Abstract
Plants acquire thermotolerance to lethal high temperatures if first exposed to moderately high temperature or if temperature is increased gradually to an otherwise lethal temperature. We have taken a genetic approach to dissecting acquired thermotolerance by characterizing loss-of-function thermotolerance mutants in Arabidopsis. In previous work, we identified single recessive alleles of four loci required for thermotolerance of hypocotyl elongation, hot1-1, hot2-1, hot3-1, and hot4-1. Completed screening of M2 progeny from approximately 2500 M1 plants has now identified new alleles of three of these original loci, along with three new loci. The low mutant frequency suggests that a relatively small number of genes make a major contribution to this phenotype or that other thermotolerance genes encode essential or redundant functions. Further analysis of the original four loci was performed to define the nature of their thermotolerance defects. Although the HOT1 locus was shown previously to encode a major heat shock protein (Hsp), Hsp101, chromosomal map positions indicate that HOT2, 3, and 4 do not correspond to major Hsp or heat shock transcription factor genes. Measurement of thermotolerance at different growth stages reveals that the mutants have growth stage-specific heat sensitivity. Analysis of Hsp accumulation shows that hot2 and hot4 produce normal levels of Hsps, whereas hot3 shows reduced accumulation. Thermotolerance of luciferase activity and of ion leakage also varies in the mutants. These data provide the first direct genetic evidence, to our knowledge, that distinct functions, independent of Hsp synthesis, are required for thermotolerance, including protection of membrane integrity and recovery of protein activity/synthesis.
Plants have many different mechanisms for surviving high temperatures in their environment, including long-term evolutionary adaptations of life history and morphology and shorter term avoidance or acclimation mechanisms involving, for example, leaf orientation, transpirational cooling, or alterations of membrane lipid composition (Berry, 1975; Turner and Kramer, 1980; Radin et al., 1994). It has also long been known that plants, like other organisms, have the ability to acquire thermotolerance rapidly, within hours, to otherwise normally lethal high temperatures (Alexandrov, 1961; Lin et al., 1984; Neumann et al., 1989; Vierling, 1991). The acquisition of thermotolerance is a cell autonomous phenomenon and results from prior exposure to a conditioning pretreatment, which can be a short, sublethal high temperature or other moderate stress treatments (Lindquist, 1986). Thermotolerance can also be induced by a gradual increase to the normally lethal temperature (Vierling, 1991), as would be experienced in the natural environment (Ansari and Loomis, 1959; Ehler, 1973). Even plants growing in their natural distribution range may experience high temperatures that would be lethal in the absence of this rapid acclimation; thus, ability to acquire thermotolerance is likely of significant importance to plants. Furthermore, because plants can experience major diurnal temperature fluctuations, the acquisition of thermotolerance may reflect a more general mechanism that contributes to homeostasis of metabolism on a daily basis.
Although acquired thermotolerance has been described and studied in plants for decades (Alexandrov, 1994), only a limited number of factors have been defined that contribute to the development of thermotolerance. Many studies have documented that heat shock protein (Hsp) synthesis is correlated with the acquisition of thermotolerance (for review, see Vierling, 1991). All major classes of Hsps are proposed to act as molecular chaperones, functioning through binding to substrate proteins that are in unstable, nonnative structural states (Hendrick and Hartl, 1993; Boston et al., 1996; Gething, 1997). By virtue of this property, the different Hsps/chaperones are able to aid in a variety of cellular processes that involve assisted protein folding, including rescue of misfolded or aggregated proteins. This latter activity is presumed to explain their important role in heat stress, a condition that leads to protein denaturation. The involvement of Hsps in heat stress tolerance is a logical model, but direct support for Hsp function in thermotolerance in plants has been difficult to obtain (Burke, 2001). Only one Hsp, Hsp101, has been shown to be essential for thermotolerance by genetic analysis. We have described both missense and protein null alleles of the Hsp101 gene (HOT1) that do not acquire thermotolerance in response to pretreatments at several stages of development (Hong and Vierling, 2000, 2001). Current models propose that Hsp101, an AAA+ chaperone ATPase (Neuwald et al., 1999), promotes ATP-dependent dissolution of cytosolic or nuclear protein aggregates formed during heat stress, consistent with a role in recovery of active proteins after heat stress (Parsell et al., 1994; Schirmer et al., 1996; Mogk et al., 1999; Motohashi et al., 1999; Zolkiewski, 1999).
Even if all Hsps contribute to thermotolerance, it is highly likely that other factors are also necessary. In addition to causing protein denaturation, high temperature also alters membrane fluidity, can disrupt the overall balance of metabolic processes, and leads to oxidative stress (Dewey, 1989; Larkindale and Knight, 2002). Heat stress also affects the cytoskeleton as assessed by cytoplasmic streaming (Alexandrov, 1994). Acquired thermotolerance can be hypothesized to moderate these types of damage through the induction of protective factors that limit the extent of damage or by enhancing processes required for recovery from damage, although these mechanisms need not be mutually exclusive.
We have taken a genetic approach to dissecting the mechanism of acquired thermotolerance and have reported the isolation of four recessive, nonallelic mutations (hot1 to 4) in Arabidopsis that are defective in acquired thermotolerance of hypocotyl elongation (Hong and Vierling, 2000). Here, we report that additional screening for loss-of-thermotolerance mutants has defined three new HOT loci and one new allele for hot1, hot2, and hot4. As described above, hot1 was previously shown to define the gene encoding Hsp101. Analysis of the chromosomal map positions of the HOT2, HOT3, and HOT4 genes, combined with further detailed phenotypic analysis of these mutants compared with hot1, provide direct genetic evidence that at least four distinct pathways/functions are necessary for acquisition of thermotolerance.
RESULTS
Isolation of New hot Mutants
We previously described recessive, ethyl methanesulfonate (EMS) mutations at four independent loci, HOT1 to 4, that fail to develop thermotolerance in response to moderate heat treatment (Hong and Vierling, 2000). In addition to these initial mutations, now designated as hot1-1, hot2-1, hot3-1, and hot4-1, six more EMS mutations were isolated after completing a screen of a total of >20,000 M2 seedlings derived from an original approximately 2,500 M1 plants. All new mutations were verified as defective in thermotolerance after propagation to the M3 generation. Genetic complementation analysis of all pairwise combinations of mutants was performed. Based on the thermotolerance phenotype (hypocotyl assay; see below) of 11 F1 plants from each pair-wise cross, three mutants were confirmed to represent new alleles of the previously isolated mutants. These new alleles have been designated hot1-4, hot2-2, and hot4-2. The three new mutants at unique loci were designated hot5-1, hot6-1, and hot7-1, and all were found to be recessive.
