Abstract
PASTICCINO (PAS) genes are required for coordinated cell division and differentiation during plant development. In loss-of-function pas mutants, plant aerial tissues showed ectopic cell division that was specifically enhanced by cytokinins, leading to disorganized tumor-like tissue. To determine the role of the PAS genes in controlling cell proliferation, we first analyzed the expression profiles of several genes involved in cell division and meristem function. Differentiated and meristematic cells of the pas mutants were more competent for cell division as illustrated by the ectopic and enlarged expression profiles of CYCLIN-DEPENDENT KINASE A and CYCLIN B1. The expression of meristematic homeobox genes KNOTTED-LIKE IN ARABIDOPSIS (KNAT2, KNAT6), and SHOOT MERISTEMLESS was also increased in pas mutants. Moreover, the loss of meristem function caused by shoot meristemless mutation can be suppressed by pas2. The KNAT2 expression pattern defines an enlarged meristematic zone in pas mutants that can be mimicked in wild type by cytokinin treatment. Cytokinin induction of the primary cytokinin response markers, ARABIDOPSIS RESPONSE REGULATOR (ARR5 and ARR6), was enhanced and lasted longer in pas mutants, suggesting that PAS genes in wild type repress cytokinin responses. The expression of the cytokinin-regulated cyclin D, cyclin D3.1, was nonetheless not modified in pas mutants. However, primary auxin response genes were down-regulated in pas mutants, as shown by a lower auxin induction of IAA4 and IAA1 genes, demonstrating that the auxin response was also modified. Altogether, our results suggest that PAS genes are involved in the hormonal control of cell division and differentiation.
Plant cell proliferation and differentiation are controlled by many factors and, in particular, by the hormones auxin and the cytokinins. High auxin/cytokinin ratios usually induce root formation, whereas low auxin/cytokinin ratios induce shoot formation. Intermediate auxin/cytokinin ratios induce cell dedifferentiation and proliferation, leading to callus development. Several cytokinin signaling and early response genes were recently found to be involved in the control of cell proliferation and differentiation (for review, see Haberer and Kieber, 2002). Cytokinin perception involves three membrane-associated receptors (AtHK2, AtHK3, and AtHK4/CRE1/WOL) similar to the lower eukaryotes two-component system, which are constituted by a sensor His kinase (also termed sensor) and one or more response regulators. An additional gene called CKI1, which is structurally related to CRE1, is also able to activate the cytokinin signaling pathway, but does not bind cytokinins, at least at physiological concentrations (for review, see Hwang and Sheen, 2001). Cytokinins induce a multistep phosphorelay transfer from the receptor to small phosphotransmitter proteins called Arabidopsis His-phosphotransfer proteins (AHP). Upon phosphorylation by one of the cytokinin-activated His kinase receptors, AHP1 and AHP2, but not AHP5, migrate from the cytosol to the nucleus where they activate B-type response regulators (ARR2 or ARR1; Hwang and Sheen, 2001). B-type response regulators are composed of a receiver domain with a C terminus output domain related to those found in the DNA-binding motifs of basic helix-loop-helix and MYB transcription factors (Lohrmann and Harter, 2002). B-type ARABIDOPSIS RESPONSE REGULATORS (ARRs) are located in the nucleus where they activate the transcription of A-type ARRs such as ARR5 or ARR6. The transcription of A-type ARRs is rapidly induced by cytokinins, whereas that of B-type ARRs has been reported to be unaffected by cytokinins (Imamura et al., 1999; Kiba et al., 1999; D'Agostino et al., 2000). Mutations in the AtHK4/CRE1/WOL gene decrease cell proliferation in response to cytokinins (Inoue et al., 2001). Overexpression of ARR2 and CKI1 leads to extensive cell proliferation and shoot regeneration in the absence of cytokinins (Kakimoto, 1996; Hwang and Sheen, 2001). Similarly, overexpression of ARR1 results in increased sensitivity to cytokinins as illustrated in a cell proliferation assay (Sakai et al., 2001). Conversely, the arr1 mutant is less sensitive to cytokinins (Sakai et al., 2001). Overexpression of the cytokinin-induced gene Enhanced Shoot Regeneration1 also confers cytokinin-independent shoot regeneration (Banno et al., 2001). Mutations in other yet-uncharacterized genes are also responsible for the deregulation of cell proliferation and regeneration in vitro. Mutations in POM1/ERH2 and IRE1 induce shoot regeneration in the presence of suboptimal concentration of cytokinins, but do not modify cytokinin endogenous levels (Cary et al., 2001). The analysis of cytokinin responses demonstrated that pom1 and ire1 mutants are cytokinin hyper-responsive rather than hypersensitive. In contrast, ckh1 and ckh2 mutants, isolated in similar screens, present typical cytokinin hypersensitivity (Kubo and Kakimoto, 2000).
Cytokinins were also involved in regulating cell division in the shoot apical meristem (Schmulling, 2002). Cell divisions in the apical meristem have been shown to be controlled by the homeobox-related factors like SHOOT MERISTEMLESS (STM; Weigel and Jurgens, 2002). STM was required for the maintenance of the meristematic stem cells. KNOTTED-like1 (KNAT1), which is related to STM, induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis (Chuck et al., 1996). Both genes were up-regulated by cytokinins (Rupp et al., 1999). The converse is also true because overexpression of the maize (Zea mays) KNOTTED1 (KN1) gene in tobacco (Nicotiana tabacum) and overexpression of KNOTTED-LIKE IN ARABIDOPSIS (KNAT1) in lettuce (Lactuca sativa) lead to cytokinin accumulation (Hewelt et al., 2000; Frugis et al., 2001). Ectopic expression of maize KN1 resulted in cytokinin-autotrophic growth of cultured tobacco tissues (Hewelt et al., 2000).
Although a large set of mutants and genes have been isolated and characterized from in vitro cell proliferation and regeneration experiments, much less is known about the genetic control of cell proliferation and differentiation in planta. Several mutant lines of Arabidopsis were directly selected for their ability to grow as callus on hormone-free medium (Frank et al., 2000). These different mutant lines were altered in their cytokinin and/or auxin sensitivity and contents. Unfortunately, these lines could not be studied further because of their sterility. Recently, three different genes TUMOROUS SHOOT DEVELOPMENT (TSD1, 2, 3) were genetically identified because their loss of function resulting in disorganized tumorous tissues instead of leaves and stems (Frank et al., 2002). Inhibitory concentrations of cytokinins for wild type enhance cell proliferation in tsd seedlings. The tsd phenotype is reminiscent of the phenotype of the pasticcino (pas) mutants (Faure et al., 1998). The pas mutants were isolated in a screen for uncontrolled growth in presence of cytokinins, leading to cell proliferation and callus formation. Pas mutants had an altered development starting at the embryo heart stage, leading to abnormal leaf and root development (Faure et al., 1998). Seedlings presented an enlarged hypocotyl and small rounded cotyledons, which did not develop further after germination. The apical meristem structure was variable, often showing a large disorganized meristematic-like zone. Organs of the plant apices showed ectopic and anarchic cell divisions, which were not observed in the root. The pas mutants represent three complementation groups (Pas1, Pas2, and Pas3). Double-mutant analysis revealed epistatic relationships between them, suggesting genetic interactions between the PAS genes. The PAS1 gene was cloned and found to encode an immunophillin-like protein of the FK506-binding protein family (Vittorioso et al., 1998; Harrar et al., 2001). FK506-binding proteins probably hold signaling proteins in “poised” states that can be triggered to active forms by protein modifications, such as phosphorylation (Rutherford and Zuker, 1994). PAS2 gene was recently identified and found to encode a protein Tyr phosphatase-like structurally conserved among eukaryotes (Bellec et al., 2002).
