Abstract
The IPO (ipomoelin) gene was isolated from sweet potato (Ipomoea batatas cv Tainung 57) and used as a molecular probe to investigate its regulation by hydrogen peroxide (H2O2) and nitric oxide (NO) after sweet potato was wounded. The expression of the IPO gene was stimulated by H2O2 whether or not the plant was wounded, but its expression after wounding was totally suppressed by the presence of diphenylene iodonium, an inhibitor of NADPH oxidase, both in the local and systemic leaves of sweet potato. These results imply that a signal transduction resulting from the mechanical wounding of sweet potato may involve NADPH oxidase, which produces endogenous H2O2 to stimulate the expression of the IPO gene. The production of H2O2 was also required for methyl jasmonate to stimulate the IPO gene expression. On the contrary, NO delayed the expression of the IPO gene, whereas NG-monomethyl-l-arginine monoacetate, an inhibitor of NO synthase, enhanced the expression of the IPO gene after the plant was wounded. This study also demonstrates that the production of H2O2 stained with 3,3′-diaminobenzidine hydrochloride could be stimulated by wounding but was suppressed in the presence of NO. Meanwhile, the generation of NO was visualized by confocal scanning microscope in the presence of 4,5-diaminofluorescein diacetate after sweet potato was wounded. In conclusion, when sweet potato was wounded, both H2O2 and NO were produced to modulate the plant's defense system. Together, H2O2 and NO regulate the expression of the IPO gene, and their interaction might further stimulate plants to protect themselves from invasions by pathogens and herbivores.
Environmental stresses may lead plants to generate reactive oxygen species (ROS), which include hydrogen peroxide (H2O2) and superoxide (O2−; Bolwell, 1999). Production of excessive ROS may damage cells. When ROS is generated at a controlled level, cells can use these reactive molecules as signals to activate certain genes against attacks by pathogens and herbivores (for review, see Van Breusegem et al., 2001). Therefore, plants have evolved highly organized mechanisms for regulating the level of ROS to maximize benefit to themselves.
H2O2 could be generated during normal cellular metabolism after various environmental stresses, such as an excess of light, drought, or cold (Dat et al., 2000). Mechanical wounding also stimulates the leaves of several plant species to produce H2O2 locally and systemically (Bergey et al., 1999; Orozco-Cárdenas and Ryan, 1999). The massive production of H2O2 could initiate a localized hypersensitive response, a form of programmed cell death, which appeared to limit and block pathogen development (Levine et al., 1994). H2O2 may further activate defense genes such as proteinase inhibitors I and II as it diffuses to adjacent cells (Alvarez et al., 1998; Orozco-Cárdenas et al., 2001). Also, the generation of H2O2 seems to be mediated by a membrane-bound NADPH oxidase complex in plants (Lamb and Dixon, 1997; Del Rio et al., 1998; Potikha et al., 1999; Pei et al., 2000), and some chemicals that inhibit NADPH oxidase in mammals also block H2O2 production in plants (Levine et al., 1994; Auh and Murphy, 1995; Alvarez et al., 1998; Orozco-Cárdenas and Ryan, 1999).
Nitric oxide (NO) regulates diverse developmental and physiological processes in plants and is involved in growth and differentiation (Gouvéa et al., 1997; Leshem et al., 1998), senescence (Leshem et al., 1998), and seed germination (Keeley and Fotheringham, 1997; Beligni and Lamattina, 2000). Also, NO was shown to act as a signal regulating defense genes to hasten disease resistance in soybean (Glycine max; Delledonne et al., 1998). Mechanical stresses, such as centrifugation, induced Arabidopsis to produce NO, which further caused DNA fragmentation (Garcés et al., 2001). Although the presence and functions of NO have been well studied in animals, the mechanism for the production of NO remains unclear in plants (Beligni and Lamattina, 2001). In animals, NO synthase (NOS) generating NO from l-Arg was identified (Bredt et al., 1991), and NOS activities have also been found in pea (Pisum sativum) and maize (Zea mays; Barroso et al., 1999; Kondo et al., 1999). However, no gene or protein with a sequence highly similar to animals' NOS has been shown in plants (Beligni and Lamattina, 2001). Also, the production of NO in plants is not restricted to NOS-like activity, and NO can be generated from NO2 either through a light-mediated conversion by carotenoids (Cooney et al., 1994) or from nitrate reductase (Yamasaki et al., 1999; Yamasaki and Sakihama, 2000).
The NO molecule contains an unpaired electron and, thus, can react with ROS to affect cellular metabolism. Under an ordinary physiological condition, superoxide dismutase rapidly converts O2− to H2O2 and an oxygen molecule. However, a large amount of NO may combine with O2− to form peroxynitrite (ONOO−), which has been reported to damage lipids, proteins, and nucleic acids (Lipton et al., 1993; Yamasaki et al., 1999). Nevertheless, O2− and H2O2 are more toxic than NO and ONOO−; therefore, NO may protect cells from destruction (Wink et al., 1993). In accordance, NO has been suggested to have dual roles, either toxic or protective, depending on its environments (Beligni and Lamattina, 1999, 2001).
