Abstract
The phototolerance of three chlP transgenic tobacco (Nicotiana tabacum) lines, affected in geranylgeranyl reductase and, hence, deficient in tocopherols (vitamin E), was estimated by in vivo luminescence and fluorescence measurements and was compared with that of the wild type (WT). Exposure of leaf discs to high light (1 mmol photon m−2 s−1) and low temperature (10°C) led to a rapid inhibition of photosystem II (PSII) photochemistry that showed little dependence on the tocopherol level. PSII photo-inhibition was followed by lipid peroxidation with a time delay of about 4 h, and this phenomenon was exacerbated in the tocopherol-deficient leaves. A linear correlation was observed in these short-term experiments between resistance to photooxidation and tocopherol content. When whole plants were exposed to the same treatment, PSII was severely photo-inhibited in mature leaves of all genotypes. Lipid peroxidation was also observed in all plants, but it occurred much more rapidly in tocopherol-deficient transgenic plants relative to WT plants. The time at which extensive lipid peroxidation occurred was correlated with the tocopherol content of the leaves. The present results show that tocopherols protect thylakoid membranes against photodestruction through lipid peroxidation. However, tocopherol deficiency was compensated in young, developing leaves that were able to photo-acclimate in the long term and did not suffer from photooxidative damage. Soluble antioxidants (glutathione and ascorbate) did not accumulate in photo-acclimated chlP transgenic leaves relative to WT leaves. In contrast, a selective accumulation of xanthophyll cycle pigments was observed in young transgenic leaves, and this could represent a compensatory mechanism for tocopherol deficiency.
Vitamin E is the collective term for a group of amphipathic compounds, the tocopherols and tocotrienols, synthesized exclusively by photosynthetic organisms (Fryer, 1992; Bramley et al., 2000; Munné-Bosch and Alegre, 2002). α-Tocopherol is the major and most active component of vitamin E. In vascular plants, α-tocopherol is found mainly in the envelope and the thylakoid membranes of chloroplasts. In vitro experiments on lipid bilayers incorporated with tocopherols have demonstrated that tocopherols have the ability to terminate chain reactions of polyunsaturated fatty acid free radicals generated by lipid oxidation. This chain-breaking action during lipid oxidation is supposed to be the most important function of tocopherols in vivo.
Vitamin E has long been recognized to be an essential antioxidant in animal cells. Tocopherols have been the subject of numerous studies that have emphasized their importance in the defense of animal or human tissues from a wide range of conditions and diseases that are mediated by oxidative degeneration (Bramley et al., 2000). Although tocopherols may be similarly important in plant tissues, their antioxidant activity in plant cells has received far less attention. No precise function, except a putative role in the maintenance of PSII function by scavenging singlet oxygen 1O2 at the PSII reaction center (Trebst et al., 2002), has been described to date. This is rather surprising because reactive O2 species are produced predominantly in the chloroplasts (Fryer et al., 2002), and thylakoid membrane fatty acids are highly unsaturated and, hence, very vulnerable to oxidative degradation. Moreover, in most plants, tocopherols seem to be the sole lipophilic photoprotectors that are present constitutively in the thylakoid membrane lipid phase. It has been suggested that, under certain environmental conditions, the antioxidant activity of tocopherols in thylakoid membranes can be supplemented by the carotenoid zeaxanthin (Havaux, 1998), by diterpenes such as carnosic acid (Hopia et al., 1996), or by isoprene (Loreto and Velikova, 2001; Afek and Yakir, 2002).
The involvement of thylakoid tocopherols in the photoprotection of plants is supported by the observation that the tocopherol level increases in plants exposed to environmental stress conditions that are susceptible to induce oxidative stress (e.g. Wildi and Lütz, 1996; Delong and Steffen, 1997; Fryer et al., 1998; Havaux et al., 2000; Munné-Bosch and Alegre, 2000). Although there have been several attempts to investigate the function of tocopherols in mutant or transgenic plants affected in tocopherol synthesis, these studies did not provide a clear answer regarding the antioxidant activity of tocopherols. Tocopherol deficiency was generated in tobacco (Nicotiana tabacum) plants transformed with antisense chlP that have a reduced activity of geranylgeranyl reductase (Tanaka et al., 1999). These plants were characterized by decreased tocopherol content and by the accumulation of geranylgeranylated chlorophyll at the expense of (phytylated) chlorophyll. However, the transgenic plants could not be distinguished from the wild type (WT) on the basis of their photosynthetic performance (Grasses et al., 2001), indicating that geranylgeranylated chlorophyll does not affect light harvesting and energy transfer in the PSs and also that tocopherol deficiency has no major effect on the photosynthetic machinery. Similarly, disruption of homogentisate phytylphytyltransferase or hydroxyphenylpyruvate dioxygenase in the cyanobacterium Synechocystis PCC 6803 caused an absence of tocopherols without apparent changes in photosynthesis and growth in low or high light (Collakova and DellaPenna, 2001; Dähnhardt et al., 2002). Very recently, Porfirova et al. (2002) isolated an Arabidopsis mutant that was deficient in tocopherol cyclase activity, resulting in a complete lack of tocopherols. Again, absence of tocopherol had no large impact on photosynthesis and had minor effects on phototolerance. This lack of deleterious effects of tocopherol deficiency was interpreted as the result of the up-regulation of other photoprotective mechanisms that compensated for the tocopherol deficit or by the stress treatments that were not severe enough to produce detectable differences between the WT and the transgenic strain. Nevertheless, a small increase in the susceptibility of PSII to photo-inhibition was observed in tocopherol-deficient chlP transgenic tobacco leaves using chlorophyll fluorescence measurements (Grasses et al., 2001). Similarly, herbicide-induced blocking of tocopherol synthesis in the green alga Chlamydomonas reinhardtii exposed to high light was associated with a loss of PSII activity (Trebst et al., 2002).
In this work, we have compared the phototolerance of WT tobacco and three chlP transgenic tobacco lines deficient in tocopherols. Plants were exposed in the short term and in the long term to excess light energy induced by low-temperature and high-light intensity, and the lipid peroxidation status was monitored by in vivo thermoluminescence measurements. The combination of chilling stress and high-light intensity is known to be particularly favorable to induce both photo-inhibition and photooxidation in higher plant leaves. During cold treatment, the enzymes of the Calvin cycle are slowed so that the incoming light energy funneled into the electron transport chain becomes more excessive. This can eventually cause overexcitation of the PSs and overreduction of the electron carriers, leading to excitation/electron “leakage” to molecular oxygen and production of activated oxygen species (Wise, 1995). Although the tocopherol deficiency in the chlP transgenic tobacco plants used in this study was not complete, lipid peroxidation was noticeably increased relative to the WT, and we observed that this increase was proportional to the decrease in tocopherol concentration. However, in the long term, young leaves of all transformants were able to acclimate to photooxidative stress, suggesting the existence of compensatory mechanisms for the tocopherol deficit. One possible mechanism was identified in this study, namely a marked increase in the pool size of the xanthophyll cycle carotenoids.