As cytokinins are involved in cell differentiation and proliferation and have been shown to enhance proliferation in the pas mutants, we have analyzed the relationship between the pas phenotype and cytokinin responses. Here, we present evidence that pas mutants have a modified competence for cell division in meristematic and differentiated tissues that is associated with the deregulation of cytokinin but also auxin primary response genes.
RESULTS
The Expression Pattern of Cell Cycle Genes Is Deregulated in pas Mutants
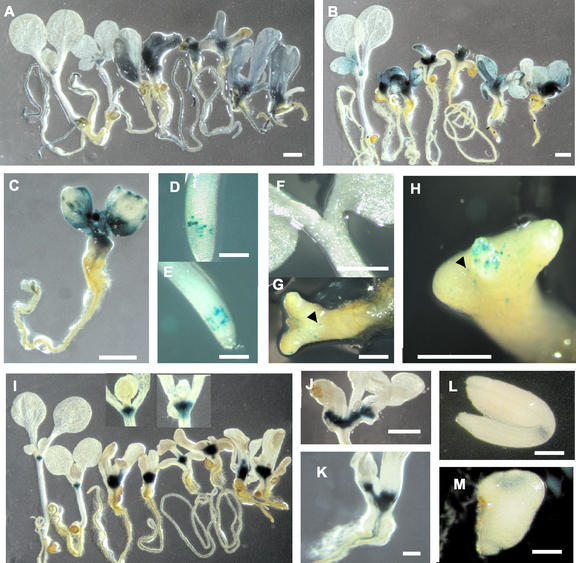
To evaluate the differentiation state of the cells in different seedling tissues, we examined the expression of CYCLIN-DEPENDENT KINASE A (CDKA), a marker of cell division competency, which is weakly expressed in differentiated cells (Hemerly et al., 1993). CDKA::β-glucuronidase (GUS) expression was analyzed in 10-d-old pas and wild-type seedlings (Fig. 1A). In wild type, CDKA was mainly expressed in the root and apical meristems as well as in the leaf primordia, but was absent or weakly expressed in differentiated tissues like hypocotyls, roots, or cotyledons. In the three pas mutants, CDKA expression was enhanced in seedling apices, particularly in the meristematic area. CDKA expression was also ectopically expressed in the hypocotyl and the cotyledons, demonstrating that the cells of these normally differentiated tissues retained some competence to divide. To examine whether CDKA expression was correlated with the expression of other cell division markers, we monitored CYCLIN B1 (CYCB1 also named Cyc1a) expression in pas mutants (Fig. 1, B and C). CYCB1 is a mitotic cyclin specifically expressed at the G2/M transition and provides a marker for cells undergoing mitotic divisions (Ferreira et al., 1994). We introduced CYCB1::GUS marker into the pas mutants to monitor the pattern of cell divisions in seedlings. Due to the stability of GUS and the absence of the cyclin “destruction box” (db) in the construct, CYCB1::GUS does not indicate the cells dividing at the time of observation, but rather the history of divisions that took place in the different tissues the days preceding the observation. Wild-type seedlings showed CYCB1 expression in roots and apical meristems as well as in young leaf primordia. The three pas mutant seedlings showed enhanced expression of CYCB1 in the meristems, but also ectopically in the hypocotyl and the cotyledons. CYCB1 expression often appeared in localized areas of pas leaves, suggesting heterogeneous activation of cell division (Fig. 1C); this patchy pattern was never observed in wild type. To monitor cell division at the time of staining, CYCB1::db::GUS was introduced into pas mutants and was analyzed in 5-d-old seedlings to check whether ectopic cell division observed with CYCB1::GUS was occurring earlier in development. In wild type, 5 d after germination CYCB1::db::GUS staining was only observed in the root meristematic zone (Fig. 1D). No staining could be observed in the seedling apical part (Fig. 1F). In the pas mutants, CYCB1::db::GUS staining in the root is similar to wild type, indicating that pas mutations did not alter directly the cell cycle and thus the expression of CYCB1. Contrary to the root, the apical part of pas seedling showed ectopic expression of CYCB1::db::GUS in differentiated tissues such as the hypocotyl and the cotyledons (Fig. 1, G and H).
Figure 1.
Expression of cell division and meristematic-associated markers in the pas mutants. (A) CDKA::GUS expression in wild-type, pas1, pas2, and pas3 seedlings (from left to right, two seedlings each) 10 d after germination. Wild-type seedlings were grown in the absence (left) or presence of 0.1 μm 6-benzyladenine (BA; right). All of the pas mutants were grown in absence of cytokinins. (B) CYCB1::GUS expression in wild-type, pas1, pas2, and pas3 seedlings (from left to right, two seedlings each) 10 d after germination. CDKA and CYCB1 were strongly expressed in the meristems and were ectopically expressed in the leaves of the pas mutants. (C) CYCB1::GUS expression in pas1 mutant 10 d after germination showing localized area of ectopic CYCB1 expression in the leaves. (D) CycB1::db::GUS expression in 5 d after germination wild-type root. (E) CycB1::db::GUS expression 5 d after germination in pas2-1 root. (F) CycB1::db::GUS expression 5 d after germination in wild-type seedling. (G and H) CycB1::db::GUS expression 5 d after germination in pas2-1 seedling. (I) KNAT2::GUS expression in wild-type, pas1, pas2, and pas3 seedlings (from left to right, two seedlings each) 10 d after germination. Wild-type seedlings were grown in the absence (left) or presence of 0.1 μm BA (right). The corresponding meristematic areas are enlarged, showing increased expression of KNAT2 in the presence of cytokinins (right insert) compared with control (left insert). The pas mutants were grown in the absence of cytokinins. (J) KNAT2::GUS expression in the pas2 mutant 18 d after germination. (K) KNAT2::GUS expression in the pas3 mutant 10 d after germination. KNAT2::GUS staining shows the existence of enlarged or doubled meristem in the pas mutants. (L) KNAT2::GUS expression in mature wild-type embryo. (M) KNAT2::GUS expression in mature pas2 embryo. Scale bar represents 1 mm except for D, E, L, and M, where it represents 200 μm.
