Abstract
The mechanical properties of plant organs depend upon anatomical structure, cell-cell adhesion, cell turgidity, and the mechanical properties of their cell walls. By testing the mechanical responses of Arabidopsis mutants, it is possible to deduce the contribution that polymers of the cell wall make to organ strength. We developed a method to measure the tensile parameters of the expanded regions of turgid or plasmolyzed dark-grown Arabidopsis hypocotyls and applied it to the fucose biosynthesis mutant mur1, the xyloglucan glycosyltransferase mutants mur2 and mur3, and the katanin mutant bot1. Hypocotyls from plants grown in the presence of increasing concentrations of dichlorobenzonitrile, an inhibitor of cellulose synthesis, were considerably weakened, indicating the validity of our approach. In order of decreasing strength, the hypocotyls of mur2 > bot1 and mur1 > mur3 were each found to have reduced strength and a proportionate reduction in modulus compared with wild type. The tensile properties of the hypocotyls and of the inflorescence stems of mur1 were rescued by growth in the presence of high concentrations of borate, which is known to cross-link the pectic component rhamnogalacturonan II. From comparison of the mechanical responses of mur2 and mur3, we deduce that galactose-containing side chains of xyloglucan make a major contribution to overall wall strength, whereas xyloglucan fucosylation plays a comparatively minor role. We conclude that borate-complexed rhamnogalacturonan II and galactosylated xyloglucan contribute to the tensile strength of cell walls.
Cell walls constrain the rate of plant cell expansion during growth and limit the final size that plant cells achieve by resisting tensile stresses generated within the wall as a consequence of turgor pressure within the cell (Carpita, 1985; Cosgrove, 2000; Darley et al., 2001). There are two major polysaccharidic structural networks in the walls of dicotyledonous plants: the cellulose network tethered by cross-linking glycans and the pectin network (McCann and Roberts, 1991; Carpita and Gibeaut, 1993). The cellulose network dominates the mechanical response of isolated cell walls in small deformation rheology measurements (Whitney et al., 1999), but the manner in which the cellulose is deposited is conditioned by the presence of other molecules, leading to gross mechanical differences (Whitney et al., 1995, 1999; Chanliaud and Gidley, 1999; Chanliaud et al., 2002). In work with onion (Allium cepa), a non-graminaceous monocot with a cell wall composition typical of a dicotyledon (Redgwell and Selvendran, 1986), Wilson et al. (2000) obtained evidence for the independence of the cellulose and pectin networks in the epidermis and showed that pectin is mechanically important in its own right as well as affecting the viscoelastic properties of the cell wall through its modification of cellulose hydration. However, the specific structural features of cell wall polysaccharides that influence mechanical properties have not been identified.
The cellular structure of plants can be modeled as a liquid-filled foam (Gibson and Ashby, 1996). Cell walls act as struts or beams: the conductivity of mechanical stresses throughout a plant tissue depends greatly on the cell corners and intervening edges, and the cells are filled with incompressible liquid that provides extra support in compression. The struts or beams are themselves structured (Carpita and Gibeaut, 1993) and may in turn be modeled as fiber-reinforced composites (Kerstens et al., 2001) as a first approximation comprising cellulose fibrils in a matrix of other wall biopolymers. However, cell-cell adhesion, which ultimately affects whether tissues fail by cell separation or breakage, is not accounted for by a foam model. To relate changes in cell wall composition to the mechanical properties of an organ, we must account for the contributions of turgor, cell-cell adhesion, the relative density of the foam (distribution of material between symplast and apoplast), and cell wall structure.
The availability of Arabidopsis mutants with cell wall phenotypes allows us to determine the contributions that individual polymers might make to the mechanical properties of plant organs. Arabidopsis inflorescence stems have been used previously for the measurement of mechanical phenotypes, appropriately so for mutants related to secondary wall formation (Turner and Somerville, 1997), fiber cell formation (Zhong et al., 1997), and lignification (Jones et al., 2001; Köhler and Spatz, 2002). However, several Arabidopsis mutants are known (including mur2 and mur3) in which polysaccharides of the primary wall are altered (Reiter et al., 1993, 1997), but no morphological or mechanical phenotype is apparent in the mature plants (Vanzin et al., 2002). These mutants may exhibit mechanical phenotypes if the plants are studied at a stage when little lignification has occurred and primary-walled tissue can dominate the mechanical response.
In the case of the Fuc biosynthesis mutant mur1, the mature plant is dwarfed and requires about one-half the tensile force as wild type to break elongating inflorescence stems (Reiter et al., 1993). The mur1 mutation affects all Fuc-containing polysaccharides, including the pectic components rhamnogalacturonan I (RG I), rhamnogalacturonan II (RG II), and xyloglucan, the main cross-linking glycan in dicotyledonous plants. The morphological phenotype can be rescued by supplying excess borate to the plants, which compensates for the lowered binding efficiency of RG II for borate to form di-di-ester cross-links (O'Neill et al., 2001), but restoration of the mechanical phenotype has not been tested.
