Abstract
Cytokinins are hormones that play an essential role in plant growth and development. The irreversible degradation of cytokinins, catalyzed by cytokinin oxidase, is an important mechanism by which plants modulate their cytokinin levels. Cytokinin oxidase has been well characterized biochemically, but its regulation at the molecular level is not well understood. We isolated a cytokinin oxidase open reading frame from maize (Zea mays), called Ckx1, and we used it as a probe in northern and in situ hybridization experiments. We found that the gene is expressed in a developmental manner in the kernel, which correlates with cytokinin levels and cytokinin oxidase activity. In situ hybridization with Ckx1 and transgenic expression of a transcriptional fusion of the Ckx1 promoter to the Escherichia coli β-glucuronidase reporter gene revealed that the gene is expressed in the vascular bundles of kernels, seedling roots, and coleoptiles. We show that Ckx1 gene expression is inducible in various organs by synthetic and natural cytokinins. Ckx1 is also induced by abscisic acid, which may control cytokinin oxidase expression in the kernel under abiotic stress. We hypothesize that under non-stress conditions, cytokinin oxidase in maize plays a role in controlling growth and development via regulation of cytokinin levels transiting in the xylem. In addition, we suggest that under environmental stress conditions, cytokinin oxidase gene induction by abscisic acid results in aberrant degradation of cytokinins therefore impairing normal development.
Active cytokinins consist of an adenine (Ade) moiety with an N6-substituted isoprene chain, isoprene derivative, or an aromatic ring (Zažímalová et al., 1999). The size of the active cytokinin pool in plants is controlled by the rate of import, biosynthesis, inactivation, and degradation. Irreversible or transient inactivation can occur through glucosyl, xylosyl, or amino acid conjugations (Mok and Martin, 1994). Irreversible degradation is catalyzed by the enzyme cytokinin oxidase whose existence was first postulated by McCalla et al. (1962). Subsequently, an enzyme activity that catalyzes the conversion of N6-(Δ2-isopentenyladenosine) (iPAR) to adenosine was detected in cultured tobacco (Nicotiana tabacum) pith tissue (Pac̀es et al., 1971) and in maize (Zea mays) kernels (Whitty and Hall, 1974). Cytokinin oxidase acts by removing the N6-substituted isoprene chain of cytokinins or their ribonucleosides to produce Ade and the corresponding aldehyde (Armstrong, 1994; Hare and van Staden, 1994). Cytokinin degradation activities have been detected in a number of different plant species including wheat (Triticum aestivum; Laloue and Fox, 1989), maize, poplar (Populus spp.), soybean (Glycine max), Alnus glutinosa, bean (Phaseolus vulgaris), and callus tissue from several plants (Armstrong, 1994). It is thought that cytokinin oxidase is the primary means of cytokinin degradation in plants. A cytokinin oxidase gene (called Ckx or CKO) was recently isolated from maize (Houba-Hérin et al., 1999; Morris et al., 1999). The open reading frame encodes a 57.4-kD protein containing a signal peptide, eight putative glycosylation sites, and a possible FAD-binding site. Expression of the cytokinin oxidase gene in either moss protoplasts or recombinant yeast led to the secretion of an active cytokinin oxidase into the medium, suggesting that in plants, the putative signal peptide targets the protein outside of the plasma membrane to the apoplast (Houba-Hérin et al., 1999; Morris et al., 1999). Seven putative Arabidopsis cytokinin oxidase proteins were identified in silico based on their similarity to the maize enzyme. Among these proteins showing between 40% to 47% identity to the maize cytokinin oxidase, three of them had cytokinin oxidase activity in vitro (Bilyeu et al., 2001). Recently, four Arabidopsis cytokinin oxidase genes were independently over-expressed in tobacco (Werner et al., 2001). The transgenic plants exhibited an increase in cytokinin oxidase activity, which resulted in a significant decrease in cytokinin levels. Interestingly, the plants also exhibited an increase in root branching as well as retardation in shoot development.