Isolating second alleles of three of the original hot mutants in this mutant population is consistent with the conclusion that a relatively small number of genes make strong contributions to the hypocotyl thermotolerance phenotype or that mutation of other functions must be lethal or redundant. The low mutant frequency and isolation of multiple alleles also indicates that the hot mutants are unlikely to represent random temperature-sensitive mutations in any gene but rather are specific to the mechanism of acquired thermotolerance. Further work reported here was performed with the original set of alleles (hot1-1, hot2-1, hot3-1, and hot4-1) after a single backcross to the wild-type Columbia parent, and the new alleles were tested (before backcrossing) in many of the assays, as discussed in the text. Further genetic and phenotypic characterization of the newly isolated mutants is continuing.
Chromosomal Map Position of Mutants Defective in Acquired Thermotolerance
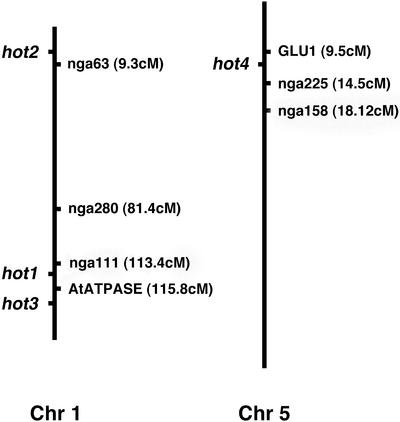
To begin to establish the identity of the hot2, hot3, and hot4 mutations, their chromosomal map positions were determined as shown in Figure 1 (see “Materials and Methods”). The HOT1 gene was mapped to bacterial artificial chromosome F9E11 and identified as Hsp101 as described previously (Hong and Vierling, 2000). The HOT2 locus was mapped to the top of chromosome 1 by scoring recombination with nF20D22 at 6.1 cM (0.09% of 318 chromosomes) and nga63 at 11.4 cM (6.4% of 236 chromosomes). HOT3 mapped to the bottom of chromosome 1 showing recombination with AtATPase at 115.7 cM (2.4% of 338 chromosomes), 0% recombination with nF22K20 (68 chromosomes), and 1.8% recombination with nF28K19 (336 chromosomes). Similarly, HOT4 mapped to the top of chromosome 5 with 3.5% recombination with Glu1 at 9.5 cM (380 chromosomes), only 0.09% recombination with nga225 at 14.31 cM (380 chromosomes), and 5.3% recombination at nga158 at 18.12 cM (280 chromosomes). Considering the known genes within the intervals defined by the markers used for gene mapping, none corresponded to Hsps of the Hsp70, Hsp101, or sHsp classes, nor to the many heat shock transcription factor (Hsf) genes (Nover et al., 2001; Scharf et al., 2001; Sung et al., 2001). Thus, the hot2, 3, and 4 mutants provide direct genetic support for the involvement of factors other than the major Hsps or Hsfs in the acquisition of thermotolerance.
Figure 1.
Chromosomal locations of the HOT loci. The vertical lines represent chromosome I and 5, respectively, with positions of selected marker loci (in centiMorgans) and the relative positions of the hot mutations as indicated.
Phenotypes of the hot Mutants under Optimal Growth Conditions
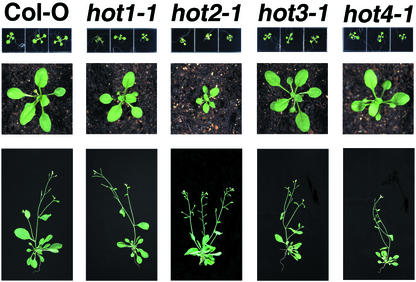
To determine if the hot mutations have strong effects on growth and development under optimal conditions, the mutants were observed through a complete life cycle. As documented in Figure 2, only the hot2-1 mutant showed consistent phenotypes significantly different from wild type. The hot2-1 plants are semidwarf and highly branched. The semidwarf phenotype of hot2-1 is also seen in hypocotyl length (see Fig. 3). These phenotypes were also observed in hot2-2 and segregated with the hypocotyl thermotolerance defect as judged from observations of approximately 600 seedlings and over 300 mature plants.
Figure 2.
Growth and development of the hot mutants under normal conditions. Ten-d-old (top), 3-week-old (middle), and 6-week-old plants (bottom) representative of wild type or the hot mutants are shown.
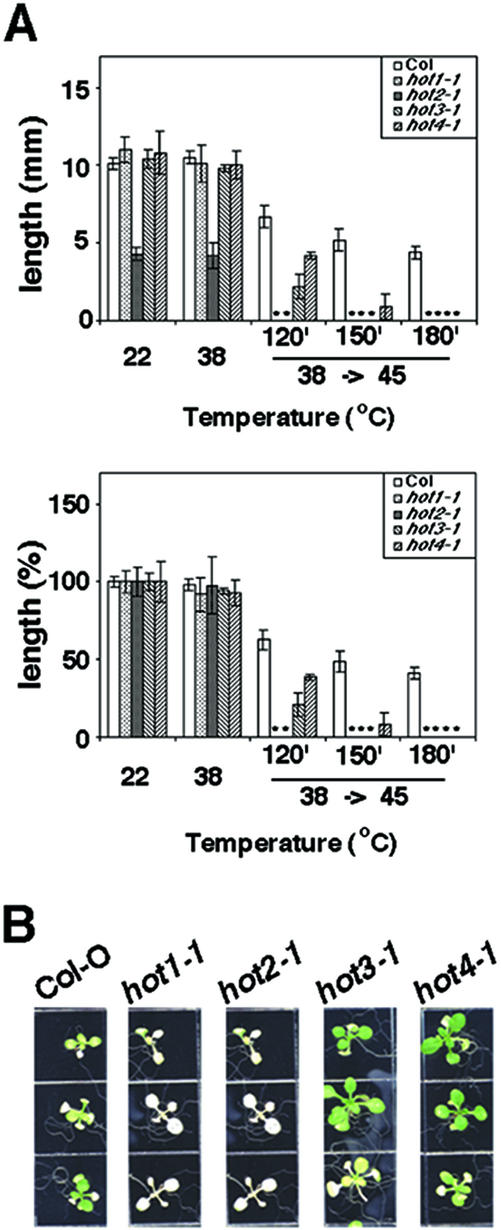
Figure 3.