RESULTS
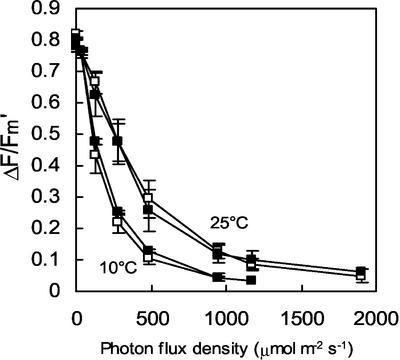
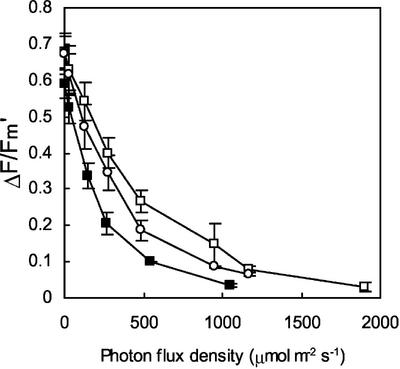
The tocopherol level (α- and γ-tocopherols) in leaves of the transgenic tobacco lines T6 and T10 was reduced to approximately 50% of the tocopherol content of WT leaves, whereas the tocopherol concentration in T20 leaves was intermediate (see “control leaves” in Table I), confirming previous analyses (Grasses et al., 2001). Using chlorophyll fluorescence measurements, we also confirmed that the photochemical activity of chloroplasts was not affected in the transgenic plants grown in low light. No significant difference in the PSII photochemical efficiency (Fv/Fm) was observed between WT and the transgenics (Table I). Similarly, photosynthetic electron transport, measured by the fluorescence ratio (ΔF/Fm′) in a wide range of photon flux densities (PFDs), was identical in WT and T6 leaves both at 25°C and at 10°C (Fig. 1). The photosynthetic electron transport characteristics of T10 and T20 were also identical to those of WT leaves (data not shown). These observations confirm that geranylgeranylated chlorophyll, which represents approximately 50% of the total chlorophyll in T6 and T10 (Tanaka et al., 1999; Grasses et al., 2001), does not restrict the photosynthetic capacity of the leaves.
Table I.
Tocopherol level (α-tocopherol + γ-tocopherol), PSII photochemical efficiency (Fv/Fm), and lipid peroxidation (thermoluminescence [TL] signal amplitude at 80°C) in tobacco leaves grown under control conditions (70 μmol photon m−2 s−1, 25°C) and in young (Y) leaves acclimated for 2 weeks to strong light stress at low temperature (1,000 μmol m−2 s−1, 10°C)
| Leaf Samples | α- + γ-Tocopherols | Fv/Fm | TL (80°C) |
|---|---|---|---|
| ng cm−2 | |||
| Control leaves | |||
| WT | 171.2 ± 30.0 | 0.791 ± 0.006 | 380 ± 75 |
| T6 | 91.8 ± 24.2 | 0.778 ± 0.009 | 375 ± 41 |
| T10 | 90.0 ± 30.0 | 0.784 ± 0.008 | 450 ± 41 |
| T20 | 125.4 ± 28.3 | 0.782 ± 0.006 | 406 ± 31 |
| Acclimated Y leaves | |||
| WT | 442.6 ± 35.0 | 0.716 ± 0.031 | 492 ± 67 |
| T6 | 189.5 ± 15.5 | 0.695 ± 0.019 | 353 ± 38 |
| T10 | 184.5 ± 6.5 | 0.696 ± 0.019 | 575 ± 25 |
| 20 | 335.4 ± 85.7 | 0.700 ± 0.041 | 646 ± 251 |
Data are mean values of at least three experiments ± sd.
Figure 1.
Photosynthetic characteristics of WT and T6 leaves at 25°C and 10°C. The quantum yield of PSII-mediated electron transport was measured at different PFDs with ΔF/Fm′. White squares, WT; black squares, T6.
Short-Term Photostress
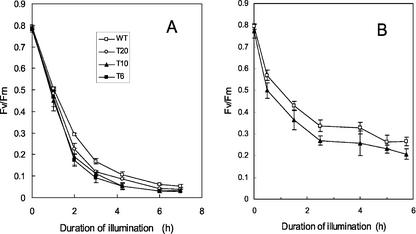
Leaf discs punched out from mature leaves (leaves 5–7 of plants aged 40 d) were exposed to strong light (1,000 μmol photon m−2 s−1) and to low temperature (10°C)—a condition that is close to photosynthetic saturation (see Fig. 1). This treatment resulted in a rapid and pronounced photo-inhibition of PSII as indicated by the decrease in the chlorophyll fluorescence parameter Fv/Fm, and this phenomenon was observed in all genotypes (Fig. 2A). WT and T20 leaves appeared to be less photo-inhibited than T6 and T10 leaves, but the difference was small. The same experiment was repeated at lower PFDs (500 μmol photon m−2 s−1, Fig. 2B; and 250 μmol photon m−2 s−1, not shown) with a similar outcome: PSII photo-inhibition was only slightly increased in the tocopherol-deficient plants relative to WT plants.
Figure 2.
A, PSII photo-inhibition in tobacco leaf discs (WT, T6, T10, and T20) exposed to a high PFD of 1,000 μmol m−2 s−1 and a low temperature of 10°C. B, PSII photo-inhibition in WT and T10 leaves exposed to 500 μmol photon m−2 s−1 at 10°C. Leaf discs were taken from plants grown in low light at 25°C. Photo-inhibition was measured by the decrease in the maximal quantum yield of PSII photochemistry as estimated by Fv/Fm. Fv/Fm was measured after 15 min of dark adaptation. White squares, WT; black squares, T6; black triangle, T10; white circle, T20.
