Abstract
Hydrogen peroxide (H2O2) induces increases, to different degrees, in transcripts, protein levels, and activity of the Ndh complex (EC 1.6.5.3). In the present work, we have compared the effects of relatively excess light, H2O2, dimethylthiourea (a scavenger of H2O2), and/or EGTA (a Ca2+ chelator) on the activity and protein levels of the Ndh complex of barley (Hordeum vulgare cv Hassan) leaf segments. The results show the involvement of H2O2 in the modulation of both the protein level and activity of the Ndh complex and the participation of Ca2+ mainly in the activity regulation of pre-existing protein. Changes in Ndh complex activity could not be explained only by changes in Ndh protein levels, suggesting posttranslational modifications. Hence, we investigate the possible phosphorylation of the Ndh complex both in thylakoids and in the immunopurified Ndh complex using monoclonal phosphoamino acid antibodies. We demonstrate that the Ndh complex is phosphorylated in vivo at threonine residue(s) of the NDH-F polypeptide and that the level of phosphorylation is closely correlated with the Ndh complex activity. The emerging picture is that full activity of the Ndh complex is reached by phosphorylation of its NDH-F subunit in a H2O2- and Ca2+-mediated action.
A plastid Ndh complex, analogous to the NADH dehydrogenase (NADH-DH) or complex I (EC 1.6.5.3) of the mitochondrial respiratory chain, which catalyzes the transfer of electrons from NADH to plastoquinone, has been purified from pea (Pisum sativum; Sazanov et al., 1998) and barley (Hordeum vulgare cv Hassan; Casano et al., 2000). Eleven polypeptides of the Ndh complex (NDH polypeptides) are encoded by respective ndh genes of plastid DNA (Maier et al., 1995). Both the Ndh complex (providing electrons) and thylakoid plastoquinol peroxidase (Zapata et al., 1998) together with the Mehler reaction and superoxide dismutase (draining electrons) might poise the redox level of the photosynthetic electron carriers. This mechanism (chlororespiration) would most likely ensure the photosynthetic electron transport under a variety of environmental conditions, which include rapid changes of light intensity caused by sunflecks and leaf movements. In addition, chlororespiration may act as a scavenging system of reactive oxygen species generated under continuous photooxidative stress or by the successions of sunflecks and light gaps (Casano et al., 2000). In fact, NDH polypeptides and NADH-DH activity of the Ndh complex increase under photooxidative stress provoked by the herbicide paraquat (Martín et al., 1996; Catalá et al., 1997; Casano et al., 1999, 2000) or bright light and chilling in field-grown barley (Teicher et al., 2000). In addition, ndh mutants show increased sensitivity to photooxidative stress (Endo et al., 1999; Horvath et al., 2000), which strongly suggests that the activity of the Ndh complex is involved in the protection against said stress.
The increases of plastid-encoded NDH polypeptides and Ndh complex activity under photooxidative stress are mediated by hydrogen peroxide (H2O2; Casano et al., 2001). Similarly, H2O2 mediates the induction of several nuclear-encoded defensive enzymes, such as cytosolic ascorbate peroxidase (Karpinski et al., 1999; Morita et al., 1999), glutathione S-transferase and catalase (Polidoros and Scandalios, 1999) under oxidative stress. The time courses of the increases of ndh mRNAs, NDH polypeptides, and NADH-DH activity of the Ndh complex after photooxidative or H2O2 treatment of the leaves have been compared (Casano et al., 2001). Approximate 100% increases in protein levels and activity of the Ndh complex during the first 4 h suggest an activation of the translation of pre-existing mRNAs, whereas further 400% to 500% increases of protein and activity between 6 to 15 h of treatment are parallel to increases of mRNA levels. Between 15 to 30 h of treatment, important differences were detected between the levels of Ndh protein and Ndh complex activity (Casano et al., 2001), which suggest an additional effect of the H2O2-mediated pathway on the activity of the Ndh complex. In addition, it has been indicated that H2O2 is a common intermediary in several signaling pathways, which include Ca2+ mobilization (Price et al., 1994), abscisic acid (Pei et al., 2000), jasmonic acid (Orozco-Cárdenas et al., 2001), gibberellins (Fath et al., 2001), and protein kinases (Kovtun et al., 2000).
In this work, we compare the levels of the Ndh protein and Ndh complex activity when barley leaf segments are treated with H2O2 and relatively excess light (photooxidative light [PhL]). The role of Ca2+ and protein phosphorylation on the photooxidative increase of the Ndh complex activity is also studied. The phosphorylation of the NDH-F polypeptide is demonstrated in thylakoid membranes and immunopurified Ndh complex. The activation of the Ndh complex by phosphorylation of the NDH-F polypeptide is assessed comparing the changes in Ndh activity, NDH-F protein, and levels of phosphorylated NDH-F. The NDH-F phosphorylation by a putative chloroplast protein kinase(s) regulated by H2O2 and Ca2+ is discussed.
