae K, Amano E, Satoh H. Changes in structure of starch and enzyme activities affected by sugary mutations in developing rice endosperm. Possible role of starch debranching enzyme (R-enzyme) in amylopectin biosynthesis. Physiol Plant. 1996;97:491–498. [Google Scholar]
- Peat S, Whelan WJ, Rees WR. The enzymic synthesis and degradation of starch: the disproportionating enzyme of potato. J Chem Soc. 1956;1956:44–53. [Google Scholar]
- Rochaix JD, Mayfield S, Goldschmidt-Clermont M, Erickson J. Molecular biology of Chlamydomonas. In: Shaw C, editor. Plant Molecular Biology: A Practical Approach. Oxford: IRL Press; 1991. pp. 253–275. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sueoka N. Mitotic replication of deoxyribonucleic acid in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1960;46:83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teste MA, Enjalbert B, Parrou JL, Francois JM. The Saccharomyces cerevisiae YPR184wgene encodes the glycogen debranching enzyme. FEMS Microbiol Lett. 2000;193:105–110. doi: 10.1111/j.1574-6968.2000.tb09410.x. [DOI] [PubMed] [Google Scholar]
- Van den Koornhuyse N, Libessart N, Delrue B, Zabawinski C, Decq A, Iglesias A, Carton A, Preiss J, Ball S. Control of starch composition and structure through substrate supply in the monocellular alga Chlamydomonas reinhardtii. J Biol Chem. 1996;271:16281–16287. doi: 10.1074/jbc.271.27.16281. [DOI] [PubMed] [Google Scholar]
- Zabawinski C, Van Den Koornhuyse N, D'Hulst C, Schlichting R, Giersch C, Delrue B, Lacroix J-M, Preiss J, Ball S. Starchless mutants of Chlamydomonas reinhardtiilack the small subunit of an heterotetrameric ADP-glucose pyrophosphorylase. J Bacteriol. 2001;183:1069–1077. doi: 10.1128/JB.183.3.1069-1077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Umemoto T, Lue WL, Au-Yeung P, Martin C, Smith AM, Chen J. A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both starch and phytoglycogen. Plant Cell. 1998;10:1699–1712. doi: 10.1105/tpc.10.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Herman P, Weeks D. Gene isolation through genomic complementation using an indexed library of Chlamydomonas reinhardtiiDNA. Plant Mol Biol. 1994;24:663–672. doi: 10.1007/BF00023562. [DOI] [PubMed] [Google Scholar]