Abstract
Powdery mildew fungi are biotrophic pathogens that form a complex interface, the haustorium, between the host plant and the parasite. The pathogen acts as an additional sink, competing with host sinks, resulting in considerable modification of photoassimilate production and partitioning within the host tissue. Here, we examine the factors that may contribute to these changes. We show for the first time in one biotrophic interaction (Arabidopsis/Erysiphe cichoracearum) all of the following responses: Glc uptake in host tissues is enhanced after fungal infection; this coincides with the induction of expression of the monosaccharide transporter gene, Arabidopsis sugar transport protein 4 (AtSTP4), in infected leaves; invertase activity and transcript levels for a cell wall invertase, Atβfruct1, increase substantially in Arabidopsis during attack by this pathogen. Before infection, Arabidopsis plants transformed with an AtSTP4 promoter-β-glucuronidase construct show expression mainly in sink tissues such as roots; after infection, AtSTP4 expression is induced in the mature leaves and increases over the 6-d time period. Sections of infected leaves stained for β-glucuronidase show that AtSTP4 expression is not confined to infected epidermal cells but is also evident in a wider range of cells, including those of the vascular tissue. The results are discussed in relation to the possible coordinated expression of hexose transporters and cell wall invertase in the host response to powdery mildew infection.
Powdery mildews are widespread biotrophic pathogens that cause major losses in crop yield. They are usually restricted to the shoot epidermal cells where they acquire essential nutrients from the living host tissues over a long period. Thus they form an additional sink, and this can lead to considerable changes in carbon transport and partitioning within the plant (see Farrar and Lewis, 1987; Ayres et al., 1996; Hall and Williams, 2000). There is still much to learn about the nutrition of these important biotrophic pathogens. For example, little is known about the molecular mechanisms involved in the transfer of essential solutes, particularly sugars, across the host/pathogen interface, or about the molecular responses induced in the host transport processes in response to such infection. There is good evidence that Glc may play a key role in such biotrophic interactions. Glc appears to be the major carbon energy source transferred from the host to cereal and pea powdery mildew (Mendgen and Nass, 1988; Clark and Hall, 1998; Sutton et al., 1999), although the route and mechanisms by which this occurs are not clear. A recent study investigating the role of haustoria in sugar supply during infection of broad bean (Vicia faba) by the biotrophic rust fungus Uromyces fabae demonstrated that a fungal gene encoding a hexose transporter was expressed abundantly in rust haustoria (Voegele et al., 2001). The protein was localized exclusively to the haustorial plasma membrane, suggesting that this hexose transporter may have a major role in carbon transfer into the fungus from the extrahaustorial matrix (Voegele et al., 2001).
An important observation in particular powdery mildew/host compatible interactions is that infection can result in an increased capacity for sugar uptake by the host tissue (Sutton, 1997; Clark and Hall, 1998). However the molecular mechanisms leading to such a response have not been defined. The increased capacity may be due to enhanced activity of existing transporters or synthesis of new transporters possibly at the epidermal cells, thus increasing the level of Glc available to the invading fungal haustoria. Alternatively, the increased capacity may be a retrieval mechanism to support the increased demand for carbon in defense mechanisms. The monosaccharide carrier AtSTP4 in Arabidopsis has been shown to be stress regulated (Truernit et al., 1996). In the present study, we investigate its role in a biotrophic interaction.
Infection may also result in a general increase in apoplastic Glc levels due to the activity of the cell wall invertase, an enzyme catalyzing the cleavage of Suc to Glc and Fru (for review, see Hall and Williams, 2000). Several types of invertases exist, differing in their pH optima and their different cellular locations. Neutral or alkaline invertases are thought to be located in the cytosol, whereas acid invertases are found either in the vacuole or cell wall. An increase in the various types of invertases has been observed in a variety of biotrophic infections, including powdery mildew; this usually concerns acid invertase (Scholes et al., 1994; Wright et al., 1995; Clark and Hall, 1998; Chou et al., 2000), although in some cases increases in alkaline invertase have been observed (Storr and Hall, 1992). However, it is not clear whether this is due to the synthesis of new isoforms or to an increase in the level of existing isoforms. The relationship between these changes clearly needs to be studied to provide a fuller understanding of the processes involved. Glc and stress appear to independently regulate source and sink metabolism and defense mechanisms in cultured Chenopodium sp. cells (Ehness et al., 1997); in particular, treatment with Glc induces the sink-specific extracellular invertase that is thought to play a key role in Suc partitioning (Tang et al., 1999).
To understand more fully the roles of Glc, monosaccharide transporters, and invertase activity in the response of plants to powdery mildew infection, we have examined the various related responses discussed above in the model plant Arabidopsis that is now widely used in the study of plant-pathogen interactions (Buell, 1998; Schulze-Lefert and Vogel, 2000). We have shown for the first time in one interaction that infection results in enhanced Glc uptake in infected host tissue, an increase in the expression of the host monosaccharide transporter gene, AtSTP4 in infected leaves, an increase in the host cell wall invertase activity, and an increase in expression of the cell wall invertase, Atβfruct1. The results are discussed in relation to the possible coordinated expression of hexose transporters and cell wall invertase in the host response to powdery mildew infection.
