Abstract
Legume seeds are heterotrophic and dependent on mitochondrial respiration. Due to the limited diffusional gas exchange, embryos grow in an environment of low oxygen. O2 levels within embryo tissues were measured using microsensors and are lowest in early stages and during night, up to 0.4% of atmospheric O2 concentration (1.1 μm). Embryo respiration was more strongly inhibited by low O2 during earlier than later stages. ATP content and adenylate energy charge were lowest in young embryos, whereas ethanol emission and alcohol dehydrogenase activity were high, indicating restricted ATP synthesis and fermentative metabolism. In vitro and in vivo experiments further revealed that embryo metabolism is O2 limited. During maturation, ATP levels increased and fermentative metabolism disappeared. This indicates that embryos become adapted to the low O2 and can adjust its energy state on a higher level. Embryos become green and photosynthetically active during differentiation. Photosynthetic O2 production elevated the internal level up to approximately 50% of atmospheric O2 concentration (135 μm). Upon light conditions, embryos partitioned approximately 3-fold more [14C]sucrose into starch. The light-dependent increase of starch synthesis was developmentally regulated. However, steady-state levels of nucleotides, free amino acids, sugars, and glycolytic intermediates did not change upon light or dark conditions. Maturing embryos responded to low O2 supply by adjusting metabolic fluxes rather than the steady-state levels of metabolites. We conclude that embryogenic photosynthesis increases biosynthetic fluxes probably by providing O2 and energy that is readily used for biosynthesis and respiration.
Growing seeds are sink organs that import assimilates from the phloem, mainly as Suc and amino acids. Their photosynthetic potential to fix carbon is rather low compared with leaves and pods (Harvey et al., 1976; Atkins and Flinn, 1978; Flinn, 1985; Eastmond et al., 1996; Asokanthan et al., 1997). Embryos are therefore predominantly heterotrophic. Accordingly, their ATP supply is mainly covered by mitochondrial respiration, the regulation of which is therefore important for storage activity and maturation. Mitochondrial respiration is regulated at different levels like carbohydrate status, light, and temperature. Especially the availability of respiratory substrates is often regarded as a limiting factor. Because the overall sugar levels within embryo tissues are quite high at all stages, its availability should not limit mitochondrial respiration. During early seed growth (prestorage phase), embryos contain high levels of hexoses due to high invertase activity (Weber et al., 1995a). Later on, invertase decreases and mainly Suc is imported. The switch from hexoses to Suc is accompanied by cell differentiation and storage product synthesis (Borisjuk et al., 1995; Weber et al., 1998). The analysis of the spatial distribution of both Glc and Suc concentrations in growing broad bean (Vicia faba) cotyledons detected specific patterns that change during development. High Glc concentrations were found in non-differentiated premature regions, whereas mature starch-accumulating regions contain particularly low concentrations (Borisjuk et al., 1998). In contrast, actively elongating and starch-accumulating cells contain highest Suc concentrations, suggesting a role for Suc in differentiation and storage processes (Borisjuk et al., 2002).
Besides sugars, oxygen is a substrate for mitochondrial respiration as well and can be rate limiting. This aspect, however, was almost neglected in previous studies on seed physiology, although there was some indirect evidence for oxygen shortage within seeds (Wager, 1974; Yeung and Blackman, 1987; Shelp et al., 1995). Porterfield et al. (1999) measured the O2 concentration within siliques of Arabidopsis and Brassica sp. using small-diameter electrodes. Mean O2 levels were well below saturation and changed upon illumination. The authors postulated that hypoxia could be an important factor to control seed development.
Recently, we measured O2 levels within seeds of pea (Pisum sativum) and faba bean using O2-sensitive microsensors with high spatial resolution (Rolletschek et al., 2002a). Across the seed coat, O2 levels fall strongly, and they reached very low concentrations at the inner surface (approximately 3% of atmospheric O2 concentration, corresponds to approximately 8 μm at room temperature). Thus, O2 influx into the seed is strongly restricted by the seed coat and occurs entirely through the micropylar region (Wager, 1974). Such a restricted gas exchange might be helpful to minimize the loss of CO2 coming from respiration, and to promote CO2 refixation via phosphoenolpyruvate carboxylase (Flinn, 1985; Golombek et al., 1999). Low O2 levels of approximately 1% (approximately 2.7 μm) were detected within the endospermal vacuole surrounding the embryo. Within the embryo, O2 concentrations were low when measured in darkness, but there was no direct evidence for hypoxia and fermentative metabolism. However, it has been shown that metabolic adaptations occur already at 5% to 10% of atmospheric O2 concentration (Geigenberger et al., 2000; Gibon et al., 2002). The O2 levels inside the embryo raised to higher values upon illumination, indicating that light supply and photosynthetic activity could be important to increase O2 contents. Two main conclusions emerged from this study: First, O2 level within growing embryos falls to critical levels thereby potentially restricting respiration, ATP production, and overall metabolic activity. Second, photosynthesis may elevate internal O2 levels, which could promote higher respiratory and biosynthetic activity.
Using a bioluminescence-based metabolite-imaging method (L. Borisjuk, unpublished data), we measured the spatial and temporal distribution pattern of ATP within growing embryos. The local ATP distribution temporally and spatially corresponded to greening pattern, chlorophyll distribution, and photosynthetic O2 production, indicating that the overall increase of the energy state is associated to the greening process. Thus, embryogenic photosynthesis may be particularly important under low O2 conditions delivering both oxygen and ATP. In this study, we provide additional experimental evidence for this model by analyzing the effects of day/night conditions on O2, metabolites, and biosynthetic activity. We found that during the early stages, embryos are ATP limited and responded by fermentation. During further differentiation, the ATP levels increased along with increasing photosynthetic capacity. Light supply and corresponding photosynthetic activity increased respiratory and biosynthetic fluxes.