High light-induced inhibition of photosynthesis at 10°C was accompanied by lipid peroxidation. The latter phenomenon was detected and quantified by thermoluminescence measurements on leaf discs. Spontaneous chemiluminescence from biological samples originates from electronically excited states such as singlet oxygen and triplet carbonyl provoked by radical chain reactions in lipid peroxidation (Boveris et al., 1983; Gutteridge and Halliwell, 1990). This luminescence emission can be thermally stimulated by slowly warming the sample, leading to the appearance of successive emission bands in thermograms. (Thermo) luminescence measurements have been applied for estimating the lipid hydroperoxide content of various plant materials from edible oil samples to organs (Mathew and Roy, 1992; Hideg and Vass, 1993; Miyazawa et al., 1994; Makino et al., 1996; Palacios et al., 1996; Vavilin et al., 1998). In plant leaves, the two lipid peroxation-related TL bands that have been characterized (at about 80°C and about 135°C) are believed to originate mostly from lipid cycloperoxides that are broken during heating, leading to the formation of carbonyl species in excited triplet state (Vavilin and Ducruet, 1998). The luminescence amplitude has been correlated with other indicators of lipid peroxidation such as the thiobarbituric acid (TBA) reactive species assay (Minamide et al., 1998; Vavilin et al., 1998; Rhoden et al., 2001; P. Müller-Moulé, M. Havaux, and K.K. Niyogi, unpublished data), the ethane assay (Havaux and Niyogi, 1999), and the HPLC determination of the lipid hydroperoxide concentration (Havaux and Niyogi, 1999).
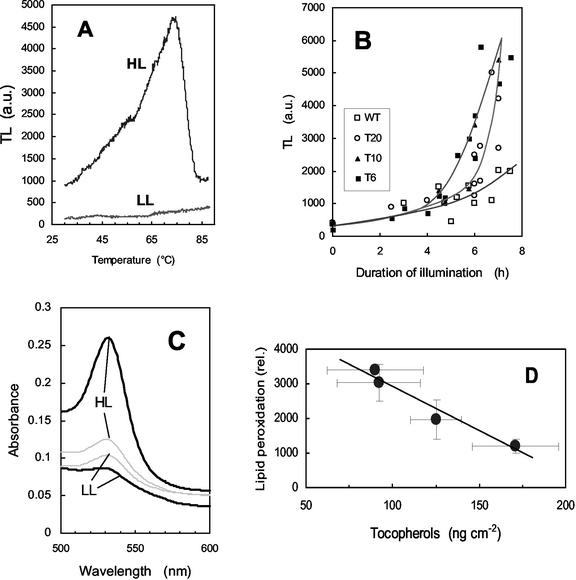
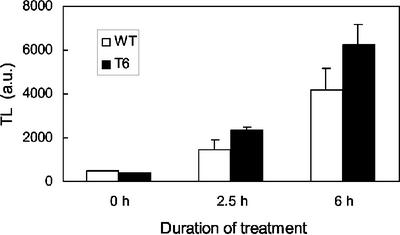
As shown in Figure 3A, lipid peroxidation manifests clearly in tobacco leaves as a TL emission peak at about 80°C. No significant lipid peroxidation was measured during the first 4 h of illumination (Fig. 3B). Subsequently, a dramatic increase in the 80°C TL emission band was measured in T6 and T10 leaves, indicating the occurrence of pronounced peroxidative damage to membrane lipids and the accumulation of lipid peroxy radicals. Pigment bleaching was also observed in T6 and T10 leaves exposed to high-light stress (not shown). In contrast, WT was resistant to lipid peroxidation, and the increase in TL emission was small and no leaf bleaching was detected. T20 appeared to be intermediate between WT and the T6 and T10 transformants; TL emission at 80°C strongly increased in T20 leaves, but this occurred later than the TL signal rise measured in T6 and T10. Lipid peroxidation was also estimated by measuring the level of MDA, a secondary end product of the oxidation of tri-unsaturated fatty acids (Gutteridge and Halliwell, 1990; Hodges et al., 1999). In acidic medium, MDA reacts with TBA, yielding a pinkish-red compound with an absorbance maximum at 532 nm. As shown in Figure 3C, the MDA level, as measured by the A532 of the MDA-TBA complex, increased markedly in T6 leaf discs exposed for 5.5 h to high light at low temperature, confirming the occurrence of lipid peroxidation in those leaves. In contrast, the MDA level was low in WT leaf discs exposed to the same light treatment and also in unstressed T6 and WT leaves. T10 leaves behaved like T6 leaves with regard to MDA accumulation after light stress at low temperature (not shown). We calculated that the MDA content of light-stressed WT and T6 leaves was 0.24 ± 0.01 and 0.62 ± 0.06 nmol cm−2, respectively. Thus, the results of the TBA reactive species assay confirm the TL data. In Figure 3D, the lipid peroxidation status of leaves after 6 h of exposure to chilling stress in high light was plotted as a function of the tocopherol concentration. A good linear (inverse) correlation was found between the two parameters.
Figure 3.
Photooxidation of tobacco leaf discs exposed to a high PFD of 1,000 μmol m−2 s−1 and a low temperature of 10°C. Leaf discs were taken from plants grown in low light at 25°C. Lipid peroxidation was measured by the amplitude of the 70°C to 80°C thermoluminescence signal as shown in A (HL, T6 leaf discs exposed for 7 h to the light stress; LL, control T6 leaf grown in low light at 25°C). B, Time course of the increase in thermoluminescence (80°C peak) during chilling stress in high light. White squares, WT; black squares, T6; black triangle, T10; white circle, T20. C, Malondialdehyde (MDA) level, as indicated by its light absorption at 532 nm after reaction with TBA in acidic medium, in T6 (thick line) and WT (thin line) leaves before (LL) and after (HL) high-light stress at low temperature. D, Plot of the lipid peroxidation status of tobacco leaves (measured by thermoluminescence after 6 h in high light at low temperature) versus the tocopherol concentration.
