Abstract
Starch granules from higher plants contain alternating zones of semicrystalline and amorphous material known as growth rings. The regulation of growth ring formation is not understood. We provide several independent lines of evidence that growth ring formation in the starch granules of potato (Solanum tuberosum) tubers is not under diurnal control. Ring formation is not abolished by growth in constant conditions, and ring periodicity and appearance are relatively unaffected by a change from a 24-h to a 40-h photoperiod, and by alterations in substrate supply to the tuber that are known to affect the diurnal pattern of tuber starch synthesis. Some, but not all, of the features of ring formation are consistent with the involvement of a circadian rhythm. Such a rhythm might operate by changing the relative activities of starch-synthesizing enzymes: Growth ring formation is disrupted in tubers with reduced activity of a major isoform of starch synthase. We suggest that physical as well as biological mechanisms may contribute to the control of ring formation, and that a complex interplay of several factors may by involved.
Starch granules from every higher plant species studied so far contain alternating regions of semicrystalline and amorphous material commonly known as growth rings. Growth rings can be observed by light microscopy, by atomic force microscopy, and by scanning and transmission electron microscopy (SEM and TEM) after treatment of granules with acid or degradative enzymes. These methods reveal that the rings represent alternating concentric layers of high/low refractive index, density, crystallinity, and resistance to chemical and enzymatic attack (Badenhuizen, 1939; Badenhuizen, 1959; Buttrose, 1960; Gallant and Guilbot, 1969; Hall and Sayre, 1973; Baker et al., 2001).
The origin of growth rings remains obscure. Previous studies have suggested that one of two biological mechanisms could regulate their formation. First, their formation could be under the control of a diurnal rhythm that is dependent on day/night variations in the environment, such as a light/dark regime or alternating temperature cycles. Meyer (1895) hypothesized that growth of the granule follows a diurnal rhythm and that one growth ring is laid down per day. Support for this comes from studies of growth rings in the starch of developing cereal endosperm. Granules from barley (Hordeum vulgare) endosperm were claimed to have one growth ring for each day after their initiation (Buttrose, 1960), and growth rings were not visible in granules from the endosperm of wheat (Triticum aestivum) and barley plants grown in constant light and temperature (Van de Sande-Bakhuyzen, 1925; Buttrose, 1960, 1962). Rings reappeared in the peripheral regions of granules when plants grown initially in constant conditions were transferred to a day-night regime during the course of granule development (Buttrose, 1962). These observations lead Buttrose (1962) to propose that growth ring formation is controlled by a diurnal rhythm that is dependent on day/night fluctuations in the supply of Suc, the precursor for starch synthesis.
Second, growth ring formation could be under the control of an endogenous or circadian rhythm (Roberts and Proctor, 1954; Buttrose, 1962). In this case, ring formation would persist in the absence of environmental cycles. In contrast to the situation in cereal endosperm, the few previous studies of potato (Solanum tuberosum) indicate that granules from the tubers of plants grown under constant light and constant temperature retain growth rings (Bünning and Hess, 1954; Mes and Menge, 1954; Roberts and Proctor, 1954; Buttrose, 1962). However, it is unclear how stringently environmental conditions were controlled in these early studies, and it remains possible that external conditions may also have influenced the granule structure.
The aim of this study was to investigate further the control of growth ring formation in the starch granules of potato tubers. To distinguish between diurnal and circadian rhythms, we subjected plant material to constant conditions and altered photoperiods, and studied transgenic plants with altered diurnal patterns of supply of substrate for starch synthesis in the tuber. We also investigate whether growth ring formation is influenced by the structure of starch polymers, and hence by variation in activities of starch-synthesizing enzymes, using transgenic plants with altered activities of major isoforms of starch synthase in the tubers.
RESULTS
Growth under Constant Conditions
To reveal growth rings, granules were subjected to mechanical damage at low temperature and then to enzymic digestion with α-amylase. Rings were examined by SEM on the inner surfaces of granules cracked along the major axis. Granules from tubers grown under 16 h of light and 8 h of dark had well-defined rings that decreased in width from the hilum (point of origin of the ring structure) to the periphery. Ring widths at the distal end were much greater than those at the proximal end (Fig. 1).
