Abstract
The formation of suberized and lignified barriers in the exodermis is suggested to be part of a suite of adaptations to flooded or waterlogged conditions, adjusting transport of solutes and gases in and out of roots. In this study, the composition of apoplasmic barriers in hypodermal cell walls and oxygen profiles in roots and the surrounding medium of four Amazon tree species that are subjected to long-term flooding at their habitat was analyzed. In hypodermal cell walls of the deciduous tree Crateva benthami, suberization is very weak and dominated by monoacids, 2-hydroxy acids, and ω-hydroxycarboxylic acids. This species does not show any morphological adaptations to flooding and overcomes the aquatic period in a dormant state. Hypodermal cells of Tabernaemontana juruana, a tree which is able to maintain its leaf system during the aquatic phase, are characterized by extensively suberized walls, incrusted mainly by the unsaturated C18 ω-hydroxycarboxylic acid and the α,ω-dicarboxylic acid analogon, known as typical suberin markers. Two other evergreen species, Laetia corymbulosa and Salix martiana, contained 3- to 4-fold less aliphatic suberin in the exodermis, but more than 85% of the aromatic moiety of suberin are composed of para-hydroxybenzoic acid, suggesting a function of suberin in pathogen defense. No major differences in the lignin content among the species were observed. Determination of oxygen distribution in the roots and rhizosphere of the four species revealed that radial loss of oxygen can be effectively restricted by the formation of suberized barriers but not by lignification of exodermal cell walls.
Suberin is a heterogeneous extracellular biopolymer closely attached to the inner primary cell wall (Schreiber et al., 1999). On the basis of chemical analysis of enzymatically isolated cell walls, the composition of suberin in the exodermis was shown to consist of long-chain aliphatic monomers esterified with aromatic compounds like ferulic and coumaric acids and cell wall carbohydrates (Zeier and Schreiber, 1997; Kolattukudy, 2001). Recently, glycerol has been identified as a new important structural element in the suberin macromolecule, which is supposed to cross-link the aliphatic and aromatic suberin domains (Moire et al., 1999; Graça and Pereira, 2000a, 2000b). The aliphatic monomers of suberin are synthesized via the fatty acid biosynthetic pathway, catalyzed by fatty acid elongases in the root cells (Domeregue et al., 1998; Schreiber et al., 2000). Hydroxylation is mediated by cytochrome P450-dependent enzymes, converting ω-hydroxyacids to either 1,ω-dicarboxylic acids or alcohols (Agrawal and Kolattukudy, 1978; Le Bouquin et al., 2001). The assembly of the aromatic moiety of suberin, in most cases cinnamic acid derivatives, proceeds via the general phenylpropanoid pathway with Phe ammonia-lyase as the central enzyme (Kolattukudy, 2001). Similar to suberin, lignin is a highly variable biopolymer synthesized in a complex pathway. The basic lignin molecule is derived from the oxidative polymerization of the monolignols p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, bearing the three aromatic residues p-hydroxyphenyl, guaiacyl, and syringyl (Freudenberg, 1965; Boudet, 1998).
The root peripheral cell layers separates the plant from the subterranean environment and plays a crucial role in root-rhizosphere interactions. The occurrence of an exodermis, a hypodermis with Casparian bands and suberized cell walls located underneath the epidermis of the root (Perumalla et al., 1990), is widespread among both herbaceous and woody plant species (Peterson and Perumalla, 1990). Deposition of suberin in anticlinal, including Casparian bands, and tangential cell walls of the exodermis equips the root with a hydrophobic barrier that contributes to the plant's overall resistance under unfavorable growth conditions, such as low oxygen levels or high salinity. A suberized exodermis seems to be well designed to prevent loss of water and stored solutes into the rhizosphere during drought periods, which may represent another important protective feature (Hose et al., 2001). The physiological function of lignin was attributed to mechanical support and compressive strength, providing a prerequisite for the development of plants adapted to a terrestrial habitat. In addition, its resistance to degradation may contribute to plant defense (Campbell and Sederoff, 1996; Önnerud et al., 2002). The role of suberin may be multiple. Investigations on rice (Miyamoto et al., 2001) and corn roots (Zimmermann et al., 2000) showed that increased amounts of suberin in the hypodermal cell layers of aeroponically grown roots reduced the hydraulic conductivity for radial water flow. In addition, suberin acts as a component of the wound- and pathogen-induced plant defense response, preventing infection by microbial pathogens (Mohan et al., 1993a, 1993b). It is supposed that a heavily suberized exodermis limits radial oxygen loss (ROL) from the root to the rhizosphere, supporting root growth in oxygen-depleted soils under flooded conditions (Colmer et al., 1998; Armstrong et al., 2000; De Simone et al., 2002a). It also appears that suberization prevents the entry of reduced phytotoxic compounds into the roots, suggesting an important function in adapting roots to waterlogged or temporarily flooded soils. However, neither the physiological function nor the composition and quantity of suberin deposited in cells of species occurring in habitats with temporarily suboptimal oxygen supply has been clearly demonstrated up to now.
Covering more than 300,000 square kilometers, the Central Amazon floodplain represents one of the largest inundation areas in the world (Junk, 1997). The Amazon river and its large tributaries are accompanied by adjacent species-rich and highly adapted floodplain forest communities, which are subjected to a monomodal floodpulse for up to 10 months with extremely high water levels, reaching an amplitude of about 10 m (Junk, 1997; Junk et al., 1989). Due to the chemical composition of the flood water, these floodplains have been classified as nutrient-rich white-water river floodplains (várzea) and nutrient-poor black-water river floodplains (igapó; Sioli, 1968; Prance, 1979). With more than 250 tree species, an uncommonly high species diversity was recorded for white-water floodplains of the Amazon basin (Junk et al., 2000). The drastic changes of the soil chemistry during inundation pose extreme constraints for plant survival and reproductivity. The oxygen depletion of the flooded soil by microbial activity is followed by a rapid decrease of the soil redox potential, leading to a build up of high levels of reduced and potentially harmful compounds (Kozlowski, 1984; Armstrong and Armstrong, 1999; Čížeková et al., 1999). Different strategies of adaptation to the long flooding period during the aquatic phase becomes obvious in the leaf-shedding behavior of the species inhabiting these forests. Várzea tree species can be divided into two groups; deciduous tree species, which reduce the transpiring area by complete defoliation during the aquatic phase, and evergreen trees, which are able to maintain their leaf system during this period (Worbes et al., 1992; Parolin et al., 1998). Shedding of leaves can be considered as a visible symptom of down-regulating metabolism during conditions of prolonged flooding, which aids in preventing loss of root energy reserves and water by reducing energy consuming fermentation processes and leaf-transpiration.
The aim of the present study was to evaluate the function of a suberized and lignified exodermis in the adaptation of plants to low oxygen levels. Consequently, the suberin and lignin composition of rhizodermal cell walls (RHCWs) was analyzed and quantified using gas chromatography/mass spectrometry and was related to the distribution of oxygen in and around the roots of four species inhabiting floodplain forests in Central Amazonia. It is shown that oxygen profiles in and around roots correlate with the degree of suberization in peripheral cell layers of the roots. Analysis of the suberin compounds further suggests a function of the exodermis in pathogen defense in some of the species and supports a multifunctional role of the exodermis in adapting plants to an extremely fluctuating environment.
