Abstract
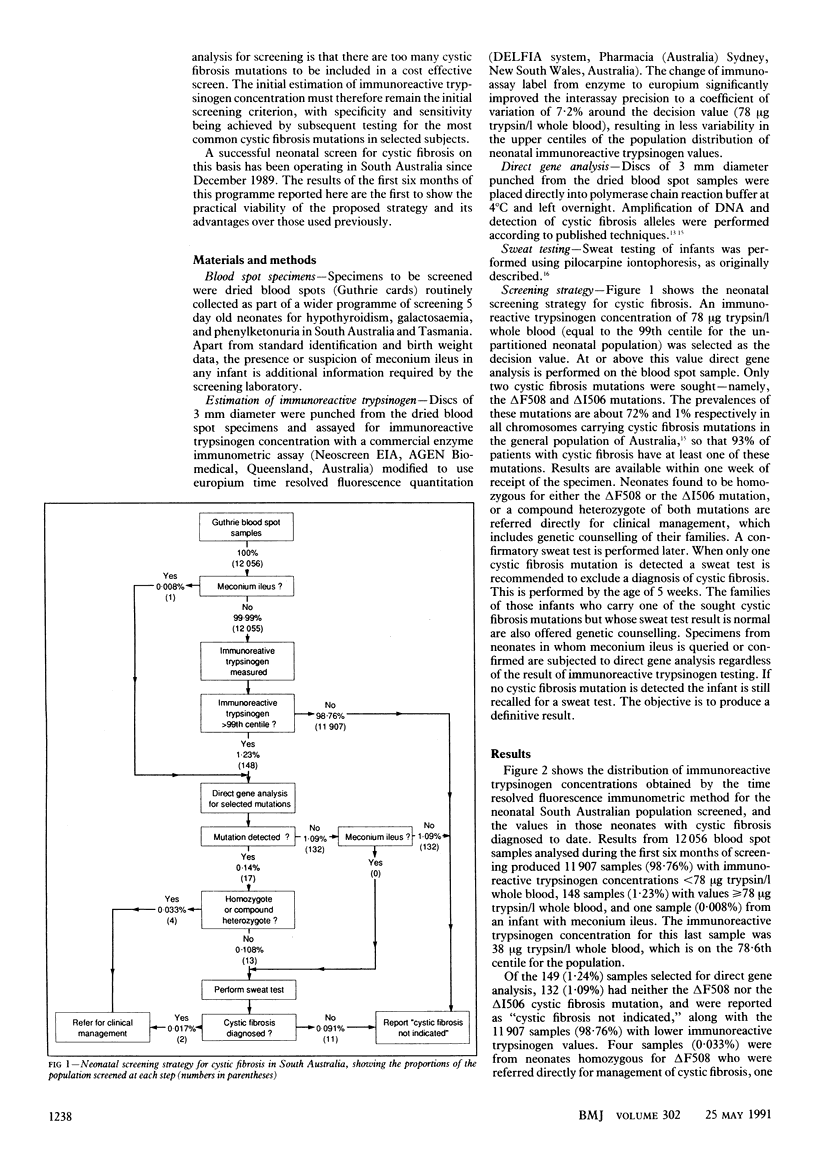
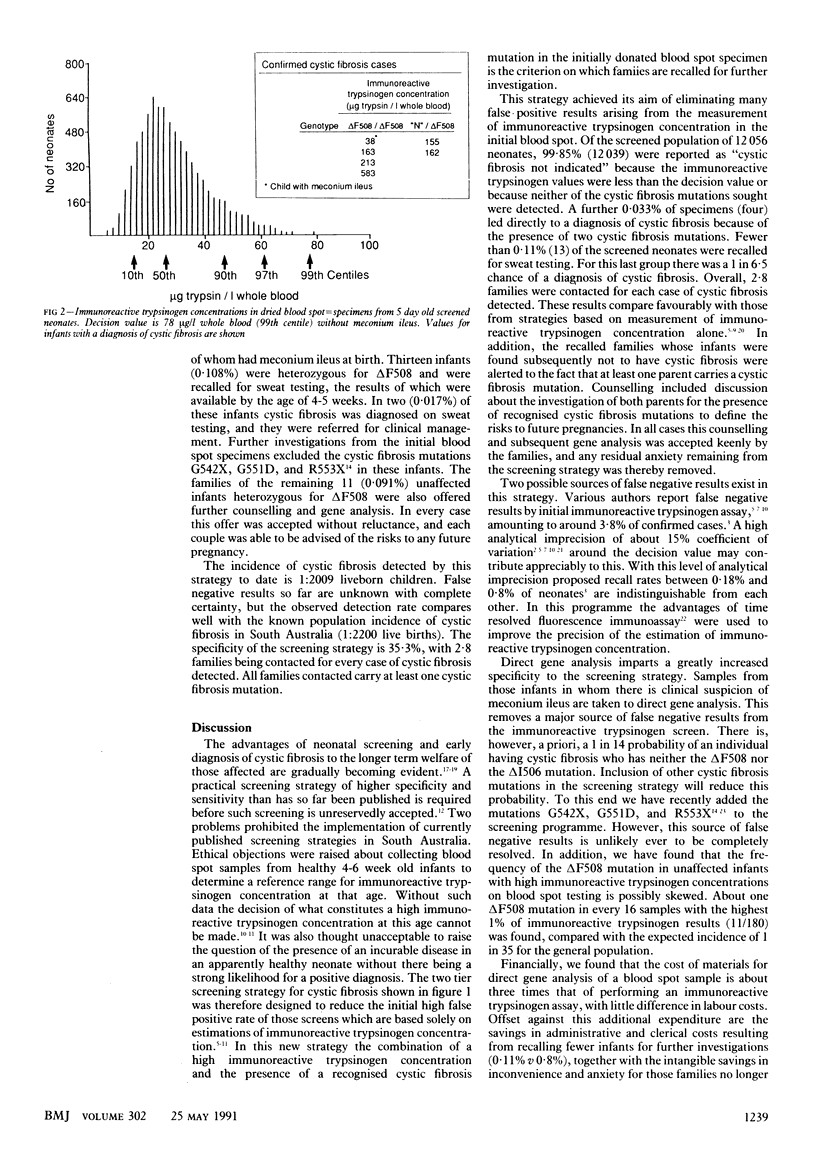
OBJECTIVE--To assess the effectiveness of a two tier neonatal screening strategy for cystic fibrosis, which combines estimation of immunoreactive trypsinogen followed by direct gene analysis in dried blood spot samples collected at age 5 days. DESIGN--Prospective study of two tier screening strategy. The first tier of testing immunoreactive trypsinogen concentration was measured in dried blood spot samples from neonates aged 4-5 days. In the second tier direct gene analysis to detect cystic fibrosis mutations deltaF508 and deltaI506 was performed in those blood spot samples which produced the highest 1% of immunoreactive trypsinogen values. Direct gene analysis was also performed on blood spot samples from infants with suspected or confirmed meconium ileus, regardless of the immunoreactive trypsinogen value. SETTING--The South Australian Neonatal Screening Programme, operating from the department of chemical pathology at Adelaide Children's Hospital. Subjects--All 12,056 neonates born in South Australia between December 1989 and June 1990. No selection criteria were applied. INTERVENTIONS--All infants found to have two recognised cystic fibrosis mutations on direct gene analysis were referred directly for clinical management, and those with one recognised cystic fibrosis mutation were recalled for a sweat test; their families were given genetic counselling. MAIN OUTCOME MEASURES--Direct or exclusion of cystic fibrosis by sweat testing of neonates identified as being at high risk of cystic fibrosis on screening and of those at minimum risk but whose subsequent clinical history raised suspicion about the disease. RESULTS--Of the 12,056 infants screened, 11,907 (98.8%) were reported as "cystic fibrosis not indicated" on the basis of low immunoreactive trypsinogen values. Of the 148 (1.23%) infants with raised immunoreactive trypsinogen values and one (0.008%) with meconium ileus, 132 (1.09%) were reported as cystic fibrosis not indicated, four (0.033%) were identified as having cystic fibrosis, and 13 (0.108%) were recalled for sweat testing after direct gene analysis for the presence of the deltaF508 and deltaI506 cystic fibrosis mutations. No cases of affected infants are known to have been missed to date. CONCLUSION--The strategy of measurement of immunoreactive trypsinogen followed by direct gene analysis is a highly specific neonatal screen for cystic fibrosis, requiring only 2.8 families to be contacted for every case of cystic fibrosis diagnosed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaenssens K., Janssens H., van Soom H. Two tier screen for cystic fibrosis. Lancet. 