Abstract
When stimulated to bend downward by being held at 45 degrees off vertical but unable to penetrate into agar-based media, Arabidopsis roots develop waving and looping growth patterns. Here, we demonstrate that ethylene modulates these responses. We determined that agar-containing plates sealed with low-porosity film generate abiotic ethylene concentrations of 0.1 to 0.3 μL L-1, whereas in plates wrapped with porous tape, ethylene remains at trace levels. We demonstrate that exogenous ethylene at concentrations as low as a few nanoliters per liter modulates root waving, root growth direction, and looping but through partly different mechanisms. Nutrients and Suc modify the effects of ethylene on root waving. Thus, ethylene had little effect on temporal wave frequency when nutrients were omitted but reduced it significantly on nutrient-supplemented agar. Suc masked the ethylene response. Ethylene consistently suppressed the normal tendency for roots of Landsberg erecta to skew to the right as they grow against hard-agar surfaces and also generated righthanded petiole twisting. Furthermore, ethylene suppressed root looping, a gravity-dependent growth response that was enhanced by high nutrient and Suc availability. Our work demonstrates that cell file twisting is not essential for root waving or skewing to occur. Differential flank growth accounted for both the extreme root waving on zero-nutrient plates and for root skewing. Root twisting was nutrient-dependent and was thus strongly associated with the looping response. The possible role of auxin transport in these responses and the involvement of circadian rhythms are discussed.
Plants, as sessile organisms, require flexible growth responses to adapt to stressful conditions, including stresses related to soil mechanical strength. To penetrate soils, roots must displace or deform soil aggregates. Even in the loosest soils, root tips experience combinations of mechanical forces, one of which is touch. Roots are very sensitive to touch, and thigmomorphogenic responses have been studied under a variety of experimental systems ranging from exogenous applications of transient forces to gravity-induced tests (e.g. Goss, 1977; Wilson et al., 1977; Okada and Shimura, 1990; Legué et al., 1997). One extensively studied morphological response is root waving. Since Okada and Shimura (1990) first described and attributed the wave-like growth in the Landsberg erecta (Ler) ecotype of Arabidopsis to a gravity-induced touch response, Simmons et al. (1995) and Mullen et al. (1998) hypothesized that gravity and/or circumnutation provided the stimuli. Although phenotypes are well described at the whole-root level, the underlying cellular mechanisms are not well understood. We recently discovered that the gaseous environment within a petri plate could override gravitational effects on root waving, although the causal agent(s) remained unidentified (Buer et al., 2000). Wrapping plates with a porous material that allows gas exchange between the atmosphere and the inside of the plate, as opposed to a low-porosity material that inhibits gas exchange, produced remarkably different root phenotypes. These phenotypes ranged from straight growth to waving or looping depending on nutrient conditions. Moreover, plants growing in sealed plates developed increased root hair densities, radial root expansion, decreased root elongation, reduced leaf expansion, and cotyledon epinasty, all phenomena associated with ethylene exposure (Baskin and Williamson, 1992; Kieber et al., 1993; Masucci and Schiefelbein, 1994; Tanimoto et al., 1995; Smalle and van der Straeten, 1997; Pitts et al., 1998). This led us to hypothesize that ethylene signaling was involved in controlling root waving and the morphological differences observed between seedlings grown in aerated and sealed plates. Ethylene gas regulates many growth processes and stress responses in plants (Mattoo and Suttle, 1991; Abeles et al., 1992). Many morphological changes in impeded roots mimic changes that occur during ethylene exposure (Masle, 2002). Ethylene is evolved during root mechanical impedance (e.g. maize [Zea mays]; Kays et al., 1974; Sarquis et al., 1991) and has been identified as one of the root signals causing reduced root and leaf elongation in impeded tomato (Lycopersicon esculentum) plants (Hussain et al., 1999, 2000). Ethylene interactions seem complex. Ethylene cross-talks with other growth regulators (abscisic acid [Beaudoin et al., 2000], auxin [Rahman et al., 2001], cytokinins [Cary et al., 1995], and gibberellins [Ghassemian et al., 2000; Rahman et al., 2000]) and is also involved in Suc signaling (Zhou et al., 1998; Gibson, 2000).
We conducted experiments to test whether ethylene acts as a modulator of root waving and associated morphological responses in Arabidopsis. We held the gravity vector constant and manipulated exogenous ethylene concentrations around seedlings growing on the surface of tilted agar plates. We demonstrate that ethylene is a key factor in the control of morphogenetic responses in roots repetitively challenged by a gravity-induced mechanical stimulus. Furthermore, we show that ethylene effects on root waving, root looping, cell file twisting, and differential flank growth are modulated by Suc and nutrient ion concentrations in the agar. Whether these interactions entirely reflect a direct influence of Suc and nutrients on ethylene signaling or also indicate Suc and ionic effects on the physical properties of the medium, including its surface characteristics, remains to be established.
RESULTS
Agar-Containing Plates Evolve Significant Levels of Ethylene
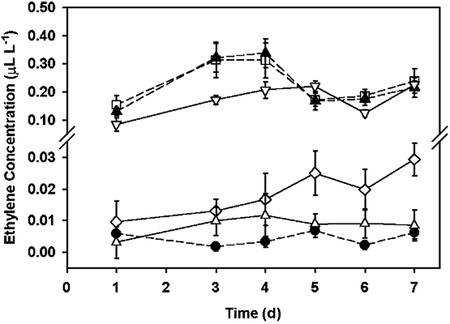
Gas chromatography was used to determine ethylene concentrations in the headspace of petri plates wrapped with Nescofilm, a paraffin-based film (hereafter sealed plates) or with surgical tape (hereafter aerated plates). Figure 1 shows these concentrations over a 7-d period with d 1 corresponding to the 2nd d after plate preparation and, for plates with seedlings, to the day when most seeds germinated. Aerated plates containing plants had nearly undetectable levels of ethylene. In sealed plates, however, ethylene concentrations varied between 0.1 and 0.3 μL L-1. Remarkably, these concentrations were not significantly affected by the presence or absence of seedlings in the plate, demonstrating that the measured ethylene was mostly of abiotic origin. Furthermore, Figure 1 indicates that larger amounts of ethylene were evolved in plates in which the agar was not supplemented with nutrients (zero nutrient plates). The addition of KMnO4 crystals lowered the ethylene concentrations within sealed plates to 0.03 μL L-1 or less.
Figure 1.
Agar-containing petri dishes wrapped with low-porosity film generate ethylene. Ethylene concentrations (microliters per liter) measured using flame-ionization detector gas chromatography in the headspace of 1.5% (w/v) Bacto-agar containing petri plates, tilted at 45° over 7 d. Treatments were: Murashige and Skoog nutrients and 3% (w/v) Suc (solid lines) or zero nutrients, zero Suc (dashed lines). White symbols denote plates without seedlings and closed symbols plates with seedlings. All plates were wrapped with Nescofilm (sealed plates) except for data points represented by a black circle corresponding to samples from aerated plates (wrapped with porous surgical tape). Sealed plates containing KMnO4 crystals (0.1 or 0.01 g) are represented by (▵) and (⋄), respectively. Day 1 corresponds to the day of germination. Error bars are se (n = 12).
Exogenous Ethylene Reduces Root Elongation and Increases the Amplitude and Spatial Frequency of Root Waving
To test the influence of exogenous ethylene on root morphogenesis and waving phenotypes, we directly manipulated ethylene concentrations within the plate headspace. Our previous study showed ionic effects on root waving and looping, suggesting ethylene: nutrient interactions (Buer et al., 2000). Conventional methods for blocking the action of ethylene or increasing its production by adding chemical compounds (e.g. silver ions or ethephon, which is extremely acidic) to the growth medium were therefore avoided. Instead, we injected known volumes of ethylene gas directly into culture chambers or oxidized the ethylene evolved by the medium by enclosing KMnO4 crystals within the plates, taking care to keep them out of contact with the agar and the seedlings.
