Abstract
Local leaf infections by a necrogenic pathogen can lead to systemic acquired resistance (SAR) in untreated leaves. We reasoned that, whatever the nature of the long-distance signal, if it is transported in the phloem, the pattern of SAR induced within the plant by treatment of a single leaf should match the pattern of translocation out of that leaf. The source-sink relationships (orthostichies) in the Arabidopsis rosette were established with [14C]Suc or phloem-mobile 3-aminotriazole at herbicidal concentrations. SAR was activated by infiltrating a single Columbia leaf with Pseudomonas syringae pv maculicola DC3000/avrRPM1, which causes a hypersensitive response. The pattern of SAR in the rosette was monitored by assessing the growth of wild-type DC3000 and by measuring the SAR markers salicylic acid and PR1 transcripts. Although the orthostichy of a single leaf was clearly limited to a row of vertically aligned leaves, SAR and SAR markers were also found outside the orthostichy. This indicates that, whatever the nature of the long-distance signal from the treated leaf to the upper responding leaves, its transport is either not limited exclusively to the phloem or the minor proportion of translocate that is not confined to the orthostichy contains enough of the SAR systemic signal to set in motion events leading to the establishment of the SAR state in the upper leaves.
Plants that are susceptible to a particular pathogen can often be induced to become systemically resistant by a predisposing treatment on lower leaves with a pathogen that causes local lesions. This phenomenon has been termed induced systemic resistance or systemic acquired resistance (SAR) and has been known for some years (Chester, 1933; Kuc, 1982). SAR is typically effective against a wide range of pathogens, including those taxonomically unrelated to the original inducing organism. The resistant state is associated with the local and systemic accumulation of pathogenesis-related (PR) proteins and has been well characterized in tobacco (Nicotiana tabacum), cucumber (Cucumis sativus), and Arabidopsis (Uknes et al., 1992). A number of mutants compromised in their ability to be induced to the SAR state have been documented, but relatively little is known about the long-distance signaling events occurring between the original inducing stimulus and the onset of resistance in systemic leaves, and the nature of the long-distance signal substance is still unclear (Uknes et al., 1992; Ryals et al., 1996; Van Loon, 2000). It is postulated that the systemic signal, produced at the lesion caused by the inducing infection, is translocated in the phloem to the upper leaves (Ross, 1966; Jenns and Kuc, 1977). White (1979) showed that acetyl salicylic acid (aspirin) could induce the accumulation of PR proteins and condition resistance in tobacco. Subsequently, Van Loon (1983) showed that the more commonly occurring plant secondary metabolites salicylic acid (SA) and 2,6-dihydroxybenzoic acid were the only other hydroxylated benzoic acid derivatives that were effective at inducing SAR. After Malamy et al. (1990) and Métraux et al. (1990) independently showed that levels of SA in the phloem rise in tobacco mosaic virus-infected tobacco and Colletotrichum lagenarium- or tobacco necrosis virus-infected cucumber, respectively, there was speculation that SA might be the systemic signal substance in SAR (Yalpani et al., 1991; Uknes et al., 1992). However, as early as 1991, Rasmussen et al. (1991) showed that removal of the inoculated leaf before SA levels in the phloem begin to rise still led to resistance induction in upper leaves of cucumber. In addition, grafting experiments using transgenic tobacco plants expressing the nahG gene encoding salicylate hydroxylase, and thus unable to accumulate SA, suggested that SA was not the systemic signal in SAR (Vernooij et al., 1994). However, the authors clearly showed that SA was necessary for the local expression of resistance (Malamy et al., 1996). Use of 18O2 in feeding experiments in tobacco showed that the majority (69%) of SA accumulating systemically was synthesized in, and exported from, the leaf harboring the inducing infection (Shulaev et al., 1995). Mölders et al. (1996) showed that in cucumber the SA accumulating in systemic leaves after an inducing treatment was the result of both import from the induced leaf and de novo synthesis. Mölders et al. (1996) interpreted their results as consistent with the hypothesis that SA could be the systemic SAR signal in cucumber. Thus, the nature of the systemic signal is still speculative, and the exact role of SA remains controversial (Cameron, 2000; Van Loon, 2000). Allografts between cucumber, muskmelon (Cucumis melo), and watermelon (Citrullis vulgaris) suggested that the systemic signal is not genus or species specific, at least within the Cucurbitaceae (Jenns and Kuc, 1979). Homografts in cucumber showed that the infected leaf alone is the source of the systemic SAR signal and that the signal is not remobilized from or produced in systemically protected leaves (Dean and Kuc, 1986).
An interesting recent development has been the characterization of the lesion in a T-DNA-tagged Arabidopsis mutant defective in long-distance SAR signaling as a mutation in the lipid transfer protein gene DIR1 (Maldonado et al., 2002). The authors speculate that DIR1 interacts with a lipid-derived molecule to promote long-distance signaling.
