Abstract
Seeds are usually stored in physiological conditions in which they gradually lose their viability and vigor depending on storage conditions, storage time, and genotype. Very little is known about the underlying genetics of seed storability and seed deterioration. We analyzed a mutant in Arabidopsis disturbed in seed storability. This mutant was isolated as a grs (green-seeded) mutant in an abi3-1 (abscisic acid 3) mutant background. Genetic and physiological characterization showed that the monogenic grs mutant was not visibly green seeded and mapped on chromosome 4. This enhancer mutation did not affect the ABA sensitivity of seed germination or seed dormancy but was found to affect seed storability and seedling vigor. Seed storability was assessed in a controlled deterioration test, in which the germination capacity of the mutant decreased with the duration of the treatment. The decrease in viability and vigor was confirmed by storing the seeds in two relative humidities (RHs) for a prolonged period. At 60% RH, the mutant lost germinability, but storage at 32% RH showed no decrease of germination although seed vigor decreased. The decrease in viability and vigor could be related to an increase in conductivity, suggesting membrane deterioration. This was not affected by light conditions during imbibition, expected to influence the generation of active oxygen species. During seed maturation, ABI3 regulates several processes: acquiring dormancy and long-term storability and loss of chlorophyll. Our results indicate that GRS is a common regulator in the latter two but not of dormancy/germination.
Seeds of good quality are undamaged seeds that produce viable and vigorous seedlings without defects under various environmental conditions also after storage (Dickson, 1980). Depending on storage conditions, storage time and genotype seeds gradually lose vigor and viability. A common interpretation of the physiology of this seed deterioration cannot be given because the causes are assumed to be due to a variety of processes. For instance, mechanical damage that occurs in the field is different than physiological damage occurring during storage, which in itself may have different components (McDonald, 1999). Seed deterioration in agronomic crops is a problem that has been unintentionally aggravated by domestication because wild species often retain a high seed quality for many years (Moore and Halloin, 2000). Little is known about the genetic basis of differences in seed quality because this trait is affected by environmental effects during seed formation, harvest, and storage and is probably controlled by several genes and, therefore, is complex in genetic studies. To investigate the genetics of this process, one can identify mutations that either have an improved or a deteriorated seed quality (Tesnier et al., 2002). Among Arabidopsis mutants that have a poor seed quality, which is obvious by their rapid loss of viability upon storage, are mutants that affect seed maturation such as lec1, lec2, and fus3 (Holdsworth et al., 1999; Finkelstein et al., 2002) and strong abi3 alleles (Nambara et al., 1992; Ooms et al., 1993). ABI3 is a B3 domain transcription factor that is assumed to control abscisic acid (ABA)-induced seed maturation and germination processes. Its effect on seed maturation is indicated by the reduced expression of many seed maturation-specific mRNAs (Parcy et al., 1994) and a reduced chlorophyll breakdown at the end of seed maturation in abi3 null alleles (Nambara et al., 1995; Parcy et al., 1997). This results in the typical green seed phenotype of strong abi3 alleles (Ooms et al., 1993). This green seed phenotype was also observed in double mutants that combined the weak abi3-1 allele with ABA deficiency due to a mutation in the ABA1 gene (Koornneef et al., 1989).
It is believed that the many, mainly seed-specific, pleiotropic effects of abi3 mutants may be because the ABI3 protein induces several pathways, which act independently after being promoted by the ABA/ABI3 system. However, some of these processes might be functionally related. For instance, it might be possible that the poor storability and the green seed phenotype have a causal relationship where the extra chlorophyll during imbibition may lead to photodamage (Thomas and Smart, 1993; Hendry, 1997). This is also suggested by the observation in cabbage (Brassica oleracea) seeds sorted based on their chlorophyll fluorescence, high amounts of chlorophyll correlated with lower quality in normal germination assays, but also in artificially deteriorated seeds (Jalink et al., 1998). It is also possible that both are processes that have functionally no relationship but have only in common that they are both occurring during the later phases of seed maturation and have ABI3 as a common inducer. When processes are independent, it might be possible to identify mutations that affect only one of these specific downstream pathways of ABI3. Such mutants can be isolated as enhancers or suppressors of specific aspects of pleiotropic mutants such as abi3. When such mutants modify all aspects of the mutant phenotype, it is expected that they directly influence the functioning of the pleiotropic gene. However, when only a subset of the phenotype is affected these modifiers may control specific processes downstream of the common regulator. Leaky mutant alleles provide good opportunities to find enhancers of specific mutants traits because they represent a sensitive background in which minor changes are easily observed (Page and Grossniklaus, 2002). In the present report, we describe a mutation that enhances the phenotype of the leaky abi3-1 allele and show that this affects only a subset of the ABI3-induced processes, including aspects of seed quality.