RESULTS
Effect of Infection on Glc Uptake
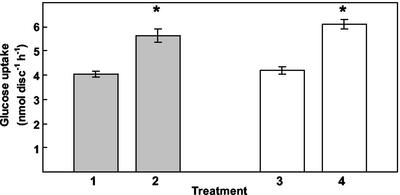
When Glc uptake was measured in mature leaves of Arabidopsis from healthy noninfected plants and plants infected with Erysiphe cichoracearum, a small but significant increase in Glc uptake was observed (Fig. 1). A sellotape treatment was carried out to remove most, if not all, of the mycelium inoculum from the surface of the leaf before uptake. Members of the Erysiphales only colonize the outer surface of the invaded tissue and do not produce hyphae that penetrate within the host tissue, with the exception of a few species of powdery mildew fungi (Alexopoulos et al., 1996). A similar level of enhanced Glc uptake was observed after removal of the mycelium by the sellotape treatment, suggesting that this was a host response rather than the fungus acting as an additional sink (Fig. 1). Glc uptake in noninfected and infected material was inhibited by the protonophore carbonylcyanide-m-chlorophenyl-hydrazone (Table I). Uptake was inhibited by the permeable sulfydryl reagent, NEM, but showed low sensitivity to the non-permeant sulfydryl reagent p-chloromercuribenzenesulfonic acid. Diethyl pyrocarbonate, a His modifier, caused moderate inhibition, as did phloridzin, a competitive inhibitor of Glc uptake in animal cells. Levels of inhibition were similar in infected and noninfected material for all the reagents tested (Table I). The inhibitor profile is consistent with a proton-coupled carrier mechanism mediating Glc uptake.
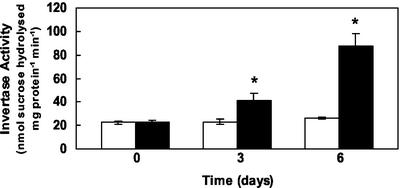
Figure 1.
Glc uptake into Arabidopsis source leaves after infection with E. cichoracearum. Glc uptake into infected (brushed with inoculum) and noninfected (brushed without inoculum) Arabidopsis leaf discs was measured using 0.5 mm [14C]Glc (1, noninfected; 2, infected). Treatments 2 and 4 were 6 d post inoculation. Measurements were also taken after removal of the mycelium with sellotape before uptake (3, noninfected; 4, infected). Results are the mean (±se) of three experiments, and the asterisk indicates significant difference from Glc uptake in corresponding control noninfected leaf discs (P ≤ 0.001).
Table I.
The effect of inhibitors on Glc uptake into leaf discs from infected and noninfected tissue
Uptake of 0.5 mm Glc was measured at 25°C after 1.5 h following incubation in reaction buffer containing 20 mm Mes-BTP (pH 5.8), 1 mm CaCl2, 500 μm Glc and 1 μCi [14C] glucose. Inhibitors were added as indicated. Values are the mean % inhibitors calculated from three replicates.
| Inhibitor
|
Inhibition of Glc Uptake
|
|
|---|---|---|
| Noninfected | Infected | |
| % | ||
| 10 μm CCCP | 67 | 63 |
| 1 mm NEM | 70 | 66 |
| 1 mm PCMBS | 17 | 20 |
| 1 mm DEPC | 42 | 35 |
| 1 mm Phloridzin | 42 | 28 |
Expression of the Monosaccharide Transporter, AtSTP4, Is Induced on Infection
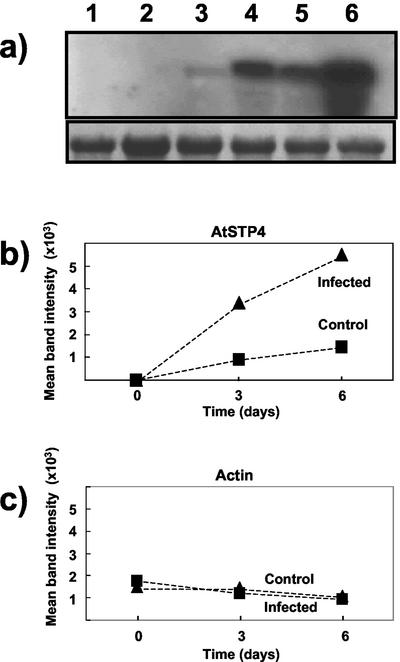
The monosaccharide transporter, AtSTP4, has been implicated in responses to abiotic and biotic stress in Arabidopsis (Truernit et al., 1996). We investigated whether this particular monosaccharide transporter has a role in the response to infection by powdery mildew by comparing its expression in noninfected and infected leaf tissue. Northern analysis was performed with total RNA from infected and noninfected leaf material using the AtSTP4 probe (Fig. 2). This was carried out under stringency conditions that did not allow cross-hybridization with AtSTP1 and -2. These results also show that there is a marked increase in AtSTP4 expression in infected material compared with noninfected leaves. Light brushing without fungal inoculum (equivalent to the brushing that was carried out to apply the fungus) also caused an increase in AtSTP4 expression, although this was far less than the expression induced by infection with the powdery mildew. In contrast, the actin control showed little change throughout infection or with brushing (Fig. 2). Similar results were obtained in a semiquantitative analysis using reverse transcriptase (RT)-PCR and gene-specific primers for AtSTP4 (data not shown). RT-PCR with gene-specific primers for AtSTP1 and AtSTP2 indicated that the former did not alter markedly in expression after infection, whereas the latter was not expressed in noninfected or infected leaf material (J.K. Pittman and L.E. Williams, unpublished data).
Figure 2.
Northern analysis of AtSTP4 expression after infection with E. cichoracearum. a, Forty micrograms of total RNA per lane was separated on a 1.2% (w/v) agarose gel and transferred to a nylon membrane. Hybridization was carried out with a digoxigenin (DIG)labeled AtSTP4 (top) and actin (bottom) probes. Blots were washed at high stringency. Tissue was treated with fungal inoculum by gently brushing with a paintbrush. Controls were brushed without inoculum. Lane 1, Noninfected, non-brushed tissue (d 0); lane 2, noninfected brushed tissue (d 0); lane 3, control tissue (d 3); lane 4, infected tissue (d 3); lane 5, control tissue (d 6); and lane 6, infected tissue (d 6). A representative blot is shown. The experiment was performed three times for AtSTP4 and twice for actin. Densitometry data were obtained for each experiment and the mean band intensity is shown for AtSTP4 (b) and actin (c).