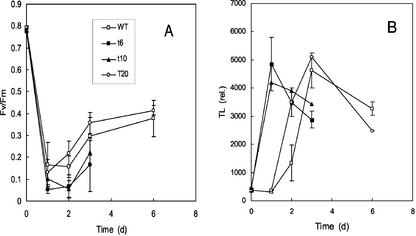
The same experiment was performed on whole plants transferred to a growth chamber at 8°C. A rapid decrease in Fv/Fm was observed in leaves of all genotypes after transfer of the plants to low temperature and high light (1,000 μmol photon m−2 s−1; Fig. 4A). PSII slightly recovered during the stress experiment because Fv/Fm increased after 1 d of stress in all lines. A similar PSII recovery was previously found in Arabidopsis plants exposed to chilling stress in high light (Havaux and Kloppstech, 2001). Thermoluminescence measurements indicated that a dramatic lipid peroxidation occurred rapidly in T10 and T6 lines; a strong TL emission was measured in leaves of those lines after 1 d of exposure to the stress conditions (Fig. 4B). Leaf bleaching also appeared after 1 d of stress. Lipid peroxidation and leaf bleaching also occurred in WT leaves, but those phenomena were much slower and were delayed by about 2 d relative to T6 or T10 leaves. Again, T20 appeared to be intermediate between WT and T6 or T10. Leaf photooxidation was accompanied by tocopherol consumption in all genotypes. After 3 d of exposure to chilling stress in high light, the α- + γ-tocopherol level fell to 44.1 ± 3.4, 30.3 ± 3.0, 8.7 ± 3.8, and 4.6 ± 3.3 ng cm−2 in WT, T20, T6, and T10 leaves, respectively.
Figure 4.
Photo-inhibition and photooxidation of mature leaves of tobacco plants exposed to a high PFD of 1,000 μmol m−2 s−1 and a low temperature of 8°C. PSII photo-inhibition (A) was measured by Fv/Fm. Photooxidation (B) was measured by the amplitude of the 80°C thermoluminescence peak. Leaf temperature was about 10°C. White squares, WT; black squares, T6; black triangle, T10; white circle, T20.
Eosin Toxicity
Eosin is a well-known generator of 1O2 in the light (Knox and Dodge, 1985). Leaf discs floating on an aqueous solution of eosin were illuminated with white light at a PFD of 400 μmol photons m−2 s−1. As expected (compare with Knox and Dodge, 1985), this treatment brought about lipid peroxidation in T6 leaves, which manifested by a rapid increase in the TL signal amplitude (Fig. 5). The luminescence increase appeared to be less pronounced in WT leaves, indicating a lower sensitivity to eosin toxicity. No lipid peroxidation was detected when the light treatment was imposed on leaf discs floating on distilled water (data not shown).
Figure 5.
Lipid peroxidation (as measured by the amplitude of the TL signal) induced by illumination of T6 and WT leaf discs floating on eosin. TL did not increase during illumination of leaf discs floating on distilled water. PFD of white light = 400 μmol m−2 s−1.
Long-Term Photostress
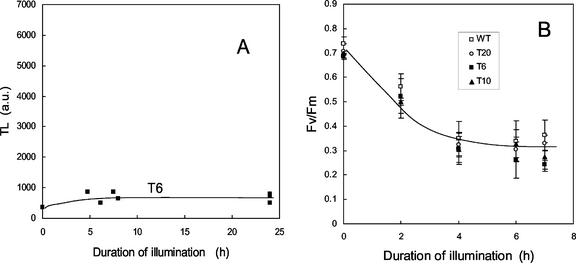
As shown above, mature tobacco leaves, and particularly those of the tocopherol-deficient chlP transgenic plants, suffered from extensive photooxidation when suddenly exposed to high light at low temperature. Lipid peroxidation was followed by bleaching of the leaves, which finally died and dried. However, we observed that very young leaves at the top of the plants did not bleach and succeeded to grow under conditions of chilling stress in high light. No lipid peroxidation was detected by thermoluminescence in those leaves, and the maximal photochemical efficiency of PSII was only slightly reduced (Table I, “acclimated Y leaves”). This tolerance of young leaves to photo-inhibition and photooxidation was found in WT and in all the transgenic lines. When discs punched out from young acclimated leaves were exposed to the same in vitro treatment as that used in the experiment of Figures 2 and 3, no lipid peroxidation was observed in T6 leaves (Fig. 6A) or in leaves of the other genotypes (data not shown). Also, PSII photo-inhibition (Fig. 6B) was similar in all types of plants and was noticeably less pronounced than that observed for nonacclimated leaves (compare with Fig. 2). Thus, young leaves that developed under chilling stress were able to photo-acclimate, independently of the tocopherol deficiency.
Figure 6.
Photo-inhibition and photooxidation of leaf discs taken from young leaves of tobacco plants acclimated for 15 d to chilling stress in high light. A, Lipid peroxidation level in leaf discs of line 6. PSII photo-inhibition (B) was measured by Fv/Fm. White squares, WT; black squares, T6; black triangle, T10; white circle, T20.
The tocopherol level is known to be environmentally regulated (Fryer, 1992). In accordance, photo-acclimation of young WT leaves was associated with a noticeable accumulation of tocopherols (Table I, “acclimated Y leaves”). The tocopherol level was also increased in the T6 and T10 transgenic lines, but it remained much lower (approximately 50%) than the level measured in WT leaves.
Previous studies have shown that, on a leaf area or leaf weight basis, the carotenoid content of T6, T10, and T20 transformants was lower than the carotenoid content of WT, the decrease being slightly more pronounced for β-carotene and lutein compared with neoxanthin and the sum violaxanthin + antheraxanthin + zeaxanthin (Tanaka et al., 1999; Grasses et al., 2001). This carotenoid decrease was also observed in this study (not shown). We have examined in detail the carotenoid composition of photo-acclimated young leaves (Table II). No significant difference was observed between WT and transgenic leaves with respect to the lutein, neoxanthin, and β-carotene concentrations. In contrast, the pool of xanthophyll cycle pigments (violaxanthin, antheraxanthin, and zeaxanthin) was noticeably increased in T6 (+38%) and T10 (+30%) relative to WT. This increase in the xanthophyll cycle carotenoids was not observed in T20. Thus, photo-acclimation of T6 and T10 leaves involved a selective increase in xanthophyll cycle pigments.
Table II.
Carotenoid concentrations in tobacco leaves acclimated for 2 weeks to strong light stress at low temperature (1,000 μmol m−2 s−1, 10°C)
| Compounds | WT | T6 | T10 | T20 |
|---|---|---|---|---|
| ng cm−2 | ||||
| Neoxanthin | 577 ± 240 | 547 ± 153 | 729 ± 39 | 643 ± 77 |
| Violaxanthin | 665 ± 195 | 1,116 ± 485 | 1,429 ± 116 | 867 ± 175 |
| Antheraxanthin | 652 ± 91 | 853 ± 155 | 662 ± 60 | 625 ± 16 |
| Lutein | 2,198 ± 377 | 2,445 ± 279 | 2,562 ± 266 | 2,433 ± 282 |
| Zeaxanthin | 583 ± 165 | 662 ± 213 | 379 ± 145 | 453 ± 70 |
| β-carotene | 983 ± 191 | 1,049 ± 137 | 1,068 ± 12 | 995 ± 121 |
| V + A + Z | 1,900 ± 176 (100) | 2,632 ± 465 (138) | 2,470 ± 88 (130) | 1,946 ± 190 (102) |
V + A + Z, Sum of violaxanthin, antheraxanthin, and zeaxanthin (the no. in parentheses indicates the V + A + Z level in the transgenics as percentage of the WT level). Data are mean values of three to four experiments ± sd.