Our earlier observations indicated there is a correlation between root elongation rate, spatial density, and amplitude of waving (Buer et al., 2000). To determine the role of ethylene on waving, independent of its effects on growth rate, we varied ethylene concentrations across a range of nutrients and Suc concentrations in the agar. This created a 4-fold variation in the average root elongation rate over 7 d. We quantified these effects by comparing temporal wave frequency (waves per day), spatial wave frequency (number of waves per millimeter of root, which integrates variations in wavelength and wave amplitude), root axis angle (the overall deviation from the vertical axis, to the right or to the left when viewing the seedling from the cotyledon to the root tip), and the incidence of looping roots (percentage of roots forming a complete loop per plate). Controls (not shown) included zero ethylene injection and zero KMnO4 addition under all conditions.
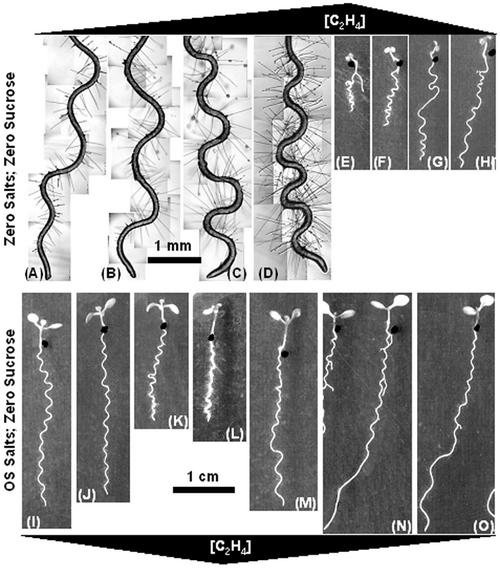
Manipulating exogenous ethylene concentrations caused a wide range of root-waving and morphogenetic phenotypes, shown pictorially in Figure 2 and quantitatively in Figure 3. Ethylene increased root hair density and root diameter (45% and 27%, respectively, under zero nutrient conditions [Fig. 2, A–D]). It stimulated cotyledon epinasty (Fig. 2, E, F, K, and L) and reduced seedling growth (Figs. 2, E–H and I–O, and 3A). Ethylene increased the tightness of root bending regardless of nutrient supplementation (Fig. 2) and, under zero nutrient conditions, it dramatically increased the spatial frequency of root waving (Fig. 3B). These data unambiguously demonstrate a role for ethylene in the modulation of root waving on a spatial scale.
Figure 2.
Ethylene affects root-waving patterns. Representative root phenotypes of Arabidopsis Ler growing on 1.5% (w/v) Bacto-agar plates tilted at 45° and exposed to varying ethylene concentrations. A through H, Zero nutrient salts without added Suc; I through O, Okada and Shimura salts without added Suc. A through D, Composite micrographs of roots in an aerated plate (A) or sealed plates with 0.1 g KMnO4 (B), 0.01 g KMnO4 (C), or no KMnO4 (D). E through O, Scans of whole seedlings grown in aerated plates injected with ethylene to final concentrations of 1 μL L-1 (E and L), 0.1 μL L-1 (F and M), 0.01 μL L-1 (G and N), or 0.001 μL L-1 (H and O) or in sealed plates containing KMnO4 to oxidize ethylene (0.1 g [I], 0.01 g [J], and 0 g [K]). Increasing and decreasing ethylene levels are indicated by the black triangles. All images represent roots as they appeared viewed from above the plate. Scale bars in A through D = 1 mm, in E through O = 1 cm.
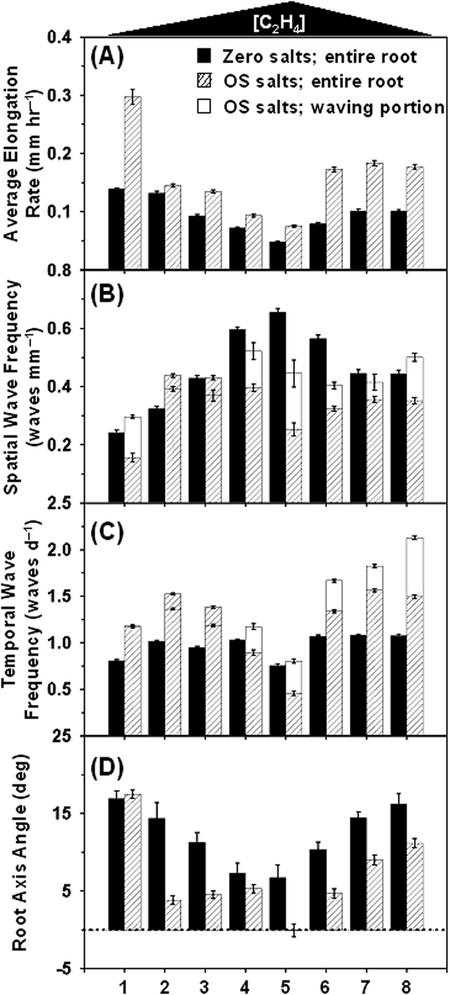
Figure 3.
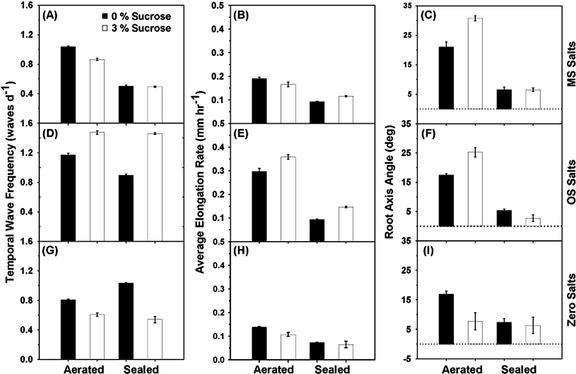
Root growth responses to ethylene differ in the presence of nutrient supplements. A, Quantitative assessment of the root-wave phenotypes on zero nutrient salts (black bars) and Okada and Shimura salts (hatched bars). The black and hatched bar measurements represent values averaged over the entire root, whereas the white bars (B and C) describe values calculated over the portion of the root where regular waving was occurring with little variation in periodicity. The triangle above the panels indicates variations in ethylene concentrations. Abscissae conditions are: 1, aerated plates; 2 to 4, sealed plates with 0.1, 0.01, and 0 g KMnO4 crystals, respectively; 5 to 8, data from aerated plates exposed to ethylene concentrations of 1 μL L-1 (5), 0.1 μL L-1 (6), 0.01 μL L-1 (7), and 0.001 μL L-1 (8). The dashed line in D indicates vertical root elongation (0°); positive angles describe righthanded deviation of the root axis from the vertical when roots are viewed downwards from the base of the hypocotyl to the root tip. Bars are the mean of at least 50 pooled roots from at least six replicated plates. Error bars are the se.