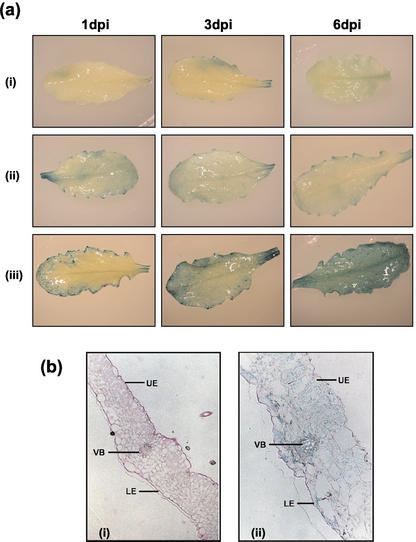
To investigate the induction of AtSTP4 expression during infection in more detail, we studied the response in transgenic plants transformed with an AtSTP4-promoter-β-glucuronidase (GUS) construct. Initially, the AtSTP4 promoter-GUS construct was in the Arabidopsis C24 ecotype (Truernit et al., 1996), which is not susceptible to E. cichoracearum. Therefore these plants were crossed with the susceptible accession Col-0, and seed was collected. Crossing was repeated three times to increase the Col-0 background. The resulting plants were susceptible to E. cichoracearum. We confirmed that the healthy uninfected Col-0 plants containing the AtSTP4-promoter GUS construct showed the same pattern of expression for AtSTP4 as originally observed in the C24 ecotype i.e. high expression in the sink organs (roots and anthers) but very low in the mature source leaves. When detected in the mature leaves, it was often found at the hydathodes (M.J. Gilbert, J.L. Hall, and L.E. Williams, unpublished data). Expression of AtSTP4, as indicated by GUS staining, was induced in leaves infected with E. cichoracearum at 1 d postinfection and increased with time after inoculation (Fig. 3a). Leaves inoculated with the incompatible Blumeria graminis spores showed some increase in staining at d 1 postinfection, but this did not increase over 6 d as observed for the compatible interaction. Similar results were found with a semiquantitative RT-PCR analysis, where no increase in expression was observed between d 1 and 6 postinfection after inoculation with B. graminis (V. Fotopoulos, unpublished data).
Figure 3.
Histochemical analysis of mature transgenic Arabidopsis leaves expressing the GUS gene under the control of the AtSTP4 promoter after inoculation with compatible and incompatible mildew strains. a, To visualize the location of GUS activity (indicated by blue color), leaves were incubated at 22°C for 15 h in the presence of 5-bromo-4-chloro-3-indolyl-β-d-glucuronide-sodium salt trihydrate. Results are shown for 1, 3, and 6 d postinoculation for (i), healthy, uninfected leaf; (ii), leaf with the incompatible B. graminis; and (iii), leaf with the compatible E. cichoracearum. b, Tissue was stained for GUS then fixed, embedded, and sectioned. Cell walls are stained pink with Schiff reagent. (i), Control healthy leaf; (ii), +6 d postinoculation with E. cichoracearum. UE, Upper epidermis; LE, lower epidermis; VB, vascular bundle. Representative leaves for each condition are shown.
To determine the cellular pattern of expression, sections were taken from the infected and noninfected leaves of the AtSTP4 promoter-GUS transformed plants. In the sections from control plants, no GUS expression was observed, whereas in those obtained from infected material, GUS staining was present in many cells of the leaf including the vascular tissue (Fig. 3b).
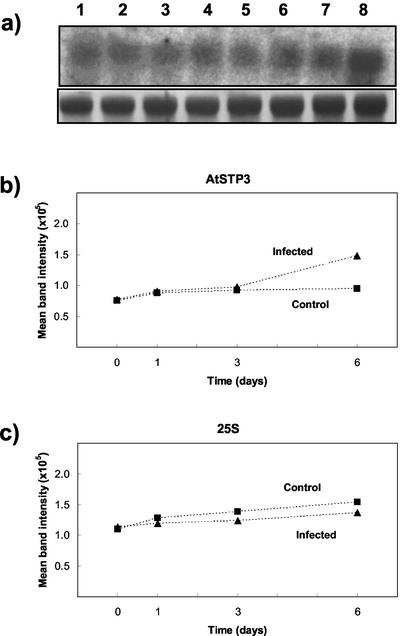
More recently another monosaccharide transporter gene, AtSTP3, has been reported, which is also expressed in leaves (Büttner et al., 2000), and therefore we investigated whether this transporter had any role in the host response to infection. Little change in expression over the first 3 d and only a small induction at 6-d postinfection was observed in northern analysis (Fig. 4) and RT-PCR (V. Fotopoulos, unpublished data). The response was minor compared with that seen for AtSTP4. No marked response of AtSTP3 was observed in the incompatible interaction as determined by RT-PCR (V. Fotopoulos, unpublished data). Therefore, of the four sugar transporters investigated here (AtSTP1–4), AtSTP4 shows the most marked response with a severalfold increase in expression after infection with a compatible mildew strain.
Figure 4.
Northern analysis of AtSTP3 expression after infection with E. cichoracearum. a, Twenty-five micrograms of total RNA per lane was separated on a 1.5% (w/v) agarose gel and transferred to a nylon membrane. Hybridization was carried out with a 32P-labeled AtSTP3 probe (top). Methylene blue staining of the 25s rRNA band is also shown (bottom). Blots were washed at moderate stringency. Tissue was treated with fungal inoculum by gently brushing with a paintbrush. Controls were brushed without inoculum. Lane 1, Noninfected tissue (d 0); lane 2, infected tissue (d 0); lane 3, control tissue (d 1); lane 4, infected tissue (d 1); lane 5, control tissue (d 3); lane 6, infected tissue (d 3); lane 7, control tissue (d 6); and lane 8, infected tissue (d 6). A representative blot is shown. The experiment was performed three times with similar results. Densitometry data were obtained for two experiments, and the mean band intensity is shown for AtSTP3 (b) and 25S rRNA (c).