In this paper, we describe a method to measure the mechanical behavior of portions of dark-grown hypocotyls to define the contributions of specific cell wall polymers as load-bearing elements of cell walls. Arabidopsis stems have been tested in tension (Reiter et al., 1993; Köhler and Spatz, 2002) and in flexure (Turner and Somerville, 1997; Bichet et al., 2001). Instrumental sensitivity is inadequate for flexure tests of hypocotyls; therefore, a tensile test needs to be performed. The measurement of uniaxial mechanical properties for a cylindrical specimen requires a length to diameter ratio of about 10 (Brown, 1981). Such a shape is provided by the basal region of dark-grown hypocotyls and by pedicels, which are geometrically much simpler than any section of the inflorescence stem. By using the basal end of the hypocotyls, we avoid both the creep-prone growing region and the light-induced development of secondary walls. The parameters of strength and modulus that we derive represent an average of the properties of primary cell walls and some secondary wall material in the central stele.
In this paper, we examine the consequences of modifications to the cellulose-xyloglucan network and borate cross-linked RG II dimers. Cellulose content was titrated by growing hypocotyls in the presence of various concentrations of 2,6-dichlorobenzonitrile (DCB), whereas xyloglucan structure was modified in the cell wall mutants mur1, 2, and 3. mur1 lacks Fuc in all aerial portions of the plant because of a defect in the de novo synthesis of GDP-l-Fuc. mur2 is impaired in a xyloglucan-specific fucosyl transferase (Vanzin et al., 2002) and mur3 in a xyloglucan-specific galactosyl transferase (Madson et al., 2003). Our results demonstrate that mechanical phenotypes are conveniently measurable in expanded regions of the dark-grown hypocotyl and that mur1, mur2, and mur3 all have mechanical phenotypes. In the case of mur1, the reduced strength of hypocotyls and inflorescence stems can be rescued by addition of borate to the plants. We conclude that the galactosyl residues of xyloglucan and the fucosyl residues of RG II are important for xyloglucan and RG II to become load-bearing elements in cell walls.
RESULTS
Development and Validation of the Method
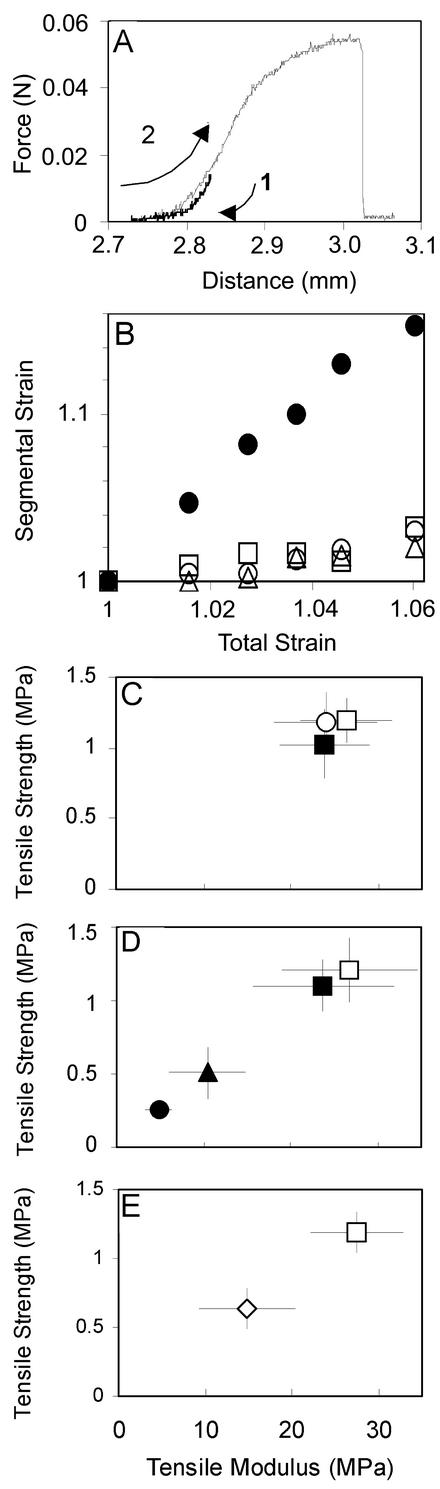
Intact seedlings were mounted with cyanoacrylate glue across two aluminum tabs held in a transfer clamp 3 mm apart to select regions along the hypocotyl. During the tensile test, the plantlet was submerged in liquid medium to maintain turgor. A force displacement curve for a wild-type hypocotyl is shown in Figure 1A. Strain is the ratio of change in length to the original length and is dimensionless. Stress is the force per unit of cross-sectional area (megapascals). Two tensile parameters are defined at failure; the maximum strain is termed the failure strain, the maximum stress is the tensile strength. The ratio of stress to strain varies during the tensile test. The tensile modulus (megapascals) is defined as the ratio of stress to strain at the point where the slope of the force displacement curve is at a maximum and linear.
Figure 1.