Several KNAT Genes are Up-Regulated in pas Mutants
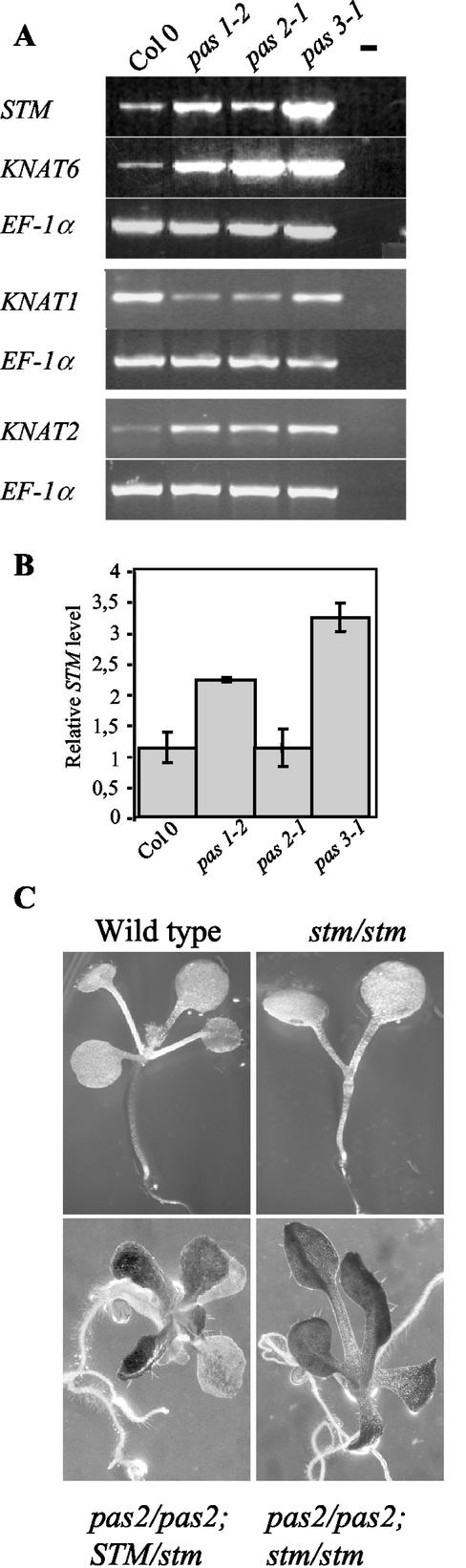
Analysis of cell division markers showed an enlarged meristematic region in the three pas mutants. Enlargement of meristematic area was associated with disorganized meristem structure, as already described (Faure et al., 1998). Thus, we analyzed the expression pattern of several KNOTTED-like in Arabidopsis (KNAT) genes, which where found to be involved in meristem function. Expression levels of KNAT1, KNAT2, KNAT6, and STM were analyzed by reverse-transcriptase (RT)-PCR and real-time quantitative PCR (for STM only). KNAT2, KNAT6, and STM genes, but not KNAT1, showed increased expression levels in pas seedlings compared with wild type, suggesting that PAS genes act as negative regulators of the expression of several KNAT genes (Fig. 2, A and B).
Figure 2.
Interaction between KNAT genes and pas phenotype. (A) RT-PCR analysis of KNAT mRNA levels in wild-type and pas mutants. (B) Real-time quantitative PCR of STM mRNA levels in wild-type and pas mutants. (C) Phenotype of wild type, stm/stm, pas2/pas2, and the double-mutant pas2/pas2;stm/stm.
KNAT expression was further analyzed in the case of KNAT2, which is specifically expressed in the inner part of the meristem that contains the founder cells that will be recruited by the peripheral and rib zones to initiate organs (Dockx et al., 1995). KNAT2 provides a marker for the most undifferentiated cells of the meristem. KNAT2::GUS was observed in 10-d-old wild-type and mutant seedlings (Fig. 1I). Although KNAT2::GUS was restricted in wild type to a small internal zone of the meristem, the zone of its expression was enlarged in the three pas mutants. In some pas2 and pas3 mutant seedlings, KNAT2::GUS staining defined several distinct but connected regions in the meristematic zone, suggesting the occurrence of meristematic activity outside the usual bounds of the shoot apical meristem (Fig. 1, J and K). The ectopic formation of shoots in pas2 and pas3 suggests that multiple sites of shoot initiation could develop from these enlarged meristematic regions. KNAT2 enlarged expression could be a consequence of the modified meristematic zone in the pas mutants. KNAT2::GUS expression was analyzed in mature embryo imbibated for 16 h (Fig. 1, L and M). The pas embryos still presented an enlarged KNAT2::GUS expression zone compared with wild type, even if the expression pattern was not as pronounced as in the seedlings. As previously shown (Hamant et al., 2002), KNAT2 expression is enhanced in wild-type apical meristems of seedlings treated with exogenous cytokinins (Fig. 1I, inserts). Cytokinin treatment also induced ectopic KNAT2 expression in the vascular tissue of wild-type roots (data not shown). The KNAT2::GUS pattern in pas mutants were similar to those observed in cytokinin-treated wild type, i.e. enlarged meristematic GUS staining and ectopic staining in root vascular bundles. In a wild-type background expressing KNAT2::GUS, GUS activity as quantified by fluorometry was 2 ± 0.2 nmol MU min–1 mg–1. In pas seedlings, GUS activity was 7.2 ± 0.2 nmol MU min–1 mg–1 for pas1, 5.6 ± 1.5 for pas2, and 8.2 ± 0.2 for pas3. This result confirmed the enhanced expression of KNAT2 in pas mutants.
To investigate how the increased cell division in pas meristems is relevant to meristem function, we analyzed the phenotype of double mutants between pas2-1 and a strong stm allele (stm dgh6), which is unable to form a shoot apical meristem (Fig. 2C). We have chosen pas2 because it has the less altered postembryonic phenotype among the pas mutants, in particular at the levels of leaves and stems (Bellec et al., 2002). Plants from the F2 population were genotyped by PCR. All the double mutants pas2/pas2; stm/stm analyzed at the seedling stage showed fused and deformed leaves characteristic of pas2 mutants. The presence of leaves in the double mutants indicated that the shoot apical meristem was functional and that pas2 mutation was able to suppress strong stm mutation, restoring an active meristem.
Cytokinin Primary Response Genes Are Up-Regulated in pas Mutants
Because KNAT expression and function were associated with cytokinins in several studies (Rupp et al., 1999; Hamant et al., 2002), an attractive hypothesis could be that pas mutations affect directly cytokinin responses. Thus, cytokinin responses were analyzed in the pas mutants compared with wild type by quantifying the expression of two A-type ARR genes, ARR5 and ARR6 (Fig. 3). We used an experimental system that allowed rapid and reproducible treatment of seedlings by hormones. Seedlings were germinated on solid media and were grown in the light for about 12 d and were then transferred to the same liquid media for 2 d and finally were directly treated with cytokinins in liquid. Liquid culture allows homogeneous seedling treatment and rapid access of the hormone to all seedling tissues.
