Abstract
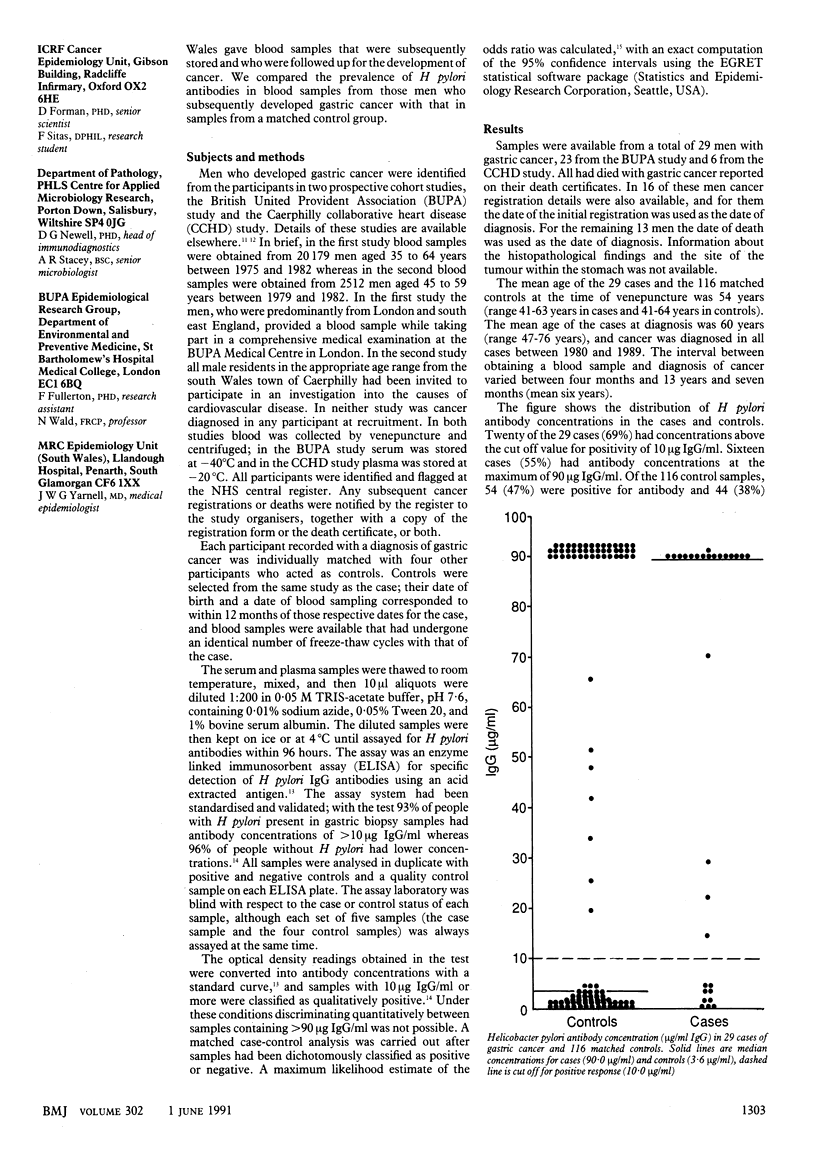
OBJECTIVE--To investigate the association between gastric cancer and prior infection with Helicobacter pylori. DESIGN--Case-control comparison of prevalence of IgG antibodies to H pylori in blood samples collected prospectively, before diagnosis of gastric cancer in the cases. Presence of H pylori antibody (greater than 10 micrograms IgG/ml) determined by enzyme linked immunosorbent assay (ELISA). SUBJECTS--29 men with a subsequent diagnosis of gastric cancer and 116 aged matched controls selected from over 22,000 middle aged men participating in two ongoing cohort studies (the British United Provident Association study and the Caerphilly collaborative heart disease study), who had provided blood samples during 1975-1982. RESULTS--20 of the 29 cases (69%) and 54 of the 116 controls (47%) were positive for H pylori specific antibody. The median specific IgG concentration was significantly higher in the cases than controls (90 micrograms/ml v 3.6 micrograms/ml, p less than 0.01). The estimated odds ratio for the risk of gastric cancer in those with a history of infection with H pylori was 2.77 (95% confidence interval 1.04 to 7.97, 2p = 0.039). CONCLUSIONS--H pylori infection may be an important cause of gastric cancer; between 35% and 55% of all cases may be associated with such an infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. J., Coggon D., Osmond C., Wickham C. Poor housing in childhood and high rates of stomach cancer in England and Wales. Br J Cancer. 1990 Apr;61(4):575–578. doi: 10.1038/bjc.1990.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. Y., Wang Y. Campylobacter pylori in patients with gastritis, peptic ulcer, and carcinoma of the stomach in Lanzhou, China. Lancet. 1988 May 7;1(8593):1055–1055. doi: 10.1016/s0140-6736(88)91877-6. [DOI] [PubMed] [Google Scholar]
- Forman D., Sitas F., Newell D. G., Stacey A. R., Boreham J., Peto R., Campbell T. C., Li J., Chen J. Geographic association of Helicobacter pylori antibody prevalence and gastric cancer mortality in rural China. Int J Cancer. 1990 Oct 15;46(4):608–611. doi: 10.1002/ijc.2910460410. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Correa P., Taylor N. S., Zavala D., Fontham E., Janney F., Rodriguez E., Hunter F., Diavolitsis S. Campylobacter pylori-associated gastritis and immune response in a population at increased risk of gastric carcinoma. Am J Gastroenterol. 1989 Jul;84(7):775–781. [PubMed] [Google Scholar]