RESULTS
Effects of Relatively Excess Light (PhL) and H2O2 on the Ndh Complex Activity and Protein Levels
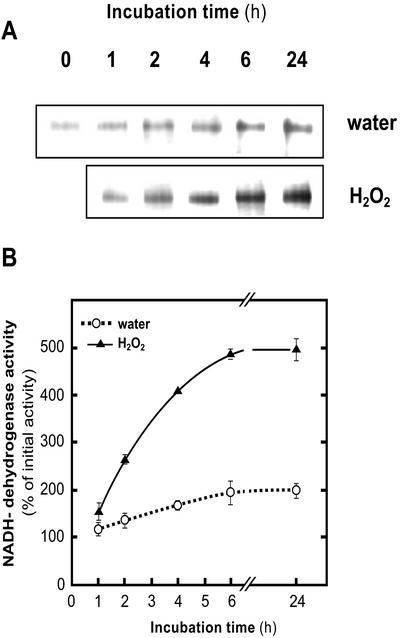
Leaf segments of barley grown under 80 μmol photon m−2 s−1 (growth light [GL]) were incubated in water under 300 μmol photon m−2 s−1 (PhL). In agreement with previous observations (Casano et al., 2001), Ndh activity progressively increased in control segments incubated under PhL (Fig. 1, A and B), reaching the maximum value at 6-h incubations. The addition of H2O2 to the incubation medium further increased the Ndh complex activity up to approximately 300% over the control at 6- and 24-h incubations. H2O2 provoked a slight decrease of chlorophyll content, which did not exceed 20% with respect to controls in GL, after 24-h incubations (data not shown).
Figure 1.
Time course of H2O2 effects under PhL on Ndh activity. A, Typical NADH-DH zymograms of the Ndh complex of crude extracts (60 μg of protein per lane) from 14-d-old barley leaf segments incubated up to 24 h under PhL (300 μmol photon m−2 s−1) and water or H2O2 (10 mm). B, Quantification of Ndh complex activity by image analysis of NADH-DH zymograms as shown in A. Activities were expressed as percentages of the value in freshly detached leaves (0 time). Each value represents the mean ± se of at least four independent experiments.
As recently reviewed (Bowler and Fluhr, 2000), Ca2+ exhibits important signaling roles in the active oxygen-mediated responses to different stresses. Therefore, the implication of H2O2 in the regulation of Ndh complex activity was further studied using dimethylthiourea (DMTU), a H2O2 scavenger (Levine et al., 1994), and the participation of Ca2+ was preliminarily assessed by the addition of EGTA, a Ca2+ chelator. Leaf segments were preincubated in water, DMTU, or EGTA, for 4 h under GL and then incubated in water or H2O2 for 20 h under PhL. Because the photooxidative induction of NADH-DH activity is a complex process including, at least, increases in the steady-state level of the corresponding transcripts and NDH polypeptides (Martín et al., 1996; Catalá et al., 1997; Casano et al., 2001), in the present study, we compared the variations of Ndh complex activity with the changes in the NDH-F polypeptide level, using the latter as an estimation of the total Ndh complex. Values in Table I are expressed as percentages of control (100%) leaves, which were incubated in water for 24 h under GL. As expected, incubation under PhL approximately duplicated Ndh activity (197%), whereas the effect on the amount of NDH-F polypeptide was less intense (162%; Table I). Under a stronger photooxidative condition, as generated by H2O2 treatment, leaf segments showed an even higher Ndh activity (495%), which was accompanied by a lower increase of NDH-F polypeptide (236%). Both DMTU and EGTA inhibited PhL- and H2O2-induced increases of Ndh activity and NDH-F polypeptide to different extents. In leaves preincubated with DMTU, Ndh activity was 30% lower than in those preincubated with water (138% versus 197% and 350% versus 495%, in water and H2O2 incubations, respectively). The impact of DMTU on the NDH-F polypeptide level was more severe, totally inhibiting PhL- and H2O2-induced increases, maintaining the amount of NDH-F within the value of control leaves. In contrast, EGTA had only a marginal effect on the NDH-F level increase produced by PhL (142% versus 162%) but a strong influence on Ndh activity (83% versus 197%). EGTA reduced the H2O2-induced increases of Ndh activity by approximately 65% (236% versus 495%) and of NDH-F polypeptide by approximately 80% (128% versus 236%). In summary, results shown in Table I indicate that H2O2 and Ca2+ are both involved in the regulation of Ndh activity, and that this regulation could not be solely explained in terms of changes in the NDH-F polypeptide level. As indicated by the Ndh activity to NDH-F level ratio (Table I), H2O2 and Ca2+ also seem to modulate the activation degree of the Ndh complex. Therefore, posttranslational modifications of certain NDH polypeptide(s) could be involved in changes of the Ndh complex activation.
Table I.