RESULTS
Amazon Tree Species Differ in Their Leaf-Shedding Behavior
Four tree species typical of Amazon floodplains that are subjected to a similar water regime at their natural habitat were chosen for the present investigations. On the basis of in situ observations in a várzea forest near Manaus, Brazil, these four trees either are deciduous or are able to maintain their leaf system during the aquatic phase (for a survey, see Table I). Tabernaemontana juruana is a late successional and shade-tolerant shrub that typically inhabits the understory of forest communities. The leaf-system of T. juruana is not affected by the rise of the water table during the aquatic phase. Laetia corymbulosa is one of the most abundant várzea tree species, reaching a stem height of up to 25 m. The leaf-system of L. corymbulosa is continuously renewed during the year. Complete leaf-shedding does not occur. Salix martiana is a light-demanding and fast growing pioneer species that inhabits open sites along the Amazon river. The stem reaches heights of up to 12 m, and the leaf-system is well developed during the flooding period. The formation of adventitious roots allows S. martiana to tolerate high sedimentation rates on sand banks. Crateva benthami is a leaf-shedding species from the lower canopy inhabiting low elevation sites. The rise of the water table results in complete defoliation of submerged individuals.
Table I.
Morphological characteristics of the four Central Amazon floodplain tree species
| Species | Leaf-Shedding | Suberization | Adventitious Roots | Aerenchyma | Intercellular Spaces |
|---|---|---|---|---|---|
| T. juruana | − | +++ | + | − | + |
| L. corymbulosa | − | ++ | − | − | − |
| S. martiana | − | + | ++ | + | + |
| C. benthami | + | − | − | − | + |
With the exception of adventitious roots, which are formed in response to low oxygen conditions, morphological traits were not affected by the oxygen level in the growth medium. The intensity of suberization and adventitious rooting is indicated by + and − symbols using the following order: − < + < ++ < +++.
The Formation of Suberin Barriers in Young Root Zones Is Restricted to Evergreen Species
Suberization in the root exodermis has been attributed to the adaptation to low oxygen availability. To elaborate whether maintenance of the leaf system during the aquatic phase is associated with changes in the root anatomy, cross sections from young root segments (30 mm behind the root tip) were analyzed by light and fluorescence microscopy, and suberin incrustations were visualized by staining with the fluorescent dye neutral red. To discover possible morphological changes induced by flooding, plants were grown either aerobically or under hypoxic conditions. T. juruana and S. martiana responded to hypoxic growth conditions by inducing the formation of a new root type from the stem basis, which can be referred to as adventitious roots. L. corymbulosa and C. benthami were not able to form such roots. Microscopic examinations did not reveal any anatomical differences between aerobically grown roots, hypoxically treated roots, and roots induced by hypoxic conditions among the four examined tree species (Fig. 1).
Figure 1.
Transverse sections of young root segments (30 mm) from Central Amazon floodplain tree species. For fluorescence microscopical investigations of suberin deposits, root sections were stained with neutral red after quenching of autofluorescence with toluidine blue. a, Aerobically grown root tips from T. juruana. Note that the suberization initiates shortly behind the root tip. Arrow shows initiation of suberization. b through d, Transverse sections of aerobically (b and c) and hypoxically (d) grown roots. Roots of T. juruana are characterized by the presence of small intercellular spaces in the root cortex (b) and a strongly suberized hypodermis (c and d). e through g, Transverse sections of aerobically (e and f) and hypoxically (g) grown roots of L. corymbulosa. e, No air spaces in the root cortex are evident. Suberin staining pattern reveals an incompletely suberized hypodermis with a high number of passage cells. h through j, Transverse sections of aerobically (h and i) and hypoxically (j) grown roots of S. martiana. Cross sections show the formation of large aerenchymatous air spaces in the root cortex (h) and a weak suberin staining of the hypodermal cell layer (i and j). k through m, Transverse sections of aerobically (k and l) and hypoxically (m) grown roots of C. benthami. k, Small intercellular air spaces are present in the root cortex. Staining reveals no suberin deposits in young root segments. Bars = 5 mm (a) and 50 μm (b–m). Eight root segments were analyzed per species and growth type (C. benthami, n = 4).
Roots of T. juruana are characterized by small, intercellular spaces in the cortex of schizogenous origin, which are present throughout the root (Fig. 1b), and by a heavily suberized hypodermal cell layer (Fig. 1, c and d). Suberization includes anticlinal and tangential cell walls and initiates about 2 mm behind the root tip (Fig. 1a). No intercellular spaces are developed in the root cortex of L. corymbulosa (Fig. 1e). Suberization is distributed along walls of the hypodermal cell layer with numerous passage cells devoid of suberin (Fig. 1, f and g). S. martiana shows large air spaces in the root cortex, arising from lysigenous degeneration of cortical cells (Fig. 1h). In roots of S. martiana, suberization is most abundant in radial cell walls of the hypodermis, suggesting Casparian bands (Fig. 1, i and j). Roots of C. benthami were found to form intercellular spaces in the cortex (Fig. 1k), but this species completely lack a visible suberized lamella in the hypodermis (Fig. 1, l and m).
The morphological characteristics were reflected by the measurements of the root porosity of the investigated species (Table II). As expected, the aerenchymatous species S. martiana showed the highest root porosity, reaching values that are 7-fold higher than those determined for roots from T. juruana. Due to the lack of air spaces, roots from L. corymbulosa exhibited almost no root porosity. In all species, no plasticity toward the formation of air spaces or lignin and suberin deposits was observed among the treatments.
Table II.
Root porosity as a percentage of the total volume of roots from four Central Amazon tree species, either grown in aerated nutrient solution or for at least 4 weeks under hypoxic conditions
| +O2 | −O2 | −O2 Adventitious Roots | |
|---|---|---|---|
| T. juruana | 2.8 ± 0.7 | 3.3 ± 0.2 | 3.5 ± 0.1 |
| L. corymbulosa | 0.2 ± 0.3 | 0.0 ± 0.0 | n.d. |
| S. martiana | 20.4 ± 6.9 | 21.4 ± 1.1 | 21.2 ± 1.2 |
| C. benthami | 3.9 ± 0.5 | 4.4 ± 0.3 | n.d. |
Root porosity was determined for the apical 5 cm of the roots. Data are means of three replicates. N.d., Not determined.
Evergreen Species Show Higher Contents of Suberin Components
For a qualitative analysis of peripheral cell walls (RHCWs), cell wall samples were enzymatically isolated from root segments derived 0 to 3 cm from the apex. Because walls of hypodermal cells cannot be separated from those of the rhizodermis, analysis of suberin and lignin compounds always comprises both cell types. RHCWs of all investigated tree species released detectable amounts of suberin monomers after chemical degradation by transesterification with BF3 in methanol. A survey over the chemical composition of the suberin compounds is given in Table III. In all four species, the aromatic domain is composed of ester-linked cis/trans-ferulic acid and a syringyl-derived lignin monomer. Para-hydroxybenzoic acid was dominating in RHCWs of L. corymbulosa and S. martiana, whereas it was not detectable in T. juruana and C. benthami (Table III). In all species, the aliphatic moiety of suberin consists of long-chain (C16-C28) monoalcohols, monocarboxylic acids, α,ω-dicarboxylic acids, ω-hydroxyacids, and 2-hydroxyacids (Table III), which were detected as their monomethylesters or trimethylsilyl-derivatives. The aliphatic suberin composition of the RHCWs from the evergreen species T. juruana, L. corymbulosa, and S. martiana is dominated by dicarboxylic acids and ω-hydroxyacids. In contrast, aliphatic suberin of RHCWs from C. benthami is mainly composed of α,ω-dicarboxylic acids, monocarboxylic acids, and 2-hydroxyacids (Table III).