Evidence that the systemic signal in SAR is propagated via the phloem comes from girdling experiments (Ross, 1966; Guedes et al., 1980). Although Guedes et al. (1980) used cotton (Gossypium hirsutum) and hot water to prevent phloem transport, Ross (1966) removed the outer layers of the stem down to (and including) the phloem. However, neither treatment can be regarded as specifically inhibiting only phloem transport. Thus, for example, an effect on the symplastic transport of a putative signal via other cells affected by such drastic treatments cannot be ruled out.
Plants generally employ one of two mechanisms to load substances into phloem for long-distance translocation. In symplastic loaders like Coleus blumei, loading is via the numerous plasmodesmatal connections between the mesophyll, bundle sheath, and phloem cells (Gamalei, 1989). In apoplastic loaders, the companion cell/sieve element complex is isolated from the symplast of the surrounding cells by a lack of plasmodesmatal connections. Thus, in apoplastic phloem loaders like Arabidopsis (Haritatos et al., 2000) and pea (Pisum sativum), Suc and presumably other phloem-transported metabolites are first exported from mesophyll cells into the apoplast and then loaded into the companion cell/sieve element complex by an energy-dependent transport system (DeWitt and Sussman, 1995); for example, the At-SUC2 Suc carrier (Stadler and Sauer, 1996). A further characteristic of apoplastic loaders is that phloem loading of Suc can be inhibited by the thiol reagent p-chloromercuribenzenesulphonic acid (PCMBS; Van Bel et al., 1994). Because the companion cell/sieve element complex in Arabidopsis is symplastically relatively isolated from the surrounding cells (Haritatos et al., 2000), presumably the systemic SAR signal must be loaded into the phloem via an apoplastic route in a similar manner to Suc. However, even in apoplastic phloem loaders, it is thought that loading of viruses into the phloem must occur symplastically through the few plasmodesmatal connections that are present (Santa Cruz, 1999). Thus, symplastic loading of other materials, e.g. the systemic SAR signal substance, must also be considered a possibility even in nominally apoplastic-loading species like Arabidopsis.
A characteristic of long-distance translocation is that not all sinks are equally supplied by source leaves and that source leaves preferentially serve sinks with a direct vascular connection forming what is known as an orthostichy (Joy, 1964; Ho and Peel, 1969; Taiz and Zeiger, 1998). Arabidopsis has a three + five spiral leaf phyllotaxy with a divergence angle between successive leaves of 137.5° (Callos and Medford, 1994), and leaves in an orthostichy are arranged in an approximately vertical line on the stem above each other in the phyllotaxy.
We reasoned that if the pathway of movement of the systemic signal in SAR is in the phloem, it might be predicted that the pattern of translocate movement and the induction of SAR and SAR markers such as SA and PR1 should coincide. To investigate the pathway of systemic signal movement in the SAR, we determined the orthostichy relationships in the Arabidopsis rosette and compared these with the pattern of the induction of SAR and SAR markers. The results are discussed in relation to the possible nature of the systemic SAR signal and the possible nature of long-distance signal transmission.
RESULTS
Translocation of 3-Aminotriazole (3-AT), [14C]SA, and [14C]Suc
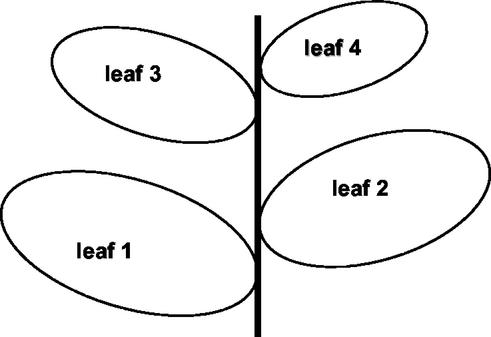
The relative positions on the Arabidopsis rosette of the treated leaf (L1) and the other investigated leaves (L2, L3, L4) are shown in Fig. 1.
Figure 1.
Relative positions of L1 (treated leaf), L2, L3, and L4 on the Arabidopsis rosette.