RESULTS
The Genetic Characterization of the grs (Green Seed) Mutant
A mutant line with mature seeds that are greener compared with the parental genotype was isolated in the selfed progeny of ethyl methanesulfonate-treated seeds, carrying the leaky abi3-1 allele. This phenotype, with a green embryo and a brown testa, resembled abi3 alleles stronger than abi3-1, such as abi3-7 and null alleles of ABI3, which have been isolated in the same mutagenesis experiment (Ooms et al., 1993; Bies-Etheve et al., 1999). The analysis of the progeny derived from the cross of this mutant with the Landsberg erecta wild type showed that only the seeds harvested on five of 168 F2 plants had a phenotype similar to that of the original green seed mutant. This ratio, which is not in conflict with a 1:15 ratio (χ2 = 3.07, P > 0.05), together with the observation that the green seed phenotype did not occur in seeds harvested on F2 plants that were wild type for the ABI3 locus, shows that the green seed phenotype is only expressed in homozygous abi3-1 mutants and in her its as a recessive mutation independently from abi3. This mutation can be considered as an enhancer of abi3-1 because it leads to a phenotype resembling strong abi3 alleles. This enhancer locus is called grs. Lines homozygous for the grs mutation in WT (non-abi3-1 background) were identified as follows. Seeds harvested on F2 plants that showed approximately 25% green seeds and that also segregated 25% of germinating seeds on 10 μm ABA were assumed to be homozygous for grs and heterozygous for abi3-1 genotypes. F3 plants from such progeny were screened for plants of which the F4 seeds were fully sensitive to the germination-inhibiting effect of ABA. These single grs mutants did not differ phenotypically from wild-type plants, and their seeds had a normal brown wild-type color with a white mature embryo.
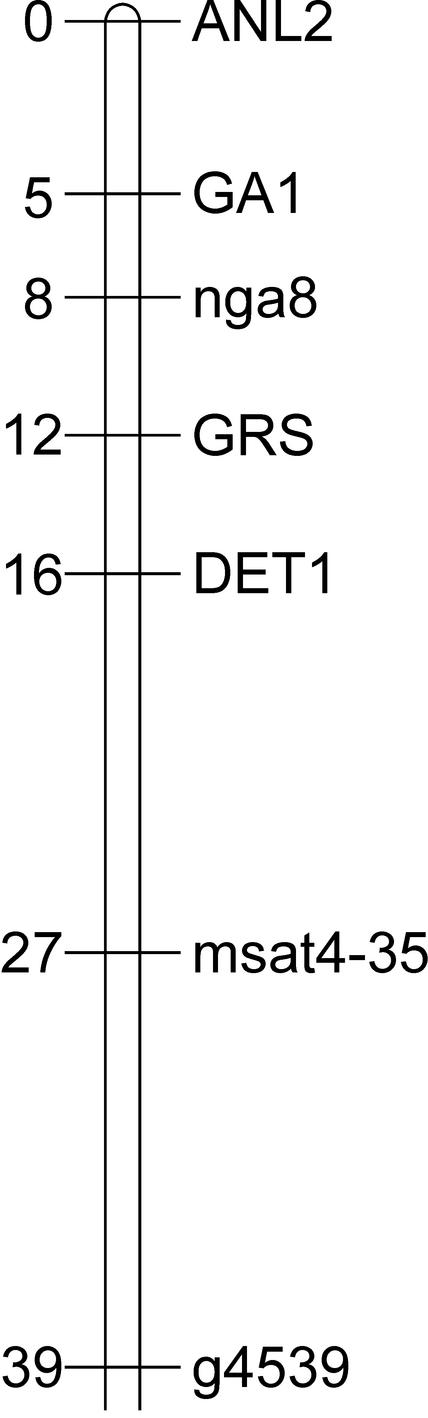
Linkage analysis using the progeny of an F2 individual homozygous for abi3-1 but segregating for green seeds showed that the GRS locus is located on chromosome 4 near DET1 (Fig. 1) at a position where no other mutants of seed development have been located so far (Meinke et al., 2003).
Figure 1.
Map position of the grs mutation on chromosome 4.
Physiological Characterization of the grs Mutant
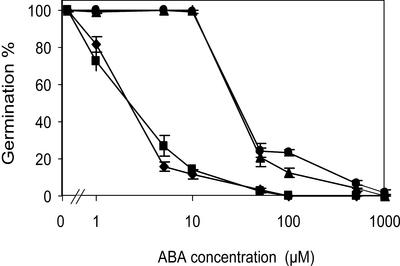
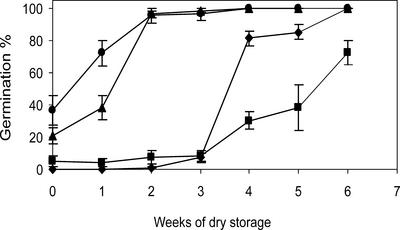
A further characterization of the genotypes homozygous for grs, either with or without the abi3-1 mutation, was performed to see if the grs mutation affects all aspects of the abi3 mutant phenotype and if it has a recognizable phenotype without the abi3-1 mutation. The four major characteristics of abi3 mutations that we analyzed are: the sensitivity to applied ABA, seed dormancy, chlorophyll content in seeds, and seed quality. The reduced germination of seeds upon storage in adverse conditions and the number of abnormal seedlings were used as criteria for seed quality. The ABA sensitivity of all four lines was tested and showed that the single grs mutant is as sensitive to ABA as wild type of which complete inhibition of germination occurs at 50 μm, whereas the inhibition of the abi3-1 and the abi3-1 grs double mutant is also similar (Fig. 2). This indicates that the grs mutation does not alter the response to ABA. In contrast to seed germination on ABA, small differences were observed in the dormancy of seeds (Fig. 3). Lines with the abi3-1 mutation are much less dormant than genotypes with the wild-type ABI3 allele as shown before (Bies-Etheve et al., 1999). Double mutants (abi3-1 grs) were slightly less dormant, indicating some enhancement of the abi3-1 phenotype. However, the single grs mutant was slightly more dormant than the wild-type Landsberg erecta, which is opposite to the effect of this mutation in the abi3-1 mutant background. We conclude that the grs mutation does not consistently affect seed dormancy.
Figure 2.
Germination in the presence of ABA of wild-type (diamonds), abi3-1 GRS (triangles), ABI3 grs (squares), and abi3-1 grs (circles). Data are averages of six replicates ± se.
