Abstract
We describe a new resource for targeted insertional mutagenesis in Arabidopsis using a maize (Zea mays) Activator/Dissociation (Ds) two-element system. The two components of the system, T-DNA vectors carrying a Ds launch pad and a stable Activator transposase source, were designed to simplify selection of transposition events and maximize their usefulness. Because Ds elements preferentially transpose to nearby genomic sites, they can be used in targeted mutagenesis of linked genes. To efficiently target all genes throughout the genome, we generated a large population of transgenic Arabidopsis plants containing the Ds launch pad construct, identified lines containing single Ds launch pad inserts, and mapped the positions of Ds launch pads in 89 lines. The integration sites of the Ds launch pads were relatively evenly distributed on all five chromosomes, except for a region of chromosomes 2 and 4 and the centromeric regions. This resource therefore provides access to the majority of the Arabidopsis genome for targeted tagging.
Arabidopsis has become a model organism for plant sciences, and the entire genome sequence of this plant is now available (The Arabidopsis Genome Initiative, 2000). The challenge facing scientists is to assign function to thousands of previously unknown genes and one effective way to do this is by generating loss of function mutations. Several methods have been developed in Arabidopsis, including T-DNA mutagenesis (for review, see Feldmann, 1991), transposon mutagenesis (for review, see Martienssen, 1998), gene replacement (Kempin et al., 1997), gene silencing via RNA interference (Waterhouse et al., 1998), and TILLing (McCallum et al., 2000).
Insertional mutagenesis in Arabidopsis using T-DNA and the maize (Zea mays) transposable elements Activator/Dissociation (Ac/Ds) and Enhancer/Suppressor-mutator (En/Spm) has been widely and successfully used for revealing gene function (for example, see Koncz et al., 1990; Bancroft et al., 1993; Meissner et al., 1999). Many resources comprising large populations with T-DNA and transposon insertions have been generated (for example, see Feldmann, 1991; Sundaresan et al., 1995; Martienssen, 1998; Krysan et al., 1999; Weigel, et al., 2000; available through The Arabidopsis Information Resource, http://www.Arabidopsis.org/). Insertions into target genes can be identified by screening these collections by PCR. More recently, databases have been established carrying the sequences flanking the T-DNA and transposon insertions (for example, TMRI/Syngenta Arabidopsis Insertion Library Project [formerly GARLIC] and SIGnAL; Parinov et al., 1999; Speulman et al., 1999; Tissier et al., 1999). In addition to providing simple gene knock-out tools, T-DNAs and transposons have been modified to act as gene traps (for review, see Springer, 2000) or gene activators (Weigel et al., 2000; Suzuki et al., 2001). These systems have the advantage of being able to uncover genes exhibiting functional redundancy, those that have multiple functions, or those that are essential during development. Due to their ease of generation, T-DNA lines represent the largest current resource for insertional mutagenesis in Arabidopsis. Although they have been extremely successful for reverse genetic studies, there are limitations in obtaining T-DNA inserts into smaller genes (approximately 0.5 kb in size), which make up 10% to 20% of all genes. To target this remaining 10% to 20% of genes using T-DNA insertional mutagenesis, more than 500,000 inserts would have to generated to have a greater than 90% chance of obtaining the desired insertion. Therefore, to efficiently target the remaining 10% to 20% of genes not tagged by the current T-DNA populations, a complementary approach is required. One strategy for targeting the remaining genes exploits the property of linked transposition of transposable elements. In Arabidopsis, the majority of Ds transpositions are within a few centi-Morgans (cM) of the starting location (Bancroft and Dean, 1993b; Carroll et al., 1995; Smith et al., 1996; Machida et al., 1997; Long et al., 1997). Therefore, use of an Ac/Ds system allows the targeting of a particular region of interest with a high frequency of Ds insertions if a linked starting point is available. The utility of the Ac/Ds system for regional insertional mutagenesis in Arabidopsis has been demonstrated by several studies (Long et al., 1997; Dubois et al., 1998; Ito et al., 1999; Parinov et al., 1999), and it has been used successfully in a targeted tagging strategy (James et al., 1995). If a donor locus is situated very close to a target gene, it is also possible to generate multiple mutant alleles. For example, in Arabidopsis, six independent Ac insertion alleles of DIF1 were generated from the same donor T-DNA (Bhatt et al., 1996). An allelic series can also be achieved by reactivating a Ds or autonomous Ac element (a procedure known as reconstitutional mutagenesis). More than 250 new Ac insertion alleles of the maize P gene were generated by this method (Moreno et al., 1992).
To be able to target genes throughout the genome using the Ac/Ds system, a collection comprising a large number of starting positions is required; however, only a limited number are currently available (Smith et al., 1996; Long et al., 1997). We have generated a resource for targeted insertional mutagenesis in Arabidopsis comprising 89 independent lines containing single-copy Ds launch pads, which have been mapped onto the genome. The Ds launch pads are relatively evenly distributed over all five chromosomes, therefore providing the ability to target genes throughout the majority of the genome. We have designed a new Ds launch pad T-DNA vector incorporating a gene trap and herbicide resistance selection for excision and re-insertion events. To transactivate these Ds elements we have generated two vectors containing stable Ac transposase sources linked to a phenotypic marker. This eases the selection of progeny where the transposase source has segregated away stabilizing the Ds element and therefore the mutant phenotype.
RESULTS AND DISCUSSION
Ds Launch Pad T-DNA Vector
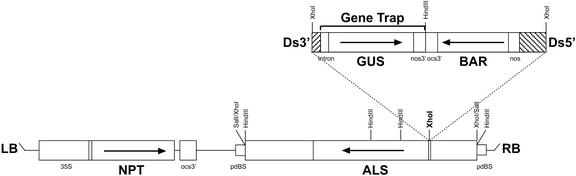
A schematic representation of the Ds launch pad T-DNA vector is shown in Figure 1. The Ds element contains a nos::BAR transcriptional fusion, conferring resistance to the herbicide phosphinothricin (Ppt, or gluphosinate ammonium), and a gene trap. The gene trap comprises a promoterless β-glucuronidase (uidA or GUS) reporter gene with a GPA1 intron sequence and splice acceptor sites in all three frames fused upstream of the GUS ATG codon (Sundaresan et al., 1995; engineered to remove an upstream ATG codon). Activation of the GUS reporter gene requires Ds insertion within a gene. When Ds inserts in the correct orientation into an intron, splicing occurs between the 5′ splice donor site of the gene and the 3′ splice acceptor site in the GUS fusion resulting in a GUS translational fusion. Insertion into an exon can also lead to a translational fusion because sequences at the 3′ end of the Ds element can act as splice donor sites. GUS expression, which is expected to mirror the expression of the native gene, can be simply visualized by histochemical staining (Jefferson et al., 1987). The presence of the gene trap extends the utility of the insertion element by enabling the expression pattern of the flanking gene to be characterized.