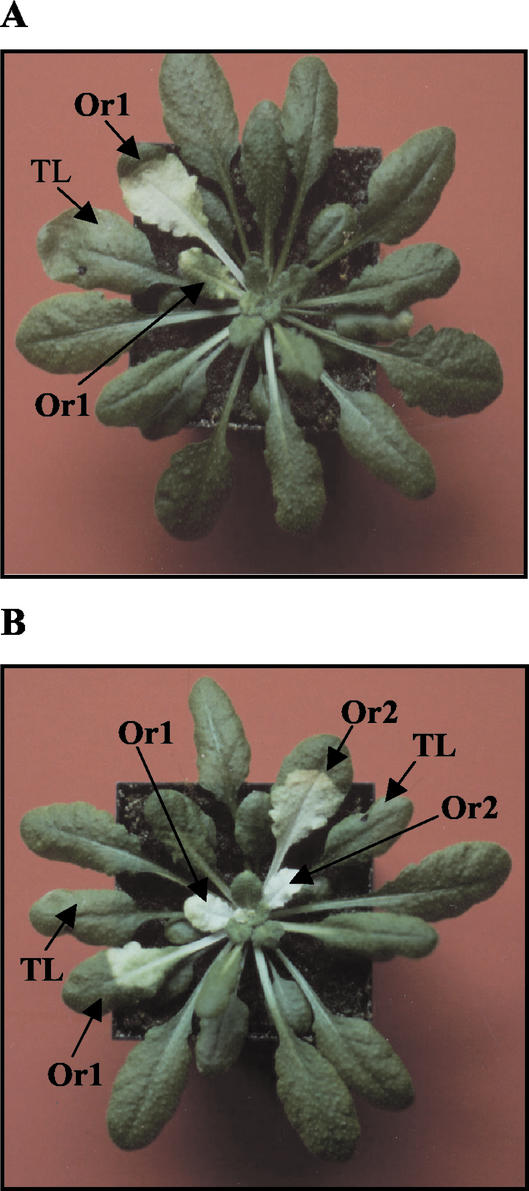
At 2 mm, the catalase inhibitor 3-AT causes lesions in leaves in a light-dependent manner. When applied to the lower leaves of an Arabidopsis rosette, 3-AT lesions occur in the treated leaf itself and in a vertical row of leaves in the phyllotaxy (Fig. 2). This vertical row of leaves in the rosette forms an orthostichy, that is, they are all connected by a contiguous row of vascular bundles (Taiz and Zeiger, 1998).
Figure 2.
Demonstration of orthostichies in Arabidopsis. The photograph shows rosettes 96 h after infiltrating 2 mm 3-AT into: A, a single lower leaf; and B, two lower leaves on opposite sides of the rosette. TL, Treated leaf. Or1 and Or2, Leaves in separate orthostichies.
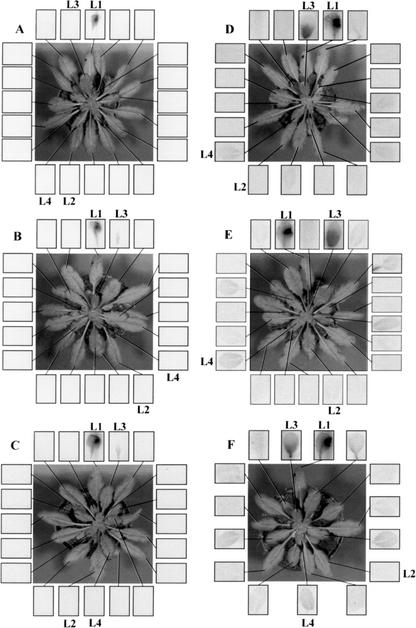
When 2 μCi [14C]SA was applied to a single lower rosette leaf (L1), a weak signal was observed on an autoradiograph in a single upper leaf in the vertically aligned orthostichy after 24 and 48 h (Fig. 3, A–C).
Figure 3.
Autoradiograph showing the translocation of [14C]SA (A, C, and E) and [14C]Suc (B, D, and F) from L1 at 6 (A and B), 24 (E and F), and 48 (C and D) h post application.
When 2 μCi [14C]Suc was applied to a single lower rosette leaf (L1), the translocation pattern defining the vertically aligned orthostichy was also observed on autoradiographs (Fig. 3, D–F) to correspond essentially to the orthostichy defined in Figure 2 by the herbicide effect of 3-AT. Interestingly, however, even as early as 6 h after application of [14C]Suc to L1, a weak signal was also seen in L4 and by 24 and 48 h, a weak signal could be seen in several upper leaves belonging to orthostichies other than L1/L3. Nevertheless, it is significant that no signal was ever observed in L2 lying opposite from L1 in the rosette and that more than 90% of the transported signal remained in the orthostichy, with the majority appearing in the young expanding L3 sink leaf (Table I).
Table I.