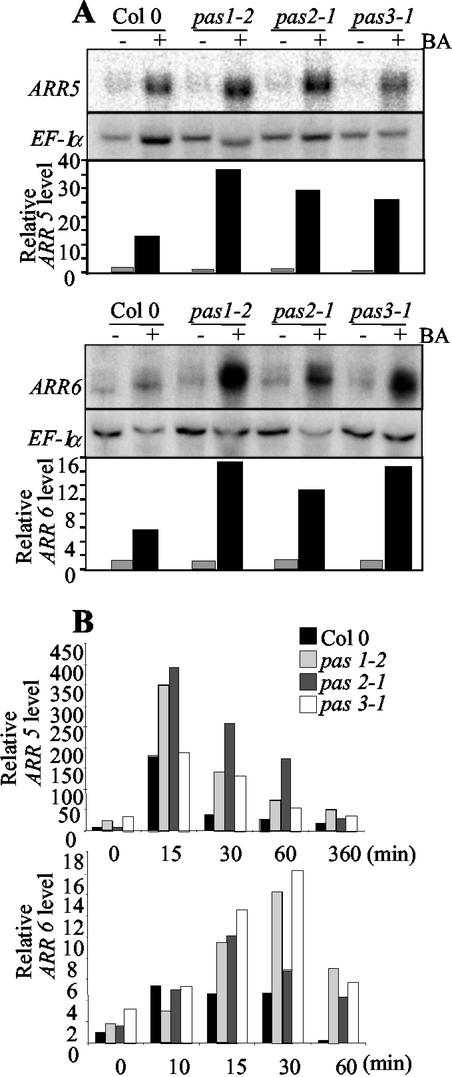
Figure 3.
Cytokinin regulation of ARR5 and ARR6 in pas mutants. (A) ARR5 and ARR6 mRNA levels in wild-type and pas mutant seedlings after 30 and 60 min cytokinin treatment, respectively. (B) Time-course expression analysis of ARR5 (top) and ARR6 (bottom) in wild-type and pas mutants. Seedlings were transferred 12 d after germination to liquid media for 2 d and treated with 10 μm BA for the time indicated. ARR hybridization signals were quantified and normalized with EF-1α as described in “Materials and Methods.”
ARR5 and ARR6 expression were first monitored in wild-type and pas mutants after 30 and 60 min, respectively, of induction with 10 μm BA (Fig. 3A). Pas mutants showed increased expression levels of both genes after cytokinin treatment compared with wild type (5-, 24-, 16-, and 23-fold for ARR5 at 30 min and 4-, 13-, 8-, and 10-fold for ARR6 at 60 min, for wild type, pas1, pas2, and pas3, respectively; Fig. 3A).
To investigate whether pas mutations modified not only the amplitude but also the timing of cytokinin response, time-course analysis of ARR5 and ARR6 expression was undertaken. ARR5 was induced by cytokinins in wild-type treated seedlings in 15 min with a 19-fold induction and then mRNA levels declined rapidly, reaching noninduced levels after 60 min (Fig. 3B). ARR6 mRNA showed a 4-fold induction by cytokinins after 10 min and then reached basal levels after 60 min. (Fig. 3B). Both genes were also induced by cytokinins in pas mutants but with a higher amplitude than in wild type. After a 15-min induction, ARR5 mRNA levels were 2-fold higher in pas1 and pas2 mutants compared with wild type, and after a 30-min induction, reached three to six times wild-type levels for the three pas mutants. Induction of ARR5 in pas3 mutants was similar to wild type after 15 min of treatment, but mRNA levels remained high after 60 min of induction. ARR6 induction was also enhanced in the three pas mutants. Pas3 mutants showed the highest response with a 4-fold wild-type level at 30 min. In all three mutants, ARR6 expression was maintained at high levels even after 60 min of treatment, whereas wild-type mRNA levels returned to almost basal level.
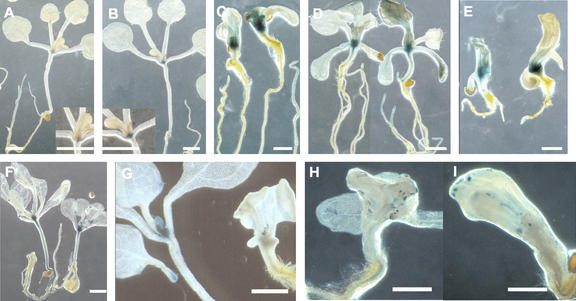
ARR5 expression was also analyzed in planta by following the expression of an ARR5::GUS construct (Fig. 4A-E). ARR5 expression in young seedlings was mainly expressed in apical and root meristems, but was also found in vascular tissues (root, hypocotyl, and cotyledons). Weak ARR5 expression was also observed in cotyledons and mature leaves after prolonged staining (Fig. 4A; D'Agostino et al., 2000). Cytokinin treatment enhanced GUS staining in the meristem, confirming the northern results (Fig. 4, A and B, inserts). In the pas mutants, ARR5::GUS expression was also mainly localized in meristems, but the staining intensity was significantly increased compared with wild type in agreement with the northern results (Fig. 1, C-E). In young pas seedlings showing a strong phenotype, enhanced ARR5::GUS expression can be found in cotyledon and hypocotyl cells (data not shown). ARR5::GUS staining was also observed in the leaf vascular tissues and in the roots of pas2 mutants and in leaves of pas3 mutants (Fig. 4, D and E).
Figure 4.
Expression of cytokinin-associated markers in pas mutants. (A) ARR5::GUS expression in a 15 d after germination wild-type seedling with an enlarged view of the corresponding meristematic zone in the insert. (B) ARR5::GUS expression in a 15 d after germination wild-type seedling treated for 2 h with 10 μm BA. An enlarged view of the corresponding meristematic zone is shown in the insert. (C) ARR5::GUS expression 15 d after germination in pas1 seedlings treated with 10 μm BA (right) compared with control (left). (D) ARR5::GUS expression 15 d after germination in pas2 seedlings treated with 10 μm BA (right) compared with control (left). (E) ARR5::GUS expression 15 d after germination in pas3 seedlings treated with 10 μm BA (right) compared with control (left). (F) CYCD3::GUS expression 15 d after germination in wild-type seedlings grown in the presence of 5 μm BA (right) compared with control (left). (G) CYCD3::GUS expression in wild-type (left) and pas1 (right) seedlings 15 d after germination. (H) CYCD3::GUS expression in pas2 mutant. (I) CYCD3::GUS expression in pas3-1 mutant. Scale bar represents 1 mm.