Changes in Ndh activity, NDH-F polypeptide level, and phosphorylation degree of the NDH-F polypeptide induced by PhL and H2O2 in barley leaves preincubated with or without DMTU or EGTA
| Preincubation under GL | Incubation under PhL (Preincubation-Incubation Combinations) | Ndh Activity | NDH-F % with Respect to Control | Phosphorylation of NDH-F | Ratios
|
|
|---|---|---|---|---|---|---|
| Ndh Activity NDH-F | Phospho-NDH-F NDH-F | |||||
| % with Resepect to Control | ||||||
| Water | Water (a) | 197 (29.5) | 162 (19.4) | 256 (35.4) | 1.2 | 1.6 |
| Water | H2O2 (b) | 495 (64.3) | 236 (40.2) | 513 (36.0) | 2.1 | 2.2 |
| DMTU | Water (c) | 138 (26.5) | 94 (12.6) | 96 (10.3) | 1.4 | 1.1 |
| DMTU | H2O2 (d) | 350 (50.4) | 95 (16.1) | 208 (24.8) | 3.6 | 2.2 |
| EGTA | Water (e) | 83 (21.6) | 142 (14.4) | 89 (12.4) | 0.6 | 0.6 |
| EGTA | H2O2 (f) | 236 (34.2) | 128 (24.3) | 234 (26.7) | 1.8 | 1.8 |
Ndh complex activity was estimated by image analysis from NADH-dehydrogenase zymograms of crude leaf extract (60 μg of protein). The polypeptide level and phosphorylation degree of NDH-F were estimated by image analysis from western blots of thylakoids (30 μg of protein) using NDH-F and phosphoamino acid antibodies, respectively. Segments of 14-d-old barley leaf were preincubated under GL (80 μmol photon m−2 s−1) with water, DMTU (5 mM), or EGTA (5 mM) for 4 h and then incubated under PhL (300 μmol photon m−2 s−1) with or without H2O2 (10 mM) for 20 h. Ndh activity, NDH-F polypeptide level, and phosphorylation of NDH-F were expressed as percentages with respect to control (100%) of leaves incubated for 24 h in water under GL. Each value represents the mean and SE (in parentheses) of at least four independent experiments. Representative examples of NADH-dehydrogenase zymograms and immunoblots of NDH-F polypeptide and phosphorylated NDH-F are shown in the upper part of the table. Letters indicate the different preincubation-incubation combinations.
Phosphorylation of the NDH-F Polypeptide and Regulation of Ndh Activity
It is well-known that abiotic stresses use Ca2+ and Ca2+-dependent changes of protein phosphorylation for signal transduction (Bowler and Fluhr, 2000). Reversible protein phosphorylation is an important regulatory mechanism that modulates the structure and function of proteins in all organisms. Therefore, we investigated the possible phosphorylation of the Ndh complex in vivo, using monoclonal phosphoamino acid antibodies; and whether changes in the level of phosphorylation could account for variations in its enzymatic activity.
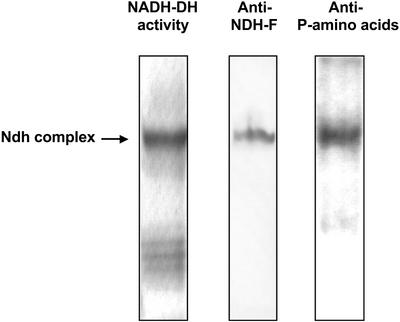
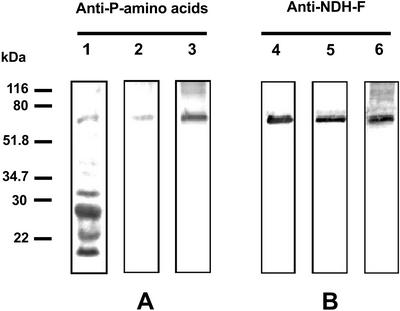
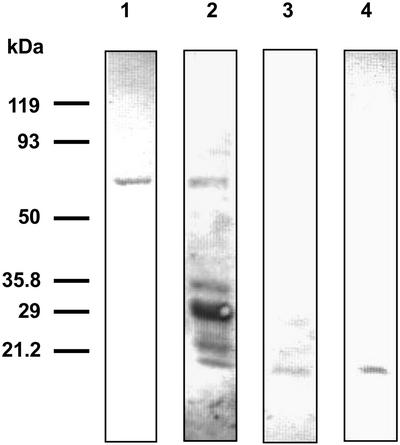
In a first approach, we performed western blots from native gels using NDH-F antibody and a mixture of phosphoamino acid antibodies (anti-phospho-Ser, anti-phospho-Thr, and anti-phospho-Tyr) after developing the NADH-DH activity stain (Fig. 2). Both NDH-F antibody and phosphoamino acid antibodies recognized the NADH-DH activity band of the Ndh complex. The analyses of sequence motifs (Bairoch et al., 1997) revealed several potential phosphorylation sites in various NDH polypeptides. To confirm and further study the phosphorylation of the Ndh complex, we carried out a series of experiments to determine which NDH polypeptide(s) were phosphorylated. Isolated thylakoids solubilized with SDS or Triton X-100 and immunopurified Ndh complex were subjected to SDS-PAGE and western analysis (Fig. 3). The mixture of phosphoamino acid antibodies recognized several polypeptides in SDS-solubilized thylakoids (lane 1) among them, one of 70 kD and the well-known phosphorylated photosystem II polypeptides of approximately 32 and 29 kD (Rintamäki et al., 1997). Only the 70-kD polypeptide was recognized by phosphoamino acid antibodies in Triton X-100-solubilized Ndh complex (lane 2). The conditions of Triton X-100 solubilization employed in these experiments removes proteins from stromal thylakoids only (Morrissey et al., 1986), which is the proposed location of the Ndh complex (Nixon, 2000). The immunopurified Ndh complex also showed an intense 70-kD phosphorylated polypeptide (lane 3). A 70-kD polypeptide corresponding to the NDH-F subunit of the Ndh complex has been described (Catalá et al., 1997). Therefore, the possible phosphorylation of the NDH-F polypeptide was assessed in a parallel set of western blots using anti-NDH-F (Fig. 3B). A single band of the expected molecular mass for NDH-F (approximately 70 kD) was detected in whole thylakoids (lane 4), in Triton X-100-solubilized Ndh complex (lane 5), and in the immunopurified Ndh complex (lane 6). In summary, a phosphorylated band with the same electrophoretic mobility as the NDH-F band was clearly observed in thylakoids and, with a higher intensity, in the immunopurified Ndh complex, indicating that the NDH-F polypeptide may be phosphorylated in vivo.
