Abstract
In response to a moisture gradient, roots exhibit hydrotropism to control the orientation of their growth. To exhibit hydrotropism, however, they must overcome the gravitropism that is dominant on Earth. We found that moisture gradient or water stress caused immediate degradation of the starch anchors, amyloplasts, in root columella cells of Arabidopsis and radish (Raphanus sativus). Namely, development of hydrotropic response was accompanied by a simultaneous reduction in starch content in columella cells. Rapid degradation of amyloplasts in columella cells also occurred in the water-stressed roots with sorbitol or mannitol. Both hydrotropically stimulated and water-stressed roots showed a reduced responsiveness to gravity. Roots of a starchless mutant, pgm1-1, showed an enhanced hydrotropism compared with that of the wild type. These results suggest that the reduced responsiveness to gravity is, at least in part, attributable to the degradation of amyloplasts in columella cells. Thus, the reduction in gravitropism allows the roots to exhibit hydrotropism.
Terrestrial plants develop their root systems in soil to avoid stressful environments such as drought, extreme temperature, and nutrient deficiency, and to establish their stand. Tropistic responses have an important role in developing the root system. Primary roots dominantly display gravitropism, growing down in response to gravity. The columella cells of the root cap have long been considered to act as a sensory apparatus involved in inducing the gravitropic response because they include amyloplasts that contain dense starch grains and sediment to the floor of the cells upon reorientation of the roots (Wilkins, 1984; Sack, 1991; Kiss, 2000). Manipulations of the columella cells or amyloplasts in recent studies have verified that columella cells and amyloplasts are necessary for graviperception in roots. For example, roots of starchless mutants or roots in which columella cells were ablated with a laser showed severely reduced gravitropism (Kiss et al., 1989; Blancaflor et al., 1998). Roots also show hydrotropism, phototropism, and thigmotropism in response to moisture gradients, unilateral light, and touch stimuli, respectively (Jaffe et al., 1985; Okada and Shimura, 1990; Takahashi, 1997; Sakai et al., 2000; Ruppel et al., 2001). That is, roots grow toward the higher water potential and toward or away from light or touch, depending on the water status, the wavelength, or the cells of stimulus perception. In Arabidopsis, roots exhibit negative phototropism growing away from the unilateral blue light and positive phototropism growing toward the unilateral red light (Sakai et al., 2000; Ruppel et al., 2001). The latter response is much weaker than the former (Ruppel et al., 2001). Although the perception mechanisms for those tropisms in roots are still obscure, the sensory cells are likely to reside in the root cap (Jaffe et al., 1985; Kiss et al., 1989; Takahashi and Suge, 1991; Takahashi and Scott, 1993; Takahashi, 1997; Sakai et al., 2000). These tropisms, including gravitropism, interact with one another in orienting the roots. Amyloplasts in the gravisensing cells are required for the full gravitropic response but are inhibitory to other tropisms because of the counteracting effect of the gravitropic response (Jaffe et al., 1985; Takahashi, 1997; Vitha et al., 2000; Ruppel et al., 2001). Among the tropisms, gravitropism and hydrotropism in particular have an important role in plant growth in areas where there is little precipitation.
Roots of agravitropic mutant or of clinorotated seedlings enabled us to separate hydrotropism from gravitropism, suggesting that gravitropism interferes with hydrotropism in peas (Pisum sativum) and cucumber (Cucumis sativus; Jaffe et al., 1985; Takahashi and Suge, 1991; Takahashi et al., 1996; Takahashi, 1997; Mizuno et al., 2002). On the other hand, we have found recently that seedling roots of Arabidopsis are super-sensitive to moisture gradient displaying strong hydrotropic response (Takahashi et al., 2002). It has not yet been elucidated, however, how Arabidopsis roots respond hydrotropically to overcome gravitropism. Here, we discovered that moisture gradient triggers the degradation of amyloplasts in the columella cells; thus, roots display hydrotropism with less interference from gravitropism.
