Abstract
It has been proposed that fw2.2 encodes a negative fruit-growth regulator that underlies natural fruit-size variation in tomato (Lycopersicon spp.) via heterochronic allelic variation of fw2.2 expression, rather than by variation in the structural protein itself. To further test the negative regulator and the transcriptional control hypotheses, a gene dosage series was constructed, which produced a wider range of fw2.2 transcript accumulation than can be found in natural tomato populations. Fruit developmental analyses revealed that fw2.2 transcript levels were highly correlated (negatively) with fruit mass, supporting the negative regulator and transcriptional regulation hypotheses. Further, the effect of fw2.2 on fruit mass was mediated by repressing three- and two-dimensional cell division in placental and pericarp tissues, respectively. Finally, fw2.2 had little effect on fertility and seed size/number, indicating that fruit size effects of fw2.2 are due largely to expression in the maternal tissues of developing fruit and not mediated through fertility or seed-setting-related processes.
Crop domestication began in several regions around the world about 7,000 to 10,000 years ago—a very recent event in the entire evolutionary history of plants (White and Doebley, 1998). In the short span of crop domestication, genetic changes followed by repeated cycles of human selection have fundamentally altered the morphology, physiology, and overall environmental adaptations of a handful of wild species, leading to the formation of modern crops (Diamond, 2002). However, when and how these events took place remains unclear.
Genetic studies have demonstrated that most traits that distinguish modern crops from their related wild species are due to quantitative trait loci (QTLs) with distinct effects (White and Doebley, 1998; Grandillo et al., 1999; Mackay, 2001; Barton and Keightley, 2002). With the advent of molecular markers in combination with statistical methodology, dissecting the genetic and molecular bases of these traits is no longer an impossible mission. During the last decade, we have witnessed the cloning of several major QTLs related to crop domestication in maize (Zea mays; Doebley et al., 1997), tomatoes (Lycopersicon esculentem; Frary et al., 2000; Fridman et al., 2000; Liu et al., 2002), and rice (Oryza sativa; Yano et al., 2000).
A key morphological change that has accompanied the domestication of many fruit and vegetable crops has been the dramatic expansion of fruit and explosion of shape variation. Tomato is a classic example. The wild forms of tomato bear small (approximately 1–2 g), round, seed dense berries—ideal for reproduction and dispersal. In contrast, cultivated tomatoes typically produce fruit that weigh anywhere from 50 to 1,000 g, come in a wide variety of shapes (e.g. round, oblate, pear-shaped, torpedo-shaped), and are not well adapted for seed dispersal in the wild. Genetic studies involving crosses of wild and cultivated tomatoes have shown that most of the variation in size and shape can be attributed to fewer than 30 QTLs, with a smaller subset of these accounting for a disproportionate amount of variation (Grandillo et al., 1999).
One of the major QTLs involved in tomato domestication, fw2.2, accounts for approximately 30% of the variance in fruit weight in many segregating populations and is attributable to a gene encoding a 22-kD protein (Frary et al., 2000; Nesbitt and Tanksley, 2001). Comparative sequencing of fw2.2 alleles suggests that the modulation of fruit size attributable to fw2.2 is due to 5′-regulatory variation among the alleles rather than to differences in the structural protein (Frary et al., 2000; Nesbitt and Tanksley, 2002). Detailed studies on the temporal expression profiles of fw2.2 alleles demonstrated that heterochronic expression of fw2.2 accounts for fruit mass variation between wild and domesticated tomato species. In addition, the levels and the timing of fw2.2 expression are concomitant with the activities of cell division in tomato fruit tissues, i.e. a higher transcript level is associated with a less active state of cell division (Cong et al., 2002). These results further support the speculation that the fw2.2 protein functions as a negative regulator of cell proliferation (Frary et al., 2000).
To test both the negative regulator and transcriptional control hypotheses, we have constructed a series of transgenic plants containing zero, one, two, three, or four copies of the small-fruit alleles of fw2.2 driven by their native promoters. By constructing this gene dosage series, we were able to create a set of lines with a wider range of steady-state transcript levels of fw2.2 than can be found in natural genetic stocks. These lines were characterized for associated changes in fruit development, fruit anatomy, cell proliferation, fertility, and other reproductive parameters. These results provided strong evidence for both the negative regulator and transcriptional control hypotheses and revealed that fw2.2 exerts its effects primarily on two- or three-dimensional growth of the pericarp and inner placental tissues, respectively, with little or no effect on seed development, seed size, or overall fertility. Therefore, fw2.2 negatively controls fruit growth in a tissue-specific manner.