1981 Apr 11;1(8224):833–833. doi: 10.1016/s0140-6736(81)92699-4. [DOI] [PubMed] [Google Scholar]
- Cassio A., Bernardi F., Piazzi S., Capelli M., Frejaville E., Villa M. P., Martelli E., Balsamo A., Salardi S., Merighi R. Neonatal screening for cystic fibrosis by dried blood spot trypsin assay. Results in 47 127 newborn infants from a homogeneous population. Acta Paediatr Scand. 1984 Jul;73(4):554–558. doi: 10.1111/j.1651-2227.1984.tb09970.x. [DOI] [PubMed] [Google Scholar]
- Chatfield S., Owen G., Ryley H. C., Williams J., Alfaham M., Goodchild M. C., Weller P. Neonatal screening for cystic fibrosis in Wales and the West Midlands: clinical assessment after five years of screening. Arch Dis Child. 1991 Jan;66(1 Spec No):29–33. doi: 10.1136/adc.66.1_spec_no.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley J. R., Elliott R. B., Smith P. A. Dried-blood spot screening for cystic fibrosis in the newborn. Lancet. 1979 Mar 3;1(8114):472–474. doi: 10.1016/s0140-6736(79)90825-0. [DOI] [PubMed] [Google Scholar]
- Cutting G. R., Kasch L. M., Rosenstein B. J., Zielenski J., Tsui L. C., Antonarakis S. E., Kazazian H. H., Jr A cluster of cystic fibrosis mutations in the first nucleotide-binding fold of the cystic fibrosis conductance regulator protein. Nature. 1990 Jul 26;346(6282):366–369. doi: 10.1038/346366a0. [DOI] [PubMed] [Google Scholar]
- Dankert-Roelse J. E., te Meerman G. J., Martijn A., ten Kate L. P., Knol K. Survival and clinical outcome in patients with cystic fibrosis, with or without neonatal screening. J Pediatr. 1989 Mar;114(3):362–367. doi: 10.1016/s0022-3476(89)80552-9. [DOI] [PubMed] [Google Scholar]
- Diamandis E. P. Immunoassays with time-resolved fluorescence spectroscopy: principles and applications. Clin Biochem. 1988 Jun;21(3):139–150. doi: 10.1016/0009-9120(88)90001-x. [DOI] [PubMed] [Google Scholar]
- GIBSON L. E., COOKE R. E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959 Mar;23(3):545–549. [PubMed] [Google Scholar]