RESULTS AND DISCUSSION
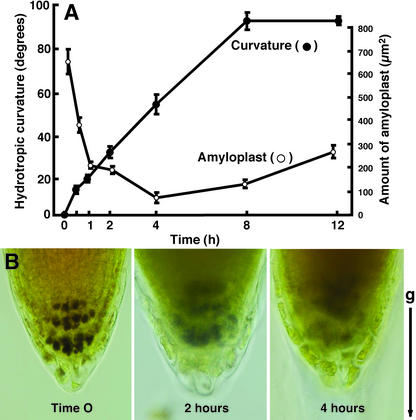
We developed an experimental system to induce hydrotropism in seedling roots of Arabidopsis and found that the roots showed a remarkable hydrotropic response by overcoming gravitropism (Takahashi et al., 2002). Using the same system, seedling roots of Arabidopsis (ecotype Columbia) were hydrotropically stimulated by a moisture gradient established between 1% (w/v) agar plate and saturated solution of KCl in a closed chamber (Fig. 1A). Hydrotropic bending of Arabidopsis roots commenced within 30 min after exposure to a gradient of moisture and further developed lineally for 4 to 8 h until the root tips reach the agar by curving greater than 90° (Fig. 1A). Under the water-saturated conditions, however, the control roots showed only small curvature that was less than 10° during the experimental period of time. When amyloplasts were visualized by staining starch with I2-KI solution, the roots substantially decreased the amount of amyloplasts in response to the moisture gradient (Fig. 1A). Hydrotropic curvature accompanying the digestion of starch in the columella cells became visible within 30 min, and the maximum curvature and the minimum level of starch were observed 4 to 8 h after the start of hydrostimulation (Fig. 1, A and B). The amount of amyloplasts in the columella cells was reduced to less than 60% and 15% of the control 30 min and 4 h, respectively, after exposure to the moisture gradient. Columella cells restore the amount of amyloplasts when the roots ultimately reach wet agar medium.
Figure 1.
Hydrotropic response of Arabidopsis roots and decrease of amyloplasts in the root cap. A, Roots were exposed to a moisture gradient to induce hydrotropism, and curvature and amount of amyloplasts were measured in the hydrotropically responding roots in a time course study. Data represent the mean ± se (n = 20). B, Reduced amyloplasts in the columella cells of Arabidopsis roots that were hydrotropically stimulated by a moisture gradient. Amyloplasts sediment in the columella cells before exposure to the moisture gradient (left). Columella cells of the root cap 2 h after the start of exposure to the moisture gradient (center). Columella cells of the root cap 4 h after the start of exposure to the moisture gradient (right). Amyloplast starch was stained with I2-KI solution and observed under a light microscope. Arrow (g) indicates the direction of gravitational force.
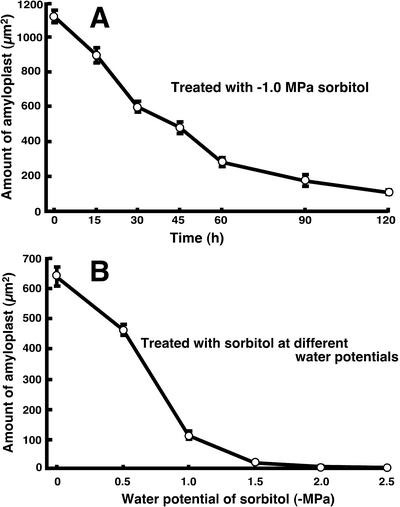
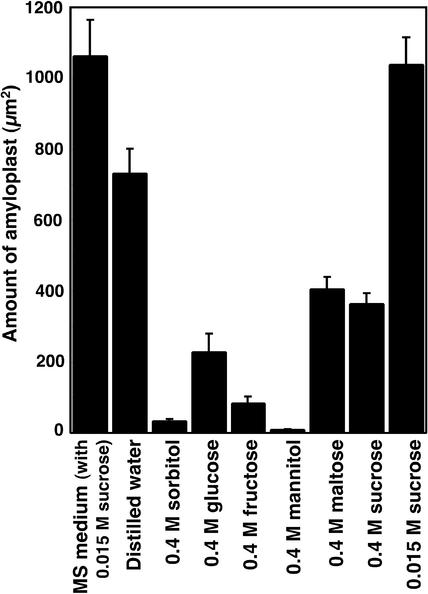