Figure 3.
Germination behavior of seeds in light harvested 22 d after flowering from mature dry siliques and subsequent dry storage until 6 weeks. Diamonds, wild type; triangles, abi3-1 GRS; squares, ABI3 grs; circles, abi3-1 grs. Data are averages of six replicates ±se.
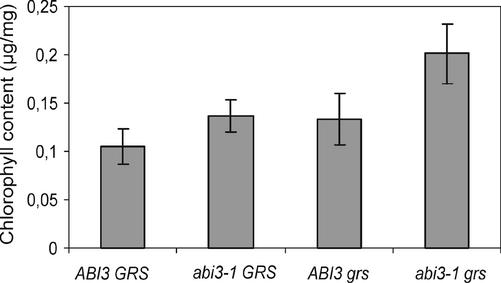
The effect on which the selection of this mutant was based is the green color of its seeds. Chlorophyll measurements in the double mutant confirmed the expected higher chlorophyll content in the double mutant compared with the other three genotypes tested (Fig. 4). Although statistically not significant and not obvious by visual inspection, abi3-1 seeds also seem to contain slightly more chlorophyll than wild type, as has been shown before by Parcy et al. (1997). A slight increase in chlorophyll content is also suggested for the single grs mutant. Jalink et al. (1998) also noticed that cabbage seeds of different maturity that have the same color to the human eye can contain different amounts of chlorophyll.
Figure 4.
Analysis of total chlorophyll content (micrograms per milligram of seed) of wild-type (ABI3 GRS), abi3-1 GRS, ABI3 grs, and abi3-1 grs seeds. Data are averages of 12 replicated extractions ± se.
The reduced breakdown of chlorophyll might be an indication that seed maturation is not completed properly in the grs mutant, this effect could be additive to a similar effect in abi3-1 and might lead to reduced desiccation tolerance of the seeds that is also observed in the abi3 allelic series. Although only null alleles of abi3 such as abi3-4, abi3-5, and abi3-6 cannot survive more than a few weeks of dry storage at room temperature (Ooms et al., 1993; Nambara et al., 1992), leaky abi3 alleles such as abi3-7 show this reduced storability when seed deterioration is enhanced in controlled deterioration (CD) tests as described for Arabidopsis by Tesnier et al. (2002). Therefore, we compared seed storability both in CD test conditions and under normal but less favorable conditions when seeds are stored at relative high humidity.
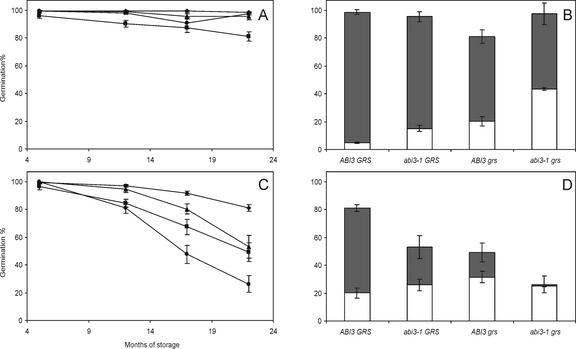
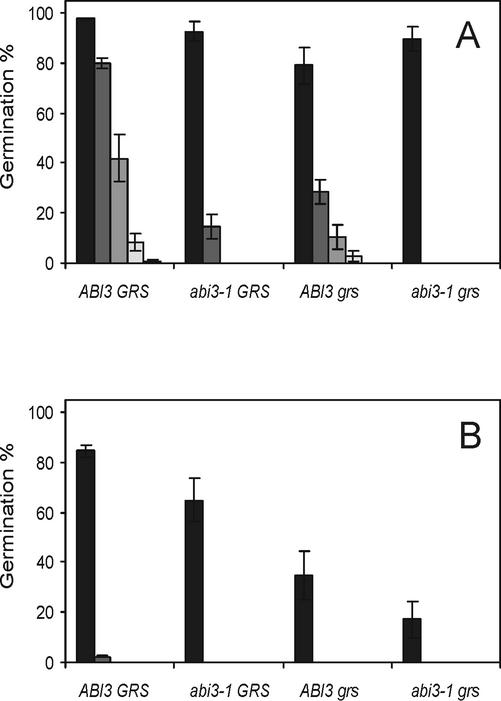
When seeds were stored at 60% relative humidity (RH) and ambient temperature, viability was lost more rapidly in the double mutants than in the single mutants, which lost viability at a slower speed (Fig. 5C). Even after 22 months of storage at 60% RH, the wild-type seeds still germinated almost 100%. The effect of storage at low humidity on preventing viability loss is shown by the absence of viability loss for all genotypes when seeds were stored for the same period at 32% RH (Fig. 5A). In the germination assay done after 22 months of storage, the number of abnormal seedlings as percentage of total germination also was determined. It appeared that although storage at 32% RH did not show a large decline in total germination in the four genotypes, the double mutant produced the highest number of abnormal seedlings (Fig. 5B). The effect is even more dramatic after storage at 60% RH (Fig. 5D), where almost all the double mutant seeds that had germinated gave rise to seedlings with an aberrant phenotype. In a CD test, simulating aging of seeds, seeds from the same batches and stored for 14 months at 32% (Fig. 6A) or 60% (Fig. 6B) RH were compared. The four genotypes showed viability loss in the order wild type, single mutants, and double mutant, although in these conditions the abi3-1 mutant seeds performed less well than the grs single mutants when seeds were stored at 32% RH. In seeds first stored at 60% RH, even the wild type is severely affected by the subsequent CD treatment, and the abi3-1 mutant again performs better then the single grs mutant. The additive effect of abi3-1 and grs is obvious in all test conditions.