Table III.
Suberin composition of rhizodermal cell walls (RHCWs) from root tip segments (0–30 mm) as a percentage of all identified suberin monomers
| T. juruana | L. corymbulosa | S. martiana | C. benthami | |
|---|---|---|---|---|
| Aromatics | ||||
| cis-Ferulic acid | 0.65 ± 0.21 | 0.29 ± 0.14 | n.d. | 1.35 ± 0.29 |
| trans-Ferulic acid | 6.56 ± 0.55 | 6.64 ± 0.65 | 4.89 ± 0.13 | 18.46 ± 1.99 |
| p-OH-Benzoic acid | n.d. | 47.13 ± 1.15 | 52.82 ± 4.05 | n.d. |
| s-Lignin | 0.66 ± 0.22 | 0.39 ± 0.09 | 0.75 ± 0.04 | 3.11 ± 0.61 |
| Total | 7.86 ± 0.98 | 54.45 ± 2.03 | 58.46 ± 4.22 | 22.92 ± 2.89 |
| Aliphatics | ||||
| Monocarboxylic acids | ||||
| C16 | n.d. | n.d. | n.d. | 3.54 ± 1.05 |
| C18 | 1.25 ± 0.37 | 1.12 ± 0.11 | n.d. | 5.27 ± 1.22 |
| C18-9-en | 2.07 ± 0.37 | 3.00 ± 0.50 | 3.15 ± 0.39 | 8.27 ± 2.64 |
| C18-9,12-dien | 2.92 ± 0.28 | 3.25 ± 0.24 | 2.38 ± 0.42 | 9.27 ± 2.63 |
| C20 | 0.42 ± 0.05 | 1.01 ± 0.20 | n.d. | n.d. |
| C22 | 1.64 ± 0.33 | 0.66 ± 0.13 | 0.66 ± 0.17 | 2.52 ± 2.27 |
| C24 | 0.98 ± 0.36 | 0.42 ± 0.02 | 0.77 ± 0.08 | 3.26 ± 3.97 |
| C26 | 1.54 ± 0.18 | n.d. | 0.53 ± 0.14 | n.d. |
| C28 | n.d. | n.d. | n.d. | 0.30 ± 0.03 |
| Total | 10.82 ± 1.94 | 9.46 ± 1.20 | 7.49 ± 1.21 | 32.44 ± 13.80 |
| α,ω-Dicarboxylic acids | ||||
| C16 | 7.74 ± 1.35 | 7.97 ± 1.67 | 4.03 ± 0.71 | 1.80 ± 0.02 |
| C18 | 1.09 ± 0.33 | 0.55 ± 0.15 | 1.32 ± 0.33 | 1.30 ± 0.04 |
| C18-9-en | 20.94 ± 3.57 | 4.71 ± 1.14 | 4.97 ± 0.79 | 3.23 ± 0.23 |
| C22 | 0.89 ± 0.31 | 0.43 ± 0.14 | n.d. | n.d. |
| C24 | 1.17 ± 0.09 | n.d. | n.d. | n.d. |
| Total | 31.83 ± 5.63 | 13.65 ± 3.09 | 10.32 ± 1.84 | 6.33 ± 0.29 |
| ω-Hydroxyacids | ||||
| C16 | 3.64 ± 0.82 | 5.45 ± 0.98 | 4.50 ± 0.59 | 3.15 ± 0.12 |
| C18 | n.d. | 0.96 ± 0.24 | 1.51 ± 0.23 | n.d. |
| C18−9-en | 26.56 ± 5.31 | 7.11 ± 1.50 | 8.29 ± 0.98 | 11.09 ± 0.30 |
| C20 | n.d. | 0.81 ± 0.24 | 0.70 ± 0.06 | 1.09 ± 0.01 |
| C22 | 4.74 ± 1.26 | 3.42 ± 0.69 | 2.46 ± 0.17 | 1.68 ± 0.15 |
| C24 | 4.18 ± 1.33 | 0.50 ± 0.11 | 1.35 ± 0.25 | n.d. |
| C26 | 0.62 ± 0.18 | n.d. | 1.51 ± 0.55 | n.d. |
| C28 | 0.68 ± 0.90 | n.d. | n.d. | n.d. |
| Total | 40.41 ± 9.79 | 18.26 ± 3.76 | 20.32 ± 2.82 | 17.02 ± 0.58 |
| 2-Hydroxyacids | ||||
| C18 | n.d. | n.d. | n.d. | 1.67 ± 0.06 |
| C22 | 1.24 ± 0.32 | 0.44 ± 0.07 | n.d. | n.d. |
| C24 | 1.75 ± 0.09 | 0.83 ± 0.05 | 1.17 ± 0.03 | 12.14 ± 1.57 |
| C26 | n.d. | 0.30 ± 0.02 | n.d. | 3.83 ± 0.67 |
| Total | 2.99 ± 0.41 | 1.57 ± 0.14 | 1.17 ± 0.03 | 17.63 ± 2.30 |
| Alcohols | ||||
| C18 | 0.63 ± 0.09 | 0.37 ± 0.10 | n.d. | 1.69 ± 0.15 |
| C20 | 2.09 ± 0.54 | 0.23 ± 0.01 | n.d. | n.d. |
| C22 | 1.47 ± 1.03 | 1.29 ± 0.10 | 0.91 ± 0.15 | 1.48 ± 0.02 |
| C24 | 0.85 ± 0.52 | 0.14 ± 0.02 | 0.42 ± 0.10 | n.d. |
| C26 | 0.35 ± 0.26 | 0.34 ± 0.00 | 0.90 ± 0.27 | n.d. |
| C28 | 0.69 ± 0.41 | 0.23 ± 0.03 | n.d. | 0.49 ± 0.16 |
| Total | 6.08 ± 2.84 | 2.61 ± 0.26 | 2.24 ± 0.52 | 3.65 ± 0.33 |
sds of three replicates are given. n.d., Not detectable.
Quantitatively, the suberin content of the isolated RHCWs differed considerably among the species. The aliphatic suberin content was 3.3- and 4-fold higher in RHCWs from T. juruana compared with RHCWs from L. corymbulosa and S. martiana, respectively, and 6.7-fold higher than in RHCWs from C. benthami (Fig. 2). These differences are mainly attributable to the characteristic C18-unsaturated suberin markers ω-hydroxycarboxylic acid and α,ω-dicarboxylic acid. Quantitative analysis of these two compounds in RHCWs of the four species is shown in Figure 3. For both monomers RHCWs from T. juruana exhibit about 6-fold higher values than those of L. corymbulosa and S. martiana, which show similar contents of these components in the peripheral cell walls. Compared with RHCWs from T. juruana, lowest amounts of C18-unsaturated ω-hydroxycarboxylic acid and α,ω-dicarboxylic acid were observed in C. benthami.