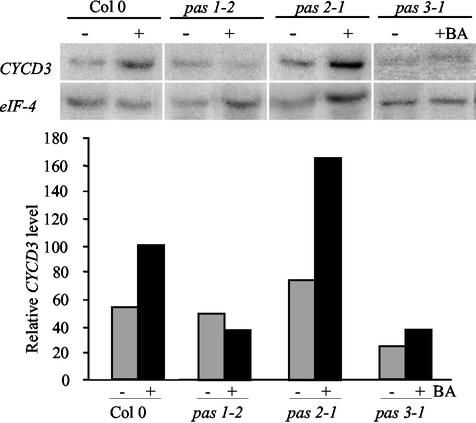
Cyclin D3.1 (CYCD3) Expression Is Not Enhanced in pas Mutants
Our results indicated that in the pas mutants, differentiated cells of the cotyledons or the hypocotyl were more competent for cell division. Moreover, we found that cytokinin primary responses were enhanced, suggesting that the PAS genes might control cell cycle regulation by cytokinins. CYCD3 is one of the most well-characterized cell cycle genes regulated by cytokinins. Constitutive expression of CYCD3 in transgenic plants led to cell proliferation in the absence of exogenous cytokinins (Riou-Khamlichi et al., 1999). In wild-type seedlings, CYCD3 expression levels showed an almost 2-fold induction after cytokinin treatment (Fig. 5). CYCD3 expression after cytokinin induction was slightly higher in the pas2 mutant and lower in pas1 and pas3 mutants compared with wild type. To confirm this result, CYCD3 expression was analyzed in planta using a CYCD3::GUS marker in wild-type and pas mutants. In wild type, CYCD3 was mainly expressed in the apical meristem, leaf primordia, young leaves, and hydrathodes of older leaves (Fig. 4, F and G). Heterogeneous and weak staining was usually observed in pas mutants (Fig. 4, G-I). Some seedlings expressed CYCD3 with a similar pattern to that of wild type, whereas most others showed no staining in apical meristems and leaf primordia but very localized foci of expression. Some stainings were localized in structures similar to the wild-type hydrathodes (Fig. 4G), whereas others were found in leaf cells clusters as for CYCB1::GUS staining (Fig. 4, H and I). CYCD3::GUS staining confirmed that CYCD3 was not overexpressed in pas mutants. CYCD3::GUS staining was maintained in mutant root meristems (data not shown), probably explaining why pas2 showed higher CYCD3 expression than other pas mutants in northern experiments because pas2 has an increased numbers of secondary root (Faure et al., 1998).
Figure 5.
Cytokinin regulation of CYCD3 expression in pas mutants. Steady-state mRNA levels of CYCD3 in wild-type and pas mutants treated with 10 μm BA in liquid for 24 h. CYCD3 hybridization signals were quantified and normalized with eIF4A as described in “Materials and Methods.”
Auxin Induction of Primary Response Genes Is Reduced in pas Mutants
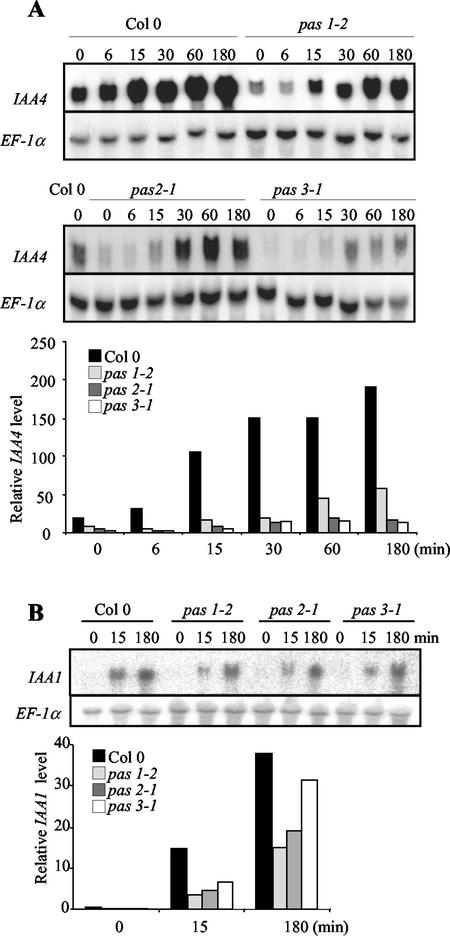
Cell dedifferentiation and proliferation are usually induced by a balanced auxin-to-cytokinin ratio. As pas mutants show an enhanced response to cytokinins, we investigated whether auxin responses were also modified. Auxin response was analyzed by the quantification of expression levels of primary response genes rapidly induced by auxin. We chose indole-3-acetic acid4 (IAA4) and IAA1 because they present a robust auxin regulation and they are among the few IAA genes expressed in shoot tissues (Abel et al., 1995).
The IAA4 mRNA level was rapidly increased in wild-type seedlings, five and 10 times, respectively, after 15 min and 3 h of auxin treatment (Fig. 6A). IAA4 mRNA basal levels were found to be lower in the three pas mutants compared with wild type. Auxin induction of IAA4 was nonetheless observed in pas mutants, but with a lower magnitude (10, 7, 3, and 6 times for wild type, pas1, pas2, and pas3, respectively, relative to noninduced levels after 3 h of treatment). A similar pattern of expression was also observed for IAA1 with lower mRNA levels in the pas mutants (Fig. 6B). After a 15-min induction, IAA1 levels were induced 82-, 62-, 49-, and 68-fold in wild type, pas1, pas2, and pas3, respectively. These results suggest that the expression of primary auxin response genes is also modified in the pas mutants.
Figure 6.
Auxin regulation of IAA4 and IAA1 gene expression in pas mutants. (A) Time-course analysis of steady-state IAA4 mRNA levels in wild-type and pas seedlings. (B) Time-course analysis of steady-state IAA1 mRNA levels in wild-type seedlings. Seedlings were transferred to liquid media 12 d after germination for 2 d and were treated with 20 μm IAA for the time indicated. IAA hybridization signals were quantified and normalized with EF-1α as described in “Materials and Methods.”
DISCUSSION
The pas mutants are characterized by ectopic cell divisions, which are specifically enhanced by cytokinins. They provide an interesting genetic model to study hormonal regulation of cell differentiation and proliferation in planta. Mutations in PAS genes resulted into developmental defects that can be tracked back to the heart stage of embryogenesis during the transition from radial to bilateral symmetry (Faure et al., 1998).