Figure 5.
Seeds were stored at two different conditions, optimal 32% (A and B) and suboptimal 60% (C and D) RH. Germination behavior was tested during several time points during prolonged storage (A and C) of wild type (diamonds), abi3-1 GRS (triangles), ABI3 grs (squares), and abi3-1 grs (circles). Bars (C and D) = total germination after 22 months of storage. In each bar, the dark-gray part of the bar indicates the normal seedlings, and the white part represents the abnormal seedlings. Each point represents six replicates of 50 to 80 seeds ± se.
Figure 6.
Effect of a CD treatment on wild-type (ABI3 GRS), abi3-1 GRS, ABI3 grs, and abi3-1 grs seeds that had first been stored for 14 months in: A, optimal, 32% RH conditions; and B, suboptimal, 60% RH conditions. Both sets were treated for 1 (dark-gray bars), 2 (gray bars), 3 (light-gray bars), and 5 (white bars) d at 40°C and 85% RH. Control seeds (black bars) were only equilibrated at 85% RH. After this treatment, all seeds were dried back to 32% RH until sowing. Each bar represents a sowing of three replicates of 90 to 100 seeds ± se.
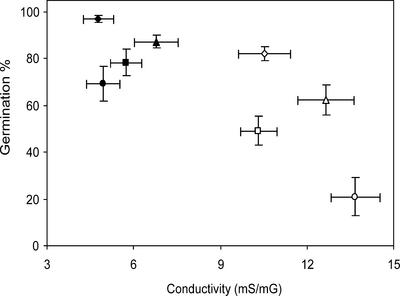
Damage during dry storage of seeds that may result in loss of viability has often been associated with damage to membranes and may result in leakage of electrolytes. The amount of electrolytes released from imbibed seeds and measured as conductivity can be used as a method to assess quality of seeds (Illipronti, 1997). In the present experiment where both genetic and environmental factors affect the loss of viability, we observed a negative correlation (r = -0.74) between both parameters when results from both storage treatments were combined (Fig. 7). However, this correlation was absent when the genotypes were compared that were stored at 32% RH.
Figure 7.
Conductivity measurement on seeds that were submerged in demiwater. The scatter diagram shows the effect of prolonged storage (19 months) on wild-type (ABI3 GRS, diamonds), abi3-1 GRS (triangles), ABI3 grs (squares), and abi3-1 grs (circles) under two conditions: favorable, 32% RH (black symbols); and unfavorable, 60% RH (white symbols), on germination and the conductivity after 24 h of imbibition. The germination assay was done with seeds from the same batches and age after a 7-d cold treatment, six replicates ± se. Conductivity was measured in three replicates of bulked seeds ± se.
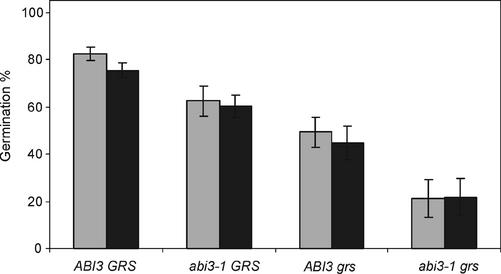
Higher chlorophyll amounts could increase the amount of reactive oxygen species in light (Heath and Packer, 1968). When this would be the main source of damage, more damage should be expected upon imbibition in light compared with germination in darkness. Seeds that had been stored for 19 months at 60% RH were compared for germination in light, respectively, in darkness (Fig. 8). The dark germinated seeds obtained only a 5-h light period to induce maximum germination. Again, differences were observed between different lines in total germination, but no significant differences could be observed due to differences in light conditions during germination.
Figure 8.
Germination of wild-type (ABI3 GRS), abi3-1 GRS, ABI3 grs, and abi3-1 grs seeds stored for 19 months at 60% RH. Maximal germination was induced by storing the seeds for 7 d in the cold before exposing the seeds to white light for 5 h (white light between 55–62 μmol m-2 s-1). Thereafter, seeds for the dark treatment (black bars) were transferred to darkness for 7 d or kept in the light (gray bars) for 7 d, after which the germination percentage was recorded. Each bar represents six replicates ± se.
DISCUSSION
The plant hormone ABA affects a wide range of processes in plants. Examples are: altered gene expression, tolerance to cold stress, inhibition of growth, and tissue-specific stress responses like the closure of stomata. Furthermore, the plant hormone plays a role in seed maturation and dormancy. The various mutants used to elucidate the role of ABA in these processes have been reviewed by Leung and Giraudat (1998), Koornneef et al. (1998), and Finkelstein et al. (2002). The many pleiotropic effects of the abi3 mutants indicate that this protein affects many processes during seed maturation and germination, including the storability of seeds. Storability under various conditions is an important aspect of seed quality. In this report, we describe the grs mutation that enhances some, but not all, aspects of the pleiotropic abi3 mutant phenotype. The observation that ABA sensitivity is not affected and that not all ABA-controlled processes such as seed dormancy and stomata closure seem affected indicates that this enhancer locus does not control ABA metabolism or the mode of action of ABA in general. For instance, this is observed in the aba1 mutant, which is also an enhancer of abi3-1 (Koornneef et al., 1989). Therefore, we conclude that the GRS locus promotes only ABI3-induced degradation of chlorophyll and the storability of seeds. Although the effects on the latter are mild, they are observed in different storage conditions that are relatively unfavorable for maintaining seed quality. Therefore, the grs mutant can be described as a mutant specifically affected in seed quality, which is part of an ABI3-induced pathway that protects seeds from deterioration in a dry state. Because this mutant also affects chlorophyll degradation, especially in the abi3-1 background, it cannot be excluded that the maintenance of a high chlorophyll content leads to this reduced seed quality.