We also have examined the concentration of two soluble antioxidants, ascorbate and glutathione, that are involved in tocopherol recycling in thylakoid membranes. No significant difference was found in the total glutathione level between young leaves of WT and transformed plants exposed for 2 weeks to high light at 10°C. The concentration found in those leaves (1,459 ± 18, 1,601 ± 253, and 1,321 ± 180 nmol g−1 fresh weight in WT, T6, and T10 leaves, respectively) corresponds approximately to the double of the glutathione level in control plants grown in low light. Similarly, no accumulation of ascorbate was observed in transgenic leaves relative to WT leaves during photo-acclimation (4,807 ± 511, 2,568 ± 273, and 4,176 ± 739 nmol total ascorbate g−1 fresh weight in WT, T6, and T10 leaves, respectively).
Figure 7 shows the light dependence plot of the quantum yield of photosynthetic electron transport in WT, T6, and T20 young leaves that developed under chilling stress conditions. Photo-/thermo-acclimation of electron transport was obvious in WT leaves (compare with Fig. 1, 10°C); electron transport at 10°C was less rapidly saturated with increasing PFD in leaves acclimated to chilling stress in strong light relative to control leaves. At a given PFD, the efficiency of photosynthetic electron transport was higher in acclimated WT leaves relative to control (nonacclimated) WT leaves. In T6, the light dependence curve of photosynthetic electron transport in acclimated leaves was not significantly modified relative to control growth conditions (Fig. 6 versus Fig. 1). T20 was intermediate between WT and T10. Thus, the phototolerance of young T6 and T10 transgenic leaves cannot be explained by a change in their photosynthetic capacities, which remained virtually constant during acclimation.
Figure 7.
Quantum yield of photosynthetic electron transport (ΔF/Fm′) measured at 10°C in tobacco leaves (WT, T6, and T20 transgenic lines) acclimated for 15 d to chilling stress in high light. White squares, WT; black squares, T6; white circles, T20.
DISCUSSION
Tocopherol Deficiency Increases the Sensitivity of Tobacco Leaves to Photooxidative Stress
Tocopherol deficiency had no detectable effect on photosynthesis of tobacco plants grown in low light (70 μmol photon m−2 s−1). This finding is in agreement with previous studies of tocopherol-deficient plants and cyanobacteria (Collakova and DellaPenna, 2001; Grasses et al., 2001; Porfirova et al., 2002). Similarly, treatment of algal cells with an inhibitor of tocopherol synthesis in low light was shown to decrease the tocopherol level without affecting PSII-mediated electron transport (Trebst et al., 2002). We found also that tocopherol-deficient leaves did not exhibit any increase in the luminescence emission at high temperature, in the MDA content, or in the concentration of antioxidants (carotenoids, glutathione, and ascorbate) relative to the WT, indicating that chronic oxidative stress did not occur. In striking contrast, the sensitivity of tocopherol-deficient tobacco plants to a sudden exposure to excess light energy at low temperature was dramatically increased, and we found that the level of lipid peroxidation under such conditions was related in a quantitative manner with the tocopherol content of the leaves. This was observed both in leaf discs and in attached leaves. In both cases, lipid peroxidation occurred much more rapidly in tocopherol-deficient leaves relative to WT leaves, confirming the idea that tocopherols are important antioxidants specifically involved in the protection of plant membrane lipids against photodestruction. To our knowledge, this is the first in vivo demonstration using genetically modified plants that tocopherols reduce photooxidative stress and lipid peroxidation in plants. This study differs from previous studies of tocopherol-deficient transgenic photosynthetic organisms, which failed to show a specific effect of tocopherols against photooxidative stress (Collakova and DellaPenna, 2001; Grasses et al., 2001; Porfirova et al., 2002). Possibly, the use of appropriate stress treatments (chilling stress + high light) and a sensitive biophysical method (thermoluminescence), which can detect and quantify in situ peroxidative damage of thylakoid membrane lipids, allowed us to observe the antioxidative activity of tocopherols in chloroplasts. The fact that tobacco is sensitive to chilling stress (compared e.g. with Arabidopsis) was also favorable to observe lipid peroxidation under the stress conditions used in this study.
As far as photo-inhibition of PSII photochemistry is concerned, we found relatively little difference between WT and the transgenics exposed to different light stress conditions. Thus, the differential lipid peroxidation observed between WT and the tocopherol-deficient transgenic plants was not associated with a similar difference in PSII photo-inhibition. Our results do not allow us to assign tocopherol a specific role in the maintenance of PSII structure and function, as suggested in green algae (Trebst et al., 2002). Grasses et al. (2001) found that the slowly reversible nonphotochemical quenching component of chlorophyll fluorescence was increased in the transgenic tobacco lines 6 and 10 after a short-term high-light stress at 20°C, suggesting an increased susceptibility to PSII photo-inhibition when tocopherols are reduced. The rate and extent of photo-inhibition in the present study (Figs. 2A and 4A) were much more severe than those reported by Grasses et al. (2001). Perhaps the stress treatment used here was adequate to reveal an increased susceptibility to photooxidation and masked possible differences in photo-inhibition. However, when the intensity of the stress treatment was lowered (Fig. 2B), the apparent rate of PSII photo-inhibition in the chlP transformants did not differ strongly from that of WT. Similarly, PSII inhibition after high-light stress was only slightly increased in the completely tocopherol-deficient vte1 Arabidopsis mutant relative to WT (Porfirova et al., 2002). The fact that PSII photo-inhibition in ChlP transgenic leaves did not differ much from that of WT leaves confirms that geranylgeranylated chlorophyll did not significantly perturb energy transfer and photochemical reactions in the PSs. Therefore, it is unlikely that photooxidation of T6 and T10 leaves was due indirectly to the partial replacement of (phytylated) chlorophyll by geranylgeranylated chlorophyll. This conclusion is supported by the increased sensitivity of T6 leaves to eosin toxicity because in the experiments of Figure 5, 1O2 was generated by direct excitation of the dye and not through chlorophyll excitation.