Figure 2.
Phosphorylation of the Ndh complex. Thylakoids were isolated from 14-d-old primary leaves incubated for 20 h under PhL (300 μmol photon m−2 s−1). After Triton X-100 solubilization, aliquots of 60 μg protein were subjected to native-PAGE, and NADH-DH zymograms were developed. After electroblotting onto polyvinylidene difluoride membranes, Ndh complex was detected with NDH-F antibody, and phosphorylated polypeptides were detected with a mixture of monoclonal phosphoamino acid (anti-phospho-Ser, anti-phospho-Thr, and anti-phospho-Tyr) antibodies.
Figure 3.
Phosphorylation of NDH-F polypeptide. Thylakoids were isolated from 14-d-old primary leaves incubated for 20 h under PhL (300 μmol photon m−2 s−1). Thylakoids solubilized with SDS (30 μg protein; lanes 1 and 4) and solubilized with Triton X-100 (30 μg protein; lanes 2 and 5) and the immunopurified Ndh complex (0.25 μg protein; lanes 3 and 6) were subjected to SDS-PAGE and western blots using the mixture of monoclonal phosphoamino acid antibodies (A) and an NDH-F antibody (B).
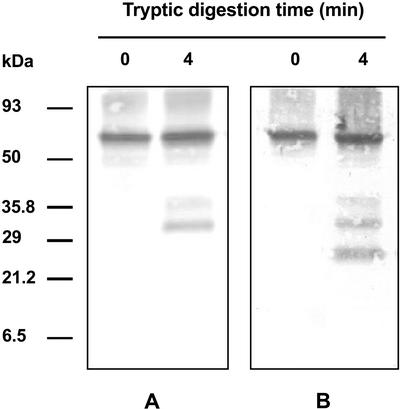
Analysis of the tryptic digestion products of immunopurified Ndh complex confirmed that the 70-kD polypeptide recognized by phosphoamino acid antibodies is the NDH-F subunit of the Ndh complex. As shown in Figure 4, both phosphoamino acids and NDH-F antibodies recognized the 70-kD polypeptide and two tryptic fragments of approximately 39 and 31 kD. One additional polypeptide of approximately 25 kD was recognized by anti-NADH-F (Fig. 4B) but not by anti-phosphoamino acids (Fig. 4A). Obviously, the two larger tryptic peptides include the NDH-F sequence selected to prepare NDH-F antibody and phosphoamino acid site(s) and are derived from digestion of the NDH-F subunit of Ndh complex. Very probably, the shortest 25-kD polypeptide is a fragment that may be derived from the former ones, still retaining the NDH-F epitope sequence but not the phosphoamino acid site(s).
Figure 4.
Tryptic pattern of phosphorylated NDH-F polypeptide. Immunopurified Ndh complex (0.5 μg) was incubated in 100 mm Tris-HCl pH 7.5 and 10 units of trypsin at 20°C. The reaction was stopped by the addition of soybean (Glycine max) trypsin inhibitor (75 ng μg−1 trypsin) in SDS sample buffer and then boiling for 10 min. Samples were subjected to Tricine-SDS-PAGE and western-blot analysis using a mixture of monoclonal phosphoamino acid antibodies (A) and an NDH-F antiserum (B).
The amino acid sequence of the barley NDH-F polypeptide deduced from the barley ndh-F gene (Gaut et al., 1997) contains several potential phosphorylation sites: three Ser residues, three Thr residues, and a Tyr (Bairoch et al., 1997). SDS-PAGE and western analysis of SDS-solubilized thylakoids performed with each anti-phosphoamino acid separately (Fig. 5) show that only the phospho-Thr antibody (lane 2) recognized the 70-kD NDH-F polypeptide (lane 1) among other phosphorylated thylakoid proteins. Therefore, the NDH-F polypeptide must be phosphorylated at a Thr residue(s).
Figure 5.