Despite the wealth of information collected on the biochemical properties of the enzyme, much less is known about cytokinin oxidase gene expression and its regulation. To address these issues, we isolated an allele of the cytokinin oxidase gene from maize called Ckx1 and used it to monitor cytokinin oxidase transcript levels in different maize organs via northern blots and in situ hybridization. In this report, we show that Ckx1 mRNA is most abundant in roots and kernels. In developing kernels, gene expression parallels enzyme activity and correlates with cytokinin levels. In situ hybridization as well as analysis of transgenic plants expressing the β-glucuronidase gene (GUS) under the control of the Ckx1 promoter shows expression of the gene in the vascular tissue of developing kernels and roots. We show that exogenously applied cytokinins induce a positive regulation on Ckx1 transcriptional activity in several organs. Our results suggest that cytokinin oxidase regulates cytokinin levels entering an organ by controlling cytokinin flux transiting the xylem via a feedback control mechanism. We also demonstrate that abscisic acid (ABA), drought, and heat stresses induce Ckx1 expression, leading us to hypothesize that maize employs cytokinin degradation as a means to control growth and development under abiotic stress conditions.
RESULTS
Isolation of a Cytokinin Oxidase Allele
The Ckx1 coding region (1,625 bp) was amplified from an 18-d after pollination (DAP) embryo cDNA library using reverse transcriptase (RT)-PCR and primers based on the published sequence. The coding region of Ckx1 shares 98.4% homology with both CKX1 (AF044603; Morris et al., 1999) and CKO (Y18377; Houba-Hérin et al., 1999). The 27 differences at the nucleotide level lead to nine changes, two insertions, and a deletion at the amino acid level. The predicted protein has an N-terminal putative signal peptide of 18 amino acids and eight putative glycosylation sites as shown for other cytokinin oxidase proteins (Houba-Hérin et al., 1999; Morris et al., 1999). However, the likelihood of N-linked glycosylation at one of these sites, AA-NST-P, might be strongly reduced by the presence of a Pro residue after the Thr amino acid (Gavel and von Heijne, 1990).
Expression Pattern of Ckx1 in Different Maize Organs
The Ckx1 full-length coding region was used as a probe in both Southern- and northern-blot experiments. Southern-blot analysis indicated that hybridization was specific for Ckx1, which we mapped, using RFLP technology, to the short arm of chromosome 3 (bin 3.02; data not shown), consistent with data found in the Maize Genomic Database (http://www.agron.missouri.edu/).
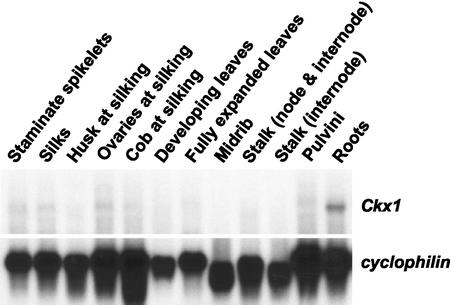
Ckx1 transcript levels were first assessed in different organs. Figure 1 shows that transcripts were detected at higher levels in roots, with very low levels being found in ovaries at silking. The blot was probed with cyclophilin (Marivet et al., 1995), which has been found to be an appropriate control when comparing expression levels across organs. Although little or no signal could be detected in other organs by northern analysis, RT-PCR analysis of the same samples with Ckx1-specific primers showed the presence of transcripts in all tissues examined (data not shown), indicating very low steady-state levels of Ckx1 transcripts in organs tested (except roots).
Figure 1.
Expression of Ckx1 in different maize organs. Northern-blot analysis of Ckx1 transcript levels in different organs of maize (B73). Poly(A) RNA (3 μg) was extracted from the organs indicated. The same blot was hybridized with cyclophilin, which served as a loading control.