Figure 2.
Aliphatic and aromatic suberin monomers released from RHCWs of young roots segments (0–30 mm) from the evergreen species T. juruana (Tj), L. corymbulosa (Lc), and S. martiana (Sm) and the deciduous species C. benthami (Cb). Data are given in nanomoles per square centimeter. Three samples per species out of pooled root tips from 30 plants were analyzed (C. benthami, n = 3 of 5 plants).
Figure 3.
Released amounts of unsaturated C18 α, ω-9en-dicarboxylic acid and ω-OH-9-en-carboxylic acid, characteristic suberin markers from enzymatically isolated RHCW. Data are given in nanomoles per square centimeter. (Tj, T. juruana; Lc, L. corymbulosa; Sm, S. martiana; and Cb, C. benthami). Three samples per species out of pooled root tips from 30 plants were analyzed (C. benthami, n = 3 of 5 plants). Error bars indicate sd among the three samples.
Lignin monomers, corresponding to the three typical lignin units p-hydroxyphenyl, syringyl, and guaiacyl, were characterized by thioacidolysis of RHCWs from T. juruana, L. corymbulosa, and S. martiana. All investigated species revealed amounts of total lignin between 22 and 28 nmol cm−2 (Fig. 4), which were 90% lower in T. juruana and 50% lower in L. corymbulosa and S. martiana than the aliphatic suberin amounts. In all species, the lignin biopolymer was composed of syringyl and guaiacyl units. Whereas lignin in T. juruana and L. corymbulosa was dominated by syringyl units, RHCWs from S. martiana exhibited higher contents of guaiacyl. The lignin monomer p-hydroxyphenyl was not detectable in the species under investigation.
Figure 4.
Total lignin and lignin monomer amounts of RHCWs (0–30 mm) from T. juruana (Tj), L. corymbulosa (Lc), and S. martiana (Sm) identified by thioacidolysis. Data are given in nanomoles per square centimeter. Three samples per species out of pooled root tips from 30 plants were analyzed. Error bars indicate sd among the three samples.
Suberin Functions as a Permeability Barrier for Gas Exchange
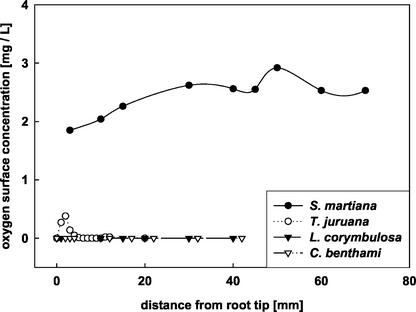
The barrier function of suberin for gas-exchange between the root and the rhizosphere was investigated by using oxygen microelectrodes controlled by a mechanical micromanipulator. Oxygen measurements were performed both on the root surface and inside the root after radial penetration of the microelectrode in roots of agar-embedded plants. No anatomical differences were observed between hydroponically grown and agar exposed roots. The species under investigation showed marked differences in ROLs and root cortex oxygen concentration. Highest ROLs were observed in adventitious roots of S. martiana, with rates remaining almost equal along the whole root (Fig. 5). A slight increase in O2 levels was noted in areas where laterals emerged (e.g. at a distance of 50 mm from the apex in Fig. 5). Adventitious roots of T. juruana showed only a thin oxygen layer in a narrow (approximately 5 mm) zone at the root tip. However, the oxygen level around the root within this zone did not by far reach the values characteristic of root tips from S. martiana. Behind the apex, no oxygen was detectable on the surface of roots from T. juruana, indicating the presence of an effective gas barrier. Surface measurements on roots of L. corymbulosa and C. benthami did not exhibit any detectable leakage of oxygen into the rhizosphere, irrespective of the root zone.
Figure 5.
Surface oxygen concentration of roots at various distances from the root apex embedded in stagnant and oxygen-free agar. Measurements on T. juruana and S. martiana were performed on 10- to 12-d-old adventitious roots. In the cases of L. corymbulosa and C. benthami, where cultivation in anoxic agar did not induce adventitious roots, pre-existing roots were used for the determinations. ROL was only observed in roots of T. juruana and S. martiana. Adventitious roots of T. juruana exhibited oxygen leakage to the rhizosphere only in a small (approximately 5 mm wide) zone at the root tip. No outward diffusion of oxygen was detectable from roots of L. corymbulosa and C. benthami. Lengths of the roots were 12 cm (S. martiana), 5 cm (T. juruana), or 6 cm (L. corymbulosa and C. benthami). Determinations were performed on 20 different roots from at least six individual plants without major deviations in the overall pattern. Data are from a representative experiment.
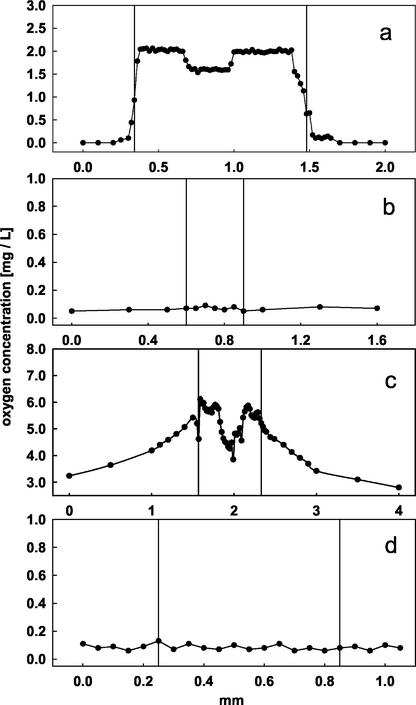
The findings described above were confirmed by determination of the oxygen levels within the roots. Root cortex oxygen concentration was only measurable in adventitious roots from T. juruana and S. martiana (Fig. 6). Oxygen concentration in the cortex of adventitious root from T. juruana did not exceed 2.0 mg L−1 (Fig. 6a). The steep increase in the oxygen level behind the root periphery indicates that the suberized exodermis can effectively prevent the efflux of oxygen from the root into the rhizosphere. The decrease in oxygen concentration in the stele is related to the absence of air spaces. The oxygen concentration in the cortex of adventitious roots from S. martiana was about 3-fold higher than that in adventitious roots of T. juruana (Fig. 6c). The gradual decline of the oxygen levels in the rhizosphere with increasing distance from the root indicates radial O2 diffusion, which corresponds to the measurements along the whole root surface. No significant amount of oxygen was detectable in roots of L. corymbulosa (Fig. 6b) and C. benthami (Fig. 6d).
Figure 6.
Oxygen profiles in the rhizosphere and in roots of the investigated species. Vertical axes indicate points of vertical penetration of the microelectrode into and out of the roots. Profiles were taken 10 to 15 mm behind the apex. a, Oxygen profile through a 12-d-old and 5-cm-long adventitious root from T. juruana. The low oxygen concentration indicates hypoxic conditions in the root cortex and stele. No ROL was detectable. b, Measurement of the oxygen distribution revealed the absence of oxygen in the rhizosphere and in roots of L. corymbulosa. c, The oxygen profiles through 10-d-old and 12-cm-long adventitious roots from S. martiana revealed high oxygen concentrations inside the roots and a several-millimeter-thick oxygenated zone around the roots. d, Roots from C. benthami did not exhibit any oxygen inside or outside the roots. Lengths of the roots were 12 cm (S. martiana), 5 cm (T. juruana), or 6 cm (L. corymbulosa and C. benthami). Determinations were performed on 20 different roots from at least six individual plants without major deviations in the overall pattern. Data are from a representative experiment.