Experiments to validate the tensile test method. A, Force displacement curve for the basal 3 mm of a 4-d-old dark-grown wild-type hypocotyl, expanded zone. 1, First movement, relieving tension; 2, second movement, the tensile test. B, Average strain distribution in: □, first (basal); ○, second; ▵, third; and •, fourth (apical) portions of two 4-d-old wild-type hypocotyls in which a strain was applied across the whole length of the hypocotyl. At the next increment, both had broken. C to E, Tensile properties of hypocotyls showing average strength and average modulus ± one sd (n = 20). C, Sections (2.5–2.8 mm) were tested from the lowest erect portion of 4-d-old wild-type hypocotyls (□) and from a position 3 mm above this (▪). Lower parts of 10-d-old dark-grown wild-type hypocotyls (○) were also tested; only the upper parts of 4-d-old hypocotyls were significantly different in strength from the lower portion at 4 d (P < 0.05). D, Four-day-old wild-type hypocotyls grown in the presence of 0 (□), 0.01 (▪), 0.1 (▴), or 0.25 (•) μm DCB. E, Four-day-old bot1-1 hypocotyls (⋄) compared with wild type (□).
The greatest variation in mechanical properties within the length of the hypocotyl is that between expanded and expanding zones, as shown by the measurement of strain distribution over the full length of the hypocotyl (Fig. 1B). The dark-grown wild-type hypocotyl at 4 d has an average length of 10.2 ± 1.3 mm. The apical quarter is tapering and extremely fragile, so tensile tests must avoid this region. The basal 6 mm is cylindrical and has an average diameter of 0.24 ± 0.03 mm. Within this region, lower portions were on average 12% stronger (P < 0.05) than upper portions (Fig. 1C), whereas moduli and failure strains did not differ significantly. This lowest erect portion of the hypocotyl, in which the cells are fully expanded by d 3 and are of approximately uniform length (Gendreau et al., 1997), was used in all subsequent experiments. The same region at 10 d showed no significant differences in diameter or tensile parameters. The mean values for the tensile parameters of the 4-d-old plants were somewhat variable between experiments. Strength varied from 0.9 to 1.2 MPa, and the modulus varied from 21.3 to 27.5 MPa.
To establish the range over which mechanical properties can be measured, populations of hypocotyls were grown in the presence of DCB to inhibit the synthesis of cellulose, the major load-bearing polymer in the wall. Radiolabeled Glc incorporation into acetic-nitric acid-insoluble material (Updegraff, 1969) by 4-d-old dark-grown hypocotyls in a 1-h period is inhibited by 40% with 0.25 μm DCB (Heim et al., 1990). In hypocotyls grown in 0.25 μm DCB, strength and modulus were reduced 5-fold (Fig. 1D). With the reduction in cellulose, there is a reduced elongation and a radial swelling of the hypocotyl, a feature of several mutants affected in cellulose synthesis or organization (Arioli et al., 1998; Nicol et al., 1998; Fagard et al., 2000).
As an example of a mutant with a known mechanical phenotype, bot1-1 hypocotyls were assayed. The alleles bot1 and fra2 have a mutation in the same gene, AtKSS, which encodes katanin p60, a microtubule severing protein (Bichet et al., 2001). In the inflorescence stem, there is an 80% reduction in bending modulus in bot1-1 (Bichet et al., 2001), and the stems break easily with a sharp breaking surface. An 83% reduction in tensile modulus was observed in bot1-7 (Köhler and Spatz, 2002), and fra2 plants showed a reduction in tensile breaking force of up to 78% and a reduction in cellulose content of 20% (Burk et al., 2001). In the 4-d-old dark-grown hypocotyls of bot1-1, the diameter (0.35 ± 0.04 mm) was 36% greater than in wild type (0.26 ± 0.02 mm), and mean strength and modulus were both reduced by 46% compared with wild type (Fig. 1E). Failure strain was unaffected, indicating that the bot1-1 mutation causes mechanical weakness but not brittleness in the hypocotyls.
Mechanical Phenotypes of mur Mutants
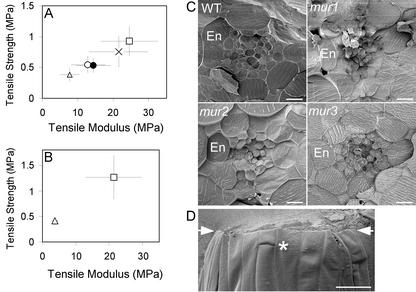
Xyloglucans can hydrogen bond to cellulose microfibrils to form networks and are hypothesized to be load bearing in the wall (Passioura and Fry, 1992). Therefore, we assayed those mur mutants affected in neutral sugar composition of xyloglucan (Reiter et al., 1997), mur1, mur2, and mur3. Testing the lower portions of hypocotyls, all mutants had a reduced average modulus compared with wild type and a proportionate reduction in strength (Fig. 2A). Failure strains did not differ significantly from wild type. Errors in strength and modulus tended to increase in proportion to strength and modulus; therefore, the statistical comparisons used the loge transformations. For mur2-1, the modulus was not significantly different from wild type, whereas the difference in strength was significant at the 5% level. Comparing strength and modulus of the other mutants with wild type, differences were highly significant (P < 0.001). Both alleles of mur1 were mechanically similar to each other, and mur3-1 was by far the weakest.