Ethylene Modulates the Temporal Frequency of Root Waving in a Nutrient-Dependent Manner
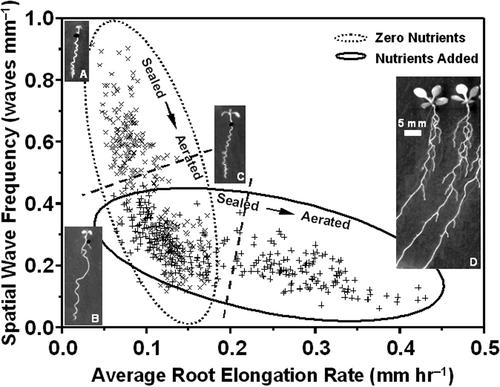
The data in Figure 3 indicated an inverse correlation between the effects of ethylene on root elongation rate and on the spatial frequency of waving (compare trends in Fig. 3, A and B). Figure 4 depicts the overall quantitative relationship between the two parameters, across the range of nutrients, Suc, and ethylene concentrations analyzed in this study. The individual values recorded for all treatments fell along a common curve, with the number of waves per unit root length decaying exponentially as roots elongated faster, either because nutrients were added to the medium, or because ethylene concentrations around the seedlings were lowered. Therefore, to determine whether ethylene had direct effects on the signaling of waving in roots, independent of growth effects, we examined the rhythm of waving (Fig. 3C). Waving frequency was calculated on a temporal basis (number of waves per unit of time). On zero nutrient plates, waves were produced at a frequency of about one wave per day (Fig. 3C, black bar). Wave production was little affected by variations in ethylene concentrations, except at the highest concentration of 1 μL L-1, which generated an approximately 25% lower waving frequency (Fig. 3C, black bar, treatment 5). In contrast, on Okada and Shimura-supplemented plates, exposure to 1 μL L-1 ethylene reduced the wave frequency almost 3-fold in comparison with wave frequencies detected at the lowest ethylene levels (Fig. 3C, hatched bar). On Murashige and Skoog-supplemented agar, the wave frequency was approximately halved in sealed plates (0.1–0.3 μL L-1 ethylene) compared with aerated plates (trace ethylene levels; data not shown). This demonstrates that ethylene can directly modulate the rhythm of root waving but that the extent of modulation is nutrient dependent.
Figure 4.
Spatial wave frequency (number of waves per unit root length) depends on root elongation rate (mm h-1) and ethylene exposure. Values on the x and y axes describe averages over the 7 d after germination. All plates were tilted at a 45° slope from the vertical. Growth was on 1.5% (w/v) Bacto-agar without or with nutrient supplements (Murashige and Skoog salts or Okada and Shimura salts). The dashed line across each set of data separates sealed versus aerated plates. The equation for the exponential fit of all data is y = 0.16 + 1.6e-20x, R = 0.84, and χ2 = 0.0097. Representative phenotypes across the range of conditions are shown, all viewed from above the plate: A, zero salts, 0% (w/v) Suc, and sealed plate; B, zero salts, 0% Suc, and aerated plate; C, Okada and Shimura salts, 0% (w/v) Suc, and sealed plate; and D, Okada and Shimura salts, 0% (w/v) Suc, and aerated plate. The scale bar = 5 mm.
Ethylene Suppresses the Rightward Skewing of Roots, and This Correlates Strongly with Its Inhibition of Root Elongation
Ethylene reduced the characteristic righthanded skewing of roots of the Ler ecotype (e.g. Fig. 2, N and O). This was observed on both nutrient-deprived and -supplemented plates (Fig. 3D), with a greater than 10° lefthanded shift (i.e. toward the vertical) in the root axis angle as ethylene concentrations increased from 1 nL L-1 to 1 μL L-1. This suppression of root skewing was correlated with the inhibition of root elongation rate by ethylene (Fig. 3, compare A and D).
Suc Influences the Waving, Skewing, and Looping Responses of Arabidopsis Roots, and These Effects Are Nutrient and Ethylene Dependent
There is compelling evidence for a direct role for nutrient ions, especially nitrogen (for review, see Forde, 2001) and Suc (e.g. Koch, 1996; Smeekens, 2000), on gene expression and the signaling of stress to roots. We therefore compared growth responses with either 0% or 3% (w/v) Suc in the agar, in sealed and aerated plates, and with or without supplementing the media with one of two defined nutrient formulations (Murashige and Skoog [1962] or Okada and Shimura [1990]), chosen for their wide use in Arabidopsis studies. The results of these comparisons are described in Figures 5 and 6. Suc had marked but complex effects on root phenotypes. The root-waving response to Suc was generally similar in aerated and sealed plates but varied considerably according to the availability and composition of nutrients (Fig. 5, A, D, and G). On zero nutrient plates, Suc consistently decreased the frequency of root waving, from about 1 wave per day without Suc to 0.5 waves per day with 3% (w/v) Suc added (Fig. 5G). In presence of Okada and Shimura nutrients, however, Suc addition increased temporal wave frequency (Fig. 5D), but on Murashige and Skoog nutrients, Suc had no clear effect on the rate of root waving (Fig. 5A).
Figure 5.
Suc and nutrient supplements affect growth responses to ethylene. Temporal wave frequency, average root elongation rate over the 7-d period following germination and average root axis angle on d 7 are shown for Ler seedlings grown on 1.5% (w/v) Bacto-agar supplemented with Murashige and Skoog salts (A–C), with Okada and Shimura salts (D–F), or under zero nutrients (G–I), in aerated or sealed plates. For each category of plates, data are shown for 0% (w/v) Suc (solid bars) or with 3% (w/v) Suc added (open bars). The dashed lines in graphs C, F, and I indicate vertical root elongation (0°), and positive angles describe righthanded skewing when seedlings are viewed from the cotyledon downwards to the root tip. Bars are the mean of at least 25 pooled roots from at least six replicated plates. Error bars are the se.
Figure 6.
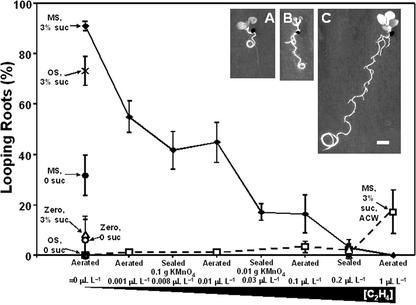
Suc stimulates root looping, and ethylene overrides this effect. Looping was always clockwise (CW, viewed from above the plate) except when stated otherwise (i.e. ACW, anticlockwise). Insets, Representative scans of seedlings in an aerated plate on 1.5% (w/v) Bactoagar supplemented with Murashige and Skoog-nutrient salts and 3% (w/v) Suc (A); the same conditions except exposure to 1 μLL-1 ethylene (B), or grown on Okada and Shimura nutrient salts and 3% (w/v) Suc, in an aerated plate (C). The bars are ses for at least 50 pooled samples from six replicates. The scale bar = 1 cm.
Ethylene clearly influenced the effects of Suc on root skewing (Fig. 5, C, F, and I). In sealed plates (0.1–0.3 μL L-1 ethylene), Suc addition did not alter root skewing. In the ethylene-free (aerated) plates, however, Suc caused roots to skew further to the right when nutrients were supplied (Fig. 5, C and F) and caused an opposite shift i.e. toward the vertical on zero-nutrient plates. Interestingly, these shifts in root axis angle with increasing Suc concentration were not always correlated with Suc effects on root elongation rate (compare Fig. 5, B and C), showing that the two parameters are not intrinsically linked.