In this study, we found that the disorganized cell divisions are associated with the deregulation of cell cycle marker genes like CDKA and CYCB1, suggesting that cells would be maintained in a state competent for cell proliferation. Ectopic figures of division are probably responsible for the increased layers of cells observed, for instance, in the cortex and the epidermis of the hypocotyl (Faure et al., 1998). Increased cell division competency in pas mutants was particularly pronounced in already undifferentiated cells such as meristematic cells as illustrated by the expression of the cell division markers CDKA and CYCB1. Previous observations found that pas apical meristems were often enlarged and presented a loose structure where the different layers and zones were difficult to distinguish (Faure et al., 1998). Alteration of pas meristem structure is correlated with the up-regulation of several KNAT genes. The loss of PAS function leads to an enlarged KNAT2 expression zone comprising almost the entire apical meristem, confirming that most of the pas meristem are constituted by L3-like type cells. This enlarged KNAT2 expression zone could be already seen in mature embryos, suggesting that an altered expression of KNAT2 is an early effect of pas mutations. Increased propensity for cell division caused by the loss of PAS function is also illustrated by the suppression of strong stm phenotype by pas2. Loss of STM function leads to improper corpus/tunica organization in the embryo meristem and a functional meristem is never organized after germination (Barton and Poethig, 1993). STM loss of function could also be suppressed by a mutation in ASYMMETRYC LEAVES 1 (AS1), which is a negative regulator of KNAT genes. Secondary suppressor screen of as1 stm double mutants revealed that KNAT1 was involved in the restoration of meristem function in stm background (Byrne et al., 2002). Similarly, a mutation in YABBY that is associated with STM and KNAT2 up-regulation led to partial suppression of stm phenotype (Kumaran et al., 2002). The suppression of STM dysfunction by pas mutations could be explained by the up-regulation of several KNAT genes and by the fact that there is some functional redundancy among the KNAT family members.
Several reports linked cytokinins to the expression of meristematic homeobox transcription factors (Rupp et al., 1999; Hamant et al., 2002). The KNAT2 expression zone in cytokinin-treated wild type was enlarged as in pas mutants, suggesting that the deregulated KNOX expression in pas mutants could be related to an altered cytokinin response. Several other characteristics of pas phenotype suggest a defect in cytokinin responses. Earlier work has shown that, associated with their cytokinin-enhanced cell proliferation, pas mutants have two-dimensional protein profiles reminiscent to cytokinin-treated wild-type (Faure et al., 1998). The three pas mutants also show significant delay of senescence (Y. Harrar, unpublished data). Finally, pas2 seedlings exhibited ectopic shoot formation, which is a phenotype observed in cytokinin-overproducing plants or cytokinin-treated callus (Bellec et al., 2002).
Cytokinin primary response of the pas mutants was enhanced as illustrated by the expression pattern of two A-type ARRs. The response of the pas mutants to cytokinins may be caused by the increased size or number of meristems in the mutants because ARR5 was mainly expressed in the meristem. However, this seems unlikely because differences in tissue expression have not been correlated with the level of cytokinin response. Although ARR5 is mainly expressed in the meristems and the vascular tissue, the RNA levels after cytokinin induction are higher in leaves and stems than in buds and young flowers (Brandstatter and Kieber, 1998). Furthermore, ARR6 that is expressed in most tissues shows the strongest induction in leaves and not in buds (D'Agostino et al., 2000). Moreover, a higher number of responsive cells, as in pas meristems of the pas mutants, would lead to increased expression levels but with kinetics similar to that of the wild type. On the contrary, pas mutants show not only an increased amplitude of ARR gene expression, but also a delay in returning to basal levels. The modification of the amplitude of ARR5 and ARR6 expression but not the timing of the maximum cytokinin response suggests that the pas response to cytokinins could not only be explained by an higher number of responsive cells. The transient cytokinin induction of A-type ARRs is explained by the existence of a negative feedback regulatory loop in which the A-type ARR genes repress their own expression (Hwang and Sheen, 2001). The enhanced cytokinin response in pas mutants was also associated with a prolonged ARR expression. Such an expression pattern could not simply be the consequence of an increased amplitude of ARRs expression because pas3 showed wild-type levels of ARR5 induction, but its expression was nonetheless maintained after a 1-h induction. The proposed model for the maintenance of A-type ARR expression after cytokinin induction in the pas mutants would be that PAS genes are required for the A-type ARR negative regulatory feedback loop.
As in A-type ARRs, CYCD3 is also inducible by cytokinins and thus provides a valuable marker for cytokinin involvement in cell cycle regulation (Soni et al., 1995; Fuerst et al., 1996). Surprisingly, its expression was not enhanced in the pas or tsd mutants (Frank et al., 2002). The absence of CYCD3 overexpression in the six known classes of mutants with tumorous development suggests that in contrast to mammals in which CYCD altered expression is very often associated with cancer (Prober and Edgar, 2001), plant CYCD3 deregulation is not the main cause of cytokinin-driven tumor development.
Cell dedifferentiation and proliferation is usually caused by a balanced ratio of cytokinins and auxin. Callus-like development of pas seedlings in the presence of cytokinins was not found to be caused by a parallel increased of auxin sensitivity as judged by the phenotypic analysis of auxin-treated seedlings (Faure et al., 1998). Typical auxin responses such as secondary root formation in light-grown seedlings or hypocotyl peeling in dark-grown seedlings can be observed in pas mutants when exposed to auxin (Faure et al., 1998; C. Bellini, unpublished data). Auxin treatment could not compensate for pas apical phenotypes nor could it induce callus-like development in seedlings. The reduced induction of the early auxin-induced genes IAA4 and IAA1 suggests that the pas mutants have a reduced primary response to auxin. As several IAA genes have been involved as negative regulators of auxin response, a decreased induction of IAA genes in pas mutants could also be interpreted as an adaptive response to an enhanced cytokinin response by increasing auxin sensitivity (Tiwari et al., 2001; Park et al., 2002). The opposite case was illustrated for axr3 mutant, which showed an increased auxin sensitivity and where an exogenous supply of cytokinins was able to complement many aspects of the mutant phenotype, demonstrating that increased cytokinin levels can compensate, to a certain degree, an increase in auxin sensitivity (Leyser et al., 1996).
Altogether, PAS genes appear as negative regulators of cell proliferation by repressing cell division or by inducing cell differentiation. The negative regulation of KNAT expression by PAS genes is probably involved in maintaining cells in a differentiated state, avoiding uncontrolled cell proliferation and tumor development. Competency for cell division is dependent on the cell response to cytokinins and auxin. PAS genes controlled the amplitude of cytokinin and auxin responses and thus represent new regulators linking hormone response to the control of cell division and differentiation. The elucidation of the biochemical function of PAS proteins and their corresponding protein networks should shed light on the mechanisms of hormonal control of cell proliferation and differentiation.
MATERIALS AND METHODS
Plant Material and Hormone Treatment
The pas mutants were isolated from an ethyl methane sulfonatemutagenized population of the Columbia ecotype (Col-0; Faure et al., 1998). Seeds were sterilized and grown in vitro as described previously (Santoni et al., 1994) in a controlled environment chamber (irradiance 200 μE m2–2 s–1, 16 h of light, 60% humidity, 20°C day temperature, and 15°C night temperature). Mutant lines expressing GUS were produced by crossing wild-type lines expressing the GUS marker with the mutant lines. Three different progeny lines were tested in GUS staining to avoid background effect on GUS expression. pas2/pas2; stm/stm double mutants were obtained by selfing of the progeny of the pas2/pas2 × stm dgh6/+ (Aida et al., 2002) cross and were genotyped with stm primers (GAGACAGCAATTGATAGGAACAAT/ATGGTG-GAGGAGATGTGATCC).
