Abstract
Hsp70s function as molecular chaperones. The protective chaperone activities of hsp70 help to confer tolerance to heat, glucose deprivation, and drought. Overexpression of hsp70s in many organisms correlates with enhanced thermotolerance, altered growth, and development. To better understand the roles of hsp70 proteins in Arabidopsis, the molecular and physiological consequences of altered expression of the major heat shock cognate, Hsc70-1, were analyzed. Extensive efforts to achieve underexpression of Hsc70-1 mRNA using a full-length antisense cDNA resulted in no viable transgenic plants, suggesting that reduced expression is lethal. Constitutive overexpression of Hsc70-1 also appeared to be deleterious to viability, growth, and development because fewer transformants were recovered, and most were dwarfed with altered root systems. Despite being dwarfed, the overexpression plants progressed normally through four selected developmental stages. Heat treatment revealed that Hsc70-1 overexpression plants were more tolerant to heat shock (44°C for 10 min). The elevated basal levels of HSC70-1 in transgenic plants led to delayed heat shock response of several heat shock genes. The data in this study suggest that tight regulation of Hsc70-1 expression is critical for the viability of Arabidopsis and that the functions of HSC70-1 contribute to optimum growth, development, thermotolerance, and regulation of the heat shock response.
Hsp70 functions as a molecular chaperone by helping newly synthesized proteins fold properly, preventing unfolded proteins from undergoing nonproductive aggregation and maintaining an extended conformation of proteins during translocation (Feldman and Frydman, 2000; Sung et al., 2001a). As a component of protein import mechanisms, hsp70 facilitates import of precursor proteins by establishing directional movement into the organelles (Matouschek et al., 2000; Jackson-Constan et al., 2001). These chaperone functions may involve not only the repetitive cycles of binding and the release of substrate peptides but also the active twisting of peptide bonds because it operates as a secondary amide peptide bond cis-trans isomerase (Schiene-Fischer et al., 2002).
Molecular chaperone functions of hsp70 are important in thermotolerance. The expression of hsp70 genes positively correlates with the acquisition of thermotolerance (Feder et al., 1996; Lee and Schöffl, 1996; Nollen et al., 1999), and the overexpression of hsp70 often results in enhanced thermotolerance (Feder et al., 1996; Nollen et al., 1999). However, the cellular mechanisms of thermoprotection by hsp70 are not fully understood. In some cases, interaction of hsp70 with individual enzymes confers enhanced stability (Anwar et al., 2002). In other cases, hsp70 modulates the activity of signal transducers and/or transcriptional factors such as HSF1, protein kinase A, protein kinase C, and protein phosphatase (Ding et al., 1998), thus potentially acting to modulate the expression of a large number of downstream genes in signal transduction pathways. Overexpression of hsp70 also confers resistance to oxygen and Glc deprivation (Papadopoulos et al., 1996), protection against neurodegeneration (Cummings et al., 2001), and hydrogen peroxide (Echave et al., 2002). It also promotes life span extension of Caenorhabditis elegans (Yokoyama et al., 2002) and human (Homo sapiens) cells (Kaula et al., 2000).
Hsp70 is involved in regulation of the general heat shock response. Interaction between hsp70 and HSF has been suggested as a negative regulatory mechanism for HSF-mediated transcriptional activation before, after, and during the heat shock response (Morimoto, 1998). In this hypothesis, hsp70 binds to HSF, thereby preventing trimerization, binding of HSF to HSE, and transcriptional activation of heat shock genes by HSF. Repression of heat shock response by overexpression of mammalian hsp70 homologs (Mosser et al., 1993; Baler et al., 1996; Shi et al., 1998) and prolonged complex formation of HSF:HSE in hsp70 underexpression plants (Lee and Schöffl, 1996) support this hypothesis.
Successful alteration of hsp70 expression level in plants has been reported. Lee and Schöffl (1996) reduced expression of cytosolic hsp70s in Arabidopsis during heat shock by introducing antisense mRNA of a tobacco (Nicotiana tabacum) hsp70 under the control of a soybean (Glycine max) heat shock promoter. Due to the reduced expression of hsp70s, transgenic plants lost their induced thermotolerance and showed a prolonged heat shock response (Lee and Schöffl, 1996). Overexpression of an endogenous endoplasmic reticulum (ER)-localized hsc70 homolog, BiP (BLP4), in tobacco alleviated ER stress imposed by treatment of tunicamycin, a protein glycosylation inhibitor (Leborgne-Castel et al., 1999), whereas overexpression of a soybean BiP gene in tobacco produced transgenic plants resistant to water stress and to tunicamycin treatment (Alvim et al., 2001). When an endogenous chloroplast-localized hsp70 (HSP70B) was overexpressed in Chlamydomonas reinhardtii, reactivation of PSII after photoinhibition was enhanced, whereas underexpression resulted in reduced reactivation of PSII after photoinhibition (Schroda et al., 1999). These studies confirm that altering expression of individual members of the hsp70 family is quite feasible in plants. The different phenotypic changes obtained in these studies suggest that hsp70s localized to different subcellular compartments participate in different cellular processes.
Arabidopsis has five full-length cytosolic hsp70s and a sixth that is truncated in the C-terminal domain (Lin et al., 2001; Sung et al., 2001b). It is expected to be a challenging task to decipher roles of individual hsp70 proteins due to the high degree of functional conservation and the high degree of substrate promiscuity of hsp70. It is possible that loss of function of a single hsp70 can be easily compensated by other colocalized hsp70s. Furthermore, even when its function is not replaceable, the high level of substrate promiscuity of hsp70 could cause pleiotropic phenotypes that make it difficult to pinpoint the immediate beneficiaries of hsp70 functions in the cell. Considering the functional redundancy of individual hsp70 proteins, the specific roles of individual hsp70 proteins in organisms are likely to be determined by their location in different subcellular compartments, by differential expression of hsp70 genes in specific cells (Michaud et al., 1997) and at different stages of development (Sung et al., 2001b), or by interacting with specific sets of hsp70-associated proteins (May and Soll, 2000).