Effect of Powdery Mildew Infection on Cell Wall Invertase
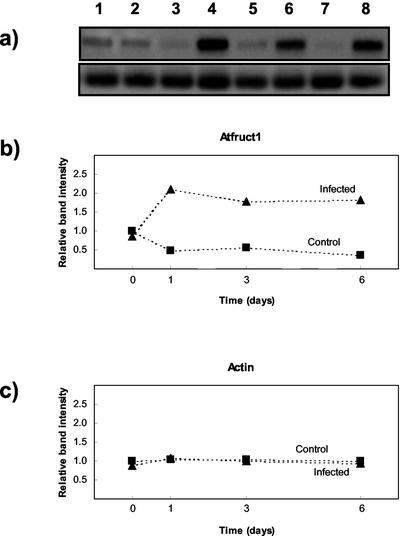
Cell wall invertase activity increased markedly 3 and 6 d postinfection (Fig. 5). The response was also investigated at the RNA level using RT-PCR. Degenerate oligonucleotide primers were designed based on highly conserved amino acid regions identified in cell wall invertases from carrot (Daucus carota), mung bean (Vigna radiata), tomato (Lycopersicon esculentum), and Arabidopsis. RT-PCR was carried out using RNA prepared from noninfected and infected material, and greater amplification was observed in infected samples. To investigate this in more detail, we cloned the RT-PCR products produced with 3-d postinfected material. Five clones were selected and sequenced, and in all cases they represented partial cDNAs of Atβfruct1, a cell wall invertase (Schwebel-Dugue et al., 1994). We therefore monitored expression of this particular gene during infection. For comparison, Atβfruct2 was included in the analysis. RT-PCR was carried out on RNA samples obtained from noninfected and infected material (1, 3, and 6 d postinfection). In these particular experiments, fungal inoculum was supplied by shaking spores onto leaves to avoid any effects of brushing. The products were separated by gel electrophoresis, probed, and blotted with probes specific for Atβfruct1 and Atβfruct2 under conditions where minimal cross-reactivity occurred. Atβfruct1 expression was detected in all samples but showed a marked increase in expression 1, 3, and 6 d postinfection (Fig. 6). In contrast, actin did not change markedly on infection. With similar exposure times, Atβfruct2 expression could not be detected in any of the samples, and this is consistent with previous reports showing that this gene is only expressed in flowers (Tymowska-Lalanne and Kreis, 1998). After prolonged exposure, we observed some binding of Atβfruct2, which also occurred to the ladder, indicating nonspecific binding (V. Fotopoulos, unpublished data). Northern analysis was also carried out with Atβfruct1, and similar results were observed to the RT-PCR experiments with an increase in expression 1, 3, and 6 d postinfection compared with controls (V. Fotopoulos, unpublished data). In contrast, in the incompatible interaction, RT-PCR showed no marked increase in the expression of Atβfruct1 up to 6 d postinoculation (V. Fotopoulos, unpublished data).
Figure 5.
Effect of infection on cell wall invertase activity in Arabidopsis source leaves. Invertase activity was assayed at pH 4.5 in extracts isolated from leaves treated with (black bars) or without (white bars) fungal inoculum. Noninfected control tissue was gently brushed without fungal inoculum. Values are the means of four replicated experiments. Asterisk indicates significant difference from control plants (P ≤ 0.001).
Figure 6.
Effect of infection on invertase expression in Arabidopsis source leaf using RT-PCR. a, RT-PCR was carried out with primers designed to generate partial-length invertase (top) or actin cDNAs (bottom). Products were run on a 1.2% (w/v) agarose gel, hybridized with DIG-labeled Atβfruct1 and actin cDNA probes, and washed at high stringency. Lane 1, Noninfected (d 0); lane 2, infected (d 0); lane 3 noninfected (d 1); lane 4, infected (d 1); lane 5, noninfected (d 3); lane 6, infected (d 3); lane 7, noninfected (d 6); and lane 8, infected (d 6). b and c, Densitometry data were obtained for each amplified product and expressed relative to the products at d 0 (healthy), which were normalized to a value of 1. The data are from a representative experiment repeated twice.
DISCUSSION
The nature of transporters involved in powdery mildew infection in the host has never been resolved at the molecular level. The Arabidopsis/E. cichoracearum interaction provides us with a useful system for investigation because Arabidopsis has been used extensively for studying the role of sugar carriers in source/sink interactions (Williams et al., 2000). cDNAs encoding monosaccharide transporters have been cloned from several species, and there is now evidence for the existence of multigene families. Arabidopsis contains a family of STP genes coding for monosaccharide transporters that appear to be tissue specific or switched on in response to particular stimuli (Büttner et al., 2000; Williams et al., 2000). Hexoses, as well as Suc, may be important signaling molecules in source/sink regulation including responses to biotic stress (Roitsch, 1999), and the transporters that regulate their distribution in stressed tissues may be an important component of this regulatory and defense activity.
Increased uptake of sugars into leaf tissues in response to abiotic and biotic stress has been observed after ozone treatment (Sutton and Ting, 1977) and pathogen infection (Sutton, 1997; Clark and Hall, 1998). In the present study, an increase in Glc uptake was also observed in the response of Arabidopsis leaf tissue to powdery mildew infection. This increased capacity for Glc transport in infected tissues may be due to the pathogen acting as an additional sink, although a similar increase is observed when the fungal mycelium is removed before measuring uptake. This indicates that there is a change in nutrient demand of the host in this plant/fungal interaction that is independent of a direct requirement of the pathogen for assimilates. The host response could be related to an increased requirement by leaf cells for carbon to support repair or defense processes. Our objective was to examine which transporters may have a role in this response. Truernit et al. (1996) have suggested that the hexose-transport response to stress may be linked to a specific member of the monosaccharide gene family, AtSTP4. We have shown, using a variety of molecular techniques (northern analysis, RT-PCR, and reporter gene technology), that AtSTP4 expression is induced after infection with E. cichoracearum, a compatible mildew strain. A significant increase in AtSTP4 expression was observed in leaf tissue 1, 3, and 6 d postinfection compared with the noninfected controls. Leaves inoculated with the incompatible B. graminis spores showed some increase in GUS staining at d 1 postinoculation but, unlike the compatible reaction, no further increase in expression was observed at d 3 and 6 postinfection. This difference presumably relates to the progression, or not (in the case of the incompatible interaction), of the disease and may reflect the change in the leaf from source to sink metabolism in the compatible reaction.