RESULTS
Oxygen Levels in Embryos Are Low during Early Development and at Night
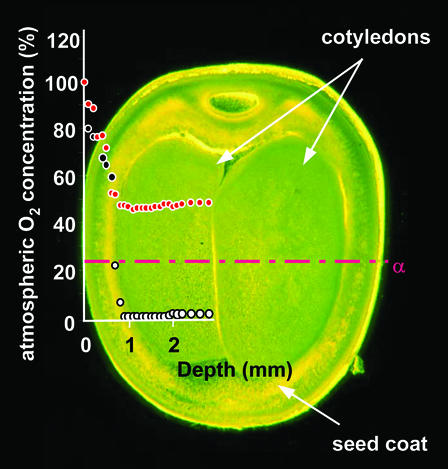
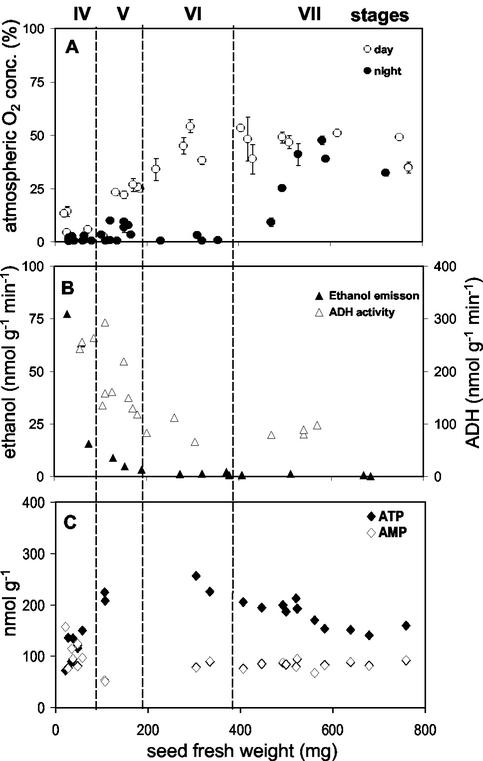
Using oxygen-sensitive microsensors, we measured the spatial O2 distribution within seeds. Figure 1 shows an O2 profile across a transect through a pea seed of 300 mg fresh weight. O2 levels dropped strongly within the seed coat with a much stronger gradient at dark compared with light conditions. O2 fell to very low levels of about 0.5% to 1.5% of atmospheric O2 concentration in darkness (1.3-4 μm). In the light, the mean level was approximately 48% (130 μm). During both light and dark conditions, the O2 level was strikingly stable without detectable gradients within the embryo. The O2 profiles measured in seeds of faba bean were similar to those of pea (data not shown). Within broad bean seeds, O2 levels were dependent on both seed age and light supply (Fig. 2A). When O2 was measured at light, the mean levels inside the embryo were low (3%-20% of saturation, which corresponds to 8.1-54 μm) at early stage IV. Later on, levels increased steadily to approximately 50% (135 μm) at the end of stage VI (300 mg seed fresh weight), and remained stable thereafter. In darkness, concentrations were very low (0.4% of atmospheric O2 concentration, which corresponds to 1.1 μm), but anoxia could never be detected. During stage VII, O2 increased to values observed also under light conditions. In summary, the O2 levels in growing embryos changed during both development and upon dark-light conditions with lowest values at early stages and in darkness.
Figure 1.
O2 concentration profile measured in light and in darkness along a transect of a pea seed (300 mg fresh weight). Red symbols measured under light conditions, black and white symbols measured under dark conditions.
Figure 2.
A, Developmental changes in O2 levels (percentage of atmospheric O2 concentration) measured in embryos of broad bean at day and night. One data point represents six measurements within a single embryo. B, Ethanol emission rate and ADH enzyme activity. C, Levels of ATP and AMP. Staging of V. faba seed development (see Borisjuk et al., 1995).
Fermentative Activity Is High during Early Embryo Growth
To analyze whether embryo metabolism is restricted by low oxygen, we measured fermentative activity and nucleotide levels in growing broad bean embryos. Both ethanol emission and alcohol dehydrogenase (ADH) activity were highest at stage IV with maximum rates of 80 and 300 nmol g–1 min–1, respectively (Fig. 2B). During stage V, ADH activity and ethanol emission decreased and remained nearly constant in seeds of >200 mg fresh weight. Ethanol emission was not detectable during stages VI and VII. Together with the low O2 levels and fermentative metabolism, the ATP concentrations were lowest at early stages, but increased to values of approximately 250 nmol g–1 in seeds of stage V to VI (Fig. 2C). From this stage onwards, ATP levels remained relatively constant. AMP decreased initially together with increasing ATP and remained constant thereafter. ADP levels were between 40 to 60 nmol g–1 throughout growth without major changes (data not shown). The profile of the adenylate energy charge (AEC = [ATP + 0.5 ADP]/[ATP + ADP + AMP]) was similar to that of ATP with minimum values of 0.38 at early stages and maximum levels of 0.76 at later stages.
In summary, at stage IV when oxygen levels are lowest, embryos also have a low energy state and high fermentative activity, indicating hypoxic metabolism. During stage V, energy levels increase and fermentative metabolism disappears.
Low Oxygen Limits Respiratory Activity
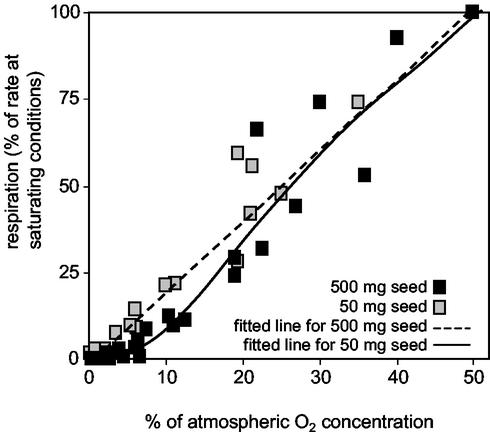
Low O2 levels within the embryo might affect respiratory activity. We therefore measured respiration related to external O2 levels. The respiration rate, given as percentage of the rate at O2-saturating condition, decreased almost linearly in response to falling O2 levels in the medium (Fig. 3). The decrease occurred already when external O2 levels were still high. This might be attributed to the high diffusional impedance of embryo tissue. With the decrease of the concentration gradient at lower external O2 levels, the driving force for diffusive influx also decreases. Therefore, from the fitted lines in Figure 3 we cannot estimate the extent to which in vivo respiration is inhibited by low O2. However, due to the very low in vivo O2 levels (Fig. 2A), it can be assumed that embryo respiration is inhibited. Remarkably, respiration of mitotically active embryos (stages IV to V) is more strongly inhibited by low O2 than at stage VII after onset of storage. This indicates that embryos of early stages are more susceptible to O2 limitation and that during maturation respiration becomes adapted to low O2.