Figure 1.
Ds launch pad T-DNA vector. Schematic representation of Ds launch pad T-DNA vector. Arrows represent the direction of open reading frames. Restriction enzyme sites relevant to vector construction and DNA gel-blot analysis are shown (for details, see “Materials and Methods”). The Ds element was cloned into the XhoI site (bold) within the 5′-untranslated leader (UTL) of the ALS gene.
The Ds element was inserted into the 5′-UTL of a chimeric tobacco (Nicotiana tabacum) ACETOLACTATE SYNTHASE (ALS) gene (Fig. 1), encoding a mutant version of ALS, which confers resistance to the herbicide chlorsulfuron (Cs, or sulfonylurea). The chimeric ALS gene is a fusion of two genes, SuRA/Hra and SuRB, from Cs-resistant mutants of tobacco (Mazur et al., 1987; Lee et al., 1988). The presence of the Ds element within the 5′-UTL inactivates expression of the ALS gene, and its activity is restored upon Ds excision, resulting in a Cs-resistant phenotype. Ds excision and re-insertion events (following introduction of a transposase source) can therefore be selected by treatment with Cs and Ppt.
Transposase Source T-DNA Vectors
For transactivation of Ds elements from the Ds launch pads, two new transposase source vectors (Fig. 2A) were constructed that incorporated the phenotypic marker Lc (Ludwig et al., 1989) and either the 35S::Ac transposase fusion (Scofield et al., 1992; Swinburne et al., 1992) or an sΔNaeIAc element, where the 3′ end of the element has been removed (from position 4,385 to the end) and a 537-bp deletion has been created within the 5′-UTL of Ac (Bancroft et al., 1992), the latter causing an increase in excision frequency. The two transposase fusions have previously been demonstrated to provide different levels of Ds transactivation and generate transposition events at different times during plant development. The 35S::Ac fusion has been shown to give rise to high levels of Ds excision (typically >30% germinal excision frequency) but a low and variable re-insertion frequency of Ds (Swinburne et al., 1992; Long et al., 1993). Also, Ds excision events occur early during vegetative growth of the F1 plant, leading to sectors encompassing many flowers, resulting in many of the F2 progeny carrying the same transposition event (Long et al., 1993). Therefore, when using the 35S::Ac transposase source many F1 hybrid and F2 families need to be generated to produce large numbers of independent transposition events. In contrast, the sΔNaeIAc transposase source results in a lower germinal excision frequency (approximately 5%; Bancroft et al., 1992), but a higher proportion of reinsertion events (approximately 50%; Bancroft and Dean, 1993a). The sΔNaeIAc transposase generally results in transposition very late in plant development (after the divergence of cell lines leading to pollen and ova), so Ds transposition events carried in F2 progeny are generally independent (Bancroft and Dean, 1993b). However, a significant proportion (approximately one-third) of the F2 progeny selected to be doubly resistant for Ds excision and integration markers do not carry transposed Ds elements but contain a combination of nonexcised Ds alleles and empty donor sites (Bancroft and Dean, 1993b). The two transposase sources thus provide different strategies to generate lines carrying new insertions, the choice depending on the difficulty of the mutant screens to be employed.
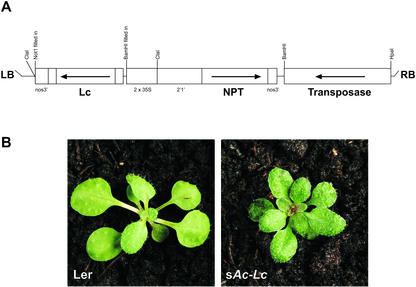
Figure 2.
sAc-Lc T-DNA vector. A, Schematic representation of sAc-Lc T-DNA vector. Arrows represent the direction of open reading frames. Restriction enzyme sites relevant to vector construction are shown (for details see “Materials and Methods”). B, Hirsute phenotype of sAc-Lc line.
To phenotypically mark plants carrying a transposase source, a 35S::Lc transcriptional fusion was cloned into the transposase T-DNA vector (Fig. 2A). Lc is a member of the maize R gene family and is involved in the regulation of anthocyanin pigmentation (Ludwig et al., 1989). In Arabidopsis, the expression of the 35S::Lc fusion increases the level of pigmentation and density of trichomes (Lloyd et al., 1992). The Lc fusion therefore provides a visual marker, and plants carrying transposase can be easily identified by their hirsute, darkly pigmented phenotype (Fig. 2B). This makes identification of plants lacking transposase very straightforward, simplifying stabilization of the Ds element. The 35S::Lc transcriptional fusion has previously been demonstrated to be a successful visual marker for sAc transposase T-DNAs and shown not to adversely affect Ds transposition (Osborne et al., 1995).
Mapping Ds Launch Pad T-DNA Vectors
To produce the required number of mapped Ds launch pads, a large population of transgenic Arabidopsis plants was generated. The Ds launch pad T-DNA vector was introduced into Arabidopsis genotype Landsberg erecta via Agrobacterium tumefaciens-mediated transformation (Valvekens et al., 1988). The NPTII gene (which confers resistance to the antibiotic kanamycin) contained within the T-DNA (Fig. 1) was used to select for transgenic plants. More than 300 independent Arabidopsis transformants were generated. It was important to identify transgenic lines that contained a single copy of the Ds launch pad T-DNA. The selection for Ds excision and re-insertion events requires a single Ds launch pad. In addition, analysis of lines containing transposed Ds elements is simplified if only one copy is present, i.e. an observed phenotype is solely due to one Ds insertion. Initially, lines containing single-locus Ds launch pad T-DNA inserts were identified by segregation of the NPTII gene. A segregation ratio of 3:1 (kanamycin resistant: kanamycin sensitive) in the T2 generation was diagnostic of a single-locus insert. DNA gel-blot analysis was subsequently performed on these single-locus lines to identify those with single-copy Ds launch pad inserts. Probes specific for the left and right T-DNA borders were used to determine Ds launch pad copy number, and single hybridizing bands with both probes indicated a single insert. An internal probe (GUS gene fragment) was also used to demonstrate the Ds launch pad was intact. More than 150 lines were identified to contain single-copy Ds launch pad T-DNA inserts (approximately 50% of transgenic lines generated). This illustrated the efficiency of the binary vector pSLJ1711 in obtaining transformants with single, simple T-DNA inserts.