NDH-F polypeptide of the Ndh complex is phosphorylated at a Thr residue. Barley thylakoids were isolated from 14-d-old primary leaves incubated for 20 h under PhL (300 μmol photon m−2 s−1). After SDS solubilization, aliquots of 30 μg protein were subjected to SDS-PAGE and western-blot analysis using an NDH-F antibody (lane 1), monoclonal phospho-Thr antibody (lane 2), monoclonal phospho-Ser antibody (lane 3), or monoclonal phospho-Tyr antibody (lane 4).
With the aim to establish the effect of NDH-F phosphorylation on the Ndh complex activity, we compared the Ndh activity and the amount of NDH-F polypeptide with the NDH-F phosphorylation level in leaf segments preincubated under GL with water, DMTU, or EGTA and then incubated under PhL with water or H2O2. Table I shows that NDH-F phosphorylation and Ndh activity increased to a higher degree than the NDH-F polypeptide level after PhL treatment (water incubation) and more strongly after H2O2 incubation. All increases were inhibited (although at different extents) by DMTU and EGTA. In all treatments that increased or decreased NDH-F phosphorylation, similar increases or decreases of the Ndh complex activity were observed, except in DMTU- and H2O2-treated leaves in which Ndh activity increased more than NDH-F phosphorylation. DMTU probably did not completely eliminate H2O2 in these leaves, and residual H2O2 might exert additional effect(s) on Ndh complex activity not mediated by NDH-F phosphorylation. In general, changes in the phosphorylation of NDH-F to NDH-F level ratio resembled the variations of the Ndh activity to NDH-F level ratio (Table I), suggesting a correlation between NDH-F phosphorylation and the activation of Ndh complex.
DISCUSSION
Active oxygen generation is a common phenomenon in all aerobic organisms and is enhanced under most stress conditions (Asada, 1999). Increasing evidence indicates that active oxygen species (especially H2O2), the intracellular redox state, and Ca2+ exhibit important signaling functions in the responses to different stress conditions (Foyer and Noctor, 1999; Karpinski et al., 1999; Bowler and Fluhr, 2000; Mullineaux and Karpinski, 2002). H2O2 induces the nuclear-encoded gene expression of antioxidant enzymes such as catalase, glutathione S-transferase and cytosolic ascorbate peroxidase (Karpinski et al., 1999; Morita et al., 1999; Polidoros and Scandalios, 1999). In a previous paper, it was demonstrated that H2O2 under GL can mimic the inductive photooxidative effect generated by PhL or PhL plus paraquat (Casano et al., 2001). In the present paper, we demonstrate the inductive effect of H2O2 on the Ndh complex activity even under PhL. PhL and above all H2O2 treatments induced stronger increases in Ndh complex activity than in NDH-F polypeptide levels. Thus DMTU, a H2O2 scavenger, strongly inhibited the increase of Ndh activity induced by PhL and partially inhibited the inductive effect of H2O2 (Table I). DMTU had a more marked effect decreasing the NDH-F polypeptide level in all treatments (Table I). These results indicate that H2O2 could act as a second messenger regulating, to different extents, both Ndh complex activity and NDH-F polypeptide levels. In addition, we found that Ca2+ participates in the Ndh complex activity regulating signal pathways driven by H2O2. Our results show that EGTA strongly inhibited Ndh activity increases generated by PhL and H2O2. However, the effect of EGTA on the NDH-F polypeptide levels was lower than on Ndh activity, suggesting that Ca2+ is mainly involved in a posttranslational regulating mechanism(s).
Protein phosphorylation is a common posttranslational modification that plays a major role mediating the intracellular responses to different stimuli. In the present work, we unambiguously demonstrated that the NDH-F polypeptide of the Ndh complex is phosphorylated at Thr residue(s) (Figs. 2–5). Thr residues 181, 468, and 496 are potential phosphorylation sites in barley NDH-F (Bairoch et al., 1997). Sequence comparison of the NDH-F polypeptide from different species indicates that Thr-181 is conserved from Synechocystis sp. to higher plants within a highly conserved sequence (Gln-Lys-Ala-Phe-Val-Thr-Aspn-Arg-Val-Gly). None of the other potential phosphorylation sites in NDH-F show such high sequence conservation, suggesting that Thr 181 could be the phosphorylation site in the NDH-F polypeptide.
The good correlation between the degree of NDH-F phosphorylation and Ndh activity (Table I) indicates that the Ndh complex could be activated by phosphorylation of the NDH-F subunit. This process seems to be stimulated by H2O2 in a Ca2+-mediated pathway. Accumulating evidence indicates that a wide range of environmental stimuli can modify intracellular Ca2+ concentrations (Reddy, 2001) and modulate the generation rate of active oxygen species (Bowler and Fluhr, 2000). H2O2 induces changes in the intracellular Ca2+ levels (Price et al., 1994; Pei et al., 2000), indicating that both active oxygen species and Ca2+ are functionally related. In plants, there are many types of Ca2+-regulated kinases that have been implicated in a variety of stress responses (Sheen, 1996; Hardie, 1999; Reddy, 2001). In addition, the redox state of the plastoquinone pool and H2O2-generation are the main signal sources in chloroplasts under photooxidative stress (Karpinski et al., 1999; Foyer and Noctor, 1999). It is well-known that the redox state of plastoquinone pool controls the thylakoid-associated kinases that phosphorylate photosystem II and other thylakoid proteins (Bennett, 1991; Allen, 1992; Rintamäki et al., 1997; Verner et al., 1997; Snyders and Kohorn, 1999, 2001). At present, there is no data regarding the role of H2O2 in the regulation of thylakoid kinases. However, the activation of MAP kinases by H2O2 has been clearly demonstrated (Desikan et al., 1999; Kovtun et al., 2000). Moreover, it has been shown that a soluble thiol-dependent mechanism can modulate the chloroplastic kinase activities (Rintamäki et al., 2000; Baena-González et al., 2001). Because H2O2 can oxidize protein thiol groups, it may be possible that thylakoid kinase activities can be directly modulated by H2O2.