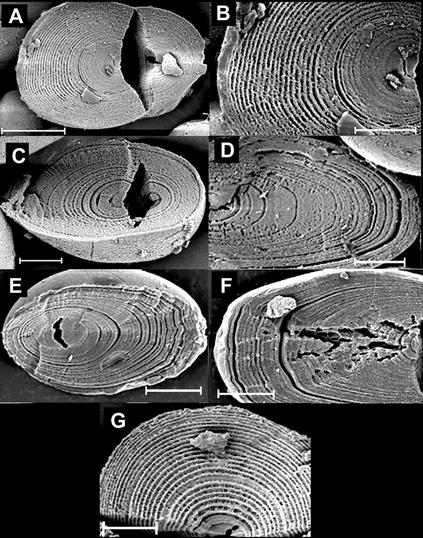
Figure 1.
Growth rings in granules developed under different environmental conditions. Scale bars on A and G represent 10 μm; all other scale bars represent 5 μm. A and B, Plant grown in 16 h of light at 18°C and 8 h of dark at 15°C. C and D, Plant grown in constant light and constant temperature (18°C). E and F, Microtuber grown in continuous darkness at 25°C for 12 to 16 weeks. G, Plant grown in 20 h of light at 18°C and 20 h of dark at 15°C.
The effect on growth rings of development in a constant environment were examined in starch from tubers of plants maintained from the point of planting in constant light, temperature, and humidity (about 3 months growth), and in microtubers developed on stem explants cultured for 12 to 16 weeks at high Suc concentrations in constant darkness at a constant temperature. In both cases, growth rings were present in all of the starch granules examined (Fig. 1). The rings differed from those in granules from plants in normal day-night conditions in that there were prominent “major” rings in which the digested zone was wide, alternating with “minor” rings with narrower digested zones. Major rings were separated by several minor rings. However, other studies show that the occurrence of major and minor rings is not specific to constant conditions: major and minor rings have been observed in granules from plants grown under normal day-night conditions by TEM after acid-etching and sectioning (Frey-Wyssling and Buttrose, 1961). Minor rings were poorly defined in starch from tubers grown under constant conditions, and ring width could not be measured accurately. In granules from microtubers, in which minor rings were better defined, the width of rings was markedly different from that in granules from tubers grown under normal conditions. There was less change in ring width from the center to the periphery of the granule, and less difference between the proximal and distal ends of the granule (Fig. 2).
Figure 2.
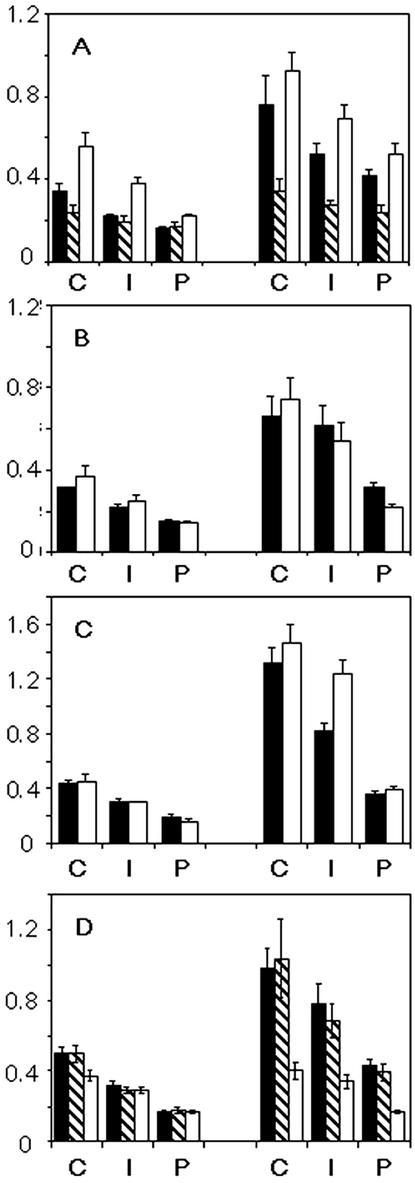
Widths of growth rings in starch granules. Growth ring width was calculated for the proximal (end closer to the hilum) and distal ends of the granule by measuring the distance from the innermost visible ring to the periphery perpendicular to the curve of the growth rings. The distance was divided into three equal segments: center (C), intermediate (I), and periphery (P). The number of rings in each segment was counted and the average ring width (micrometers, shown on the y-axis) was calculated. Measurements were conducted on 10 granules from each sample, and bars show ses. A, Comparison of granules from tubers of plants grown under a normal 16-h light/8-h dark regime (black bars), from microtubers grown in constant conditions (shaded bars), and from tubers of plants grown under a 20-h light/20-h dark regime (white bars). B, Comparison of granules from transgenic plants with reduced cytosolic FBPase activity (white bars), and control plants with normal FBPase grown under the same conditions at the same time (black bars). C, Comparison of granules from transgenic plants with reduced SPS activity (white bars), and control plants with normal SPS grown under the same conditions at the same time (black bars). D, Comparison of granules from untransformed plants (black bars), transgenic plants with reduced granule-bound starch synthase (GBSS) activity (shaded bars), and transgenic plants with reduced activity of GBSS and SSIII (white bars).