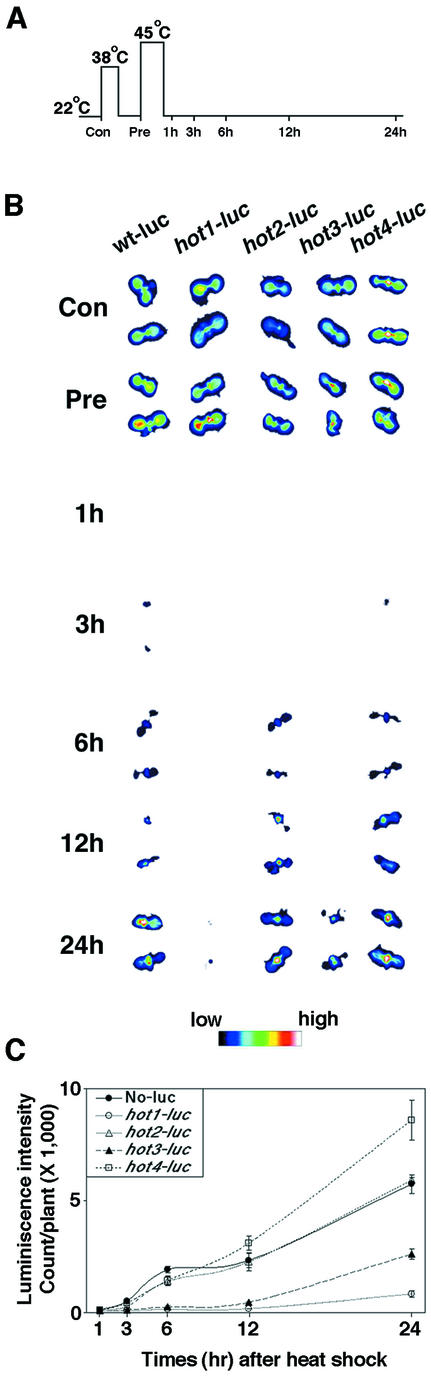
Thermotolerance phenotypes of the hot mutants. A, Quantitative assessment of acquired thermotolerance in the hot mutants using the hypocotyl elongation assay. Dark-grown, 2.5-d-old seedlings were pre-adapted at 38°C for 90 min, followed by 120 min of recovery at 22°C. Seedlings were then either returned to 22°C or further stressed at 45°C for 120, 150, or 180 min as indicated. Total hypocotyl elongation after the heat stress was measured after an additional 2.5 d growth in the dark. Mean and SD were derived by measurement of at least 11 seedlings, and values plotted as growth (millimeters; top) or as a percentage of the value of seedlings grown at 22°C (bottom). B, Thermotolerance of 10-d-old seedlings after pretreatment and subsequent heat treatment at 45°C for 150 min. Three seedlings of each of the indicated genotypes are shown, photographed 5 d after heat treatment.
Thermotolerance Phenotypes of the hot Mutants
The hot mutants were all isolated based on their failure to show acquired thermotolerance in a hypocotyl elongation assay (Hong and Vierling, 2000). The hot1-1 mutant is also defective in basal heat tolerance of imbibed seeds and in acquired thermotolerance of 7- to 10-d-old seedlings (Hong and Vierling, 2000, 2001). To determine if all of the hot mutants have the same thermotolerance phenotypes, thermotolerance was tested in all three assays, using the defined hot1-1 mutant as a reference. When tested for hypocotyl elongation, the mutants showed different degrees of severity (Fig. 3A). hot1-1 and hot2-1 were completely blocked in hypocotyl elongation after 120 min of 45°C heat treatment. In contrast, hot3-1 and hot4-1 were less severe. hot3-1 and 4-1 showed tolerance to 120 min at 45°C equivalent to approximately 30% or 60% of wild type, respectively. The hot4-1 mutant even retained some ability to elongate after 150 min at 45°C and required a full 180 min at 45°C to completely block elongation. Note that all hot mutants and wild type failed to elongate after treatment at 45°C without a pretreatment (data not shown; see Hong and Vierling, 2000). Because we do not know if these mutations, with the exception of hot1-1 (Hong and Vierling, 2001), are completely null for activity, the quantitative differences observed may be allele rather than gene specific. However, tests of the other alleles (hot1-4, hot2-2, and hot4-2) revealed similar differences in tolerance between the mutants (data not shown).
In contrast to the small quantitative differences between the mutants in the hypocotyl assay, the ability of 10-d-old seedlings to acquire thermotolerance differed dramatically between the mutants (Fig. 3B). The hot1-1 and hot2-1 mutants failed to acquire thermotolerance at this stage; they ceased production of additional leaves and existing leaves and cotyledons turned white. In the same assay, hot3-1 and hot4-1 behaved like wild type. In fact, hot4-1 consistently appeared more robust than wild type after either a 38°C pretreatment alone (not shown) or after the pretreatment followed by a 45°C stress (Fig. 3B). Again, results with the other alleles were the same (not shown), arguing that this stage specificity of thermotolerance is gene specific.
Before germination, imbibed Arabidopsis seeds have greater temperature tolerance than seedlings. That is, without any acclimating pretreatment, imbibed seeds can be heated at 45°C for at least 150 min and still survive and grow (Hong and Vierling, 2000, 2001). Seeds of the hot1-1 mutant lost this temperature tolerance and were growth arrested after germination when treated at 45°C for 120 min or more. When tested for the same phenotype, all alleles of the other hot mutants behaved essentially like wild type and were not growth arrested (not shown).
Another phenotype that can be scored for the development of thermotolerance is the ability of dark-grown seedlings to accumulate chlorophyll (develop chloroplasts). In this assay, which has been used by Burke et al. (2000) to isolate other mutants defective in the acquisition of thermotolerance, dark-grown seedlings were pretreated to induce thermotolerance and then given the severe heat treatment before exposure to light. Chlorophyll accumulation during light incubation was then quantified. When hot1-1, hot2-1, hot3-1, and hot4-1 were tested in this chlorophyll accumulation assay, only hot1-1 exhibited a mutant phenotype (data not shown).