Ckx1 Expression and Cytokinin Oxidase Activity during Kernel Development
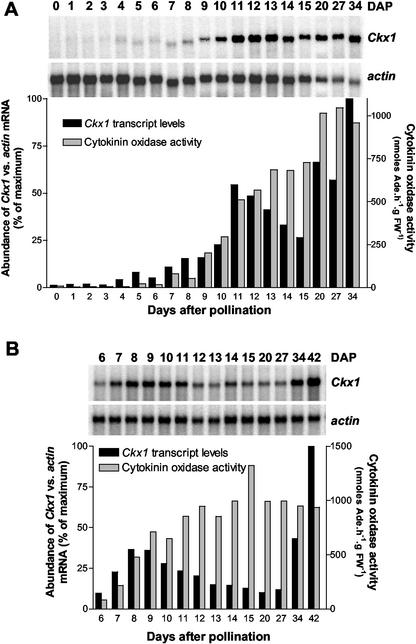
Although low levels of Ckx1 transcripts were found in young ovaries, developing kernels have been shown to have high levels of cytokinin oxidase activity. For example, cytokinin oxidase activity in the maize inbred line B73 has been shown to increase during the lag phase of kernel development (Jones et al., 1992). To determine whether this increase was associated with transcriptional control of gene expression, we measured Ckx1 steady-state levels, cytokinin oxidase activity, and cytokinin levels in developing kernels of the inbred B73. On the basis of previous data showing a higher cytokinin oxidase activity in the pedicel/placental-chalazal (Jones et al., 1992; Jones and Setter, 2000), we decided to analyze this region and the rest of the kernel separately. From 0 to 5 DAP, transcript levels of Ckx1 were measured in whole kernels. Starting at 6 DAP the pedicel (pedicel/placental-chalazal region) was separated from the rest of the kernel and the two were analyzed independently (Fig. 2). Transcript levels of Ckx1 were quantified relative to abundance of actin transcripts. Ckx1 transcripts are detected at low levels in kernels from 0 to 4 DAP, and then from 5 to 11 DAP, expression gradually increases (Fig. 2A). The steady-state level of Ckx1 transcripts then decreases slightly until 15 DAP before increasing again in the later stages of kernel development (20–34 DAP). This same pattern of expression was measured in pedicels (Fig. 2B); however, the first peak was forward phase-shifted from that of the kernel, whereas the second peak in the pedicel exhibited a backward phase-shift relative to that of the kernel.
Figure 2.
Relative abundance of Ckx1 transcripts and cytokinin oxidase activity during B73 kernel development. A, Ckx1 transcript levels were measured in 0- to 5-DAP kernels with pedicels and 6- to 34-DAP kernels without pedicels using [α-32P]dCTP labeled Ckx1 DNA probe. The same nylon membrane was stripped and probed with actin1-9. Black bars indicate the relative abundance of Ckx1 versus actin transcripts. Gray bars indicate cytokinin oxidase activity measured in the same samples. B, Ckx1 transcript levels were measured in pedicel samples of 6- to 42-DAP kernels. Transcripts (black bars) were detected, quantified, and normalized to actin transcript levels using the same procedure as in A. Cytokinin oxidase activity (gray bars) was measured as indicated in A. Poly(A) RNA (3 μg) were used for each northern-blot experiment.
Cytokinin oxidase activity in both pedicel (Fig. 2B) and the rest of the kernel (Fig. 2A) parallels the increase in Ckx1 transcript levels. Enzyme activity was more prominent in the pedicel, where it reached a plateau around 14 DAP, than the rest of the seed, where a plateau was observed at 20 DAP. The activity values for the plateau in each tissue were very similar. The decrease in Ckx1 transcript levels in both tissues during kernel development was not associated with a diminution in activity, suggesting a slow turnover of the protein.
Changes in Cytokinin Levels during Kernel Development
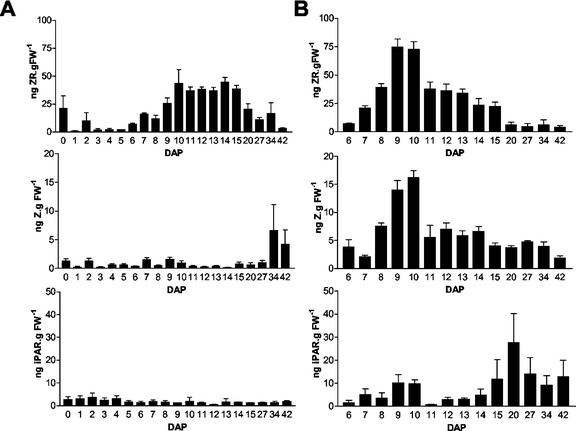
To study possible correlations between levels of different cytokinins and Ckx1 transcript levels, we determined the amount of three different cytokinins in the same B73 samples used for northern and enzyme activity measurements. Figure 3 shows zeatin riboside (ZR), zeatin (Z), and iPAR levels in kernels (Fig. 3A) and pedicels (Fig. 3B). In general, cytokinin levels measured in the developing kernel were in the range of concentration detected in other studies (Cheikh and Jones, 1994; Dietrich et al., 1995, and refs. therein). Overall, cytokinin levels were higher in the pedicel region than the rest of the seed. As expected, ZR was the most abundant cytokinin present in both the pedicel and the rest of the kernel. In the rest of the kernel, the amount of ZR increases from 6 to 10 DAP, reaches a plateau that is maintained until 15 DAP, and then becomes lower at later developmental stages (Fig. 3A). The initial increase in ZR levels (from 6 to 10 DAP) parallels the elevation in Ckx1 transcript levels (compare Fig. 2A with Fig. 3A). Interestingly, levels of Z were relatively constant throughout kernel development but were sharply increased at later stages of development. This increase could be responsible for the rise in Ckx1 transcripts observed at 34 DAP. Relative to the rest of the kernel, a sharper peak of ZR was observed in the pedicel (Fig. 3B) with levels reaching a maximum at 9 to 10 DAP. Unlike what was observed for the rest of the seed, Z levels in the pedicels showed an increased accumulation early in development that then leveled off after 10 DAP (Fig. 3B). iPAR levels in the pedicels showed two peaks: one at 9 DAP and the second one a 20 DAP. Compared with northern-blot results, these data suggest that cytokinin levels strongly correlate with Ckx1 transcript levels during kernel development. Moreover, transcript accumulation might be induced by different cytokinins depending on the part of the kernel considered and its developmental stage.