Plants with Alterations in the Pattern of Suc Supply to the Tuber
The diurnal rhythm most likely to influence growth ring formation is that displayed by the supply of Suc from the leaves to the tubers. The rate of supply of Suc—the substrate for starch synthesis—is higher during the day than during the night. The rate of starch synthesis in the tuber is influenced by this pattern: It is about twice as high at the end of the day than at the end of the night (Geigenberger and Stitt, 2000).
Alterations in the rate of supply of substrate for starch synthesis, and in the rate of synthesis itself, could potentially affect the organization of the granule matrix in three ways. First, changes in the concentration of ADP-Glc can affect the relative activities of isoforms of starch synthase, and hence starch structure (Van den Koornhuyse et al., 1996; Clarke et al., 1999). GBSS has a much lower affinity for ADP-Glc than soluble isoforms, and there appear to be differences between the soluble isoforms in their affinities for this substrate (Clarke et al., 1999; Edwards et al., 1999a; Lloyd et al., 1999a). There is indirect evidence that changes in ADP-Glc concentrations in vivo do affect the composition and structure of starch polymers in potato tubers (Geigenberger et al., 2001). Second, changes in the availability of Suc in a starch-synthesizing organ are likely to have far-reaching effects on a wide range of metabolite concentrations and potentially on concentrations of other cellular components. These changes in the chemical environment could influence the organization of newly formed amylopectin molecules. Third, it is theoretically possible that the rate of synthesis of amylopectin determines the manner in which it becomes organized to form the granule matrix.
To investigate directly whether diurnal variation in substrate supply has any impact on growth ring formation, we used two types of transgenic potato in which the diurnal pattern of supply of Suc to the tuber is altered. Reduction in cytosolic Fru 1,6-bisphosphatase (FBPase, an enzyme involved in synthesis of Suc from triose phosphate in the leaf) reduces the rate of Suc synthesis from products of photosynthesis during the day, resulting in accumulation of starch in the chloroplast (Zrenner et al., 1996). At night, this starch is degraded to Glc, which is converted to Suc via a pathway that does not involve FBPase. These and other plants with reductions in the capacity to convert triose phosphate to Suc during the day show large changes in the diurnal pattern of export of Suc from the leaf (Heineke et al., 1994; Kehr et al., 1998). Thus, the supply of Suc to the tuber in FBPase antisense plants is reduced during the day and is greatly enhanced at night relative to that in normal plants. Reduction in Suc phosphate synthase (SPS) reduces Suc synthesis in the leaf during the day and the night, resulting in an altered pattern of Suc export from the leaf. This is known to affect the diurnal pattern of tuber starch synthesis: The rates at the end of the day and the end of the night are almost identical in these plants (Geigenberger et al., 2000). We found that starch granules from tubers of transgenic plants with reduced FBPase or reduced SPS had growth rings apparently identical in structure to those of control plants grown under identical conditions (Fig. 3). The widths of rings in the two sets of plants were also very similar (Fig. 2).
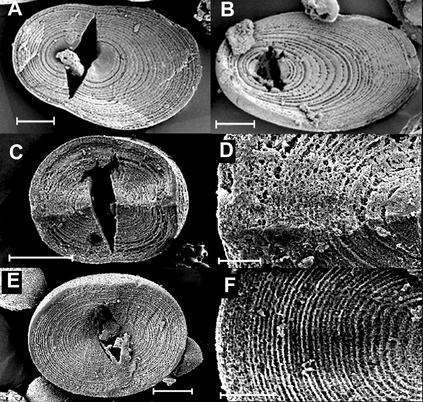
Figure 3.
Growth rings in granules from tubers of transgenic plants with altered carbohydrate supply or altered activities of starch-synthesizing enzymes. Scale bars on A, B, and E represent 5 μm, the scale bar on C represents 10 μm, and the scale bars on D and F represent 2 μm. A, Plant with reduced cytosolic FBPase activity (line F-70), grown in 16 h of light and 8 h of dark. B, Plant with reduced SPS activity (line 1–74), grown in 16 h of light and 8 h of dark. C and D, Plant with reduced activity of SSIII. E and F, Plant with reduced activity of GBSS and SSIII.