Relative proportions (%) of [14C] label in leaves L2, L3, and L4 calculated from the integral signal values from the autoradiograph in Figure 3
| Leaf
|
Time
|
||
|---|---|---|---|
| 6 h | 24 h | 48 h | |
| L2 | 0.0 | 1.1 | 3.2 |
| L3 | 93.1 | 97.5 | 92.9 |
| L4 | 6.9 | 1.3 | 3.9 |
SA Accumulation
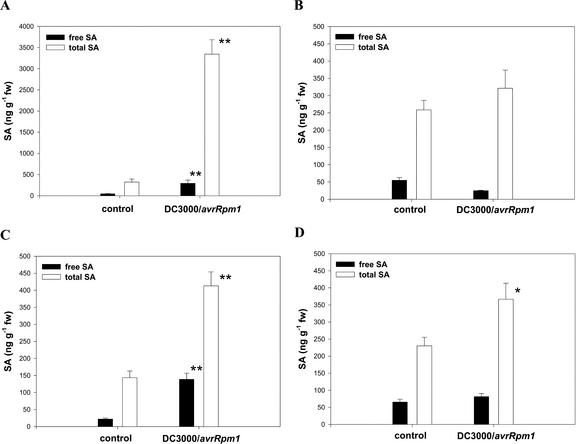
Free and total SA levels in L1 to L4 48 h after inoculation of L1 with either 10 mm MgSO4 or 5 × 107 cells mL–1 DC3000/avrRPM1 are shown in Figure 4. The general pattern observed was an increase in both the free and total SA levels in leaves from inoculated plants when compared with buffer-treated controls. Compared with the controls significant increases in both free and total SA were observed in L1 and L3 (Student's t test, P < 0.001), with the largest magnitude of change in L1. Thus, in L1, approximately 6.5× and 10× increases in free and total SA, respectively, were observed, whereas in L3, 6.5× and 3× increases in free and total SA were recorded. The total SA in L4 showed a small (1.6×) but nevertheless statistically significant increase from mean 230 ng g–1 fresh weight to mean 370 ng g–1 fresh weight (P < 0.05). The slight increase in free SA in L4 (from a mean of 60 ng g–1 fresh weight to mean 70 ng g–1 fresh weight) was not significant. There were no statistically significant changes in either free or total SA in L2 (50–25 ng g–1 fresh weight and 260–310 ng g–1 fresh weight, respectively) over the period of the experiment.
Figure 4.
Changes in SA in individual Arabidopsis rosette leaves. Free (squlf) and total (□) SA levels are shown for leaves L1 (A), L2 (B), L3 (C), and L4 (D) 48 h after treatment of L1 with either 10 mm MgSO4 (control) or 5 × 107 colony forming units (cfu) mL–1 Pseudomonas syringae pv maculicola DC3000/avrRPM1. * and **, Values significantly different from the control at the P = 0.05 and 0.01 levels (Student's t test), respectively. Note the use of a different concentration scale for L1.
PR1 Transcript Accumulation
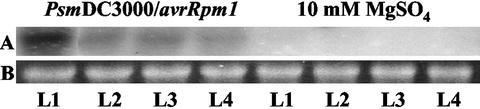
Steady-state levels of PR1 transcripts increased in all leaves, i.e. L1 to L4 by 48 h after inoculation of L1 with DC3000/avrRPM1 (Fig. 5).
Figure 5.
A, Steady-state levels of PR1 transcripts in leaves L1 to L4 48 h after inoculation with DC300/avrRpm1 or 10 mm MgSO4. B, Ethidium bromide-stained loading control.
SAR
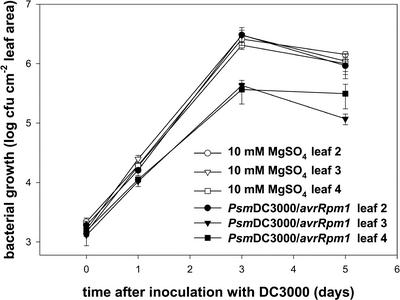
When a single, lower rosette leaf (L1 in Fig. 1) was treated with P. syringae pv maculicola DC3000/avrRPM1 to induce SAR, subsequent growth of virulent DC3000 cells was suppressed by 48 h after the inducing treatment in both of the upper, opposite rosette leaves tested (L3 and L4 in Fig. 1) but not in the L2 leaf opposite L1 in the lower rosette (Fig. 6). Multiplication of DC3000 cells in L2 matched the growth observed in control plants mock inoculated with 10 mm MgSO4 (Fig. 6).
Figure 6.
Induction patterns of SAR in Arabidopsis rosettes. Two days after treatment of L1 with either 10 mm MgSO4 (control) or 5 × 107 cfu mL–1 P. syringae pv maculicola C3000/avrRPM1, leaves L2, L3, and L4 were inoculated with 1 × 105 cfu mL–1 DC3000 (virulent) and bacterial growth in the individual leaves monitored. ○, [invtrio], and □, Growth of DC3000 in L2, L3, and L4, respectively, in the controls. •, [invtrif], and ▪, Growth of DC3000 in L2, L3, and L4, respectively, in DC3000/avrRPM1-inoculated plants.