For RNA analysis, mutant and wild-type plants were grown in vitro for 12 d and were then transferred to liquid Arabidopsis medium. After 2 d in liquid culture to avoid stress effects, culture medium was supplemented with 10 μm BA or 20 μm IAA for treated plants and dimethyl sulfoxide or ethanol, respectively, for control plants. Plants were harvested and stored in liquid nitrogen after different times of hormone induction before RNA extraction.
For GUS analysis, plants were grown for 10 to 15 d with or without 0.1 μm BA. A short 2-h induction was performed in liquid medium for ARR5::GUS-containing plants.
RNA Methods
Total RNA was extracted from seedlings as described previously (Verwoerd et al., 1989). Approximately 20 μg of RNA was separated in a denaturing 1.5% (w/v) agarose-formaldehyde gel and then transferred to GeneScreen (NEN Life Science Products, Boston) nylon membranes. Northern hybridization was performed in church buffer at 65°C with probes obtained by PCR: EF-1α (CATTTGGCACCCTTCTTCAC, CCACGAGTCTGTTCTTGAGGC), or by enzymatic digestion of plasmids: ARR5, ARR6, CYCD3, IAA1, and IAA4. Blots were washed at 55°C in 2× SSC, 0.2% (w/v) tetra-sodium diphosphate, and 0.5% (w/v) SDS, and were exposed to an Imaging Plate (FUJI, Tokyo) or to film (Eastman-Kodak, Rochester, NY). Quantification was performed with a phosphorimager (BAS 1500; FUJI), which has a higher sensitivity and a broader linear range of measurement. As the quality of images was not as good as x-ray films, each blot was also exposed to film (Eastman-Kodak) and the corresponding image was used for the illustration. Hybridization signals from different samples in a blot were normalized with EF-1 αA4 (Liboz et al., 1989) or eIF4A (Metz et al., 1992). To compare samples from different blots, a wild-type Col-0 control sample was loaded in each blot. Northern experiments were performed at least twice with independent plant samples.
RT-PCR Methods
RTs were performed from total mRNA or poly(A) mRNA after DNase treatment, using superscript II enzyme (Qiagen, Valencia, CA) according to standard protocol. Poly(A) mRNA was obtained from total mRNA using the oligotex mRNA Mini kit (Qiagen). RTs were tested and normalized with EF-1αA4 primers. Each PCR was done on the same quantity of cDNA according to EF-1 αA4 amplification product intensity. For each gene tested by RT-PCR, EF-1 αA4 amplification was done from the same mix as an internal control. PCRs were performed using STM (CTTATGTCAATTGTCAGAAGG, ATGGTGGAGGAGATGTGATCC), KNAT1 (TTCTTCTCTTCCATGTCACTTC, CTGTTGTCATGCTGGTATTCTT), KNAT2 (CTTTTGTGTTTCTTCATATTCT, CGATTTTGGATTTGATGACACT), and KNAT6 (GATAAGTCGGTTCTGATGATG, TATCTTATCTCCTTCAGTAGGGT) primers. PCR programs were chosen for each gene to recover PCR products during the exponential phase. RT-PCRs were done several times on two or three independent experiments.
Real-time PCR was done using STM (AGAGAATAGGCAGGAGCACAA, TGATGGTCCGATGTGTCCTATG), EF-1αA4 (CGAAGGGTGGTGAAAGCAAGA, CTGGAGGTTTTGAGGCTGGTAT) primers. The PCR efficiency calculated for each couple of primers was similar, allowing us to express STM mRNA quantity as a percentage of EF-1αA4 mRNA quantity.
GUS Staining
Histochemical analysis of the GUS reporter enzyme was performed as described previously (Molier et al., 1995). Sample tissues were fixed in 80% (w/v) ice-cold acetone for 10 min, washed three times with water, and placed under vacuum to increase the penetration of reaction buffer into the tissue. Samples were incubated at 37°C for 1 to 16 h in reaction buffer. Staining time was 1, 16, 16, 3, 1, and 16 h for CDKA::GUS, CYCB1::GUS, CYCB1::db::GUS KNAT2::GUS, ARR5::GUS, and CYCD3::GUS transgenic lines, respectively. Plant samples were destained in 70% (w/v) ethanol before observation. GUS fluorometric assay was performed as described by Elmayan et al. (1996) on 9 μg of total protein.
Acknowledgments
We thank Dirk Inzé (University of Gent, Gent, Belgium) for CDKA::GUS and CYCB1::GUS lines, Joe Kieber (University of North Carolina, Chapel Hill, NC) for ARR5 and ARR6 cDNAs and the ARR5::GUS transgenic line, Athanasios Theologis (Plant Gene Expression Center, Albany, NY) for IAA4 and IAA1 cDNAs, John Murray (University of Cambridge, Cambridge, UK) for the CYCD3::GUS transgenic line, and Véronique Pautot and Olivier Hamant (Institut National de la Recherche Agronomique, Versailles, France) for KNAT2::GUS transgenic line and the different KNAT oligonucleotides. We would also like to thank Céline Sorin and Hervé Vaucheret for their technical help.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.019026.