Sweet potato (Ipomoea batatas cv Tainung 57) is an important crop and a major source of starch worldwide; therefore, there is a wide interest in studying the mechanisms it uses to protect against environmental stresses. The expression of the IPO (ipomoelin) gene in sweet potato was shown to be enhanced by the application of methyl jasmonate (MeJA) and mechanical wounding (Imanishi et al., 1997). However, regulations of the expression of this gene and its physiological roles remain unclear. Because wounding could enhance the expression of the IPO gene, the effects of H2O2 and NO on its expression were studied. Also, the interaction between H2O2 and NO in regulating the expression of the IPO gene was investigated.
RESULTS
Stimulation of IPO Gene Expression by H2O2
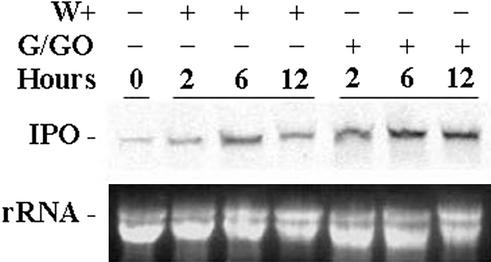
The expression of the IPO gene is induced by environmental stress such as mechanical wounding locally and systemically (Imanishi et al., 1997). Mechanical wounding induces plants to produce H2O2 (Bergey et al., 1999; Orozco-Cárdenas and Ryan, 1999). Therefore, it is an interesting topic to investigate whether oxidative stress like H2O2 can influence the expression of the IPO gene and also whether H2O2 is involved in the signal transduction to systemic leaves. Glc together with Glc oxidase (G/GO) was used to provide plant tissues with a continuous generation of H2O2 (Levine et al., 1994; Alvarez et al., 1998). The cut petioles of leaves of sweet potato were immersed in 1× Murashige and Skoog solutions for 12 h to reduce the wounding effect due to the separation of leaf petiole cuttings from plants. Then, G/GO was added to generate H2O2, or the leaves were wounded for comparison. For another 2, 6, and 12 h, their total RNA was isolated and analyzed by northern blotting (Fig. 1). High-level expression of the IPO gene was observed 2 h later and remained for up to 12 h after H2O2 was generated. Also, the amount of IPO mRNA accumulation stimulated by H2O2 was greater than that of the stimulation caused by mechanical wounding at every time point tested (Fig. 1). Because neither Glc nor G/GO alone could enhance the expression of the IPO gene (data not shown), the stimulation of the expression of the IPO gene was presumably attributable to H2O2.
Figure 1.
H2O2 induces the expression of the IPO gene. The second and third fully expanded leaves counting from the terminal bud of a sweet potato were excised, and their cut petioles were immersed in 1× Murashige and Skoog solution for 12 h. Glc and G/GO were added to generate H2O2, or leaves were wounded (W+) using forceps, and 2, 6 and 12 h later, their total RNA was isolated and analyzed by northern blotting to detect the amount of IPO mRNA. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Results for untreated leaves are included for comparison.
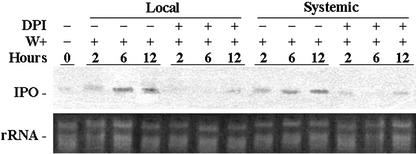
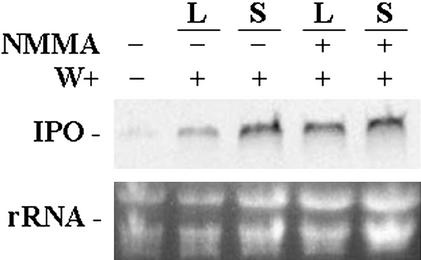
The activation of NADPH oxidase complex is believed to participate in the accumulation of H2O2 in plants (Lamb and Dixon, 1997; Del Rio et al., 1998; Potikha et al., 1999; Pei et al., 2000). Diphenylene iodonium (DPI), a chemical inhibitor of NADPH oxidase, could inhibit the generation of ROS and the accumulation of H2O2 after plants were wounded or pathogen infected (Orozco-Cárdenas et al., 2001) and was used to examine further the role of H2O2 in stimulating the expression of the IPO gene. Plants with six to eight fully developed leaves were excised at the base of their stems, and DPI was added for 12 h. The third and fourth fully expanded leaves from the terminal bud were then wounded using forceps as local injuries. The first and second fully expanded leaves, which were not wounded on the same plant, were treated as the systemic leaves. Plants without DPI treatment were operated in the same way for comparison (Fig. 2). After the plants were incubated with DPI, the expression of their IPO genes was significantly decreased. DPI inhibited the expression of the IPO gene not only in the local but also in the systemic leaves. Without DPI treatment, the expression of the IPO gene was stimulated by mechanical wounding locally and systematically (Fig. 2). This result suggests that the signal transduction due to the mechanical wounding of sweet potato might include the activation of NADPH oxidase, which produces endogenous H2O2 to stimulate the IPO gene. Also, the ability of DPI to block the systemic expression of the IPO gene suggests that either H2O2 participates in the production of systemic signals, or H2O2 by itself is the systemic signal inducing the expression of the IPO gene in the systemic leaves.
Figure 2.
DPI inhibits the expression of the IPO gene induced by mechanical wounding. Plants with six to eight fully developed leaves were excised at the base of their stems, and the cut stems were immersed in 1× Murashige and Skoog for 12 h. The cut stems were treated with or without DPI, inhibiting NADPH oxidase to produce H2O2, at the final concentration of 0.2 mm, and plants were incubated for another 12 h. The third and fourth fully expanded leaves counting from the terminal bud were then wounded (W+) using forceps to inflict local injuries. The first and second unwounded fully expanded leaves in the same plant were treated as systemic leaves. For another 2, 6, and 12 h, their total RNA was isolated and analyzed by northern blotting to detect the amount of IPO mRNA. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Results from leaves without any treatment are included for comparison.