DISCUSSION
Vascular connections defining orthostichies in the Arabidopsis rosette were clearly demonstrated using three test substances: 3-AT, [14C]SA, and [14C]Suc (Figs. 2 and 3). The endogenous transport substance Suc was more amenable to phloem transport than [14C]SA, as evidenced by a detectable autoradiographic signal in sink leaves of the orthostichy by as early as 6 h after label application (Fig. 3). Although the orthostichy was clearly defined as the major route of [14C]Suc transport, a weak autoradiographic signal was apparent in other leaves in the rosette, but, interestingly, label was virtually absent from L2 directly opposite the treated leaf until the later sampling time in the experiments (48 h; Fig. 3). It must be noted that the autoradiographic signal observed may be partly due to metabolites derived from the labeled Suc rather than Suc itself. However, this is not relevant to the questions posed in the work reported here.
Available evidence suggests that phloem loading in Arabidopsis is apoplastic (Haritatos et al., 2000). There are very few plasmodesmatal connections between the companion cell/sieve element complex and surrounding cells in Arabidopsis, but there are numerous plasmodesmata between companion cells and sieve elements themselves (Imlau et al., 1999) Interestingly, it appears that proteins are unloaded symplastically in sink tissues in Arabidopsis (Oparka et al., 1994). However, this observation does not preclude the possibility of carrier-dependent apoplastic unloading of other substances.
If the systemic SAR signal follows the assimilate transport pathway, presumably it must be loaded apoplastically in leaf L1 into the companion cell/sieve element complex and will be unloaded symplastically or possibly apoplastically. Because apoplastic loading of Suc is inhibited by PCMBS (Van Bel et al., 1994), we reasoned that it would be interesting to see if the systemic SAR signal cannot be translocated out of an induced leaf in the presence of PCMBS. However, P. syringae proved to be sensitive to PCMBS in the range 0.02 to 0.2 mm, which is well below the 0.5 to 2.5 mm working concentration routinely used to inhibit apoplastic loading; thus, this experiment could not be performed. In addition, PCMBS is usually supplied to a detached leaf by placing the cut end of the petiole in the PCMBS solution (Van Bel et al., 1994) and monitoring Suc loading. We used the cut flap procedure to supply substances into the L1 leaf, and we were unable to demonstrate any effect of PCMBS in our system on the systemic transport of [14C]Suc to other leaves.
Pattern of SAR and SAR Marker Accumulation
When a single lower rosette leaf (L1) was treated with an SAR-inducing inoculum of P. syringae pv maculicola DC3000/avrRPM1, the pattern of SA accumulation coincided approximately with the biological induction of SAR (Figs. 4 and 6). Thus, SAR developed not only in L3 in the same orthostichy as L1 but also in L4. However, neither SAR nor a statistically significant SA accumulation were induced in L2 on the opposite side of the lower rosette to L1 (Figs. 4 and 6). Interestingly, PR1 transcripts accumulated in all leaves, even L2, which did not show SAR or SA accumulation.
The pattern of phloem translocation of [14C]Suc does not correspond exactly with the induction of SAR or the pattern of SAR markers such as SA and PR1 transcripts. That is, after an inducing treatment of a single leaf (L1), SAR was induced beyond the orthostichy defined for phloem transport. Orthostichies define the major route of assimilate transport along physically connected vascular bundles. However, lateral transport between different orthostichies is known to occur, and our results show this in so far as a small amount of label was observed outside the L1/L3 orthostichy in L4 (Fig. 3). Because Dean and Kuc (1986) demonstrated that the systemic signal is not remobilized from, or produced in, systemically protected leaves, this implies that the small amount of signal that leaks into sink leaves in other orthostichies must be sufficient to induce SAR in those sink leaves. Alternatively, one could postulate other systemic signal routes, e.g. an electrical depolarization signal as was proposed for the induction of PI proteins in tomato (Lycopersicon esculentum) seedlings (Wildon et al., 1992). The induction of PR1 transcript accumulation in all leaves suggests that multiple cues might be working together to achieve the SAR state and that SA accumulation alone is only part of the complex.
In a conceptually similar investigation of wound-induced systemic resistance to leaf-feeding insects in cottonwood (Populus deltoides), Jones et al. (1993) found an exact correlation with the vascular architecture. Resistance of systemic cottonwood leaves to herbivores was presumably based on protease inhibitors regulated similarly as reported for the wound-induced systemin signaling reported in tomato and potato (Solanum tuberosum; Bergey et al., 1996). These results emphasize the multiplicity of signaling cues and mechanisms involved in systemic responses in different plant species and specific situations.
In conclusion, our results show clearly that the induction of SAR and SAR markers extends beyond the route of assimilate movement along an orthostichy and that some markers themselves are induced in a non-overlapping way. This has implications for the mechanism of action of the hypothetical SAR signaling substance and will be of interest when considering potential candidates for this role.