RESULTS
Steady-State Levels of fw2.2 Transcripts Are Positively Correlated with the Number of fw2.2 Alleles in a Gene Dosage Series
The small-fruit allele of fw2.2 has been shown to be associated with overall higher total levels of fw2.2 transcripts in developing fruit (Frary et al., 2000; Cong et al., 2002). To maximize the range of steady-state transcript levels, a gene dosage series was created with zero, one, two, three, or four copies of the small-fruit alleles (Table I). To assure proper spatial/temporal expression, all copies were driven by their native promoters. This gene dosage series was then subjected to real-time PCR analyses (Bustin, 2000) to accurately quantify the fw2.2 transcript levels in 9-d-after-pollination (DAP) fruit. At 9 DAP, fruit tissues undergo active cell division and cell enlargement (Gillapsy et al., 1993; Joubès et al., 1999) and fw2.2 transcripts accumulate at a higher level (Cong et al., 2002). In addition, the levels of fw2.2 transcripts are inversely associated with the amount of cell division in developing fruit (Cong et al., 2002). Therefore, the possible relationship between fw2.2 gene dosage and its transcript levels would most likely be clearly manifested at this stage.
Table I.
Genotypes of the F2 segregants
| No. of the fw2.2 small-fruit alleles | 4 | 3 | 2 | 1 | 0 |
| Genotype (Endo_Trans) (n) | PP_PP (6) | PP_P (5) | PP_ (5) | PE_ (5) | EE_ (3) |
| PE_PP (4) | PE_P (8) | EE_P (8) | |||
| EE_PP (2) |
P, fw2.2 small-fruit allele of L. pennellii; E, fw2.2 large-fruit allele of L. esculentum; Endo, endogenous allele; Trans, transgenic allele; n, no. of F2 plants.
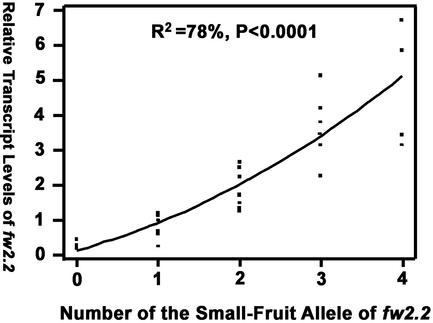
Our results indicated that fw2.2 transcript levels increased in a near-linear manner as the number of small-fruit alleles increased (Fig. 1). The experiment was successful in the sense that we were able to produce a set of genotypes with a 7-fold range in steady-state transcript levels –a prerequisite for assessing the effects of fw2.2 transcription on various aspects of fruit growth, anatomy, seed size, and fertility.
Figure 1.
Relationship between fw2.2 small-fruit alleles and steady-state transcript levels of fw2.2. Total RNAs from three 9-DAP fruit of each plant were individually extracted. Subsequently, fw2.2 transcript levels of each sample were determined by real-time PCR and normalized by endogenous18S rRNA levels. Each plant was genotyped for its endogenous and transgenically introduced fw2.2 loci. Data presented are the averages (three repeats) of the relative transcript levels of fw2.2 from each line. The line represents a quadratic linear regression model.
Increased Levels of fw2.2 Transcripts Are Associated with Reduced Fruit Growth in Tomato without Affecting Fruit Shape
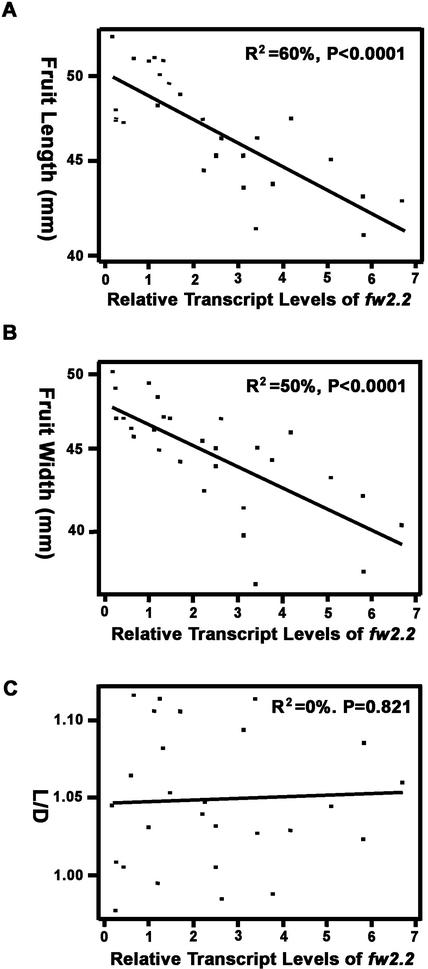
Analyses of the gene dosage series indicated that the mass of mature fruit was highly correlated (negatively) with fw2.2 transcript levels in immature, 9-DAP fruit (r = −0.77, P < 0.0001). Sixty-two percent of the variation in final fruit mass could be attributed to fw2.2 transcription (Fig. 2). Increased accumulation of fw2.2 transcripts was also negatively correlated with both fruit length and width (r = −0.78, P < 0.0001; r = −0.73; P < 0.0001, respectively; Fig. 3, A and B). However, the overall fruit shape (length/diameter) was not significantly affected (r = 0.04; P = 0.82; Figs. 3C and 4A), indicating that fw2.2 largely affects fruit growth in all dimensions.
Figure 2.
Relationship between fw2.2 steady-state transcript levels and mature-fruit weight. Data represented are the average mass of the three largest mature fruit from each plant plotted against its relative levels of fw2.2 transcript levels in 9-DAP fruit. Note that the y axis is in log scale.