The first step toward mapping the Ds launch pads onto the Arabidopsis genome was to isolate DNA flanking the Ds launch pad T-DNA insertion. For the majority of lines this was achieved using thermal asymmetric interlaced (TAIL) PCR (Liu and Whitter, 1995; Liu et al., 1995), using either right border (RB) or left border (LB) specific primer sets (for details, see “Materials and Methods”). Generally, TAIL PCR was more successful when using the LB primers. In cases where T-DNA flanking fragments were not amplified by TAIL PCR, inverse PCR (IPCR) was used (Ochman et al., 1988; Triglia et al., 1990).
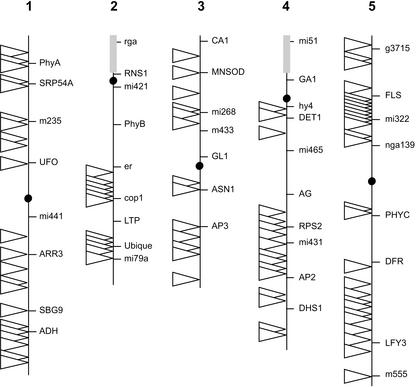
To map the Ds launch pad T-DNA insertion sites amplified by TAIL PCR or IPCR onto the Arabidopsis genome, two strategies were adopted. Initially, the Ds launch pad flanking fragments were used as probes to screen YAC and, to a lesser extent, bacterial artificial chromosome (BAC) library filters (for details, see “Materials and Methods”; Muskett et al., 2002). This approach was extremely useful before the availability of the entire Arabidopsis genome sequence. However, when the genome sequence became available, the second approach was to sequence the TAIL PCR or IPCR products and map them simply by using the BLAST alignment program (Altschul et al., 1990). Sequencing of the PCR products also confirmed the presence of expected T-DNA border sequences, eliminating the need to perform DNA gel-blot analysis to confirm that the amplified products truly flanked the Ds launch pads in each line. By combining the two mapping strategies, 89 Ds launch pads were accurately positioned onto the Arabidopsis genome (Fig. 3). Mapping revealed the distribution of Ds launch pads to be fairly even over the five Arabidopsis chromosomes. With the exception of centromeric and nucleolar organizer regions (insertions into these repetitive DNA regions could not be mapped by the techniques employed), few chromosomal regions contained no Ds launch pads. The two most notable regions lacking mapped Ds launch pads included an approximately 24-cM region between m421 and B68 on chromosome 2 and an approximately 22-cM region between g4108 and g13838 on chromosome 4. Conversely, there were no obvious “hotspots” of Ds launch pad insertions (the most clustered region being between FLS and mi322 on chromosome 5). Therefore, the 89 mapped Ds launch pads provide starting locations to target genes throughout the majority of the genome. Details of all 89 lines, including line number, amplification of Ds launch pad flanking fragment, hybridizing YAC or BAC clones, GenBank accession number of Ds launch pad insertion site sequence, and BLAST hits are shown in Table I.
Figure 3.
Positions of mapped Ds launch pad T-DNA inserts on the Arabidopsis genome. Genomic locations of Ds launch pad T-DNA inserts in 89 independent lines (one insert per line) are shown. Ds launch pads are represented by triangles. Circles represent centromeric regions, gray boxes represent nucleolar organizer regions. Each chromosome is labeled with a number of genetic markers to give an approximate reference position.
Table I.
Mapping details of Ds launch pad T-DNA insertions
The flanking fragment column lists which method of PCR was used, TAIL PCR or IPCR, and from which T-DNA border the fragment was amplified: LB or RB. For TAIL PCR-generated fragments, the degenerate primer used is listed, AD2 or AD5 (see “Materials and Methods”). For IPCR-generated fragments, the restriction enzyme used is listed (BstY1, HincII, or Bg/II). —, not used for screening.