Figure 2.
Tensile properties and cellular phenotype of the hypocotyls of the mur mutants. A, Lower portions of 4-d-old wild-type hypocotyls and of the mur mutants (n = 20). □, Columbia (Col-0); ○, mur1-1; •, mur1-2; ×, mur2-1; ▵, mur3-1. Average tensile strength and modulus ± one sd are shown. B, Flaccid hypocotyls plasmolyzed in a 0.4 m mannitol-supplemented medium for 6 h: ▵, mur3-1; and □, Col-0. C, Field emission scanning electron microscopy (FESEM) of fractured hypocotyls showing the anatomy of the endodermis (En) of wild type, mur1-1, mur2-1, and mur3-1 (bar = 10 μm). D, The fractured surface of a mur3-1 hypocotyl broken in a tensile test is shown between the arrows. A transverse cell wall is labeled with an asterisk (bar = 50 μm).
Turgor, Structural Anatomy, and Cell-Cell Adhesion
The large differences in mechanical phenotypes among the mur mutants could arise from differences in structural features other than cell wall composition. First, we measured hypocotyl strength in the absence of turgor. Turgor is essential to the rigidity of a primary tissue. Plasmolyzed hypocotyls have no resistance to bending, and the effect of turgor on tensile properties may be considerable. Hypocotyls of the wild type and the mur mutant with the most extreme phenotype, mur3-1, were plasmolyzed in mannitol and then tested in tension. Greater differences were observed in strength and modulus of the flaccid hypocotyls than the turgid hypocotyls (Fig. 2B), differences that were highly significant (P < 0.001).
Second, we verified that the mutants did not have aberrant anatomical phenotypes or wall thicknesses. Hypocotyls of wild type, mur1-1, mur2-1, and mur3-1 were cryofixed and fractured in the FESEM for analysis of tissue anatomy (Fig. 2C) and measurements of the inner and outer epidermal walls. mur2-1 and mur3-1 were very similar to wild type. The cells of the endodermis of mur1-1 were less expanded in the radial direction than in wild type, although all of the other cells appeared normal. The radial widths of the endodermal cells in six fractured hypocotyls were as follows: wild type, 15.6 ± 3.4 μm; mur1-1, 13.4 ± 4.1 μm; mur2-1, 15.8 ± 3.6 μm; and mur3-1, 16.0 ± 4.1 μm. Only mur1-1 was significantly different from wild type (Dunnett's, P < 0.05). Cell wall thicknesses were variable but not significantly different between wild type and the three mur mutants (data not shown).
Third, we verified that the mode of failure was by cell breakage rather than loss of cell-cell adhesion. Wild-type and mur3-1 hypocotyls broken in a tensile test showed no evidence of cell separation at the fracture surface (Fig. 2D). Therefore, we can assume that the mechanical properties of the cell walls will contribute more to the mechanical properties of the organ than domains of cell-cell adhesion between cells, the pectin-rich middle lamella.
Effect of Borate on Hypocotyl and Stem Strengths of mur1 and Wild Type
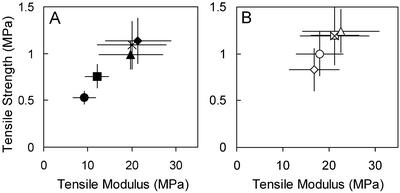
In mur1-1, the mature leaf is dwarfed and only 56% of its RG II is dimerized, compared with 95% in wild type. By watering with boric acid, the growth phenotype of the mur1 rosette can be rescued and the proportion of RG II that is dimerized increases to 78% (O'Neill et al., 2001). The possibility of rescuing the phenotype in the mur1-1 hypocotyl or of further impairing its tensile properties by manipulating borate concentrations was investigated. Tensile tests were performed on mur1-1 and wild-type hypocotyls grown on Murashige and Skoog medium with five different concentrations of boric acid: 0, 0.1, 1.3, 2.6, and 5 mm. The normal Murashige and Skoog medium contains 0.1 mm boric acid. In medium with no added boric acid, modulus and strength of mur1-1 were further reduced (Fig. 3A) with the respective statistical significances of P < 0.05 and P < 0.001). With 1.3 mm boric acid, the mur1-1 phenotype was rescued to within 93% of the modulus and 82% of the strength of wild type in 0.1 mm boric acid. With 2.6 mm, strength and modulus of mur1-1 were rescued to within 99% of wild-type. Wild type in medium with no added boric acid was slightly weaker (P < 0.05) than normal (Fig. 3B). At 1.3 and 2.6 mm boric acid, wild-type properties were not significantly different from that at 0.1 mm. At 5 mm boric acid, wild type showed a significant reduction in strength (P < 0.001). The normalized tensile breaking force of the inflorescence stem of mur1 increased from 16.2 ± 0.53 to 24.0 ± 0.8 n mg-1 cell wall material by watering the plants every 3 d with 1 mm borate-containing medium. No differences were seen between borate-watered and control wild-type plants.