MATERIALS AND METHODS
Arabidopsis
Seeds of the ecotype Columbia were stratified in damp potting compost for 2 to 3 d at 4°C. Plants were grown in controlled environment chambers with an 8-h photoperiod (58 μmol m–2 s–1) with day and night temperatures of 20°C to 23°C and 18°C to 20°C, respectively. Five-week-old rosettes in individual pots were used in the experiments, and all experiments were repeated at least three times unless otherwise stated.
In the present work, the treated leaf was designated L1 and the next approximately opposite leaf in the phyllotaxy further up the rosette spiral was designated L2. Leaf 3 (L3) is a test leaf in the same orthostichy as L1 further up the rosette spiral, and L4 is the leaf on the opposite side of the rosette to L3 (Fig. 1).
Bacteria
Pseudomonas syringae pv maculicola DC3000(pCR105) and DC3000(pCR105: avrRPM1), both resistant to kanamycin (Kanr) and rifampicin (Rifr) (Debener et al., 1991; Grant et al., 1995) and virulent and avirulent on Columbia, respectively, were stored as glycerol stocks at –80°C and working plates on King's B agar (King et al., 1954) containing 50 μg mL–1 Rif and 30 μg mL–1 Kan prepared 48 h before inoculation of liquid shake cultures (King's B with Rif and Kan) and cultivation at 28°C 250 rpm on an orbital shaker overnight. Inoculum was prepared by harvesting cells from shake culture by centrifugation (700g for 5 min) and resuspending and washing the pellets two times in 10 mm MgSO4 and resuspending in 10 mm MgSO4 for inoculation. The concentration of cells was adjusted to 5 × 107 and 1 × 105 cfu mL–1, respectively, for the inducing and challenge inoculations. Bacteria were infiltrated locally into one leaf half under pressure using a 1-mL syringe without a needle. The other one-half of the leaf lamina was marked with a spot from an “Edding” permanent marker pen.
Leaf Treatments with 3-AT, [14C]Suc, [14C]SA, and Autoradiography
Approximately one leaf half was infiltrated with 2 mm 3-AT using a 1-mL syringe without a needle. The other half of the leaf lamina was marked with a spot from an “Edding” permanent marker pen.
To apply radiolabeled compounds, a small area of the epidermis over the central midrib of a lower rosette leaf was sliced away with a razor blade, and 20 μL of test solution was applied to the site. In experiments with [14CU]Suc, 20 μL (2 μCi) containing 1.14 μg of Suc dissolved in ethanol:water (2:98 [v/v]; Moravek Biochemicals, Inc., Brea, CA) was applied and in the case of SA, 20 μL (2 μCi) containing 3 μg of SA dissolved in methanol (Sigma-Aldrich, St. Louis). At 6, 24, and 48 h after application of radionuclides, rosette leaves were detached and laid out systematically on a phosphor imaging plate and scanned using an FLA3000 (Fuji, Tokyo) fluorescent image analyzer. Results were recorded photographically and quantified using the manufacturer's integration software. Experiments with [14C] compounds were repeated twice and with 3-AT three times.
PR1 Transcript Determination
Leaves (five per treatment, approximately 0.5 g) were snap frozen in liquid nitrogen, ground to a powder, and resuspended in 300 μL of RNA extraction buffer (10 mm EDTA and 100 mm LiCl in 100 mm Tris/HCl [pH 8] to which 300 μL of Tris/HCl [pH 8]-saturated phenol was added immediately and the sample vortexed to mix the phases). The mixture was extracted (300 μL of 24:1 [v/v] chloroform:isoamyl alcohol) and centrifuged repeatedly in a microfuge until no more denatured protein was visible at the interface. The upper, aqueous phase was removed and 0.25 volumes of 10 m LiCl was added before overnight incubation at 4°C to precipitate the RNA. The precipitate was collected by centrifugation (14,000 rpm in a microfuge), the pellet was redissolved in 250 μL of diethyl pyrocarbonate-treated water and reprecipitated in 0.3 m sodium acetate with ethanol. The pellet collected after centrifugation was redissolved in 20 μL of diethyl pyrocarbonate-treated water, and the concentration was determined spectrophotometrically (Sambrook et al., 1989). RNA was separated electrophoretically under denaturing conditions (formaldehyde) in 1.2% (w/v) agarose gels according to standard protocols (Sambrook et al., 1989). After capillary blotting, RNA gel blots were hybridized overnight at 65°C with 3,000 μCi [α-32P]dCTP-labeled Arabidopsis PR1 probe (prepared with a Decalabel kit from MBIFermentas, St. Leon-Rot, Germany). After hybridization, blots were washed to a final stringency of 0.1% (w/v) SDS in 0.2× SSC at 65°C, wrapped in plastic foil, and autoradiographed at –80°C using enhancer screens and Hyperfilm MP (Amersham Biosciences, Freiburg, Germany).