In tissue sections of E. cichoracearum-infected leaves (prepared from AtSTP4 promoter-GUS transformed plants), GUS appeared to be present in many cells of the leaf, whereas the fungal haustoria are restricted to the epidermis in this infection (Adam and Somerville, 1996). In contrast, in some biotrophic rust infections, gene up-regulation is restricted to the infection sites (Chou et al., 2000; Ayliffe et al., 2002). The initial penetration events during the early stages of powdery mildew infection could be expected to induce AtSTP4, because it is a wound-inducible carrier (Truernit et al., 1996). However, the findings that AtSTP4 expression can also be induced by pathogen elicitors (Truernit et al., 1996) and the fact that we observed AtSTP4 induction in cells other than those penetrated by the fungus in the epidermis suggest this is not the case. Therefore it is possible that a general retrieval response may be induced in all leaf cells to retain carbon for defense purposes and may be linked to the increased invertase activity that also accompanies infection (see below).
Although AtSTP4 showed a marked increase in expression, there was a less marked effect on Glc uptake. However, in the latter experiments, the net uptake is affected by a number of parameters including both influx and efflux. The predominant role of AtSTP4 in this response was supported by analysis of other monosaccharide transporters. AtSTP1 expression did not change, whereas AtSTP2 was not expressed. AtSTP3, a sugar transporter expressed in green leaves (Büttner et al., 2000), showed some increase in expression with infection but this was small and not apparent until 6 d postinfection. We cannot rule out that some of the other AtSTPs may also be involved in this response, but further study is required to determine this.
In addition to investigating changes in host monosaccharide transporters, our work has focused on the role of the enzyme invertase. Increased invertase activity has been recorded for a number of biotrophic infections, although the precise form, location, and role of the activity are not clear (for reviews, see Ayres et al., 1996; Hall and Williams, 2000). Wright et al. (1995) observed that acid invertase activity increased in wheat (Triticum aestivum) leaves infected with powdery mildew even when treated with a fungicide to reduce the size of the fungal sink; this indicated that a signal from the fungus caused an increase in invertase activity that was not directly related to the size of the fungal sink. The present study has focused on cell wall invertase and shows that there is a marked increase in activity of invertase upon infection. This coincides with an increase in the expression of the Atβfruct1 isoform. Although Atβfrut2 does not seem to be involved in this response, we cannot rule out the possibility that other invertase isoforms may also have a role.
An induction of cell wall invertase has been reported for tobacco (Nicotiana tabacum) leaves infected with potato (Solanum tuberosum) virus Y (Herbers et al., 2000) and for Atβfruct1 in Arabidopsis leaves infected with white blister rust (Chou et al., 2000). In the latter report, increased invertase activity was confined to the parts of the leaves invaded by the fungal mycelium. Such increases in cell wall invertase during infection could have a profound effect on the source-sink balance within the plant. For example, transgenic tobacco plants expressing yeast-derived invertase in the apoplast showed an accumulation of soluble sugars and an inhibition of photosynthesis (Stitt et al., 1990; von Schaewen et al., 1990) that presumably resulted in a decrease in translocation from the leaf. Again, ectopic expression of invertase in transgenic plants leads to the activation of defense responses and appears to be regulated by sugar levels in the tissue (Herbers et al., 1996; Ehness et al., 1997). In the powdery mildew interaction, it is not clear whether as a result of this increase in invertase activity, the increased hexose levels act to support the defense responses of the host tissue or serve to provide a supply of hexoses for the growing pathogen. It is interesting that earlier studies showed that the degree of fungal attack may be related to the sugar content of the infected leaf tissue; if sinks, such as fruits, were removed from some plants then this resulted in an increase in sugars in the leaves and increased resistance to certain diseases. This led to the concept of low sugar disease and sink-induced loss of resistance (see Vanderplank, 1984). However, in mildews and rusts, increased sugar content in the leaves increases the susceptibility to the disease, although this reverses at still higher levels of sugar (Vanderplank, 1984). More information is required in this area to investigate the delicate balance between sugar content, pathogen invasion, and defense responses in fungal biotrophs.
The finding that infection by powdery mildew induces AtSTP4 expression in a wide range of host cells despite the fungus being confined to the epidermis implicates the role of signaling mechanisms in this response. There is evidence that defense response genes are induced by elevated sugar levels (Ehness et al., 1997; Herbers et al., 2000), and hexose transport into the host cells may be enhanced to cope with this increased energy demand and/or to reduce availability of sugars to the pathogen. Enhanced cell wall invertase reduces Suc loading (Stitt et al., 1990; von Schaewen et al., 1990) and an increase in hexose availability as a consequence could increase the concentration gradient of hexoses to the fungus. The transfer of carbon to the fungus, primarily as Glc, is thought to occur down a concentration gradient created by fungal solute uptake and use. The nature of the sugar transporters at the haustorial membrane in the powdery mildew fungi is unknown, but by analogy with rust, carriers may exist at this membrane to facilitate transport into the mycelium. A major goal will be to dissect the signaling pathway in a compatible interaction that results in the diversion of host resources to the fungus.