DISCUSSION
The response of plants to flooding is complex, involving an array of physiological, biochemical, and morphological adaptations. Anatomical changes, such as the development of aerenchyma and the formation of apoplastic barriers in the peripheral cell layers, are thought to be part of complex strategies to withstand periods of low oxygen availability. Deposition of suberin and lignin compounds in the outer cell layers of roots has been described for many wetland species, either as being induced by flooding (Colmer et al., 1998) and/or phytotoxins (Armstrong and Armstrong, 1999) or as being constitutively present. A comparison of the suberin content of the investigated species with those of other taxa is hampered by the lack of available data. In comparison with herbaceous species, the várzea tree species reached multifold amounts of aliphatic suberin monomers (Schreiber et al., 1999; Zimmermann et al., 2000). Qualitatively, the aliphatic moiety of RHCWs is dominated by ω-hydroxycarboxylic acid and diacids, representing typical suberin substance classes (Kolattukudy, 2001). Only in C. benthami, the composition of the suberin lamellae is dominated by monoacids, 2-hydroxy acids, and ω-hydroxy acids, which resembles the suberin composition in roots of Picea abies (Matzke and Riederer, 1991). The detection of a syringyl-derived monomer in the analyzed samples confirms the findings of Zeier and Schreiber (1997), showing that aromatic lignin-related monomers can be released from the suberin polymer by transesterification, and indicates the presence of lignin in the isolated RHCWs of the investigated tree species.
On the basis of their anatomical distribution, the function of suberized cell walls has been primarily attributed to water retention and to a restriction of solute transport through the apoplast. In wetland plants, the hypodermis was assumed to act as a gas diffusion barrier, restricting the radial loss of oxygen into the rhizosphere (Armstrong et al., 1994). Plants subjected to flooding rely on transport of oxygen from aerial plant parts to the roots. The effectiveness of internal gas transport depends on the resistance to diffusion within the plant, the plant's respiratory demand for oxygen, and the rate of O2 diffusion out of the roots. The presence of a barrier to ROL is obviously of advantage in terms of maintenance of energy metabolism. On the other hand, the diffusion of oxygen out of the roots restores ion uptake (Engelaar, 1993) and helps to re-oxidize toxic compounds of flooded soils to nontoxic compounds. Although the restriction of gas exchange by cell wall deposits in wetland plant roots has been demonstrated polarographically using Clark-type microelectrodes (Armstrong et al., 2000; Visser et al., 2000), the nature of potential barrier-biopolymers in hypodermal walls of such species has yet not been determined. Staining techniques developed to localize aliphatic and aromatic material in plant cell walls are not always sensitive enough to allow for a qualitative estimation of the substances involved in forming such barriers. Although, in general, quantitative differences in suberin content among the investigated species were revealed by microscopic analysis, deviations between the intensity of morphological staining and the suberin amount of isolated RHCWs from roots of L. corymbulosa and S. martiana imply that quantitative chemical analysis of root sections is more reliable and more sensitive than microscopic techniques. The inaccuracy of the suberin staining also becomes obvious in roots of C. benthami. No suberin was visualized by the staining procedure, but suberin monomers could be detected in isolated RHCWs, implying that the suberin amount in RHCWs from C. benthami does not reach the threshold value for becoming detectable by histochemical probing.
Combining the data collected from the chemical analysis of RHCWs with the ROL- measurements, we are able to confirm the postulated role of suberin as a permeability barrier for gas-exchange between the root and the rhizosphere. The results validate the presence of such a barrier in roots of T. juruana, consisting of a heavily suberized hypodermis with depositions in tangential and radial cell walls. Despite the strong resistance to oxygen, water transport may not be severely restricted by suberin. Water transfer across the root peripheral cell layers may occur via the middle lamellae, as suggested for the outermost phellems in various tree species (Groh et al., 2002). A 3-fold lower aliphatic suberin amount was recorded in roots of S. martiana in which high ROLs were evident. This suggests that suberin in these concentrations is not an absolute air-tight barrier and should rather be described as a resistor whose permeability depends on its chemical composition and on the root oxygen concentration. In general, a higher content of suberin components was observed in evergreen species (this study; De Simone et al., 2002b), suggesting that suberin barriers are of advantage to prevent leaf shading during the aquatic phase. No major difference in lignin content was observed between the two species, suggesting that lignin does not contribute to the resistance to oxygen loss in the species under investigation.
Suberin polymers have been suggested to be involved in pathogen defense, either by a breakdown of polymers by enzymes of microbial origin and subsequent release of toxic phenols or by acting as a mechanical barrier (Peterson, 1997). A point of major deviation in the composition of suberin among the species is the high percentage of aromatics in RHCWs of L. corymbulosa and S. martiana, which is dominated by para-hydroxybenzoic acid (> 85% of total aromatics). Similar to salicylic acid, para-hydroxybenzoic acid was shown to be involved in pathogen defense, either by their direct antimicrobial action or by induction of a subset of pathogenesis-related genes (Ryals et al., 1996; Smith-Becker et al., 1998). In roots of L. corymbulosa and S. martiana, suberization did not start shortly behind the root tip, emphasizing the adaptive value of this feature to defend the roots from microbiological attack during the aquatic period.
In summary, our data show that the composition of the hypodermis can contribute significantly to the adaptation of plants to long-term flooding conditions. Suberin barriers may determine important processes that in turn aid in withstanding low oxygen availability such as pathogen defense, re-oxidation of reduced phytotoxins, and the restriction of oxygen loss to the rhizosphere. We show here for the first time, to our knowledge, that suberin, and not lignin, represents the barrier for the diffusion of oxygen. Our data also show that different adaptive strategies can be realized in plant species facing similar environmental constraints.
MATERIALS AND METHODS
Plants and Growth Conditions
Experiments were carried out with 3- to 4-month-old cuttings of Tabernaemontana juruana [Markgr.] Schumann ex J.F. Macbride (Apocynaceae), Laetia corymbulosa Spruce ex Bent. (Flacourtiaceae), Salix martiana Leyb. (Salicaceae), and Crateva benthami Eichl. In Mart.(Capparaceae), which were grown hydroponically in a climate-controlled greenhouse under the following conditions: 70% to 90% relative humidity, day/night regime of 16 h/8 h (150 μmol m−2 s−1 supplied by IP 23 lamps [Philips, Eindhoven, The Netherlands]) and 30°C/22°C day/night temperature. The cuttings were derived from 1- to 2-year-old young trees. Basal shoot ends were spread with 2% (w/v) Rhizopon AA (Rhizopon bv, Hazerswonde, The Netherlands) and incubated in peat to induce root formation. After initiation of rooting, the cuttings were transferred to aerated nutrient solution containing 3.00 mm NH4NO3, 0.50 mm MgSO4, 1.50 mm CaCl2, 1.50 mm K2SO4, 1.50 mm NaH2PO4, 25.0 μm H3BO3, 1.00 μm MnSO4, 0.50 μm ZnSO4, 0.05 μm (NH4)6Mo7O24, 0.30 μm CuSO4, and 40.0 μm FeEDTA, pH 6.0, and grown for an additional 2 months. Nutrient solution was changed weekly, pH values ranged from 5.5 to 6.0 within this period. The cuttings were then transferred into the experimental aerobic and hypoxic treatments in 1.5-L plastic pots with four cuttings per container for 1 to 4 weeks. Hypoxic conditions were induced by continuous flushing of the nutrient solution with nitrogen through a gas permeable S6/2 Accurel tube (Membrana, Wuppertal, Germany). Oxygen concentration did not exceed 0.1 mg L−1 in the plastic pots during the whole experimental period. Aerobic controls were grown in 1.5-L pots in aerated nutrient solution for the same period of time.
