Abstract
We have used genotypic variation in birch (Betula pendula Roth) to investigate the roles of ozone (O3)-induced ethylene (ET), jasmonic acid, and salicylic acid in the regulation of tissue tolerance to O3. Of these hormones, ET evolution correlated best with O3-induced cell death. Disruption of ET perception by transformation of birch with the dominant negative mutant allele etr1-1 of the Arabidopsis ET receptor gene ETR1 or blocking of ET perception with 1-methylcyclopropene reduced but did not completely prevent the O3-induced cell death, when inhibition of ET biosynthesis with aminooxyacetic acid completely abolished O3 lesion formation. This suggests the presence of an ET-signaling-independent but ET biosynthesis-dependent component in the ET-mediated stimulation of cell death in O3-exposed birch. Functional ET signaling was required for the O3 induction of the gene encoding β-cyanoalanine synthase, which catalyzes detoxification of the cyanide formed during ET biosynthesis. The results suggest that functional ET signaling is required to protect birch from the O3-induced cell death and that a decrease in ET sensitivity together with a simultaneous, high ET biosynthesis can potentially cause cell death through a deficient detoxification of cyanide.
The concentration of tropospheric ozone (O3) has increased during the past decades due to human activities, and it has been estimated that in year 2100, 50% of global forest area will be exposed to potentially phytotoxic O3 concentrations (Fowler et al., 1999). In the leaves of O3-sensitive plants, symptoms of high O3 are observed as rapid lesion formation. Traditionally, formation of reactive oxygen species (ROS), such as superoxide (O2·−) and hydrogen peroxide (H2O2) from the degradation of O3 in the apoplast has been thought to alter the integrity of the plasma membrane and thus the integrity of the cell (Laisk et al., 1989; Heath, 1994). However, O3 also induces an active and controlled apoplastic oxidative burst, the production of O2·− and H2O2 in the leaves affected, which may initiate programmed cell death analogous to that induced by ROS in an incompatible plant-pathogen interaction (Schraudner et al., 1998; Pellinen et al., 1999, 2002; Overmyer et al., 2000; Moeder et al., 2002; Wohlgemuth et al., 2002).
Activation of ethylene (ET) biosynthesis by induction of the genes encoding 1-aminocyclopropane-1-carboxylate synthase (ACS) is one of the fastest and most obvious biochemical responses to O3 and has been mechanistically linked to the regulation of O3 lesion formation (Schlagnhaufer et al., 1997; Tuomainen et al., 1997; Vahala et al., 1998; Overmyer et al., 2000; Moeder et al., 2002). ET is perceived by a two-component His kinase receptor family (Chang et al., 1993a; Hua et al., 1995, 1998; Sakai et al., 1998). The ET receptor can be pharmacologically blocked with a competitive inhibitor of ET action, norbornadiene, or with 1-methylcyclopropene (MCP; Serek et al., 1995), which prevents the binding of ET to the receptor. The known dominant ET receptor mutations also prevent ET perception and thus cause ET insensitivity (Hall et al., 1999). This has been used to create stable ET insensitivity in petunia (Petunia hybrida), tomato (Lycopersicon esculentum), and tobacco (Nicotiana tabacum) by transforming them with the Arabidopsis etr1-1 mutant allele (Wilkinson et al., 1997; Knoester et al., 1998).
Inhibition of ET biosynthesis with inhibitors of ACS has significantly reduced O3-induced lesion formation in the leaves of O3-exposed plants (Mehlhorn and Wellburn, 1987; Mehlhorn et al., 1991; Schlagnhaufer et al., 1995; Wenzel et al., 1995; Tuomainen et al., 1997; Moeder et al., 2002). Similarly, defective ET signaling in the ET-insensitive ein2 mutant (Overmyer et al., 2000) or inhibition of ET perception with exogenous norbornadiene reduced O3-induced cell death in tomato (Bae et al., 1996; Moeder et al., 2002) and the spread of O3-induced programmed cell death in an O3-sensitive Arabidopsis radical induced cell death1 (rcd1) mutant (Overmyer et al., 2000). Furthermore, in tomato, both ET biosynthesis and the subsequent H2O2 accumulation, which was required for the O3 lesion formation, were highly colocalized, and inhibition of ET synthesis or perception also significantly reduced H2O2 accumulation and O3 lesion formation (Moeder et al., 2002). Taken together, in tomato and Arabidopsis, both ET synthesis and signaling are required in the processes that result in O3 lesion formation.
In addition to ET, jasmonic acid (JA), and salicylic acid (SA) are involved in the regulation of oxidative stress responses in plants. JA is commonly regarded as a protective compound (Creelman and Mullet, 1995; Berger, 2001) and may have an essential role in the control of proper lesion containment upon pathogen and O3 challenge (Thomma et al., 1998; Overmyer et al., 2000; Berger, 2001). SA is a central component in plant pathogen defense. It is involved in the regulation of the oxidative burst, cell death in hypersensitive response, defense signaling, and systemic acquired resistance (Godiard et al., 1994; Staskawicz et al., 1995; Draper, 1997; Shirasu et al., 1997; Ciardi et al., 2000; Dangl and Jones, 2001). SA has a dual role in the control of plant O3 responses; SA promotes the oxidative cell death during a short-term O3 challenge but is also required for the up-regulation of defenses during a long-term oxidative challenge (Rao and Davis, 1999, 2001).
The mechanisms of stress resistance and acclimation in trees are not well understood; for example, there are only a few studies on the hormonal interactions during oxidative stress in trees. In hybrid poplar (Populus maximowizii × P. trichocarpa), sensitivity to acute high O3 correlated with deficiencies in both JA- and SA-signaling pathways (Koch et al., 1998, 2000). However, the role of ET was unexplored. We have explored the role of ET under low chronic O3 in the accompanying study (Vahala et al., 2003), which shows that ET also modifies O3 sensitivity in hybrid aspen. In the experiments reported here, we have characterized the involvement of all three signaling pathways, ET, JA, and SA, in cell death induced by a short exposure to high O3 concentration in silver birch (Betula pendula Roth) genotypes differing in their O3 sensitivity. The role of ET was elucidated in detail using transgenic ET-insensitive birch lines and inhibitors. We show that ET insensitivity in birch reduced but did not completely eliminate O3-induced cell death when inhibition of ET biosynthesis abolished the lesion formation. This suggests the presence of an ET-signaling-independent but ET biosynthesis-dependent component in the ET-mediated stimulation of cell death in O3-exposed birch.
RESULTS
Kinetics of O3-Induced ET, JA, and SA Production among Three Wild-Type Birch Clones
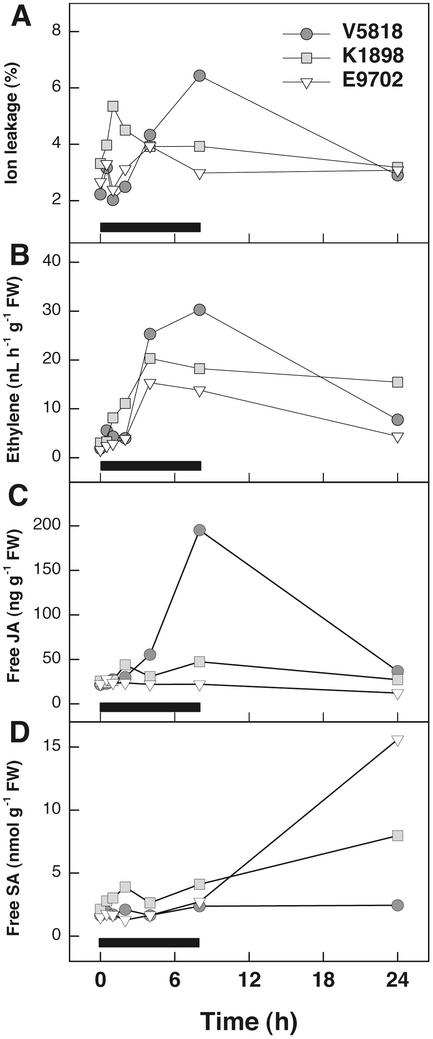
Three birch clones, the O3-tolerant E9702, the moderately sensitive K1898 and the sensitive V5818 were selected from 17 genotypes differing in their O3 sensitivity. One-year-old copies of the clones (ramets) were exposed to 200 nL L−1 O3 for 8 h. Over the whole experimental period, the clones did not differ statistically significantly in O3-induced ion leakage but displayed a differential pattern in O3-induced ion leakage (Fig. 1A).