Figure 3.
Relationship between external O2 levels and in vitro respiratory activity of broad bean embryos. A polynomial regression line is fitted to the data derived from measurements with 10 embryos.
Varying O2 Supply Affects Metabolite Pools
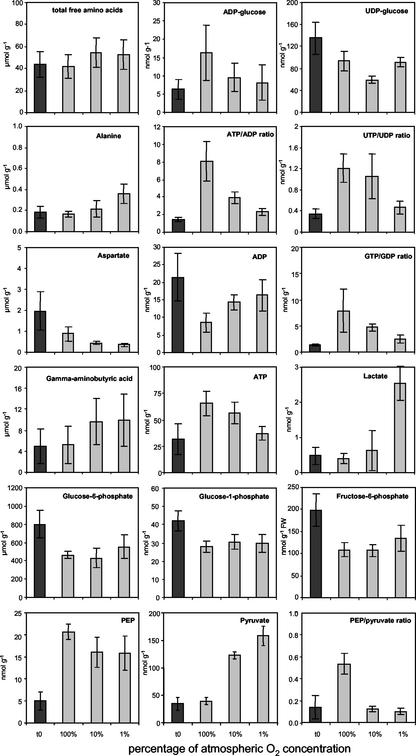
To further characterize the metabolic adaptations to O2 limitation, we changed experimentally the oxygen supply to isolated pea embryos of approximately 100 mg seed fresh weight from 100% to 10% and 1% of the atmospheric O2 concentration (270, 27, and 2.7 μm). Characteristic changes in the metabolite pattern are shown in Figure 4. The t0 values represent levels in freshly harvested embryos. Decreasing O2 from 100% to 1% of atmospheric O2 concentration did not change the total free amino acid pool. Ala and γ-aminobutyric acid increased gradually, whereas Asp decreased. Nucleotide sugars ADP-Glc and UDP-Glc decreased. The ratio of ATP to ADP decreased significantly due to both increasing ADP and decreasing ATP contents. Similar patterns were obvious for the UTP to UDP and the GTP to GDP ratios. Lowering the O2 to 1% of atmospheric O2 concentration increased lactate by 5-fold. The PEP to pyruvate ratio decreased due to the 4-fold elevated pyruvate levels. These changes indicate a partial inhibition of respiration and glycolysis. Hexose phosphates and the soluble sugars Glc, Fru, and Suc (data not shown) did not respond to varying O2 supply.
Figure 4.
Changes in metabolite levels measured in pea embryos (t0 means in vivo level) after in vitro incubation at different oxygen levels (100%, 10%, and 1% saturation of atmospheric level, respectively; n = 4-6).
Metabolite levels in immediately frozen embryos represent the in vivo values, assigned as t0. Comparing these to the values of isolated embryos, treated with 100% of atmospheric O2 concentration, reveals the response of the metabolite pools to elevated O2. This is because the embryo is normally surrounded by the seed coat, which is a strong diffusional barrier and will never come in contact to 100% of atmospheric O2 concentration. Upon increased O2 supply, ADP-Glc, ATP, ATP to ADP ratio, UTP to UDP ratio, GTP to GDP ratio, and PEP increased, whereas ADP, hexose phosphates, and pyruvate decreased (Fig. 4). This indicates that increasing O2 supply leads to a higher overall energy state.
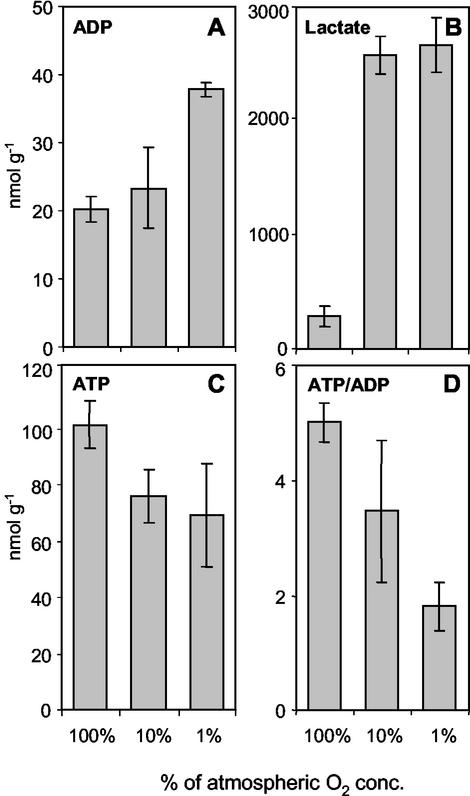
The in vitro experiments are potentially affected by artifacts. Therefore, we performed near in vivo experiments where intact pods, still attached to the plant, were treated with an air stream containing 100%, 10%, and 1% of atmospheric O2 concentration, respectively. Thereby, the ambient O2 concentration was lowered followed by reduced diffusive O2 uptake. In principle, the results were very similar to the in vitro experiments (Fig. 5). Upon lowering O2 levels, ADP and lactate increased (Fig. 5, A and B), whereas ATP and the ATP to ADP ratio decreased (Fig. 5, C and D). Levels of ADP- and UDP-Glc and the GTP to GDP ratio decreased (data not shown). Lactate accumulated strongly already at 10% of atmospheric O2 concentration. However, within freshly harvested embryos accumulation of lactate had never been detected. Obviously, the treatment with 1% (and 10% in vivo) led to unphysiological low O2 conditions or anoxia.
Figure 5.
Levels of ADP (A), lactate (B), ATP (C), and ATP to ADP ratios (D) measured in pea embryos aerated in vivo with oxygen levels, 100%, 10%, and 1% of atmospheric O2 concentration (n = 4-6).
The results show that lowering the oxygen supply decreases the energy state of the embryo. On the other hand, an experimentally increased O2 supply elevates the energy state. This indicates that in vivo the embryo metabolism is O2 limited.