The loss of chlorophyll as seeds mature is referred to as degreening. Papers by Johnson-Flanagan et al. (1994) and Ward et al. (1995) describe problems with green seeds in relation to canola (Brassica napus) seed oil where this residual amount of chlorophyll promotes oxidation of the oil leading to rancidity. This chlorophyll content is usually due to immature seeds present in a harvest (Johnson-Flanagan and Thiagarajah, 1990; Ward et al., 1995). Immature seeds are known to have a reduced viability and vigor as was shown by Bailly et al. (2001), and the number of normal seedlings increased as the seeds matured. Steckel et al. (1989) showed that the germination performance of carrot (Daucus carota) seeds improved with decreasing amounts of chlorophyll and viability increased as seeds matured. The same negative relationship could also be found for geranium and soybean (Glycine max) seeds (Kwong, 1991; Illipronti, 1997). In one seed lot of cabbage seeds that was sorted based on their chlorophyll fluorescence and tested for their seed quality, high amounts of chlorophyll, as measured by chlorophyll fluorescence, correlated with lower quality in normal germination assays and in artificially deteriorated seeds (Jalink et al., 1998). Senescence of the grs mutant fruits occurs at the same time after pollination as in wild-type control plants (data not shown), but this does not exclude the possibility of a reduced speed of seed maturation of the grs mutant within a normally maturing silique. It also cannot be excluded that the grs mutation affects specifically chlorophyll degradation, normally occurring at the end of the seed maturation process. A loss of chlorophyll breakdown is also described in many plants as a “tay-green”phenotype as reviewed by Thomas and Smart (1993), in which they also describe some crop plants that display this phenotype in their seeds. Chlorophyll breakdown in leaves occurs in several sequential steps: hydrolysis of chlorophyll into chlorophyllide and phytol by the action of chlorophyllase; removal of Mg2+ from chlorophyllide to yield phaeophorbide by Mg-dechelatase; and cleavage of phaeophorbide by phaeorphorbide a oxygenase to get red chlorophyll catabolite (RCC), which in turn is reduced by RCC reductase, and the final products are then transported over the tonoplast (Thomas et al., 2002). A number of enzymes involved in the breakdown of chlorophyll have been cloned in Arabidopsis: chlorophyllase (Tsuchiya et al., 1999), RCC-reductase (Wüthrich et al., 2000), and the ABC-type tonoplast transporter (Lu et al., 1998). However, none of them maps to the position found for grs.
The increased damage in the grs and abi3-1 mutant and especially their double mutant could be related to their higher chlorophyll content because photosynthesis is a source of active oxygen species (Thomas and Smart, 1993; Hendry, 1997) that can damage biological systems. The problem could be 2-fold, either an increase in active oxygen during storage decreased the seed quality, which could be possible because certainly for the storage at a higher RH, it is unlikely that all metabolic processes are stopped. Heath and Packer (1968) showed that light is required to generate radicals in chloroplasts and to prevent this effect, seeds were stored in excicators in darkness. Only upon sowing were seeds exposed to light for a short while. The other possibility is a lack of repair during the imbibition process; this would also be more damaging in light than in darkness. However, when seeds stored for 19 months at 60% RH were compared for germination in light or in darkness, no significant differences were observed, suggesting that imbibition in light does not increase viability loss.
Therefore, our physiological experiments do not suggest a causal relationship between chlorophyll retention and storability defects. Genetics studies with other species also fail to support a causal relationship. Mutants that retain chlorophyll in mature seeds have been described for soybean, but these d1d2 and cyt-G1 mutants have no impaired desiccation tolerance (Chao et al., 1995) such as the green-seeded severe abi3 alleles (Nambara et al., 1995) and abi3-1 aba-1 double mutants (Koornneef et al., 1989). Furthermore, Mendel's peas (Pisum sativum), disturbed in the enzyme phaeorphorbide a oxygenase and, therefore, remaining green (Thomas et al., 1996), have not been reported as having more problems with longevity compared with the yellow-seeded peas. Furthermore desiccation intolerance can occur together with chlorophyll degradation because the lec (leafy cotyledon) and fus3 (fusca) mutants (Meinke et al., 1994) do not acquire desiccation tolerance but degrade their chlorophyll during seed maturation. ABI3 regulates several processes in maturation such as acquiring dormancy and long-term storability (desiccation tolerance) and chlorophyll breakdown. All these processes could be sequential processes. The existence of among others rdo (reduced dormancy) and dog (delay of germination) mutants, not green and desiccation tolerant (Léon-Kloosterziel et al., 1996; Peeters et al., 2002; Bentsink, 2002), shows that acquiring dormancy can be separated from the other two. Therefore, we hypothesize that the green seed mutant described here is disturbed in a common step before chlorophyll breakdown and acquiring desiccation tolerance.
Both the grs and the abi3-1 mutations resulted in seeds that exhibited increased electrolyte leakage upon imbibition after storage, and the effects of the mutations are additive. Increased leakage of electrolytes indicates a problem with cellular membrane integrity. High levels of seed electrolyte leakage can be because of a rapid inrush of water due to a high water potential gradient between dry seeds and the imbibition medium (Pandey, 1992) or to the damage caused by radicals either generated by excited chlorophyll or in the mitochondria. The first reason was prevented by pre-incubation of the seeds at 100% RH overnight. The second cause is unlikely because germination in light and darkness showed no difference. The last reason cannot be excluded, and McDonald (1999) suggests it as one of the causes for poor germination of aged seeds. The direct relation between the function of the wild-type ABI3 and GRS genes in protection of the membranes during seed storage remains to be elucidated.