Expression of Hsps in the hot Mutants
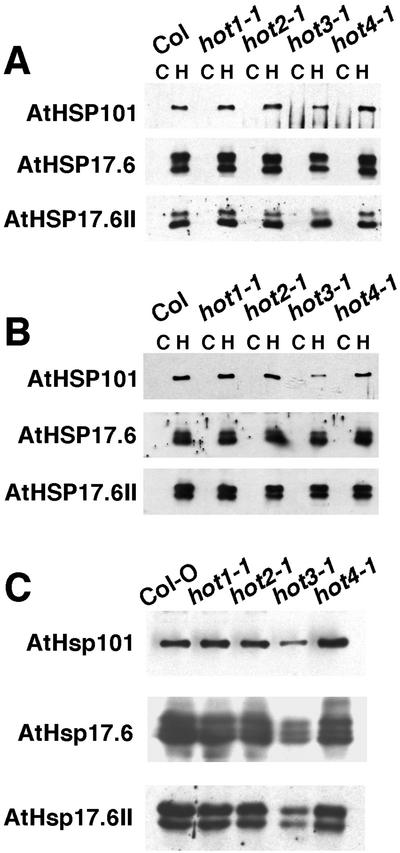
Because the expression of Hsp101 is essential for thermotolerance, and expression of other Hsps is likely to also be essential, the level of Hsp101 and selected sHsps was estimated by western analysis in the hot mutants as shown in Figure 4. In all of the mutants, the Hsps were not present at significant levels before heat stress. Accumulation of Hsps after the 38°C conditioning pretreatment in 2.5- and 10-d-old seedlings revealed a wild-type pattern of expression in hot1-1, as seen previously (Hong and Vierling, 2000). The hot1-1 allele has a missense mutation in Hsp101 and accumulates normal levels of Hsp101, but shows a phenotype essentially equivalent to that of an Hsp101 protein null allele (Hong and Vierling, 2001). Wild-type levels of Hsp expression were also observed in hot2-1 and hot4-1. There was, however, a notable decrease in the accumulation of Hsp101 in the hot3-1 mutant. Despite this decrease, 10-d-old hot3-1 plants showed normal acquired thermotolerance (Fig. 3), indicating that the level of Hsp101 must still be sufficient for thermotolerance at this growth stage. Differences in the level of sHsps in hot3-1 did not appear to be significant in these samples.
Figure 4.
Accumulation of Hsp101 and sHsp proteins in heat-stressed seedlings of the hot mutants. A, Hsp protein levels in 2.5-d-old dark-grown hypocotyls. Total proteins were isolated from control or heat-stressed (38°C, 90 min, followed by 2 h at 22°C) hypocotyls. Equal quantities of total protein (0.5 μg for Hsp101 antibodies, 15 μg for sHsp antibodies) from each of the mutants and the wild type were separated on 7.5% (Hsp101) or 15% (sHsps) (w/v) polyacrylamide-SDS gels, and protein blots were probed as indicated with anti-Arabidopsis Hsp101, anti-Arabidopsis class I sHsp (Hsp17.6), or anti-Arabidopsis class II sHsp (Hsp17.6II) antibodies. B, Control and heat-stressed leaves of 10-d-old plants. Samples were analyzed as described for A. C, Accumulation of Hsps in 2.5-d-old dark-grown seedlings of the hot mutants or wild type treated at 40°C for 90 min, followed by 2 h of recovery at 22°C. Samples were analyzed as described for A.
To investigate further the decreased expression of Hsp101 in hot3-1, a 40°C 90-min treatment, rather than a 38°C treatment, was used to induce Hsp accumulation in 2- or 10-d-old seedlings. At this increased temperature, the induction of all Hsps tested was significantly reduced in hot3-1 (Fig. 4), whereas induction remained unchanged in all the other genotypes. Analysis of a dilution series (not shown) indicates that Hsp101 and class II sHsps (Hsp17.6II) are reduced by 50%, and class I sHsps (Hsp17.4) are reduced by about 70%. Thus, the defect in hot3-1 is not restricted to the expression of Hsp101, but has a more general effect on accumulation of Hsps that is more severe at higher temperatures. When 10-d-old seedlings given the 40°C pretreatment were examined for acquired thermotolerance to a 45°C treatment, hot3-1 was now found to be unable to acquire tolerance, similar to hot1-1 and hot2-1, whereas wild-type and hot4-1 seedlings still exhibited thermotolerance as before (not shown). The reduced thermotolerance of hot3-1 under these treatment conditions may be related to the greater reduction in Hsp induction.
Hsp levels were also tested in seeds, which are known to store significant levels of Hsp101 and specific class I and class II cytosolic sHsps (Wehmeyer et al., 1996; Hong and Vierling, 2001). These Hsps were present in seeds of all of the mutants at wild-type levels (not shown). The high level of Hsp101 in all the other hot mutants was expected based on the observation that none of these mutants is defective in basal thermotolerance of germinating seeds.
Activity of a Reporter Enzyme Provides Another Test for Acquired Thermotolerance
In total, results of the above thermotolerance tests indicate that each of the hot mutants must be defective in different processes required for the development of thermotolerance. To begin to dissect the thermotolerance mechanism, assays that measure biochemical functions, rather than only survival and growth, were necessary. For this purpose, an assay for the heat tolerance of a reporter enzyme, firefly luciferase (Luc), was developed. Luc is a very thermolabile protein that has been used extensively both in vitro and in vivo to examine the effects of heat treatment and the mechanism of chaperone action (Pinto et al., 1991; Buchberger et al., 1996; Forreiter et al., 1997; Michels et al., 1997; Lee and Vierling, 2000). A transgenic Arabidopsis line (Nössen [No] background) expressing Luc under control of the constitutive UBQ10 promoter (Worley et al., 2000) was crossed to the original allele of each of the hot mutants to introduce the Luc transgene. Importantly, the Luc gene was engineered to remove the three C-terminal amino acids that are necessary and sufficient for peroxisomal targeting, so that Luc would serve as a reporter enzyme in the cytosolic compartment of the cell. The recovery of Luc activity after heat acclimation was then followed in vivo by luminescence imaging in intact seedlings.
The results in Figure 5 demonstrate that pretreatment of 7-d-old seedlings at 38°C has little effect on Luc activity in any of the mutants, whereas subsequent treatment at 45°C reduced activity basically to zero in wild type and all of the mutants. However, Luc activity begins to recover within 3 h after 45°C treatment in wild type and is essentially fully recovered after 24 h. In comparison, the hot1-1 and hot3-1 mutants show dramatically reduced ability to recover Luc activity, with hot1-1 exhibiting the most severe phenotype. The phenotype of hot3-1, which is delayed in recovery but still shows significant recovery after 24 h, is consistent with the observation that thermotolerance of 10-d-old hot3-1 seedlings pretreated at 38°C appears similar to wild type as assessed by growth 5 d after heat stress (Fig. 3B). The hot2-1 mutant recovers Luc activity as rapidly as wild type, despite the fact that hot2-1 seedlings are severely damaged or die from the treatment (Fig. 3). Luc activity in hot4-1 actually achieves higher levels after 24 h of recovery than what is seen for wild type.