Requirement of H2O2 for MeJA to Stimulate IPO Gene Expression
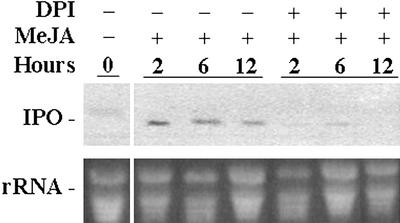
To evaluate the effect of H2O2 on stimulation of the IPO gene by MeJA, DPI was added for 12 h before MeJA was put into solution for 2, 6, and 12 h, respectively (Fig. 3). Northern analysis revealed that the expression of the IPO gene was blocked at all times in the presence of DPI. Without DPI treatment, MeJA stimulated the expression of the IPO gene normally (Fig. 3). Therefore, the action of MeJA in inducing the IPO gene appeared to require the generation of H2O2. Also, in the signal transduction pathway after wounding, the position of H2O2 is downstream of that of MeJA.
Figure 3.
DPI inhibits the expression of the IPO gene induced by MeJA. Leaf petiole cuttings were immersed in 1× Murashige and Skoog for 12 h, and some cuttings were supplied with 0.2 mm DPI, an NADPH inhibitor, for another 12 h. All leaves were then treated with 50 μm MeJA, and their total RNA was extracted 2, 6, and 12 h later before analysis by northern blotting to detect the amount of IPO mRNA. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Results for untreated leaves are included for comparison.
Interference of NO in the Expression of the IPO Gene
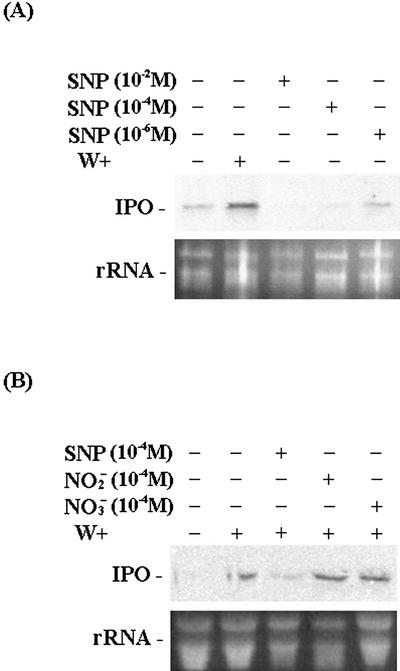
Oxidative stress produced by environments includes not only H2O2 but also NO. Sodium nitroprusside (SNP), a nonenzymatic NO donor, was used to examine the function of NO in regulating the IPO gene. The cut petioles of the excised leaves were put into 1× Murashige and Skoog for 12 h. SNP was added to the concentration of 10−2, 10−4, and 10−6 m for 12 h. Northern analysis of IPO mRNA level indicates that SNP by itself cannot induce the expression of the IPO gene (Fig. 4A). Furthermore, SNP, sodium nitrite, and sodium nitrate were also applied to the leaf petioles of sweet potato, respectively, for 12 h. Then, leaves were wounded for another 6 h before RNA was isolated for northern analysis (Fig. 4B). The presence of SNP decreased the expression of IPO after sweet potato was wounded. Therefore, the application of sodium nitrate or sodium nitrite did not influence the accumulation of IPO mRNA induced by mechanical wounding. Therefore, SNP, the NO donor, but not sodium nitrate and sodium nitrite reduced the expression of the IPO gene.
Figure 4.
Effects of NO donor SNP, nitrite, and nitrate on the expression of the IPO gene after wounding. Leaf petiole cuttings were immersed in 1× Murashige and Skoog for 12 h and were then treated with 10−2, 10−4, or 10−6 m SNP (A) or 10−4 m SNP, 10−4 m sodium nitrite, 10−4 m sodium nitrate, or water as a control for 12 h (B). Leaves in B were then wounded (W+) using forceps for another 6 h. Their total RNA was analyzed by northern blotting to detect the amount of IPO mRNA. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Results for untreated leaves are also included for comparison.
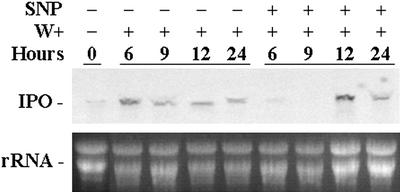
The period of time between SNP application and wounding was extended to elucidate further the impact of NO on the expression of the IPO gene. The cut petioles of the excised leaves were placed in 1× Murashige and Skoog for 12 h, and SNP was added to a concentration of 0.1 mm for 12 h. Leaves were then wounded for another 6 to 24 h before RNA was isolated for analysis. Although the IPO gene was not expressed in the presence of SNP at the time points of 6 and 9 h, it was surprising that it appeared at the time points of 12 and 24 h (Fig. 5). Without SNP treatment, the IPO gene was expressed from the time point of 6 to 24 h (Fig. 5). These results imply that NO did not totally inhibit the expression of the IPO gene but rather delayed it.
Figure 5.