Sorbitol-induced water stress also produced a dramatic decrease in amyloplasts in Arabidopsis root cap cells (Fig. 2, A and B). When treated with –1.0 MPa sorbitol, the amount of amyloplasts decreased by one-half within 30 min and to less than 20% of the initial level 1 to 2 h after the start of water stress (Fig. 2A). Water stress with –0.5 to –1.0 MPa sorbitol for 2 h caused a substantial decrease in amyloplasts in the root cap (Fig. 2B). When Arabidopsis roots were treated with different sugars, all sugars (sorbitol, Glc, Fru, mannitol, maltose, and Suc) used at 0.4 m caused a decrease in starch content, although sorbitol and mannitol were most effective (Fig. 3). There was a slight reduction of starch in the distilled water-treated roots, but it was notable that Suc at low concentration (0.015 m) prevented the reduction of starch in the root cap (Fig. 3). Root columella cells may use Suc for synthesis or maintenance of starch unless it is present at high concentration. At present, we do not know why water stress results in degradation of amyloplasts in columella cells. However, degradation of the starch amyloplast could occur as an adaptive response to water stress by supplying an osmoregulating component. Also, the root system could eventually adapt to drought conditions and restore amyloplasts in the root caps.
Figure 2.
Water stress-induced decrease of amyloplasts in the root cap of Arabidopsis roots. A, Roots were treated with –1.0 MPa sorbitol, and the amount of amyloplasts in the root caps was measured in a time course study. B, Roots were treated with sorbitol at different water potentials for 2 h for the measurement of the amount of amyloplasts in the root caps. Data represent the mean ± se (n = 14–15).
Figure 3.
Effects of different sugars on the degradation of amyloplasts in the root cap cells of Arabidopsis seedlings. Roots were treated with 0.4 m (1 MPa) solution of sorbitol, Glc, Fru, mannitol, maltose, or Suc. Roots were also treated with 0.015 m Suc solution. Two hours after the treatment, roots were fixed with (5% formaldehyde:5% acetic acid: 45% ethanol) for the measurement of amyloplasts. Vertical bar indicates se (n = 12).
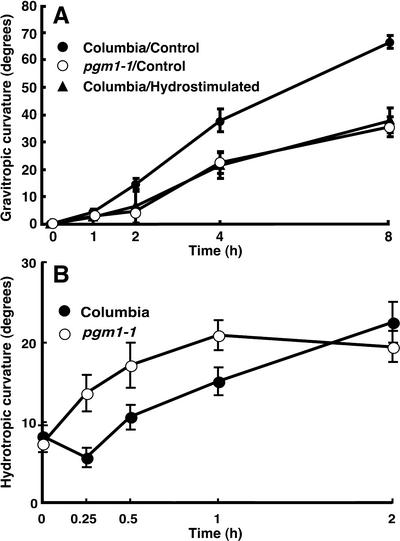
Arabidopsis roots that lost amyloplasts due to stimulation by a moisture gradient exhibited a reduced gravitropic response (Fig. 4A). The responsiveness of the hydrostimulated roots to gravity was similar to that of the starchless mutant, pgm1-1. Also, we compared the kinetics of gravitropism of the sorbitol-treated roots and that of the control by recording curvature development every 10 min. We found that curvatures 10, 20, 30, 40, 50, and 60 min after gravistimulation were 3.9°, 16.6°, 30.3°, 39.4°, 48.8°, and 51.1°, respectively, in the control and 1.6°, 3.8°, 8.0°, 11.7°, 14.4°, and 20.7°, respectively, in the sorbitol-treated roots. A time course study for hydrotropic response of pgm1-1 roots revealed that the starchless roots showed a greater responsiveness to moisture gradient for the induction of hydrotropism compared with that of the wild type (Fig. 4B). The pronounced responsiveness of pgm1-1 roots to moisture gradient was evident at an early stage of the response, although degrees of curvature ultimately became similar to that of the wild type.
Figure 4.