Figure 1.
Ion leakage, ET evolution, and JA and SA concentrations in three 1-year-old wild-type birch clones, V5818, K1898, and E9702, in response to O3. Clones were subjected to 200 nL L−1 O3 for 8 h, and leaves were harvested for ion leakage and hormone determinations after the beginning of the exposure at the times indicated. Black bar indicates the duration of O3 fumigation. Measurements were conducted from three independent ramets at each time point/clone. Two-way ANOVA (clone and time as factors) followed by Tukey's honestly significant difference (HSD) mean-separation test was used to detect statistically significant differences between the clones. A, Ion leakage (P = 0.163); B, ET evolution (P = 0.074); C, free JA concentrations (ANOVA, P < 0.0005; E9702 had lower JA accumulation than V5818 and K1898, Tukey's HSD mean-separation test P < 0.0005 and P = 0.001, respectively); D, free SA concentrations (ANOVA, P < 0.0005; K1898 had higher SA accumulation than V5818 and E9702, Tukey's HSD mean-separation test P < 0.0005 and P = 0.006, respectively).
ET evolution was highest in the O3-sensitive V5818 and lowest in the tolerant E9702. ET evolution was maximal at 4 to 8 h and correlated with ion leakage in all three clones (Table I). All of the changes in ET evolution were caused by O3 because ET production did not change in air-grown plants throughout the experiments (data not shown). O3 induced a significant increase in the accumulation of JA at 8 h in the O3-sensitive clone V5818, whereas only a slight increase was observed in K1898 and no increase was observed in the O3-tolerant E9702 (Fig. 1C). SA accumulated in significant amounts only 24 h after the onset of O3 exposure, especially in the O3 tolerant clone E9702. Clone K1898 accumulated intermediate levels of SA, whereas no SA accumulation was evident in the O3-sensitive V5818 (Fig. 1D). O3-induced ion leakage, JA and SA concentrations, and ET evolution increased more rapidly in clone K1898 than in the two other clones.
Table I.
Correlation analysis of ion leakage, ET, JA, and SA in one-year-old clones V5818, K1898, and E9702 in response to O3.
| Clone | Hormone | Ion Leakage | ET | JA |
|---|---|---|---|---|
| V5818 | ET | 0.643** | ||
| JA | 0.552** | 0.582** | ||
| SA | 0.173 | 0.121 | 0.392 | |
| K1898 | ET | 0.477* | ||
| JA | 0.512* | 0.376 | ||
| SA | −0.004 | −0.016 | 0.371 | |
| E9702 | ET | 0.469* | ||
| JA | −0.053 | −0.022 | ||
| SA | 0.110 | 0.438 | −0.001 |
The clones were subjected to 200 nL L−1 of O3 for 8 h, and leaves were harvested for ion leakage and hormone determinations at 0, 0.5, 1, 2, 4, 8, and 24 h from the beginning of the O3-exposure. Correlation analysis was conducted with the nonparametric Spearman's p. The statistical differences were considered significant at the level of P < 0.05. **P < 0.01; *P < 0.05.
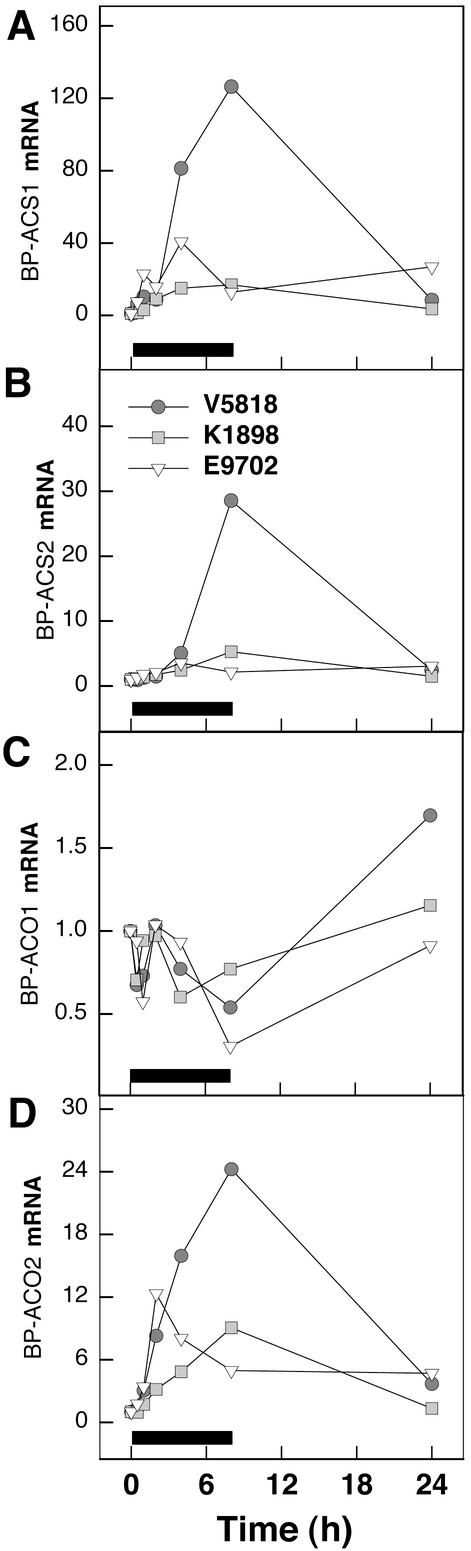
Kinetics of Transcript Accumulation for Genes of ET Biosynthesis Correlates with O3 Tolerance
Three birch ACS cDNAs were isolated to study whether the expression kinetics of ACS genes differed among the clones. In E9702, transcript levels for BP-ACS1 (AY120897) and BP-ACS2 (AY120898) reached the maximum at 4 h, whereas in V5818 and K1898, the maximum was reached at 8 h (Fig. 2, A and B). In V5818, the steady-state transcript levels for BP-ACS1 and BP-ACS2 were approximately 140- and 30-fold higher, respectively, than the initial level at 8 h, whereas in E9702, transcripts for BP-ACS2 were almost undetectable at 8 h. BP-ACS3 (AY120899) expression was not affected by O3 (data not shown). In all clones, BP-ACO1 (l-aminocyclopropane-l-carboxylate oxygenase; Y10749) transcript levels decreased between 0.5 and 1 h, increased back to the initial level at 2 h, and thereafter again decreased (Fig. 2C). The increase in transcript accumulation for BP-ACO2 (AY154649) was among the fastest responses to O3 and attained the maximum in clone E9702 at 2 h, and in clones K1898 and V5818 at 8 h (Fig. 2D).