NO postpones the expression of the IPO gene induced by mechanical wounding. Cut petioles of the excised leaves were immersed in 1× Murashige and Skoog for 12 h, and some were treated with 0.1 mm SNP for another 12 h. All these leaves were then wounded (W+) using forceps, and their total RNA was analyzed by northern blotting to detect the amount of IPO mRNA 6, 9, 12, and 24 h later. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Results for untreated leaves are included for comparison.
NO Generation after Mechanical Wounding
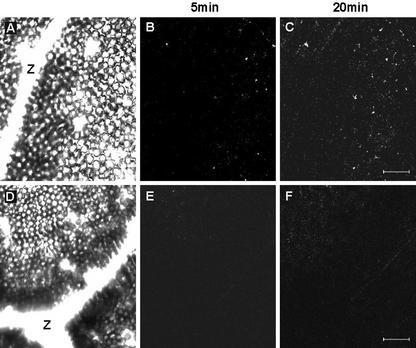
The application of exogenous NO affected the expression of IPO gene; hence, it is important to understand whether sweet potato produces NO by itself. The fluorescent probe 4,5-diaminofluorescein diacetate (DAF-2DA) is highly specific for NO and does not react with other ROS (Foissner et al., 2000). Therefore, the leaves of sweet potato were treated with or without NG-monomethyl-l-Arg monoacetate (NMMA) and were probed by DAF-2DA. Then, the leaves of sweet potato were wounded using a needle, and images of the NMMA-treated (Fig. 6, A–C) and -untreated (Fig. 6, D–F) leaves were taken by confocal scanning microscopy 5 and 20 min later. Compared with the image of leaves without NMMA treatment taken at 5 min, the image at 20 min showed that significant amounts of NO were produced 20 min after wounding (Fig. 6, B and C). Also, in the presence of NMMA, the induction of NO was not observed (Fig. 6, E and F). These images clearly indicate that mechanical wounding stimulates the production of NO through the activation of NOS, and also that NO is a transduction signal for wounding in sweet potato.
Figure 6.
Mechanical wounding induces NO generation. Leaf pieces (0.5 cm2) of sweet potato were immersed in loading buffer for 16 h and were added with or without 0.5 mm NMMA, an NADPH oxidase inhibitor, for another 6 h. DAF-2DA was then added at a final concentration of 10 μm. After being washed by loading buffer, leaf pieces were wounded using a needle and examined by confocal scanning laser microscope, whose wavelength for excitation is 488 nm and for emission is 515 nm. Bright-field image of the wounded leaf without NMMA treatment is shown in A, and images of this area were taken by confocal scanning microscope at 5 (B) and 20 (C) min after wounding. Bright-field image of the wounded leaf treated with NMMA is shown in D, and images of this area were taken by confocal scanning microscope at 5 (E) and 20 (F) min after wounding. Z, Position of a vein. Bar = 40 μm.
Effects of NO on the Production and Function of H2O2
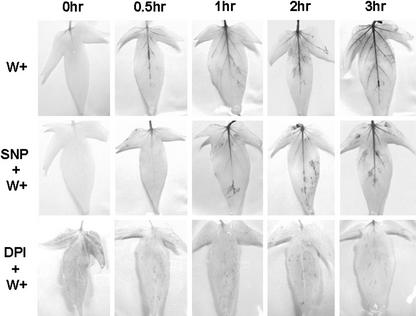
The action of H2O2 in sweet potato was visualized by 3,3′-diaminobenzidine hydrochloride (DAB) staining. DAB binding to H2O2 undergoes a polymerization reaction to yield a dark-brown color (Thordal-Christensen et al., 1997). Without SNP and DPI treatment, the dark-brown color was first observed at the midrib 0.5 h after wounding and spread to the lateral and minor veins 1 h later (Fig. 7). This finding implies that mechanical wounding induced the production of H2O2 in sweet potato within 0.5 h. However, the midrib of the leaves treated with SNP became dark brown at least 1 h after leaves were wounded and was lighter in color than leaves without SNP treatment at every time point tested (Fig. 7). In the presence of DPI, the color of the midrib in leaves did not change color at all, and this indicated that the dark color from DAB comes from H2O2. Therefore, NO appeared to reduce and delay the production of H2O2, thereby postponing the expression of the IPO gene.
Figure 7.
NO reduces the production of the wound-induced H2O2. The first and second fully developed leaves counting from the terminal bud of sweet potato were immersed in 1× Murashige and Skoog for 12 h and then placed in solution with 0.1 mm SNP, 0.2 mm DPI, or 1× Murashige and Skoog for another 12 h. After DAB was added, leaves were wounded (W+) using forceps 6 h later. For another 0, 0.5, 1, 2, and 3 h, the whole leaves were immersed in 96% (w/v) boiling ethanol for 10 min to decolorize the chloroplast. After cooling, the leaves were stored in ethanol and photographed.
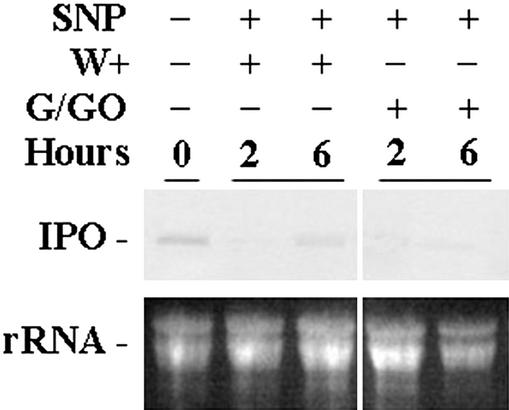
NO not only reduces the production of H2O2 but also inhibits the function of H2O2. The cut petioles of the excised leaves were treated with the NO donor SNP first, and then G/GO releasing H2O2 was added for 2 and 6 h before RNA was isolated. The result shows that in the presence of NO, the ability of H2O2 to stimulate the expression of the IPO gene was totally inhibited (Fig. 8). Conceivably, NO not only reduced the production of H2O2 but also decreased the function of H2O2 in stimulating the IPO gene expression.