| Line | Flanking Fragment | YAC CIC Hits | BAC Hits | Chromosome | Nearest Genetic Marker | Accession No. | Sequence BLAST Hits |
|---|---|---|---|---|---|---|---|
| 268A | TAIL PCR (RBAD2) | Unsuccessful | F24L23, F4D20, F25B4, F27C15, F25J17, F10K1 | 1 | m488 | AL672132 | BAC F10K1 |
| 419B | TAIL PCR (LBAD2) | — | — | 1 | ve005, nga63 | AL672133 | BAC F21M12 |
| 10A | TAIL PCR (RBAD5) | 4C8, 10E2 | — | 1 | m214A | AL672134 | BAC F14N23 |
| 255B | TAIL PCR (RBAD2) | 10E2, 3C3 | — | 1 | m214A | AL672135 | BAC T19D16 |
| 491A | TAIL PCR (LBAD2) | — | — | 1 | NCC1 | AL672136 | BAC F12F1 |
| 306A | TAIL PCR (LBAD5) | — | — | 1 | mi265 | AL672137 | BAC F316 |
| 385A | TAIL PCR (LBAD5) | — | — | 1 | mi163 | AL683885 | BAC F21J9 |
| 413A | TAIL PCR (RBAD5) | — | — | 1 | ve008 | BX088701 | BAC F13K9 |
| 126A | TAIL PCR (RBAD2) | 11B7, 5D9, 9C8, 10B6 | — | 1 | m271, m201 | BX119327 | BAC F3M18 |
| 202A | TAIL PCR (RBAD2) | 3H12, 12F3 | — | 1 | m402, m254 | BX119328 | BAC T518 |
| 265A | IPCR (LB/BstYI) | Unsuccessful | Unsuccessful | 1 | CIC9A2 | AL672131 | BAC F12M16 |
| 339A | TAIL PCR (LBAD2) | — | — | 1 | nga280 | BX119329 | BAC F14G9, F13N6 |
| 250A | IPCR (LB/HincII) | 6G10, 5D1 | — | 1 | g4026 | BX119330 | BAC T13D8 |
| 364B | TAIL PCR (LBAD2) | — | — | 1 | m305 | BX119331 | BAC F24D7 |
| 32B | TAIL PCR (RBAD2) | — | — | 1 | m305 | BX119332 | BAC F15H21 |
| 384A | TAIL PCR (LBAD2) | — | — | 1 | m315 | BX119333 | BAC F1E22 |
| 309A | TAIL PCR (RBAD2) | - - | F1B12, F28P5, F15H19, T5N1, T22F10, T22G18, T15G23, T10O9 | 1 | mi462 | BX119334 | BAC F28P5 |
| 130A | IPCR (LB/BstYI) | 2F9, 12H9, 6H1 | — | 1 | ve116 | BX119335 | BAC T9L24 |
| 316A | TAIL PCR (RBAD2) | — | — | 1 | mi425 | BX119336 | BAC F2P9 |
| 272A | TAIL PCR (LBAD2) | 4F1, 11F4 | — | 1 | m532 | BX119337 | BAC F10A5 |
| 27A | TAIL PCR (LBAD5) | Unsuccessful | F5I24, T10K12, T10K14, T5M16 | 1 | agp15 | BX119338 | BAC T5M16 |
| 264A | IPCR (LB/BstYI) | Unsuccessful | — | 1 | agp15 | BX119339 | BAC T5M16 |
| 262A | TAIL PCR (RBAD2) | Unsuccessful | — | 2 | B68 | BX119340 | BAC F12C20 |
| 86B | TAIL PCR (RBAD2) | 3H8, 4B4, 12B1 | — | 2 | mi54 | BX119341 | BAC F16P2 |
| 492A | TAIL PCR (LBAD2) | — | — | 2 | mi54 | BX119342 | BAC F16P2 |
| 60A | TAIL PCR (RBAD5) | 3H8, 12B1 | — | 2 | mi54 | BX119343 | BAC T27A16 |
| 345B | TAIL PCR (LBAD5) | — | — | 2 | m283C | BX119344 | BAC T6B20 |
| 437A | TAIL PCR (LBAD2, RBAD2) | — | — | 2 | nga361 | BX119345 | BAC F20M17, T9H9 |
| 453A | TAIL PCR (LBAD2) | — | — | 2 | COR15 | BX119346 | BAC F14N22, T9E20 |
| 313B | TAIL PCR (LBAD2, RBAD5) | — | — | 2 | m336 | BX119347 | BAC F411 |
| 43A | TAIL PCR (LBAD2) | 2E5, 2E7 | — | 2 | m336 | BX119348 | BAC F411 |
| 340B | TAIL PCR (LBAD2) | — | — | 2 | ve019 | BX119349 | BAC F11C10, T3F17 |
| 402B | TAIL PCR (LBAD5) | — | — | 2 | mi79A | BX119350 | BAC T30B22 |
| 141B | TAIL PCR (LBAD5) | 11E4 | — | 3 | mi335 | BX119351 | BAC F2O10, F10A16 |
| 317A | TAIL PCR (LBAD5, RBAD5) | — | — | 3 | MS2 | BX119352 | BAC F9F8 |
| 276A | IPCR (LB/Bg/II) | 6G10, 11G11 | F10H8, F2F18, F9O23, F4N22, F6M2, F17E7, F17A12, T27L22, T15C3, T14I11, T9M12, T13K13, T6A16 | 3 | B26 | BX119353 | P1 MGL6 |
| 196A | IPCR (LB/Bg/II) | Unsuccessful | F8C15, F2A16, F1507, F11K22, F3P10, F3B8, F22B10, F22H17, T4K14, T25J17 | 3 | mi268 | No sequence | — |
| 92A | IPCR (LB/BstYI) | Unsuccessful | F3K10, F25J12, F19G21, F2P4, T7A12, T4J23, T4M20, T22F20, T17M20, T23K2 | 3 | mi268 | No sequence | — |
| 143A | TAIL PCR (LBAD2) | Unsuccessful | F5C8, F2I14, F2D23, F15M23, F12, H18, F13O5, F12O8, T9E21, T18I20 | 3 | mi225 | BX119354 | P1 MYM9 |
| 146A | TAIL PCR (LBAD5) | — | — | 3 | ve021 | BX119355 | BAC T22K7 |
| 70A | TAIL PCR (RBAD2) | 3C4, 4D9, 4D10, 6F10, 3H10 | — | 3 | ve021 | BX119356 | BAC T14D3 |
| 380A | TAIL PCR (LBAD2) | — | — | 3 | m457 | BX119357 | BAC T16K5, T9C5 |
| 19E | TAIL PCR (RBAD5) | 10A11, 11G7, 7A4, 6F4 | — | 3 | mi456 | BX119358 | AtEm1 |
| 151A | TAIL PCR (RBAD2) | 11B6, 11G5 | — | 3 | ve042 | BX119359 | BAC F24B22 |
| 208A | IPCR (LB/HincII) | 7F7 | — | 3 | ve022 | BX119360 | BAC T8M16, T26M13 |
| 473A | TAIL PCR (LBAD5) | — | — | 3 | m424 | BX119361 | BAC F21F14 |
| 101A | IPCR (LB/HincII) | 1D5 | F15G24, T28D5, T16H12 | 4 | mi167 | BX119362 | BAC T28D5 |
| 438A | TAIL PCR (RBAD2) | — | — | 4 | m518 | BX119363 | BAC F17A8, T5L19 |
| 55A | TAIL PCR (RBAD2) | 10F9, 10G11, 3G3, 3H5, 9G5 | — | 4 | m518 | BX119364 | BAC T22B4 |
| 66A | TAIL PCR (RBAD5) | Unsuccessful | F2O7, F9F13, T4C8, T8K8, T2H15 | 4 | g13838 | BX119365 | BAC F9F13 |