Growth in a 40-h Photoperiod
The persistence of ring formation in constant conditions suggests that a circadian rhythm could be involved. To investigate this possibility, we grew plants under a 20-h light/20-h dark regime and compared rings in these tuber starch granules with those of plants grown under normal 16-h light/8-h dark conditions. Circadian rhythms in plants can usually be entrained to periods of between 18 and 30 h (Bünning, 1967). If the period is greater than 30 h, entrainment does not occur and the organism shows a natural rhythm of about 24 h even after several weeks or months. Thus, if growth ring formation is dependent on a circadian rhythm, the width of rings should be unaffected by growth in the abnormal 40-h regime.
Growth rings in tuber starch granules from plants grown under a 40-h regime looked like those from plants grown under the 24-h regime. The width of the rings at the distal end was approximately the same in granules from the two sets of plants. However, the width of rings at the proximal end was about 1.7-fold greater in granules from plants grown under the 40-h regime. Thus, the hilum was more central in granules grown under the longer period (Figs. 1 and 2).
Plants with Altered Starch Synthase Activity
A circadian rhythm could potentially regulate growth ring formation by causing periodic changes in the relative activities of starch-synthesizing enzymes. Starch synthases are responsible for elongation of the chains of amylose and amylopectin, the two glucans that make up the granule. Reductions in activity of specific isoforms have distinct, well-documented effects on polymer structure and composition (Edwards et al., 1999b; Lloyd et al., 1999b; Fulton et al., 2002). Thus, diurnal variations in the relative activities of these enzymes could potentially generate periodic variations in the structure and/or composition of matrix of the granule, leading to the formation of zones with different levels of organization and crystallinity.
To discover whether the relative activities of isoforms of starch synthase can influence growth ring formation, and, hence, whether a circadian mechanism might operate via these enzymes, we examined starch from tubers of transgenic potatoes with altered activities of one or both of the two isoforms of highest activity in the tuber, GBSS and starch synthase III (SSIII).
GBSS is exclusively responsible for the synthesis of the amylose component of starch. Granules from transgenic potatoes with reduced activity of GBSS (GBSS antisense lines) contain amylose in the center of the granule but not at the periphery (Kuipers et al., 1994; Tatge et al., 1999). Growth ring width and general appearance was normal in the amylose-free regions of granules from a GBSS antisense line (Fulton et al., 2002; Fig. 2D; data not shown). This result indicates that amylose is not necessary for growth ring formation in potato starch granules: The periodic change in organization of the granule matrix is a function of a change in the amylopectin component of the granule.
SSIII is the major isoform responsible for amylopectin synthesis in the tuber (Abel et al., 1996; Marshall et al., 1996). Amylopectin in transgenic lines with reduced activity of SSIII (SSIII antisense lines) differs from normal amylopectin in the distribution of lengths of its shorter chains. Very long chains are also more abundant than normal, the size of amylose molecules is increased, and granules are deeply lobed and fissured (Fulton et al., 2002). As we reported previously, individual growth rings in starch granules from the tubers of an SSIII antisense line were much less distinct and regular in appearance than those of control plants (Fulton et al., 2002; Fig. 3 and supplemental material available at www.plantphysiol.org). The lack of distinct growth rings in the SSIII antisense granules is unlikely to be due to an overall increase in resistance to enzymatic attack, as these granules were often more digested overall than those of untransformed tubers.
In potatoes in which activity of GBSS and SSIII is reduced (SSIII/GBSS antisense lines), the distribution of lengths of the shorter chains of amylopectin is like that of SSIII antisense lines. However, very long chains are no more abundant than normal, amylose molecular mass is also normal, and granules are not fissured (Fulton et al., 2002). Unlike those of the SSIII antisense line, growth rings in a SSIII/GBSS antisense line were indistinguishable in appearance from those of normal plants (Fig. 3). The distribution of rings was different from that in normal plants in that ring width was similar at the proximal and distal ends: In other words, the hilum was more central and the granules were more spherical than in normal plants (Figs. 2 and 3; Fulton et al., 2002).
Overall, these results indicate that growth ring formation is susceptible to changes in the structures of starch polymers. The very long amylopectin chains and/or larger amylose molecules present in the starch of SSIII antisense plants appear to disrupt the long-range organization of the matrix resulting in fissuring and the disruption of growth ring development.