Previously, we assessed functions of hsp70s in Arabidopsis by analyzing expression patterns of the hsp70 gene family using a quantitative reverse transcriptase (RT)-PCR analysis (Sung et al., 2001b). In the present study, we generated transgenic Arabidopsis over-/underexpressing the major cytosolic hsc70, Hsc70-1, as a first step to identify functions of HSC70-1 by analyzing molecular and physiological consequences of altered expression of Hsc70-1. The findings indicate that tight regulation of Hsc70-1 is necessary in Arabidopsis because altering expression of Hsc70-1 brought negative consequences to plant growth and viability. A delayed heat shock response and enhanced thermotolerance in Hsc70-1 overexpression (Hsc70-1 OE) plants revealed a protective function against heat stress and a regulatory role in heat shock gene expression during heat stress.
RESULTS
Plant Transformation
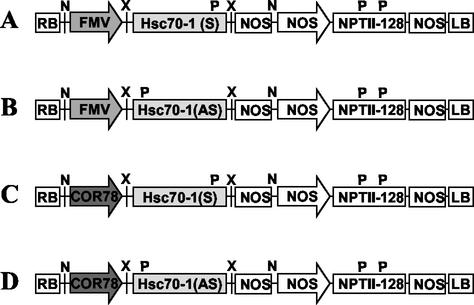
Plants were transformed with four different constructs (Fig. 1). PCR screening for primary transformants revealed that the frequency of transformation is distinct for each construct (Table I). Empty vector was transformed with an efficiency of 0.62%. However, no primary transformants for constitutive underexpression of Hsc70-1 were obtained after extensive kanamycin screening. This suggests that constitutive expression of a full-length Hsc70-1 antisense RNA may be lethal. The inducible overexpression construct, however, showed an equal efficiency to the empty vector control (Table I). This suggests that the harmful effects of the constitutive overexpression construct resulted from ectopic expression of Hsc70-1, not from a cosuppression mechanism due to the presence of the Hsc70-1 construct in the genome. In addition, low transformation efficiency for the constitutive overexpression construct (approximately 30 times lower compared with the empty vector construct) indicates overexpression of Hsc70-1 is also detrimental. Inducible overexpression/underexpression of Hsc70-1 was attempted by using a cold-inducible promoter (Cor78/Rd29A). Transformants for inducible overexpression were easily obtained with transformation efficiency comparable with that of the empty vector (Table I). However, growing plants at low temperature (4°C) to induce the transgene expression complicated plant growth and development, making assessment of phenotypic consequences of inducible expression of transgenes unreliable. A search for a more suitable inducible system is currently under way.
Figure 1.
Transformation constructs for Hsc70-1. A, pHK-Hsc70-1 (S); B, pHK-Hsc70-1 (AS); C, pHKCOR-Hsc70-1 (S); D, pHKCOR-Hsc70-1 (AS). RB, Right border of T-DNA; FMV, figwort mosaic virus promoter; Cor78, the promoter of a cold-inducible gene, Cor78/rd29A; Hsc70-1 (S) and Hsc70-1 (AS), the full-length Hsc70-1 cDNA in sense or antisense orientation, respectively; NOS, NOS terminator or promoter; NPTII-128, an NPT gene; LB, left border of T-DNA; K, KpnI, N, NotI, P, PstI, X, XbaI.
Table I.
Transformation efficiency of Hsc70-1 constructs
| Construct | No. of Seedlings Screeneda | No. of PCR-Positive Plants | Transformation Efficiency |
|---|---|---|---|
| % | |||
| pHK-Hsc70-1 (S) | 50,000 | 8 | 0.02 |
| pHK-Hsc70-1 (AS) | 52,500 | 0 | 0.00 |
| pHKCOR-Hsc70-1 (S) | 15,000 | 95 | 0.63 |
| pHKCOR-Hsc70-1 (AS) | 15,000 | 37 | 0.25 |
| pHK (vector control) | 5,000 | 31 | 0.62 |
Seeds harvested from transformed plants were germinated on Murashige and Skoog medium with 30 μg mL-1 kanamycin. Green seedlings on kanamycin plates were then subjected to PCR screening for the presence of T-DNA
Integration and Expression of Transgenes
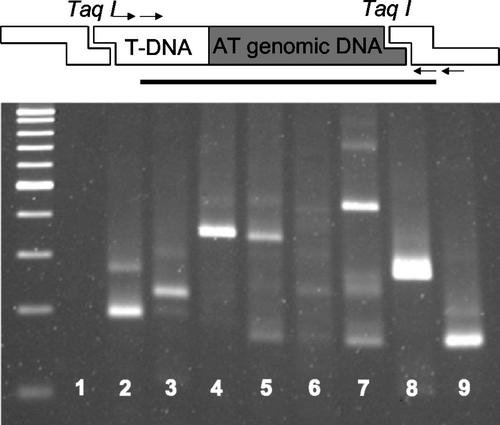
A modified adaptor ligation PCR technique was used to confirm integration of T-DNA into the Arabidopsis genome as an alternative to Southern-blot analysis (Spertini et al., 1999). This technique yields a different length of PCR product for each insertion event because the length of the genomic DNA fragment flanking T-DNA on a restriction fragment is specific to the integration site (Fig. 2). Except for the wild-type Arabidopsis, all lines showed single or multiple bands of different sizes, suggesting independent T-DNA integration events for each line.
Figure 2.
Adaptor ligation PCR for Hsc70-1 overexpression (Hsc70-1 OE) lines. Lane 1, Wild-type Arabidopsis; lanes 2 to 9, lines 8-2, 8-3, 8-4, 8-5, 8-7, 8-9, 8-10, and 8-11, respectively. Arrows indicate the positions of primers. The solid line under the diagram indicates PCR product after two rounds of adaptor ligation PCR.