Figure 8.
NO inhibits the expression of the IPO gene induced by H2O2. Cut petioles of the excised leaves were placed in 1× Murashige and Skoog for 12 h, and SNP was added at a concentration of 0.1 mm for another 12 h. These leaves were wounded (W+) or treated with Glc and G/GO to produce H2O2. Their total RNA was isolated 2 and 6 h later to detect the amount of IPO mRNA by northern blotting. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Results for untreated leaves are included for comparison.
Effects of NO Deprivation on IPO Gene Expression
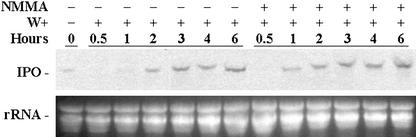
No NOS in plants has been found, but NOS activity was reported in plants (Barroso et al., 1999; Kondo et al., 1999). NMMA is an NOS inhibitor and is widely used for studying the function of NO within cells (Oddis et al., 1994). Plants with six to eight fully developed leaves were excised at the base of their stems and incubated in a solution with NMMA. The third and fourth fully expanded leaves from the terminal bud were then wounded using forceps as local injuries, and the first and second unwounded fully expanded leaves in the same plant were treated as the systemic leaves. Northern analysis indicates that the IPO gene was expressed in both local and systemic leaves with or without NMMA treatment (Fig. 9). Also, local leaves treated with NMMA had more IPO mRNA than those without NMMA treatment. This result implies that wounding the leaves of sweet potato might activate an NOS-like enzyme, which produces NO. Therefore, inhibiting NO production during wounding promotes the expression of the IPO gene, and this result is constant with that in Figure 5, which shows that the presence of NO reduced the expression of the IPO gene. This conclusion is further supported by the time point assays after wounding in the presence of NMMA (Fig. 10). In the leaves treated with NMMA, IPO mRNA was produced 1 h after wounding; however, without NMMA treatment, their IPO mRNA appeared in the leaves 2 h later after wounding (Fig. 10). This finding agrees with that of Figure 9 and indicates that inhibition of the generation of NO accelerated the expression of the IPO gene after wounding.
Figure 9.
Effect of NMMA on the expression of IPO gene in local and systemic leaves. Plants with six to eight fully developed leaves were excised at the base of their stems, and their cut stems were immersed in 1× Murashige and Skoog for 12 h. NMMA, inhibiting NOS-producing NO, was added to a final concentration of 0.5 mm for another 12 h, and the third and fourth fully expanded leaves counting from the terminal bud were then wounded (W+) using forceps as local (L) injuries. The first and second unwounded fully expanded leaves in the same plant were treated as systemic (S) leaves. Their total RNA was isolated and analyzed by northern blotting to detect the amount of IPO mRNA 6 h later. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Result for untreated leaves is included for comparison.
Figure 10.
NOS inhibitor NMMA accelerates the expression of the IPO gene. Cut petioles of the excised leaves were placed in 1× Murashige and Skoog for 12 h, and some petioles were treated with 0.5 mm NMMA to inhibit NOS producing NO for another 12 h. Leaves were then wounded (W+), and 0, 0.5, 1, 2, 3, 4 and 6 h later, their total RNA was isolated to detect the amount of IPO mRNA by northern blotting. Ethidium bromide-stained agarose gel presents ribosome RNA (rRNA) as a loading control. Results for untreated leaves are included for comparison.
DISCUSSION
Wounding has been shown to stimulate the production of H2O2 in tobacco (Nicotiana tabacum) and cassava (Manihot esculenta Crantz.) plants (Repka, 1999). This study also demonstrates that H2O2 was generated in mechanically wounded leaves of sweet potato (Fig. 7). Furthermore, among 18 plant species from six families examined, 14 of them produced H2O2 in the wounded leaves (Orozco-Cárdenas and Ryan, 1999). Also, DAB staining revealed the presence of wound-induced H2O2 in the systemic leaves of tomato (Lycopersicon esculentum) plant (Orozco-Cárdenas and Ryan, 1999). Thus, the generation of H2O2 after wounding seems to be widespread in the plant kingdom and protects plants from attack by herbivores.
In the presence of DPI, which inhibits the generation of H2O2from NADPH oxidase, the expression of the IPO gene in sweet potato was blocked not only in local but also in systemic leaves (Fig. 2). Two possible explanations exist. First, a systemin-like protein may be present in sweet potato and signals H2O2 to activate the IPO gene in systemic leaves. In the presence of DPI, even though wounding could stimulate the production of a systemin-like protein, DPI inhibited the production of H2O2 and, thus, suppressed the systemic expression of the IPO gene. This deduction is supported by the finding that systemin functions as a first messenger of the wounding signal to the systemic leaves, and H2O2 acts downstream of systemin and is considered to be a second messenger in tomato (Orozco-Cárdenas et al., 2001). Second, DPI blocking the systemic expression of the IPO gene may suggest that H2O2 participates in the production of a systemic signal, or that H2O2 itself or a related compound derived from H2O2 is the systemic signal that stimulates the expression of the IPO gene in systemic leaves. This claim is supported by a recent finding that the production of MeJA in response to wounding or systemin was required to produce a long-distance signal to systemic leaves (Li et al., 2002). Also, the function of MeJA to stimulate the IPO gene was dependent on the production of H2O2 (Fig. 3). These results perhaps imply that the production of H2O2 was required for the generation of a systemic signal to be sent to the distal leaves.