| 22A4 | TAIL PCR (LBAD5) | 5C2, 1E5, 6B12, 1G9 | F2C22, F24K5, F2L2, F5L19, F22K18, T6I22, T24D6, T19P12, T11C5, T13C3 | 4 | mi475 | BX119366 | BAC F22K18 |
| 436A | TAIL PCR (LBAD2) | — | — | 4 | RPS2 | BX119367 | BAC T25K17 |
| 475A | TAIL PCR (LBAD2) | — | — | 4 | mi123 | BX119368 | BAC T27E11 |
| 423A | TAIL PCR (LBAD2) | — | — | 4 | mi232 | BX119369 | BAC F26K10 |
| 431A | TAIL PCR (LBAD5) | - | - | 4 | mi232 | BX119370 | BAC F25024 |
| 368A | TAIL PCR (LBAD5) | - | - | 4 | g8300 | BX119371 | BAC F28M20 |
| 93A | IPCR (LB/Hincll) | 11C6, 10E9 | - | 4 | mi431 | BX119372 | BAC F10N7 |
| 245A | TAIL PCR (RBAD5) | 1F9, 10E9, 11D9 | - | 4 | mi431 | BX119373 | BAC F4D11 |
| 162A | TAIL PCR (RBAD2) | Unsuccessful | Unsuccessful | 4 | HLS1 | BX119374 | BAC F19F18 |
| 100A | IPCR (LB/Hincll) | 6C9 | - | 4 | CIC3H2 | BX119375 | BAC F20M13 |
| 137A | TAIL PCR (RBAD2) | Unsuccessful | - | 4 | mi369 | BX119376 | BAC T19P19 |
| 312B | TAIL PCR (LBAD2) | - | - | 4 | mi369 | BX119913 | BAC T5J17 |
| 350C | TAIL PCR (LBAD5) | - | - | 5 | m447 | BX119377 | BAC F8F6 |
| 458C | TAIL PCR (LBAD2) | - | - | 5 | fad8, PAI2 | BX119378 | P1 MOP10 |
| 480A | TAIL PCR (LBAD2) | - | - | 5 | nga158 | BX119379 | P1 MJJ3 |
| 37A | TAIL PCR (LBAD2) | 12E4 (not mapped) | - | 5 | mi97 | BX119380 | P1 MXM12 |
| 157A | IPCR (LB/BstYI) | 2A5, 1B8, 12E1 | - | 5 | ve033 | BX119381 | P1 MYH9 |
| 50A2 | TAIL PCR (RBAD2) | 7F6 | F2E7, F3J8, F5H2, T27G13, T24P18, T3E23 | 5 | CHS | BX119382 | BAC T24H18 |
| 449A | TAIL PCR (LBAD2) | - | - | 5 | CHS | BX119383 | BA T19L5 |
| 5A2 | TAIL PCR (RBAD2) | 5H3, 7F6 | - | 5 | CHS | BX119384 | P1 MSH12 |
| 365B | TAIL PCR (LBAD2) | - | - | 5 | CHS | BX119385 | P1 MSH12 |
| 263A | TAIL PCR (LBAD5) | Unsuccessful | - | 5 | CHS | BX119386 | P1 MSH12 |
| 46A | TAIL PCR (LBAD2) | 8E12, 9F1 | - | 5 | nga106 | No sequence | - |
| 247A | TAIL PCR (LBAD5) | 6H3 | - | 5 | nga106 | BX119387 | TAC K3M16 |
| 341A | TAIL PCR (LBAD5) | - | - | 5 | NIT4 | BX119388 | BAC F22D1 |
| 337A | TAIL PCR (LBAD5) | - | - | 5 | mi90, mi433 | BX119389 | P1 MDJ22 |
| 94A | TAIL PCR (RBAD2) | 4B3 | F17C11, F10L15, F28E12, F19J5 F10E22, F10A10, T32E23, T3K10, T3M12 | 5 | PhyC | BX119390 | P1 MXH1 |
| 256A | TAIL PCR (LBAD2) | 4B3 | F16L17, F2G12, F8J23, F2A3, F5O7, F19J5, F10E22, F10A10, F17C11, T13E1, T10C22, T10D24 | 5 | PhyC | BX119391 | P1 MXH1 |
| 343A | TAIL PCR (LBAD5) | - | - | 5 | g4028 | BX119392 | P1 MBK23 |
| 203A | TAIL PCR (RBAD2) | 10G6 | F21H14, F22A6, F15F8, F19K24, F24K21 | 5 | ve027 | BX119393 | P1 MNJ7 |
| 373A | TAIL PCR (RBAD2) | - | - | 5 | nga129 | BX119394 | TAC K9P8 |
| 412A | TAIL PCR (LBAD5) | - | - | 5 | KG8 | BX119395 | TAC K10D11 |
| 115B | TAIL PCR (RBAD2) | 11F9, 11F10 | F2111, F6J7, T32G8, T30P9 | 5 | KG8 | BX119396 | BAC T4M5 |
| 150B | TAIL PCR (LBAD2) | 5H10, 1C5 | - | 5 | m435 | BX119397 | P1 MJP23 |
| 188A | TAIL PCR (LBAD5) | 1C5, 1C7, 1C8, 1C9, 1D7 | - | 5 | m435 | No sequence | - |
| 1A | TAIL PCR (RBAD2) | Unsuccessful | - | 5 | mi69 | BX119398 | P1 MCD7 |
| 398A | TAIL PCR (RBAD2) | - | - | 5 | mi70 | BX119399 | P1 MRI1 |
| 270B | IPCR (LB/BstYI) | - | F11A13, F24L17, F23P19, F22F21, F23L2, F4114, F6A13, T6D17, T5G15, T1L17, T2O2, T20M17 | 5 | g2368, mi335 | No sequence | - |
| 15B | TAIL PCR (LBAD2) | Unsuccessful | - | 5 | m555 | BX119400 | TAC K919 |
Ds Excision and Re-Insertion
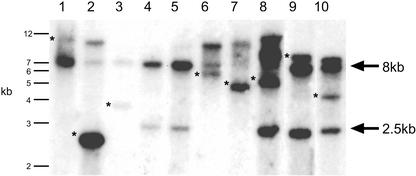
To demonstrate excision and re-insertion of the Ds element from the Ds launch pads, DNA gel-blot analysis was performed on F2 progeny (generated from a cross to a line containing the 35S::Ac fusion) selected for double herbicide resistance, and the Ds integration site was analyzed using the GUS gene or BAR gene as a hybridization probe. This analysis detects both intact Ds launch pads (i.e. with a nonexcised Ds element) and re-inserted Ds elements (Fig. 4). DNA gel-blot analysis revealed hybridizing fragments, corresponding to re-inserted Ds elements, of various sizes (Fig. 4, asterisks). This demonstrated the ability of Ds to excise and re-insert into different genomic locations in the independent lines. Single hybridizing fragments corresponding to re-inserted Ds elements also demonstrated germinal excision events (i.e. transposition events inherited from the previous generation). In lines that did not contain a re-inserted Ds element (Fig. 4, lanes 4 and 5), Ppt resistance was conferred by an intact Ds launch pad revealed by a hybridizing fragment of expected size, 2.8 kb. Further analysis of the double herbicide-resistant seedlings also showed that a small proportion did not contain an empty donor site, suggesting some leakiness of the Cs selection.