Figure 3.
Relationship between fw2.2 steady-state transcript levels and the size/shape of mature fruit. A, Fruit length (L). B, Fruit diameter (D). C, Fruit shape index, length/diameter (L/D). Note that the y axis in A and B is in log scale.
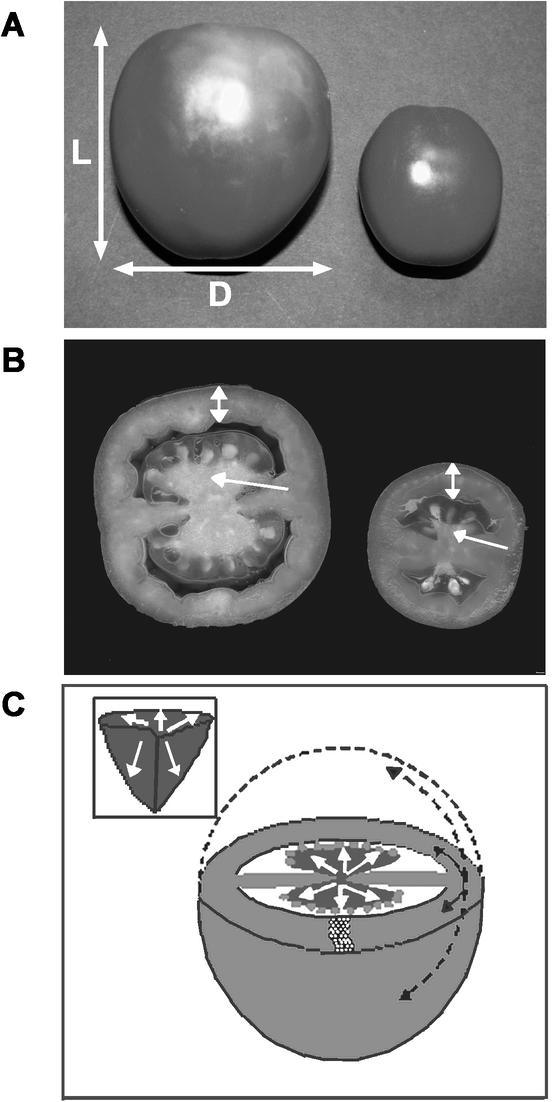
Figure 4.
Typical phenotypes of mature fruit. A, Fruit from a plant with lower (left) or higher (right) levels of fw2.2 transcripts in immature (9-DAP) fruit. B, Median transverse sections of the corresponding fruit described in A. Notice a significant difference in the size of the triangle-like placental tissue (pointed by the single arrow), but not in the thickness of pericarp (delimited by the double arrow) between both fruit. Moreover, both fruit apparently had similar seed size and normal seed setting. C, Schematic model of a transversely sectioned mature tomato fruit showing repression of fruit-tissue growth/cell division by fw2.2 in a two (double arrow)- or three (single arrow)-dimensional manner in the pericarp or placental tissues (also see the inset), respectively. Inset, Diagram of the fruit placental tissue. Arrows point the directions of tissue growth/cell division suppressed by fw2.2. White circles illustrate cell layers of the pericarp tissue in a non-proportional manner.
Fruit Growth Suppression by fw2.2 Is Not Mediated by Fertilization and Seed Set
Fertilization and subsequent seed development stimulate the formation and release of plant growth hormones, such as gibberellins and auxins, which are essential for full development of ovaries into fruit (Sastry and Muir, 1963; Nitsch, 1970; Varoquaux et al., 2000). Without applications of exogenous plant growth hormones, poor seed setting has been shown to be associated with under-developed and small tomato fruit (Varoquaux et al., 2000). To test whether fruit growth reduction associated with fw2.2 is mediated by fertilization and seed set, average seed number and seed weight were evaluated for all plants. Neither trait showed a significant correlation with transcript levels of fw2.2 (seed number versus fw2.2 transcript levels, r = −0.25, P = 0.192; seed weight versus fw2.2 transcript levels, r = −0.06, P = 0.777). These results suggested that the effects of fw2.2 on fruit weight are not related to fertility or seed development and that fw2.2 has an effect restricted to maternal tissues of fruit.
fw2.2 Differentially Affects Growth Patterns of Placental and Pericarp Tissues
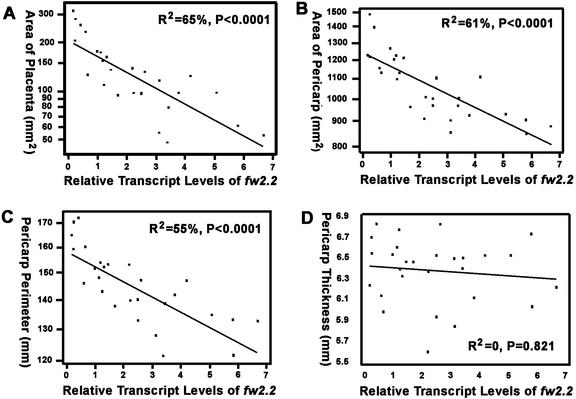
Although evidence presented in previous sections indicated that fw2.2 transcript levels in immature fruit are associated with ultimate fruit size, the question remains as to what specific tissues of the developing fruit are affected by fw2.2. To address this question, mature fruit from each of the gene dosage lines were transversely sectioned and imaged (Fig. 4B), and the areas of the placental and pericarp tissues were measured and plotted against fw2.2 transcript levels. Our results indicated that the growth (size) of both tissues was highly (and negatively) correlated with fw2.2 transcript levels in developing fruit (r = −0.76, P < 0.0001 and r = −0.77, P < 0.0001, respectively; Fig. 5, A and B).