Suc Stimulates Looping, and Ethylene Suppresses That Response
The frequency of looping was plotted against ethylene concentrations for all conditions under which it was recorded (Fig. 6). Manipulating Suc and nutrient concentrations in the agar within both aerated and sealed plates revealed their role and interactions with ethylene in the signaling of root looping. Suc promoted root looping, but ethylene overrode these effects. Looping was rarely observed on zero-nutrient plates (Fig. 6, ≈0 μL L-1 on x axis) even after Suc addition. In fact, the most obvious Suc effect on zero-nutrient plates was a decrease in seed germination and retarded seedling development (data not shown). In ethylene-free plates supplemented with Murashige and Skoog or Okada and Shimura nutrients, adding Suc caused a majority of roots to describe a complete circular clockwise loop before penetrating the semisolid medium (Fig. 6, A and C). On Okada and Shimura medium, looping was observed on 3% (w/v) Suc only and occurred after a lag of about 2 d (Fig. 6C) whereas looping occurred sooner on Murashige and Skoog nutrients supplemented with Suc. Increasing ethylene concentrations reduced the incidence of looping and at 0.2 μL L-1, suppressed looping almost completely (Fig. 6). This trend, however, was reversed on Murashige and Skoog salts at the highest ethylene concentration of 1 μL L-1. Under these conditions, approximately 20% of roots looped, although the direction was anticlockwise and occurred after a time lag (Fig. 6B).
Root Waving and Skewing Do Not Depend on Cell File Twisting
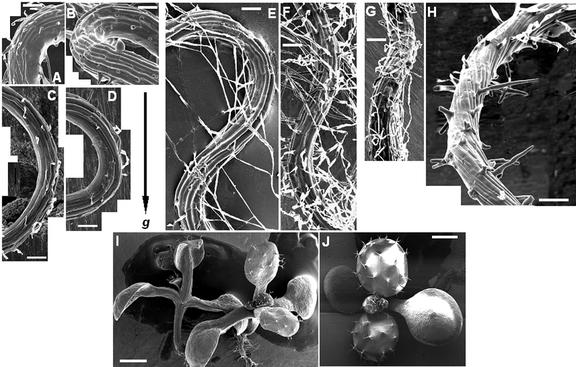
Previous studies have consistently reported cell file twisting in roots that either wave or skew across tilted agar plates. To determine whether root twisting is an essential component of root-waving, -skewing, and -looping phenotypes, cryo-stage scanning electron micrographs were taken of roots grown under the various conditions described in this study. Under zero-nutrient conditions, no cell file rotation could be detected in wavy roots irrespective of plate aeration and Suc concentration (Fig. 7, A–D). Roots growing on nutrient-supplemented agar often showed alternating lefthanded and righthanded twisting of epidermal cell files according to the direction of root elongation, (Fig. 7, E and G). This was not always the case, however, as exemplified in Figure 7F, even in roots that traversed across the agar surface while continuing to wave (Fig. 8). All looping roots were characterized by pronounced cell file twisting (Fig. 7H); that twisting was always lefthanded in clockwise loops. Interestingly, in sealed plates or plates in which high concentrations of ethylene were injected (1 μL L-1), a slight righthanded cell file twisting was visible on petioles (Fig. 7I). No such response could be detected in aerated plates (Fig. 7J) nor in any of the plates from which nutrients had been omitted (not shown).
Figure 7.
Waving does not require cell file twisting. Cryo-stage scanning electron micrographs show Ler roots grown on various nutrient salt concentrations and plate aeration. All roots were grown in 1.5% (w/v) Bacto-agar tilted plates (45° angle from the vertical). Treatments are: A and B, zero salts, 0% (w/v) Suc, and sealed plates; C and D, zero salts, 0% (w/v) Suc, and aerated plates; E, Okada and Shimura salts, 0% (w/v) Suc, and sealed plates; F and G, Okada and Shimura salts, 3% (w/v) Suc, and sealed plates; H, cell file twisting in a looping root on Murashige and Skoog salts, 3% (w/v) Suc, and aerated plates; I, righthanded twisting in petioles on Okada and Shimura salts, 3% (w/v) Suc, and sealed plates; J, the absence of twisting in petioles of Okada and Shimura seedlings grown on 3% (w/v) Suc, and aerated plates. The petiole phenotypes for the other media formulations were similar (i.e. showed no twisting) irrespective of Suc or nutrients addition. Scale bars in A through G = 100 μm, in I through J = 1 mm.
Figure 8.
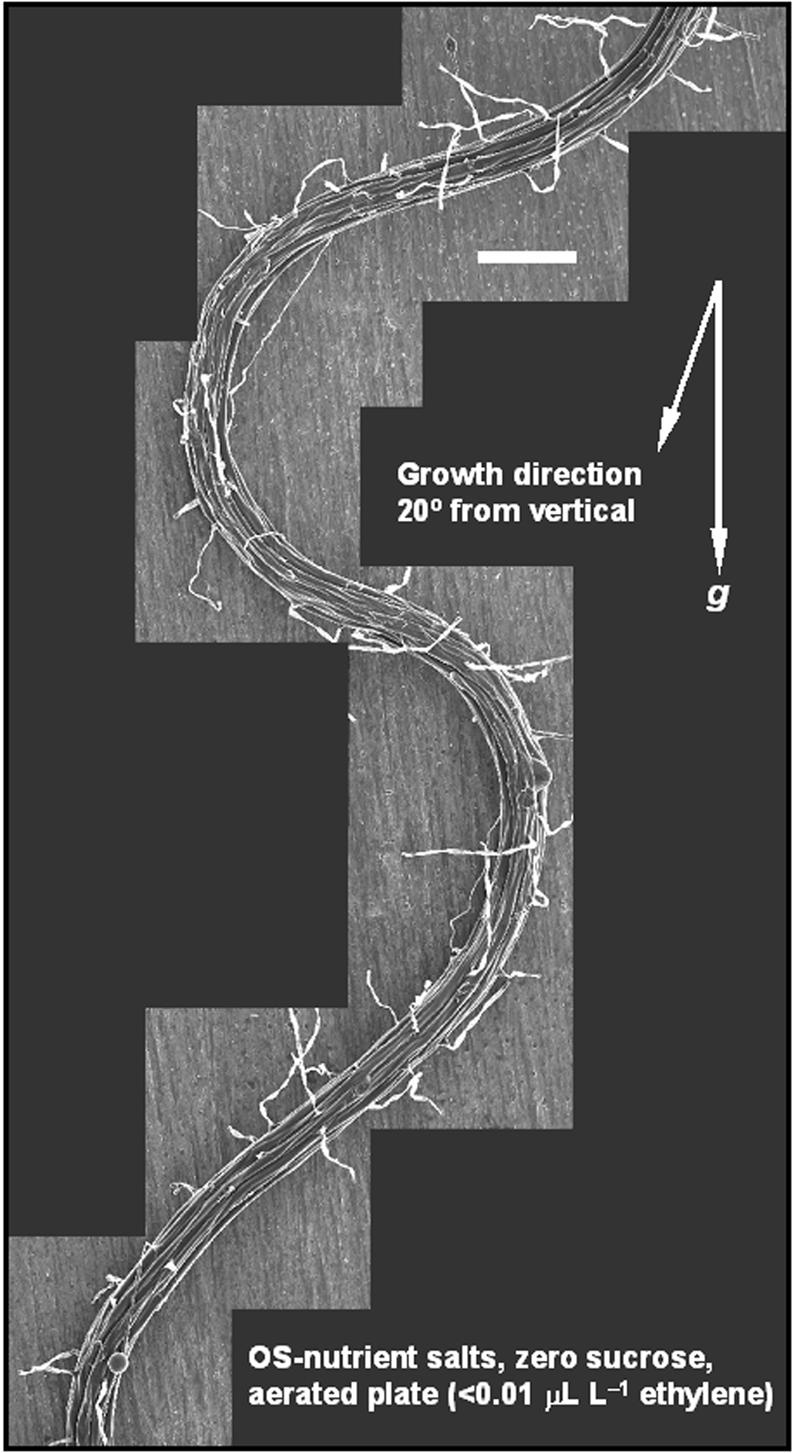
Skewing does not require cell file twisting. A composite cryo-stage scanning electron micrograph of a typical waving root skewing across the agar surface. The root was grown on 1.5% (w/v) Bacto-agar, Okada and Shimura salts, 0% (w/v) Suc, and aerated plates. Note that cell file twisting is mostly absent and limited to the inception of a change in direction. The arrows indicate the gravity vector and 20° from vertical for the growth direction. Scale bar = 200 μm.