- Heeley A. F., Heeley M. E., King D. N., Kuzemko J. A., Walsh M. P. Screening for cystic fibrosis by died blood spot trypsin assay. Arch Dis Child. 1982 Jan;57(1):18–21. [PMC free article] [PubMed] [Google Scholar]
- Kerem B. S., Zielenski J., Markiewicz D., Bozon D., Gazit E., Yahav J., Kennedy D., Riordan J. R., Collins F. S., Rommens J. M. Identification of mutations in regions corresponding to the two putative nucleotide (ATP)-binding folds of the cystic fibrosis gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8447–8451. doi: 10.1073/pnas.87.21.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- King D. N., Heeley A. F., Walsh M. P., Kuzemko J. A. Sensitive trypsin assay for dried-blood specimens as a screening procedure for early detection of cystic fibrosis. Lancet. 1979 Dec 8;2(8154):1217–1219. doi: 10.1016/s0140-6736(79)92336-5. [DOI] [PubMed] [Google Scholar]
- Nelson P. V., Carey W. F., Morris C. P., Pollard A. C. The frequency of the common (delta F508) cystic fibrosis mutation in the Australian population. Med J Aust. 1990 Mar 19;152(6):328–328. [PubMed] [Google Scholar]
- Rock M. J., Mischler E. H., Farrell P. M., Wei L. J., Bruns W. T., Hassemer D. J., Laessig R. H. Newborn screening for cystic fibrosis is complicated by age-related decline in immunoreactive trypsinogen levels. Pediatrics. 1990 Jun;85(6):1001–1007. [PubMed] [Google Scholar]
- Ryley H. C., Deam S. M., Williams J., Alfaham M., Weller P. H., Goodchild M. C., Carter R. A., Bradley D., Dodge J. A. Neonatal screening for cystic fibrosis in Wales and the West Midlands: 1. Evaluation of immunoreactive trypsin test. J Clin Pathol. 1988 Jul;41(7):726–729. doi: 10.1136/jcp.41.7.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley A. W., Smith P. A., Elliott R. B. Experience with neonatal screening for cystic fibrosis in New Zealand using measurement of immunoreactive trypsinogen. Aust Paediatr J. 1989 Jun;25(3):151–155. doi: 10.1111/j.1440-1754.1989.tb01440.x. [DOI] [PubMed] [Google Scholar]
- Wilcken B., Brown A. R., Urwin R., Brown D. A. Cystic fibrosis screening by dried blood spot trypsin assay: results in 75,000 newborn infants. J Pediatr. 1983 Mar;102(3):383–387. doi: 10.1016/s0022-3476(83)80653-2. [DOI] [PubMed] [Google Scholar]
- Wilcken B., Chalmers G. Reduced morbidity in patients with cystic fibrosis detected by neonatal screening. Lancet. 1985 Dec 14;2(8468):1319–1321. doi: 10.1016/s0140-6736(85)92623-6. [DOI] [PubMed] [Google Scholar]