The regulation of Ndh complex activity by NDH-F phosphorylation opens new perspectives on the functional role of this complex within the context of chlororespiration. Rapid changes of Ndh activity, as can be expected by reversible phosphorylation, may have influence on the dynamic levels of the redox state of plastoquinone and H2O2, two important elements implicated in the perception of photooxidative conditions and in modulation of the adaptative responses. As far as we know, this is the first report of a chloroplast protein phosphorylation regulated by H2O2 and Ca2+.
MATERIALS AND METHODS
Plant Material
Barley (Hordeum vulgare cv Hassan) was grown on vermiculite under controlled conditions at 23°C ± 1°C and a 16-h photoperiod of 80 μmol photon m−2 s−1 white light. Subapical segments (3 cm in length) of the primary leaf of 14-d-old plants were used for the different treatments.
Leaf segments were incubated at 23°C ± 1°C at different times up to 24 h with 0 or 10 mm H2O2, under relatively excess light (PhL, 300 μmol photon m−2 s−1). In other experiments, leaf segments were preincubated with 5 mm DMTU and 5 mm EGTA under GL (80 μmol photon m−2 s−1) and then treated as described above.
Leaf Crude Extracts and Thylakoid Isolation
Whole-leaf extracts were obtained by homogenization of 10 leaf segments with liquid nitrogen in a mortar with 2 mL of 50 mm potassium phosphate, pH 7.0, 1 mm l-ascorbic acid, 1 mm EDTA, 1% (w/v) polyvinylpirrolidone, and 2% (w/v) Triton X-100. The suspensions were gently stirred for 30 min and then centrifuged at 20,000g for 30 min. Thylakoid isolation was carried out as described by Casano et al. (2000). Unless otherwise stated, thylakoid solubilization was achieved with Triton X-100 using a chlorophyll to detergent ratio of 1:20 (w/w) that mainly solubilized the thylakoid lamellae and the Ndh complex (Morrisey et al., 1986; Casano et al., 2000). After being gently stirred for 30 min, unsolubilized membranes were separated by centrifugation at 20,000g for 30 min. All steps were carried out at 4°C. The supernatants of both whole extracts and thylakoids were used for zymograms and western-blot assays.
Immunoaffinity Matrix Preparation and Immunopurification of Ndh Complex
NDH-F antibody was produced by Sigma Genosys Co. (Cambridge, UK) using a synthetic peptide as antigen, which was established by the protein sequence analysis of barley NDH-F polypeptide (Gaut et al., 1997). The amino acid sequence of the antigen peptide is WSKDEILSNSWLYS and corresponds to amino acids 414 to 427 of the NDH-F protein. Before injection of rabbits, the antigen peptide was coupled to the carrier protein keyhole limpet hemocyanin.
NDH-F or preimmune antisera were bound to a Protein A-Sepharose CL-4B matrix (Sigma-Aldrich, St. Louis), cross-linked with dimethyl pimelimidate (Schneider et al., 1982), and then equilibrated with 50 mm Tris-HCl, pH 8.3, 150 mm NaCl, 1 mm EDTA, and 0.5% (w/v) Triton X-100. Triton X-100 solubilized thylakoid samples were incubated in batch with the preimmune matrix at 4°C for 1 h with gentle agitation. The supernatants were then incubated with the NDH-F immunoaffinity matrix under the same conditions. The immunoaffinity matrix was pelleted by a short pulse in microfuge, and washed twice with 10 volumes of 50 mm Tris-HCl, pH 8.3, 150 mm NaCl, 1 mm EDTA, and 0.5% (w/v) Triton X-100. The Ndh complex was eluted with 50 mm diethylamine, pH 11.5, containing 0.5% (w/v) Triton X-100. The eluted samples were immediately neutralized with 1 m NaH2PO4.
Gel Electrophoresis, Zymograms, and Immunoassays
Native PAGE was carried out at 5°C in a linear gradient gel of 3% to 10% (w/v) polyacrylamide (2.5% [w/v] bis-acrylamide) containing 0.1% (w/v) Triton X-100 (Casano et al., 1999). NDH-DH zymograms were developed by incubating the gel for 20 to 30 min at 30°C in darkness with 50 mm potassium phosphate pH 8.0, 1 mm EDTA, 0.2 mm NADH, and 0.5 mg mL−1 nitroblue tetrazolium. In the control without NADH, no stain developed. The activity band corresponding to the Ndh complex was identified by immunoblotting.