Tocopherols and Xanthophyll Carotenoids
The low tocopherol concentration in T6 and T10 leaves did not prevent long-term acclimation of the plants to excess light energy. Young T6 and T10 leaves succeeded in acclimating and growing under excess light energy and low temperature conditions. They did not exhibit any lipid peroxidation, showed limited photo-inhibition, and were resistant to the in vitro light stress that caused strong photooxidative damage in nonacclimated leaves. It is likely that the tocopherol deficit was compensated in the long term by other photoprotective mechanisms. One can exclude from the chlorophyll fluorescence measurements presented in this study (Fig. 7) that a change in the photosynthetic electron transport efficiency was involved in the photo-acclimation of young T6 and T10 leaves. Although the tocopherol content increased during photo-acclimation, it remained much lower than that measured in photo-acclimated WT leaves, and it was comparable with that measured in nonacclimated WT leaves, which were sensitive to a sudden exposure to chilling stress in the light (Fig. 4). Moreover, young acclimated leaves were thicker than nonacclimated leaves; the leaf-specific weight increased from around 15 mg fresh weight mm−2 on average (in low light) to around 25 mg mm−2 (in high light) in all genotypes. Thus, in reality, the increase in tocopherol content (per leaf weight unit) was much less pronounced than suggested by the data of Table I. When expressed per milligram of leaf fresh weight, the tocopherol level in T6 and T10 was increased by only 20% to 25% during photo-acclimation at low temperature, whereas the tocopherol concentration in WT leaves increased by about 50% to 60%. It is clear, however, that the drawback of our ChlP transgenic plants is that the tocopherol deficiency is not complete (although this allowed us to correlate leaf phototolerance with tocopherol content, Fig. 3D). We cannot exclude that the capacity of T6 and T10 to acclimate in the long term to high-light stress is related to the residual level of tocopherols.
Neither glutathione nor ascorbate (which are both involved in α-tocopherol regeneration from α-tocopheroxyl radicals) accumulated in the transgenics (relative to WT) during photo-acclimation. A similar phenomenon was observed previously in the high alpine plant Eriophorum angustifolium (Lütz, 1996). Strong irradiation after a frost period resulted in a destruction of the thylakoid membranes with massive losses of α-tocopherol and β-carotene, whereas ascorbate and glutathione remained unaffected, suggesting that the latter compounds were not consumed in a defense reaction. In contrast to soluble antioxidants, a marked increase in the xanthophyll pigment content was found in T6 and T10 leaves relative to WT leaves, and we believe that this phenomenon has a photoprotective value. It is known that one of the functions of zeaxanthin is to prevent accumulation of reactive oxygen species that lead to oxidative damage of membrane through lipid peroxidation (Havaux and Niyogi, 1999; Havaux et al., 2000). In Arabidopsis, overexpression of the chyB gene that encodes β-carotene hydroxylase has been shown to cause a specific increase in the size of the xanthophyll cycle pool and a concomitant enhancement of photooxidative stress tolerance (Davison et al., 2002). Similarly, transgenic tobacco plants overexpressing a bacterial carotene hydroxylase gene synthesized more zeaxanthin in high light than the WT and were more tolerant to UV- and rose bengal-induced lipid peroxidation (Götz et al., 2002). Moreover, it seems that minor amounts of zeaxanthin in the chloroplasts are sufficient for protection against photooxidative damage (Baroli et al., 2003). The exact mechanism by which zeaxanthin inhibits lipid peroxidation is not yet established. Zeaxanthin could block lipid peroxidation by a direct antioxidant action within the membrane lipid phase (Lim et al., 1992; Havaux, 1998), where it could have a synergetic effect on the action of tocopherol (Palozza and Krinsky, 1992). Alternatively, zeaxanthin could inhibit photooxidation by efficient quenching of 1O2 produced in the light-harvesting complexes (Mathews-Roth et al., 1974). Based on the observations reported above, one can interpret the selective accumulation of xanthophyll cycle pigments in T6 and T10 leaves as an adaptive response that compensates for the tocopherol deficiency. Interestingly, photo-acclimation of young leaves of the zeaxanthin-deficient npq1 Arabidopsis mutant was associated with a substantial increase in tocopherols relative to the WT (Havaux et al., 2000). Accumulation of tocopherols when zeaxanthin is absent and vice versa supports the idea that both compounds could have overlapping functions. It is clear that the function of tocopherols must be considered as part of an interconnected network of antioxidants, detoxificative enzymes, and physiological responses. The use of completely tocopherol-deficient mutants and of multiple mutants affected in several components of this network (e.g. mutants deficient in both vitamin E and zeaxanthin) will probably be the next step in the study of tocopherol function and functional redundancy by other antioxidative systems.
MATERIALS AND METHODS
Plant Material and Treatments
Three tobacco (Nicotiana tabacum) lines (named T6, T10, and T20) transformed with antisense chlP were analyzed (Tanaka et al., 1999). These plants are characterized by a reduced activity of geranylgeranyl reductase, the enzyme that catalyzes the reduction of geranylgeranyldiphosphate to phytylphytyl diphosphate in chloroplasts. Because the latter compound is required for tocopherol synthesis, the chlP transgenics are deficient in tocopherols (Tanaka et al., 1999). The effects of the genetic transformation on the pigment content and growth of the plants have been described elsewhere (Tanaka et al., 1999; Grasses et al., 2001). WT tobacco cv Samsun NN and chlP transgenic tobacco plants were grown under controlled conditions in a phytotron: The temperature was 25°C, PFD was 70 μmol m−2 s−1, and photoperiod was 12 h. Light stress was imposed by transferring plants aged 40 d to a growth chamber at 8°C/6°C (day/night air temperature) and under a PFD of 1,000 μmol m−2 s−1. Leaf temperature, measured with an infrared thermometer, was about 10°C. “Mature” leaves corresponded to leaves 5 to 7 (from bottom), and “young” leaves corresponded to the leaves (of approximately 10 cm in length) at the top of the plants. Light stress was also imposed on leaf discs of 16 mm in diameter. The discs were punched out from mature leaves. Leaf discs placed on wet filter paper were exposed to white light (PFD = 1,000 μmol m−2 s−1) produced by 150-W metal halide lamps equipped with two infrared suppressor filters. Leaf temperature was maintained constant at 10°C. Eosin treatments of leaf discs were done as described previously (Havaux et al., 2000). In brief, leaf discs of 1 cm diameter, floating on an aqueous solution of 0.5% (w/v) eosin Y, were illuminated with white light at a PFD of 400 μmol photon m−2 s−1. Temperature of eosin was maintained constant at 22°C.