Figure 5.
Relationship between fw2.2 steady-state transcript levels and the tissue size of mature fruit. A, Areas of placental tissues. B, Areas of pericarp tissues. C, Perimeter of pericarp tissues. D, Thickness of pericarp tissues. Note that the y axis in A through C is in log scale.
However, a closer examination revealed differential growth patterns in placental and pericarp tissues. Despite the changes in placental size, the shape index (the ratio of height to width) of the triangle-like tissues (Fig. 4, B and C) in both transverse and longitudinal sections was not correlated with fw2.2 transcript levels (r = 0.18, P = 0.423; r = 0.13, P = 0.432, respectively). These results suggested that fw2.2 inhibits placental tissue growth in a three-dimensional manner.
In contrast, the shape of pericarp tissues was not proportionally changed with respect to the fw2.2 transcript levels. Although the pericarp perimeter of mature fruit was highly (and negatively) correlated with fw2.2 transcript levels in immature fruit (r = −0.75, P < 0.0001; Fig. 5C), the thickness was not (r = −0.12, P = 0.53; Figs. 4B and 5D), leading to a disproportional change in pericarp tissues. These results, in combination with the fact that fruit length is associated with the levels of fw2.2 transcripts (Figs. 3A and 4A), indicated that fw2.2 controls two-dimensional growth in pericarp tissues (Fig. 4C).
fw2.2 Influences Two- and Three-Dimensional Cell Division Patterns in Pericarp and Placental Tissues, Respectively
Fruit growth is a consequence of defined cell division and cell enlargement (Gillapsy et al., 1993). To gain insights into the mechanisms by which fw2.2 controls two- or three-dimensional growth in maternal tissues, comparisons were made for cell size and/or cell number of different developing fruit tissues between plant stocks with zero and two copies of fw2.2 small-fruit alleles, which represents lower (0.0–0.5) and higher (1.1–3.0) relative accumulation of fw2.2 transcripts in 9-DAP fruit, respectively (Fig. 1).
Developmental studies have revealed that throughout fruit developmental stages, cell size in both placental and pericarp tissues was not significantly different between tomato stocks with zero and two copies of the fw2.2 small-fruit alleles (Cong et al., 2002). This result suggests that it is the amount of cell division, rather than the extent of cell enlargement, that causes fw2.2-induced fruit size variation (Cong et al., 2002).
On the basis of above observations, the variation in fruit placental size, which is associated with the level of fw2.2 transcripts (Figs. 4B and 5A), can be attributed to different amounts of cell division. Because placental shape is independent of the action of fw2.2 transcript levels (see the previous sections), our results suggested that fw2.2 controls cell division in placental tissues in a three-dimensional manner. Because the placental size was negatively correlated with the levels of fw2.2 transcripts in developing fruit (Fig. 5A), the accumulation of fw2.2 transcripts might be a limiting factor for the suppression of cell division in fruit placental tissues.
The growth pattern of fruit pericarp tissues affected by fw2.2 was quite different from that observed in placental tissues. Although both fruit (pericarp) length and pericarp perimeter were negatively correlated with the levels of fw2.2 transcripts, the thickness of pericarp was not (Figs. 3A and 5C). Microscopic examinations did not revealed significant differences in the number of pericarp cell layers between fruit with the lower and higher accumulation of fw2.2 transcripts in developing fruit (Table II). Between 0 and 6 DAP, the number of pericarp cell layers doubled, indicating that pericarp cells underwent active periclinal cell division at this stage. However, after 6 DAP, the periclinal cell division entered an inert phase as the number of pericarp cell layers increased slightly until 12 DAP and remained unchanged after (Table II). The patterns of periclinal cell division were comparable between the two groups. So, we concluded that the periclinal cell division in pericarp tissues is not subjected to the influence of fw2.2 transcript levels. Because pericarp cell size is independent of fw2.2 (Cong et al., 2002), fw2.2-associated decreases in length and perimeter of pericarp tissues (Figs. 3A and 5C) can be explained by fw2.2-induced reductions in the amounts of both transversely and longitudinally anticlinal cell division. Taken together, fw2.2 affects cell division in fruit pericarp tissues in a two-dimensional manner.
Table II.