Interactive Effects of Ethylene and Auxin Transport Inhibition
The above results demonstrated the involvement of differential flank growth in root waving, especially in roots in which cell file rotation could not be detected. To explore the possibility that this could be caused by changes in auxin distribution, we examined the influence of the polar auxin transport inhibitor 1-N-naphthylphthalamic acid (NPA; Lomax et al., 1995) on root waving and looping. Results are shown in Supplementary Figure 1 (supplementary data can be viewed at http://www.plantphysiol.org) for roots grown on zero nutrient agar (Supplementary Fig. 1, A–D) or on Murashige and Skoog nutrient-supplemented agar (Supplementary Fig. 1, E–H). Under ethylene-accumulating conditions, auxin transport inhibition by NPA caused slower root elongation but had no clear effects on the periodicity of waving (Supplementary Fig. 1, B and F compared with A and E) and only occasionally induced a reduced gravity response (Supplementary Fig. 1F). For aerated plates, however, where ethylene gas was hardly detectable, similar additions of NPA had marked effects on root gravitropism (Supplementary Fig. 1, D and H), promoting root looping and at higher doses, causing roots to elongate in random, unpredictable directions.
DISCUSSION
Exogenous Ethylene Modulates Root Tropisms and Can Override Effects of Gravity-Induced Mechanical Stimulus
Roots growing in soil face complex conditions. They are continuously exposed to gravity, encounter mechanical forces that constrain their growth, and experience unpredictable changes in nutrient, carbon, and water availability. The aim of this work was to uncouple and create controlled variations of these parameters to assess their role in root morphogenesis. Our specific goal was to determine how ethylene affects the movement of Arabidopsis roots, which include waving or looping and, in the Ler ecotype, a tendency to skew to the right as they elongate along a hard agar surface. The possibility that ethylene was involved arose from our recent observations (Buer et al., 2000) that these responses, although widely attributed to a gravity-induced mechanical stimulus, could be overridden by complex interactions between the gaseous environment of the seedlings within plates and the chemical and structural properties of the growth medium. In the experiments described here, we directly varied ethylene concentrations while keeping the culture plates at a constant 45° slope from the vertical. We show for the first time that ethylene modulates the waving, looping, and skewing phenotypes that are characteristic of Arabidopsis roots challenged with an impenetrable agar barrier during gravistimulated downward bending (Buer et al., 2000; Migliaccio and Piconese, 2001). We also show that ethylene can induce righthanded petiole twisting. Nevertheless, our analysis indicates that root waving and skewing are not wholly dependent on organ twisting and can, under certain conditions, be accomplished by differential flank growth.
Our experiments demonstrate that the buildup of ethylene in sealed plates can account for the differences in root phenotypes we reported earlier (Buer et al., 2000). From the quantitative changes measured in aerated plates upon addition of known amounts of ethylene, it can be inferred that the ethylene concentrations in the headspace of sealed plates were between 0.1 and 1 μL L-1. Using gas chromatography, we determined that within 2 d of the agar-containing plates being sealed with low-porosity film, significant ethylene levels are detectable within the headspace, with maximum values of 0.2 to 0.3 μL L-1 being reached on d 5, consistent with the above estimates. There have been reports of ethylene evolution by impeded roots (Kays et al., 1974; Sarquis et al., 1991). Remarkably, however, the ethylene buildup measured in our experiments was not significantly affected by the presence or absence of seedlings (Fig. 1), demonstrating that it was mostly of abiotic origin. Because ethylene was undetectable in empty sealed plates (data not shown), we conclude that roots were affected by ethylene evolved from the agar itself rather than from the sealing film or the plate walls (Mensuali-Sodi et al., 1992) and that the reactions involved were influenced by the agar ionic content (Fig. 1). Beyond its role in the present experiments, these results suggest that ethylene is likely to be a frequent confounding factor in the interpretation of in vitro experiments with Arabidopsis, at least when agar is used as a gelling agent within sealed plates.
By injecting ethylene into the headspace of aerated plates, or by oxidizing the ethylene evolved in sealed plates with KMnO4, we obtained reversion of root phenotypes, between straight growth and waving or looping or between vertical growth and skewing, despite no change in gravity or mechanical stimulus to the growing root tip. These effects occurred across a range of ethylene concentrations, from a few nanoliters per liter to about 100 nL L-1. Previous observations that ethylene causes roots to coil (Ipomoea pentaphylla [Vyas et al., 1973] and tomato [Woods et al., 1984]), or to inconsistently undulate (bean [Phaseolus vulgaris] and pea [Pisum sativum; Goeschl and Kays, 1975]) are likely to relate to the phenomena we have identified, although these earlier studies used much higher ethylene concentrations. Similarly, Burg and Kang (1993) used 100 μL L-1 ethylene to revert the gravitropic response of pea stems. Recent reports, however, have revealed significant ethylene effects on hypocotyl growth (Arabidopsis [Larsen and Chang, 2001]) or gravitropic curvature (tomato [Madlung et al., 1999] and Arabidopsis [Lu et al., 2002]) in the range of low concentrations shown in our study to affect root elongation rates and tropisms. The role of ethylene that we demonstrate in the present experiments adds to the mounting evidence that this hormone mediates local and systemic responses to physical stresses in general (Jaffe and Forbes, 1993; Botella et al., 1995; Jaffe et al., 2002), in both leaves and roots.
Evidence for Interactions between Ethylene-, Sugar-, and Nitrogen-Signaling Pathways in the Control of Root Waving and Root Looping
Root waving, looping, and skewing all respond to ethylene but in different ways. Ethylene corrects for the ecotype-specific righthanded skewing of roots across the agar surface and does this consistently across a range of nutrient and Suc conditions. In contrast, the inhibitory effect of ethylene on root looping requires both Suc and nutrients. Root-waving responses to ethylene are more complex. Ethylene generally promotes dense waving (Figs. 2 and 3) but partly through a reduction on root elongation rates. When these effects are accounted for, and temporal waving frequencies are examined, complex interactions are revealed between ethylene, Suc concentration, nutrient availability, and composition (Figs. 3 and 5). Ethylene slows down waving in the presence of nutrients while having little effect in their absence (Figs. 3 and 5). Suc addition reduced this response or completely overrode it (Murashige and Skoog medium and Okada and Shimura medium, respectively). These observations demonstrate for the first time an antagonism between ethylene and Suc in the control of the root waving and looping phenotypes and add to the increasing evidence for cross-talk between the ethylene- and Suc-signaling cascades. Furthermore, they indicate that this ethylene: Suc antagonism is nutrient dependent. Recent studies of sugar-insensitive mutants indicate that a sugar: ethylene antagonism also operates in cotyledons and shoots for the control of many developmental transitions (Zhou et al., 1998; Gibson, 2000). A number of stress-related genes are induced by sugars (Smeekens, 2000). Conversely, many sugar response mutants have altered responses to phytohormones (Sheen et al., 1999), suggesting that genes involved in sugar sensing and signaling could be part of a stress-response network and act in the same pathways as ethylene-regulated genes or in convergent pathways (e.g. Gibson, 2000; Smeekens, 2000).