Figure 2.
Relative increases in transcript abundance for the enzymes of ET biosynthesis in three 1-year-old wild-type birch clones, V5818, K1898, and E9702, in response to O3. Clones were subjected to 200 nL L−1 O3 for 8 h, and leaves for RNA isolation were harvested at the times indicated. Hybridization signals of each transcript are indicated as -fold increase of the initial level at 0 h. Black bars indicate the duration of O3 fumigation. A, mRNA levels of BP-ACS1; B, mRNA levels of BP-ACS2; C, mRNA levels of BP-ACO1; and D, mRNA levels of BP-ACO2.
Generation of Transgenic, ET-Insensitive Birch Lines
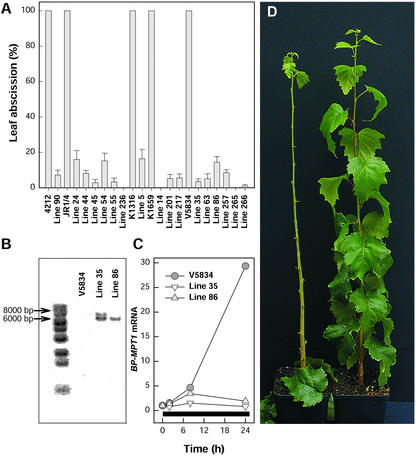
Of the hormones investigated, ET evolution had the highest correlation with O3-induced cell death in all three clones (Table I). The role of ET in O3-induced cell death was studied further with inhibitors of ACS and ET perception and by generating in five different genetic backgrounds several transgenic birch lines that carry the dominant etr1-1 mutant allele of the Arabidopsis ET receptor gene ETR1. The degree of ET insensitivity in the transgenic lines was assessed by ET-induced leaf abscission and induction of an ET-regulated gene. After a 3-d exposure to ET, most of the wild type but none of the transgenic trees had freely abscised their leaves (Fig. 3D). When all of the remaining leaves were pulled with a rather strong force, a greatly attenuated leaf abscission was evident in 17 of the 23 independent transgenic lines examined (Fig. 3A). Southern analysis with the Arabidopsis ETR1 revealed that the number of etr1-1 inserts varied between the transgenic lines. For example, line 35 had two etr1-1 inserts, and line 86 had one (Fig. 3B).
Figure 3.
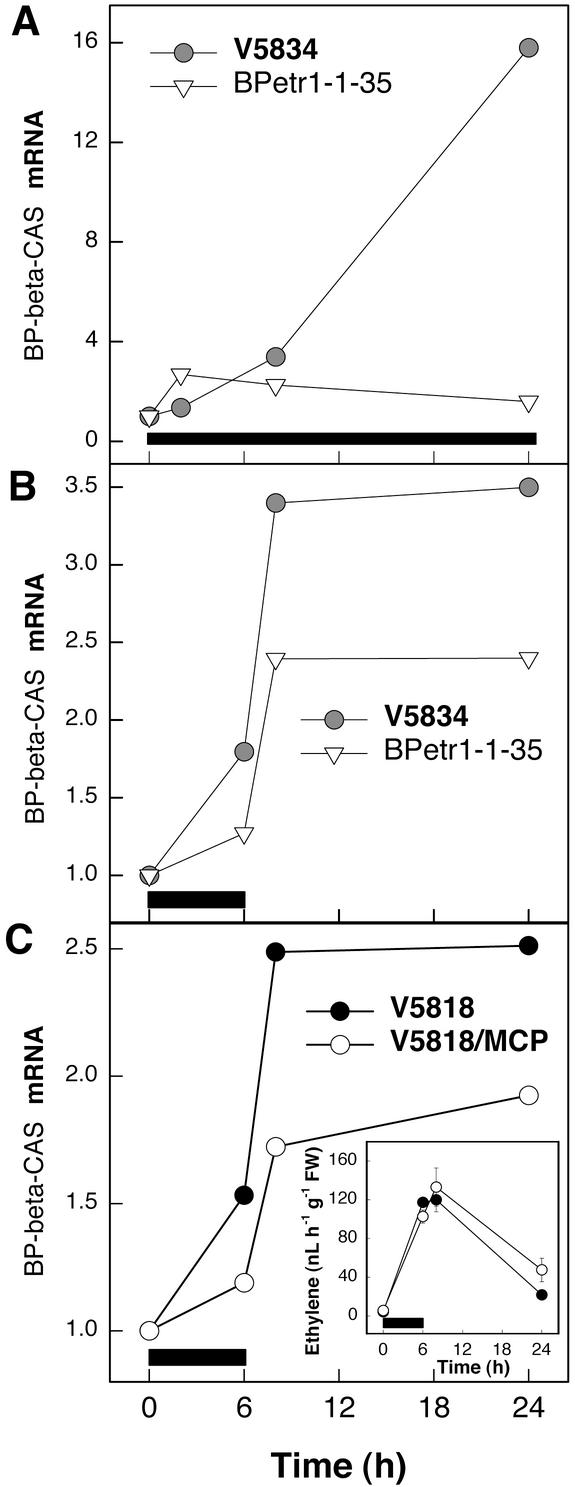
Evidence for ET insensitivity in the transgenic birch lines carrying Arabidopsis ET receptor gene with the dominant etr1-1 mutation. A, Leaf abscission in response to 50 μL L−1 ET for 3 d in wild-type clones 4212, JR1/4, K1316, K1659, and V5834 and in 17 transgenic birch lines. Error bars indicate ±se (n = 6–11). Leaf abscission in each clone was assessed by pulling with similar force every leaf of the trees exposed to ET. B, Southern analysis for the presence of the Arabidopsis etr1-1 gene in the wild-type clone V5834 and in transgenic birch lines 35 and 86. Birch genomic DNA was digested with KpnI and was hybridized with an Arabidopsis ETR1-specific probe. C, Accumulation of birch mitochondrial phosphate translocator (BP-MPT1) transcript in the wild-type clone V5834 and in the transgenic birch lines 35 and 86 in response to 50 μL L−1 ET. Black bar indicates the duration of ET treatment. D, Leaf abscission in response to a 3-d ET treatment in the wild type (left) and etr1-1 transformed transgenic line (right) of birch.
ET insensitivity was also visible as impaired ET induction of gene expression in the transgenic lines. A birch mitochondrial phosphate translocator gene (BP-MPT1 [Y08499]; Kiiskinen et al., 1997) was strongly induced by ET in the wild-type birch clones but not in the transgenic lines. This was tested in five wild-type clones and 12 independent transgenic lines with similar results; the results for two of the transgenic lines (35 and 86) and the corresponding wild-type clone V5834 are shown in Figure 3C. We selected line 35, denoted hereafter as BPetr1-1-35, for further studies on the role of ET perception in O3-induced cell death. The O3 sensitivity of V5834 is similar to clone V5818 used in the other experiments (J. Vahala, H. Tuominen, and J. Kangasjärvi, unpublished data).