References
- Abel S, Nguyen MD, Theologis A (1995) The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251: 533-549 [DOI] [PubMed] [Google Scholar]
- Aida M, Vernoux T, Furutani M, Traas J, Tasaka M (2002). Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 129: 3965-3974 [DOI] [PubMed] [Google Scholar]
- Banno H, Ikeda Y, Niu QW, Chua NH (2001) Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 13: 2609-2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton MK, Poethig RS (1993). Formation of the shoot apical meristem in Arabidopsis thaliana: an analysis of development in the wild-type and in the shoot meristemless mutant. Development 119: 823-831 [Google Scholar]
- Bellec Y, Harrar Y, Bréard C, Darnet D, Bellini C, Faure JD (2002) PASTICCINO2 is a protein tyrosine phosphatase-like involved in cell proliferation and differentiation in Arabidopsis. Plant J 32: 713-722 [DOI] [PubMed] [Google Scholar]
- Brandstatter I, Kieber JJ (1998) Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell 10: 1009-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME, Simorowski J, Martienssen RA (2002). ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 129: 1957-1965 [DOI] [PubMed] [Google Scholar]
- Cary A, Uttamchandani SJ, Smets R, Van Onckelen HA, Howell SH (2001) Arabidopsis mutants with increased organ regeneration in tissue culture are more competent to respond to hormonal signals. Planta 213: 700-707 [DOI] [PubMed] [Google Scholar]
- Chuck G, Lincoln C, Hake S (1996) KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell 8: 1277-1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124: 1706-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockx J, Quaedvlieg N, Keultjes G, Kock P, Weisbeek P, Smeekens S (1995). The homeobox gene ATK1 of Arabidopsis thaliana is expressed in the shoot apex of the seedling and in flowers and inflorescence stems of mature plants. Plant Mol Biol 28: 723-737 [DOI] [PubMed] [Google Scholar]
- Elmayan T, Vaucheret H (1996) Expression of single copies of a strongly expressed 35S transgene can be silenced post-transcriptionally. Plant J 9: 787-797 [Google Scholar]
- Faure JD, Vittorioso P, Santoni V, Fraisier V, Prinsen E, Barlier I, Van Onckelen H, Caboche M, Bellini C (1998) The PASTICCINO genes of Arabidopsis thaliana are involved in the control of cell division and differentiation. Development 125: 909-918 [DOI] [PubMed] [Google Scholar]
- Ferreira PC, Hemerly AS, Engler JD, van Montagu M, Engler G, Inze D (1994) Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell 6: 1763-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Guivarc'h, A., Krupkova E, Lorenz-Meyer I, Chriqui, D. Schmülling T (2002) TUMOROUS SHOOT DEVELOPMENT (TSD) genes are required for coordinated plant shoot development. Plant J 29: 73-85 [DOI] [PubMed] [Google Scholar]
- Frank M, Rupp HM, Prinsen E, Motyka V, Van Onckelen H, Schmulling T (2000) Hormone autotrophic growth and differentiation identifies mutant lines of Arabidopsis with altered cytokinin and auxin content or signaling. Plant Physiol 122: 721-729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugis G, Giannino D, Mele G, Nicolodi C, Chiappetta A, Bitonti MB, Innocenti AM, Dewitte W, Van Onckelen H, Mariotti D (2001) Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot-like indeterminate growth associated with an accumulation of isopentenyl-type cytokinins. Plant Physiol 126: 1370-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst RA, Soni R, Murray JA, Lindsey K (1996) Modulation of cyclin transcript levels in cultured cells of Arabidopsis thaliana. Plant Physiol 112: 1023-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer G, Kieber JJ (2002) Cytokinins: new insights into a classic phytohormone. Plant Physiol 128: 354-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamant O, Nogué F, Belles-Boix E, Jublot D, Grandjean O, Traas J, Pautot V (2002) The KNAT2 homeodomain protein interacts with ethylene and cytokinin signalling. Plant Physiol 130: 657-665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrar Y, Bellini C, Faure JD (2001) FKBPs: at the crossroads of folding and transduction. Trends Plant Sci 6: 426-431 [DOI] [PubMed] [Google Scholar]
- Hemerly AS, Ferreira P, de Almeida Engler J, Van Montagu M, Engler G, Inze D (1993) CDKAa expression in Arabidopsis is linked with competence for cell division. Plant Cell 5: 1711-1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewelt A, Prinsen E, Thomas M, Van Onckelen H, Meins F Jr (2000) Ectopic expression of maize knotted1 results in the cytokinin-autotrophic growth of cultured tobacco tissues. Planta 210: 884-889 [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413: 383-389 [DOI] [PubMed] [Google Scholar]
- Imamura A, Hanaki N, Nakamura A, Suzuki T, Taniguchi M, Kiba T, Ueguchi C, Sugiyama T, Mizuno T (1999) Compilation and characterization of Arabidopsis thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol 40: 733-742 [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: 1060-1063 [DOI] [PubMed] [Google Scholar]
- Kakimoto T (1996) CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science 274: 982-985 [DOI] [PubMed] [Google Scholar]
- Kiba T, Taniguchi M, Imamura A, Ueguchi C, Mizuno T, Sugiyama T (1999) Differential expression of genes for response regulators in response to cytokinins and nitrate in Arabidopsis thaliana. Plant Cell Physiol 40: 767-771 [DOI] [PubMed] [Google Scholar]
- Kubo M, Kakimoto T (2000) The cytokinin-hypersensitive genes of Arabidopsis negatively regulate the cytokinin-signaling pathway for cell division and chloroplast development. Plant J 23: 385-394 [DOI] [PubMed] [Google Scholar]
- Kumaran MK, Bowman JL, Sundaresan V (2002). YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell 14: 2761-2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser HM, Pickett FB, Dharmasiri S, Estelle M (1996) Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J 10: 403-413 [DOI] [PubMed] [Google Scholar]
- Liboz T, Bardet C, Le Van Thai A, Axelos M, Lescure B (1989) The four members of the gene family encoding the Arabidopsis thaliana translation elongation factor EF-1α are actively transcribed. Plant Mol Biol 14: 107-110 [DOI] [PubMed] [Google Scholar]
- Lohrmann J, Harter K (2002) Plant two-component signaling systems and the role of response regulators. Plant Physiol 128: 363-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz AM, Timmer RT, Browning KS (1992) Sequences for two cDNAs encoding Arabidopsis thaliana eukaryotic protein synthesis initiation factor 4A. Gene 120: 313-314 [DOI] [PubMed] [Google Scholar]
- Molier P, Montoro P, Delarue M, Bechtold N, Bellini C, Pelletier G (1995) Promoterless gusA expression in a large number of Arabidopsis thaliana transformants obtained by the in planta infiltration method. C R Acad Sci Paris 318: 465-474 [Google Scholar]
- Park JY, Kim HJ, Kim J (2002). Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J 32: 669-683 [DOI] [PubMed] [Google Scholar]
- Prober DA, Edgar BA (2001) Growth regulation by oncogenes: new insights from model organisms. Curr Opin Genet Dev 11: 19-26 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541-1544 [DOI] [PubMed] [Google Scholar]
- Rupp HM, Frank M, Werner T, Strnad M, Schmulling T (1999) Increased steady-state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J 18: 557-563 [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Zuker CS (1994) Protein folding and the regulation of signaling pathways. Cell 79: 1129-1132 [DOI] [PubMed] [Google Scholar]
- Sakai H, Honma T, Aoyama T, Sato S, Kato T, Tabata S, Oka A (2001) ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 294: 1519-1521 [DOI] [PubMed] [Google Scholar]
- Santoni V, Bellini C, Caboche M (1994) Use of two-dimensional electrophoresis protein-pattern analysis for the characterization of Arabidopsis thaliana mutants. Planta 192: 557-566 [Google Scholar]
- Schmulling T (2002) New insights into the functions of cytokinins in plant development. J Plant Growth Regul 21: 40-49 [DOI] [PubMed] [Google Scholar]
- Soni R, Carmichael JP, Shah ZH, Murray JA (1995) A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 7: 85-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SB, Wang XJ, Hagen G, Guilfoyle TJ (2001) AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 13: 2809-2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17: 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittorioso P, Cowling R, Faure JD, Caboche M, Bellini C (1998) Mutation in the Arabidopsis PASTICCINO1 gene, which encodes a new FK506-binding protein-like protein, has a dramatic effect on plant development. Mol Cell Biol 18: 3034-3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Jurgens G (2002) Stem cells that make stems. Nature 415: 751-754. [DOI] [PubMed] [Google Scholar]