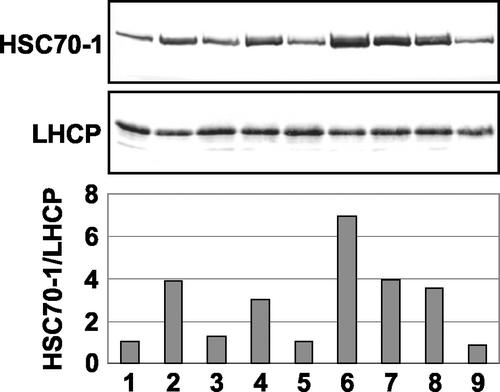
Western-blot analysis of eight Hsc70-1 OE lines identified several lines containing a higher level of HSC70-1 protein compared with empty vector-transformed plants (pHK; Fig. 3). Overexpression lines 8-2, 8-4, 8-9, and 8-10 showed an approximately 4-fold increase in the amount of protein. Line 8-7 had the highest level of protein, 8-fold compared with wild-type plants. Lines 8-3, 8-5, and 8-11 showed little or no difference in the amount of protein compared with empty vector-transformed plants.
Figure 3.
Western-blot analysis for Hsc70-1 OE lines. Lane 1, Empty vector-transformed plant; lanes 2 to 9, lines 8-2, 8-3, 8-4, 8-5, 8-7, 8-9, 8-10, and 8-11, respectively. A light-harvesting complex protein (LHCP) was used as a loading control. The level of HSC70-1 was determined using a monoclonal antibody 5B7, and the level of LHCP was determined using a polyclonal antibody from Dr. Kenneth C. Cline. The intensity of protein bands was quantified using Scion Image for Windows (http://www.scioncorp.com), and the ratio of HSC70-1 to LHCP was calculated using Excel (Microsoft, Redmond, WA). A representative blot is shown.
Increased Thermotolerance of Hsc70-1 OE Plants
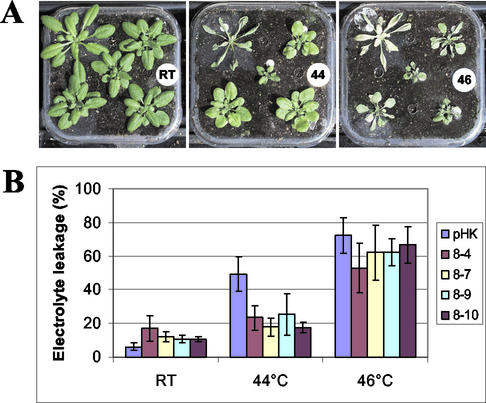
Four strong Hsc70-1 OE lines (8-4, 8-7, 8-9, and 8-10) were selected for further analysis. Thermotolerance of the four lines was tested by giving a 10-min heat shock in water at temperatures ranging from 42°C to 48°C. Only the samples treated with room temperature, 44°C, and 46°C were shown in Figure 4B. These four lines showed lower electrolyte leakage and survived a 10-min heat shock at 44°C (Fig. 4, A and B). Empty vector-transformed plants showed greater electrolyte leakage and showed no sign of survival 3 d after heat shock (Fig. 4B). At the temperatures below or above 44°C, no difference in thermotolerance was observed in the four overexpression lines compared with empty vector-transformed plants (Fig. 4, A and B).
Figure 4.
Thermotolerance of Hsc70-1 OE lines. A, Deli pot culture system is shown. The plant in the top left corner of each picture is wild-type Arabidopsis. The other four plants are randomly arrayed from the four selected Hsc70-1 overexpression lines. B, Thermotolerance of Hsc70-1 OE lines assessed by electrolyte leakage assay. pHK, Empty vector-transformed Arabidopsis. Means and sd are indicated (n = 8).
Altered Development of Hsc70-1 OE Plants
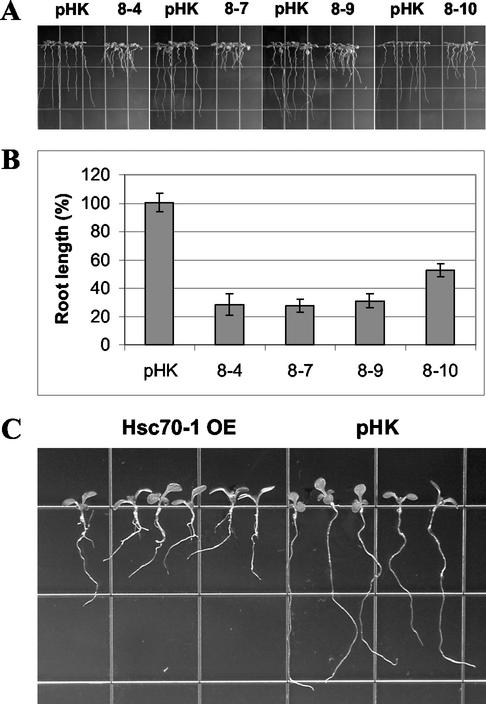
It was noticed during screening of primary transformants that numerous kanamycin-resistant seedlings of Hsc70-1 OE plants had shorter and more branched root systems than empty vector-transformed plants (pHK). To recapitulate this phenomenon without kanamycin treatment, seeds of the overexpression lines were planted on agar plates and grown vertically to monitor root growth and development. One week after growing on vertical plates, root length was measured. The length of the primary root was reduced in Hsc70-1 OE plants compared with empty vector-transformed plants (Fig. 5). Also, the overexpression lines showed early branching of lateral roots (Fig. 5A). Early lateral root formation primarily occurred in the upper part of the root system (Fig. 5A). The identical results were obtained when seedlings were grown on horizontal Murashige and Skoog media indicating that alterations of root system in the overexpression lines are robust (Fig. 5C).
Figure 5.
Root growth of Hsc70-1 OE lines. A, Two-week-old seedlings grown vertically on Murashige and Skoog media were digitally photographed. pHK represents the empty vector-transformed Arabidopsis. B, The percentage of root length was calculated based on the root length of the empty vector-transformed Arabidopsis (pHK). Means and sd are indicated (n = 8). C, Ten-day-old seedlings grown on horizontal Murashige and Skoog media were picked out and laid on the Murashige and Skoog media for photography. Note shorter primary roots and early branching of lateral roots in Hsc70-1 OE compared with the empty vector-transformed plants (pHK).