ET Insensitivity Reduces But Does Not Prevent O3-Induced Cell Death
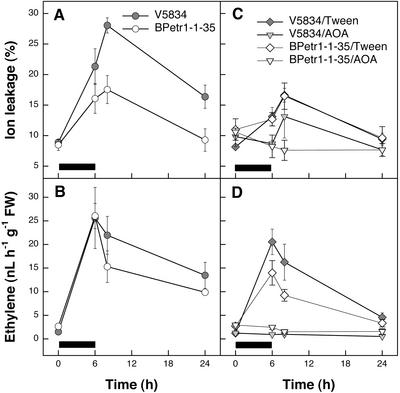
The ET-insensitive BPetr1-1-35 had less O3 damage (two-way ANOVA; genotype and time as factors; P = 0.001; Fig. 4A) but similar ET evolution (two-way ANOVA; P = 0.944; Fig. 4B) when compared with the wild-type clone V5834. The remaining O3 lesions were mainly localized near the veins. Because the transgenic lines are insensitive to ET throughout their development and thus might lack some ET-dependent constitutive defenses, we elucidated the significance of ET signaling in the wild-type birch during a short-term O3 exposure by blocking of ET perception with MCP only during the exposure. Similarly, as in the transgenic BPetr1-1-35, inhibition of ET perception in the wild-type clones partially reduced, but did not fully prevent visible O3 lesion formation in the leaves of 1-year-old V5818 trees (Fig. 5A). Likewise, blocking of ET perception reduced the O3-induced visible cell death in 3-month-old leaves of clone V5818 (Fig. 5B).
Figure 4.
O3-induced ion leakage, ET evolution, and the effect of blocking of ET biosynthesis in 3-month-old birch clone V5834 and in the transgenic line BPetr1-1-35 carrying Arabidopsis ET receptor gene with the dominant etr1-1 mutation. Ramets were sprayed either with 0.05% (v/v) Tween 20 or with 1.5 mm aminooxyacetic acid (AOA) in 0.05% (v/v) Tween 20 to block ET biosynthesis and were exposed to 200 nL L−1 O3 for 6 h. Leaves were harvested for ET and ion leakage determinations at 0, 6, 8, and 24 h. Error bars indicate ±se (n = 3). Black bars indicate the duration of O3 exposure. A, Ion leakage in the wild-type clone V5834 and in the transgenic line BPetr1-1-35. B, ET evolution in the wild-type clone V5834 and in the transgenic line BPetr1-1-35. C, Ion leakage in the wild-type clone V5834 and in the transgenic line BPetr1-1-35 with and without the AOA treatment. D, ET evolution in the wild-type clone V5834 and in the transgenic line BPetr1-1-35 with and without the AOA treatment.
Figure 5.
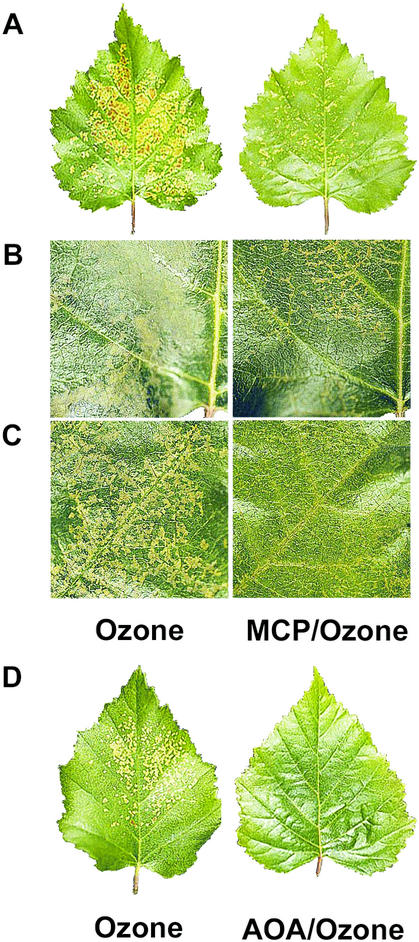
Visual damage in response to O3 in birch after the inhibition of ET perception or biosynthesis. ET perception was blocked with 300 nL L−1 MCP and ET biosynthesis with 1 mm AOA. Ramets were exposed to 200 nL L−1 O3 for 6 h. Photographs were taken 48 h after the beginning of the O3 exposure. A, An O3-treated leaf of the 1-year-old clone V5818 (left) and a leaf pretreated with MCP and then with O3 (right). B, A detail of an O3-treated leaf of the 3-month-old clone V5818 (left) and a leaf pretreated with MCP and then with O3 (right). C, A detail of an O3-treated leaf of the 3-month-old clone V5818 (left) and a leaf treated with MCP (right) after the O3 pulse. D, An O3-treated leaf of the 1-year-old clone V5818 (left) and a leaf treated with AOA and O3 (right).
To demonstrate that the MCP that could potentially be remaining in the cells did not cause any unexpected effects (for example, by possibly reacting with O3 during the exposure), we treated two wild-type birch clones, V5818 (Fig. 5C) and K1898 (not shown), with MCP immediately after a 6-h O3 pulse. The blocking of ET perception with MCP after the O3 treatment, again, only reduced cell death in both clones. This also indicates that ET is required for the O3 lesion formation after the actual contact of O3 with the apoplastic structures. In the transgenic BPetr1-1-35, O3 damage was not reduced any further by a MCP treatment, whereas in the wild-type V5834, the extent of visual O3 lesions was reduced by 32% (data not shown). Thus, the transformation of the genomic clone of the Arabidopsis etr1-1 allele caused such a strong ET insensitivity in birch that it could not be further increased by exogenous MCP.
Inhibition of ET Biosynthesis Prevents O3-Induced Cell Death
The ACS inhibitor AOA was used to determine how prevention of ET biosynthesis affects O3 lesion formation in wild-type clone V5834 and the transgenic line BPetr1-1-35. AOA abolished ET production (three-way ANOVA; genotype, time and treatment as factors; Tukey's HSD mean-separation test; P < 0.0005) and prevented the O3-dependent cell death completely in both wild-type and transgenic trees (Tukey's HSD mean-separation test; P = 0.001; Fig. 4, C and D). Similarly, AOA treatment of either 3-month-old (data not shown) or 1-year-old V5818 ramets abolished O3-induced formation of visible lesions (Fig. 5D) and eliminated O3-induced ion leakage (three-way ANOVA; genotype, time and treatment as factors; P < 0.0005) and ET evolution completely (three-way ANOVA; P < 0.0005; data not shown). For an unknown reason, treatment with Tween 20 alone also reduced slightly the O3-induced ion leakage and ET evolution. Measured in wild-type clone V5818, the stomatal conductance in AOA-treated leaves was approximately 2-fold higher than either in O3 or O3/MCP-treated leaves (data not shown). Thus, differential O3 lesion formation under MCP and AOA treatments was not based on higher entry of O3 to the leaf.
The O3-Induced Activation of β-Cyano-Ala Synthase (β-CAS) Is Dependent on ET
The results above suggest that the ET-dependent cell death is differentially affected by ET synthesis and signaling and prompts the question of what is the basis of cell death in the O3-exposed ET-insensitive plants that can be prevented by inhibition of ET biosynthesis. The ACO-catalyzed oxidation of 1-aminocyclopropane-1-carboxylic acid (ACC) to ET produces an equimolar amount of cyanide (HCN), which is normally rapidly detoxified to β-cyano-Ala by β-CAS (Akopyan et al., 1975; Wurtele et al., 1985; Yip and Yang, 1988). We examined whether the birch β-CAS gene (AY154650) is ET regulated. Results in Figure 6A show that when in the wild-type clone V5834 β-CAS transcript accumulation increased over 16-fold by exposure to ET, in the ET-insensitive BPetr1-1-35 β-CAS was not induced. In response to O3, β-CAS transcript accumulation was also lower in BPetr1-1-35 than in the wild-type V5834 (Fig. 6B), whereas ET evolution was equal (Fig. 4B). Similarly, blocking of ET receptors with MCP reduced β-CAS transcript accumulation in response to O3, but ET evolution was not affected (Fig. 6C). Thus, it can be concluded that functional ET signaling is required for the O3-induced activation of β-CAS in birch and that as a result of decreased ET sensitivity, HCN detoxification can be compromised.