Embryo Greening during Maturation Is Correlated to Increasing Oxygen and ATP Levels
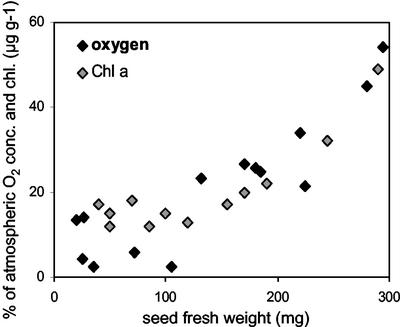
To estimate the photosynthetic capacity, we measured the chlorophyll content in developing embryos. Chlorophyll a content was constant in embryos up to 120 mg fresh weight, but increased significantly thereafter (Fig. 6). Chlorophyll b showed a similar time course, but the chlorophyll a to b ratio increased from approximately 0.5 in 30 mg of seed to 2 in 400 mg of seed (data not shown). The increase of chlorophyll was significantly correlated to that of O2 levels measured under light but not dark conditions. It indicates that photosynthetic capacity increased during differentiation leading to elevated O2 levels. The relationship between chlorophyll content, photosynthetic production of oxygen, and ATP concentration are also in accordance with their spatial distribution pattern (L. Borisjuk, unpublished data). The increase of chlorophyll as well as of oxygen was accompanied by increasing ATP and AEC levels (Fig. 2C), indicating that embryo photosynthesis improves the energy state.
Figure 6.
Relationship between mean O2 levels (measured at light) and chlorophyll a content measured after extraction of broad bean embryos.
Carbon Flux into Starch Increases upon Light versus Dark Conditions
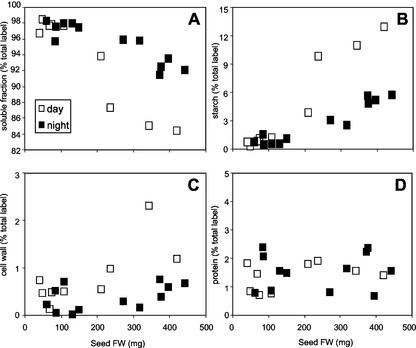
Embryogenic photosynthesis contributes to O2 and ATP production, which should increase metabolic fluxes. We therefore measured partitioning of [14C]Suc during day and night for embryos of different stages under near in vivo conditions. [14C]Suc was injected into the liquid endosperm surrounding the embryo, and label uptake as well as partitioning was monitored. The overall uptake of [14C] label per embryo fresh weight was not different (data not shown). However, at light conditions, embryos from 200 mg of seed fresh weight onward partitioned less label into the soluble fraction (Fig. 7A), but about 3-fold more label into starch (Fig. 7B), and slightly more into the cell wall fraction (Fig. 7C) compared with darkness. The flux of label into the protein fraction was unaffected by day or night conditions (Fig. 7D). Different fluxes might be caused by changing Suc levels within the liquid endosperm (isotope dilution effects). However, Suc was constant during the day/night cycle (50 ± 15 and 67 ± 14 mm, respectively).
Figure 7.
Partitioning of [14C]Suc given to embryos of broad bean into soluble compounds (A), starch (B), cell wall (C), and protein (D).
These results indicate that the carbon flux into starch is increased upon light versus dark conditions possibly due to the higher O2/energy supply by photosynthetic activity of the embryo.
Metabolite Pools Are Constant during the Day/Night Cycle
Changing O2 levels and respiratory activities as well as light-dark regulation of biosynthetic fluxes may affect steady-state levels of metabolites. Therefore, we measured nucleotides, nucleotide sugars, glycolytic intermediates, lactate, soluble sugars, and free amino acids upon dark or light conditions. Samples were taken at the mid and end of the night phase, the following day and the following night (total of six sampling times). Because samples derived from distinct time points during night and day were not significantly different, we pooled them to one night (n = 16) and day (n = 10) value. Data are summarized in Table I. Nucleotides, hexosephosphates, nucleotide sugars, PEP, pyruvate, lactate, and free sugars and amino acids were not different. The 3-P-glycerate pool increased significantly upon light conditions, most likely caused by photosynthetic CO2 fixation. Thus, light versus dark conditions did not change steady-state levels of metabolites but altered metabolic fluxes as shown by [14C]Suc-partitioning experiments.
Table I.
Level of nucleotides, nucleotide sugars, glycolytic intermediates, lactate, soluble sugars, and free amino acids in broad bean embryos (about 150 mg seed fresh wt) sampled during day (n = 10) and night (n = 16; means ± SD)
| Metabolites | Day | Night |
|---|---|---|
| ADPa | 55 ± 27 | 43 ± 13 |
| ATPa | 81 ± 15 | 85 ± 9 |
| ATP/ADP ratio | 1.5 ± 0.6 | 2.1 ± 0.6 |
| ADP-Glca | 13 ± 5 | 12 ± 3 |
| UDP-Glca | 113 ± 10 | 109 ± 12 |
| Glc-6-Pa | 287 ± 54 | 297 ± 35 |
| Glc-1-Pa | 26 ± 10 | 27 ± 8 |
| Fru-6-Pa | 74 ± 13 | 67 ± 8 |
| 3-P-glyceratea | 448 ± 169c | 117 ± 48 |
| Phosphoeno/pyruvatea | 17 ± 7 | 19 ± 9 |
| Pyruvatea | 38 ± 13 | 42 ± 17 |
| Lactatea | 651 ± 259 | 934 ± 479 |
| Glcb | 42 ± 17 | 41 ± 6 |
| Frub | 42 ± 18 | 39 ± 6 |
| Sucb | 70 ± 33 | 68 ± 30 |
| Free amino acidsb | 130 ± 17 | 124 ± 31 |
Values are given in nmol g-1 fresh wt. b Values are given in μmol g-1 fresh wt. c Significant differences according to t test; P < 0.05.
DISCUSSION
The aim the work reported in this paper is to analyze the energy state and its control on legume embryogenesis. Growing embryos are predominantly heterotrophic and require not only carbon and nitrogen precursors but also ATP from mitochondrial respiration. Due to the limited diffusional O2 influx across the seed coat, embryos grow in an environment of low O2 availability. We show here that young embryos respond with hypoxic growth including fermentative metabolism. During maturation, embryos become adapted to low O2 supply and able to increase their energy state. The adaptation process is evident at different levels and part of the differentiation program. It may therefore be regarded as a prerequisition to perform storage product biosynthesis. Greening and the gain of photosynthetic activity in maturing embryos contribute significantly to O2 supply thereby improving the embryo's energy state and metabolic fluxes.