Reduced responsiveness to gravity in the hydrostimulated roots and roots of starchless mutant of Arabidopsis seedlings. A, Roots exposed to moisture gradient for 4 h and roots of starchless mutant, pgm1-1, were gravistimulated for comparison with graviresponsiveness of the roots of non-hydrostimulated wild type. Data represent the mean ±se (n = 18). B, Hydrotropic responsiveness of pgm1-1 roots was compared with that of the wild type (Columbia) in a time course study. Data represent the mean ± se (n = 20).
The results mentioned above demonstrate that the reduced gravitropism of Arabidopsis roots is, at least in part, attributable to the loss of amyloplasts, which occurs in response to moisture gradients or water stress. Recently, Wolverton et al. (2002) proposed that roots respond to gravity by dual motors and sensors. That is, gravisensors possibly reside not only in the root cap but also in the distal elongation zone proximal to the root cap. Although the non-cap sensing accounts for only about 20% of the total rate of gravicurvature (Wolverton et al., 2002), moisture gradient or water stress could also affect the second sensing system for reducing gravitropism.
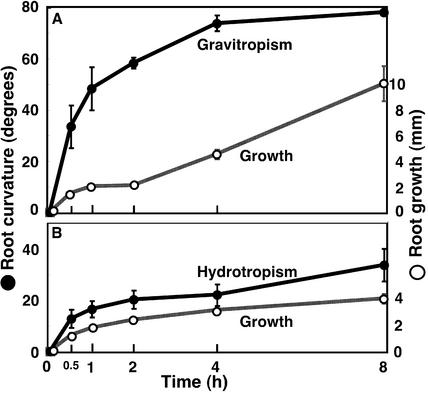
Using the same system as in hydrotropism of Arabidopsis roots, we found that radish (Raphanus sativus) roots also responded hydrotropically to moisture gradient by overcoming gravitropism (Fig. 5). That is, the roots in a vertical position bent sideways toward the higher moisture. Hydrotropic curvature in radish roots commenced as early as in Arabidopsis roots, although the ultimate responsiveness was weaker than that of Arabidopsis roots (Fig. 5).
Figure 5.
Gravitropism, hydrotropism, and growth in the roots of radish seedlings. Roots were gravitropically or hydrotropically stimulated as described for Arabidopsis roots. Vertical bar = se (n = 6–9).
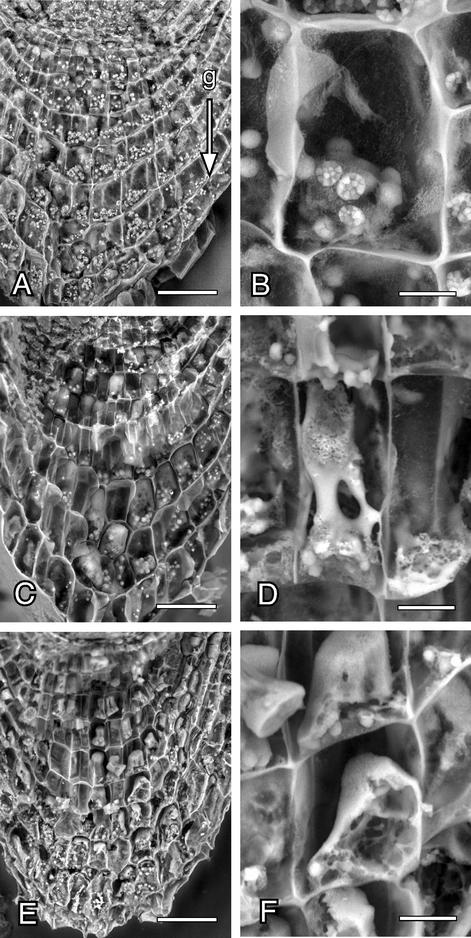
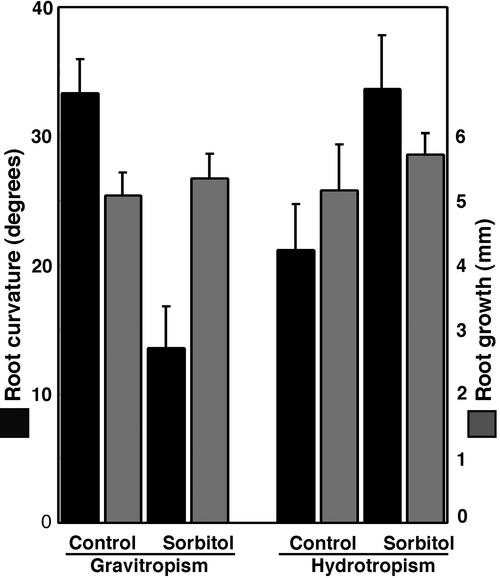
To observe the columella cells of the hydrotropically responding roots by scanning electron microscopy (SEM), we used radish roots because, compared with Arabidopsis roots, it was much easier to divide radish root tip specimens into halves. Figure 6 shows the scanning electron micrographs of the columella cells of the radish roots that are hydrotropically responding to a gradient of moisture. In the control roots, amyloplasts are normal, and they sediment to the floor of the columella cells (Fig. 6, A and B). On the other hand, digestion of amyloplasts is obvious 2 h after exposure to the moisture gradient (Fig. 6, C and D). After 5 h, the amount of amyloplasts was severely reduced, and the dense cytoplasm had become colloidal (Fig. 6, E and F). When roots were released from water stress by reaching the agar medium due to hydrotropic response, the initial level of amyloplasts and the normal structural conformation were restored in the columella cells. As in the Arabidopsis roots, the radish roots exposed to water stress showed a severe reduction in the gravitropic response without substantial reduction in elongation growth (Fig. 7). It is interesting that the water-stressed roots showed even greater hydrotropic response (Fig. 7).