Figure 6.
Accumulation of β-CAS transcript in response to ET or O3. Birch clone V5834, the transgenic BPetr1-1-35 line carrying the Arabidopsis ET receptor gene with the dominant etr1-1 mutation, and clone V5818 with blocked ET perception were exposed to O3 or ET, and the transcript levels of β-CAS were analyzed. Ramets were subjected to 50 μL L−1 ET for 24 h or 200 nL L−1 O3 for 6 h. ET perception was blocked with 300 nL L−1 MCP. Black bars indicate the duration of treatments. A, β-CAS mRNA accumulation in response to ET treatment in the wild-type clone V5834 and in the transgenic BPetr1-1-35 line. B, β-CAS mRNA accumulation in response to O3 in the wild-type clone V5834 and in the transgenic line BPetr1-1-35. C β-CAS mRNA accumulation in response to O3 in the control and MCP-treated wild-type clone V5818. Inset, ET evolution in the control and MCP-treated ramets in response to O3.
DISCUSSION
O3-Induced Cell Death Correlates with ET and JA Accumulation
According to the recent results in several studies (Rao and Davis, 2001), plant responses to high concentrations of O3 share common features with pathogen attack due to the function of ROS as signaling molecules. In this study, we employed high O3 concentrations as a tool to probe the interactions of hormonal responses and the high level of ROS in birch. The results of these experiments address more the interactions and roles of SA, JA, and ET with ROS in the regulation of ROS-dependent cell death, and thus are not directly applicable to describe or represent plant responses to O3 in natural environments. The latter we have evaluated in the accompanying paper (Vahala et al., 2003; this issue), where the roles of same hormones in response to chronic O3 concentrations have been studied in hybrid aspen (Populus tremula × P. tremuloides) under more realistic levels of O3 found in natural environments.
The timing and magnitude of ET evolution correlated with the extent of cell death in the wild-type birch clones. As could be expected, blocking of ET biosynthesis in the O3-sensitive clone V5818 reduced O3-induced cell death significantly, indicating the requirement for ET. The O3-sensitive clone V5818 displayed high O3-induced ET accumulation without the late SA accumulation, whereas the O3-tolerant clone E9702 had low O3-induced ET and highly induced late SA accumulation. This suggests that the early high ET production may antagonize the late SA accumulation, or vice versa, increased SA production may down-regulate ET accumulation and thus prevent the ET-dependent cell death.
Similarly, the basal SA level of the ET-insensitive Arabidopsis mutant ein2 was higher than in wild type, suggesting that ET can inhibit SA biosynthesis (H. Tuominen, K. Overmyer, M. Keinänen, and J. Kangasjärvi, unpublished data). The same interaction works also in the opposite direction; O3-induced, EIN2-dependent gene expression was suppressed in the SA-deficient NahG plants. These results suggest similar regulation and interaction between ET and SA during oxidative stress in both Arabidopsis and birch. The role of JA is, however, more complicated. In birch, both ET and JA accumulation correlated with O3-induced cell death in the O3-sensitive clones V5818 and K1898, whereas in the O3-tolerant clone E9702, JA concentration did not increase. Similarly, in the O3-sensitive Arabidopsis mutants rcd1 and jar1, high JA accumulation was evident. It has been shown that JA is involved in lesion containment (Overmyer et al., 2000), which at first may seem contradictory to the results of JA accumulation in the sensitive clones and not in the tolerant ones. However, because the biosynthesis of JA appears to be limited by substrate availability (Laudert et al., 2000; Ziegler et al., 2001), it is also possible that the accumulation of JA is a consequence of the ET-dependent cell death: Substrate for JA synthesis (α-linolenic acid or 13-(S)-hydroperoxylinolenic acid) could be released from the dying cells, thus resulting in increased JA synthesis, which then halts the ET-dependent lesion propagation.
A Dual Role for ET in O3 Responses
ET can have opposite roles in O3-exposed plants. Several reports have shown an essential pro-death role for ET in O3-exposed plants. However, depending on the temporal pattern of biosynthesis ET may also have a pro-survival role. In mung bean (Vigna radiata) and pea (Pisum sativum), pretreatment of plants with ET before O3 exposure promoted O3 tolerance (Mehlhorn, 1990). Involvement of ET-dependent protection from oxidative stress may require a sufficient level of rapid but transient ET induction, however, at the intensity where it does not exceed the rate that induces prolonged spreading cell death. In the O3-sensitive clone V5818, timing and degree of the late ET accumulation was most likely beyond the limit to provide protection against the O3 challenge. This is also supported by the fact that blocking of ET perception after the O3 exposure decreased O3 damage, which indicates that the ET action for the promotion of cell death is required late. Although the early transcript accumulation of BP-ACS1 was evident in all birch clones in response to O3, BP-ACS2 transcript levels increased considerably only in the O3-sensitive clone V5818 at 8 h. Therefore, in these three wild-type clones, differential ACS transcript accumulation and ET production suggest a dual role for ET. Depending on the magnitude of synthesis and its temporal pattern, ET can serve as a mediator of either survival or cell death. The results presented here suggest that in the O3-sensitive birch clone ET was required to promote cell death at the time of lesion development and that the requirement of ET, for example, for the induction of antioxidant defenses had less significance.
Transformation of Arabidopsis etr1-1 Allele Causes Strong ET Insensitivity in Birch
The dominant Arabidopsis etr1-1 mutation causes ET insensitivity (Chang et al., 1993a). When the genomic clone of Arabidopsis etr1-1 was transformed to tobacco, it caused ET insensitivity in the transgenic plants and strongly reduced basic pathogenesis-related gene expression and non-host resistance against Pythium spp. (Knoester et al., 1998). Here, we show that transformation of birch with the genomic copy of the Arabidopsis etr1-1 mutant allele under its own promoter resulted in ET insensitivity in birch. Furthermore, the ET insensitivity was strong, which is demonstrated by the fact that MCP treatment of the transgenic trees did not decrease the ET-dependent O3 lesion formation any further. Therefore, regulation of ETR1 is most likely conserved among various plant species because apparently birch transcription factors recognized the Arabidopsis ETR1 promoter and regulated its expression properly.
O3 Lesions in ET-Insensitive Plants May Be Due to Defective HCN Removal
Insensitivity to ET provided only a partial protection against O3, when the prevention of ET biosynthesis in the ET-insensitive transgenic birch blocked cell death completely. This suggests that ET modifies O3-induced ET-dependent cell death downstream of ACS and both upstream and downstream of ET receptors. When ACC is oxidized to ET, stoichiometric amounts of ET and HCN are produced. Under normal circumstances, this HCN is efficiently detoxified by β-CAS. However, it has been suggested (Grossmann, 1996) that under specific conditions, strongly stimulated ET biosynthesis may result in necrotic cell death due to insufficient HCN detoxification. Infection of tobacco with tobacco mosaic virus caused elevated HCN and ACC levels, concomitant with a decrease in β-CAS activity (Siefert et al., 1995), and HCN accumulation was suggested to contribute to the cell death. Our results indicate that the birch β-CAS gene is ET-regulated. The β-CAS transcript accumulation was also considerably lower in response to O3 when ET perception was disrupted. However, this was still accompanied by equal O3-induced ET accumulation when compared with the wild-type birch, thus ACO was still producing HCN at an equal rate as ET. Hence, the HCN formed could be a likely candidate to mediate the O3-induced cell death.