Photosynthetic Electron Transport
Chlorophyll fluorescence emission from the upper surface of the leaves was measured with a PAM-2000 fluorometer (Walz, Effeltrich, Germany), as previously described (Havaux and Kloppstech, 2001). The maximal quantum yield of PSII photochemistry was measured in dark-adapted sample from the initial fluorescence level (Fo) and the maximal fluorescence level (Fm) as Fv/Fm = (Fm − Fo)/Fm. The quantum yield of PSII-mediated electron transport was measured in illuminated leaves by the ΔF/Fm′ ratio, where Fm′ is the maximal fluorescence level and ΔF is the difference between Fm′ and the steady state fluorescence level (Fs).
Lipid Peroxidation
Thermoluminescence measurements were performed on leaf discs with a custom-built apparatus, as described previously (Havaux and Kloppstech, 2001). In brief, the leaf sample was slowly heated from 25°C to 150°C at a rate of 6°C min−1. Leaf temperature was measured with a tiny K-type thermocouple. Heat-induced luminescence emission was measured with a photomultiplier tube, the current of which was amplified by a transimpedance amplifier. Both leaf temperature and thermoluminescence were recorded by a computer using a DaqPad-1200 data acquisition system (National Instruments, Austin, TX). The amplitude of the 80°C thermoluminescence band was used as an index of lipid peroxidation (Hideg and Vass, 1993; Havaux et al., 2000).
For comparison purposes, MDA, a decomposition product of the oxidation of polyunsaturated fatty acids, was also used as an index of lipid peroxidation (Van Hasselt, 1974; Hodges et al., 1999). The MDA assay is based on the fact that MDA reacts with two molecules of TBA via an acid-catalyzed nucleophilic addition reaction, yielding a pinkish-red chromagen with an absorbance maximum at 532 nm. TBA reactivity was determined according to Van Hasselt (1974). One leaf disc of 1.6 cm in diameter was ground in 1 mL of chilled reagent (0.25% [w/v] TBA in 10% [w/v] trichloroacetic acid). After incubation at 90°C to 95°C for 20 min, the extracts were cooled at room temperature and then centrifuged. TBA reactivity was determined in the supernatant by measuring the A532. Nonspecific turbidy was determined at 600 nm.
Soluble Antioxidants
Total glutathione (reduced + oxidized) was determined by HPLC as described elsewhere (Carrier et al., 2003). Total ascorbate was determined by HPLC using the method described by Wildi and Lütz (1996).
Lipophilic Antioxidants
Leaf discs (1-cm diameter) were frozen in liquid nitrogen and kept at −80°C before analysis. Carotenoids and tocopherols were extracted in 275 μL of pure methanol. After centrifugation and filtration of the extracts, photosynthetic pigments were separated by HPLC with a reverse phase C18 column (Nova Pak, 60 A, 4 μm, 3.9 × 300 mm, Waters, Milford, MA) protected by a Bondapak C18 guard column, using the method developed by Lagarde et al. (2000). The chromatography system consisted of a Waters 600E system controller and a Waters 996 Photodiode Array detector. Pure carotenoids used for calibration were bought from Extrasynthèse (Genay, France) or were prepared by thin-layer chromatography with n-hexane:isopropanol (100:10; v/v) as solvent system. Tocopherols were detected with a Waters 474 scanning fluorescence detector. Excitation wavelength was 295 nm, and emission wavelength was 340 nm. Tocopherol standards were obtained from Sigma (St. Louis).
ACKNOWLEDGMENTS
We thank M. Péan (CEA/Cadarache, Saint-Paul-lez-Durance) and the members of the Groupe de Recherches Appliquées en Phytotechnologie Laboratory (CEA/Cadarache, Saint-Paul-lez-Durance) for help in growing tobacco plants under stress conditions, J. Massimino (CEA/Cadarache) for taking care of the plants, S. Cuiné (CEA/Cadarache) for help in glutathione determination, and A. Haniss (GSF-National Research Center for Environment and Health, Munich) for ascorbate determinations. We would like also to thank P. Jahns (University of Dusseldorf, Germany) for helpful discussion.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017178.
LITERATURE CITED
- Afek HP, Yakir D. Protection by isoprene against singlet oxygen in leaves. Plant Physiol. 2002;129:269–277. doi: 10.1104/pp.010909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroli IM, Do AD, Yamane T, Niyogi KK (2003) Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell (in press) [DOI] [PMC free article] [PubMed]
- Boveris A, Varsavsky A, Goncalves da Silva S, Sanchez RA. Chemiluminescence of soybean seeds: spectral analysis, temperature dependence and effects of inhibitors. Photochem Photobiol. 1983;40:99–104. [Google Scholar]
- Bramley PM, Elmadfa I, Kafatos A, Kelly FJ, Manios Y, Roxborough HE, Schuch W, Sheehy PJA, Wagner K-H. Vitamin E. J Sci Food Agric. 2000;80:913–938. [Google Scholar]
- Carrier P, Baryla A, Havaux M (2003) Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta (in press) [DOI] [PubMed]
- Collakova E, DellaPenna D. Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol. 2001;127:1113–1124. [PMC free article] [PubMed] [Google Scholar]
- Dähnhardt D, Falk J, Appel J, van der Kooij TAW, Schulz-Friedrich R, Krupinska K. The hydroxyphenylpyruvate dioxygenase from Synechocystis sp. PCC 6803 is not required for plastoquinone biosynthesis. FEBS Lett. 2002;523:177–181. doi: 10.1016/s0014-5793(02)02978-2. [DOI] [PubMed] [Google Scholar]
- Davison PA, Hunter CN, Horton P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature. 2002;418:203–206. doi: 10.1038/nature00861. [DOI] [PubMed] [Google Scholar]
- Delong JM, Steffen KL. Photosynthetic function, lipid peroxidation and α-tocopherol content in spinach leaves during exposure to UV-B radiation. Can J Plant Sci. 1997;77:453–459. [Google Scholar]
- Fryer MJ. The antioxidant effects of thylakoid vitamin E (α-tocopherol) Plant Cell Environ. 1992;15:381–392. [Google Scholar]
- Fryer MJ, Andrews JR, Oxborough K, Blowers DA, Baker NR. Relationship between CO2 assimilation, photosynthetic electron transport, and active O-2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 1998;116:571–580. doi: 10.1104/pp.116.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. J Exp Bot. 2002;53:1249–1254. [PubMed] [Google Scholar]