Comparison of cell layers of pericarps between plants with higher or lower fw2.2 transcript levels in immature fruit
DISCUSSION
fw2.2 Controls Fruit Weight through Variation in Transcript Levels
There is significant evidence that changes in transcriptional regulation have fueled much of the morphological variation observed in nature (Doebley and Lukens, 1998; Purugganan, 1998; Carroll, 2000; Cronk, 2001). At least three mechanisms can lead to transcriptional variation in nature. These include variation in (a) the expression of transcriptional factors that regulate the target components (Doebley and Lukens, 1998; Wang et al., 1999), (b) the protein sequences of transcription factors, which can affect the specificity of the DNA binding or the interactions of the transcription factors with other regulators (Galant and Carroll, 2002; Ronshaugen et al., 2002), and (c) the cis-regulatory sequences that control the patterns of gene expression (Weatherbee et al., 1999). Modulation of fruit size by the fw2.2 QTL apparently belongs to this latter category for the reasons described below.
Transgenic experiments clearly verified that the fruit size variation caused by the fw2.2 QTL is due to allelic variation on fw2.2 locus rather than variation in other components such as transcription factors that regulate fw2.2 (the target component; Frary et al., 2000). This result rules out the first mechanism mentioned above to explain the fw2.2 action. Sequence analysis of multiple alleles of the fw2.2 locus revealed no consensus sequence diversities in the coding regions between a variety of wild small-fruit alleles and the large-fruit domestication allele. These results suggested that the allelic variation of fw2.2 locus is due to 5′-regulatory regions and gene expression patterns rather than variation in protein sequences of different alleles (Frary et al., 2000; Nesbitt et al., 2002). The most striking evidence in support of this notion came from the fact that the coding sequence of a small-fruit wild tomato species (Lycopersicon cheesmanii) is identical to that of the large-fruit domestication species (Lycopersicon esculentum), indicating that the fw2.2 coding sequences cannot be the reason for fruit size variation (Nesbitt et al., 2002).
It has been hypothesized that fw2.2 regulates fruit size via variation in transcript levels of the gene (Nesbitt and Tanksley, 2002). Detailed studies on gene expression profiles have revealed that differences in the levels and timing of fw2.2 expression account for fruit mass variation between the large- and the small-fruit alleles, providing a strong support for the transcriptional regulation hypothesis (Cong et al., 2002). The gene dosage series described herein provided the raw material for further testing this hypothesis.
This dosage series represents a set of genetically defined plants in which transcript levels of fw2.2 (under its native promoter) were modulated over a 7-fold range. The reason for having transcription of fw2.2 driven by its native promoter was to create a broader range of variation in steady-state transcript levels while assuring that the spatial/temporal expression patterns of fw2.2 would be similar to what is normally experienced by developing fruit.
At stage of 9 DAP, fw2.2 transcripts remain at a high level relative to other fruit developmental stages (Cong et al., 2002) and no signs of co-suppression (Baulcombe, 1999) were observed in lines accumulating high transcript levels of fw2.2 (Fig. 1). The fw2.2 transcript levels in 9-DAP fruit were highly correlated (negatively) with final fruit size, which could account for 62% of fruit size variation in mature fruit (Fig. 2)—a very large percentage considering that fruit size is a quantitative trait and is affected not only by genetic, but also environmental factors.
It is also worth noting that plants with higher gene dosage of fw2.2 and hence higher levels of transcripts produced fruit substantially smaller than is otherwise observed in non-transgenic lines of the same genetic stocks. For example, in the genetic background used for this study, plants containing two copies of the small-fruit alleles of fw2.2 produced fruit averaging 51 g—a 29% decrease compared with average fruit weight (72 g) of plants homozygous for the large-fruit allele. However, plants with four copies of the small-fruit alleles produced fruit averaging only 42 g, an 18% further decrease in fruit mass as compared with those with two copies of the small-fruit alleles of fw2.2. The fact that fruit size is sensitive to the levels of fw2.2 transcripts indicates that the levels of fw2.2 transcripts are not saturated in fruit cells of naturally occurring plants as well as the gene dosage lines. It will be interesting to see the effects of fw2.2 on fruit mass by further expansion of the range of the transcript levels through overexpression or gene knock-out techniques.
fw2.2 is expressed at a very low level in both reproductive and vegetative organs of tomato plants (Frary et al., 2000; Cong et al., 2002). The effects of small differences in the expression patterns between different fw2.2 alleles could be amplified by the regulation of the cell division machinery, leading to final large variation of fruit size.
Mechanisms by Which fw2.2 Controls Fruit Growth
Although the size of fruit maternal parts including pericarp and placental tissues is negatively correlated with the levels of fw2.2 transcripts in immature fruit (Fig. 5, A and B), the cell size of the corresponding tissues is not (Cong et al., 2002). These results further supported the notion that fw2.2 suppresses cell division rather than cell enlargement in the fruit maternal tissues (Frary et al., 2000; Cong et al., 2002). Although sequence alignments revealed no sequence similarity of fw2.2 to any known genes involved in cell cycle regulation, fw2.2 shows predicted similarity with RAS, an oncogene, at three-dimensional protein structure levels (Frary et al., 2000). However, its real biochemical function still remains to be determined experimentally. Identifying the other cellular components that interact and cofunction with FW2.2 in regulation of fruit growth will help dissect the genetic networks involved in fw2.2-mediated signaling pathways and will provide more insights into the previously uncharacterized molecular and biochemical mechanisms underlying the regulation of fruit development.