Figure 3.
Effects of borate on the primary wall properties of mur1-1 hypocotyls. Tensile properties of the lower portions of 4-d-old hypocotyls grown on media with various concentrations of borate: ○•, 0; □▪, 0.1 mm; □▪, 1.3 mm; ××, 2.6 mm; and ⋄♦, 5 mm boric acid. A, Black symbols, mur1-1; B, white symbols, Col-0.
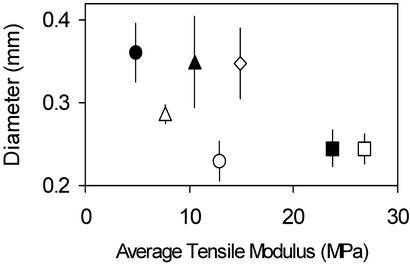
Strength and modulus scale similarly in the mur mutants as in the DCB-treated hypocotyls and bot1-1, and there are no significant differences in failure strains. However, the mur mutants exhibit considerable reductions in strength and modulus for rather slight changes in diameter, which are shown in Figure 4. The modulus and strength of bot1-1 is similar to that of mur1-1, but its diameter is much greater.
Figure 4.
An illustration of the difference in radial swelling among hypocotyls affected in wall composition. Average diameter of the basal 3-mm sections ± one sd and average moduli (n = 20) are shown for: wild type grown in: □, 0; ▪, 0.01; ▴, 0.1; and •, 0.25 μm DCB; and the mutants: ○, mur1-1; ▵, mur3-1; and ⋄, bot1-1.
Assay of Pedicels of mur Mutants
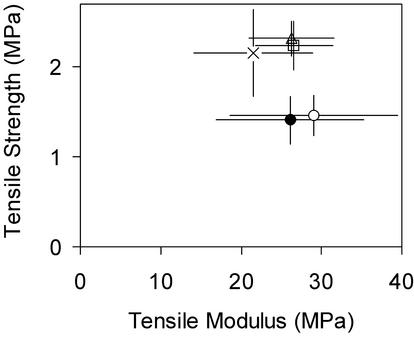
Testing of the pedicel allows an organ of equivalent dimensions to the hypocotyl but with a greater extent of secondary wall formation to be examined. When the silique was 3 mm long, specimens of wild-type pedicels had a mean diameter of 0.24 ± 0.01 mm, a tested length of 3.17 ± 0.37 mm, and a similar mean modulus, 26.55 ± 4.83 MPa. The strength was about twice as high as that of the hypocotyls, 2.24 ± 0.27 MPa, and the failure strain was much greater, 0.35 ± 0.10. The modulus, strength, and failure strain of wild type, mur2-1, and mur3-1 did not differ significantly (Fig. 5). The pedicels of mur1-1 and mur1-2 had a strength and failure strain about one-half that of wild type (P < 0.001 and P < 0.001), but there was no significant difference in modulus.
Figure 5.
Mechanical phenotypes of the pedicels of the mur mutants. Tensile properties of pedicels: □, Col-0; ○, mur1-1; •, mur1-2; ×, mur2-1; and ▵, mur3-1.
DISCUSSION
The hypocotyl has a simple and consistent anatomy in which all growth is a consequence of cell expansion without division (Gendreau et al., 1997). Although DCB treatment and the bot1-1 mutation induced radial swelling, the three mur mutants had a reasonably consistent anatomical structure, wall thickness, and diameter. Thus, we have accounted for the two parameters in the foam model of porosity and edge/face distribution of material. We also used FESEM to demonstrate that the mode of failure involves fracture across cells rather than differences in cell-cell adhesion. Finally, we showed that, although turgor pressure makes a significant contribution to organ mechanical properties, the differences between wild type and mutant can be observed in its absence, i.e. in plasmolyzed tissues. We conclude that differences in organ strength can be reasonably interpreted as a consequence of the changes to the composition of the matrix of the foam, i.e. the cell walls. DCB-treated hypocotyls with decreased cellulose content showed correspondingly decreasing tensile strength and modulus. The bot1-1 mutant is already known to be mechanically weaker, and we established that this phenotype could also be measured in hypocotyls. Thus, we used DCB treatment and a known mutant to establish that our tensile test would be sensitive to compositional and mechanical changes in hypocotyls, themselves already fragile.