Figure 5.
Time course recovery of Luc activity after heat stress in 10-d-old wild-type or hot mutant seedlings. A, Temperature treatment protocol. Luc activity was imaged at the times indicated. B, Images of two seedlings per time point for each genotype. Note that the wild-type plants are No ecotype (No-luc). C, Quantitation of Luc signal intensity during recovery. Data were acquired from 18 plants in three different experiments.
Wild-type Luc activity in hot4-1 could reflect the absence of a thermotolerance defect in hot4-1 mutants at this growth stage (see Fig. 3B). To test if Luc activity in hot4-1 was compromised in 2-d-old heat-treated seedlings, Luc activity was also monitored in the mutants under the conditions used for the hypocotyl elongation assay. Even at this growth stage, where hypocotyl elongation is blocked by heat stress, Luc activity recovered as well or better than in wild type in hot4-1, and results with the other mutants were also similar to the those seen with 10-d-old seedlings (not shown).
The Luc reaction requires ATP and, therefore, is expected to be sensitive to intracellular ATP concentrations. To confirm that the in vivo luminescence measurements reflect the level of active Luc rather than the level of intracellular ATP, selected samples were also extracted for analysis of Luc activity in vitro with added ATP (see “Materials and Methods”). Extractable Luc activity paralleled the activity assessed by in vivo imaging (not shown), indicating the in vivo measurements are a valid measure of Luc activity, rather than a measure of ATP levels.
In total, these data indicate that ability to recover Luc activity, which could reflect ability to reactivate denatured enzyme or to recover normal transcriptional and translational activities, is necessary (hot1-1 and hot3-1) but not sufficient (hot2-1 and hot4-1) for the development of thermotolerance.
Acquired Thermotolerance of Heat-Induced Ion Leakage
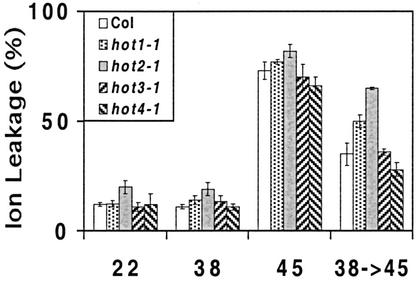
Heat is also predicted to alter membrane transport properties, possibly through effects on membrane fluidity or the activity of membrane channels and transporters (Levitt, 1980). To determine if the hot mutations affected membrane stability, an ion leakage test of acquired thermotolerance was developed. As in the other thermotolerance tests, ion leakage (as measured by solution conductivity) from 38°C pretreated seedlings was not significantly different from untreated (22°C) controls (Fig. 6). A lethal treatment at 45°C had the expected result of causing virtually complete ion loss from seedling tissues. A moderate but significant protection from severe ion loss could be effected by the usual 38°C pretreatment before the 45°C stress; ion leakage was on the order of 50% lower in wild-type seedlings after this acclimation. Only hot2-1 showed a dramatic reduction in ability to develop tolerance against ion leakage. hot3-1 and 4-1 behaved as wild type, and hot1-1 showed a small increase in conductivity. It may also be significant that hot2-1 reproducibly showed a higher level of ion leakage under control conditions than the other mutants or wild type (Fig. 6).
Figure 6.
Acquired thermotolerance of membrane permeability in the hot mutants. Ten-dayold seedlings grown on minimal medium under normal conditions were heat treated as indicated and then incubated in deionized water for 1 d. Conductivity was measured for at least 10 seedlings and plotted as a percentage of the total conductivity measured after seedlings were autoclaved. The data are from a representative experiment. Results were confirmed in multiple experiments.
DISCUSSION
Our analysis of loss-of-function mutants in Arabidopsis has defined seven loci, the HOT genes, required for the acquisition of thermotolerance. Four of these hot mutations have been mapped to the Arabidopsis genome and exhibit differences in thermotolerance at different life stages. Analysis of biochemical phenotypes of hot1, hot2, hot3, and hot4, including production of Hsps, and thermotolerance of Luc activity and of ion leakage provide the first direct genetic evidence that at least four distinct functions are required for thermotolerance. These include production of Hsps, specifically Hsp101, protection of membrane integrity, recovery of protein activity/synthesis, and at least one other undefined function. Disruption of any one of these functions is sufficient to eliminate the ability of plants to acclimate to high temperature, underscoring the fact that engineering increased plant heat tolerance will require manipulation of multiple cellular characteristics.
We have considered the possibility that temperature-sensitive mutations in any gene could lead to a loss-of-thermotolerance phenotype that is unrelated to the mechanism of acquired thermotolerance. However, several lines of evidence indicate that the hot mutations represent specific lesions affecting functions required for thermotolerance, rather than unrelated temperature-sensitive mutations. First, the conditions under which the hot mutations reveal their phenotype are short-term high-temperature treatments, as opposed to continuous growth at elevated temperatures, as is typically used for identification of temperature-sensitive mutations. It seems unlikely that a brief period at 45°C, but not 38°C, would irreversibly inactivate all of these proteins and that their function could not be replaced by new protein synthesis during the recovery period. Second, our screen identified the Hsp101 gene (HOT1), which had been established previously in bacteria and yeast (Saccharomyces cerevisiae) as essential for acquired thermotolerance (Schirmer et al., 1996). In addition to the EMS-generated hot1-1 point mutation studied in this report, a protein null mutation of the Hsp101 gene (hot1-3) has an identical phenotype, indicating loss of gene function rather than temperature sensitivity of gene function leads to the hot1 thermotolerance phenotype (Hong and Vierling, 2001). Finally, if the screen uncovered random temperature-sensitive mutations in any gene, the frequency of mutant recovery would be predicted to be high, and the screen would be difficult to saturate. However, from screening progeny of an approximately 2,500 EMS mutagenized M1 plants, only 10 mutants were recovered, seven of which represent alleles of the four HOT genes studied in detail here. Based on all of the above observations, we conclude that the hot mutations disrupt specific processes required for the acquisition of thermotolerance in plants.
The stage-specific effects on seed, 2.5-, and 10-d-old seedling thermotolerance in the hot mutants are quite striking. Although all the mutants are defective in acquired thermotolerance of hypocotyl elongation, only hot1 is defective in all three growth assays. These data, along with the hot1 defect in thermotolerance of chlorophyll accumulation and Luc recovery, emphasize that many processes are dependent on the function of this single chaperone protein. The ability of the hot4 mutants to acquire thermotolerance after 10 d, but not 2.5 d of growth, might be explained by activation of redundant stress recovery pathways during seedling growth.