The observed interactions between ethylene and nutrient ions in modulating root waving and looping constitute a novel finding. Whether nutrients act directly on the ethylene-signaling cascade and/or indirectly by changing the chemical and mechanical properties of the agar and the amounts of ethylene it releases (Fig. 1) needs to be established. Further experiments are also required to ascertain which ions are involved. The observed differences between roots grown on Murashige and Skoog or Okada and Shimura media, however, suggest nitrogen ions may be the key element. The two media differ in composition, notably in their nitrogen contents. The Okada and Shimura formulation is low in nitrate (4.5 mm) and contains no ammonium ions (Somerville and Ogren, 1982), whereas the Murashige and Skoog formulation (Murashige and Skoog, 1962) is rich in both nitrate and ammonium (39.6 and 20.6 mm, respectively). These differences alter the balance between Suc and nitrogen with likely effects on the Suc:ethylene signaling operating in our experiments. Recent studies have demonstrated the role of C-N balance on root morphogenesis (Malamy and Ryan, 2001; Kaiser et al., 2002; Martin et al., 2002), and there is mounting evidence for intimate interactions between carbon, nitrogen, and the major plant hormones (e.g. Sheen et al., 1999). Besides nitrogen, micronutrients such as copper, which is a cofactor for ethylene binding (Rodríguez et al., 1999), could also be involved.
Root Waving, Skewing, and Looping Can Be Uncoupled and Do Not Necessarily Involve Cell File Twisting
It was recently proposed that root-waving and -skewing patterns in Arabidopsis roots are both the consequence of the intrinsic root righthandedness and pronounced chirality seen in certain ecotypes (Migliaccio and Piconese, 2001). According to this unifying theory, when free to elongate in air, the root tip follows a righthanded helical path. If grown on tilted hard-agar plates, the root tip hits the flat agar surface each time it describes a half circle. This causes a “negative” thigmotropic reaction in which the root tip switches direction and starts describing a half circle in the opposite direction until it hits the agar again, and so on. Migliaccio and Piconese (2001) suggest this explains “the sinusoidal and slanting pattern of Arabidopsis roots” and also the lefthanded cell file twisting that would be required to allow flattening of the righthanded root helix against the agar surface. Several features of our results suggest a more complex picture: (a) Our data demonstrate that waving does not necessarily cause skewing. In fact, the tightest waves were observed in roots growing with no net angular deviation from the vertical (Fig. 2), and the portions of roots with most pronounced twisting were generally those where the temporal frequency of waving was lower. (b) Furthermore, we show that the waving, skewing, and also looping phenotypes are not intrinsically linked and can be uncoupled. (c) In our experiments, cell file rotation (twisting) was unnecessary for waving or skewing to occur. An obvious lefthanded cell file twisting was seen in clockwise-looping roots as previously reported (Okada and Shimura, 1990; Simmons et al., 1995; Sedbrook et al., 2002), but in many of our waving roots twisting could not be detected and root cell files remained parallel, even when skewing (Fig. 8).
Overall, these results lead to the conclusion that root waving and skewing may result from two categories of processes driven by partly separate mechanisms: twisting and differential flank growth. Differential flank growth was clearly sufficient to drive waving and skewing in zero nutrient plates and on Okada and Shimura medium free of Suc, whereas some amount of root twisting may assist bending under nutrient- and Suc-supplemented conditions. Ethylene has been shown to influence cell wall extensibility (Smalle et al., 1997), directly or via interactions with auxins. Whether the exaggerated twisting phenotypes seen especially with Murashige and Skoog nutrients and added Suc are generated by unequal cell elongation in the epidermis and cortex as observed by Furutani et al. (2000) for the right-twisting spr1 mutant remains to be ascertained. Several recent studies have linked twisting phenotypes to the organization of cortical microtubules, with the righthanded spiral mutants (Furutani et al., 2000), the lefthanded twist-inducing mor1 (Whittington et al., 2001), lefty (Thitamadee et al., 2002), and wvd2-1 (Yuen et al., 2003) mutants all having abnormal microtubule patterns. Immunostaining microtubules in waving roots, however, failed to show any obvious differences in microtubule organization associated with the growth responses observed in our study (data not shown). Other recent studies also failed to show a link between twisting and microtubule organization (sku5; Sedbrook et al., 2002). Moreover, in relation to its involvement in many root growth responses including polar auxin transport (Muday and DeLong, 2001; Friml et al., 2002), the actin cytoskeleton is also likely to be involved (maize; Hasenstein et al., 1999; Yoder et al., 2001; for review, see Kiss, 2000).
Under conditions that enhanced skewing and looping responses (nutrient and Suc supplements), there was a clear bias for lefthanded helical twisting of roots. In those conditions, ethylene diminished, abolished, or even reversed looping and skewing and also generated righthanded twisting of petioles. One possible interpretation of these observations is that ethylene's modulation of looping and skewing is in fact a stimulation of righthanded twisting rather than a moderation of the nutrient and Suc-stimulated lefthanded twisting. If so, however, one would expect to see righthanded twisting in roots grown on zero-nutrient plates, which was not the case. One cannot therefore here separate between a direct ethylene signaling on root twisting or an indirect signaling, via effects on nutrient and Suc signaling pathways.
A Circadian Clock in the Temporal Control of Root Waving?
Our data suggest an endogenous circadian rhythm is involved in root waving, especially in the differential flank growth component. On zero-nutrient plates, where the most pronounced waving occurred in the absence of cell file twisting, the periodicity of waving was remarkably stable over a wide range of ethylene concentrations (Fig. 3C) and root elongation rates (Fig. 3A). A new wave was formed approximately every 24 h in the absence of photoperiodic clues, as seedlings were grown under continuous light. The expression of this free-running rhythm appears to depend on complex nutrient:Suc interactions (Fig. 5) with Suc addition causing a dramatic decrease in the wave period in the presence of nutrients, but a marked increase in period when nutrient supplements were omitted. Such interactions are consistent with increasing evidence for the involvement of circadian clocks in the control of ethylene, nitrogen, and sugar signaling (McClung, 2001), which our study demonstrates influences root waving. The present study sets the foundation for future experiments in which the proposed endogenous free-running rhythm can be confirmed and the mechanisms by which it may be modified by environmental cues can be investigated.
MATERIALS AND METHODS
Plant Culture
Arabidopsis (ecotype Ler) seeds were surface-sterilized and sown on petri plates (φ = 9 cm, polystyrene) at 8-mm intervals in two rows 2.5 cm apart onto the medium surface polymerized with 1.5% (w/v) Bacto-agar (Difco Laboratories, Detroit). The medium contained no nutrient supplements (“zero nutrient” plates) or was supplemented with Murashige and Skoog basal salts (M-056 and M-052, Sigma-Aldrich, St. Louis) or the salt formulation used by Okada and Shimura (1990; 0.5× as listed by Somerville and Ogren [1982]). For Suc treatments, no Suc or 3% (w/v) was used. Vitamins (M-6896, Sigma-Aldrich) were added in Murashige and Skoog plates at the recommended level. MES (0.05%, w/v) was added to all media as a buffer and the pH was adjusted to 5.7 with 1 n NaOH before adding the gelling agent and autoclaving.
The plates were wrapped with a low-porosity paraffin-based film similar to Parafilm (Nescofilm, Azwell Inc., Osaka; labeled “sealed plates”) or with gas permeable surgical tape (Micropore, 3M Health Care, St. Paul; labeled “aerated”). In all experiments, plates were placed in a growth chamber (Conviron, Controlled Environments, Pembina, ND) fitted with cool-white fluorescent tubes and incandescent bulbs providing 100 μmol quanta m-2 s-1 continuous light and maintained at 21°C temperature and 80% relative humidity. The plates were placed in racks where they were maintained at a 45° angle between the root growth axis and the gravity vector.