Fertilization and following seed development are independent of the levels of fw2.2 transcripts, suggesting that the function of fw2.2 is limited to fruit maternal tissues that are not directly involved in reproductive processes. In support of this notion, results from in situ hybridization indicated that the detectable fw2.2 expression is mainly located in the placental tissues (Cong et al., 2002) where the growth suppression is more severe than other parts of fruit tissues (Figs. 4B and 5, A and B).
Patterned cell division and cell enlargement are essential for the development of all organisms (Meyerowitz, 1997; Knoblich, 2001). In Arabidopsis, both SCARCROW (Di Laurenzio et al., 1996) and SHORT-ROOT (Helariutta et al., 2000) have been identified as determinants for promoting the asymmetric periclinal cell division in the daughter cells of root cortex/endodermal initials. However, in general, our knowledge about the plant cellular components that specifically control cell division planes is still very limited.
FW2.2 seems to influence cell division patterns in pericarp tissues of tomato fruit: the overall anticlinal, but not periclinal, cell division in the pericarp is associated with the levels of fw2.2 transcripts (see “Results” and Table II). We postulate that, like SCARCROW and SHORT-ROOT in Arabidopsis, FW2.2 might be able to directly recognize and selectively suppress the anticlinal cell division in pericarp tissues. Alternatively, FW2.2 indirectly controls cell division patterns in pericarp tissues as a result of nonoverlapping of the timing of fw2.2 expression (cell division suppression) with the timing of periclinal cell division events. The latter hypothesis seems more plausible because the major events of periclinal cell division occur early in fruit development (Table II, 0–6 DAP), whereas fw2.2 is expressed at relatively low levels at this stage (Cong et al., 2002). In support of this notion, fw2.2 influences cell division in placental tissues in a three-dimensional manner.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Genotyping Assays
TA1589, from tomato (Lycopersicon esculentum), is a nearly isogenic line homozygous for the small-fruit allele from Lycopersicon pennellii, LA716, at the endogenous fw2.2 locus (Alpert et al., 1995); TA1616 is homozygous for the large-fruit allele at the endogenous fw2.2 locus and homozygous for the small-fruit allele, also from L. pennellii, introduced into an unlinked site through transgenesis (Frary et al., 2000). A series of stocks carrying zero, one, two, three, or four copies of the small-fruit alleles were selected from the progeny of a cross between TA1589 and TA1616.
A total of 78 F2 plants from the above cross were individually genotyped for both their endogenous and transgenically introduced fw2.2 small-fruit alleles. TG167, a cleaved amplified polymorphic sequence marker tightly linked (<0.13 cM or <150 kb), was used to infer the genotype of the endogenous fw2.2 alleles (Alpert and Tanksley, 1996). A 50-μL volume of the PCR contained 50 to 100 ng of genomic DNA, 2.5 units of Taq polymerase in its recommended buffer, 0.5 mm of each primer 5′-GCG AGA GCG AGT TGA GTG TAT ATC-3′ and 5′-CAG AAG AGA GAA GCT GCA AAG CAG-3′, and 0.1 mm of each dNTP. PCR conditions were 1-min denature at 94°C followed by 30 cycles of 45 s at 94°C, 45 s at 60°C, and 1 min at 72°C. Five microliters of PCR products was digested with TaqI in its recommended buffer in a reaction volume of 20 μL, incubated at 37°C over night, and scored on agarose gels. Individuals homozygous for the presence or absence of the cut site were scored as EE and PP representing L. esculentum and L. pennellii, respectively, whereas heterozygous plants were labeled as PE (Table I).
The genotypes at the transgenically introduced fw2.2 locus were inferred by the presence or absence of a tightly linked NPTII gene that can be detected by PCR with the NPTII-specific primers: 5′-TGG AGA GGC TAT TCG GCT AT-3′ and 5′-CTC TTC AGC AAT ATC ACG GGT A-3′. The PCR conditions were the same as above except that the annealing temperature was 55°C. Templates homozygous/heterozygous for the fw2.2 transgene produced 300-bp amplicons; those of non-transgenic insertions had no PCR products (Table I).
Forty-six selected F2 plants were potted to soil, and grown in a greenhouse in a completely randomized design. Each plant was progeny tested with F3 to verify their genotypes for both endogenous and transgenically introduced fw2.2 alleles (Table I).
Phenotypic Analyses
Fruit Measurements
The three largest fruit from each plant were used for gathering mature-fruit data. Data collected included fruit weight and fruit-shape index—the ratio of longitudinal diameter (L) to equatorial diameter (D). The equatorial sections of each fruit were also scanned by Vistascan (UMAX technologies, Inc., Dallas) and the thickness of pericarp (fruit wall), the areas of pericarp, and placental regions of each fruit were determined by measuring the scanned images with the Scion Software (Scion Corporation, Frederick, MD). Seeds from individual fruit were extracted and counted; the dry weight of 100 seeds from each plant was recorded.