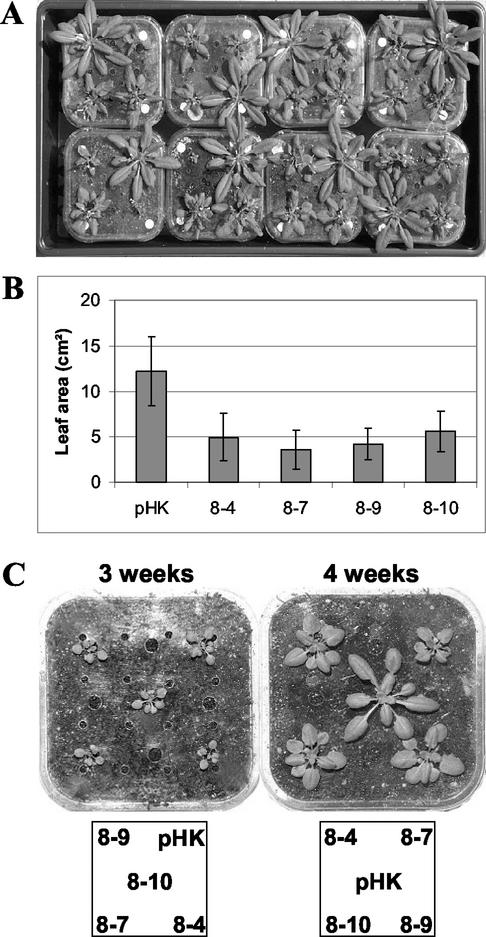
In addition to short and branched root systems, the Hsc70-1 OE plants had significantly smaller aerial parts (leaf, stem, and flower) compared with empty vector-transformed plants (Fig. 6). However, this difference only became apparent at 4 weeks after imbibition (Fig. 6B). These alterations in growth and development appear specific to Hsc70-1 because overexpression of an ER luminal hsc70, BiP-2 (data not shown), and Hsp101 (Queitsch et al., 2000) in Arabidopsis did not show any of these phenotypes.
Figure 6.
Comparative size of Hsc70-1 OE lines. A, The largest plant in each pot is the empty vector-transformed Arabidopsis. The other three plants are randomly arrayed of the four Hsc70-1 OE lines. B, Leaf area of each line at 5 weeks after imbibition. Mean and sd are indicated (n = 8). C, Pictures were taken at 3 and 4 weeks after imbibition. The squares below the pictures illustrate the location of each line in the pot. Note larger size of the empty vector-transformed plant compared with Hsc70-1 OE at 4 weeks after imbibition.
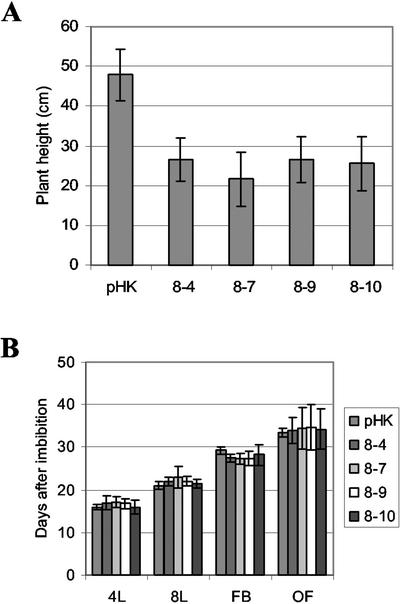
The overexpression plants have smaller stature compared with empty vector-transformed plants (Fig. 7A). However, the number of days to reach each of four selected stages was indistinguishable from the empty vector-transformed plants showing the aerial parts of Hsc70-1 OE plants, and empty vector-transformed plants progressed through different stages of development at the same rate (Fig. 7B). To test whether smaller plant size resulted from any defects in electron transfer of photosynthesis, chlorophyll fluorescence was analyzed with the same plants shown in Figure 6A. Almost identical values of maximum photochemical efficiency of PSII in the dark-adapted state (0.86 ± 0.01, n = 18–20) were obtained from the empty vector-transformed plants and Hsc70-1 OE plants, indicating electron transfer of photosynthesis in Hsc70-1 OE plants was not compromised.
Figure 7.
Growth and development of Hsc70-1 OE lines. A, Plant height measured at 8 weeks after imbibition. pHK, Empty vector-transformed Arabidopsis. 4L, 8L, FB, and OF represent defined stages of plant development at four true leaves, eight true leaves, the first bolting, and the first open flower, respectively. The number of days taken to reach each stage is plotted in B. Means and sd are indicated (n = 25–30).
Delayed Heat Induction in Hsc70-1 OE Plants
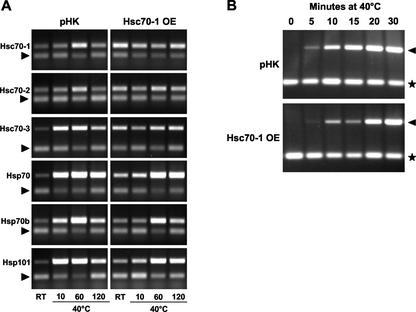
To analyze the effect of Hsc70-1 overexpression on other closely related heat shock genes, the expression of five cytosolic members of the hsp70 gene family and Hsp101 was monitored in Hsc70-1 OE plants. All five members showed maximum induction within 60 min of heat treatment at 40°C (Fig. 8A). Two significant differences between the empty vector-transformed plants (pHK) and Hsc70-1 OE plants (8-7) were observed. First, Hsc70-1 OE plants showed moderately elevated expression of four other cytosolic hsp70 genes and Hsp101 in the absence of heat shock (Fig. 8A). Second, the induction after 10 min of heat treatment at 40°C was reduced in Hsc70-1 OE plants compared with the empty vector-transformed plants (Fig. 8A). The expression of Hsp101 was further analyzed with more time points during the first 30 min of heat treatment to study the induction kinetics of the heat shock response in Hsc70-1 OE plants in detail. Total RNA samples from empty vector-transformed plants and another Hsc70-1 OE line (8-10) were isolated at 0, 5, 10, 15, 20, and 30 min at 40°C and subjected to RT-PCR analysis. The expression of Hsp101 in empty vector-transformed plants (pHK) was strongly induced even after 5 min at 40°C and reached peak expression between 20 and 30 min at 40°C (Fig. 8B). However, the induction of Hsp101 was slower in the Hsc70-1 OE plant (8-10), but the time required to reach the peak expression remained unchanged (Fig. 8B).
Figure 8.