Figure 6.
Scanning electron micrographs of the columella cells and amyloplasts in the hydrotropically responding roots of radish seedlings. Roots were exposed to the moisture gradient as in the case of Arabidopsis. The root tip was longitudinally divided and observed under a scanning electron microscope. A and B, Root cap cells of the roots not exposed to a moisture gradient. C and D, Root cap cells 2 h after the exposure to moisture gradient. E and F, Root cap cells 5 h after the exposure to moisture gradient. A, C, and E, Root cap cells (horizontal bar = 50 μm). B, D, and F, Enlargement of the columella cells (horizontal bar = 10 μm). Arrow (g) indicates the direction of gravitational force.
Figure 7.
Gravitropism, hydrotropism, and growth of the sorbitoltreated roots of radish seedlings. Seedlings were placed on agar plate in a vertical position with the root tips (approximately 0.5 mm long) suspended in humid air. A 15-μL droplet of 1 MPa of sorbitol solution or MES buffer was applied to the root tips. Two hours after the treatment, roots were gravitropically or hydrotropically stimulated for 4 h. Vertical bar = se (n = 18).
Thus, roots are highly responsive to moisture gradient, exhibiting hydrotropism, if gravitropism is reduced. Water stress that causes degradation of the gravisensor, amyloplasts, in the columella cells could partly be responsible for reducing gravisensitivity and thereby displaying hydrotropism. These findings are the first to our knowledge to show that columella cells are highly susceptible to moisture gradient that cause immediate degradation of amyloplasts. How this tropistic stimulus causes the degradation of amyloplasts remains to be clarified, but the phenomenon is an important clue to understanding the root sensory mechanism that functions for integrating multiple stimuli in the root cap cells. Systemic water stress and moisture gradients result in the degradation of amyloplasts (Figs. 1, 2, 3). Thus, there might be two independent mechanisms: one for the perception of moisture gradients for hydrotropism and the other for the perception of water stress for starch degradation. These two events might occur simultaneously in the columella cells of the hydrotropically stimulated roots. Alternatively, the water stress-induced degradation of starch could be the first step for the perception of the moisture gradient. However, this is unlikely because a starchless mutant, pgm1-1, shows hydrotropic response even greater than the wild type (Fig. 4).