DISCUSSION
Our data provide several independent lines of evidence that growth ring formation in tuber starch granules is not primarily controlled by diurnal rhythms. First, ring formation is not abolished by growth of plants under constant conditions of light, temperature, and humidity, or in microtubers that develop in darkness with constant temperature and carbohydrate supply. Second, growth of plants under a 40-h photoperiod with 20 h of light and 20 h of dark does not result in a 1.7-fold increase in ring width, as would be expected if ring formation were under diurnal control. Third, there are no differences in ring formation and appearance in tubers of plants in which the diurnal pattern of supply of Suc to the tuber has been radically altered (FBPase antisense plants). Finally, growth rings are present in tubers in which the normal diurnal variation in the rate of starch synthesis is known to have been abolished (SPS antisense plants). These results confirm and expand on those of early studies of growth ring formation in tubers in which growth of plants in constant conditions did not abolish rings observed by light microscopy and by TEM of acid-digested granules (Bünning and Hess, 1954; Mes and Menge, 1954; Roberts and Proctor, 1954; Buttrose, 1962). Our results also confirm that mechanisms governing growth ring formation in potato tubers are different from those in cereal endosperm. Several reports show that growth of cereal plants in constant conditions abolishes growth ring formation, and it has been speculated that in this case, ring formation is governed by diurnal variation in supply of substrate from the leaves (Van de Sande-Bakhuysen, 1925; Buttrose, 1962).
An alternative explanation for the formation of growth rings in potato starch, suggested by earlier work, is some form of circadian rhythm. The existence of a circadian rhythm is consistent with the observation that ring formation continues during growth in constant conditions, and when diurnal variation in substrate supply and in the rate of starch synthesis is abolished. A circadian rhythm could bring about growth ring formation via periodic changes in the relative activities of starch-synthesizing enzymes. We have shown that changes in the relative activities of isoforms of starch synthase have a marked impact on growth ring formation. However, our results as a whole indicate that factors other than a circadian rhythm are also involved in growth ring formation. The differences in ring widths between granules from plants in normal conditions, plants grown under a 40-h photoperiod, and material grown in constant conditions cannot be explained if a circadian rhythm alone determines ring formation.
We suggest three factors that might account for the alterations in ring periodicity in constant conditions or in a 40-h photoperiod. First, although diurnal rhythms appear to have no impact on growth ring formation in normal conditions, it remains possible that they interact with and modify the impact of a circadian rhythm under a 40-h photoperiod. Second, ring periodicity under abnormal growth conditions may be influenced by altered levels of expression of starch-synthesizing enzymes under these conditions. We have shown that a simultaneous decrease in SSIII and GBSS (in SSIII/GBSS antisense plants) causes ring widths at the proximal and distal ends to become more similar. Thus, the changes in ring periodicity in constant conditions and in 40-h photoperiods might be attributable to modification of the impact of a circadian rhythm by alterations in starch-synthesizing enzymes.
Third, growth ring formation might be determined, at least in part, by a physical mechanism rather than a circadian rhythm. The semicrystalline nature of the matrix is attributable to the arrangement of the branch points of amylopectin at regular intervals along the axis of the molecules, leading to periodic clustering of the shorter chains of the molecule (Hizukuri et al., 1983; Hizukuri, 1986). Within the clusters, double helices form between adjacent chains. These pack in ordered arrays within the granule, giving rise to alternating crystalline and amorphous lamellae (representing the packed helices and the intervening regions containing branch points, respectively) with a periodicity of 9 nm (Jenkins et al., 1993). Recent analyses suggest that amylopectin may be a side chain liquid-crystalline polymer: a self-organizing structure in which the double helices are the mesogens that align to form the matrix (Waigh et al., 1998). One growth ring contains a semicrystalline zone consisting of some tens of lamellar repeats, and an amorphous zone in which the amylopectin is in less-ordered form. It can be speculated that the branching structure of amylopectin results in a progressive change in the packing of double helices, and hence a progressively less-ordered lamellar structure, as successive lamellae crystallize during granule synthesis. If this is the case, a point may be reached at which the regular packing of double helices is no longer energetically the most favorable arrangement, the semicrystalline packing breaks down, and an amorphous zone is formed. This would release stresses on the matrix and allow the development of a semicrystalline zone to resume. It is interesting to note that the organization of the crystallites in cereal starches, in which a diurnal rhythm apparently controls growth ring formation, is very different from that in potato starch (Gidley, 1987; Jane et al., 1997). Our present understanding does not allow the implications of these differences for growth ring formation to be assessed, but we speculate that any physical constraints on the growth of the semicrystalline zone will be different in cereal and potato starches.