Young Undifferentiated Embryos Have a Hypoxic Metabolism
Growing embryos are mainly heterotrophic and dependent on mitochondrial respiration. However, oxygen concentrations within growing seeds of broad bean and pea are very low. This seems to be a general phenomenon in seeds and has also been suggested for Brassica sp., soybean (Glycine max), and Arabidopsis (Quebedeaux and Hardy, 1975; Kuang et al., 1998; Porterfield et al., 1999). The low O2 conditions are a consequence of the morphological characteristics of the maternal seed tissues. The particular morphology is required to prevent loss of CO2 from the highly respiring embryo (Harvey et al., 1976; Flinn, 1985). On the other hand, it restricts diffusional O2 influx. Respiratory activity measured in vitro is highest for young embryos (L. Borisjuk, unpublished data). However, under in vivo conditions, O2 levels are low and limit respiration, which lowers the energy state and induces fermentation. Using in vitro and in vivo experiments, we could show that lowering the O2 supply to embryos decreases the overall energy state of nucleotide pools, especially of ATP, but increases pyruvate, fermentation products (lactate and Ala), and γ-aminobutyric acid levels. Thus, legume embryos respond to O2 depletion by typical metabolic responses (Drew, 1997; Gibon et al., 2002). When O2 supply is increased, metabolite patterns show inverse changes indicating that respiration and glycolysis are inhibited by low O2 under in vivo conditions.
We show that ethanol emission and ADH activity within embryos are high during the early growth stages, whereas at later stages, levels are constantly low (Fig. 2B). We conclude therefore that only the young embryo responds to hypoxic conditions by inducing fermentative metabolism. Accordingly, ATP concentrations are lowest at stage IV but increase to higher values in seeds of 150 mg onward (Fig. 2C). Thus, metabolic responses upon hypoxia and low ATP levels are characteristic only for the young undifferentiated stage and disappear in the course of further differentiation. In embryos from 150 to 200 mg fresh weight onward, fermentative metabolism is no more evident. An increased diffusional uptake of O2 is unlikely the reason for overcoming hypoxic metabolism because O2 concentrations remain low in embryos up to 400 mg fresh weight in darkness (Fig. 2A).
Low O2 could be sensed and the signal translated into hypoxic responses (López-Barneo et al., 2001). The Km for plant mitochondrial cytochrome oxidase is as low as 0.013% of atmospheric O2 concentration or approximately 0.035 μm (Drew, 1997). Physiological O2 sensing may therefore be distinct from mitochondrial inhibition, explaining the onset of hypoxic responses at relatively high O2 levels, at 5% to 10% saturation, which corresponds to approximately 13.5 to 27 μm (Geigenberger et al., 2000; Gibon et al., 2002). Hemoglobins are regarded as putative oxygen sensors, the expression of which is altered by either oxygen or ATP availability (Hill, 1998). The physiological significance of these sensors in growing seeds is still unknown.
During Differentiation, Embryos Become Adapted to Low Oxygen
Embryo respiration rates gradually decrease in response to falling O2 levels when oxygen is already relatively high (Fig. 3). This suggests that embryonic respiration is apparently O2 limited at all stages. Furthermore, after experimentally increasing oxygen supply, the ATP and AEC levels rise (Fig. 5), indicating that O2 is rate limiting for ATP production. Although the O2 supply during early and later stages is suboptimal and thus embryonic respiration is assumed to be restricted, there is an apparent lack of hypoxic responses or fermentative metabolism at later stages. We hypothesize therefore that embryos become adapted to the low O2 environment during differentiation mainly due to a drastic decrease of the overall respiration. As a consequence, they can regulate their metabolism and maintain their energy state at a constant high level. The early embryo has a high respiration and consequently becomes fermentatively active. However, fermentation is very inefficient with respect to ATP synthesis and produces potentially toxic metabolites. The metabolic and physiological adaptation may therefore be regarded as a precondition to perform storage product biosynthesis. A similar decrease of respiration from the early cell division to the maturation phase was reported for wheat (Triticum aestivum) endosperm (Emes et al., 2003).
We here show that the metabolic and physiological adaptations to the low O2 conditions are embedded in differentiation program of the embryo and are evident on different levels: First, respiration is more strongly inhibited by low O2 during earlier than later stages (Fig. 3), and overall rates decrease during embryo growth (L. Borisjuk, unpublished data). This indicates that during maturation, embryonic respiration becomes tightly controlled and adapted to the low O2 conditions. Second, ATP concentrations and AEC are lowest in embryos of early stages but increase later on (Fig. 2C). This indicates that embryos acquire the ability to elevate and stabilize ATP and AEC levels. Third, during maturation, embryos switch from an invertase and hexose-based metabolism to one that is Suc-based and controlled by a Suc synthase pathway (Weber et al., 1995a, 1996). Suc synthase catalyzes a readily reversible reaction (Geigenberger and Stitt, 1993) and, compared with invertase, saves ATP. Flux through Suc synthase depends upon high Suc levels and upon removal of the cleavage products. Thus, at maturation, the embryonic metabolism becomes energetically more economic and tightly controlled. Fourth, legume embryos are getting green during maturation and acquire photosynthetic activity with important consequences for O2 supply and energy state.
Embryonic Photosynthesis Is an Important Element of Maturation
Broad bean and pea embryos become green and photosynthetically active during differentiation. Spatial distribution of photosynthetic activity corresponds well to the chlorophyll pattern (L. Borisjuk, unpublished data). We could show that the increase of chlorophyll within the embryo is correlated to the O2 concentration under light conditions (Fig. 3). Accordingly, in oilseed rape (Brassica napus) seeds, chlorophyll content is correlated to the photosynthesis-dependent O2 evolution (Eastmond et al., 1996). Using microsensors, we have shown that oxygen immediately increased upon illumination (Rolletschek et al., 2002a). Therefore, light supply and the corresponding photosynthesis elevate oxygen levels within the embryo. The O2 increase during light is relatively low at earlier but higher at later stages, indicating that photosynthetic capacity increases during maturation. The gain of photosynthetic capacity is an integral part of the differentiation process. It correlates to the increase of the overall energy state during maturation and especially to the ATP pattern both on a spatial (L. Borisjuk, unpublished data) and a temporal scale.