As shown previously, the hot1 mutations demonstrate that production of a specific Hsp, Hsp101, is required for thermotolerance (Hong and Vierling, 2000, 2001). Based on analogy with yeast and bacterial systems, the role of Hsp101 is likely to involve renaturation of damaged cellular proteins (Parsell et al., 1994; Glover and Lindquist, 1998; Mogk et al., 1999; Motohashi et al., 1999; Zolkiewski, 1999). The failure of hot1-1 plants to reactivate Luc after heat stress could be a direct measure of inability of these plants to reactivate damaged proteins. Despite the measured changes in Luc activity, the Luc protein level does not change significantly over the time course of the stress and recovery (S.-W. Hong, unpublished data), consistent with the interpretation that protein reactivation is responsible for recovery of Luc activity. However, direct measurement of rates of Luc degradation and synthesis are required to rule out the model that recovery involves Hsp101 effects on reactivation of Luc transcription and translation. The ability of the hot1-1 mutant to acquire almost wild-type levels of membrane protection in the electrolyte leakage assay also suggests that membrane factors required for thermotolerance are not major targets of Hsp101 activity.
The hot2 and hot4 mutations unambiguously demonstrate that functions independent of Hsp101, Hsp70, and sHsps are required for thermotolerance. These mutants produce normal levels of Hsp101 and sHsps despite their failure to develop thermotolerance. Map positions of the hot2 and hot4 mutations also fall outside intervals containing Hsp70 or other sHsp genes. It remains highly possible that induction of Hsp70 and sHsps are both critical to thermotolerance, as implicated in other studies (Waters et al., 1996; Malik et al., 1999). However, Hsp70 and sHsp genes are found in small gene families in Arabidopsis; therefore, it is not surprising that our screen did not uncover sHsp or Hsp70 mutants.
The phenotype of the hot2-1 mutant provides evidence that modulation of membrane properties is an essential aspect of acquired thermotolerance. The hot2-1 mutant shows reproducibly higher levels of electrolyte leakage compared with wild-type plants under control conditions, and increased electrolyte leakage at 45°C is not protected by a pretreatment at 38°C as it is in wild type and the other hot mutants. Lin et al. (1985) reported that heat shock-induced electrolyte leakage could be protected by prior heat treatment of soybean (Glycine max) seedlings and suggested protection was effected by Hsps. As discussed above, HOT2 does not encode a major Hsp and expresses normal levels of those Hsps that we have tested. Although we cannot strictly eliminate the possibility that HOT2 indirectly alters regulation of specific Hsps that were not measured in our study, we suggest that the membrane protection loss in hot2 is due to a defect distinct from Hsp expression. It is also interesting to note that Luc activity recovers normally in hot2-1, despite severe damage to the plants. Therefore, reactivation of proteins or protein synthesis is also not sufficient for thermotolerance. There are no obvious candidate genes for membrane functions in the region to which HOT2 maps, and it cannot necessarily be concluded that the ion leakage defect is the primary cause of lost thermotolerance. However, we have noted recently that HOT2 maps to the same region as the chitinase-like protein AtCTL1 (Zhong et al., 2002) and that the hot2 mutants and a mutant of AtCTL1 have similar phenotypes in the absence of stress, including semidwarfism and increased flowering stalks. Because the mutation of AtCTL1 also has effects on cell wall and cell structure, it may indirectly perturb membrane functions required for thermotolerance. We are currently testing whether HOT2 and AtCTL1 are allelic as we continue efforts to clone HOT2 using map-based approaches.
The hot3-1 mutation clearly affects accumulation of Hsp101 and sHsps, with the defect increasing at higher temperatures. The reduced level of Hsp101 could account for the slow recovery of Luc activity in 2.5- and 10-d-old seedlings of this mutant. However, it seems unlikely that decreased synthesis of Hsp101 is solely responsible for the thermotolerance defect of hot3. In 2.5-d-old seedlings, at least 50% wild-type levels of Hsp101 are produced, which appear sufficient for thermotolerance as demonstrated in Hsp101 antisense experiments (Queitsch et al., 2000). Furthermore, a similar reduction in Hsp101 accumulation occurred in 10-d-old plants pretreated at 38°C, and these plants showed normal acquisition of thermotolerance. Only when pretreatment was increased to 40°C was acquired thermotolerance lost in hot3-1, in parallel with reduced expression of additional Hsps. Rather then interpreting the failure of Luc recovery as arising from decreased Hsps, it is also possible that reduced Hsps and reduced Luc recovery are caused by the same defect, such as a failure of some aspect of transcription or translation in hot3-1. Both transcriptional and translational activities have been shown to acquire thermotolerance.
At this time, it is difficult to interpret further the phenotype of hot4. This mutant showed normal Hsp synthesis, Luc recovery, and adaptation of electrolyte leakage. It also acquired full thermotolerance as 10-d-old seedlings and showed no difference in basal thermotolerance of seeds. Thus, the only thermotolerance phenotype yet identified in hot4 is restricted to acquired thermotolerance of hypocotyl elongation. In fact, despite the obvious sensitivity of 2.5-d-old seedlings, we reproducibly observe 10-d-old hot4-1 seedlings perform much better than wild type after heat stress. Further assays are required to determine what defects underlie growth arrest at the hypocotyl stage after heat treatment of hot4.
Cloning the different HOT genes will provide new insight into mechanisms essential for acquired thermotolerance. Equally of interest is to use the hot mutants to investigate if any of the same genes are involved in tolerance to chronic heat stress or to other forms of abiotic stress. The screen in which the hot mutants were identified is unlikely to identify many more genes. Additional genes are no doubt required for thermotolerance but are either redundant, or stage specific in function, or would result in lethality in a thermotolerance screen. Using the hot mutations to isolate enhancer and suppressor mutations will provide a further genetic approach to dissecting thermotolerance.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis ecotypes Columbia and No were used where indicated. Plants were grown in lighted growth chambers (approximately 100 μmol m–2 s–1) on a 22°C/18°C, 16-h day/night cycle. All seeds used for testing thermotolerance and Hsp levels during seed development were derived from plants grown under these controlled conditions.
Genetic Analysis and Gene Mapping
Genetic complementation tests and analysis of dominance were performed as described previously, using the hypocotyl assay of thermotolerance (Hong and Vierling, 2000).