The mur2 and mur3 Mutants Indicate a Load-Bearing Role for the Xyloglucan Cellulose Network
Artificial constructs of bacterial cellulose and plant hemicellulose (Whitney et al., 1999) have allowed the mechanical properties of the cellulose:hemicellulose network to be investigated independently from other wall polymers. Cellulose microfibrils possess great tensile strength (Mark, 1967) but are quite brittle. Addition of an unfucosylated xyloglucan results in a composite that has both a reduced strength and a reduced modulus. It was suggested that the decrease in modulus was due to controlled slippage of the cellulose fibers relative to each other, facilitated by the breakage and reformation of H bonds between the cellulose and cross-linking xyloglucans (Whitney et al., 1999). The specific loss of terminal Fuc from the xyloglucan in dark-grown mur2-1 hypocotyls causes only a slight impairment of tensile properties. It is possible that any loss of strength resulting from lack of Fuc on xyloglucan could have been compensated by, for example, a change in the pattern of acetylation or a change in average Mr of the xyloglucan. However, the loss of Gal from xyloglucan of mur3-1 hypocotyls (N. Carpita, personal communication) causes a gross impairment of mechanical properties. Reid et al. (1988) showed that degalactosylation of tamarind xyloglucan changed the rheological properties of the polymer in vitro. A change in xyloglucan solubility or gelation characteristics may affect the interaction between cellulose and xyloglucan in the wall. The tensile properties of mur3 are impaired without causing much cell bulging, the wall is still capable of resisting a biaxial strain (Chanliaud et al., 2002), but a wall with defective cellulose bulges has reduced tensile properties. In contrast, no defect was observed in pedicels of either mur2 or mur3, which indicates that the secondary wall that develops in the light is not impaired by the presence of a defective xyloglucan.
The mur1 Mutants Indicate a Role for Borate Cross-Linked RG II in Organ Strength
mur1 has less than 2% of the normal amounts of Fuc in aerial parts of the plant (Reiter et al., 1997), but the hypocotyl is a root-like organ, and the neutral sugar composition of mur1 hypocotyls resembles that of the root with 60% of the wild-type levels of Fuc (N. Carpita, personal communication). mur1 has a tensile strength of elongating inflorescences less than 50% that of wild type and exhibits a slightly dwarfed growth habit (Reiter et al., 1993; Bonin et al., 1997). In various cell and plant systems, boron deficiency has been shown to result in reduced mechanical strength (Hu and Brown, 1994; Findeklee and Goldbach, 1996; Fleischer et al., 1998; Ishii et al., 2002). In mur1, the growth phenotype can be rescued by supplying excess borate to the plants, which compensates for the lowered binding efficiency of RG II for borate to form di,di-ester cross-links (O'Neill et al., 2001). The mur1 mutants contain normal amounts of RG II, but the two fucosyl residues present in RG II are replaced by α-l-Galp residues (O'Neill et al., 2001). As a consequence of this, the rate of RG II dimer formation and the stability of this cross-link are impaired. The tensile strength of mur1 in the hypocotyl and the stem is completely rescued with boron demonstrating that the lack of Fuc in RG II rather than in xyloglucan is important for the mechanical phenotype. It also suggests that as well as having a role in the expanded primary wall, RG II-borate complexes are important for secondary wall structure or assembly. We conclude that tensile properties of the cell wall depend both on a xyloglucan cross-linked microfibrillar network and RG II-borate complexes.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of Arabidopsis (Col-0) and mutants in the Col-0 background were surface sterilized in a mixture of 3% (v/v) hydrogen peroxide and 50% (v/v) ethanol for 2 min. After rinsing in sterilized water, seeds were plated on a nutrient-solidified medium (0.44% [w/v] Murashige and Skoog [Duchefa, Haarlem, The Netherlands], 1% [w/v] Suc, and 0.5% [w/v] Gellan gum [Phytagel, Sigma, Gillingham, Dorset, UK] at pH 5.5). Some plates were supplemented with DCB at up to 0.5 μm, and others with 1.2, 2.5, and 4.9 mm boric acid. Medium (Murashige and Skoog recipe) was prepared without borate salts and using Analar water for experiments using borate-free plates. Plates were sealed with laboratory film and placed horizontally in the dark at 25°C for 2 d, exposed to light for 4 h, and then left in the dark for 4 to 10 d. Seeds of Col-0, mur1-1, mur1-2, mur2-1, and mur3-1 were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus), and botero1-1 was kindly supplied by Dr Herman Höfte (Institut National de la Recherche Agronomique, Versailles, France).
Measurement of Strain Distributions along the Length of the Hypocotyl
Two wild-type hypocotyls were glued at their extremities to a small stretching device. The hypocotyls were wetted and sprinkled with coal dust. Strain was applied slowly in increments. The hypocotyls were imaged (Image Pro, Media Cybernetics, Silver Spring, MD, at 40× magnification), and the strains in quarter portions of the hypocotyls were measured.
Mechanical Testing of the Hypocotyls
At 4 d, wild-type hypocotyls were 11 mm high. Hypocotyls turned erect within 1 mm of the root boundary. Whole plants were fixed with cyanoacrylate (Resist H20, Holdtite, Gateshead, UK) across two aluminum tabs held in a brass transfer clamp about 3 mm apart. The adhesive was cured rapidly with 20 μL of an activator (Cyanolit Plus, Eurobond, Sittingbourne, UK). The glued specimens were submerged in liquid Murashige and Skoog medium + 1% (w/v) Suc, and the section of the hypocotyl between the aluminum tabs was imaged using a microscope (Image-Pro, Media Cybernetics), and the length of the specimen and 10 diameters were measured. Specimens were tested in tension submerged in the same medium using a TA-XT2i texture analyzer (Stable Microsystems, Godalming, Surrey, UK) with a load cell sensitive to 1 mn. Hypocotyls were also plasmolyzed in a 0.4 m mannitol-supplemented liquid Murashige and Skoog-Suc medium (Wu et al., 2000) for 6 to 8 h. They were then tested in tension in the mannitol-supplemented medium. Hypocotyls grown in borate-supplemented media and media with no added borate were tested in the corresponding liquid medium.