For immunoblot analyses, samples were subjected to native PAGE, SDS-PAGE, or Tricine-SDS-PAGE (Schägger and Von Jagow, 1987) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA).
NDH-F polypeptide levels, or its derived tryptic peptides, were estimated using the NDH-F antibody described above. Phosphorylated polypeptides were immunodetected with mouse monoclonal phosphoamino acid (anti-phospho-Ser, anti-phospho-Thr, and anti-phospho-Tyr) antibodies (Sigma-Aldrich). The different immunocomplexes were detected with the alkaline phosphatase western-blot analysis system (Roche Diagnostics, Mannheim, Germany).
In quantitative experiments (Table I), zymograms and immunoblots were performed with 60 or 30 μg of protein per treatment, respectively. These protein amounts are within the linear dose to response range for NADH-DH activity and for immunodetected NDH-F polypeptide and phosphorylated NDH-F. Each zymogram and immunoblot was scanned and quantified with a UVP Easy Digital Image analyzer five to seven times, and measurements with more than 30% deviation were discarded.
Trypsin Treatment of Immunopurified Ndh Complex
Immunopurified Ndh complex (0.5 μg) was incubated in 100 mm Tris-HCl, pH 7.5, and 10 units of trypsin for 4 min at 20°C. Reactions were stopped by the addition of soybean (Glycine max) trypsin inhibitor (75 ng μg−1 trypsin) in SDS sample buffer and then boiling for 10 min. Samples were subjected to Tricine-SDS-PAGE and western-blot analysis, as described above.
Other Determinations
Total protein levels were determined by the Bradford method (Bradford, 1976) using bovine serum albumin as standard. Chlorophyll content was measured as described by Arnon (1949). All experiments were repeated at least four times.
ACKNOWLEDGMENT
We thank Patricia Hauke for reading and editing the manuscript.
Footnotes
This work was supported by the Spanish Dirección General de Investigación Científica y Técnica (grant no. BFI2000–0781). H.R.L. has a Postdoctoral Fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas (Argentina).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.020321.
LITERATURE CITED
- Allen JF. Protein phosphorylation in regulation of photosynthesis. Biochem Biophys Acta. 1992;1098:275–335. doi: 10.1016/s0005-2728(09)91014-3. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Baginski S, Mulo P, Summer H, Aro E-M, Link G. Chloroplast transcription at different light intensities. Glutathione-mediated phosphorylation of the major RNA polymerase involved in redox-regulated organellar gene expression. Plant Physiol. 2001;127:1044–1052. doi: 10.1104/pp.010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1997. Nucleic Acid Res. 1997;25:217–221. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. Protein phosphorylation in green plant chloroplasts. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:281–311. [Google Scholar]
- Bowler C, Fluhr R. The role of calcium and activated oxygens as signals for controlling cross-tolerance. Trends Plant Sci. 2000;5:241–246. doi: 10.1016/s1360-1385(00)01628-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casano LM, Martín M, Sabater B. Hydrogen peroxide mediates the induction of chloroplastic Ndh complex under photooxidative stress in barley. Plant Physiol. 2001;125:1450–1458. doi: 10.1104/pp.125.3.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casano LM, Martín M, Zapata JM, Sabater B. Leaf age- and paraquat-dependent effects on the levels of enzymes protecting against photooxidative stress. Plant Sci. 1999;149:13–22. [Google Scholar]
- Casano LM, Zapata JM, Martín M, Sabater B. Chlororespiration and poising of cyclic electron transport: plastoquinone as electron transporter between thylakoid NADH dehydrogenase and peroxidase. J Biol Chem. 2000;275:942–948. doi: 10.1074/jbc.275.2.942. [DOI] [PubMed] [Google Scholar]
- Catalá R, Sabater B, Guéra A. Expression of the plastid ndhF gene product in photosynthetic and non-photosynthetic tissues of developing barley seedlings. Plant Cell Physiol. 1997;38:1382–1388. doi: 10.1093/oxfordjournals.pcp.a029133. [DOI] [PubMed] [Google Scholar]
- Desikan R, Clarke A, Hancock JT, Neil ST. H2O2 activates a MAP kinase-like enzyme in Arabidopsis thaliana suspension culture. J Exp Bot. 1999;341:1863–1866. [Google Scholar]
- Endo T, Shikanai T, Takabayashi A, Asada K, Sato F. The role of chloroplastic NAD(P) H dehydrogenase in photoprotection. FEBS Lett. 1999;457:5–8. doi: 10.1016/s0014-5793(99)00989-8. [DOI] [PubMed] [Google Scholar]
- Fath A, Bethke P, Jones RL. Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol. 2001;126:156–166. doi: 10.1104/pp.126.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Leaves in the dark see the light. Science. 1999;284:599. doi: 10.1126/science.284.5414.599. [DOI] [PubMed] [Google Scholar]