For genetic mapping, the hot2, hot3, and hot 4 mutants were outcrossed with wild-type plants of the Landsberg erecta ecotype. The resulting F1 plants were allowed to self, and homozygous hot mutants in the segregating F2 population were selected on the basis of their phenotype in the hypocotyl thermotolerance assay. Genomic DNA was extracted according to Klimyuk et al. (1993). Both initial mapping to individual chromosomes and further fine mapping was carried out by scoring cosegregation with simple sequence length polymorphism markers. Markers used for mapping are indicated in Figure 1 and in the text. In addition to publicly available markers (http://www.Arabidopsis.org), three new markers were developed. For mapping hot2, marker nF20D22 (7 cM on chromosome I) was detected with the following primers: CCCAAGTGACGTCTGGTTTC and AACAAAATGAGTTTCTCTGCAT. For mapping hot3, two new markers were developed in the region of AtATPase on chromosome I: nF28K19 detected with primers GTGACACTAACAACCAAAACC and CGAAAGGAAGAGACAAGCGTC, and nF22K20 detected with primers TTTTTGGTGAGATTTTAAGCCC and ATATCTCCATCGCTGCAACC. The exact position of the latter marker relative to hot3 is not known because no recombinants were obtained with this marker; therefore, it was not placed on the map in Figure 2.
Thermotolerance Assays
Thermotolerance assays of seeds, 2.5-d dark-grown and 10-d-old light-grown seedlings were performed basically according to Hong and Vierling (2000). Seeds were surface sterilized, plated on nutrient medium (Haughn and Somerville, 1986) containing 0.5% (w/v) Suc, and kept at 4°C for a minimum of 3 d. For the seed thermotolerance test, seeds were heat treated immediately after removal from the cold and then allowed to grow an additional 3 d before measurement. For hypocotyl elongation, seedlings were grown for 2.5 d in the dark and then heat stressed and measured after an additional 2.5 d in the dark. Only growth after the heat treatment was measured and compared with seedlings receiving no heat treatment. The assays with 10-d-old seedlings were photographed 5 d after the heat stress.
SDS-PAGE and Western Analysis
Total protein from seed or seedlings was extracted in SDS sample buffer (60 mm Tris-HCl [pH 8.0], 60 mm dithiothreitol, 2.0% [w/v] SDS, 15% [w/v] Suc, 5 mm ε-amino-N-caproic acid, and 1.0 mm benzamidine) in a ground-glass homogenizer. Protein concentration was determined using a Coomassie Blue dye-binding assay (Ghosh et al., 1988) with bovine serum albumin as a standard. All protein samples were also examined by SDS-PAGE and Coomassie Blue staining, and protein amounts were found to be consistent with the protein assay.
Standard methods were used for SDS-PAGE separation of protein samples on 7.5% or 15% (w/v) polyacrylamide gels. For western analysis, proteins were blotted to nitrocellulose and processed for detection using chemiluminescence (Amersham, Piscataway, NJ) as described previously (Wehmeyer et al., 1996). Anti-Hsp101 antiserum was used at a dilution of 1:1,000 (v/v). Antiserum against class I sHsps (Wehmeyer et al., 1996) was used at a dilution of 1:1,000 (v/v). Antiserum against class II sHsps was produced against Arabidopsis Hsp17.6II (X63443) (N. Buan and E. Vierling, unpublished data) and used at a dilution of 1:1,000.
Ion Leakage Test
To measure ion leakage caused by high temperature, 10-d-old light-grown seedlings were removed from the plates after different treatments, rinsed briefly with deionized water, and immediately placed in a tube with 5 mL of deionized water. The tubes were placed at 22°C overnight before conductivity was measured using an Electroconductivity Meter (model 1054, VWR Scientific, Phoenix). Results represent the average from measurements of ten seedlings for each condition.
Luc Activity
Seven-day-old light-grown seedlings were used to measure the recovery of Luc activity after heat shock. Seedlings were pretreated at 38°C, allowed to recover for 2 h at 22°C, and then heat shocked at 45°C for 2 h. For luminescence imaging, plants were sprayed uniformly with 1.0 mm luciferin in 0.01% (v/v) Triton X-100 at different times and kept in the dark for 10 min before imaging. All images were obtained with 5 min of exposure time on a CCD camera system (Roper Scientific, Princeton). The luminescence intensity of each seedling was quantified with WinView software system (Roper Scientific).
Luc activity in plant extracts was determined in parallel to the in vivo luminescence measurements. Samples were prepared from control seedlings, seedlings pretreated for 90 min at 38°C, and seedlings after 1, 6, or 24 h of recovery from 45°C heat stress. Seedlings were homogenized with 50 μL of extraction buffer containing 100 mm K2HPO4/KH2PO4 (pH 7.8), 1.0 mm dithiothreitol, 1.0 mm benzamidine, and 5 mm ε-amino n-caproic acid. Luc activity was determined by adding 3 μL of extract to luciferin substrate mixture (20 mm Tricine, 2.7 mm MgSO4.7H2O, 1.1 mm (MgCO3)4Mg(OH) 2.5H2O, 0.1 mm EDTA, 33 mm dithiothreitol, 0.5 mm ATP, 200 μg mL–1 coenzyme A, and 150 μg mL–1 luciferin) and measuring light emission with a luminometer. Protein concentration of the extracts was determined, and specific activity was plotted.
Acknowledgments
We would like to thank Nicole Buan for her work to produce Arabidopsis class II sHsp antibodies, Dr. Judy Callis for her gift of the UBQ10::Luc transgenic plants, Dr. Jian-Kang Zhu for use of his imaging facilities, and Dr. John Burke and his laboratory for performing assays of the thermotolerance of chlorophyll accumulation. We also thank Chris Borchert, Sarah Ryan, and Shannon Parrington for help with planting and screening of Arabidopsis seedlings. Drs. Frans Tax and Ramin Yadegari provided helpful comments on this manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017145.
This work was supported by the U.S. Department of Agriculture (National Research Initiative Competitive Grants Program grant no. 99–35100–7618) and by the Department of Energy (Energy Biosciences grant no. DE–FG03–99ER20338) to E.V.