CD tests simulate aging of seeds under controlled conditions and can be used to predict seeds storage potential (Hampton and TeKrony, 1995). Our results here confirm this because the double mutant that showed a decrease of germination during storage at 60% RH and an increase in the number of abnormal seedlings upon storage under favorable (32% RH) conditions also showed the largest response to the CD treatment. This is in agreement with results described by Tesnier et al. (2002), who indicated that differences in the same CD treatments can be due to genotype and not to variation in the treatment itself.
MATERIALS AND METHODS
Genotypes
Four genotypes were used in all experiments wild type: the Landsberg erecta (ABI3 ABI3 GRS GRS) abi3-1 mutant (abi3-1 abi3-1 GRS GRS), the new single mutant, ABI3 ABI3 grs grs (brown seeded), and the double mutant abi3-1 abi3-1 grs grs.
Culture and Storage Conditions
Seeds were sown in petri dishes on water-saturated filter paper and incubated in the growth chamber at 25°C. After 2 d of incubation, germinated seeds were transferred into soil and cultivated in an air-conditioned greenhouse (18°C–23°C) in a 16-h photoperiod. Plants were grown in two plots each with three randomized replicates of 12 plants per genotype. Eight plants were harvested in bulk per replicate for physiological analysis. Seeds harvested from mature dry siliques were stored in darkness 2 months after harvest in incubators containing saturated solutions of different salts CaCl2 (20°C; 32% RH) and Ca(NO3) (20°C; 60% RH). Seeds were left to age and sown 5, 12, 17, and 22 months after harvest. Germination was scored after 7 d of incubation in a growth chamber (25°C, 16-h light period). With the germination test performed after storage for 22 months, the abnormal seedlings were also counted: seedlings with an altered of root growth, dwarfed, not fully unfolded cotyledons (cotyledons trapped in remains of the seed coat), or those that showed yellow or necrotic lesions on cotyledons.
Mapping
To map the grs locus, a mapping population was made by crossing the abi3-1 abi3-1 grs grs line to Columbia. An F2 of 400 plants was generated, and F3 seeds were harvested per F2 plant. By germinating these F3 seeds on 10 μm ABA, the abi3-1 homozygote plants were selected (approximately 25%). Only the DNA of these abi3 mutant plants was used for molecular marker analysis, and the green seed phenotype was determined by eye in all F3 lines. When there was any doubt about the phenotype, a next generation was grown, and the F3 seed phenotype was determined based on the F4 progeny. The map was created with the program Joinmap (version 3.0, Plant Research Intrenational, Wageninjon, The Netherlands) and is based on 88 individuals.
DNA Isolation and PCR Conditions
DNA was isolated from greenhouse-grown plants. The Bernatzky and Tanksley (1986) protocol was adapted for rapid extraction of small quantities. Flower buds were harvested in liquid nitrogen and grinded in 330 μL of a preheated (65°C) extraction solution (125 μL of extraction buffer, 0.35 m sorbitol, 100 mm Tris, 5 mm EDTA [pH 7.5], and HCl) together with 175 μL of lysis buffer (200 mm Tris, 50 mm EDTA, 2 m NaCl, and 2% [w/v] cetyl-trimethyl-ammonium bromide) to which 30 μL of 10% (w/v) Sarkosyl was added. The mixture of crude plant material and extraction solution was incubated for 30 min at 65°C during which period occasional shaking was applied. Hereafter, a solution of 400 μL of chloroform:isoamyl alcohol (24:1 [v/v]) was added and vortexed. After centrifuging for 5 min at maximum speed in an Eppendorf centrifuge (Eppendorf Scientific, Westbury, NY), the water phase was transferred to a new tube. An equal amount of isopropanol was added to the tube and to precipitate the DNA, it was inverted carefully several times. After 10 min of centrifugation at maximum speed in an Eppendorf centrifuge, the water-alcohol mixture was discarded, and the pellet was washed with 70% (v/v) cold ethanol. The pellet was left to dry and dissolved in water containing RNAse A and incubated 30 min at 37°C; thereafter, it was stored in the refrigerator. The CAPS and microsatellite markers were either found in The Arabidopsis Information Resource database (http://www.Arabidopsis.org) or taken from http://www.inra.fr/qtlat/msat. The PCR conditions for the CAPS markers were adapted to the primer pair for the microsatellite markers using a standard protocol of 30 s 94°C, 30 s 50°C, and 30 s 72°C (35 cycles).
Seed Germination Assays
To determine the sensitivity of germination to ABA, 40 to 80 seeds harvested from siliques of approximately the same age and all six replicates were sown on filter paper soaked with a range of ABA concentrations in 6-cm plastic petri dishes. The imbibed seeds were stored for 4 d at 4°C and subsequently incubated in a growth chamber (25°C, 16-h light period). Germination was scored 7 d after the start of incubation at 25°C. For the dormancy release experiments, seeds were harvested from siliques of flowers that were tagged on the day of anthesis. Germination was scored by sowing 30 to 60 seeds harvested on each of the six replicates on petri dishes containing filter paper soaked with water. Germination was scored after 7 d of incubation in a growth chamber (25°C, 16-h light period). This was done six times with 1-week intervals. To compare germination in light and darkness, seeds from mature dry siliques, stored for 19 months at 60% RH as described before, were sown in the dark in two batches of six replicates and stored for 7 d at 4°C. Thereafter, they were placed in light for 5 h (55–62 μmol m-2 s-1) to induce maximum germination; then, they were further imbibed in either darkness or a 25°C, 16-h light period for 7 d when germination was determined.