SA Determination
SA was extracted and quantified after the modified method of Meuwly and Métraux (1993). Leaf material (50–200 mg) was ground in liquid nitrogen and resuspended in 0.7 mL of 90% (v/v) methanol, to which 500 pmol o-anisic acid had been added as a recovery and internal standard. After addition of 1.4 mL of 100% methanol, the sample was centrifuged (14,000 rpm in a microfuge), and the methanol was removed in a speedvac at 43°C. The residue was brought up to 1 mL with 5% (v/v) trichloroacetic acid on ice and divided into two 500-μL aliquots. One aliquot was used to measure free SA, and the other was hydrolyzed by adding 70 μL of concentrated HCl (12 m) and heating at 96°C for 60 min. The above aliquots were partitioned twice against 1 mL of ethylacetate:cyclohexane:isopropanol (50:50:0.5 [v/v]), the organic phase was taken to dryness in the speedvac, and the residue was redissolved in 200 μL of methanol for HPLC analysis. A sample (20 μL) was chromatographed under isocratic conditions with water:methanol:acetic acid (45:50:5 [v/v]) at 0.8 mL min–1 using an LG-980–02 fitted with an FP920 fluorescence detector (Jasco, Gross-Umstadt, Germany) set at excitation and detection wavelengths of 313 and 405 nm, respectively. The results of a single representative experiment are shown.
SAR
To assess SAR induction, bacterial growth was measured in test leaves by re-isolating bacteria and plating out on selective antibiotic-containing medium. Half leaves were infiltrated with DC3000 cells in 10 mm MgSO4 (105 cfu), and plants were returned to the growth chamber until sampling. Leaf discs (5-mm diameter) were cut from infected leaves using a cork borer and were ground using a mortar and pestle in 10 mm MgSO4. A series of 10-fold dilutions was prepared in 10 mm MgSO4, and aliquots were plated out on King's B (50 μg mL–1 Rif and 30 μg mL–1 Kan) and incubated at 28°C. Experiments were repeated three times.
Acknowledgments
We thank Professor Aart van Bel for providing us with a gift of PCMBS.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021709.
This work was supported by the Deutsche Forschungsgemeinschaft in the Schwerpunktprogramm “Schaderreger und Nutzorganismen” (SPP 716 grant no. JA 830/2–1), and “Genetische und molekulare Aufklärung von Prozessen der Merkmalsausprägung von Nutzpflanzen” (SPP 1005 grant no. Sl 30/1–3) and by the Rheinisch-Westfälische Technische Hochschule Aachen.
References
- Bergey DR, Howe GA, Ryan CA (1996) Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA 93: 12053–12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callos JD, Medford JI (1994) Organ positions and pattern formation in the shoot apex. Plant J 6: 1–7 [Google Scholar]
- Cameron RK (2000) Salicylic acid and its role in plant defense responses: what do we really know? Physiol Mol Plant Pathol 56: 91–93 [Google Scholar]
- Chester KS (1933) The problem of acquired physiological immunity in plants. Q Rev Biol 8: 275–324 [Google Scholar]
- Dean RA, Kuc J (1986) Induced systemic protection in cucumbers: the source of the “signal.” Physiol Plant Pathol 28: 227–233 [Google Scholar]
- Debener T, Lehnackers H, Arnold M, Dangl JL (1991) Identification and molecular mapping of a single Arabidopsis thaliana locus determining resistance to a phytopathogenic Pseudomonas syringae isolate. Plant J 1: 289–302 [DOI] [PubMed] [Google Scholar]
- DeWitt ND, Sussman R (1995) Immunocytological localization of an epitope-tagged plasma membrane proton pump (H+-ATPase) in phloem companion cells. Plant Cell 7: 2053–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamalei Y (1989) Structure and function of leaf minor veins in trees and herbs: a taxonomic review. Trees 3: 96–110 [Google Scholar]
- Grant ME, Godiard L, Straube E, Ashfield T, Lewald J, Sattler A, Innes RW, Dangl JL (1995) Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269: 843–846 [DOI] [PubMed] [Google Scholar]
- Guedes MEM, Richmond S, Kuc J (1980) Induced systemic resistance to anthracnose in cucumber as influenced by the location of the inducer inoculation with Colletotrichum lagenarium and the onset of flowering and fruiting. Physiol Plant Pathol 17: 229–233 [Google Scholar]
- Haritatos E, Medville R, Turgeon R (2000) Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211: 105–111 [DOI] [PubMed] [Google Scholar]
- Ho LC, Peel AJ (1969) Transport of 14C-labelled assimilates and 32P-labelled phosphate in Salix viminalis in relation to phyllotaxis and leaf age. Ann Bot 33: 743–751 [Google Scholar]