During the polymerization of the cyanoacrylate, an elastic strain of up to 4% can develop in the specimens; therefore, specimens were first relaxed by lowering the crosshead by 0.1 mm at 0.03 mm s-1, and then they were extended at 0.03 mm s-1 until failure (Fig. 1A). From the force displacement curve, the maximum slope, the decrease in force on breaking, the extension on breaking, and the slack length were recorded (Cleland, 1967). Taking specimens to be cylindrical, tensile modulus, tensile strength, and failure strain were derived:
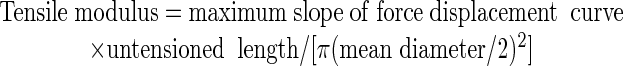 |
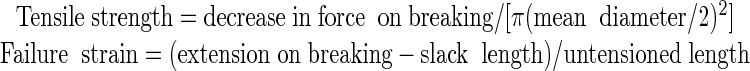 |
where untensioned length = measured length + slack length - 0.1-mm crosshead correction.
Statistical comparisons, either Dunnett's or Tukey's tests, used the loge transformations (Minitab, 1994).
Mechanical Testing of Pedicels and Inflorescence Stems
Pedicels were taken with siliques 3 mm long from near the apex of the main inflorescence stems of 41- to 48-d-old plants. Whole pedicels were glued across the aluminum tabs to test the segment between 1 mm from the silique and 2 mm from the stem and imaged. Pedicels were tested in tension in air at an extension rate of 0.05 mm s-1, and strengths were calculated as for the hypocotyls. Breaking forces of inflorescence stems were determined as described by Reiter et al. (1993).
FESEM
Hypocotyls were frozen by plunging into liquid nitrogen slush at -210°C and then fractured in the SEM (Burton et al., 2000). The SEM micrographs were taken at 5 kV or below using an XL30 FESEM (Philips, Eindhoven, The Netherlands) fitted with a CT1500HF cryo-system (Oxford Instruments Abingdon, Oxford, UK). Samples were routinely sputter coated with 2 to 4 nm of platinum before imaging. Measurements of cell size and epidermal cell wall thicknesses were made directly on samples in the microscope using the Philips imaging software. Between five and 10 individuals were measured for each mutant line and the wild type.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Julia Corsar and Nicola Stacey for growth of materials and Sue Bunnewell for media preparation. We are grateful to Nick Carpita and Keith Roberts for helpful discussions.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021873.
This work was supported by the Biotechnology and Biological Sciences Research Council (competitive strategic grants to P.R., A.C.S., and M.C.M.), by a Royal Society University Research Fellowship (to M.C.M.), by the U.S. Department of Energy (grant to W.-D.R.), and by the National Science Foundation (grant to W.-D.R.).
References
- Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, et al. (1998) Molecular analysis of cellulose biosynthesis. Science 279: 717-720 [DOI] [PubMed] [Google Scholar]
- Bichet A, Desnos T, Turner S, Grandjean O, Höfte H (2001) BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J 25: 137-148 [DOI] [PubMed] [Google Scholar]
- Bonin CP, Potter I, Vanzin GF, Reiter WD (1997) The MUR1 gene of Arabidopsis thaliana encodes an isoform of GDP-d-mannose-4,6-dehydratase, catalysing the first step in the de novo synthesis of GDP-l-fucose. Proc Natl Acad Sci USA 94: 2085-2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RP (1981) Handbook of Plastics Test Methods, Ed 2. George Godwin Ltd., London
- Burk DH, Liu B, Zhong R, Morrison WH, Ye ZH (2001) A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell 13: 807-827 [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Gibeaut DM, Bacic A, Findlay K, Roberts K, Hamilton A, Baulcombe DC, Fincher GB (2000) Virus-induced silencing of a plant cellulose synthase gene. Plant Cell 12: 691-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC (1985) Tensile-strength of cell-walls of living cells. Plant Physiol 79: 485-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary-cell walls in flowering plants: consistency of molecular structure with the physiological properties of the wall during growth. Plant J 3: 1-30 [DOI] [PubMed] [Google Scholar]
- Chanliaud E, Burrows KM, Jeronimidis G, Gidley MJ (2002) Mechanical properties of primary plant cell wall analogues. Planta 215: 989-996 [DOI] [PubMed] [Google Scholar]
- Chanliaud E, Gidley MJ (1999) In vitro synthesis and properties of pectin/Acetobacter xylinum cellulose composites. Plant J 20: 25-35 [DOI] [PubMed] [Google Scholar]
- Cleland R (1967) Extensibility of isolated cell walls: measurement and changes during cell elongation. Planta 74: 197-209 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38: 109-124 [DOI] [PubMed] [Google Scholar]
- Darley CP, Forrester AM, McQueen-Mason SJ (2001) The molecular basis of plant cell wall extension. Plant Mol Biol 47: 179-195 [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, McCann MC, Rayon C, Vernhettes S, Höfte H (2000) PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12: 2409-2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeklee P, Goldbach HE (1996) Rapid effects of boron deficiency on cell wall elasticity modulus in Cucurbita pepo roots. Bot Acta 109: 463-465 [Google Scholar]