CD Tests
Seeds that had been stored for 14 months, as described before at 32% RH and 60% RH, were used in a CD assay. The CD assay (Tesnier et al., 2002) was performed as follows: Seeds were equilibrated at 85% RH (15°C), and 0-d controls were immediately dried back at 32% RH. Treatment is done by storing the seeds (at 85% RH) for a number of days at 40°C (1, 2, 3, and 5 d here); after this, these seeds were also dried back at 32% RH (20°C) and stored at approximately 4°C until the germination was tested. Four replicates of 90 to 100 seeds were germinated on water-saturated thick filter paper placed on a “Copenhagen”germination table, at 25°C and with an 8-h light period per day. To improve detection of radical protrusion, a black filter membrane (Schleicher & Schull, Dassel, Germany, reference no. 409712) was placed between the seeds and the filter paper. The seeds were covered with a transparent bell-shaped cover to prevent drying, and the final germination was evaluated 14 d after transferring the seeds to the “Copenhagen” germination table.
Chlorophyll Extraction
All genotypes grown in one plot as six randomized replicates were harvested as mature dry seeds. Five milligrams was left to stand overnight in 200 μL of dimethyl sulfoxide at 65°C, and a second extraction was done in 150 μL of dimethyl sulfoxide; therefore, in total, each genotype was measured 12 times. Absorption was measured at two wavelengths (649 and 665 nm) in a Beckman DU-64 spectrophotometer (Beckman Instruments, Fullerton, CA). Chlorophyll a content was calculated according to the following formula: (13.95 × A665) - (6.88 × A649). Chlorophyll b was calculated according to the following formula: (24.69 × A649) - (7.32 × A665).
Conductivity Measurement
Seeds from mature dry siliques were harvested and stored at 32% and 60% RH as described above. Nineteen months after harvest, 5 mg of two batches (one from 32% RH and one from 60% RH) of three replicates was weighed. Germination of the two batches was scored by sowing them and incubating at 4°C for 7 d. Final germination was scored after 7 d at 25°C in a 16-h light period. They were placed at 100% RH overnight to reduce imbibitional damage. After that, conductivity of all samples was measured in a CM100 conductivity meter (Reid & Associates, Durban, South Africa).
Acknowledgments
We would like to thank Karin Léon-Kloosterziel for isolating the original mutant, Soazig Guyomarc'h for her help with the germination assays and the mapping, Dr. Folkert Hoekstra for the use of the conductivity meter, and colleagues at the Laboratories of Plant Physiology and Genetics and the Stichting Technische Welenschappen Supervision Committee for useful suggestions and discussions.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.022715.
This work was supported by the Technology Foundation STW, by the Applied Science Division of NWO, and by the Technology Program of the Ministry of Economic Affairs.
References
- Bailly C, Audigier C, Ladonne F, Wagner M-H, Coste F, Corbineau F, Côme D (2001) Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J Exp Bot 52: 701-708 [DOI] [PubMed] [Google Scholar]
- Bentsink L (2002) Genetic analysis of seed dormancy and seed composition in Arabidopsis thaliana using natural variation. PhD thesis. Wageningen University, The Netherlands
- Bernatzky R, Tanksley SD (1986) Genetics of actin-related sequences in tomato. Theor Appl Genet 72: 314-324 [DOI] [PubMed] [Google Scholar]
- Bies-Etheve N, Silva Conceicao Ad, Giraudat J, Koornneef M, Léon-Kloosterziel KM, Valon C, Delseny M (1999) Importance of the B2 domain of the Arabidopsis ABI3 protein and 2S gene regulation. Plant Mol Biol 40: 1045-1054 [DOI] [PubMed] [Google Scholar]
- Chao WS, Liu V, Thomson WW, Platt K, Walling LL (1995) The impact of chlorophyll-retention mutations, d1d2 and cyt-G1, during embryogeny in soybean. Plant Physiol 107: 253-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MH (1980) Genetic aspects of seed quality. Hortic Sci 15: 771-774 [Google Scholar]
- Finkelstein R, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell Suppl 14: S15-S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton JG, TeKrony DM (1995) Handbook of Vigour Test Methods. The International Seed Testing Association, Zurich, pp 117
- Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys 125: 189-198 [DOI] [PubMed] [Google Scholar]
- Hendry GAF (1997) Free radicals in seeds: moving the debate forward. In RH Ellis, M Black, AJ Murdoch, TD Hong, eds, Basic and Applied Aspects of Seed Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 657-663
- Holdsworth M, Kurup S, McKibbin R (1999) Molecular and genetic mechanisms regulating the transition from embryo development to germination. Trends Plant Sci 4: 275-382 [Google Scholar]
- Illipronti RA Jr (1997) Variation in quality of individual seeds within a seed lot of soybean [Glycine max (L.) Merrill]. PhD thesis. Wageningen University, The Netherlands
- Jalink H, van der Schoor R, Frandas A, van Pijlen JG, Bino RJ (1998) Chlorophyll fluorescence of Brassica oleracea seeds as a non-destructive marker for seed maturity and seed performance. Seed Sci Res 8: 437-443 [Google Scholar]