- Gaut BS, Clark LG, Wendell JF, Muse SV. Comparisons of the molecular evolutionary process at rbcL and ndh-F in grass family (Poacea) Mol Biol Evol. 1997;14:769–777. doi: 10.1093/oxfordjournals.molbev.a025817. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Plant protein serine/threonine kinases: classification and functions. Annu Rev Plant Physiol Mol Biol. 1999;50:97–131. doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- Horvath EM, Peter SO, Joet T, Rumeau D, Cournac L, Horvath GV, Kavanagh TA, Schafer C, Peltier G, Medgyesy P. Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol. 2000;123:1337–1349. doi: 10.1104/pp.123.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux PM. Systemic signalling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- Kovtun Y, Chiu W-L, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon RA, Lamb CJ. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Maier RM, Neckermann K, Igloi GL, Kössel H. Complete sequence of the maize chloroplast genome: gene content, hotspots of divergence and fine tuning of genetic information by transcript editing. J Mol Biol. 1995;251:614–628. doi: 10.1006/jmbi.1995.0460. [DOI] [PubMed] [Google Scholar]
- Martín M, Casano LM, Sabater B. Identification of the product of ndhA gene as a thylakoid protein synthesized in response to photooxidative treatment. Plant Cell Physiol. 1996;37:293–298. doi: 10.1093/oxfordjournals.pcp.a028945. [DOI] [PubMed] [Google Scholar]
- Morita S, Kaminaka H, Masumura T, Tanaka K. Induction of rice cytosolic ascorbate peroxidase mRNA by oxidative stress: the involvement of hydrogen peroxide in oxidative stress signalling. Plant Cell Physiol. 1999;40:417–422. [Google Scholar]
- Morrisey PJ, McCauley SW, Melis A. Differential detergent-solubilization of integral thylakoid membrane complexes in spinach chloroplasts: localization of photosystem II, cytochrome b6/f complex and photosystem I. Eur J Biochem. 1986;160:389–393. doi: 10.1111/j.1432-1033.1986.tb09983.x. [DOI] [PubMed] [Google Scholar]
- Mullineaux P, Karpinski S. Signal transduction in response to excess light: getting out of the chloroplast. Curr Opin Plant Biol. 2002;5:43–48. doi: 10.1016/s1369-5266(01)00226-6. [DOI] [PubMed] [Google Scholar]
- Nixon P. Chlororespiration. Philos Trans R Soc Lond B. 2000;355:1541–1547. doi: 10.1098/rstb.2000.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vazquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Pei Z, Murata N, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cell. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Polidoros AN, Scandalios JG. Role of hydrogen peroxide and different classes of antioxidants in the regulation of catalase and glutathione S-transferase gene expression in maize (Zea mays L.) Physiol Plant. 1999;106:112–120. [Google Scholar]
- Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR. Oxidative signals in tobacco increase cytosolic calcium. Plant Cell. 1994;6:1301–1310. doi: 10.1105/tpc.6.9.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ASN. Calcium: silver bullet in signalling. Plant Sci. 2001;160:381–404. doi: 10.1016/s0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- Rintamäki E, Martinsuo P, Pursiheimo S, Aro E-M. Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.180054297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E, Salonen M, Suoranta U-M, Calberg I, Anderson B, Aro E-M. Phosphorylation of light-harvesting complex II and photosystem II core protein shows different irradiance-dependent regulation in vivo. J Biol Chem. 1997;272:30476–30482. doi: 10.1074/jbc.272.48.30476. [DOI] [PubMed] [Google Scholar]
- Sazanov L, Burrows PA, Nixon PJ. The plastid ndh genes code for a NADH-specific dehydrogenase: purification and characterization of a mitochondrial-like complex I from pea thylakoid membranes. Proc Natl Acad Sci USA. 1998;95:1319–1324. doi: 10.1073/pnas.95.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schneider C, Roland A, Newman D, Sutherland DR, Asser U, Greaves MF. A one-step purification of membrane protein using a high efficiency immunomatrix. J Biol Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
- Sheen J. Ca2+-dependent protein kinases and stress signal transduction in plants. Science. 1996;274:1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Snyders S, Kohorn BD. TAKs, thylakoid membrane protein kinases associated with energy transduction. J Biol Chem. 1999;274:9137–9140. doi: 10.1074/jbc.274.14.9137. [DOI] [PubMed] [Google Scholar]
- Snyders S, Kohorn BD. Disruption of thylakoid-associated kinase 1 leads to alteration of light harvesting in Arabidopsis. J Biol Chem. 2001;274:32169–32176. doi: 10.1074/jbc.M102539200. [DOI] [PubMed] [Google Scholar]
- Teicher HB, Moller BL, Scheller HV. Photoinhibition of photosystem I in field-grown barley (Hordeum vulgare L.): induction, recovery and acclimation. Photosynth Res. 2000;64:53–61. doi: 10.1023/A:1026524302191. [DOI] [PubMed] [Google Scholar]
- Verner AV, van Kan PJM, Rich PR, Ohad I, Anderson B. Plastoquinol at the quinol oxidation site of reduced cytochrome b/f mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single-turnover flash. Proc Natl Acad Sci USA. 1997;94:1585–1590. doi: 10.1073/pnas.94.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata JM, Sabater B, Martín M. Identification of a thylakoid peroxidase which oxydized hydroquinone. Phytochemistry. 1998;48:1119–1123. [Google Scholar]