The primary effect of embryonic photosynthesis is to increase internal O2 contents. A rate of oxygen production of 25 to 40 nmol g–1 min–1 should be sufficient for a 50% of atmospheric O2 concentration (135 μm) within the embryo within approximately 5 min. Changing O2 concentrations upon light/dark transitions are neither reflected at the level of ATP and AEC (Borisjuk et al., unpublished results) nor on the level of several other metabolites—both remain remarkably stable (Table I). Therefore, the increase of the overall energy state during maturation is not directly related to the embryonic photosynthetic activity or the corresponding O2 production. Instead, the adjustment of energy pools is developmentally regulated and coupled to the photosynthetic capacity of the embryo. However, we cannot exclude a change of nucleotide pools or AEC within the plastidial compartment upon light/dark conditions.
Embryonic Photosynthesis Provides Energy Supply and Increases Biosynthetic Fluxes
Chloroplasts in seeds are characterized by high rates of uncoupled electron transport, a high chlorophyll a to b ratio, and abundant proteins associated with photosystem II (Banerji and Rauf, 1979; Eastmond et al., 1996; Asokanthan et al., 1997). On the other hand, photosynthetic CO2 fixation has been reported to be low in embryos of oilseed rape (Asokanthan et al., 1997), pea (Flinn, 1985), and soybean (Saito et al., 1989). Therefore, embryonic photosynthesis may primarily provide energy rather than photoassimilates. This could be important in respect to storage product synthesis because plastids of heterotrophic tissues are generally energy limited (Neuhaus and Emes, 2000). Plastids of soybean embryos perform a higher biosynthetic rate in the light, and products synthesized in the light are much more reduced (Willms et al., 1999).
An important question is what can be the contribution of embryo photosynthesis to biosynthetic activity and metabolic fluxes? Photosynthetic activity obviously does not modulate energy and metabolite levels but could contribute to O2 and ATP supplies. If there is a significant contribution, metabolic fluxes should change upon light/dark transitions. Using [14C]Suc-partitioning experiments under near in vivo conditions, we could show that upon light, embryos partitioned approximately 3-fold more label into starch (Fig. 7). This light-dependent increase of flux into starch synthesis is developmentally dependent. From these results two important conclusions can be drawn: First, embryogenic photosynthesis increases biosynthetic fluxes probably by providing O2 and ATP which is readily used for respiration and biosynthesis, although it is difficult to estimate the in vivo contribution in absolute terms. Second, the strategy of maturing embryos to respond to low O2 supply is to adjust metabolic fluxes rather than the steady-state levels of metabolites. Our results show that young undifferentiated embryos are not able to perform this mode of regulation which has to be acquired in the course of the differentiation process. The light-dependent increase in the respiratory and biosynthetic flux might also be related to redox signals coming from photosynthesis. These are known to alter both gene expression in leaves (Pfannschmidt et al., 2001) and the activity of biosynthetic enzymes in potato (Solanum tuberosum) tubers. We cannot exclude the regulation of ADP-Glc pyrophosphorylase by the redox state (Tiessen et al., 2002). Our own unpublished results show that the redox status increases immediately upon illumination (L. Borisjuk, unpublished data), but nothing is known about target enzymes at the moment. At least Vicia sp. embryo ADP-Glc pyrophosphorylase is unlikely the target of metabolic regulation compared with the leave isoform, because we have shown that it is nearly insensitive against 3-PGA activation (Weber et al., 1995b). The role of redox signals during seed growth will be addressed to during future investigations.
MATERIALS AND METHODS
Plant Material
Broad bean (Vicia faba L. var minor cv Fribo) and pea (Pisum sativum cv Erbi) were grown in growth chambers under a light/dark regime of 16 h of light and 8 h of dark at 20°C/18°C. For the isolation of embryos, pods were tagged according to days after pollination, collected, and processed further. For metabolite measurements and enzyme assays, seeds were harvested, and embryos were immediately isolated and frozen in liquid nitrogen.
Determination of Oxygen Concentration in Seeds Using Microsensors
Oxygen concentration inside broad bean and pea seeds was determined using microsensors (Presens, Neuburg, Germany) as described in detail by Rolletschek et al. (2002a). In brief, the microsensor (about 30-μm tip diameter) and the intact pod were inserted in a micromanipulator. A small window was cut into the pod wall, and the microsensor was driven into the seed at 100-μm intervals. Measurements were done at light and dark conditions. Light intensity was adjusted to 400 to 450 μmol m–2 s–1 (measured at seed surface) using the lighting system of the microscope. This value corresponds to those measured inside the pod when plants are exposed to sunlight and mimics the shading effect of intact pods. The O2 levels are given in percentage of at atmospheric O2 concentration (approximately 21 kPa = 100% corresponds to approximately 270 μm).
Respiration Measurements
To measure respiration, freshly isolated, intact broad bean embryos were transferred into a 10 mL-measuring chamber equipped with a magnetic stirrer and the inserted microsensor. Incubation buffer was prepared according to Millerd et al. (1975), modified as followed: 250 mm Suc, 30 mm Gln, 30 mm Asn, 20 mm KCl, 4 mm CaCl2, 2 mm K2SO4, 2 mm KH2PO4, 3 mm MgSO4, and 10 mm MES/KOH. Final pH of the medium was 5.6. After 3 to 5 min of preincubation, respiration was measured as O2 uptake in darkness within 5 min. To measure the dependence of respiration on external oxygen level, the incubation medium was aerated with helium until the desired O2 level was reached.