MATERIALS AND METHODS
Plant and Fungal Material
Seeds of Arabidopsis cv Columbia (ecotype Col-0) were sown in Irish moss peat, John Innes No. 2, and medium graded vermiculite (1:1:1). After sowing, the seeds were vernalized for 24 h at 4°C and then grown at 22°C, 75% humidity on a 16-h photoperiod. Arabidopsis GUS transformants were grown on Murashige and Skoog media (Murashige and Skoog, 1962) supplemented with 2% (w/v) Suc and 50 μg mL–1 kanamycin. When germinated, the seedlings were transplanted into soil and grown in identical conditions to those described above. Erysiphe cichoracearum was maintained by growing on squash (Cucurbita maxima cv Autumn Queen). Arabidopsis rosette leaves were infected with E. cichoracearum by tapping spores from an infected squash leaf directly onto the uninfected Arabidopsis leaf surface. When the squash were less heavily infected, Arabidopsis leaves were infected using a paintbrush to gently brush the spores (obtained from squash leaves) onto the surface of the Arabidopsis leaf; in this case, control tissue was obtained by performing the gentle brushing in the absence of spores. As an incompatible control, plants were infected with Blumeria graminis f.sp. hordei maintained on barley (Hordeum vulgare cv Golden Promise). Measurements were normally made 3 and 6 d after inoculation. Fungal mycelia were visible by microscopy 3 d post inoculation after staining with lactophenoltrypan blue, whereas powdery mildew colonies could be observed by eye 6 d post inoculation. In some experiments, an earlier time point of 1 d was also used as by this time most conidia had germinated (Adam and Somerville, 1996).
Plants containing the AtSTP4 promoter-GUS construct were initially of the C24 ecotype (Truernit et al., 1996), which is not susceptible to infection. Therefore these plants were crossed with the susceptible accession Col-0 and seed collected. Crossing was repeated three times and a backcross performed to increase Col-0 genetic background. At each stage, the collected seed was selected on kanamycin plates for the presence of the GUS-construct. At each stage of crossing, the mature plants were challenged with powdery mildew infection, and only susceptible plants were chosen.
RNA Extraction
Total RNA was isolated from Arabidopsis leaf tissue using phenol-SDS extraction and lithium chloride precipitation based on the method of Verwoerd et al. (1989). Extraction was performed in 0.1 m LiCl, 0.1 m Tris-HCl (pH 8.0), 10 mm EDTA, 1% (w/v) SDS, and 50% (v/v) phenol:chloroform. After extraction, the RNA was precipitated in 4 m LiCl at 4°C.
DIG-Labeled Probes
cDNA clones encoding two cell wall invertases, Atβfruct1 and Atβfruct2 (Mercier and Gogarten, 1995; Schwebel-Dugue et al., 1994) and the monosaccharide transporter AtSTP4 were used as template for the preparation of DIG-labeled probes. DNA probes were prepared with the degenerate invertase primers (see later) on the corresponding cDNA clone using the DIG labeling kit (Roche Diagnostics, Mannheim, Germany) according to the instructions provided by the manufacturer. The DIG-labeled actin probe was prepared in a similar way, using an RT-PCR product amplified from healthy, mature leaves as template.
32P-Labeled Probes
The cDNAs to be labeled were removed from plasmid vectors by digestion with the appropriate enzyme(s) and isolated using gel electrophoresis. The DNA was additionally purified using the QIAquick PCR Purification Kit (Qiagen, Dorking, Surrey, UK), according to the manufacturer's instructions. Template DNA (25 ng) in 45 μL of Tris/EDTA buffer was labeled by primer extension (Feinberg and Vogelstein, 1983) using 1.85 MBq of Redivue [32P]dCTP (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) and a Rediprime II random prime labeling system (Amersham Biosciences) according to the manufacturer's instructions.
RT-PCR
First-strand cDNA was prepared from 5 μg of total RNA using an oligo(dT) primer and Superscript reverse transcriptase (Invitrogen, Carlsbad, CA). The reaction was incubated at 37°C for 1 h and stopped by heating to 94°C for 10 min. To amplify invertase products, degenerate oligonucleotide primers were designed corresponding to highly conserved amino acid regions identified in Arabidopsis, carrot(Daucus carota), mung bean (Vigna radiata), and tomato (Lycopersicon esculentum; forward primer, 5′-CCGGAATTCTTYTAYCARTRBAAYCC-3′; reverse primer, 5′-CTAGTCTAGARTCRWARAANGGYTTNGAYGCRTA-3′) where Y = C,T; R = A,G; B = G,T,C; W = A,T; and n = G,A,T,C. For actin, oligonucleotide primers were designed corresponding to highly conserved amino acid regions found in all 10 known Arabidopsis actin genes (McDowell et al., 1996; Robinson et al., 1999; forward primer, 5′-GGTAACATTGTGCTCAGTGGT-3′; reverse primer, 5′-CTCGGCCTTGGAGATCCACAT-3′). PCR products were amplified from 300 to 500 ng of first-strand cDNA using the appropriate primers and Taq DNA polymerase (Hybaid, Ashford, UK). All oligonucleotide primers were obtained from MWG-Biotech (Ebersberg, Germany).
Reactions to amplify invertases were cycled at 94°C for 30 s, 48°C for 1 min, and 72°C for 2 min. A range of amplification cycle numbers (e.g. 25, 30, 35, 40, and 50) were carried out, and the densitometry data that are shown represents results obtained during the exponential phase of PCR. For actin, reactions were cycled at 94°C for 30 s, 60°C for 1 min, and 72°C for 1 min 30 s. Integrated density values were taken using the AlphaImager 1220 program (Alpha Innotech, San Leandro, CA).
Cloning and Sequencing of RT-PCR Products
The RT-PCR fragments obtained using the degenerate invertase primers (see above) were cloned into pGEM-T Easy vector (Promega, Southampton, UK) following the manufacturer's instructions. Clones were sequenced using the ABI PRISM BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA) in an ABI PRISM 377 DNA sequencer (Applied Biosystems).
Blotting and Hybridization
The RT-PCR products were separated on a 1.2% (w/v) agarose gel and transferred to Hybond-N nylon membrane (Amersham Biosciences). Hybridization with DIG-labeled probes and subsequent washing was carried out at moderate- to high-stringency conditions. DIG was detected using the DIG Luminescent Detection Kit (Roche Diagnostics) according to the instructions provided.