Figure 4.
Analysis of Ds excision and reinsertion. DNA gel-blot analysis to detect Ds excision and re-insertion. Genomic DNA from 10 independent F2 families was digested with HindIII. A GUS probe was used to detect Ds excision and re-insertion. A Ds launch pad with a nonexcised Ds element resulted in a hybridizing band of expected size, 2.8 kb. Excised and re-inserted Ds elements gave rise to hybridizing bands of variable sizes, marked by asterisks. The GUS probe also hybridized to an unknown fragment, resulting in a background band of 8 kb in all lanes.
CONCLUSIONS
Eighty-nine new Ds launch pads have been mapped onto the Arabidopsis genome. Their average spacing of approximately 5 cM or 1 Mb should enable high-frequency targeting of specific loci given that between 25% and 50% of Ds transpositions occur within 1 to 1.7 Mb of their starting point (Smith et al., 1996; Machida et al., 1997). Insertions into specific genes from linked Ds elements have been identified by examining as few as 200 F2 families (Bhatt et al., 1996; Parinov et al., 1999). The mapped elements in the lines can also be used for targeted insertional mutagenesis in different genetic backgrounds or accessions by introgression of the Ds launch pad. This opens up forward or reverse screens, either for phenotype or expression pattern as part of enhancer or suppressor analyses in different mutant backgrounds or for exploring functional redundancy. All the lines containing the Ds launch pad T-DNAs and Lc transposase sources are available from the Nottingham Arabidopsis Stock Centre (Nottingham, UK; http://nasc.nott.ac.uk/).
MATERIALS AND METHODS
DNA Constructs
The Ds launching pad T-DNA vector (Fig. 1) was constructed as follows: A XhoI site was introduced into the 5′-untranslated region of the tobacco, chimeric ALS gene by site-specific mutagenesis, as described by Kunkel (1985; shown in bold in Fig. 1). The ALS gene was excised from pSBAD (kindly supplied by Barbara Mazur [DuPont, Wilmington, DE]) as a BamHI/PstI fragment and ligated into the same restriction sites in pBluescript (pKSII). The ALS gene was then cloned as a SalI fragment from pKSII into the XhoI site of the pdBS polylinker in the binary vector pSLJ1711 (Jones et al., 1992). Finally, the “gene trap” Ds element (from PS116; P.S. Springer and R. Martienssen, unpublished data) was cloned into the introduced XhoI site in the 5′-untranslated region of the tobacco, chimeric ALS gene.
The transposase source T-DNA vectors (Fig. 2A) were constructed in the following way: Three modifications were made to the double-35S::Lc::nos3′ transcriptional fusion (Osborne et al., 1995). Plasmid BIO167 (kindly supplied by Brian Osborne, Cognia, New York, and Barbara Baker, Plant Gene Expression Center, University of California, Berkely, and US Department of Agriculture, Albany, CA) was cut with NotI and treated with the Klenow fragment of DNA polymerase I to result in a blunt-ended linear plasmid. ClaI linkers (Promega, Madison, WI) were ligated to the blunt ends, and the plasmid was recircularized. Plasmid BIO167 was then cut with BamHI, treated with the Klenow fragment, and recircularized, therefore removing the BamHI site. The double-35S::Lc::nos3′ transcriptional fusion was cloned as a ClaI fragment into the ClaI site of the binary vector SLJ3869 (Jones et al., 1992). Each one of the two transposase sources was then cloned into the BamHI and HpaI sites of SLJ3869. The 35S::Ac transposase fusion (Scofield et al., 1992; Swinburne et al., 1992) was excised as a BamHI/SmaI fragment from SLJ1101 (kindly supplied by Jonathan Jones [Sainsbury Laboratory, Norwich, UK]) and ligated into SLJ3869. The sΔNaeIAc transposase source (Bancroft et al., 1992) was excised from SLJ1941 (kindly supplied by Jonathan Jones) in two stages. Plasmid SLJ1941 was cut with SstI, treated with the Klenow fragment (to result in a blunt end) and then cut with BglII. This fragment was then ligated into SLJ3869.
Plant Transformation
The T-DNA constructs were introduced into Arabidopsis ecotype Landsberg erecta by Agrobacterium tumefaciens-mediated transformation (Valvekens et al., 1988) using the strain A. tumefaciens C58C1 pGV2260. Transgenic plants were selected by resistance to the antibiotic kanamycin (conferred by the NPTII gene contained in the T-DNA). Antibiotic resistance analysis was performed by growing plants under sterile conditions on growth medium supplemented with appropriate antibiotics as described by Bancroft et al. (1992).
Preparation of Plant Genomic DNA
Small-scale preparation of Arabidopsis genomic DNA was performed using a modification of the method described by Carroll et al. (1995), using a sap extractor (Erich Pollarne, Hannover, Germany).
DNA Gel-Blot Analysis
DNA gel-blot analysis was carried out as described by Jarvis et al. (1997). Probes used to detect the T-DNA borders and internal fragment were: RB probe, HindIII/EcoRI fragment from pCL0622 comprised of two RB sequences; LB probe, SphI/XbaI fragment from pCL0481 comprising 2′1′ promoter and NPTII coding region sequences; and GUS probe, XbaI/SacI fragment from pGUS (kindly supplied by Keith Lindsey, Department of Biological Sciences, University of Durham, UK) comprising the GUS coding region. T-DNA copy number was ascertained by hybridizing HindIII digested genomic DNA from each line with probes specific for LB and RB of T-DNA (for HindIII restriction sites within T-DNA, see Fig. 1). Lines that exhibited single hybridizing bands with LB and RB probes contained single T-DNA inserts. The completeness of the T-DNA was also tested by hybridizing the same blots with an internal fragment (GUS) probe. If the T-DNA copy was whole, the hybridization would result in a single, predicted size fragment (2.8 kb).