Measurement of Root Porosity
Porosity of roots was determined after the method of Raskin (1983), using the equations modified by Thomson et al. (1990). The apical 0 to 5 cm of 20 to 40 roots from each species, grown for 4 weeks under aerobic or hypoxic growth conditions, were detached with a razor blade and cut into segments of approximately 1.5 cm. The root systems of S. martiana and T. juruana were divided into hypoxic roots and adventitious roots which emerged in the flood water. The fresh weight of the samples was determined after carefully removing surface water by blotting with tissue paper. Buoyancy of root samples before and after vacuum infiltration with water was measured using a balance with a water-filled flask containing the root samples attached to the under-carriage and submerged in a beaker of water under the balance. The porosity was calculated from the difference in weight of the samples before and after infiltration (volume of airspace), divided by the difference between fresh weight and weight under water before infiltration (volume of the segments).
Light and Fluorescence Microscopy
After 4 weeks of cultivation, roots were detached and prepared for morphological investigations. Root structure was investigated by light microscopy and fluorescence microscopy of root segments. For light microscopy analysis, root segments were fixed in 3.7% (w/v) paraformaldehyde in 100 mm phosphate buffer, pH 7.0, dehydrated in an upgrading ethanol series, infiltrated with London Resin White (London Resin Co. Ltd. London), and polymerized for 24 h at 55°C and 250 mbar. Cross sections (1 μm) were cut with a microtome (Ultracut E, Reichert, Vienna) stained with 0.05% (w/v) toluidine blue O for 1 min, and viewed in dark field. Fluorescence microscopy investigations were carried out on transverse free-hand sections. To visualize suberization of the epidermal and subepidermal cell walls, sections were stained with 0.1% (w/v) neutral red in 100 mm phosphate buffer, pH 6.0, for 1 min and washed twice with tap water. The specificity of the neutral red technique for the hydrophobic/lipid domain of suberin was shown previously (Lulai and Morgan, 1992). For quenching of autofluorescence, sections were previously incubated for 2 h in 0.05% (w/v) toluidine blue O in 100 mm phosphate buffer, pH 6.0. Blue-violet excitation (exciter filter EX 420–490, dichromatic beamsplitter DM 505, barrier filter BA 520, Nikon, Tokyo) was used for all investigations of suberin deposits. Photographs were taken with a digital camera (Nikon Coolpix 990, 3.34 megapixels).
Qualification and Quantification of Suberin
Isolation and Purification of Cell Wall Materials
Cell walls from root tip segments (0–30 mm) were isolated enzymatically similar to the procedure described by Schreiber et al. (1994). The segments were vacuum infiltrated with a solution containing cellulase (Onozuka, R-10, Serva, Heidelberg) and pectinase (Macerozyme R-10, Serva) dissolved in 0.01 m acetate buffer at pH 4.5. After 8 weeks of maceration in the enzyme solution, the hypodermal cylinder and the central cylinder were separated mechanically using two precision forceps and a stereo microscope. Because rhizodermal and the attached hypodermal/exodermal cell walls could not be separated from each other, the isolated cell wall fraction was termed RHCWs. The isolated RHCWs fraction was washed several times with borate buffer (0.01 m Na2B4O7, pH 9.0) and distilled water, followed by subsequent extraction of the dry material with chloroform:methanol (1:1; v/v) to remove soluble lipids. Finally, samples were dried again and stored over silica gel until further use. To determine the RHCWs dry weight-root surface relation, RHCWs of root segments with known surface area were isolated, dried, and weighted separately.
Chemical Degradation and Chromatographic Analyses of Isolated Cell Walls
Purified cell wall material was subjected to chemical degradation methods specific for the detection of the biopolymers suberin and lignin. For suberin analysis, the extracted RHCWs were depolymerized using a BF3 catalyzed methanolic transesterification (Kolattukudy and Agrawal, 1974) as described in detail by Zeier and Schreiber (1997, 1998). Extracted RHCWs (0.5–1 mg) were added to 1 mL of a 10% (w/v) BF3/methanol solution (Fluka, Deisenhofen, Germany) and heated to 70°C for 16 h. After cooling, solid residues were removed, and the remaining solution was extracted three times with 1 mL of CHCl3 containing 20 μg of dotriacontane (Fluka) as an internal standard. The combined extracts were washed with 2 mL of a saturated sodium chloride solution and 1 mL of distilled water. The organic phase of chloroform was separated and dried over Na2SO4.
Thioacidolysis was used for the detection of lignin according to Lapierre et al. (1991). Forty microliters of BF3 etherate (Merck, Darmstadt, Germany) and 160 μL of ethanethiol (Fluka) were dissolved under argon in 300 μL of dioxane in a tube fitted with a Teflon-lined screw cap. The solution was adjusted to a volume of 1.6 mL with 1.1 mL of dioxane. The sample (0.5–1.0 mg) was added, and the mixture was stirred at 100°C. After 4 h, the reaction mixture was ice-cooled, diluted with 2 mL of water, and extracted three times with 3 mL of CHCl3, containing 20 μg of dotriacontane (Fluka) as an internal standard. The combined organic phases were dried over Na2SO4.
Gas chromatography and mass spectroscopy were used for quantification and identification of the released suberin and lignin monomers. Before injection, samples were derivatized by N,N-bis-trimethylsilyltrifluoroacetamide (Machery-Nagel, Düren, Germany) catalyzed with pyridine to convert free hydroxyl and carboxyl groups to their respective trimethylsilyl esters and ethers. Qualitative sample analyses were performed by gas chromatography (Agilent 6890N gas chromatograph, Agilent Technologies, Böblingen, Germany) combined with a quadropole mass selective detector (Agilent 5973N mass selective detector, Agilent Technologies). Quantitative sample analysis was carried out with a HP 5890 Series II gas chromatograph (Hewlett Packard, Palo Alto, CA), equipped with a flame ionization detector.
Root Surface Determination
The suberin content of rhizodermal cell walls (micrograms per milligram) was related to the root surface (micrograms per square centimeter). Fresh transversal sections, cut 5 and 20 mm behind the root apex, were prepared for light microscopy and transferred with a camera system on a Mini-MOP picture digitizer (Kontron, Munich). The circumference was related to the segment length (30 mm) for calculation of the root surface. Five root segments from five individual plants were analyzed.