- Johnson-Flanagan AM, Maret LLD, Pomeroy MK (1994) Humidification of green canola seed leads to pigment degradation in the absence of germination. Crop Sci 34: 1618-1623 [Google Scholar]
- Johnson-Flanagan AM, Thiagarajah MR (1990) Degreening in canola (Brassica napus cv Westar) embryos under optimum conditions. J Plant Physiol 136: 180-186 [Google Scholar]
- Koornneef M, Hanhart CJ, Hilhorst HWM, Karssen CM (1989) In vivo inhibition of seed development and reserve protein accumulation in recombinants of abscisic acid biosynthesis and responsiveness mutants in Arabidopsis thaliana. Plant Physiol 90: 463-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Léon-Kloosterziel KM, Schwartz SH, Zeevaart JAD (1998) The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol Biochem 36: 83-89 [Google Scholar]
- Kwong FY (1991) Research needs in the production of high quality seeds. In J Prakash, RLM Pierik, eds, Horticulture: New Technologies and Applications. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 13-20
- Léon-Kloosterziel KM, van de Bunt GA, Zeevaart JAD, Koornneef M (1996) Arabidopsis mutants with a reduced seed dormancy. Plant Physiol 110: 233-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49: 199-222 [DOI] [PubMed] [Google Scholar]
- Lu Y-P, Li Z-S, Drozdowicz YM, Hörtgensteiner S, Martinoia E, Rea PA (1998) AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell 10: 267-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MB (1999) Seed deterioration: physiology, repair and assessment. Seed Sci Technol 27: 177-237 [Google Scholar]
- Meinke DW, Franzmann LH, Nickle TC, Yeung EC (1994) Leafy Cotyledon mutants of Arabidopsis. Plant Cell 6: 1049-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke DW, Meinke LK, Showalter TC, Schissel AM, Mueller LA, Tzafrir I (2003) A sequence-based map of Arabidopsis genes with mutant phenotypes. Plant Physiol 131: 409-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SH, Halloin JM (2000) Genetic resistance to seed deterioration. In SH Moore, RW Yaklich, eds, Genetic Improvement of Seed Quality, Ed 31, Vol XVII. Crop Science Society of America, Madison, WI, pp 21-37 [Google Scholar]
- Nambara E, Keith K, McCourt P, Naito S (1995) A regulatory role for the ABI3 gene in the establishment of embryo maturation in Arabidopsis thaliana. Development 121: 629-636 [Google Scholar]
- Nambara E, Naito S, McCourt P (1992) A mutant of Arabidopsis which is defective in seed development and storage protein accumulation is a new abi3 allele. Plant J 2: 435-441 [Google Scholar]
- Ooms JJJ, Léon-Kloosterziel KM, Bartels D, Koornneef M, Karssen CM (1993) Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana: a comparative study using abscisic acid-insensitive abi3 mutants. Plant Physiol 102: 1185-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DR, Grossniklaus U (2002) The art and design of genetic screens: Arabidopsis thaliana. Nat Rev Genet 3: 124-136 [DOI] [PubMed] [Google Scholar]
- Pandey DK (1992) Conductivity testing of seeds. In HF Linskens, JF Jackson, LA Amberger, JC Autran, D Bullock, eds, Seed Analysis, Vol 14. Springer, Berlin, pp 273-304 [Google Scholar]
- Parcy F, Valon C, Kohara A, Miséra S, Giraudat J (1997) The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTOLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell 9: 1265-1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J (1994) Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6: 1567-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters AJM, Hanhart CJH, Leon-Kloosterziel KM, Zeevaart JAD, Koornneef M (2002) Characterization of mutants with reduced seed dormancy at two novel rdo loci and a further characterization of rdo1 and rdo2 in Arabidopsis. Physiol Plant 115: 604-612 [DOI] [PubMed] [Google Scholar]
- Steckel JRA, Gray D, Rowse HR (1989) Relationships between indices of seed maturity and carrot seed quality. Ann Appl Biol 114: 177-183 [Google Scholar]
- Tesnier K, Strookman-Donkers HM, van Pijlen JG, van der Geest AlHM, Bino RJ, Groot SPC (2002) A controlled deterioration test for Arabidopsis thaliana reveals genetic variation in seed quality. Seed Sci Technol 30: 149-165 [Google Scholar]
- Thomas H, Ougham H, Canter P, Donnison I (2002) What stay-green mutants tell us about nitrogen remobilization in leaf senescence. J Exp Bot 53: 801-808 [DOI] [PubMed] [Google Scholar]
- Thomas H, Schellenberg M, Vicentini F, Matile P (1996) Gregor Mendel's green and yellow pea seeds. Bot Acta 109: 3-4 [Google Scholar]
- Thomas H, Smart CM (1993) Crops that stay green. Ann Appl Biol 123: 193-219 [Google Scholar]
- Tsuchiya T, Ohta H, Okawa K, Iwamatsu A, Shimada H, Masuda T, Takamiya K-I (1999) Cloning chlorophyllase, the key enzyme in chlorophyll degradation: finding of a lipase motif and the induction by methyl jasmonate. Proc Natl Acad Sci USA 96: 15362-15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward K, Scarth R, Daun JK, Vessey JK (1995) Chlorophyll degradation in summer oilseed rape and summer turnip during seed ripening. Can J Plant Sci 75: 413-420 [Google Scholar]
- Wüthrich KL, Bovet L, Hunziker PE, Donnison I, Hörtgensteiner S (2000) Molecular cloning, functional expression and characterization of RCC reductase involved in chlorophyll catabolism. Plant J 21: 189-198 [DOI] [PubMed] [Google Scholar]