RT-PCR analysis of Hsc70-1 OE lines. Three-week-old plants were given heat shock for indicated time (minutes) at 40°C. A, Gel pictures for the empty vector-transformed plant (pHK) and an Hsc70-1 OE line (8-7). In each picture, the upper band is the RT-PCR amplicon from each heat shock gene tested, and the lower band is the 18S rRNA RT-PCR amplicon as an internal loading control (arrow). 18S rRNA amplicons were generated by using a primer mix with the normal primer pairs:3′-modified primer pairs (2:8 [v/v]). B, Three-week-old plants were given heat treatment of 0, 5, 10, 15, 20, and 30 min at 40°C, and then total RNA was isolated for RT-PCR analysis. The expression patterns of wild-type Arabidopsis and a strong overexpresser (Hsc70-1 OE; 8-10) are shown. In each gel image, the upper band is the Hsp101 RT-PCR amplicon (triangle) and the lower band is the 18S rRNA RT-PCR amplicon (star) as a loading control.
DISCUSSION
Maintenance of the Level of HSC70-1 in the Cell Is Critical to Cell Viability
Transformation of pHK-Hsc70-1 (AS) yielded no viable transformants. This suggests that constitutive antisensing of Hsc70-1 alone or, alternatively, that global suppression of all cytosolic members resulted in the lethal phenotype. We have identified several hsp70 T-DNA insertion mutants, including one for Hsc70-1 (SALK_135531) in the Salk T-DNA insertion stocks. T-DNA in SALK_135531 is inserted in the second exon of Hsc70-1 and should render the gene product nonfunctional. Seed for SALK_135531 is not available yet. Once we obtain homozygous lines (or inability to obtain homozygous lines) for SALK_135531, we can address whether the knockout of Hsc70-1 alone is sufficient to render a lethal phenotype. Considering that the transformation constructs were generated using a full-length cDNA of Hsc70-1 encoding highly conserved domains of all hsp70s (Sung et al., 2001a), it is possible that global antisensing on several, if not all, cytosolic hsp70s may have produced the lethal phenotype.
Constitutive overexpression of Hsc70-1 appears to be deleterious to plant viability given the lower transformation efficiency compared with that of the empty vector control. Similar findings have been obtained from overexpression of bacterial hsc70, DnaK, in Escherichia coli (Blum et al., 1992) and Synechococcus sp. strain PCC7942 (Nimura et al., 2001). In these systems, overexpression of DnaK resulted in growth arrest and inhibition of cell septation at permissive temperatures (Blum et al., 1992; Nimura et al., 2001). Therefore, prolonged interaction of hsp70 with substrates may be equally harmful to optimal cell functioning as inadequate or premature termination of the interaction.
Increased Level of HSC70-1 in the Overexpression Lines Resulted in Enhanced Thermotolerance
Thermotolerance is a quantitative trait (Ottaviano et al., 1991). However, knockout mutant studies of Hsp101 in Arabidopsis (Hong and Vierling, 2000) and maize (Zea mays; Nieto-Sotelo et al., 2002) demonstrated that alteration of a single gene could result in significant changes in thermotolerance. In the present study, we showed that overexpression of Hsc70-1 promotes enhanced thermotolerance at the whole-plant level. However, some of the enhanced thermotolerance may have been contributed from the activation of other heat shock genes. This is suggested by elevated basal expression of other cytosolic hsp70 genes and Hsp101 in Hsc70-1 OE lines.
Increased Level of HSC70-1 Altered Plant Growth and Development
Altered root systems and smaller aerial parts of the overexpression plants suggest HSC70-1 plays important roles in plant growth and development. The modification of the root system in the overexpression plants indicates potential perturbation of hormonal signal transduction or nutrient utilization. Enhanced lateral root formation is frequently observed when Arabidopsis is grown in a low-phosphate or a high-nitrate environment (Zhang and Forde, 2000; López-Bucio et al., 2002). Further studies are necessary to determine whether nutrient uptake and/or utilization is altered in Hsc70-1 OE plants. Alteration in root growth and development was obvious 1 week after imbibition, whereas smaller plant size became evident at 4 weeks after imbibition. Whether the smaller plant size is a consequence of an altered root system is not clear, but it is a possibility.
Delayed Heat Shock Response in Hsc70-1-OE Arabidopsis
A delayed heat shock response was observed in the expression pattern of Hsp101 and five hsp70 genes in overexpression lines. This suggests that the delay of the heat shock response did not result from a sequence-based cosuppression (i.e. gene silencing) limited to Hsc70-1-related genes but resulted from alteration of a common regulatory mechanism (likely HSFs). The repression of the heat shock response by hsp70 has been shown in overexpression studies of mammalian hsp70 homologs (Mosser et al., 1993; Baler et al., 1996; Shi et al., 1998), and it is suggested that the delayed heat shock response results from the negative regulation of HSF by HSC70-1 in Arabidopsis (Lee and Schöffl, 1996; Kim and Schöffl, 2002). The presence of the HSF:HSE complex was detected for a longer period of time in inducible lines for Hsp70/Hsc70 underexpression compared with wild-type Arabidopsis plants, indicating that a reduced level of Hsp70 rendered more HSF available for binding to HSE (Lee and Schöffl, 1996). More recently, a physical interaction between HSC70-1 and HSF1 protein was shown in vivo through electrophoretic mobility shift assay and yeast two-hybrid assay (Kim and Schöffl, 2002). Therefore, it is tempting to speculate that the delayed heat shock response most likely comes from prolonged interaction between HSF and HSC70-1. This would explain the lower induction of heat shock genes during the first 20 min of heat shock. However, the same level of the maximum induction of heat shock genes was achieved by 30 min of heat shock in Hsc70-1 OE plants. This shows that a sustained heat shock (>20 min) can override the repression of heat shock response by overexpression of Hsc70-1.