Oxygen Measurements
Measurements on the oxygen distribution in and around roots were carried out with oxygen microelectrodes on 2-month-old cuttings that were previously grown in customary potting soil to induce root formation. The roots of the cuttings were rinsed with tap water to remove soil residues and immersed in a stagnant agar (0.5%, w/v) nutrient solution in a narrow glass basin (width 20 cm, height 8 cm, depth 5 cm). To simulate natural conditions of flooding and to prevent drying of the agar, a layer of nutrient solution (about 1 cm) covered the agar surface. The plants were cultivated in a climate chamber under the following conditions: 70% to 80% relative humidity, day/night regime of 12 h/12 h, 32°C/28°C day/night temperature. The agar medium was changed once a week. Illumination of the roots was prevented by wrapping the glass basin in black paper. For oxygen measurements plants were immersed into a fresh agar medium that first had been flushed and subsequently overflown with nitrogen for at least 20 h before insertion of the plantlets. During the measurements, the basin was sealed with a thin foil, and water-saturated nitrogen flew above the agar surface during. The agar medium for the oxygen measurements consisted of two layers: a lower layer of solid agar (2%, w/v) to build up a horizontal overlay to fix the roots by means of thin glass hook at the surface, and an upper layer of liquid, but stagnant agar (0.1%, w/v) in which the roots were immersed and the oxygen profiles were recorded. The upper layer was 5 to 6 mm thick. A hole in the lower solid agar filled with liquid agar contained the bulk roots. For observing the apex of the microelectrode approaching the root surface—by means of a precision magnifying glass—the root was placed close to the glass wall of the basin. The temperature during the measurements was about 30°C. Oxygen microsensors (MasCom, Bremen, Germany) were used to measure oxygen profiles in the agar medium approaching to the root surface and in the outer tissues of the root. These sensors correspond to a miniaturized Clark oxygen electrode (Revsbech, 1989) which is sealed with a thin silicon membrane. The permeating oxygen reacts at the negatively charged microcathode (−0.8 V), and causes a current signal that is proportional to the oxygen concentration in solution. These oxygen microelectrodes operate with a response time lower than 2 s, extremely low oxygen consumption, a very stable signal, and a detection limit of 0.1 to 0.2 mg L−1oxygen. Due to the high linearity in response to oxygen concentration, an easy 2-point calibration in nitrogen-bubbled water and air-saturated water at the measuring temperature was possible. The microelectrodes were calibrated once a day. The current output of the employed microelectrodes varied between 1 and 5 pA in nitrogen-bubbled water and between 80 and 200 pA in air-saturated stagnant water. Air in the laboratory (relative humidity 50%) gave maximum 5% higher readings depending on the respective electrode. Water-saturated air would minimize these differences (Revsbech and Ward, 1983). Thus, the error caused by the change between liquid phase (root cells) and gas phase (aerenchyma) in the cortex of the adventitious root is negligible. The tip diameters of the electrodes were smaller than 20 μm. The microelectrodes were gradually driven into the agar or into the root by means of a mechanical micromanipulator in steps of 50 μm in the rhizosphere and 20 μm in the roots. The direction of the electrode tip was right-angled to the position of the root. The current signals were converted to voltage signals by the electronic sensor, digitized in the computer, and saved as an ASCII file.
ACKNOWLEDGMENTS
The study is part of the research which is carried out jointly by the tropical Ecology Group of the Max-Planck-Institute of Limnology (Plön, Germany) and the Instituto Nacional de Pesquisas da Amazônia (Manaus, Brazil).
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.014902.
LITERATURE CITED
- Agrawal VP, Kolattukudy PE. Purification and characterization of a wound-induced ω-hydroxy fatty acid: NADP oxidoreductase from potato tuber disks. Arch Biochem Biophys. 1978;191:452–465. doi: 10.1016/0003-9861(78)90384-3. [DOI] [PubMed] [Google Scholar]
- Armstrong J, Armstrong W. Phragmites die-back: toxic effects of propionic, butyric, and caproic acids in relation to pH. New Phytol. 1999;142:201–217. [Google Scholar]
- Armstrong W, Brandle R, Jackson MB. Mechanisms of flood tolerance in plants. Acta Bot Neerl. 1994;43:307–358. [Google Scholar]
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot. 2000;86:687–703. [Google Scholar]
- Boudet A-M. A new view of lignification. Trends Plant Sci. 1998;3:67–71. [Google Scholar]
- Campbell MM, Sederoff RR. Variations in lignin content and composition: mechanisms of control and implications for the genetic improvement of plants. Plant Physiol. 1996;110:3–13. doi: 10.1104/pp.110.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čížeková H, Brix H, Kopeckı J, Lukavská J. Organic acids in the sediments of wetlands dominated by Phragmites australis: evidence of phytotoxic concentrations. Aquat Bot. 1999;64:303–315. [Google Scholar]
- Colmer TD, Gibberd MR, Wiengweera A, Tinh TK. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J Exp Bot. 1998;49:1431–1436. [Google Scholar]
- De Simone O, Haase K, Müller E, Junk WJ, Gonsior G, Schmidt W. Impact of root morphology on metabolism and oxygen distribution in roots and rhizosphere from two Central Amazon floodplain tree species. Funct Plant Biol. 2002a;29:1025–1035. doi: 10.1071/PP01239. [DOI] [PubMed] [Google Scholar]
- De Simone O, Müller E, Junk WJ, Schmidt W. Adaptations of Central Amazon tree species to prolonged flooding: root morphology and leaf-longevity. Plant Biol. 2002b;4:515–522. [Google Scholar]
- Domeregue F, Bessoule J-J, Moreau P, Lessire R, Cassagne C. Recent advances in plant fatty acid elongation. In: Harwood JL, editor. Plant Lipid Biosynthesis. Vol. 67. Cambridge: Cambridge University Press; 1998. pp. 185–220. [Google Scholar]
- Engelaar WMHG. Root porosities and radial oxygen losses of Rumex and Plantago species as influenced by soil pore diameter and soil aeration. New Phytol. 1993;125:565–574. doi: 10.1111/j.1469-8137.1993.tb03904.x. [DOI] [PubMed] [Google Scholar]
- Freudenberg K. Lignin: its constitution and formation from p-hydroxycinnamyl alcohols. Science. 1965;148:595–600. doi: 10.1126/science.148.3670.595. [DOI] [PubMed] [Google Scholar]
- Graça J, Pereira H. Methanolysis of bark suberin: analysis of glycerol and acid monomers. Phytochem Anal. 2000a;11:45–51. [Google Scholar]
- Graça J, Pereira H. Suberin in potato periderm: glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J Agric Food Chem. 2000b;48:5476–5483. doi: 10.1021/jf0006123. [DOI] [PubMed] [Google Scholar]
- Groh B, Hübner C, Lendzian KJ. Water and oxygen permeance of phellems isolated from trees: the role of waxes and lenticels. Planta. 2002;215:794–801. doi: 10.1007/s00425-002-0811-8. [DOI] [PubMed] [Google Scholar]