Figure 3.
Cytokinin levels measured in developing B73 kernels. Levels of ZR, Z, and iPAR were measured in the same samples used for analysis in Figure 2. A, Cytokinin levels in developing kernels from 0 to 5 DAP and in kernels of which the pedicel (pedicel/placental-chalazal region) was separated from the rest of the kernel starting at 6 DAP until 42 DAP. B, Cytokinin levels in pedicels samples collected from 6- to 42-DAP kernels.
In Situ Localization of Cytokinin Oxidase Transcripts
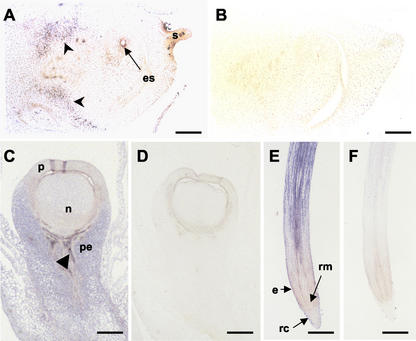
To gain a better understanding of how cytokinin oxidase temporal expression relates to its spatial distribution, we performed a series of in situ experiments on various maize organs, with an emphasis on the female flower. A subclone of Ckx1, corresponding to the last 1 kb of the amplified coding region, was used to produce labeled antisense RNA probes that were used for in situ hybridizations. Two weeks before silk emergence, a low level of label was detected throughout the ear tissue and developing female flower (Fig. 4, A and C). As shown in Figure 4A, the signal was more abundant in the tissue subtending the flower (arrow-heads) compared with the flower itself. Some label was also detected in the megaspore mother cell (embryo sac). Figure 4C shows signal detected in the female flower at silking. Transcripts were still low in the developing ovule (nucellus and upper carpel walls), but labeling was much stronger in the pedicel and parts of the vascular bundles in the pedicel (arrowhead). In roots (Fig. 4E), strong Ckx1 transcript levels were seen in all tissues of the cell elongation zone. Signal was strongest in vascular bundles, even in those that extend into the region toward the root tip where a lower signal was detected. Signal is low or absent in the root meristem and root cap, except for the outermost layer (epidermis). None of the controls showed detectable signal (Fig. 4, B, D, and F).
Figure 4.
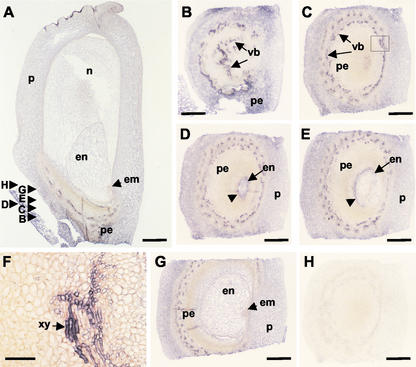
In situ localization of Ckx1 transcripts in developing ovules and roots. A, An antisense RNA probe was used to detect Ckx1 sense transcripts on tissue sections of developing ovules 7 weeks after planting in genotype An1Bz2. B, Negative control probed with a sense Ckx1 probe. Bars = 350 μm. C, Detection of Ckx1 transcripts in developing ovules at silking (9 weeks after planting) in genotype An1Bz2. D, Negative controls probed with Ckx1 sense probe. Bars = 500 μm. E, Localization of Ckx1 transcripts in roots of plants at the V6 stage of inbred N46. F, Negative control probed with Ckx1 sense probe. Bars = 750 μm. Arrowheads (A and C) indicate signal detected in the vasculature. es, Embryo sac; n, nucellus; P, pericarp; pe, pedicel; e, epidermis; rc, root cap; rm, root meristem; and s, silk.