- Imlau A, Truernit E, Sauer N (1999) Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenns AE, Kuc J (1977) Localized infection with tobacco necrosis virus protects cucumber against Colletotrichum lagenarium. Physiol Plant Pathol 11: 207–212 [Google Scholar]
- Jenns AE, Kuc J (1979) Graft transmission of systemic resistance of cucumbers to anthracnose induced by Colletotrichum lagenarium and tobacco necrosis virus. Phytopathology 69: 753–756 [Google Scholar]
- Jones CG, Hopper RF, Coleman JS, Krischik VA (1993) Control of systemically induced herbivore resistance by plant vascular architecture. Oecologia 93: 452–456 [DOI] [PubMed] [Google Scholar]
- Joy KW (1964) Translocation in sugar beet: I. Assimilation of 14CO2 and distribution of materials from leaves. J Exp Bot 15: 485–494 [Google Scholar]
- King EO, Ward MK, Raney DR (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301–307 [PubMed] [Google Scholar]
- Kuc J (1982) Induced immunity to plant disease. BioScience 32: 854–860 [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I (1990) Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250: 1002–1004 [DOI] [PubMed] [Google Scholar]
- Malamy J, Sanchez-Casas P, Hennig J, Guo A, Klessig DF (1996) Dissection of the salicylic acid signaling pathway in tobacco. Mol Plant-Microbe Interact 9: 474–482 [Google Scholar]
- Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK (2002) A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419: 399–403 [DOI] [PubMed] [Google Scholar]
- Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverard B (1990) Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 250: 1004–1006 [DOI] [PubMed] [Google Scholar]
- Meuwly P, Métraux JP (1993) Ortho-anisic acid as internal standard for the simultaneous quantitation of salicylic acid and its putative biosynthetic precursors in cucumber leaves. Anal Biochem 214: 500–505 [DOI] [PubMed] [Google Scholar]
- Mölders W, Buchala A, Métraux JP (1996) Transport of salicylic acid in tobacco necrosis virus-infected cucumber plants. Plant Physiol 112: 787–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Duckett CM, Prior DAM, Fisher DB (1994) Real-time imaging of phloem unloading in the root tip of Arabidopsis. Plant J 6: 759–766 [Google Scholar]
- Rasmussen JB, Hammerschmidt R, Zook MN (1991) Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae. Plant Physiol 97: 1342–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF (1966) Systemic effects of local lesion formation. In ABR Beemster, J Dijkstra, eds, Viruses of Plants. North-Holland, Amsterdam, pp 127–150
- Ryals JA, Neuenschwander UH, Willitis MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Santa Cruz S (1999) Perspective: phloem transport of viruses and macro-molecules: what goes in must come out. Trends Microbiol 7: 237–240 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Leon J, Raskin I (1995) Is salicylic acid a transported signal of systemic acquired resistance in tobacco? Plant Cell 7: 1691–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Sauer N (1996) The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Bot Acta 109: 299–306 [Google Scholar]
- Taiz L, Zeiger E (1998) Translocation in the phloem. In Plant Physiology. Sinauer Associates, Sunderland, MA, pp 251–285
- Uknes S, Mauch-Mani B, Moyer M, Potter S, Williams S, Dincher S, Chandler D, Slusarenko A, Ward E, Ryals J (1992) Acquired resistance in Arabidopsis. Plant Cell 4: 645–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel AJE, Ammerlaan A, van Dijk AA (1994) A three-step screening procedure to identify the mode of phloem loading in intact leaves. Planta 192: 31–39 [Google Scholar]
- Van Loon LC (1983) The induction of pathogenesis-related proteins by pathogens and specific chemicals. Neth J Plant Pathol 89: 265–273 [Google Scholar]
- Van Loon LC (2000) Systemic induced resistance. In AJ Slusarenko, RSS Fraser, LC van Loon, eds, Mechanisms of Resistance to Plant Diseases. Kluwer Academic Press, Dordrecht, The Netherlands, pp 521–574
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J (1994) Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. Plant Cell 6: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF (1979) Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology 99: 410–412 [DOI] [PubMed] [Google Scholar]
- Wildon DC, Thain JF, Minchin PEH, Gubb IR, Reilly AJ, Skipper YD, Doherty HM, O'Donnell PJ, Bowles DJ (1992) Electrical signalling and systemic proteinase inhibitor induction in the wounded plant. Nature 369: 62–65 [Google Scholar]
- Yalpani N, Silverman P, Wilson TMA, Kleier DA, Raskin I (1991) Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell 3: 809–818 [DOI] [PMC free article] [PubMed] [Google Scholar]