Ethylene Manipulation
The growth racks were either open and in direct contact with the chamber atmosphere or were enclosed in sealed Perspex boxes (i.d. 115 × 125 × 275 mm, volume ≈ 4 L) where the ethylene concentrations around the plates could be varied over a desired range and maintained as constant as possible. Ethylene (chemically pure 99.5%, BOC Gases, Sydney) was injected through a Suba-Seal fitting (no. 33, 2 cm diameter) every 24 h after opening the boxes to equilibrate them to ambient air. The box lids were fitted with a mixing fan (model FP-108 HX/DC, 0.08 A, 12V, 4 × 4 × 1 cm, Jaycar, Concord, Australia) to eliminate ethylene pooling above the petri plates. The box lids were sealed with a thin layer of silicon grease. Four ethylene concentrations around the plates were tested: 0.001, 0.01, 0.1, and 1 μL L-1. The irradiance and temperature measured inside the boxes were similar to those in the chamber. To vary the ethylene concentrations within sealed plates, potassium permanganate crystals, which oxidize ethylene gas to ethylene glycol and MnO2 (Kays et al., 1974; Abeles et al., 1992; Jackson et al., 1991; Morrison and Boyd, 1992), were used. Lightly ground crystals (0.1, 0.01, or 0 g, depending on plates) were dispensed at sowing in a tiny sterile container fitted in each plate after pouring the medium. The container was open to the plate headspace but prevented direct contact between the KMnO4 crystals, the agar, or the seedlings.
Gas Chromatography
Ethylene concentrations in the plate headspace (zero nutrient plates and a subset of Murashige and Skoog plates) were quantified using a flame-ionization detector gas chromatograph (Series 15, Dohrmann Envirotech, Santa Clara, CA). A standard curve was developed using serially diluted C2H4 gas. A 1-mL aliquot of plate headspace gases was sampled (through a small hole drilled into the plate's sidewall before pouring media) using a 1-mL syringe and was immediately injected into the gas chromatograph. Measurements were made on the day of germination (d 1), the 2nd d after germination (d 3), and subsequent days until d 7.
Auxin Transport
NPA was solubilized under alkaline conditions with 1 n NaOH. Aliquots from a sterile-filtered stock solution were added to still molten autoclaved Bacto-agar solutions (final concentrations of 0.1 or 1 μm).
Morphometric Measurements and Data Acquisition
Seedling morphometric measurements were made from Adobe PhotoShop images (600 pixels inch-1; Adobe Systems, Mountain View, CA) captured 7 d after germination by scanning the individual plates on a flatbed scanner. Photomontages of roots were assembled from captured video images (microscope: Diaphot-TMD with a 4× objective, Nikon, Tokyo; black and white camera: SPT M102 CE, Sony, Tokyo) using Scion Frame Grabber (National Institutes of Health, Bethesda, MD). Quantification of root length (distance from base of hypocotyl to root tip), wavelength (distance along root axis between the crests of two successive waves), wave spatial frequency (number of waves per mm of root length), and root axis angle (angle at which a root grew across the agar surface, where vertically down the plate is 0°, toward the right is >0° and toward the left is <0° when viewing the root from above the plate) were measured using the National Institutes of Health Scion Image software program (Windows vBeta 3b; http://www.scioncorp.com). The average root axis angle was measured by tracing the root in the segmented line mode, avoiding those portions of roots that were looping or had penetrated into the medium. Average root elongation was calculated from the increase in root length between d 1 and 7 after germination, and the average temporal wave frequency (number of waves per h) was calculated as the product of elongation rate and spatial wave frequency. All experiments included three replicated plates for each individual treatment (defined by nutrient, Suc, NPA supplements, ethylene injections, or amount of KMnO4 crystals placed in the plate), and experiments were independently repeated at least twice. Seedlings were observed over the 7-d period beyond germination, which usually occurred 2 d after plating.
Scanning Electron Microscopy
Scanning electron microscopy micrographs were generated using a Cambridge StereoScan 360 scanning electron microscope equipped with a cryostage. Seedlings were removed from the petri plate, glued to a stub, immediately immersed in liquid nitrogen slush, inserted into the microscope vacuum chamber, etched, gold coated, and photographed at 15 or 20 kV. Composite micrographs were assembled with MicroSoft PowerPoint software (Microsoft, Redmond, WA) and exported as TIFF files.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permission will be the responsibility of the requestor.
Supplementary Material
Acknowledgments
We thank Drs. Jean Yong and Chin Wong and Prof. Haruko Kazama for helpful suggestions and technical assistance; Dr. Jake Jacobsen for use of the flame-ionization detector-gas chromatograph; the Australian National University-Electron Microscopy Unit staff for assistance with the cryoscanning electron microscopy; Dr. Ulrike Mathesius for donation of NPA; and Dr. Gloria Muday for comments on the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019182.
This work was funded by a Research School of Biological Sciences Strategic Development Initiative Grant (to J.M. and G.O.W.).
The online version of this article contains Web-only data. The supplemental material is available at http://www.plantphysiol.org.
Present address: Department of Biology, Wake Forest University, Winston-Salem, NC 27109–7325.
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology. Academic Press, New York
- Baskin TI, Williamson RE (1992) Ethylene, microtubules, and root morphology in wild-type and mutant Arabidopsis seedlings. In D Randall, R Sharp, A Novaky, D Blevins, eds, Current Topics in Plant Biochemistry and Physiology, Vol 11. Interdisciplinary Plant Biochemistry and Physiology Program, Columbia, MO, pp 118-130 [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103-1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella JR, Arteca RN, Frangos JA (1995) A mechanical strain-induced 1-aminocyclopropane-1-carboxylic acid synthase gene. Proc Natl Acad Sci USA 92: 1595-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Masle J, Wasteneys GO (2000) Growth conditions modulate root-wave phenotypes in Arabidopsis. Plant Cell Physiol 41: 1164-1170 [DOI] [PubMed] [Google Scholar]
- Burg SP, Kang BG (1993) Gravity dependent ethylene action. In JC Pech, A Latache, C Balague, eds, Cellular and Molecular Aspects of the Plant Hormone Ethylene. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 335-341
- Cary AJ, Liu W, Howell SH (1995) Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol 107: 1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG (2001) The role of long-distance signalling in plant responses to nitrate and other nutrients. J Exp Bot 53: 39-43 [PubMed] [Google Scholar]
- Friml J, Wiœniewska J, Benková E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806-809 [DOI] [PubMed] [Google Scholar]
- Furutani I, Watanabe Y, Prieto R, Masukawa M, Suzuki K, Naoi K, Thitamadee S, Shikanai T, Hashimoto T (2000) The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Development 127: 4443-4453 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI (2000) Plant sugar-response pathways: part of a complex regulatory web. Plant Physiol 124: 1532-1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeschl JD, Kays SJ (1975) Concentration dependencies of some effects of ethylene on etiolated pea, peanut, bean, and cotton seedlings. Plant Physiol 55: 670-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss MJ (1977) Effects of mechanical impedance on root growth in barley (Hordeum vulgare L.): I. Effects on the elongation and branching of seminal root axes. J Exp Bot 28: 96-111 [Google Scholar]