- Fleischer A, Titel C, Ehwald R (1998) The boron requirement and cell wall properties of growing and stationary suspension-cultured Chenopodium album L. cells. Plant Physiol 118: 1103-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson LJ, Ashby MF (1996) Cellular Solids: Structure and Properties, Cambridge University Press, Cambridge, UK
- Heim DR, Skomp JR, Tschabold EE, Larrinua IM (1990) Isoxaben inhibits the synthesis of acid insoluble cell wall materials in Arabidopsis thaliana. Plant Physiol 93: 695-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HI, Brown PH (1994) Localization of boron in cell-walls of squash and tobacco and its association with pectin: evidence for a structural role of boron in the cell-wall. Plant Physiol 105: 681-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Matasunaga T, Iwai H, Satoh S, Taoshita J (2002) Germanium does not substitute for boron in cross-linking of rhamnogalacturonan II in pumpkin cell walls. Plant Physiol 130: 1967-1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Ennos R, Turner S (2001) Cloning and characterization of irregular xylem4 (irx4): a severely lignin deficient mutant of Arabidopsis. Plant J 26: 205-216 [DOI] [PubMed] [Google Scholar]
- Kerstens S, Decraemer WF, Verbelen J-P (2001) Cell walls at the plant surface behave mechanically like fiber-reinforced composite materials. Plant Physiol 127: 381-385 [PMC free article] [PubMed] [Google Scholar]
- Köhler L, Spatz HC (2002) Micromechanics of plant tissues beyond the linear-elastic range. Planta 215: 33-40 [DOI] [PubMed] [Google Scholar]
- Madson M, Dunand C, Li X, Verma R, Vanzin GF, Caplan J, Shoue DA, Carpita NC, Reiter W-D (2003) The MUR3 gene of Arabidopsis thaliana encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. Plant Cell (in press) [DOI] [PMC free article] [PubMed]
- Mark RE (1967) Cell Wall Mechanics of Tracheids. Yale University Press, New Haven, CT
- McCann MC, Roberts K (1991) Architecture of primary walls. In CW Lloyd, ed, The Cytoskeletal Basis of Plant Growth and Form. Academic Press, London, pp 109-129
- Minitab (1994) Release 10 Reference Manual. Minitab, State College, PA
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Höfte H (1998) A plasma membrane-bound putative endo-1,4-beta-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J 17: 5563-5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846-849 [DOI] [PubMed] [Google Scholar]
- Passioura JB, Fry SC (1992) Turgor and cell expansion: beyond the Lockhart equation. Aust J Plant Physiol 19: 565-576 [Google Scholar]
- Redgwell RJ, Selvendran RR (1986) Structural features of cell wall polysaccharides of onion Allium cepa. Carbohydr Res 157: 183-199 [Google Scholar]
- Reid JSG, Edwards M, Dea ICM (1988) Enzymic modification of natural seed gums. In GO Philips, PA Williams, DJ Wedlock, eds, Gums and Stabilizers for the Food Industry 4. IRL Press, Oxford, pp 391–398
- Reiter WD, Chapple CCS, Somerville CR (1993) Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science 261: 1032-1035 [DOI] [PubMed] [Google Scholar]
- Reiter WD, Chapple C, Somerville CR (1997) Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J 12: 335-345 [DOI] [PubMed] [Google Scholar]
- Turner SR, Somerville CR (1997) Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell 9: 689-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff DM (1969) Semi-micro determination of cellulose in biological materials. Anal Biochem 32: 420-424 [DOI] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, Raikel NV, Keegstra K, Reiter WD (2002) The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proc Natl Acad Sci USA 99: 3340-3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SEC, Brigham JE, Darke AH, Reid JSG, Gidley MJ (1995) In vitro assembly of cellulose/xyloglucan networks: ultrastructural and molecular aspects. Plant J 8: 491-504 [Google Scholar]
- Whitney SEC, Gothard MGE, Mitchell JT, Gidley MJ (1999) Role of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiol 121: 657-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH, Smith AC, Kacurakova M, Saunders PK, Wellner N, Waldron KW (2000) The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier transform infrared spectroscopy. Plant Physiol 124: 397-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Locy RD, Shaw JJ, Cherry JH, Singh NK (2000) Mutation in Arabidopsis HIT1 locus causing heat and osmotic hypersensitivity. J Plant Physiol 157: 543-547 [Google Scholar]
- Zhong R, Taylor JJ, Ye ZH (1997) Disruption of interfascicular fiber differentiation in an Arabidopsis mutant. Plant Cell 9: 2159-2170 [DOI] [PMC free article] [PubMed] [Google Scholar]