Enzyme Assay
For the preparation of crude extracts, broad bean embryos were homogenized on ice by precooled pestle and mortar in six volumes of cold extraction buffer (125 mm MES, 100 mm NaCl, 2.5 mm MgCl2, 0.5 mm thiamine pyrophosphate, 1 mm EDTA, and 2 mm dithiothreitol, pH 6.8). Homogenates were kept at 4°C and centrifuged for 10 min at 4°C and 10,000g. ADH (EC 1.1.1.1) was measured spectrophotometrically as given by Waters et al. (1991). Activity measurements were corrected by subtracting values obtained in the absence of substrate.
Extraction and Determination of Metabolites and Pigments
Frozen plant material was extracted with trichloroacetic acid according to Rolletschek et al. (2002b). Aliquots of snap-frozen extracts were subsequently used for metabolite analyses. Free amino acids, nucleotides, and their sugars were determined by HPLC as described in detail previously (Rolletschek et al., 2002a). Soluble sugars and lactate were measured spectrophotometrically as by Heim et al. (1993) and Rolletschek et al. (2002a), respectively. Recovery rates were already documented (Rolletschek et al., 2002a, 2002b). For pigment analysis, frozen material (20-100 mg fresh weight) was extracted once with 600 μL of acetone and 4 μL of 10 μm KOH and centrifuged for 5 min at 4°C and 10,000g. The pellet was resuspended with 300 μL of acetone and 2 μL of KOH. After centrifugation, the supernatants were combined and immediately measured spectrophotometrically according to Porra et al. (1989).
[14C] Suc Partitioning
To estimate in vivo biosynthetic fluxes of broad bean embryos, we measured the incorporation of [14C] label. Intact plants with attached pods were illuminated (approximately 400 μmol m–2 s–1) or kept in darkness. A small window was cut into the pod wall to get access to the seed. Using a 10-μL syringe, 3 μL of [U-14C]Suc (7.4 MBq mL–1, Amersham-Buchler, Braunschweig, Germany) was injected carefully through the seed coat into the endospermal cavity of the seed surrounding the embryo without injuring the latter. The injection site of the seed coat and the incision site of the pod wall was covered with silicone grease to avoid both water loss and gas exchange. After a 4-h incubation period, the embryo was removed from the seed, rinsed two times in buffer (250 mm Suc and 10 mm MES/KOH, pH 5.6), weighed, and frozen in liquid nitrogen. Only embryos without obvious damage of their surface were used for subsequent analyses. Extraction was carried out once with 80% (v/v) ethanol at 60°C, and in two subsequent steps with 60% (v/v) ethanol (combined supernatants were assigned as soluble fraction). Insoluble material was washed twice in 1 mL of water and homogenized. To hydrolyze starch, an aliquot was incubated with 14 units of amyloglucosidase in 50 mm sodium acetate (pH 4.8) for 24 h at 55°C. After centrifugation (15 min, 14,000g), the pellet was washed once in 1 mL of water, and the combined supernatants were counted. To dissolve protein, the residue was incubated for 24 h at 30°C in 1 mL of 50 mm Tris-HCl (pH 7.4) containing 0.08% (w/v) pronase. The homogenate was centrifuged and washed once in 1 mL of water, and the combined supernatants were counted. The remaining pellet (assigned as cell wall) was resuspended in 1 mL of water and counted. Radioactivity was determined by liquid scintillation counting (Rotiszint, Roth, Germany). Counts were corrected for background and quenching by external standards.
Ethanol Emission Rate
Intact broad bean seeds were removed from the pod and enclosed in 12-mL headspace vials containing a small portion of wet cotton wool (to conserve high humidity). After a 4-h incubation period, the amount of ethanol (released by the seed) within the headspace was measured gas chromatographically (6890 Series, Agilent Technologies, Palo Alto, CA). Chromatographic conditions were as follows: column DB23 (J&W Scientific, Folsom, CA) 30-m × 0.25-mm (i.d.) × 0.25-μm film; injector, 5-μL injection volume; split ratio 5:1, 250°C; oven, 50°C for 5 min; detector, flame ionization detector, 275°C, He makeup gas 25 mL min–1; carrier gas, He with 1 mL min–1. Emission rate was calculated from the amount of ethanol released during the incubation time related to the fresh weight of the incubated embryo.
Hypoxic Treatment
To study the effect of diminished oxygen supply, pea seeds were aerated with premixed gases (Messer-Griesheim, Germany). For the in vitro experiment, embryos (about 100 mg fresh weight) were harvested and incubated in a nutrient solution (as used for respiration studies) in 50-mL conical flasks in darkness. The medium was aerated with gases containing the atmospheric oxygen level (about 21 vol%, referred to as to 100%), 2 vol% O2 (= 10%, balanced by N2), and 0.2 vol% O2 (= 1%, balanced by N2), respectively. After 5 h of incubation, embryos were rinsed shortly two times in distilled water, frozen in liquid nitrogen, weighed, and stored at –80°C until used. For the in vivo experiment, pea pods attached to the plant were coated with aluminum foil and aerated with premixed gases (100%, 10%, and 1% O2 of atmospheric O2 concentration, respectively) for 5 h. Thereafter, embryos were removed, frozen in liquid nitrogen, weighed, and stored at –80°C.
Acknowledgments
We are grateful to Ulrich Wobus for continuous support and discussions. Many thanks to Katrin Blaschek for excellent technical assistance and Heiko Weichert for help with ethanol measurements.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017376.
This work was supported by the Deutsche Forschungsgemeinschaft.