Within cells, H2O2 can be generated via several different metabolite pathways (Dat et al., 2000). However, the inhibition of NADPH oxidase by DPI blocked the expression of the IPO gene in both local and systemic leaves after wounding (Fig. 2). This finding implies that NADPH oxidase regulates the production of H2O2 in the local and systemic leaves of sweet potato and that the wound signal must pass through the sole transducer, H2O2, to stimulate the expression of the IPO gene. Similarly, the presence of DPI reduced the effectiveness of MeJA (Fig. 3), indicating that the signal transduction pathway from MeJA to stimulate IPO gene must go via H2O2. In addition, DAB staining revealed that MeJA stimulated the production of H2O2 in tomato (Orozco-Cárdenas and Ryan, 1999). Therefore, MeJA might activate the membrane-bound NADPH oxidase to produce H2O2, which further induced the expression of the IPO gene. Also, this pathway occurred in both local and systemic leaves.
NO has also been demonstrated to regulate plants' defense systems against pathogens (Dangl, 1998; Durner and Klessig, 1999). The IPO gene was induced by mechanical wounding and MeJA (Fig. 3); therefore, it might be related to the defense system in sweet potato. SNP, an NO donor, delayed the expression of the IPO gene after sweet potato was mechanically wounded (Fig. 5), and at the same time the presence of NMMA, an NOS inhibitor, accelerated the expression of the IPO gene (Fig. 10). These results may suggest that the presence of NO interferes with the wounding signal in stimulating the IPO gene. Also, NOS-like proteins have been identified in plants by animal anti-NOS antiserum (Kuo et al., 1995; Sen and Cheema, 1995; Barroso et al., 1999), and the accelerated expression of the IPO gene by NMMA may further indicate that NO was generated from an NOS-like protein after the sweet potato was wounded. Furthermore, NO was produced after sweet potato was wounded (Fig. 6). Therefore, sweet potato after wounding generated both H2O2 and NO within cells. H2O2 induced the expression of the IPO gene, whereas NO interfered in its expression.
The NO donor SNP inhibited the expression of the IPO gene at the first few hours, and delayed its expression until 12 h after sweet potato was wounded (Fig. 5). The inhibition of SNP in the wound-inducible protein was also observed in tomato plants. Synthesis of proteinase inhibitor I was repressed by SNP after tomato was wounded or treated with systemin (Orozco-Cárdenas and Ryan, 2002). However, SNP only blocked the production of H2O2 induced by systemin and MeJA in tomato but not the H2O2 generated from G/GO (Orozco-Cárdenas and Ryan, 2002). As a consequence, the production of proteinase inhibitor I induced by H2O2 generated from G/GO was not affected by SNP; therefore, it was concluded that NO influenced the signal pathway downstream from MeJA synthesis and upstream of H2O2 synthesis (Orozco-Cárdenas and Ryan, 2002). However, SNP reduced the production of H2O2 generated from mechanical wounding (Fig. 7) and inhibited the expression of the IPO gene induced by both mechanical wounding and G/GO (Figs. 5 and 8). Therefore, NO appeared to affect both the production and function of H2O2, and interacted with the components in the signal pathway upstream and downstream of H2O2 synthesis in sweet potato.
Upon wounding, both H2O2 and NO were produced in sweet potato (Figs. 6 and 7), and then both might participate in plants' defense system. The interaction between H2O2 and NO generates at least two effects. First, H2O2 and NO may react synergistically to initiate a hypersensitive response, which promotes cell death in the cells infected with pathogens and limits further invasion by the pathogens (Delledonne et al., 1998). Second, NO can react with O2−, which can change to H2O2 within cells to form ONOO−; this may, in turn, damage proteins, lipids, and nucleic acids (Lipton et al., 1993) to generate antimicrobial effects (Durner and Klessig, 1999). Also, the presence of NO not only reduced the amount of H2O2 produced by sweet potato after wounding (Fig. 7) but also postponed the expression of the IPO gene (Fig. 5). Thus, the decline in the amount of H2O2 might be due to the interaction between H2O2 and NO to form ONOO−. The expression of the IPO gene might not be the first priority in protecting sweet potato from invasion by pathogens and herbivores. In accordance, NO cooperating with H2O2 modulates the plant's defense system and delays the expression of the IPO gene.
Mechanical wounding causes sweet potato to produce MeJA, which activates NADPH oxidase to generate H2O2. Wounding simultaneously causes the NOS-like protein to generate NO. H2O2 and NO cooperatively and quickly initiate the defense system, including programmed cell death at the wounded or infected cells to limit the possibility of further attack on neighboring healthy cells by pathogens or herbivores. Also, ONOO−, formed by H2O2 and NO, may damage the pathogens' proteins, lipids, and nucleic acids. Later, H2O2 activates a slow defense system, which may induce the systemic expression of genes, such as the IPO gene, to protect plants from further invasion. Thus, plants have developed delicate defense systems to survive in nature.