Overall, our data raise the possibility that circadian rhythms, physical mechanisms, and perhaps diurnal rhythms could all contribute to the control of growth ring formation in starch granules of potato tubers. We suggest that a complex interplay of several factors may well be involved.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Microtubers
Potato (Solanum tuberosum) plantlets were derived from sterilized stem sections (3 cm in length and containing auxiliary meristems) of greenhouse-grown plants of cv Desiree. The plantlets were grown under 16 h of light/8 h of darkness at 20°C for 3 to 5 weeks on Murashige and Skoog medium containing 8 g L−1 Difco Bacta Agar and 30 g L−1 Suc. Stem pieces with a single node were then transferred to Murashige and Skoog medium containing 80 g L−1 Suc, 2.5 mg L−1 benzylaminopurine, and 8 g L−1 Difco Bacta Agar and were kept in continuous darkness at 25°C for 12 to 16 weeks before harvest of microtubers.
Potato Plants
All potato plants were cv Desiree and transgenic lines derived from this cultivar. They were grown from plantlets propagated as described above. Tubers expressing antisense mRNA for cytosolic FBPase (line F-70; Zrenner et al., 1996) and SPS (line 1–74; Geigenberger and Stitt, 2000) were kind gifts from Dr. Uwe Sonnewald (Institut für Pflanzengenetik und Kulturpflanzenforschung, Gatersleben, Germany) and Dr. Peter Geigenberger (Max-Planck Institut für Molekulare Pflanzenphysiologie, Golm, Germany), respectively. Plants expressing antisense mRNA for SSIII and/or GBSS were as described by Fulton et al. (2002). Plants expressing antisense mRNA for SPS and appropriate controls were grown by Dr. Peter Geigenberger under precisely the conditions described in Geigenberger and Stitt (2000), and tubers were supplied for starch extractions. All other plants were grown in Norwich in 25-cm pots of soil-based compost. Minimum temperature in the greenhouse was 12°C, and supplementary lighting was supplied in winter. Pots in the controlled environment room were on a bed of damp dry-weave matting to maintain constant water supply. Three sets of conditions were used: constant light, constant temperature (18°C); 16 h of light at 18°C, 8 h of darkness at 15°C; and 20 h of light at 18°C, 20 h of darkness at 15°C. In all cases, the humidity was 70% and the light was approximately 400 μmol quanta m−2 s−1.
Extraction of Starch
Starch was extracted from potato tubers according to Edwards et al. (1995). Starch was extracted from freshly harvested microtubers by the same method except that 30 g of tissue was extracted in 25 mL of extraction medium using a mortar and pestle. The resuspension and centrifugation steps were carried out in 50 mL of extraction medium. The final pellet was washed three times with acetone at −20°C, dried, and stored at −20°C.
Preparation of Granules for SEM
To crack starch granules, dry starch (0.3 g for potato tuber and 0.1 g for microtuber) was suspended in 1 mL of water, frozen in liquid N2, and then ground in a mortar until the slurry began to thaw. The slurry was frozen and ground three additional times.
Cracked starch granules were suspended at 100 mg mL−1 in 100 mm MES-NaOH, pH 6.0, and 100 to 200 units of α-amylase (EC 3.2.1.1; from porcine pancreas; Roche Molecular Biochemicals, Lewes, East Sussex, UK) at 37°C for 16h. The samples were then centrifuged at 10,000g and the pellet was washed three times in acetone at −20°C, dried, and stored at −20°C.
Observation of Granules by SEM
Dry starch samples were brushed onto the surface of double-sided, carbonated sticky stills (Leit-tabs) attached to SEM stubs. The stills and stubs were from Agar Scientific (Cambridge, UK). Mounted samples were coated with platinum for 2.5 min at 10 mA in an argon atmosphere, using a cryotransfer system (CT1500 HFl Oxford Instruments, Oxford) attached to the SEM. The coated stubs were transferred to a field emission gun SEM (XL30; Phillips, Eindhoven, The Netherlands) and imaged at 3 kV.
Determination of Growth Ring Distribution
Ten granules from each SEM sample, all cracked along their major axis, were analyzed from digital images obtained from the SEM. Growth ring distribution was measured at the proximal and distal ends of the granule (Fig. 2). For both ends, the distance from the innermost visible ring to the periphery was measured perpendicular to the curve of the growth ring and was divided into three equal segments: center, intermediate, and periphery. The number of rings in each segment was counted and the average ring width (in micrometers) was calculated.