Northern-Blot Analysis
Northern-blot analysis was carried out as described by Sambrook et al. (1989). RNA was denatured and separated on a 1.2% or 1.5% (w/v) agarose denaturing gel and transferred to Hybond-N membrane. In certain cases, RNA was visualized on the nylon membrane with methylene blue (Herrin and Schmidt, 1988). Hybridization with DIG-labeled probes or 32P-labeled probes and subsequent washing was carried out using moderate- to high-stringency conditions. DIG was detected using the DIG-Luminescent Detection Kit (Roche Diagnostics) according to the instructions provided. 32P was detected by exposing blots to Kodak Biomax MS film (Eastman Kodak, Rochester, NY) at –70°C with intensifying screens for up to 5 d or a phosphor screen (Molecular Dynamics, Sunnyvale, CA) at room temperature for up to 3 d.
Histochemical Localization of GUS Activity
Leaf tissue samples were placed into a solution containing 5 mg of 5-bromo-4-chloro-3-indolyl-β-d-glucuronide sodium salt trihydrate, 20 μL of 0.5 m Na-EDTA, 50 μL of Triton X-100, and 50 μL of dimethylformamide made up to a final volume of 10 mL with 50 mm Na2HPO4 (pH 7.0) and incubated at 28°C for 18 h. Plant samples were then fixed in a solution containing 14.2 mL of 100% (v/v) ethanol, 14.2 mL of distilled water, 1.66 mL of glacial acetic acid, and 3.3 mL of formaldehyde at 22°C for at least 2 h. Tissues were then cleared in multiple incubations of 70% (v/v) ethanol.
Leaf Sections Were Obtained after GUS Coloration
Leaf tissue samples were washed in 100 mm phosphate buffer (pH 7.0) and fixed in 100 mm sodium phosphate buffer (pH 7.0), 2% (v/v) formaldehyde, and 0.5% (v/v) glutaraldehyde at 4°C for 2 h. After a wash in 100 mm phosphate buffer (pH 7.0), chlorophyll pigments were eliminated by consecutive washes in 50%, 60%, 70%, 80%, and 90% (v/v) ethanol for 5 min. Tissues were embedded in a methacrylate resin (Fluka Biochemika, Buchs, Switzerland) following the manufacturer's instructions. Semifine sections (6 μm) were cut with a LKB microtome, and the cell walls were stained pink with Schiff reagent (100 mm HCl, 0.2 g L–1 fuschine, and 0.4 g L–1 anhydrous sodium metabisulfite). Sections were placed in 1% (v/v) periodic acid for 5 min at 22°C and then in Schiff reagent for 10 min at 22°C. Sections were washed in water to eliminate the excess dye on the resin. Sections were viewed using light microscopy.
Invertase Activity
Invertase enzymes were extracted using a method based on Tang et al. (1996). Healthy or infected Arabidopsis leaves (0.5 g) were ground in liquid nitrogen with a mortar and pestle in 2 mL of ice-cold extraction buffer consisting of 50 mm HEPES-KOH (pH 8.0), 5 mm MgCl2, 2 mm EDTA, 1 mm MnCl2, 1 mm CaCl2, 1 mm benzamidine, 1 mm dithiothreitol, and 0.1 mm phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 13,000g for 15 min, and the pellet was washed three times in ice-cold extraction buffer before being suspended in 2 mL of extraction buffer. The suspended pellet contained the cell wall invertase activity.
To measure invertase activity, 25 μL of the pellet was incubated with 50 mm phosphate-citrate buffer (pH 4.5) and 100 mm Suc to give a total volume of 200 μL at 30°C for 1 h. Two hundred and fifty microliters of 500 mm Na2HPO4 was added to stop the reaction, and the mixture was vortexed with 0.5 mL of DNSa reagent consisting of 44 mm 3,5-DNSa, 250 mm NaOH, 21 mm phenol, and 4 mm Na2SO3. The mixture was incubated at 100°C for 15 min, cooled to room temperature, and 0.5 mL of 40% (w/v) sodium potassium tartrate was added to each assay. Absorbance was read at 540 nm.
Glc Uptake into Leaf Discs
Discs were cut from noninfected and infected leaves and placed lower epidermis down in a 30-mm petri dish containing equilibration buffer (20 mm MES-BTP [pH 5.8] and 1 mm CaCl2) and left for 1 h to equilibrate. Uptake was started by placing the discs in Glc buffer (20 mm MES-BTP [pH 5.8], 1 mm CaCl2, 500 μm Glc, and 1 μCi of [14C]Glc) where they were mixed and placed in a shaker at 25°C for 1.5 h. Leaf discs were removed, blotted dry on filter paper, and washed with 20 mL of ice-cold equilibration buffer before being transferred to 5 mL of 80% (v/v) ethanol and incubated in a water bath at 80°C for 1 h. After incubation, all of the contents were placed into scintillation vials with 5 mL of scintillation fluid. All samples were counted by liquid scintillation counting. For certain experiments the fungal mycelium was removed from the leaf surface using sellotape. Control plants were also treated with sellotape.
Acknowledgments
We thank Sue Nelson (University of Southampton) for excellent technical assistance and Drs. A. Lecharny (University of Paris, Paris) and R.W. Mercier (University of Connecticut) for the gifts of the invertase clones.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021428.
This work was supported by the Biotechnology and Biological Science Research Council (grant no. 51/P14477), by the European Commission DGXII Biotechnology Program (contract no. BIO4 CT96–05831), and by The Royal Society.