Amplification of T-DNA Insert Sites
TAIL PCR procedure was carried out as described by Liu et al. (1995), the only exception being that a 1-μL aliquot of the secondary reaction was diluted in 20 μL of water, and the tertiary reaction was performed in a 50-μL volume. Two sets of three nested specific primers were synthesized that corresponded to T-DNA LB and RB sequences. The LB set consisted of LB1 (5′-TGG GTA TCT GGG AAT GGC GAA ATA-3′; average Tm = 61.0°C), LB2 (5′-CAA GGC ATC GAT CGT GAA GTT T-3′; average Tm = 58.4°C), and LB3 (5′-AAT GTA GAC ACG TCG AAA TAA AGA-3′; average Tm = 55.9°C). The RB set consisted of RB1 (5′-GGG GCA TCG CAC CGG TGA GTA AT-3′; average Tm = 66.0°C), RB2 (5′-AGC GAA TTT GGC CTG TAG ACC TCA-3′; average Tm = 62.7°C), and RB3 (5′-TAT TCG GGC CTA ACT TTT GGT GTG-3′; average Tm = 61.0°C). LB1 and RB1 primers were used for the primary reactions, LB2 and RB2 for the secondary reactions, and LB3 and RB3 for the tertiary reactions. Two arbitrary degenerate primers were used, AD2 (Liu et al., 1995) and AD5 (Tsugeki et al., 1996).
IPCR procedure was carried out as described by Tissier et al. (1999). The primers used for amplification from the LB were LB1 and inverse (5′-CCT CAC ATA ATT CAC TCA AAT GCT A-3′; average Tm = 58.1°C).
YAC and BAC Library Filter Hybridizations
Gel-purified TAIL PCR or IPCR products were used as probes. CIC YAC library filters were prepared and hybridizations performed as described by Schmidt and Dean (1996). BAC filter hybridizations were performed as described by Bent et al. (1998), using the gel-purified T-DNA flanking fragments in place of YAC DNA. Institut für Genbiologische Forschung, Berlin and Texas A&M University BAC library filter sets were a kind gift of Ian Bancroft (John Innes Centre).
DNA Sequencing
Gel-purified TAIL PCR or IPCR products were sequenced using the ABI BigDye terminator reaction kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. To sequence LB-generated TAIL PCR or IPCR products, a primer positioned closer to the LB repeat was used, LB4 (5′-CGC TGC GGA CAT CTA CAT TTT TGA-3′; average Tm = 61.0°C).
Herbicide Selection
Selection of Ppt and Cs doubly resistant seedlings was performed by germinating seeds on GM supplemented with 20 ng mL–1 Cs (kindly supplied by DuPont) and 10 μg mL–1 Ppt (kindly supplied by Aventis UK, West Malling, Kent, UK). Only plants that had true leaves and well-developed roots were selected for further analysis.
Acknowledgments
We thank Richard Macknight and Clare Lister for undertaking the initial cloning steps in the Ds launch pad construction and Jonathan Jones for hosting P.S.S. during construction of the Ds nos::BAR. We thank Mervyn Smith for looking after the Arabidopsis plants and Evonne Waterman, Tania Page, and Bonnie Smart for initial Arabidopsis transformations. We also thank Jonathan Clarke and Jenn Conn for submitting flanking sequences.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.016535.
This work was supported by the Biotechnology and Biological Sciences Research Council (grant no. PAG04435) and by the European Commission (grant no. BIO4 CT98 5004).
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Bancroft I, Bhatt AM, Sjodin C, Scofield S, Jones JDG, Dean C (1992) Development of an efficient two-element transposon tagging system in Arabidopsis thaliana. Mol Gen Genet 233: 449–457 [DOI] [PubMed] [Google Scholar]
- Bancroft I, Dean C (1993a) Factors affecting the excision frequency of the maize transposable element Ds in Arabidopsis thaliana. Mol Gen Genet 240: 65–72 [DOI] [PubMed] [Google Scholar]
- Bancroft I, Dean C (1993b) Transposition pattern of the maize element Ds in Arabidopsis thaliana. Genetics 134: 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft I, Jones JDG, Dean C (1993) Heterologous transposon tagging of the DRL1 locus in Arabidopsis. Plant Cell 5: 631–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent E, Johnson S, Bancroft I (1998) BAC representation of two low-copy regions of the genome of Arabidopsis thaliana. Plant J 13: 849–955 [DOI] [PubMed] [Google Scholar]
- Bhatt AM, Page T, Lawson EJR, Lister C, Dean C (1996) Use of Ac as an insertional mutagen in Arabidopsis. Plant J 9: 935–945 [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Klimyuk VI, Thomas CM, Bishop GJ, Harrison K, Scofield SR, Jones JDG (1995) Germinal transpositions of the maize element Dissociation from T-DNA loci in tomato. Genetics 139: 407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois P, Cutler S, Belzile FJ (1998) Regional insertional mutagenesis on chromosome III of Arabidopsis thaliana using the maize Ac element. Plant J 13: 141–151 [DOI] [PubMed] [Google Scholar]
- Feldmann KA (1991) T-DNA insertion mutagenesis in Arabidopsis: mutational spectrum. Plant J 1: 71–82 [Google Scholar]
- Ito T, Seki M, Hayashida N, Shibata D, Shinozaki K (1999) Regional insertional mutagenesis of genes on Arabidopsis thaliana chromosome V using the Ac/Ds transposon in combination with a cDNA scanning method. Plant J 17: 433–444 [DOI] [PubMed] [Google Scholar]
- James DW Jr, Lim E, Keller J, Plooy I, Ralston E, Dooner HK (1995) Directed tagging of the Arabidopsis FATTY ACID ELONGATION1 (FAE1) gene with the maize transposon Activator. Plant Cell 7: 309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P, Belzile F, Page T, Dean C (1997) Increased Ac excision (iae): Arabidopsis thaliana mutations affecting Ac transposition. Plant J 11: 907–919 [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Shlumukov L, Carland F, English J, Bishop GJ, Harrison K (1992) Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Res 1: 285–297 [DOI] [PubMed] [Google Scholar]
- Kempin SA, Liljegren SJ, Block LM, Rounsley SD, Yanofsky MF, Lam E (1997) Targeted disruption in Arabidopsis. Nature 389: 802–803 [DOI] [PubMed] [Google Scholar]
- Koncz C, Mayerhofer R, Koncz-Kalman Z, Nawrath C, Reiss B, Redei GP, Schell J (1990) Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J 9: 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 82: 488–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Townsend J, Tepperman J, Black M, Chui CF, Mazur B, Dunsmuir P, Bedbrook J (1988) The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J 7: 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-G, Mitsukawa N, Oosumi T, Whitter RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8: 457–463 [DOI] [PubMed] [Google Scholar]