HCN may also relate to the ROS formation in the mitochondria: It has been shown that the HCN-resistant respiration decreased mitochondrial ROS formation in cultured tobacco cells via the alternative oxidase (AOX; Maxwell et al., 1999). It has also been shown with the Arabidopsis etr1-1 mutant that AOX activation is ET dependent (Simons et al., 1999). The AOX gene is also induced by H2O2 (Robson and Vanlerberghe, 2002). Pellinen et al. (1999, 2002) have shown that O3 induces H2O2 accumulation in birch. This suggests that AOX was most likely induced as well. Thus, attenuated induction of the HCN-resistant respiration together with defective HCN removal in ET-insensitive plants may result in inhibition of the normal mitochondrial respiration by HCN and thus cause increased ROS production in the mitochondria, which could be involved in the regulation of cell death. When the subcellular compartmentalization of O3-induced H2O2 formation was studied in birch (Pellinen et al., 1999), increased ROS accumulation temporally coinciding with cell death was observed also in the mitochondria.
In this study, the defect in HCN detoxification may be considered an “artificial” effect caused by the strong ET insensitivity obtained with etr1-1 transformation or MCP treatment. This strong ET insensitivity may be regarded as unlikely to be observed in wild-type plants. However, our results in Arabidopsis suggest that modulation of ET sensitivity takes place in O3-exposed plants (H. Tuominen, K. Overmyer, M. Keinänen, and J. Kangasjärvi, unpublished data). Furthermore, it has been shown that in tomato, the genes encoding ET receptors are differentially induced by O3 (Moeder et al., 2002) and during pathogen infection (Ciardi et al., 2000). The increased synthesis of “fresh,” unoccupied receptors has been proposed to decrease ET sensitivity and to be involved in the desensitization of plants to ET when the ET responses need to be shut down (Bleecker and Kende, 2000; Ciardi and Klee, 2001). In tomato, the O3-induced ET synthesis, ET-dependent H2O2 accumulation, and subsequent cell death were highly colocalized to the vicinity of the veins (Moeder et al., 2002). The ET-signaling-independent lesion formation in birch was also near the vascular system. Thus, it is completely possible that the simultaneous and extremely localized high ET biosynthesis in connection with decreased ET sensitivity caused by the induction of ET receptor genes can cause cell death through increased HCN formation and mitochondrial responses.
The deficient HCN removal in transgenic, ET-insensitive plants also has implications in other species that have been made insensitive to ET by transformation of mutant ET receptors; under conditions that cause highly elevated ET synthesis, sufficient HCN detoxification may not take place due to the ineffective induction of β-CAS.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Three birch (Betula pendula Roth) genotypes (V5818, K1898, and E9702) and ET-insensitive birch lines were propagated by in vitro tissue culture as described previously (Lemmetyinen et al., 1998). Copies of the clones (ramets) were planted and grown in peat:sand:vermiculite (6:1:1, v/v), fertilized with nitrogen:phosphorus:potassium (11:4:25) under greenhouse conditions, and used for experiments during either the 1st (3 month old) or 2nd year of their growth. Ramets were transferred into growth chambers with the photoperiod of 22 h of light/2 h of dark, light intensity of 300 μmol m−2 s−1 photosynthetically active radiation, temperature of 20°C/16°C (light/dark), and relative humidity of 50%/70% (light/dark) and allowed to acclimate to the chamber conditions at least for 4 d before the O3, ET, and inhibitor treatments.
ET-insensitive birch were created by genetic transformation with a construct (pCGN1547) carrying the Arabidopsis ET receptor gene ETR1 with the dominant etr1-1 mutation with its own promoter (Chang et al., 1993a). Different birch clones (4212, E9678, E9702, JR1/4, K1659, K1898, V5818, and V5834) were chosen for Agrobacterium sp.-mediated (C58C1 pGV2260) transformation as described (Lemmetyinen et al., 1998). Clones V5818, K1898, and E9702 used in the other experiments reported here could not be successfully transformed (data not shown).
O3, Chemical, and ET Treatments
The O3 exposures were conducted in growth chambers. O3 was produced from pure O2 by electric discharge, and the delivery of O3 to the chambers was computer-controlled based on continuous measurements of O3 concentration inside the chamber with an ozone analyzer (Dasibi 1008-RS, Amko Systems Inc., Ontario, Canada) as described by Pellinen et al. (1999). Wild-type birch clones (V5818, K1898, and E9702) were exposed to 200 nL L−1 O3 for 8 h, and leaves were harvested at 0, 0.5, 1, 2, 4, 8, and 24 h. ET-insensitive birch ramets were exposed to 200 nL L−1 O3 for 6 h, and leaves were harvested at 0, 6, 8, and 24 h. Wild-type birch ramets of the same clone were used as controls.
ET receptors were blocked in the wild-type clones (V5818 and K1898) and ET-insensitive transgenic birch line BPetr1-1-35 with 1-MCP (EthylBloc, Laboratorium Van der Sprong bv, Netherlands). The treatments were carried out for 12 h in a sealed growth chamber with 300 nL L−1 MCP according to manufacturer's instructions, before or after the O3 treatment. After each MCP pretreatment, the chamber was flow-through ventilated for 2 h before the O3 exposure to avoid any possible chemical reactions between O3 and MCP.
ET biosynthesis was blocked in the wild-type clone V5818 and transgenic birch line BPetr1-1-35 with 1 mm or 1.5 mm AOA (Sigma-Aldrich) with 0.1% or 0.05% (v/v) Tween 20 (Fluka, Buchs, Switzerland), respectively, by spraying the abaxial side of the leaves. The control plants for AOA treatment received 0.1% or 0.05% (v/v) Tween 20. Spraying was conducted 1.5 h before the onset of O3 exposure and was repeated four times during the O3 exposure.
ET treatment was conducted to test the ET sensitivity of the 3-month-old transgenic etr1-1 clones by subjecting ramets to 50 μL L−1 ET for 3 d in a growth chamber.
ET, Ion Leakage, JA, and SA Determinations
The fully expanded leaves 1, 2, and 5 from the apex were pooled for ET evolution and ion leakage measurements from three ramets per clone per treatment. The ET and ion leakage determinations were carried out as described by Vahala et al. (2003).
The fully expanded leaves 3, 4, and 6 from the apex were used for JA and SA determinations at each time point. JA and SA were extracted and quantified with [1,2-13C]JA and [13C]SA as internal standards by gas chromatography-mass spectrometry as described by Baldwin et al. (1997), with the following modifications. The frozen tissues were ground in 2-mL microfuge tubes, spiked with an internal standard, extracted overnight with 1.5 mL of acetone:50 mm citric acid (7:3, v/v) at 4°C with vigorous shaking, and re-extracted for 15 min with 1 mL of the extraction solvent. After centrifugation (13,000 rpm for 8 min), the combined supernatants were divided in two aliquots (for free and conjugated compounds) and evaporated to an aqueous residue under vacuum at 30°C. For the analysis of conjugated SA, the sample was adjusted to 400 μL with deionized water, acidified with 100 μL of concentrated HCl (37%, w/v), and hydrolyzed for 1 h at 80°C. After hydrolysis, the aqueous solutions were extracted twice with 1 mL of diethyl ether and evaporated to dryness under vacuum at ambient temperature. The residues were redissolved in 1 mL of diethyl ether and loaded onto 100 mg of Supelclean LC-NH2 SPE columns (Supelco, Bellefonte, PA). The columns were washed with 1.2 mL of chloroform:2-propanol (3:1, v/v), and the compounds were eluted with 1.5 mL of diethylether:formic acid (98:2, v/v). The eluates were evaporated to dryness under vacuum at ambient temperature, dissolved in 100 μL of diethyl ether, derivatized with ethereal diazomethane, and reconstituted in 40 μL of hexane.