- Götz T, Sandmann G, Römer S. Expression of a bacterial carotene hydroxylase gene (crtZ) enhances UV tolerance in tobacco. Plant Mol Biol. 2002;50:129–142. doi: 10.1023/a:1016072218801. [DOI] [PubMed] [Google Scholar]
- Grasses T, Grimm B, Koroleva O, Jahns P. Loss of α-tocopherol in tobacco plants with decreased geranylgeranyl reductase activity does not modify photosynthesis in optimal growth conditions but increase sensitivity to high-light stress. Planta. 2001;213:620–628. doi: 10.1007/s004250100532. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC, Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998;3:147–151. [Google Scholar]
- Havaux M, Bonfils J-P, Lütz C, Niyogi KK. Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol. 2000;124:273–284. doi: 10.1104/pp.124.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Kloppstech K. The protective functions of carotenoid and flavonoid pigments against excess visible radiation at chilling temperature investigated in Arabidopsis npq and tt mutants. Planta. 2001;213:953–966. doi: 10.1007/s004250100572. [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photo-oxidative damage by more than one mechanism. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Vass I. The 75°C thermoluminescence band of green tissues: chemiluminescence from membrane-chlorophyll interaction. Photochem Photobiol. 1993;58:280–283. [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- Hopia AI, Huang S, Schwarz K, German JB, Frankel EN. Effect of different lipid systems on antioxidant activity of rosemary constituents carnosol and carnosic acid with and without α-tocopherol. J Agric Food Chem. 1996;44:2030–2036. [Google Scholar]
- Knox JP, Dodge AD. The photodynamic action of eosin, a singlet-oxygen generator. Planta. 1985;164:22–34. doi: 10.1007/BF00391021. [DOI] [PubMed] [Google Scholar]
- Lagarde D, Beuf L, Vermaas W. Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. Strain PCC 6803. Appl Environ Microbiol. 2000;66:64–72. doi: 10.1128/aem.66.1.64-72.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta. 1992;1120:178–184. doi: 10.1016/0005-2760(92)90288-7. [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]
- Lütz C. Avoidance of photoinhibition and examples of photodestruction in high alpine Eriophorum. J Plant Physiol. 1996;148:120–128. [Google Scholar]
- Makino T, Kato K, Lyozumi H, Honzawa H, Tachiiri Y, Hiramatsu M. Ultraweak luminescence generated by sweet potato and Fusarium oxysporum interactions associated with a defense response. Photochem Photobiol. 1996;64:953–956. doi: 10.1111/j.1751-1097.1996.tb01860.x. [DOI] [PubMed] [Google Scholar]
- Mathew BG, Roy D. Weak luminescence from the frozen-thawed root tips of Cicer Arietinum L. J Photochem Photobiol B Biol. 1992;12:141–150. [Google Scholar]
- Mathews-Roth MM, Wilson T, Fujimori E, Krinsky NI. Carotenoid chromophore length and protection against photosensitization. Photochem Photobiol. 1974;19:217–222. doi: 10.1111/j.1751-1097.1974.tb06501.x. [DOI] [PubMed] [Google Scholar]
- Minamide Y, Horie T, Tomaru A, Awazu S. Spontaneous chemiluminescence production, lipid peroxidation, and covalent binding in rat hepatocytes exposed to acetaminophen. J Pharmacol Sci. 1998;87:640–646. doi: 10.1021/js9701014. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Fujimoto K, Kinoshita M, Usuki R. Rapid estimation of peroxide content of soybean oil by measuring thermoluminescence. J Am Oil Chem Soc. 1994;71:343–345. [Google Scholar]
- Munné-Bosch S, Alegre L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta. 2000;210:925–931. doi: 10.1007/s004250050699. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L. The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci. 2002;21:31–57. [Google Scholar]
- Palacios A, Piergiacomi VA, Catala A. Vitamin A supplementation inhibits chemiluminescence and lipid peroxidation in isolated rat liver microsomes and mitochondria. Mol Cell Biochem. 1996;154:77–82. doi: 10.1007/BF00248464. [DOI] [PubMed] [Google Scholar]
- Palozza P, Krinsky NI. β-carotene and α-tocopherol are synergistic antioxidants. Arch Biochem Biophys. 1992;297:184–187. doi: 10.1016/0003-9861(92)90658-j. [DOI] [PubMed] [Google Scholar]
- Porfirova S, Bergmüller E, Tropf S, Lemke R, Dörmann P. Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA. 2002;99:12495–12500. doi: 10.1073/pnas.182330899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoden EL, Pereira-Lima L, Telöken C, Lucas ML, Bello-Klein A, Rhoden CR. Beneficial effect of α-tocopherol in renal ischemia-reperfusion in rats. Jpn J Pharmacol. 2001;87:164–166. doi: 10.1254/jjp.87.164. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Oster U, Kruse E, Rüdiger W, Grimm B. Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol. 1999;120:695–704. doi: 10.1104/pp.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A, Depka B, Holländer-Czytko H. A specific role for tocopherol and of chemical singlet oxygen quenchers in the maintenance of photosystem II structure and function in Chlamydomonas reinhardtii. FEBS Lett. 2002;516:156–160. doi: 10.1016/s0014-5793(02)02526-7. [DOI] [PubMed] [Google Scholar]
- Van Hasselt PR. Photo-oxidation of unsaturated lipids in Cucumis leaf discs during chilling. Acta Bot Neerl. 1974;23:159–169. [Google Scholar]
- Vavilin DV, Ducruet J-M. The origin of 115–130°C thermoluminescence bands in chlorophyll containing material. Photochem Photobiol. 1998;68:191–198. [Google Scholar]
- Vavilin DV, Ducruet J-M, Matorin DN, Venediktov PS, Rubin AB. Membrane lipid peroxidation, cell viability and Photosystem II activity in the green alga Chlorella pyrenoidosa subjected to various stress conditions. J Photochem Photobiol B-Biol. 1998;42:233–239. [Google Scholar]
- Wildi B, Lütz C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996;19:138–146. [Google Scholar]
- Wise RR. Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res. 1995;45:79–97. doi: 10.1007/BF00032579. [DOI] [PubMed] [Google Scholar]