ACKNOWLEDGMENTS
We thank Prof. Athene Donald (Department of Physics, University of Cambridge, UK) and Dr. Ruth Bastow (John Innes Centre) for valuable discussions.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (United Kingdom).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018044.
LITERATURE CITED
- Abel GJW, Springer F, Willmitzer L, Kossmann J. Cloning and functional analysis of a cDNA encoding a novel 139-kDa starch synthase from potato (Solanum tuberosum L.) Plant J. 1996;10:981–991. doi: 10.1046/j.1365-313x.1996.10060981.x. [DOI] [PubMed] [Google Scholar]
- Badenhuizen NP. Growth and corrosion of the starch granule in connection with our present knowledge of the microscopical and chemical organization. Z für Bot. 1939;33:140–468. [Google Scholar]
- Badenhuizen NP. Chemistry and biology of the starch granule. In: Heiltrun LV, Weber F, editors. Handbuch der Protoplasmaforschung. Berlin: Springer-Verlag; 1959. pp. 1–73. [Google Scholar]
- Baker AA, Miles MJ, Helbert W. Internal structure of the starch granule revealed by AFM. Carbohydr Res. 2001;330:249–256. doi: 10.1016/s0008-6215(00)00275-5. [DOI] [PubMed] [Google Scholar]
- Bünning E. The Physiological Clock. New York: Longmans; 1967. [Google Scholar]
- Bünning E, Hess C. Schichtenbildung im Stärkekorn bei Konstanten Aussenbedingungen. Naturwiss. 1954;41:339. [Google Scholar]
- Buttrose MS. Submicroscopic development and structure of starch granules in cereal endosperms. J Ultrastruct Res. 1960;4:231–257. doi: 10.1016/s0022-5320(60)80021-4. [DOI] [PubMed] [Google Scholar]
- Buttrose MS. The influence of environment on the shell structure of starch granules. J Cell Biol. 1962;14:159–167. doi: 10.1083/jcb.14.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BR, Denyer K, Jenner CJ, Smith AM. The relationship between the rate of starch synthesis, the adenosine 5′-diphosphoglucose concentration and the amylose content of starch in developing pea embryos. Planta. 1999;209:324–329. doi: 10.1007/s004250050639. [DOI] [PubMed] [Google Scholar]
- Edwards A, Borthakur A, Bornemann S, Venail J, Denyer K, Waite D, Fulton D, Smith AM, Martin C. Specificity of starch synthase isoforms of potato. Eur J Biochem. 1999a;266:724–736. doi: 10.1046/j.1432-1327.1999.00861.x. [DOI] [PubMed] [Google Scholar]
- Edwards A, Fulton DC, Hylton CM, Jobling SA, Gidley M, Rössner U, Martin C, Smith AM. A combined reduction in activity of starch synthases II and III of potato has novel effects on the starch of tubers. Plant J. 1999b;17:251–261. [Google Scholar]
- Edwards A, Marshall J, Sidebottom C, Visser RGF, Smith AM, Martin C. Biochemical characterisation of a novel starch synthase from potato tubers. Plant J. 1995;8:183–294. doi: 10.1046/j.1365-313x.1995.08020283.x. [DOI] [PubMed] [Google Scholar]
- Frey-Wyssling A, Buttrose MS. Makromolekulare Feinstlamellen in der Körnen der Kartoffel Stärke. Makromol Chem. 1961;44:173–178. [Google Scholar]
- Fulton DC, Edwards A, Pilling E, Robinson HL, Fahy B, Seale R, Kato L, Donald AM, Geigenberger P, Martin C et al. Role of granule-bound starch synthase in determination of amylopectin structure and starch granule morphology in potato. J Biol Chem. 2002;277:10834–10841. doi: 10.1074/jbc.M111579200. [DOI] [PubMed] [Google Scholar]
- Gallant DJ, Guilbot A. Étude de l'ultrastructure du grain d'amidon à l'aide de nouvelles méthodes de préparation en microscopie électronique. Starch-Stärke. 1969;6:156–163. [Google Scholar]
- Geigenberger P, Stamme C, Tjaden J, Schulz A, Quick PW, Betsche T, Kersting HJ, Neuhaus HE. Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol. 2001;125:1667–1678. doi: 10.1104/pp.125.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M. Diurnal changes in sucrose, nucleotides, starch synthesis and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J. 2000;23:795–806. doi: 10.1046/j.1365-313x.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- Gidley MJ. Factors affecting the crystalline type (A-C) of native starches and model compounds: a rationalisation of observed effects in terms of polymorphic structures. Carbohydr Res. 1987;161:301–304. [Google Scholar]