References
- Adam L, Somerville SC (1996) Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J 9: 341–356 [DOI] [PubMed] [Google Scholar]
- Alexopoulos CJ, Mims CW, Blackwell M (1996) Introductory Mycology, Ed 4. Wiley, New York
- Ayliffe MA, Roberts JK, Mitchell HJ, Zhang R, Lawrence GJ, Ellis JG, Pryor TJ (2002) A plant gene up-regulated at rust infection sites. Plant Physiol 129: 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres PG, Press MC, Spencer-Phillips PTN (1996) Effects of pathogens and parasitic plants on source-sink relationships. In E Zamski, AA Schaffer, eds, Photoassimilate Distribution in Plants and Crops. Marcel Dekker, New York, pp 479–499
- Buell CR (1998) Arabidopsis: A weed leading the field of plant-pathogen interactions. Plant Physiol Biochem 36: 177–186 [Google Scholar]
- Büttner M, Truernit E, Baier K, Scholz-Starke J, Sontheim M, Lauterbach C, Huss VAR, Sauer N (2000) AtSTP3, a green leaf-specific, low affinity monosaccharide H+-symporter of Arabidopsis thaliana. Plant Cell Environ 23: 175–184 [Google Scholar]
- Chou H-M, Bundock N, Rolfe SA, Scholes JD (2000) Infection of Arabidopsis thaliana leaves with Albugo candida (white blister rust) causes a reprogramming of host metabolism. Mol Plant Pathol 1: 99–113 [DOI] [PubMed] [Google Scholar]
- Clark JIM, Hall JL (1998) Solute transport into healthy and powdery mildew-infected leaves of pea and uptake by powdery mildew mycelium. New Phytol 140: 261–269 [DOI] [PubMed] [Google Scholar]
- Ehness R, Ecker M, Godt DE, Roitsch T (1997) Glucose and stress independently regulate source and sink metabolism and defence mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 9: 1825–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JF, Lewis DH (1987) Nutrient relations in biotrophic infections. In GF Pegg, PG Ayres, eds, Fungal Infection of Plants. Cambridge University Press, Cambridge, UK, pp 92–132
- Feinberg AP, Vogelstein B (1983) A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 [DOI] [PubMed] [Google Scholar]
- Hall JL, Williams LE (2000) Assimilate transport and partitioning in fungal biotrophic interactions. Aust J Plant Physiol 27: 549–560 [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Métraux J-P, Sonnewald U (1996) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Takahata Y, Melzer M, Mock H-P, Hajirezaei M, Sonnewald U (2000) Regulation of carbohydrate partitioning during the interaction of potato virus Y with tobacco. Mol Plant Pathol 1: 51–59 [DOI] [PubMed] [Google Scholar]
- Herrin DL, Schmidt GW (1988) Rapid, reversible staining of northern blots prior to hybridisation. Biotechniques 6: 196. [PubMed] [Google Scholar]
- McDowell JM, Huang SR, McKinney EC, An YQ, Meagher RB (1996) Structure and evolution of the actin gene family in Arabidopsis thaliana. Genetics 142: 587–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendgen K, Nass P (1988) The activity of powdery-mildew haustoria after feeding the host cells with different sugars, as measured by potentiometric cyanine dye. Planta 174: 283–288 [DOI] [PubMed] [Google Scholar]
- Mercier RW, Gogarten JP (1995) A second cell-wall acid invertase gene in Arabidopsis thaliana. Plant Physiol 107: 659–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferricchelate reductase for iron uptake from soils. Nature 397: 694–697 [DOI] [PubMed] [Google Scholar]
- Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2: 198–206 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Scholes JD, Lee PJ, Horton P, Lewis DH (1994) Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytol 126: 213–222 [Google Scholar]
- Schulze-Lefert P, Vogel J (2000) Closing the ranks to attack by powdery mildew. Trends Pharmacol Sci 5: 1360–1385 [DOI] [PubMed] [Google Scholar]
- Schwebel-Dugue N, Mtili NE, Krivitzky M, Jean-Jacques I, Williams JHH, Thomas M, Kreis M, Lecharny A (1994) Arabidopsis gene and cDNA encoding cell-wall invertase. Plant Physiol 104: 809–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, von Schaewen A, Willmitzer L (1990) “Sink” regulation of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase in their cell wall involves a decrease of the Calvin-cycle enzymes and an increase in glycolytic enzymes. Planta 183: 40–50 [DOI] [PubMed] [Google Scholar]
- Storr T, Hall JL (1992) The effect of infection by Erysiphe pisi DC. on acid and alkaline invertase activities and aspects of starch biochemistry in leaves of Pisum sativum L. New Phytol 121: 535–543 [Google Scholar]
- Sutton PN (1997) Solute transport in the interaction between wheat and powdery mildew. PhD thesis. University of Southampton, UK
- Sutton PN, Henry MJ, Hall JL (1999) Glucose, and not sucrose, is transported from wheat to wheat powdery mildew. Planta 208: 426–430 [Google Scholar]
- Sutton R, Ting IP (1977) Evidence for the repair of ozone-induced membrane injury. Am J Bot 64: 404–411 [DOI] [PubMed] [Google Scholar]
- Tang G-Q, Lüscher M, Sturm A (1999) Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 11: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Ruffner H-P, Scholes JD, Rolfe SA (1996) Purification and characterisation of soluble invertases from leaves of Arabidopsis thaliana. Planta 198: 17–23 [DOI] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress regulated Arabidopsis gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8: 2169–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymowska-Lalanne Z, Kreis M (1998) Expression of the Arabidopsis thaliana invertase gene family. Planta 207: 259–265 [DOI] [PubMed] [Google Scholar]
- Vanderplank JE (1984) Disease Resistance in Plants, Ed 2. Academic Press, London
- Verwoerd TC, Dekker BMM, Hoekema A (1989) A small scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17: 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegele RT, Stuck C, Hahn M, Mendgen K (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci USA 98: 8133–8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schaewen A, Stitt M, Schmidt R, Sonnewald U, Willmitzer L (1990) Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J 9: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants: a diversity of roles and complex regulation. Trends Pharmacol Sci 5: 283–290 [DOI] [PubMed] [Google Scholar]
- Wright DP, Baldwin BC, Shephard MC, Scholes JD (1995) Source-sink relationships in wheat leaves infected with powdery mildew: 1. Alterations in carbohydrate metabolism. Physiol Mol Plant Pathol 47: 237–253 [Google Scholar]