Cell Size Measurements
Three ovaries/fruit at 0, 6, 12, and 18 DAP were collected from each plant, fixed for 24 h in 4% (v/v) formaldehyde buffer with 0.1% (v/v) Tween 20 and 0.1% (v/v) Triton X-100 in 1× PBS, pH 7.0, processed, embedded in paraffin, and sectioned transversely 10 μm thick. From microscopic section images of the pericarp or placental tissues, the areas of all cells within a unit region (1 mm2) were measured with Scion Software (Scion Corporation). The data of average cell size were obtained by dividing the value of the total cell area with corresponding cell number within the same unit area region. The number of cell layers counted from epidermis to endodermis of pericarps was also recorded.
RNA Extractions and Reverse Transcription (RT) Reactions
Fresh tissues from 9-DAP fruit were collected from each plant, frozen in liquid nitrogen immediately, and ground to a fine powder with a mortar and pestle. Total RNAs were isolated with the Trizol Reagent (Invitrogen, Carlsbad, CA). One microgram of the DNaseI-treated total RNAs from each sample was used for the first-stranded cDNA synthesis with the Taqman Reverse Transcription Reagent Kit (Applied Biosystems, Foster City, CA). The cycling condition was 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C.
Quantification of Relative fw2.2 mRNA Levels by Taqman Real-Time RT-PCR
Levels of the fw2.2 RNAs from each sample were quantified by the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Three microliters of the 1:1 diluted cDNAs were used as templates for the PCRs with conditions recommended by the manufacturer. The forward and reverse primers, as well as the probe specific to fw2.2, were designed with Primer Express software v1.0 (Applied Biosystems). They are 5′-CAA CCT TAT GTT CCT CCT CAC TAT GTA T-3′, 5′-GGG TCA TCA AAA CAA TGA CAA AGA-3, and 6FAM-5′-TGC CCC CGG CAC CAC CA-3′-TRMRA, respectively. PCR amplifications were carried out in a 28- μL reaction volume containing 1× Taqman buffer A, 5.5 mm MgCl2, 900 mm of each primer, 200 mm of the probe, 200 μm of each deoxynucleoside triphosphate (dATP, dCTP, and dGTP), 400 μm of dUTP, 0.7 unit of AmpliTaq Gold (0.0025 unit μL−1), and 0.28 unit of AmpErase uracil-N-glycosylase (0.01 unit μL−1). Distilled water or products of RT reactions without reverse transcriptase were used as negative controls.
The levels of fw2.2 mRNA from each sample were normalized by endogenous 18S RNAs with the Taqman Ribosomal RNA Control Reagents (Applied Biosystems). The sequences of the primers and a probe accompanied with the kit completely match a tomato 18S RNA gene (data not shown).
Because the CT (threshold cycle) value of a real-time PCR reaction is correlated with the amount of target (fw2.2) RNAs present in each PCR reaction, the relative quantity of the fw2.2 mRNAs present in each sample was reported as 2−ΔΔCT, where ΔΔCT = [CT(fw2.2) − CT(18S RNA)] − [CT(calibrator) − CT(18S RNA)] (User Bulletin no. 2, ABI Prism 7700 sequence detection system; http://www.appliedbiosystems.com). Each set of experiments was repeated three times, and the final quantification of fw2.2 mRNAs was the average of the three repeats.
Statistical Tests
Pearson correlation analyses, Student's t tests, ANOVA, linear regression analyses, and fitted line plots were performed by the Minitab program (Minitab Inc., State College, PA).
ACKNOWLEDGMENTS
We thank Yimin Xu for excellent technical assistance and Dr. Esther van der Knaap for critical comments on this manuscript.
Footnotes
This work was supported by the National Science Foundation (grant no. DBI–0116076), by the U.S. Department of Agriculture Plant Genome Program (grant no. 97–35300–4384), and by the U.S.-Israel Binational Agriculture Research and Development Fund (grant no. IS–3009–98C).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018143.