- Liu Y-G, Whitter RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25: 674–681 [DOI] [PubMed] [Google Scholar]
- Lloyd AM, Walbot V, Davis RW (1992) Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1. Science 258: 1773–1775 [DOI] [PubMed] [Google Scholar]
- Long D, Goodrich J, Wilson K, Sundberg E, Martin M, Puangsomlee P, Coupland G (1997) Ds elements on all five Arabidopsis chromosomes and assessment of their utility for transposon tagging. Plant J 11: 145–148 [DOI] [PubMed] [Google Scholar]
- Long D, Swinburne J, Martin M, Wilson K, Sundberg E, Lee K, Coupland G (1993) Analysis of the frequency of inheritance of transposed Ds elements in Arabidopsis after activation by CaMV 35S promoter fusion to the Ac transposase gene. Mol Gen Genet 241: 627–636 [DOI] [PubMed] [Google Scholar]
- Ludwig SR, Habera LF, Dellaporta SL, Wessler SR (1989) Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc Natl Acad Sci USA 86: 7092–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida C, Onouchi H, Koizumi J, Hamada S, Semiarti E, Torikai S, Machida Y (1997) Characterization of the transposition pattern of the Ac element in Arabidopsis thaliana using endonuclease I-SceI. Proc Natl Acad Sci USA 94: 8675–8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen RA (1998) Functional genomics: probing plant gene function and expression with transposons. Proc Natl Acad Sci USA 95: 2021–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JB, Chui CF, Smith JK (1987) Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol 85: 1110–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeting induced local lesions IN genomes (TILLING) for plant functional genomics. Plant Physiol 123: 439–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner RC, Jin H, Cominelli E, Denekamp M, Fuertes A, Greco R, Kranz HD, Penfield S, Petroni K, Urzainqui A et al. (1999) Function search in a large transcription factor gene family in Arabidopsis: assessing the potential of reverse genetics to identify insertional mutations in R2R3 MYB genes. Plant Cell 11: 1827–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno MA, Chen J, Greenblatt I, Dellaporta SL (1992) Reconstitutional mutagenesis of the maize P gene by short-range Ac transpositions. Genetics 131: 939–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskett PR, Bancroft I, Dean C (2002) Arabidopsis YAC, BAC and cosmid libraries. In P Gilmartin, C Bowler, eds, Molecular Plant Biology. A Practical Approach, Vol 1. Oxford University Press, Oxford, pp 137–158 [Google Scholar]
- Ochman H, Gerber AS, Hartl DL (1988) Genetic applications of an inverse polymerase chain reaction. Genetics 120: 621–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne BI, Wirtz U, Baker B (1995) A system for insertional mutagenesis and chromosomal rearrangement using the Ds transposon and Cre-lox. Plant J 7: 687–701 [DOI] [PubMed] [Google Scholar]
- Parinov S, Sevugan M, Ye D, Yang W-C, Kumaran M, Sundaresan V (1999) Analysis of flanking sequences from Dissociation insertion lines: a database for reverse genetics in Arabidopsis. Plant Cell 11: 2263–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Dean C (1996) Hybridization analysis of YAC clones. Methods Mol Cell Biol 5: 309–318 [Google Scholar]
- Scofield SR, Harrison K, Nurrish SJ, Jones JDG (1992) Promoter fusions to the Activator transposase gene cause distinct patterns of Dissociation excision in tobacco cotyledons. Plant Cell 4: 573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Yanai Y, Lui Y-G, Ishiguro S, Okada K, Shibata D, Whitter RF, Fedoroff NV (1996) Characterization and mapping of Ds-GUS-T-DNA lines for targeted insertional mutagenesis. Plant J 10: 721–732 [DOI] [PubMed] [Google Scholar]
- Speulman E, Metz PLJ, van Arkel G, te Lintel Hekkert B, Stiekma WJ, Pereira A (1999) A two-component Enhancer-Inhibitor transposon mutagenesis system for functional analysis of the Arabidopsis genome. Plant Cell 11: 1853–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer PS (2000) Gene traps: tools for plant development and genomics. Plant Cell 12: 1007–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JD, Dean C, Ma H, Martienssen R (1995) Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev 9: 1797–1810 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Uemura S, Saito Y, Murofushi N, Schmitz G, Theres K, Yamaguchi I (2001) A novel transposon tagging element for obtaining gain-of-function mutants based on a self-stabilizing Ac derivative. Plant Mol Biol 45: 123–131 [DOI] [PubMed] [Google Scholar]
- Swinburne J, Balcells L, Scofield S, Jones JDG, Coupland G (1992) Elevated levels of Activator transposase mRNA are associated with high frequencies of Dissociation excision in Arabidopsis. Plant Cell 4: 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier AF, Marillonnet S, Klimyuk V, Patel K, Torres MA, Murphy G, Jones JDG (1999) Multiple independent defective Suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell 11: 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T, Peterson MG, Kemp DJ (1990) A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acid Res 16: 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugeki R, Kochieva EZ, Fedoroff NV (1996) A transposon insertion in the Arabidopsis SSR16 gene causes an embryo-defective lethal mutation. Plant J 10: 479–489 [DOI] [PubMed] [Google Scholar]
- Valvekens D, van Montagu M, van Lijsebettens M (1988) Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 85: 5536–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse PM, Graham MW, Wang MB (1998) Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc Natl Acad Sci USA 95: 13959–13964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM et al. (2000) Activation tagging in Arabidopsis. Plant Physiol 122: 1003–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]