References
- Asokanthan P, Johnson RW, Griffith M, Krol M (1997) The photosynthetic potential of canola embryos. Physiol Plant 101: 353-360 [Google Scholar]
- Atkins CA, Flinn AM (1978) Carbon dioxide fixation in the carbon economy of developing seeds of Lupinus albus (L.). Plant Physiol 62: 486-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji D, Rauf A (1979) Comparative growth and biochemical studies on seed development: 3. Chlorophyll development and Hill activity in developing seeds of Pisum sativum and Vicia faba. Plant Biochem J 6: 31-35 [Google Scholar]
- Borisjuk L, Walenta S, Rolletschek H, Mueller-Klieser W, Wobus U, Weber H (2002) Spatial analysis of plant development: Sucrose imaging within Vicia faba cotyledons reveals specific developmental patterns. Plant J 29: 521-530 [DOI] [PubMed] [Google Scholar]
- Borisjuk L, Walenta S, Weber H, Mueller-Klieser W, Wobus U (1998) High resolution histographical mapping of glucose concentrations in developing cotyledons of V. faba in relation to mitotic activity and storage processes: glucose as a possible developmental trigger. Plant J 15: 583-591 [Google Scholar]
- Borisjuk L, Weber H, Panitz R, Manteuffel R, Wobus U (1995) Embryogenesis of Vicia faba L.: histodifferentiation in relation to starch and storage protein synthesis. J Plant Physiol 147: 203-218 [Google Scholar]
- Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48: 223-250 [DOI] [PubMed] [Google Scholar]
- Eastmond P, Kolacna L, Rawsthorne S (1996) Photosynthesis by developing embryos of oilseed rape. J Exp Bot 47: 1763-1769 [Google Scholar]
- Emes MJ, Bowsher CG, Hedley C, Burrell MM, Scarse-Field ESF, Tetlow IJ (2003) Starch synthesis and carbon partitioning in developing endosperm. J Exp Bot 54: 569-575 [DOI] [PubMed] [Google Scholar]
- Flinn AM (1985) Carbon dioxide fixation in developing seeds. In PD Hebblethwaite, MC Heath, TCK Dawkins, eds, The Pea Crop: A Basis for Improvement. Butterworths, London, pp 349-358
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M (2000) Metabolic activity decreases as an adaptive response to low internal O2 in growing potato tubers. Biol Chem 381: 723-740 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M (1993) Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189: 329-339 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221-235 [DOI] [PubMed] [Google Scholar]
- Golombek S, Heim U, Horstmann C, Wobus U, Weber H (1999) Phosphoenolpyruvate carboxylase in developing seeds of Vicia faba L.: gene expression and metabolic regulation. Planta 208: 66-72 [DOI] [PubMed] [Google Scholar]
- Harvey DM, Hedley CL, Keely R (1976) Photosynthetic and respiratory studies during pod and seed development in Pisum sativum L. Ann Bot 40: 993-1001 [Google Scholar]
- Heim U, Weber H, Bäumlein H, Wobus U (1993) A sucrose-synthase gene of V. faba L.: expression pattern in developing seeds in relation to starch synthesis and metabolic regulation. Planta 191: 394-401 [DOI] [PubMed] [Google Scholar]
- Hill RD (1998) What are hemoglobins doing in plants? Can J Bot 76: 707-712 [Google Scholar]
- Kuang A, Crispi M, Musgrave ME (1998) Control of seed development in Arabidopsis thaliana by atmospheric oxygen. Plant Cell Environ 21: 71-78 [DOI] [PubMed] [Google Scholar]
- López-Barneo J, Pardal R, Ortega-Sáenz P (2001) Cellular mechanisms of oxygen sensing. Annu Rev Physiol 63: 259-287 [DOI] [PubMed] [Google Scholar]
- Millerd A, Spencer D, Dudman F, Stiller M (1975) Growth of immature cotyledons in culture. Aust J Plant Physiol 2: 51-59 [Google Scholar]
- Neuhaus HE, Emes MJ (2000) Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51: 111-140 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T, Allen JF, Oelmüller R (2001) Principles of redox control in photosynthesis gene expression. Physiol Plant 112: 1-9 [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with different solvents-verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975: 384-394 [Google Scholar]
- Porterfield DM, Kuang A, Smith PJS, Crispi ML, Musgrave ME (1999) Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot 77: 1439-1446 [PubMed] [Google Scholar]
- Quebedeaux B, Hardy RWF (1975) Reproductive growth and dry matter production of Glycine max (L.) Merr. in response to oxygen concentration. Plant Physiol 55: 102-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002a) Legume embryos develop in a hypoxic environment. J Exp Bot 53: 1099-1107 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Hajirezaei M, Wobus U, Weber H (2002b) Antisense-inhibition of ADP-glucose pyrophosphorylase in Vicia narbonensis seeds increases soluble sugars, causes higher uptake of water and amino acids which leads to higher protein content. Planta 214: 954-964 [DOI] [PubMed] [Google Scholar]
- Saito GY, Chang YC, Walling LL, Thompson WW (1989) A correlation in plastid development and cytoplasmic ultrastructure with nuclear gene expression during seed ripening in soybean. New Phytol 113: 459-469 [Google Scholar]
- Shelp BJ, Walton CS, Snedden WA, Tuin LG, Oresnik IJ, Layzell DB (1995) Gaba shunt in developing soybean seeds is associated with hypoxia. Physiol Plant 94: 219-228 [Google Scholar]
- Tiessen A, Hendriks JHM, Stitt M, Branscheid A, Gibon Y, Farre EM, Geigenberger P (2002) Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell 14: 2191-2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager HG (1974) The effect of subjecting peas to air enriched with carbon dioxide: I. Respiration and the metabolism of the major acids. J Exp Bot 2: 338-351 [Google Scholar]
- Waters I, Morrell S, Greenway H, Colmer TD (1991) Effect of anoxia on wheat seedlings: II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation and sugar levels. J Exp Bot 4: 1437-1447 [Google Scholar]
- Weber H, Borisjuk L, Heim U, Buchner P, Wobus U (1995a) Seed coat associated invertases of fava bean control both unloading and storage functions: cloning of cDNAs and cell type-specific expression. Plant Cell 7: 1835-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U (1996) Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Comparative analysis of a small and a large-seeded genotype. Plant J 10: 823-824 [Google Scholar]
- Weber H, Heim U, Borisjuk L, Wobus U (1995b) Cell-type specific, coordinate expression of two ADP-glucose pyrophosphorylase genes in relation to starch biosynthesis during seed development of Vicia faba. Planta 195: 352-361 [DOI] [PubMed] [Google Scholar]
- Weber H, Heim U, Golombek S, Borisjuk L, Wobus U (1998) Assimilate uptake and the regulation of seed development. Seed Sci Res 8: 331-345 [Google Scholar]
- Willms JR, Salon C, Layzell DB (1999) Evidence for light-stimulated fatty acid synthesis in soybean fruit. Plant Physiol 120: 1117-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EC, Blackman SJ (1987) Histochemical localization of alcohol dehydrogenase in developing bean seeds. Am J Bot 74: 1461-1465 [Google Scholar]