References
- Alexandrov V (1964) Cytophysiological and cytoecological investigations of heat resistance of plant cells toward the action of high and low temperature. Q Rev Biol 39: 35–77 [Google Scholar]
- Alexandrov V (1994) Functional aspects of cell response to heat shock. Int Rev Cytol 148: 171–227 [DOI] [PubMed] [Google Scholar]
- Ansari AQ, Loomis WE (1959) Leaf temperatures. Am J Bot 46: 713–717 [Google Scholar]
- Berry JA (1975) Adaptation of photosynthetic processes to stress. Science 188: 644–650 [DOI] [PubMed] [Google Scholar]
- Boston RS, Viitanen PV, Vierling E (1996) Molecular chaperones and protein folding in plants. Plant Mol Biol 32: 191–222 [DOI] [PubMed] [Google Scholar]
- Buchberger A, Schröder H, Hesterkamp T, Schönfeld HJ, Bukau B (1996) Substrate shuttling between the DnaK and GroEL systems indicates a chaperone network promoting protein folding. J Mol Biol 261: 328–333 [DOI] [PubMed] [Google Scholar]
- Burke JJ (2001) Identification of genetic diversity and mutations in higher plant acquired thermotolerance. Physiol Plant 112: 167–170 [Google Scholar]
- Burke JJ, O'Mahony PJ, Oliver MJ (2000) Isolation of Arabidopsis mutants lacking components of acquired thermotolerance. Plant Physiol 123: 575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey WC (1989) The search for critical cellular targets damaged by heat. Radiat Res 120: 191–204 [PubMed] [Google Scholar]
- Ehler WL (1973) Cotton leaf temperatures as related to soil water depletion and meteorological factors. Agron J 65: 404–409 [Google Scholar]
- Forreiter C, Kirschner M, Nover L (1997) Stable transformation of an Arabidopsis cell suspension culture with firefly luciferase providing a cellular system for analysis of chaperone activity in vivo. Plant Cell 9: 2171–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M-J (1997) Guidebook to Molecular Chaperones and Protein Folding Catalysts. Oxford University Press, New York, pp 1–554
- Ghosh S, Hepstein S, Heikkila J, Dumbroff E (1988) Use of a densitometer or ELISA plate reader for measurement of nanogram amounts of proteins in crude extracts from biological tissues. Anal Biochem 169: 227–233 [DOI] [PubMed] [Google Scholar]
- Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 94: 73–82 [DOI] [PubMed] [Google Scholar]
- Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Hendrick JP, Hartl F-U (1993) Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem 62: 349–384 [DOI] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2001) Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant J 27: 25–35 [DOI] [PubMed] [Google Scholar]
- Klimyuk VI, Carroll BJ, Thomas CM, Jones JDG (1993) Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J 3: 493–494 [DOI] [PubMed] [Google Scholar]
- Larkindale J, Knight MR (2002) Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol 128: 682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, Vierling E (2000) A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol 122: 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J (1980) Responses of Plants to Environmental Stresses. Academic Press, New York
- Lin C-Y, Chen,Y-M, Key JL (1985) Solute leakage in soybean seedlings under various heat shock regimes. Plant Cell Physiol 26: 1493–1498 [Google Scholar]
- Lin C-Y, Roberts J, Key JL (1984) Acquisition of thermotolerance in soybean seedlings: synthesis and accumulation of heat shock proteins and their cellular localization. Plant Physiol 74: 152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S (1986) The heat shock response. Annu Rev Biochem 45: 39–72 [DOI] [PubMed] [Google Scholar]
- Malik MK, Slovin JP, Hwan CH, Zimmerman JL (1999) Modified expression of a carrot small heat shock protein gene, Hsp17.7, results in increased or decreased thermotolerance. Plant J 20: 89–99 [DOI] [PubMed] [Google Scholar]
- Michels AA, Kanon B, Konings AWT, Ohtsuka K, Bensaude O, Kampinga HH (1997) Hsp70 and Hsp40 chaperone activities in the cytoplasm and the nucleus of mammalian cells. J Biol Chem 272: 33283–33289 [DOI] [PubMed] [Google Scholar]
- Mogk A, Tomoyasu T, Goloubinoff P, Rüdiger S, Röder D, Langen H, Bukau B (1999) Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J 18: 6934–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi K, Watanabe Y, Yohda M, Yoshida M (1999) Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones. Proc Natl Acad Sci USA 96: 7184–7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Nover L, Parthier B, Rieger R, Scharf K-D, Wollgiehn R, Nieden UZ (1989) Heat shock and other stress response systems of plants. Biol Zentbl 108: 1–156 [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV (1999) AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res 9: 27–43 [PubMed] [Google Scholar]
- Nover L, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf K-D (2001) Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperones 6: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Kowal AS, Singer MA, Lindquist S (1994) Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372: 475–478 [DOI] [PubMed] [Google Scholar]
- Pinto M, Morange M, Bensaude O (1991) Denaturation of proteins during heat shock: in vivo recovery of solubility and activity of reporter enzymes. J Biol Chem 266: 13941–13946 [PubMed] [Google Scholar]
- Radin JW, Lu Z, Percy RG, Zeiger E (1994) Genetic variability for stomatal conductance in Pima cotton and its relation to improvements of heat adaptation. Proc Natl Acad Sci USA 91: 7217–7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf K-D, Siddique M, Vierling E (2001) The expanding family of small Hsps and other proteins containing an α-crystallin domain. Cell Stress Chaperones 6: 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer EC, Glover JR, Singer MA, Lindquist S (1996) HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci 21: 289–296 [PubMed] [Google Scholar]
- Sung DY, Vierling E, Guy CL (2001) Comprehensive expression profile analysis of the Arabidopsis hsp70 gene family. Plant Physiol 126: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NC, Kramer PJ (1980) Adaptation of Plants to Water and High Temperature Stress. John Wiley & Sons, New York
- Vierling E (1991) The roles of heat shock proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 42: 579–620 [Google Scholar]
- Waters ER, Lee GJ, Vierling E (1996) Evolution, structure and function of the small heat shock proteins in plants. J Exp Bot 47: 325–338 [Google Scholar]
- Wehmeyer N, Hernandez LD, Finkelstein RR, Vierling E (1996) Synthesis of small heat-shock proteins is part of the developmental program of late seed maturation. Plant Physiol 112: 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley CK, Zenser N, Ramos J, Rouse D, Leyser O, Theologis A, Callis J (2000) The degradation signal of Aux/IAA proteins is important in auxin signaling. Plant J 21: 553–562 [DOI] [PubMed] [Google Scholar]
- Zhong RQ, Kays SJ, Schroeder BP, Ye ZH (2002) Mutation of a chitinase-like gene causes ectopic deposition of lignin, aberrant cell shapes, and overproduction of ethylene. Plant Cell 14: 165–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolkiewski M (1999) ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation: a novel multi-chaperone system from Escherichia coli. J Biol Chem 274: 28083–28086 [DOI] [PubMed] [Google Scholar]