MATERIALS AND METHODS
Plant Materials and Treatments
Sweet potato (Ipomoea batatas cv Tainung 57) plants were vegetatively propagated from cuttings and grown in a controlled environment (16-h/25°C day, 8-h/22°C night, humidity 70%, light 100 μmol photons m−2 s−1). Plants with six to eight fully developed leaves were used. For the experiments involving local and systemic leaves, plants were excised at the base of the stem with a razor blade, and their cut stems were used. Local injury was defined as mechanical wounding at the third and fourth fully expanded leaves counting from the terminal bud. In addition, the first and second fully expanded leaves, which were not wounded on the same plant, were taken as the systemic leaves. For the experiments using a single leaf, the second or third fully expanded leaves from the terminal bud was excised, and its cut petioles was used. Plants were wounded by pressing leaves with forceps.
For those experiments involving one treatment with chemical reagents, cut stems or cut petioles were incubated in 1× Murashige and Skoog (Murashige and Skoog, 1962; pH 5.8) for 12 h. Then, plants were wounded or treated with 50 μm Glc with 2.5 units mL−1 G/GO, SNP (10, 0.1, or 0.001 mm), 0.1 mm sodium nitrite, or 0.1 mm sodium nitrate for the time indicated in each assay. For those experiments involving two chemical treatments, cut stems or cut petioles were also incubated in 1× Murashige and Skoog for 12 h. After 0.2 mm DPI, 0.1 mm SNP or 0.5 mm NMMA was added for another 12 h, and plants were wounded or treated with 50 μm MeJA or 50 μm Glc with 2.5 units mL−1 G/GO for the time indicated in each assay. All reagents were from Sigma (St Louis). DPI is an inhibitor for NADPH oxidase, SNP is a nonenzymatic NO donor, and NMMA is an NOS inhibitor.
RNA Isolation and Analysis
Total RNA was isolated from liquid N2-ground leaves following the procedure described by Chomzynski and Sacchi (1987) except that guanidium-HCl rather than guanidium thiocyanate was used. The quantity of RNA was estimated using a spectophotometer, and its quality was determined by agarose gel electrophoresis with formaldehyde. Total RNA (10 μg) was loaded and separated on formaldehyde-agarose gels and transferred to nylon membranes before hybridization with radiolabeled probes (Sambrook et al., 1989). The radiolabeled IPO was produced in PCR using the IPO cDNA template isolated from a subtraction library (Y.-C. Chen and S.-T. Jeng, unpublished result). Prehybridization was undertaken in 5× SSPE (0.05 m NaH2PO4 [pH 6.8], 0.9 m NaCl, and 5 mm EDTA), 0.5% (w/v) SDS, 5× Denhard's solution (0.1% [w/v] Ficoll, 0.1% [w/v] bovine serum albumin, and 0.1% [w/v] polyvinyl pyrrolidine) at 65°C for 1 h. After the radiolabeled probe was added, hybridization was performed under the same conditions for 16 h. Blots were washed twice in 0.1× SSPE and 0.1% (w/v) SDS at 65°C for 15 min. Radioactive blots were displayed on the PhosphorImager (Molecular Dynamics, Sunnyvale, CA), and their autoradiographs were printed on an XLS 8600 PS printer (Eastman-Kodak, Rochester, NY). All experiments were repeated at least three times, and similar results were obtained.
H2O2 Detection by DAB Staining
H2O2 was visualized by staining with DAB (Thordal-Christensen et al., 1997). DAB undergoes polymerization reaction to yield a dark-brown color once it encounters H2O2 (Thordal-Christensen et al., 1997). The first and second fully developed leaves counting from the terminal bud of a sweet potato were excised, and the leaf petiole cuttings were immersed in 1× Murashige and Skoog for 12 h. The Murashige and Skoog solution was then added with or without 0.1 mm SNP, an NO donor, or 0.2 mm DPI, an NADPH oxidase inhibitor, for 12 h before 1 mg mL−1 DAB was put into solution for another 6 h. Leaves were then wounded using forceps, and 0, 0.5, 1, 2, and 3 h later, leaves were immersed in 96% (w/v) boiling ethanol for 10 min to decolorize the chloroplast but not the deep-brown polymerization product formed by DAB with H2O2. After cooling, the leaves were kept in the ethanol and photographed.
Visualization of NO
Fully expanding leaves were cut into small pieces of 0.5 cm2 in area and immersed in loading buffer (10 mm Tris-KCl [pH 7.2]) for 16 h. Leaf pieces were then transferred to the loading buffer with or without 0.5 mm NMMA for another 6 h. After adding DAF-2DA (Calbiochem, La Jolla, CA) at a final concentration of 10 μm, leaf pieces were incubated in the dark for 1 h (Foissner et al., 2000; Pedroso et al., 2000). The extra DAF-2DA was removed by washing leaf pieces with loading buffer for 30 min. Leaf pieces were wounded with a needle before images were taken using a TCS-SP2 confocal laser scanning microscope (Leica Lasertechnik GmbH, Heidelberg) 5 and 20 min later (excitation = 488 nm and emission = 515 nm).
ACKNOWLEDGMENT
We are grateful to Dr. Chia-Yin Tsai (Department of Botany, National Taiwan University) for helpful discussion.