The methyl esters of JA and SA were separated by gas chromatography (HP 6890, Agilent Technologies, Avondale, PA) on an Rtx-5MS column (30-m × 0.25-mm. i.d., 0.25-μm film thickness; Restek Corp., Bellefonte, PA) with a helium flow rate of 1 mL min−1 and injector temperature of 250°C, and detected by mass spectrometry (HP 5973) in a single-ion-monitoring mode (m/z 224 and 226 for JA, m/z 152 and 153 for SA). The temperature program was as follows, 1.5 min at 55°C, 10°C min−1 to 200°C, 20°C min−1 to 300°C, and hold at 300°C for 7 min.
cDNA Probes and Northern and Southern Analysis
Several degenerate oligonucleotide primers based on conserved amino acid domains were used to isolate cDNAs for the ET biosynthetic enzymes from birch. Reverse transcription of total RNA or poly(A+) mRNA was conducted with the avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI), and the subsequent PCR was conducted with Dynazyme (Finnzymes, Espoo, Finland), Taq, or Pfu (Promega) DNA polymerases. To isolate birch ACS cDNA sequences, OLE-4 and OLE-5 upstream primers and OLE-6 downstream primer were used as described (Botella et al., 1992).
Young leaf tissue collected from the apex of shoots was used for Southern analyses. Total DNA was extracted as described (Lodhi et al., 1994) and digested with KpnI and EcoRI. For northern analysis, the fully expanded leaves 3, 4, and 6 from the apex were excised from three individuals per clone per treatment and frozen in liquid N2 at each time point. Samples were stored at −70°C or −80°C until analyzed. The three independent samples were pooled, and total RNA was isolated as described (Chang et al., 1993b). Ten micrograms of total RNA was fractionated on 1% (w/v) formaldehyde agarose gels in MOPS buffer. RNA and DNA were capillary blotted overnight onto positively charged nylon membranes (Roche Diagnostics GmbH, Mannheim, Germany), and baked at 120°C for 30 min. [α-32P]dCTP-labeled probes were prepared with High Prime random-priming labeling system (Roche Diagnostics) and purified on G-50 columns (Amersham Biosciences, Piscataway, NJ). Prehybridizations and hybridizations were carried out at 68°C in a solution containing 0.5 m NaHPO4 and 7% (w/v) SDS. After hybridization, the membranes were washed under high-stringency conditions as described (Church and Gilbert, 1984). Hybridization signals were quantified with a phosphor imager and an image analysis program (Bas 1500, Fujifilm, Tokyo) and normalized against the 18S rDNA hybridization signal.
Photosynthesis Measurements
The net photosynthesis, stomatal conductance, and transpiration measurements were performed at 5 to 6 h after the onset of O3 exposure with the LI-6400 photosynthesis system (LI-COR, Lincoln, NE). Measurements were performed on the third and fourth fully expanded leaves of three ramets per treatment. The mean value of two leaves measured from each individual ramet was calculated. All of the measurements were made under a 6400-02B red/blue LED light source at saturating light of 1,000 μmol m−2 s−1 photosynthetically active radiation, temperature of 22°C, constant input of CO2 (400 μL L−1) from the minicartridges, constant air flow rate of 500 μmol s−1, and relative humidity of 30% to 40%.
Statistical Analysis and Quantification of Visual Damage
ANOVA was used to detect significant differences among clones and treatments. All data were checked for normality and heterogeneity of variances. Hormone concentrations were log10(X) transformed and ion leakage arcsin transformed to meet the assumptions of ANOVA. Multiple comparisons of individual means and levels of factors were analyzed with a Tukey's HSD test, where appropriate. Correlations were analyzed with non-parametric Spearman's ρ test. Analyses were conducted with the SPSS v8.0 software package (SPSS Inc., Chicago). The O3-induced visual damage was estimated as a percentage of the leaf area that was damaged 48 h after the onset of the O3 exposure. All fully emerged leaves were counted. Two people conducted the quantification independently.
ACKNOWLEDGMENTS
We acknowledge Prof. Elliot Meyerowitz for providing the etr1-1 genomic clone. We thank Kari Vaahtomeri, Sari Eräluoto, Mirva Lehtinen, Airi Tauriainen, and Sari Möttönen for technical help. We acknowledge Kirk Overmyer for helpful discussions and Simo Harju and Mika Korva for the nursing of the birch ramets. We thank Prof. Ian Baldwin and Dr. Günter Brader for JA and SA standards, respectively.
Footnotes
This work was supported by the Maj and Tor Nessling Foundation, by the Finnish Society of Forest Sciences, by the Leo and Regina Wainstein's Foundation, and by the Academy of Finland, Centre of Excellence Program 2000–2005. M.K. was supported by Academy of Finland Postdoctoral grant no. 48640.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018887.
LITERATURE CITED
- Akopyan T, Braunstein A, Goryachenkova E. Beta-cyanoalanine synthase: purification and characterization. Proc Natl Acad Sci USA. 1975;72:1617–1621. doi: 10.1073/pnas.72.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae GY, Nakajima N, Ishizuka K, Kondo N. The role in ozone phytotoxicity of the evolution of ethylene upon induction of 1-aminocyclopropane-1-carboxylic acid synthase by ozone fumigation in tomato plants. Plant Cell Physiol. 1996;37:129–134. [Google Scholar]
- Baldwin IT, Zhang Z-P, Diab N, Ohnmeiss TE, McCloud ES, Lynds GY, Schmelz EA. Quantification, correlations and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta. 1997;201:397–404. [Google Scholar]
- Berger S. Jasmonate-related mutants of Arabidopsis as tools for studying stress signals. Planta. 2001;214:497–504. doi: 10.1007/s00425-001-0688-y. [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Botella JR, Schlagnhaufer CD, Arteca RN, Phillips AT. Identification and characterization of three putative genes for 1-aminocyclopropane-1-carboxylate synthase from etiolated mung bean hypocotyl segments. Plant Mol Biol. 1992;18:793–797. doi: 10.1007/BF00020022. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of products to two-component regulators. Science. 1993a;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney C. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993b;11:113–116. [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J, Klee H. Regulation of ethylene-mediated responses at the level of receptor. Ann Bot. 2001;88:813–822. [Google Scholar]
- Ciardi JA, Tieman DM, Lund ST, Jones JB, Stall RE, Klee HJ. Response to Xanthomonas campestris pv. vesicatoria in tomato involves regulation of ethylene receptor gene expression. Plant Physiol. 2000;123:81–92. doi: 10.1104/pp.123.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl JL, Jones JDG. Plant pathogens and integrated defence responses to infection. Nature. 2001;411:826–833. doi: 10.1038/35081161. [DOI] [PubMed] [Google Scholar]
- Draper J. Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 1997;2:162–165. [Google Scholar]
- Fowler D, Cape NJ, Coyle M, Flechard C, Kuylenstierna J, Hicks K, Derwent D, Johnson C, Stevenson D. The global exposure of forest to air pollutants. Water Air Soil Pollut. 1999;116:5–23. [Google Scholar]
- Godiard L, Grant MR, Dietrich RA, Kiedrowski S, Dangl JL. Perception and response in plant disease resistance. Curr Opin Genet Dev. 1994;4:662–671. doi: 10.1016/0959-437x(94)90132-m. [DOI] [PubMed] [Google Scholar]