- Howson C. P., Hiyama T., Wynder E. L. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- Jaskiewicz K., Louwrens H. D., Woodroof C. W., van Wyk M. J., Price S. K. The association of Campylobacter pylori with mucosal pathological changes in a population at risk for gastric cancer. S Afr Med J. 1989 May 6;75(9):417–419. [PubMed] [Google Scholar]
- Kwa H. G., Cleton F., Wang D. Y., Bulbrook R. D., Bulstrode J. C., Hayward J. L., Millis R. R., Cuzick J. A prospective study of plasma prolactin levels and subsequent risk of breast cancer. Int J Cancer. 1981 Dec;28(6):673–676. doi: 10.1002/ijc.2910280603. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pylori: its link to gastritis and peptic ulcer disease. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S87–S93. doi: 10.1093/clinids/12.supplement_1.s87. [DOI] [PubMed] [Google Scholar]
- Perez-Perez G. I., Taylor D. N., Bodhidatta L., Wongsrichanalai J., Baze W. B., Dunn B. E., Echeverria P. D., Blaser M. J. Seroprevalence of Helicobacter pylori infections in Thailand. J Infect Dis. 1990 Jun;161(6):1237–1241. doi: 10.1093/infdis/161.6.1237. [DOI] [PubMed] [Google Scholar]
- Robey-Cafferty S. S., Ro J. Y., Cleary K. R. The prevalence of Campylobacter pylori in gastric biopsies from cancer patients. Mod Pathol. 1989 Sep;2(5):473–476. [PubMed] [Google Scholar]
- Sitas F., Forman D., Yarnell J. W., Burr M. L., Elwood P. C., Pedley S., Marks K. J. Helicobacter pylori infection rates in relation to age and social class in a population of Welsh men. Gut. 1991 Jan;32(1):25–28. doi: 10.1136/gut.32.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siurala M., Sipponen P., Kekki M. Campylobacter pylori in a sample of Finnish population: relations to morphology and functions of the gastric mucosa. Gut. 1988 Jul;29(7):909–915. doi: 10.1136/gut.29.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald N., Idle M., Boreham J., Bailey A. Low serum-vitamin-A and subsequent risk of cancer. Preliminary results of a prospective study. Lancet. 1980 Oct 18;2(8199):813–815. doi: 10.1016/s0140-6736(80)90169-5. [DOI] [PubMed] [Google Scholar]
- Yang P. C., Davis S. Epidemiological characteristics of adenocarcinoma of the gastric cardia and distal stomach in the United States, 1973-1982. Int J Epidemiol. 1988 Jun;17(2):293–297. doi: 10.1093/ije/17.2.293. [DOI] [PubMed] [Google Scholar]