In conclusion, Arabidopsis and radish roots are hydrotropically highly sensitive because in response to a moisture gradient, they reduce gravitropism by degrading amyloplasts in columella cells. Although elongation and bending responses of all root tropisms occur at the elongation zone proximal to the root cap, our findings imply that columella cells in the root cap are able to differentiate each response from the complex network of tropisms and display a suitable growth response depending upon their environmental circumstances.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of Arabidopsis, Columbia ecotype, or radish (Raphanus sativus L. cv White Icicle) were sterilized with 5% (v/v) sodium hypochlorite containing 0.05% (v/v) Tween 20 for 12 min, washed with distilled water, and sown on a 0.2% (w/v) Gellan Gum (Sigma, St. Louis) containing 0.4% (w/v) Murashige and Skoog Salt Mixture (GIBCO BRL, Grand Island, NY), 0.2% (w/v) Murashige and Skoog vitamin (GIBCO BRL), and 2% (w/v) Suc in a plastic container (10 × 14 × 1.5 cm). They were placed at 4°C in the dark for 48 h. For germination, the plastic plate was placed in a vertical position so that seedlings grew straight on the surface of the medium. The plates were then incubated at 23°C under a 24-h photoperiod for 96 h. Seedlings with relatively straight roots, 1.0 to 1.5 cm in length, were used for experiments.
Stimulation by Moisture Gradient and Gravity
Roots were hydrotropically stimulated according to the method of Takahashi et al. (2002). To establish a moisture gradient for the induction of hydrotropism, a 1% (w/v) agar plate was placed on a plastic plate (14 × 10 × 1 cm), and a 1-cm-wide portion of the edge of the agar plate was removed. Arabidopsis or radish seedlings were placed on the agar plates such that approximately 0.5 mm of each root tip was suspended freely from the edge of the agar into the surrounding air. The plastic plates holding the agar and seedlings were attached to the inner surface of an acrylic chamber (29 × 24 × 21 cm) using double-sided tape. Thus, seedling roots were positioned vertically. A moisture gradient was established between the agar plates, and a saturated solution of KCl was placed on the floor of the closed acrylic chamber. All seedlings were photographed using a stereomicroscope to measure root growth and curvature. The magnified photographic images were used to measure growth and curvature with the help of a goniometer and ruler. Root curvature was measured as the angle of deviation from the initial straight line of the seedling root. Roots were also exposed to water stress by placing the seedlings on a filter paper wetted with different concentrations of sorbitol solutions for 2 h. The gravitropic response of seedling roots exposed to a moisture gradient for 4 h was examined by reorienting the plastic plate of the seedlings 90° and comparing with that of seedling roots of the starchless mutant, pgm1-1 (Caspar et al., 1985; Kiss et al., 1989). Roots of pgm1-1 mutant were also subjected to a moisture gradient for comparing the hydrotropic response with that of the wild type.
Light Microscopy
To observe the amyloplasts in the columella cells of the root cap, seedlings were soaked in a fixative (5% [v/v] formaldehyde, 5% [v/v] acetic acid, and 45% [v/v] ethanol) at 4°C for 48 h. The fixed seedlings were stained with I2-KI solution (0.15% [w/v] I2 and 0.45% [w/v] KI) for 5 min. Then, the root tips were observed under a light microscope (BX50F, Olympus, Tokyo), and the amount of amyloplasts was determined by measuring the area of the stained amyloplasts using a computer-assisted image analysis with the software Mac Scope version 2.5 (Mitani Co., Fukui-ken, Japan). For this measurement, averaged density of the stained amyloplasts of the control was calculated and adopted as a threshold of gray scale so that the areas of only objects denser than the threshold were extracted and summed.
SEM
Amyloplasts and columella cells were observed with low-vacuum SEM (JSM-5800LV, JEOL Ltd., Tokyo). A freeze substitution method suitable for observation of organelles such as amyloplasts was used for fixation and dehydration of the materials (Robards and Sleytr, 1985). The root tips excised from the radish seedlings were frozen in absolute acetone containing 0.4% (v/v) glutaraldehyde in a vial placed in liquid nitrogen. The specimens in the fixative were stored at –80°C for 24 h. After the solvent was removed, absolute acetone cooled at –80°C in a deep freezer was added and stored at –80°C for 24 h. The dehydrated specimens were then gradually warmed and dried in liquid CO2 on a critical point dryer (JCPD-3, JEOL Ltd.). The root tip specimen was placed between two polycarbonate holders with double-sided tape, fractured longitudinally into halves, and observed with the low-vacuum SEM operated at 15 kV in accelerating voltage in a 36-Pa vacuum.