- Hall DM, Sayre JG. A comparison of starch granules as seen by both scanning and ordinary light microscopy. Starch-Stärke. 1973;25:292–297. [Google Scholar]
- Heineke D, Kruse A, Flügge UI, Frommer WB, Riesmeier JW, Willmitzer L, Heldt HW. Effect of antisense repression of the chloroplast triose-phosphate translocator on photosynthetic metabolism in transgenic potato plants. Planta. 1994;193:174–180. [Google Scholar]
- Hizukuri S. Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydr Res. 1986;147:342–347. [Google Scholar]
- Hizukuri S, Kaneko T, Takeda Y. Measurement of the chain length of amylopectin and its relevance to the origin of crystalline polymorphism of starch granules. Biochim Biophys Acta. 1983;760:188–191. [Google Scholar]
- Jane JL, Wong K, McPherson AE. Branch-structure difference in starches of A- and B-type X-ray patterns revealed by their Naegeli dextrins. Carbohydr Res. 1997;300:219–227. [Google Scholar]
- Jenkins PJ, Cameron RE, Donald AM. A universal feature in the structure of starch granules from different botanical sources. Starch-Stärke. 1993;45:417–420. [Google Scholar]
- Kehr J, Hustiak F, Walz C, Willmitzer L, Fisahn J. Transgenic plants changed in carbon allocation pattern display a shift in diurnal growth pattern. Plant J. 1998;16:497–503. doi: 10.1046/j.1365-313x.1998.00318.x. [DOI] [PubMed] [Google Scholar]
- Kuipers AG, Jacobsen E, Visser RGF. Formation and deposition of amylose in the potato tuber are affected by the reduction of granule-bound starch synthase gene expression. Plant Cell. 1994;6:43–52. doi: 10.1105/tpc.6.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Landschütze V, Kossmann J. Simultaneous antisense inhibition of two starch synthase isoforms in potato tubers leads to accumulation of grossly modified amylopectin. Biochem J. 1999b;338:515–521. [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR, Springer F, Buléon A, Müller-Röber B, Willmitzer L, Kossmann J. The influence of alterations in ADP-glucose pyrophosphorylase activities on starch structure and composition in potato tubers. Planta. 1999a;209:230–238. doi: 10.1007/s004250050627. [DOI] [PubMed] [Google Scholar]
- Marshall J, Sidebottom C, Debet M, Martin C, Smith AM, Edwards A. Identification of the major starch synthase in the soluble fraction of potato tubers. Plant Cell. 1996;8:1121–1135. doi: 10.1105/tpc.8.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes MG, Menge I. Potato shoot and tuber cultures in vitro. Physiol Plant. 1954;7:637. [Google Scholar]
- Meyer A. Untersuchungen über die Stärkekörner. Jena, Germany: Fischer; 1895. [Google Scholar]
- Roberts EA, Proctor BE. The appearance of starch grains of potato tubers of plants grown under constant light and temperature conditions. Science. 1954;119:509–510. doi: 10.1126/science.119.3094.509. [DOI] [PubMed] [Google Scholar]
- Tatge H, Marshall J, Martin C, Edwards EA, Smith AM. Evidence that amylose synthesis occurs within the matrix of the starch granules in potato tubers. Plant Cell Environ. 1999;22:543–550. [Google Scholar]
- Van de Sande-Bakhuysen HL. The structure of starch grains from wheat grown under constant conditions. Proc Soc Exp Biol Med. 1925;23:302–304. [Google Scholar]
- Van den Koornhuyse N, Libessart N, Delrue B, Zabawinski C, Decq A, Iglesias A, Carton A, Preiss J, Ball S. Control of starch composition and structure through substrate supply in the monocellular alga Chlamydomonas reinhardtii. J Biol Chem. 1996;271:16281–16287. doi: 10.1074/jbc.271.27.16281. [DOI] [PubMed] [Google Scholar]
- Waigh TA, Perry P, Reikel C, Gidley M, Donald AM. Chiral side-chain liquid-crystalline polymeric properties of starch. Macromolecules. 1998;31:7980–7984. [Google Scholar]
- Zrenner R, Krause KP, Apel P, Sonnewald U. Reduction of the cytosolic fructose-1,6-bisphosphatase in transgenic potato plants limits photosynthetic sucrose biosynthesis with no impact on plant growth and tuber yield. Plant J. 1996;9:671–681. doi: 10.1046/j.1365-313x.1996.9050671.x. [DOI] [PubMed] [Google Scholar]