- Grossmann K. A role for cyanide, derived from ethylene biosynthesis, in the development of stress symptoms. Physiol Plant. 1996;97:772–775. [Google Scholar]
- Hall AE, Chen QG, Findell JL, Schaller GE, Bleecker AB. The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol. 1999;121:291–299. doi: 10.1104/pp.121.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL. Possible mechanisms for the inhibition of photosynthesis by ozone. Photosynth Res. 1994;39:439–451. doi: 10.1007/BF00014597. [DOI] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene-insensitivity conferred by Arabidopsis ERS gene. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiskinen M, Korhonen M, Kangasjärvi J. Isolation and characterization of cDNA for a plant mitochondrial phosphate translocator (Mpt1): Ozone stress induces Mpt1 mRNA accumulation in birch (Betula pendula Roth) Plant Mol Biol. 1997;35:271–279. doi: 10.1023/a:1005868715571. [DOI] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst JM. Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR. Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid: the role of programmed cell death in lesion formation. Plant Physiol. 2000;123:487–496. doi: 10.1104/pp.123.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JR, Scherzer AJ, Eshita SM, Davis KR. Ozone sensitivity in hybrid poplar is correlated with a lack of defense-gene activation. Plant Physiol. 1998;118:1243–1252. doi: 10.1104/pp.118.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk A, Kull O, Moldau H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol. 1989;90:1163–1167. doi: 10.1104/pp.90.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudert D, Schaller F, Weiler EW. Transgenic Nicotiana tabacum and Arabidopsis thaliana plants overexpressing allene oxide synthase. Planta. 2000;211:163–165. doi: 10.1007/s004250000316. [DOI] [PubMed] [Google Scholar]
- Lemmetyinen J, Keinonen-Mettälä K, Lännenpää M, von Weissenberg K, Sopanen T. Activity of the CaMV 35S promoter in various parts of transgenic early flowering birch clones. Plant Cell Rep. 1998;18:243–248. doi: 10.1007/s002990050564. [DOI] [PubMed] [Google Scholar]
- Lodhi MA, Ye G-N, Weeden NF, Reisch BI. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep. 1994;12:6–13. [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhorn H. Ethylene-promoted ascorbate peroxidase activity protects plants against hydrogen peroxide, ozone and paraquat. Plant Cell Environ. 1990;13:971–976. [Google Scholar]
- Mehlhorn H, O'Shea JM, Wellburn AR. Atmospheric ozone interacts with stress ethylene formation by plants to cause visible plant injury. J Exp Bot. 1991;42:17–24. [Google Scholar]
- Mehlhorn H, Wellburn AR. Stress ethylene formation determines plant sensitivity to ozone. Nature. 1987;327:417–418. [Google Scholar]
- Moeder W, Barry CS, Tauriainen AA, Betz C, Tuomainen J, Utriainen M, Grierson D, Sandermann H, Langebartels C, Kangasjärvi J. Ethylene synthesis regulated by bi-phasic induction of 1-aminocyclopropane-1-carboxylic acid synthase and 1-aminocyclopropane-1-carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone-exposed tomato. Plant Physiol. 2002;130:1918–1926. doi: 10.1104/pp.009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr, Kangasjärvi J. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen R, Palva T, Kangasjärvi J. Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J. 1999;20:349–356. doi: 10.1046/j.1365-313x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- Pellinen RI, Korhonen M-S, Tauriainen AA, Palva ET, Kangasjärvi J. H2O2 activates cell death and defense gene-expression in birch (Betula pendula) Plant Physiol. 2002;130:549–560. doi: 10.1104/pp.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR. The physiology of ozone-induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- Robson CA, Vanlerberghe GC. Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiol. 2002;129:1908–1920. doi: 10.1104/pp.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:5812–5817. doi: 10.1073/pnas.95.10.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagnhaufer CD, Arteca RN, Pell EJ. Sequential expression of two 1-aminocyclopropane-1-carboxylate synthase genes in response to biotic and abiotic stresses in potato (Solanum tuberosum L.) leaves. Plant Mol Biol. 1997;35:683–688. doi: 10.1023/a:1005857717196. [DOI] [PubMed] [Google Scholar]
- Schlagnhaufer CD, Glick RE, Arteca RN, Pell EJ. Molecular cloning of an ozone-induced 1-aminocyclopropane-1-carboxylate synthase cDNA and its relationship with a loss of rbcS in potato (Solanum tuberosum L.) plants. Plant Mol Biol. 1995;28:93–103. doi: 10.1007/BF00042041. [DOI] [PubMed] [Google Scholar]
- Schraudner M, Moeder W, Wiese C, van Camp W, Inzé D, Langebartels C, Sandermann H., Jr Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Serek M, Tamari G, Sisler EC, Borochov A. Inhibition of ethylene-induced cellular senescence symptoms by 1-methylcyclopropane, a new inhibitor of ethylene action. Physiol Plant. 1995;94:229–232. [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefert F, Kwiatkowski J, Sarkar S, Grossmann K. Changes in endogenous cyanide and 1-aminocyclopropane-1-carboxylic acid levels during the hypersensitive response of tobacco mosaic virus-infected tobacco leaves. Plant Growth Regul. 1995;17:109–113. [Google Scholar]
- Simons BH, Millenaar FF, Mulder L, Van Loon LC, Lambers H. Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiol. 1999;120:529–538. doi: 10.1104/pp.120.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JDG. Molecular genetics of plant disease resistance. Science. 1995;268:661–667. doi: 10.1126/science.7732374. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomainen J, Betz C, Kangasjärvi J, Ernst D, Yin ZH, Langebartels C, Sandermann H., Jr Ozone induction of ethylene emission in tomato plants: regulation by differential transcript accumulation for the biosynthetic enzymes. Plant J. 1997;12:1151–1162. [Google Scholar]
- Vahala J, Keinänen M, Schutzendubel A, Polle A, Kangasjärvi J. Differential effects of elevated ozone on two hybrid aspen genotypes predisposed to chronic ozone fumigation: role of ethylene and salicylic acid. Plant Physiol. 2003;132:196–205. doi: 10.1104/pp.102.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahala J, Schlagnhaufer CD, Pell EJ. Induction of an ACC synthase cDNA by ozone in light-grown Arabidopsis thaliana leaves. Physiol Plant. 1998;103:45–50. [Google Scholar]
- Wenzel AA, Schlautmann H, Jones CA, Küppers K, Mehlhorn H. Aminoethoxyvinylglycine, cobalt and ascorbic acid all reduce ozone toxicity in mung beans by inhibition of ethylene biosynthesis. Physiol Plant. 1995;93:286–290. [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Langebartels C, Sandermann H. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 2002;25:717–726. [Google Scholar]
- Wurtele ES, Nikolau BJ, Conn EE. Subcellular and developmental distribution of β-cyanoalanine synthase in barley (Hordeum vulgare) leaves. Plant Physiol. 1985;78:285–290. doi: 10.1104/pp.78.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W-K, Yang SF. Cyanide metabolism in relation to ethylene production in plant tissues. Plant Physiol. 1988;88:473–476. doi: 10.1104/pp.88.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J, Keinänen M, Baldwin IT. Herbivore-induced allene oxide synthase transcripts and jasmonic acid in Nicotiana attenuata. Phytochemistry. 2001;58:729–738. doi: 10.1016/s0031-9422(01)00284-9. [DOI] [PubMed] [Google Scholar]