If the delayed heat shock response indeed resulted from the prolonged interaction between HSC70-1 and HSF, one puzzling question is: “How was the elevated basal expression of other heat shock genes possible if HSF was tied up with HSC70-1 in the absence of heat shock?” A subfamily of HSFs (HsfBs) in Arabidopsis has no transactivation activity. HsfBs are suspected to act as transcriptional repressors by occupying HSEs in the promoter (Czarnecka-Verner et al., 2000) under non-heat shock condition. One possible explanation is that a prolonged complex formation between HSC70-1 and HsfBs in Hsc70-1 OE plants makes the promoters of the heat shock genes more accessible for other transcription factors, including active HSFs. Increased interaction between HsfBs and HSC70-1, therefore, could result in the elevated basal expression of the heat shock genes. Whether interaction of HsfBs with HSC70-1 was prolonged in Hsc70-1 OE plants needs to be determined. Overexpression of Hsc70-1 also resulted in reduced accumulation of heat-induced SUMO-conjugated proteins (Kurepa et al., 2003). A detailed mechanism of SUMO conjugation during stress is not firmly established yet but presumed to protect stress-denatured proteins by transiently tagging stress denatured proteins, thereby preventing ubiquitin-mediated degradation of the proteins (Kurepa et al., 2003). Their study suggests that overexpression of Hsc70-1 may affect protein stability during stress and the activities of HSFs.
CONCLUSION
Constitutive overexpression of Hsc70-1 resulted in several distinct phenotypes. At minimum, this study confirmed that altered expression of a single hsp70 gene could penetrate the potentially highly redundant functional background of cytosolic hsp70s and produce phenotypic consequences. Finding no viable transgenic lines for constitutive underexpression of Hsc70-1 suggests that reduction of Hsc70-1 expression is detrimental to plant viability. Constitutive overexpression of Hsc70-1 enhanced basal thermotolerance, affected plant size, and altered the root system. The overexpression also delayed the heat shock response of several heat-inducible genes probably through prolonged interaction with Arabidopsis HSFs. This study revealed that HSC70-1 is linked with enhanced thermotolerance and influences regulatory functions on the expression of multiple genes during heat stress. The small stature and altered root system of Hsc70-1 overexpression plants revealed that the functioning of HSC70-1 is pivotal in normal growth and development under non-stress conditions.
MATERIALS AND METHODS
Plant Growth
Arabidopsis (ecotype Columbia) plants were grown on an autoclaved commercial soil mix (Fafard mix no. 2) containing Canadian sphagnum peat, perlite, and vermiculite. Seeds were sown through holes made in the lids of clear delicatessen containers (a gift from Publix Super Markets Inc., Lakeland, FL). Plants grew better in this container due to fewer impediments on plant growth from fungus gnats and mold on the soil. It was also convenient to administer heat treatment where only the aerial portion of whole plants was immersed in a water bath. Plants were grown at 20°C with a photoperiod of 15 h of light/9 h of dark in Percival growth cabinets (Percival Scientific, Inc., Perry, IA). Irradiance was approximately 150 μmol m-2 s-1 at canopy height and was provided by incandescent bulbs and cool-white fluorescent tubes.
Plant Transformation
The proprietary transformation vector (pHK1001) obtained from Dr. Harry Klee with consent from Monsanto Co. (St. Louis) was used to generate Hsc70-1 constitutive and inducible expression constructs (Fig. 1). A full-length expressed sequence tag (G9H1T7) for Hsc70-1 was used to prepare the constructs. The final constructs were then transferred to an Agrobacterium tumefaciens strain (ABI) by a triparental mating method. Escherichia coli (DH5α) with pRK2013 was used as the helper cell. For plant transformation, a modified version of the vacuum infiltration method was used (Bent et al., 1994). Pots with infiltrated plants were laid on their side in a plastic flat, then covered with clear plastic wrap for 1 d. After 1 d, pots were set upright and covered with a gallon-sized freezer bag to maintain humidity and prevent cross-pollination. After 3 to 4 weeks, seeds from the same pot were harvested together. Seeds were surface sterilized and 100 μL of seeds (approximately 2,500 seeds) was plated on selection media. After 7 to 14 d, transformants were evident as green seedlings.
PCR Screening of Transformants
Putative transformants obtained after kanamycin selection were subjected to PCR screening. Primers specific to promoters (figwort mosaic virus) and Hsc70-1 were designed to amplify the segments of the transgenes in kanamycin-resistant plants. A primer pair for each promoter and construct produced a distinct-sized PCR product. Genomic DNA from each transformant was extracted as described (Li and Chory, 1998). Three microliters of genomic DNA was amplified with 10 pmol of each primer by 35 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min. Each PCR-positive line was identified by gel electrophoresis. The numbers 4 and 8 were assigned to designate pHK-Hsc70-1 (AS) and pHK-Hsc70-1 (S) transgenic lines, respectively. All transgenic lines analyzed in this study were either T3 or T4 generation. The line 8-9 was confirmed as a homozygote, and the rest of the lines were hemizygotes for the transgene.
Adaptor Ligation PCR
Genomic DNA (1 μg) was digested with a 4-bp restriction enzyme, TaqI, to generate smaller restriction fragments. Two adaptor primers (CG336, 5′-ctaatacgactcactatagggctcgagcggccgggcaggt-3′; and CG337, 5′-gcacctgcccaa-3′) were ligated by heating the primer solution at 80°C for 2 min followed by a stepwise cool down over 40 min to produce the adaptor that ligates to TaqI digested genomic DNA. Ligation of genomic DNA and the adaptor was carried out as described (Spertini et al., 1999). Adaptor-ligated genomic DNA was then subjected to two rounds of PCR. In the first round of PCR, adaptor-ligated genomic DNA was amplified with an adaptor-specific primer CG338 (5′-atcctctaatacgactcactatagggc-3′) and T-DNA left border primer LB1 (5′-cgcctataaatacgacgga-3′) by seven cycles of 94°C for 30 s, 52°C for 1 min, and 72°C for 3 min followed by 32 cycles of 94°C for 30 s, 56°C for 1 min, and 72°C for 3 min. The PCR product was diluted 50 times and an aliquot of 1 μL was subjected to the second round of PCR. In the second round of PCR, the final PCR amplicons were produced with CG339 (5′-tatagggctcgagcggc-3′) and LB2 (5′-cgctgcggacatctacat-3′), a nested primer set to CG338 and LB1, by 30 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 3 min. PCR products were separated in a 1.5% (w/v) agarose gel.