- Hasenstein KH, Blancaflor EB, Lee JS (1999) The microtubule cytoskeleton does not integrate auxin transport and gravitropism in maize roots. Physiol Plant 105: 729-738 [DOI] [PubMed] [Google Scholar]
- Hussain A, Black CR, Taylor IB, Roberts JA (1999) Soil compaction: a role for ethylene in regulating leaf expansion and shoot growth in tomato? Plant Physiol 121: 1227-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Black CR, Taylor IB, Roberts JA (2000) Does an antagonistic relationship between ABA and ethylene mediate shoot growth when tomato (Lycopersicon esculentum Mill.) plants encounter compacted soil? Plant Cell Environ 23: 1217-1226 [Google Scholar]
- Jackson MB, Abbott AJ, Belcher AR, Hall KC, Butler R, Cameron J (1991) Ventilation in plant tissue cultures and effects of poor aeration on ethylene and carbon dioxide accumulation, oxygen depletion and explant development. Ann Bot 67: 229-237 [Google Scholar]
- Jaffe MJ, Forbes S (1993) Thigmomorphogensis: the effect of mechanical perturbation on plants. J Plant Growth Regul 12: 313-324 [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Leopold AC, Staples RC (2002) Thigmo responses in plants and fungi. Am J Bot 89: 375-382 [DOI] [PubMed] [Google Scholar]
- Kaiser BN, Rawat SR, Siddiqi MY, Masle J, Glass ADM (2002) Functional analysis of an Arabidopsis T-DNA “knockout” of the high-affinity NH4+ transporter AtAMT1;1. Plant Physiol 130: 1263-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays SJ, Nicklow CW, Simons DH (1974) Ethylene in relation to the response of roots to physical impedance. Plant Soil 40: 565-571 [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427-441 [DOI] [PubMed] [Google Scholar]
- Kiss JZ (2000) Mechanisms of the early phase of gravitropism. Crit Rev Plant Sci 19: 551-573 [DOI] [PubMed] [Google Scholar]
- Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 509-540 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Chang C (2001) The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiol 125: 1061-1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S (1997) Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 114: 789-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH (1995) Auxin transport. In PJ Davies, Ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 509-530
- Lu B, Yu HY, Pei LK, Wong MY, Li N (2002) Prolonged exposure to ethylene stimulates the negative gravitropic responses of Arabidopsis inflorescence stems and hypocotyls. Funct Plant Biol 29: 987-997 [DOI] [PubMed] [Google Scholar]
- Madlung A, Behringer FJ, Lomax TL (1999) Ethylene plays multiple nonprimary roles in modulating the gravitropic response in tomato. Plant Physiol 120: 897-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899-909 [PMC free article] [PubMed] [Google Scholar]
- Martin T, Oswald O, Graham IA (2002) Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiol 128: 472-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masle J (2002) High soil strength: mechanical forces at play on root morphogenesis and in root:shoot signaling. In Y Waisel, A Eshel, U Kafkafi, eds, Plant Roots, The Hidden Half, Ed 3. Marcel Dekker, New York, pp 807–819
- Masucci JD, Schiefelbein JW (1994) The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol 106: 1335-1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Suttle JC (1991) The Plant Hormone Ethylene. CRC Press, Boca Raton, FL
- McClung CR (2001) Circadian rhythms in plants. Annu Rev Plant Physiol Plant Mol Biol 52: 139-162 [DOI] [PubMed] [Google Scholar]
- Mensuali-Sodi A, Panizza M, Tognoni F (1992) Quantification of ethylene losses in different container-seal systems and comparison of biotic and abiotic contributions to ethylene accumulation in cultured vessels. Physiol Plant 84: 472-476 [Google Scholar]
- Migliaccio F, Piconese S (2001) Spiralizations and tropisms in Arabidopsis roots. Trends Plant Sci 6: 561-565 [DOI] [PubMed] [Google Scholar]
- Morrison RT, Boyd RN (1992) Organic Chemistry, Ed 6. Prentice Hall, Englewood Cliffs, NJ
- Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6: 535-542 [DOI] [PubMed] [Google Scholar]
- Mullen JL, Turk E, Johnson K, Wolverton C, Ishikawa H, Simmons C, Söll D, Evans ML (1998) Root-growth behavior of the Arabidopsis mutant rgr1: roles of gravitropism and circumnutation in the waving/coiling phenomenon. Plant Physiol 118: 1139-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473-479 [Google Scholar]
- Okada K, Shimura Y (1990) Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250: 274-276 [DOI] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M (1998) Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J 16: 553-560 [DOI] [PubMed] [Google Scholar]
- Rahman A, Ahamed A, Amakawa T, Goto N, Tsurumi S (2001) Chromosaponin I specifically interacts with AUX1 protein in regulating the gravitropic response of Arabidopsis roots. Plant Physiol 125: 990-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Tsurumi S, Amakawa T, Soga K, Hoson T, Goto N, Kamisaka S (2000) Involvement of ethylene and gibberellin signalings in chromosaponin I-induced cell division and cell elongation in the roots of Arabidopsis seedlings. Plant Cell Physiol 41: 1-9 [DOI] [PubMed] [Google Scholar]
- Rodríguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283: 996-998 [DOI] [PubMed] [Google Scholar]
- Sarquis JI, Jordan WR, Morgan PW (1991) Ethylene evolution from maize (Zea mays L.) seedling roots and shoots in response to mechanical impedance. Plant Physiol 96: 1171-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Carroll KL, Hung KF, Masson PH, Somerville CR (2002) The Arabidopsis SKU5 gene encodes an extracellular glycosyl phosphatidylinositol-anchored glycoprotein involved in directional root growth. Plant Cell 14: 1635-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang J-C (1999) Sugars as signaling molecules. Curr Opin Plant Biol 2: 410-418 [DOI] [PubMed] [Google Scholar]
- Simmons C, Söll D, Migliaccio F (1995) Circumnutation and gravitropism cause root waving in Arabidopsis thaliana. J Exp Bot 46: 143-150 [Google Scholar]
- Smalle J, Haegman M, Kurepa J, van Montagu M, van der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, van der Straeten D (1997) Ethylene and vegetative development. Physiol Plant 100: 593-605 [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 49-81 [DOI] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL (1982) Growth conditions of Arabidopsis. In M Edelman, RB Hallick, N-H Chua, eds, Methods in Chloroplast Biology. Elsevier Biomedical Press, Amsterdam, p 131
- Tanimoto M, Roberts K, Dolan L (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8: 943-948 [DOI] [PubMed] [Google Scholar]
- Thitamadee S, Tuchihara K, Hashimoto T (2002) Microtubule basis for left-handed helical growth in Arabidopsis. Nature 417: 193-196 [DOI] [PubMed] [Google Scholar]
- Vyas SP, Bohra SP, Sankhla N (1973) Antagonism between morphactin and ethylene in root-coiling of Ipomoea pentaphylla. Z Pflanzenphysiol 69: 185-188 [Google Scholar]
- Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO (2001) MOR1 is essential for organizing cortical microtubules in plants. Nature 411: 610-613 [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Robards AW, Goss MJ (1977) Effects of mechanical impedance on root growth in barley, Hordeum vulgare L.: II. Effects on cell development in seminal roots. J Exp Bot 28: 1216-1227 [Google Scholar]
- Woods SL, Roberts JA, Taylor IB (1984) Ethylene-induced root coiling in tomato seedlings. Plant Growth Regul 2: 217-225 [Google Scholar]
- Yoder TL, Zheng H, Todd P, Staehelin CA (2001) Amyloplast sedimentation dynamics in maize columella cells supports a new model for the gravity-sensing apparatus of roots. Plant Physiol 125: 1045-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen CYL, Pearlman RS, Silo-suh L, Hilson P, Carroll KL, Masson PH (2003) WVD2 and WDL1 modulate helical organ growth and anisotropic cell expansion in Arabidopsis. Plant Physiol 131: 493-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294-10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.