- Hose E, Schreiber L, Clarskon D, Steudle E, Hartung W. The exodermis: a variable apoplastic barrier in roots. J Exp Bot. 2001;52:2245–2264. doi: 10.1093/jexbot/52.365.2245. [DOI] [PubMed] [Google Scholar]
- Junk WJ. Distribution and size of neotropical floodplains. In: Junk WJ, editor. The Central Amazon Floodplain. Ecological Studies 126. Berlin: Springer; 1997. pp. 12–16. [Google Scholar]
- Junk WJ, Bayley PB, Sparks RE. The flood pulse concept in river-floodplain systems. Can J Fish Aquat Sci. 1989;106:110–127. [Google Scholar]
- Junk WJ, Ohly JJ, Piedade MTF, Soares MGM. The Central Amazon Floodplain: Actual Use and Options for a Sustainable Management. Leiden, The Netherlands: Backhys Publishers; 2000. [Google Scholar]
- Kolattukudy PE. Polyesters in higher plants. Adv Biochem Eng Biotechnol. 2001;71:1–49. doi: 10.1007/3-540-40021-4_1. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE, Agrawal VP. Structure and composition of aliphatic constituents of potato tuber skin (suberin) Lipids. 1974;9:682–691. [Google Scholar]
- Kozlowski TT. Plant responses to flooding of soil. Bioscience. 1984;34:162–167. [Google Scholar]
- Lapierre C, Pollet B, Monties B. Thioacidolysis of spruce lignin: GC-MS analysis of the main dimers recovered after Raney nickel desulphuration. Holzforschung. 1991;45:61–68. [Google Scholar]
- Le Bouquin R, Skrabs M, Kahn R, Beneviste I, Salaün J-P, Schreiber L, Durst F, Pinot F. CYP94A5, a new cytochrome P450 from Nicotiana tabacum is able to catalyze the oxidation of fatty acids to the ω-alcohol and to the corresponding diacid. Eur J Biochem. 2001;268:3083–3090. doi: 10.1046/j.1432-1327.2001.02207.x. [DOI] [PubMed] [Google Scholar]
- Lulai EC, Morgan WC. Histochemical probing of potato periderm with neutral red: a sensitive cytofluorochrome for the hydrophobic domain of suberin. Biotechnol Histochem. 1992;67:185–195. doi: 10.3109/10520299209110065. [DOI] [PubMed] [Google Scholar]
- Matzke K, Riederer M. A comparative study into the chemical constitution of cutins and suberins from Picea abies (L.) Karst., Quercus robur L., and Fagus sylvatica L. Planta. 1991;185:233–245. doi: 10.1007/BF00194066. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R. Hydraulic conductivity of rice roots. J Exp Bot. 2001;52:1835–1846. doi: 10.1093/jexbot/52.362.1835. [DOI] [PubMed] [Google Scholar]
- Mohan R, Bajar MA, Kolattukudy PE. Induction of a tomato anionic peroxidase gene (tap1) by wounding in transgenic tobacco and activation of tap1/GUS and tap2/GUS chimeric gene fusions in transgenic tobacco by wounding and pathogen attack. Plant Mol Biol. 1993a;21:341–354. doi: 10.1007/BF00019949. [DOI] [PubMed] [Google Scholar]
- Mohan R, Vijayan P, Kolattukudy PE. Developmental and tissue-specific expression of a tomato anionic peroxidase (tap1) gene by a minimal promoter with wound and pathogen induction by an additional 5′-flanking region. Plant Mol Biol. 1993b;22:475–490. doi: 10.1007/BF00015977. [DOI] [PubMed] [Google Scholar]
- Moire L, Schmutz A, Buchala A, Yan B, Stark R, Ryser U. Glycerol is a suberin monomer: new experimental evidence for an old hypothesis. Plant Physiol. 1999;119:1137–1146. doi: 10.1104/pp.119.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önnerud H, Zhang L, Gellerstedt G, Henriksson G. Polymerization of monolignols by redox shuttle-mediated enzymatic oxidation: a new model in lignin biosynthesis I. Plant Cell. 2002;14:1953–1962. doi: 10.1105/tpc.001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin P, Ferreira LV, Junk WJ. Central Amazonian floodplains: effect of two water types on the wood density of trees. Int Assoc Theor Appl Limnol. 1998;26:1106–1112. [Google Scholar]
- Perumalla CJ, Peterson CA, Enstone DE. A survey of angiosperm species to detect hypodermal Casparian bands: I. Roots with a uniserate hypodermis or exodermis. Bot J Linn Soc. 1990;103:93–112. [Google Scholar]
- Peterson CA. The exodermis and its interactions with the environment. In: Flores HE, Lynch JP, Eissenstadt D, editors. Radial Biology: Advances and Perspectives on the Function of Plant Roots. Rockville, MD: American Society of Plant Physiologists; 1997. pp. 131–138. [Google Scholar]
- Peterson CA, Perumalla CJ. A survey of angiosperm species to detect hypodermal Casparian bands: II. Roots with a multseriate hypodermis or exodermis. Bot J Linn Soc. 1990;103:113–125. [Google Scholar]
- Prance GT. Notes on the vegetation of Amazonia: III. Terminology of Amazonian forest types subjected to inundation. Brittonia. 1979;31:26–38. [Google Scholar]
- Raskin I. A method for measuring leaf volume, density, thickness, and internal gas volume. Hortscience. 1983;18:698–699. [Google Scholar]
- Revsbech NP. An oxygen microsensor with a guard cathode. Limnol Oceanogr. 1989;34:474–478. [Google Scholar]
- Revsbech NP, Ward DM. Oxygen microelectrode that is insensitive to medium chemical composition: use in an acid microbial mat dominated by Cyanidium caldarium. Appl Environ Microbiol. 1983;45:755–759. doi: 10.1128/aem.45.3.755-759.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Mollina A, Steiner H-Y, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L, Breiner HW, Riederer M, Düggelin M, Guggenheim R. The Casparian band of Clivia miniata Reg.: isolation, fine structure and chemical nature. Bot Acta. 1994;107:353–361. [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot. 1999;50:1267–1280. [Google Scholar]
- Schreiber L, Skrabs M, Hartmann K, Becker D, Cassagne C, Lessire R. Biochemical and molecular characterization of corn (Zea mays L.) root elongases. Biochem Soc Trans. 2000;28:647–649. [PubMed] [Google Scholar]
- Sioli H. Hydrochemistry and geology in the Brasilian Amazon region. Amazoniana. 1968;1:267–277. [Google Scholar]
- Smith-Becker J, Marois E, Huguet EJ, Midland SL, Sims JJ, Keen NT. Accumulation of salicylic acid and 4-hydroxybenzoic acid in phloem fluids of cucumber during systemic acquired resistance is preceded by a transient increase in phenylalanine ammonia-lyase activity in petioles and stems. Plant Physiol. 1998;116:231–238. doi: 10.1104/pp.116.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CJ, Armstrong W, Waters I, Greenway H. Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant Cell Environ. 1990;13:395–403. [Google Scholar]
- Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ. Changes in growth porosity and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ. 2000;23:1237–1245. [Google Scholar]
- Worbes M, Klinge H, Revilla JD, Martius C. On the dynamics, floristic subdivision and geographical distribution of Várzea forests in Central Amazonia. J Veg Sci. 1992;3:553–564. [Google Scholar]
- Zeier J, Schreiber L. Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata. Plant Physiol. 1997;113:1223–1231. doi: 10.1104/pp.113.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Schreiber L. Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledoneous species: chemical composition in relation to fine structure. Planta. 1998;206:349–361. [Google Scholar]
- Zimmermann HM, Hartmann K, Schreiber L, Steudle E. Chemical composition of apoplastic barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.) Planta. 2000;210:302–311. doi: 10.1007/PL00008138. [DOI] [PubMed] [Google Scholar]