Footnotes
This work was supported by the National Science Council (grant no. 90–2311–B–002–039 to S.-T. J.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.015701.
LITERATURE CITED
- Alvarez ME, Penell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Auh C-K, Murphy TM. Plasma membrane redox enzyme is involved in the synthesis of O2 and H2O2 by phytophthrona elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, Lupiánez JA, del Río LA. Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem. 1999;274:36729–36733. doi: 10.1074/jbc.274.51.36729. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Is nitric oxide toxic or protective? Trends Plant Sci. 1999;4:299–300. doi: 10.1016/s1360-1385(99)01451-x. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide induces seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide in plants: the history is just beginning. Plant Cell Environ. 2001;24:267–278. [Google Scholar]
- Bergey DR, Orozco-Cárdenas ML, de Monra DS, Ryan CA. A wound- and systemic-inducible polygalacturonase in tomato leaves. Proc Natl Acad Sci USA. 1999;96:1756–1760. doi: 10.1073/pnas.96.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP. Role of active oxygen species and NO in plant defence responses. Curr Opin Plant Biol. 1999;2:287–294. doi: 10.1016/S1369-5266(99)80051-X. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351:714–717. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Chomzynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooney RV, Harwood PJ, Custer LJ, Franke AA. Light-mediated conversion of nitrogen dioxide to nitric oxide by carotenoids. Environ Health Perspect. 1994;102:460–462. doi: 10.1289/ehp.94102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangl J. Innate immunity: Plants just say NO to pathogens. Nature. 1998;394:525–527. doi: 10.1038/28958. [DOI] [PubMed] [Google Scholar]
- Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F. Dual action of the Active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Del Rio LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jiménez A, López-Huertas E, Hernandez JA. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998;116:1195–1200. doi: 10.1104/pp.116.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Klessig DF. Nitric oxide as a signal in plants. Curr Opin Plant Biol. 1999;2:369–374. doi: 10.1016/s1369-5266(99)00007-2. [DOI] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 2000;23:817–824. doi: 10.1046/j.1365-313x.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Garcés H, Durzan D, Pedroso MC. Mechanical stress elicits nitric oxide formation and DNA fragmentation in Arabidopsis thaliana. Ann Bot. 2001;87:567–574. [Google Scholar]
- Gouvéa CMCP, Souza JF, Magalhâes ACN, Martins IS. NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul. 1997;21:183–187. [Google Scholar]
- Imanishi S, Kito-Nakamura K, Matsuoka K, Morikami A, Nakamura K. A major jasmonate-inducible protein of sweet potato, ipomoelin, is an ABA-independent wound-inducible protein. Plant Cell Physiol. 1997;38:643–652. doi: 10.1093/oxfordjournals.pcp.a029216. [DOI] [PubMed] [Google Scholar]
- Keeley JE, Fotheringham CJ. Trace gas emissions and smoke-induced seed germination. Science. 1997;276:1248–1250. [Google Scholar]
- Kondo R, Tikunova SB, Cho MJ, Johnson JD. A point mutation in a plant calmodulin is responsible for its inhibition of nitric-oxide synthase. J Biol Chem. 1999;274:36213–36218. doi: 10.1074/jbc.274.51.36213. [DOI] [PubMed] [Google Scholar]
- Kuo WN, Kuo TW, Jones DL, Baptiste J., Jr Nitric oxide synthase immunoreactivity in baker's yeast, lobster and wheat germ. Biochem Arch. 1995;11:73–78. [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Phys. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Leshem Y, Wills RBH, Ku VV. Evidence for the function of the free radical gas, nitric oxide (NO.), as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem. 1998;36:825–833. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Li L, Li C, Lee GI, Howe GA. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci USA. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Yun-Beom CH, Pan Z-H, Le SZ, Vincent Chen H-S, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Oddis CV, Simmons RL, Hattler BG, Finkel MS. Chronotropic effects of cytokines and the nitric oxide synthase inhibitor, l-NMMA, on cardiac myocytes. Biochem Biophys Res Commun. 1994;205:992–997. doi: 10.1006/bbrc.1994.2764. [DOI] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan CA. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 1999;96:6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan CA. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 2002;130:487–493. doi: 10.1104/pp.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso MC, Magalhaes JR, Durzan D. A nitric oxide burst precedes apoptosis in angiosperm and gymnosperm callus cells and foliar tissues. J Exp Bot. 2000;51:1027–1036. doi: 10.1093/jexbot/51.347.1027. [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JJ. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999;119:849–858. doi: 10.1104/pp.119.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka V. Improved histochemical test for in situ detection of hydrogen peroxide in cells undergoing oxidative burst or lignification. Biol Plant. 1999;42:599–607. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sen S, Cheema IR. Nitric oxide synthase and calmodulin immunoreactivity in plant embryonic tissue. Biochem Arch. 1995;11:221–227. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Van Breusegem F, Vranová E, Dat JF, Inzé D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001;161:405–414. [Google Scholar]
- Wink DA, Hanbauer I, Krishna MC, DeGraff W, Gamson J, Mitchell JB. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Nat Acad Sci USA. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci. 1999;4:128–129. doi: 10.1016/s1360-1385(99)01393-x. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000;468:89–92. doi: 10.1016/s0014-5793(00)01203-5. [DOI] [PubMed] [Google Scholar]