Western-Blot Analysis on HSC70-1
Total cell extract was prepared by grinding fresh leaf samples from 4-week-old plant in 2× SDS-loading buffer (plant sample:buffer, 1:3 [w/v]). The supernatant obtained after centrifugation at 5,000g for 5 min was separated by SDS-PAGE. The proteins were then transferred onto a polyvinylidene difluoride membrane using a semidry blot transfer cell (Transblot SD, Bio-Rad Laboratories, Hercules, CA) and immunoreacted with a monoclonal antibody raised against spinach (Spinacia oleracea) cytosolic hsc70 (SPA-817, Stressgen, Victoria, BC, Canada). The same polyvinylidene difluoride membrane was cut in the middle and incubated with a polyclonal antibody (a gift from Dr. Kenneth C. Cline, University of Florida, Gainesville, FL) against an LHCP and then simultaneously developed as a loading control. The antibody used for HSC70-1 is specific to cytosolic hsp70 proteins; however, it has not been established whether this antibody reacts specifically to HSC70-1 or to several or all cytosolic hsp70 proteins.
RNA Isolation and RT-PCR
Tissue samples were ground in liquid nitrogen, and total RNA was isolated according to the manufacturer's protocol using the RNeasy plant mini-kit (Qiagen USA, Valencia, CA). The amount of total RNA was determined by UV spectrophotometry. Using commercial RT-PCR beads (Amersham Biosciences, Piscataway, NJ), aliquots of total RNA were reverse transcribed into cDNA with 0.5 μg of random primer, d(N)6, by incubating the reactions at 42°C for 30 min, then amplified with gene-specific primers (10 pmol each) in a 25-μL reaction through 25 cycles of PCR. For each RT-PCR reaction, primers for an 18S rRNA internal standard were included as a loading control. 18S rRNA signals were adjusted by using either 3:7 mix (normal pair of primers: 3′-modified primer pairs) or 2:8 mix to best balance the 18S rRNA signal to that of the target mRNA. PCR parameters were as follows: 94°C for 4 min for initial denaturation, 25 cycles of 94°C for 30 s, 60°C for 1 min, 72°C for 2 min, and 72°C for 10 min for the final extension before holding the reaction at 6°C. The annealing temperature for Hsc70-2 was 52°C and 60°C for Hsc70-1 and all other genes analyzed in this study. The forward primer for Hsp101 is 5′-agcaatctctagtgccggtg-3′, and the reverse primer is 5′-aagcgttgtagcaccaatgc-3′. Primers for Hsc70-1, Hsc70-2, Hsc70-3, Hsp70, and Hsp70b were previously described (Sung et al., 2001b).
Phenotypic Characterization Assays. Thermotolerance, Leaf Area, Root Growth, Chlorophyll Fluorescence, and Growth and Development Monitoring
Thermotolerance assays were conducted by inverting the containers over water baths allowing the aerial portion of 4-week-old plants to be immersed to the soil line at temperatures of 42°C, 44°C, 46°C, 48°C, and 50°C for 10 min. Electrolyte leakage of the aerial portion of a plant was measured 3 d after heat treatment. The aerial portion of a plant was cut and immersed in a vial with 10 mL of distilled, deionized water. Samples were shaken for 1 h at room temperature, and ion leakage measurements were taken. Samples were then heated to boiling in a microwave for 2 min, shaken for 1 h at room temperature, and total ion leakage measurements were taken. With the two measurements, percentage of electrolyte leakage was determined for each sample.
For leaf area assays, 5-week-old plants were digitally photographed and the aerial portion (in pixels) was quantified using Adobe Photoshop (Adobe Systems, San Jose, CA). The roots of plants vertically grown on Murashige and Skoog media for 2 weeks were measured for root growth. To monitor growth and development of plants, the number of days taken to reach four selected parameters (four true leaves, eight true leaves, first bolting, and first open flower) was recorded.
Electron transfer in PSs was measured by recording chlorophyll fluorescence parameters with the Plant Efficiency Analyser (Hansatech Instruments, King's Lynn, UK). Leaves of 5-week-old plants were given a 10-min dark adaptation period, then measurements were taken over a 5-s interval after exposure at the 100% illumination level by high-intensity light-emitting diodes. Five replicates, each from a different plant, were averaged for each time point. Variable fluorescence was determined as the difference between the maximal fluorescence signal and the initial darkness fluorescence level.
Acknowledgments
We thank Dale Haskell for developing the thermotolerance assay system. We appreciate Drs. William B. Gurley, David Clark, and Kiljae Lee providing critical reviews of this manuscript. Special thanks go to Dr. Kenneth C. Cline for providing access to the gel documentation system and LHCP antibody and to the Arabidopsis Biological Resource Center for providing a full-length expressed sequence tag clone for Hsc70-1.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019398.
This work was supported by the Florida Agricultural Experiment Station, by the University of Florida Plant Molecular and Cellular Biology Program, and by the U.S. Department of Agriculture National Research Initiative (grant nos. 98–35100–6147 and 00–35100–9532 to C.L.G.). This is Florida Agricultural Experiment Station Journal Series No. R–09359.