Acknowledgments
We thank Dr. Nobuharu Goto (Miyagi College of Education, Sendai, Japan) for providing us with pgm1-1Arabidopsis seeds. We also thank Dr. Kiyotaka Okada (Kyoto University) and Dr. Tadashi Hirasawa (Tokyo University of Agriculture and Technology) for their critical reading of our manuscript and Dr. Nobuharu Fujii (Tohoku University, Japan) and Dr. Nori Kurata (National Institute of Genetics, Mishima, Japan) for their helpful discussion.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018853.
This work was supported by the Ministry of Education, Science, Sports, Culture, and Technology of Japan (grant to H.T.), by the Institute of Space and Astronautical Science (grant to H.T.), by the National Space Development Agency (grant to H.T.), and by the Japan Space Forum (grant to H.T.). This work was carried out as a part of the National Institute of Genetics Cooperative Research Program (grant no. 2000–54).
References
- Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the role of cap cells in root gravitropism. Plant Physiol 116: 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe MJ, Takahashi H, Biro RL (1985) A pea mutant for the study of hydrotropism in roots. Science 230: 445–447 [DOI] [PubMed] [Google Scholar]
- Kiss JZ (2000) Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci 19: 551–573 [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Hertel R, Sack FD (1989) Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 177: 198–206 [PubMed] [Google Scholar]
- Mizuno H, Kobayashi A, Fujii N, Yamashita M, Takahashi H (2002) Hydrotropic response and expression pattern of auxin-inducible gene, CS-IAA1, in the primary roots of clinorotated cucumber seedlings. Plant Cell Physiol 43: 793–801 [DOI] [PubMed] [Google Scholar]
- Okada K, Shimura Y (1990) Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250: 274–276 [DOI] [PubMed] [Google Scholar]
- Robards AW, Sleytr UB (1985) Freeze-substitution and low temperature embedding. In AM Glauert, ed, Practical Methods in Electron Microscopy, Vol 10. North-Holland Publishing Co., Amsterdam, pp 461–499 [Google Scholar]
- Ruppel NJ, Hangarter RP, Kiss JZ (2001) Red-light-induced positive phototropism in Arabidopsis roots. Planta 212: 424–430 [DOI] [PubMed] [Google Scholar]
- Sack FD (1991) Plant gravity sensing. Int Rev Cytol 127: 193–252 [DOI] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H (1997) Hydrotropism: the current state of our knowledge. J Plant Res 110: 163–169 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Okada K, Goto N, Takahashi H (2002) Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta 216: 203–211 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Takano M, Fujii N, Yamashita M, Suge H (1996) Induction of hydrotropism in clinorotated seedling roots of Alaska pea, Pisum sativum L. J Plant Res 109: 335–337 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Scott TK (1993) Intensity of hydrostimulation for the induction of root hydrotropism and its sensing by the root cap. Plant Cell Environ 16: 99–103 [DOI] [PubMed] [Google Scholar]
- Takahashi H, Suge H (1991) Root hydrotropism of an agravitropic pea mutant, ageotropum. Physiol Plant 82: 24–31 [DOI] [PubMed] [Google Scholar]
- Vitha S, Zhao L, Sack FD (2000) Interaction of root gravitropism and phototropism in Arabidopsis wild-type and starchless mutants. Plant Physiol 122: 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MB (1984) Gravitropism. In MB Wilkins, ed, Advanced Plant Physiology. Pitman Publications Ltd., London, pp 163–185
- Wolverton C, Ishikawa H, Evans ML (2002) The kinetics of root gravitropism: dual motors and sensors. J Plant Growth Regul 21: 102–112 [DOI] [PubMed] [Google Scholar]