LITERATURE CITED
- Alpert KB, Grandillo D, Tanksley SD. fw2.2: a major QTL controlling fruit weight is common to both red- and green-fruited tomato species. Theor Appl Genet. 1995;91:994–1000. doi: 10.1007/BF00223911. [DOI] [PubMed] [Google Scholar]
- Alpert KB, Tanksley SD. High-resolution mapping and isolation of a yeast artificial chromosome contig containing fw2.2: a major fruit weight quantitative trait locus in tomato. Proc Natl Acad Sci USA. 1996;93:15503–15507. doi: 10.1073/pnas.93.26.15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe DC. Gene silencing: RNA makes RNA makes no protein. Curr Biol. 1999;9:R599–R601. doi: 10.1016/s0960-9822(99)80383-2. [DOI] [PubMed] [Google Scholar]
- Barton NH, Keightley PD. Understanding quantitative genetic variation. Nat Rev Genet. 2002;3:11–21. doi: 10.1038/nrg700. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Endless forms: the evolution of gene regulation and morphological diversity. Cell. 2000;101:577–580. doi: 10.1016/s0092-8674(00)80868-5. [DOI] [PubMed] [Google Scholar]
- Cong B, Liu J, Tanksley SD. Natural alleles of a tomato QTL modulate fruit size through heterochronic regulatory mutations. Proc Natl Acad Sci USA. 2002;99:13606–13611. doi: 10.1073/pnas.172520999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk QCB. Plant evolution and development in a post-genomic context. Nat Rev Genet. 2001;2:607–619. doi: 10.1038/35084556. [DOI] [PubMed] [Google Scholar]
- Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418:700–707. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Doebley J, Lukens L. Transcriptional regulators and the evolution of plant form. Plant Cell. 1998;10:1075–1082. doi: 10.1105/tpc.10.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley J, Stec A, Hubbard L. The evolution of apical dominance in maize. Nature. 1997;386:485–488. doi: 10.1038/386485a0. [DOI] [PubMed] [Google Scholar]
- Frary A, Nesbitt TC, Frary A, Grandillo S, van der Knaap E, Cong B, Liu J, Meller J, Elber R, Alpert KB et al. fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science. 2000;289:85–88. doi: 10.1126/science.289.5476.85. [DOI] [PubMed] [Google Scholar]
- Fridman E, Pleban T, Zamir D. A recombination hotspot delimits a wild species quantitative trait locus for tomato sugar content to 484 bp within an invertase gene. Proc Natl Acad Sci USA. 2000;97:4718–4723. doi: 10.1073/pnas.97.9.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galant R, Carroll SB. Evolution of a transcriptional repression domain in an insect Hox protein. Nature. 2002;415:910–913. doi: 10.1038/nature717. [DOI] [PubMed] [Google Scholar]
- Gillapsy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandillo S, Ku HM, Tanksley SD. Identifying the loci responsible for natural variation in fruit size and shape in tomato. Theor Appl Genet. 1999;99:978–987. [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser M-T, Benfey P. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–567. doi: 10.1016/s0092-8674(00)80865-x. [DOI] [PubMed] [Google Scholar]
- Joubès J, Phan T-H, Just D, Rothan C, Bergounioux C, Raymond P, Chevalier C. Molecular and biochemical characterization of the involvement of cyclin-dependent kinase A during the early development of tomato fruit. Plant Physiol. 1999;121:867–869. doi: 10.1104/pp.121.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J. Asymmetric cell division during animal development. Nat Rev Mol Cell Biol. 2001;2:11–20. doi: 10.1038/35048085. [DOI] [PubMed] [Google Scholar]
- Liu J, Van Eck J, Cong B, Tanksley SD. A new class of regulatory genes underlying the cause of pear-shape tomato fruit. Proc Natl Acad Sci USA. 2002;99:13302–13306. doi: 10.1073/pnas.162485999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TFC. The genetic architecture of quantitative traits. Annu Rev Genet. 2001;35:303–339. doi: 10.1146/annurev.genet.35.102401.090633. [DOI] [PubMed] [Google Scholar]
- Meyerowitz EM. Genetic control of cell division patterns in developing plants. Cell. 1997;88:299–308. doi: 10.1016/s0092-8674(00)81868-1. [DOI] [PubMed] [Google Scholar]
- Nesbitt TC, Tanksley SD. fw2.2 directly affects the size of developing tomato fruit, with secondary effects on fruit number and photosynthate distribution. Plant Physiol. 2001;127:575–583. [PMC free article] [PubMed] [Google Scholar]
- Nesbitt TC, Tanksley SD. Comparative sequencing in the genus Lycopersicon: implication for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics. 2002;162:365–379. doi: 10.1093/genetics/162.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch J. Hormonal factors in growth development. In: Hulme AC, editor. The Biochemistry of Fruits and Their Products. Vol. 2. New York: Academic Press; 1970. pp. 427–472. [Google Scholar]
- Purugganan MD. The molecular evolution of development. BioEssays. 1998;20:700–711. doi: 10.1002/(SICI)1521-1878(199809)20:9<700::AID-BIES3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ronshaugen M, McGinnis N, McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- Sastry K, Muir R. Gibberellin: effect on diffusible auxin in fruit development. Science. 1963;140:494–495. doi: 10.1126/science.140.3566.494. [DOI] [PubMed] [Google Scholar]
- Varoquaux F, Blanvillain R, Delseny M, Gallois P. Less is better: new approaches for seedless fruit production. Trends Biotechnol. 2000;18:233–242. doi: 10.1016/s0167-7799(00)01448-7. [DOI] [PubMed] [Google Scholar]
- Wang RL, Stec A, Hey J, Lukens L, Doebley J. The limits of selection during maize domestication. Nature. 1999;398:236–239. doi: 10.1038/18435. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Nijhout HF, Grunert LW, Halder G, Galant R, Selegue J, Carroll S. Ultrabithorax function in butterfly wings and the evolution of insect wing patterns. Curr Biol. 1999;9:109–115. doi: 10.1016/s0960-9822(99)80064-5. [DOI] [PubMed] [Google Scholar]
- White S, Doebley J. Of genes and genomes and the origin of maize. Trends Genet. 1998;14:327–332. doi: 10.1016/s0168-9525(98)01524-8. [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell. 2000;12:2299–2301. doi: 10.1105/tpc.12.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]