References
- Alvim FC, Carolino SM, Cascardo JC, Nunes CC, Martinez CA, Otoni WC, Fontes EP (2001) Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol 126: 1042-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar A, Siegel D, Kepa JK, Ross D (2002) Interaction of the molecular chaperone Hsp70 with human NAD(P) H:quinone oxidoreductase 1. J Biol Chem 277: 14060-14067 [DOI] [PubMed] [Google Scholar]
- Baler R, Zou J, Voellmy R (1996) Evidence for a role of hsp70 in the regulation of the heat shock response in mammalian cells. Cell Stress Chaperones 1: 33-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ (1994) RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 265: 1856-1860 [DOI] [PubMed] [Google Scholar]
- Blum P, Ory J, Bauernfeind J, Krska J (1992) Physiological consequences of DnaK and DnaJ overproduction in Escherichia coli. J Bacteriol 174: 7436-7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY (2001) Over-expression of inducible hsp70 chaperone suppresses neuropathology and improves motor function in SCA mice. Hum Mol Genet 10: 1511-1518 [DOI] [PubMed] [Google Scholar]
- Czarnecka-Verner E, Yuan CX, Scharf KD, Englich G, Gurley WB (2000) Plants contain a novel multi-member class of heat shock factors without transcriptional activator potential. Plant Mol Biol 43: 459-471 [DOI] [PubMed] [Google Scholar]
- Ding XZ, Tsokos GC, Kiang JG (1998) Overexpression of HSP-70 inhibits the phosphorylation of HSF1 by activating protein phosphatase and inhibiting protein kinase C activity. FASEB J 12: 451-459 [DOI] [PubMed] [Google Scholar]
- Echave P, Esparza-Ceron MA, Cabiscol E, Tamarit J, Ros J, Membrillo- Hernandez J, Lin EC (2002) DnaK dependence of mutant ethanol oxidoreductases evolved for aerobic function and protective role of the chaperone against protein oxidative damage in Escherichia coli. Proc Natl Acad Sci USA 99: 4626-4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Cartano NV, Milos L, Krebs RA, Lindquist SL (1996) Effect of engineering hsp70 copy number on hsp70 expression and tolerance of ecologically relevant heat shock in larvae and pupae of Drosophila melanogaster. J Exp Biol 199: 1837-1844 [DOI] [PubMed] [Google Scholar]
- Feldman DE, Frydman J (2000) Protein folding in vivo: the importance of molecular chaperones. Curr Opin Str Biol 10: 26-33 [DOI] [PubMed] [Google Scholar]
- Hong SW, Vierling E (2000) Mutants of Arabidopsis thaliana defective in the acquisition of tolerance to high temperature stress. Proc Natl Acad Sci USA 97: 4392-4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Constan D, Akita M, Keegstra K (2001) Molecular chaperones involved in chloroplast protein import. Biochim Biophys Acta 1541: 102-113 [DOI] [PubMed] [Google Scholar]
- Kaula SC, Reddelb RR, Sugiharac T, Mitsuia Y, Wadhwac R (2000) Inactivation of p53 and life span extension of human diploid fibroblasts by mot-2. FEBS Lett 474: 159-164 [DOI] [PubMed] [Google Scholar]
- Kim BH, Schöffl F (2002) Interaction between Arabidopsis heat shock transcription factor 1 and 70 kDa heat shock proteins. J Exp Bot 53: 371-375 [DOI] [PubMed] [Google Scholar]
- Kurepa J, Walker JM, Smalle J, Gosink MM, Davis SJ, Durham TL, Sung DY, Vierstra RD (2003) The SUMO protein modification system in Arabidopsis: accumulation of SUMO1 and 2 conjugates is increased by stress. J Biol Chem 278: 6862-6872 [DOI] [PubMed] [Google Scholar]
- Leborgne-Castel N, Jelitto-Van Dooren EPWM, Crofts AJ, Denecke J (1999) Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell 11: 459-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Schöffl F (1996) An Hsp70 antisense gene affects the expression of HSP70/HSC70, the regulation of HSF and the acquisition of thermotolerance in transgenic Arabidopsis thaliana. Mol Gen Genet 252: 11-19 [DOI] [PubMed] [Google Scholar]
- Li J, Chory J (1998) Preparation of DNA from Arabidopsis. In JM Martinez-Zapater, J Salinas, eds, Methods in Molecular Biology: Arabidopsis Protocols. Humana Press, Totowa, NJ, pp 55-60 [DOI] [PubMed]
- Lin BL, Wang JS, Liu HC, Chen RW, Meyer Y, Barakat A, Delseny M (2001) Genomic analysis of the hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones 6: 201-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129: 244-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouschek A, Pfanner N, Voos W (2000) Protein unfolding by mitochondria: the hsp70 import motor. EMBO Rep 1: 404-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T, Soll J (2000) 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell 12: 53-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Marin R, Westwood JT, Tanguay RM (1997) Cell-specific expression and heat-shock induction of hsps during spermatogenesis in Drosophila melanogaster. J Cell Sci 110: 1989-1997 [DOI] [PubMed] [Google Scholar]
- Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12: 3788-3796 [DOI] [PubMed] [Google Scholar]
- Mosser DD, Duchaine J, Massie B (1993) The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by hsp70. Mol Cell Biol 13: 5427-5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Sotelo J, Martinez LM, Ponce G, Cassab GI, Alagon A, Meeley RB, Ribaut JM, Yang R (2002) Maize hsp101 plays important roles in both induced and basal thermotolerance and primary root growth. Plant Cell 14: 1621-1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimura K, Takahashi H, Yoshikawa H (2001) Characterization of the dnaK multigene family in the cyanobacterium Synechococcus sp. strain PCC7942. J Bacteriol 183: 1320-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH (1999) In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol 19: 2069-2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviano E, Sari-Gorla M, Pé E, Frova C (1991) Molecular markers (RFLPs and HSPs) for the genetic dissection of thermotolerance in maize. Theor Appl Genet 81: 713-719 [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Sun Xy, Cao J, Mivechi NF, Giffard RG (1996) Overexpression of Hsp-70 protects astrocytes from combined oxygen-glucose deprivation. Neuroreport 31: 429-432 [DOI] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12: 479-492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiene-Fischer C, Habazettl J, Schmid FX, Fischer G (2002) The hsp70 chaperone DnaK is a secondary amide peptide bond cis-trans isomerase. Nat Struct Biol 9: 419-424 [DOI] [PubMed] [Google Scholar]
- Schroda M, Vallon O, Wollman FA, Beck CF (1999) A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell 11: 1165-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Mosser DD, Morimoto RI (1998) Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev 25: 654-666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini D, Beliveau C, Bellemare G (1999) Screening of transgenic plants by amplification of unknown genomic DNA flanking T-DNA. Biotechnology 27: 308-314 [DOI] [PubMed] [Google Scholar]
- Sung DY, Kaplan F, Guy CL (2001a) Plant Hsp70 molecular chaperones: protein structure, gene family, expression and function. Physiol Plant 113: 443-451 [Google Scholar]
- Sung DY, Vierling E, Guy CL (2001b) Comprehensive expression profile analysis of the Arabidopsis hsp70 gene family. Plant Physiol 126: 789-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S (2002) Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS Lett 516: 53-57 [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde BG (2000) Regulation of Arabidopsis root development by nitrate availability. J Exp Bot 51: 51-59 [PubMed] [Google Scholar]