Detailed in situ results from 8-DAP kernels are shown in Figure 5. Label is present in the pericarp, nucellus, pedicel, and endosperm (Fig. 5A). Signal is weak or absent in the embryo and placental-chalazal region. Vascular strands of the pedicel show a particularly strong signal. Analysis of transverse sections probed with Ckx1 antisense transcripts confirmed the strong labeling in the vascular strands of the pedicel (Fig. 5, B–E). Higher magnification of the vascular strand region (Fig. 5F) revealed that the strongest signal occurs in vascular elements, especially in the xylem (arrow). A strong signal was also observed in the basal region of the endosperm (Fig. 5D). Figure 5E shows that this signal was limited to the posterior side of the endosperm in sections below the embryo, but the signal was much less in sections including or above the embryo (Fig. 5G). The Ckx1 sense probe did not produce any recognizable signal (Fig. 5H).
Figure 5.
In situ localization of Ckx1 transcripts in 8-DAP kernels. A, A longitudinal section of an 8-DAP kernel (inbred N46) probed with an antisense Ckx1 probe. B through G, Transverse sections as indicated in A probed with an antisense Ckx1 probe. F, Close-up of C identified by the square; scale bar = 50 μm. H, Transverse section using sense probe as a control. en, Endosperm; em, embryo; n, nucellus; p, pericarp; pe, pedicel; vb, vascular bundles; and xy, xylem. Scale bar = 500 μm, except for F.
Expression of a Ckx1::GUS Fusion Construct in Transgenic Maize
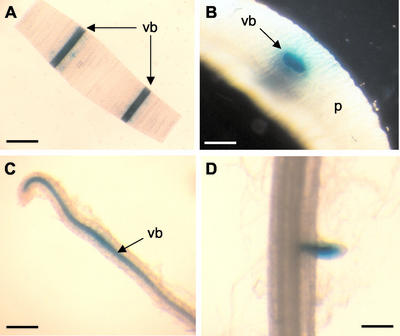
To further characterize the cytokinin oxidase gene, we isolated the Ckx1 promoter from the inbred B73 and fused it to the GUS gene of Escherichia coli. Maize was stably transformed with the Ckx1::GUS construct via Agrobacterium sp. as previously described (Zhao et al., 1998). After regeneration, T0 plants were out-crossed, and kernels were assayed for GUS activity. GUS staining was detected in the pedicel of 8-DAP kernels of three independent transgenic events (data not shown), and the event showing the strongest signal was used for further analysis. As shown in Figure 6, A and B, vascular staining in T1 seedlings was observed in coleoptile sections. In secondary roots of the same seedlings, staining was primarily found in the vasculature (Fig. 6C). Staining was stronger in the root elongation zone and decreased in the upper region of the root. Weak or no staining was detected in the vasculature of primary roots, but occasional signal was detected in developing secondary root primordia (Fig. 6D). The Ckx1 promoter was also found to be active in the base of tassel spikelets, in pedicels of 8-DAP kernels, and in the vasculature of tassel spikelet glumes (data not shown).
Figure 6.
Characterization of transgenic maize plants expressing a fusion of the Ckx1 promoter and the GUS gene. A, GUS staining of a coleoptile cross-section showing strong labeling in the vascular bundles (vb). Bar = 3 mm. B, Close-up of a transverse coleoptile section showing intense labeling in the vascular bundles. Bar = 500 μm. C, Staining of lateral root showing GUS staining in the vascular bundle. Bar = 250 μm. D, Staining of a primary root showing activity in emerging lateral root. Bar = 250 μm. vb, Vascular bundle; and P, parenchyma.
Ckx1 Expression Is Induced by Cytokinins
Cytokinin oxidase transcript levels and activity patterns (Fig. 2) suggest transcriptional control of Ckx1 gene expression by cytokinins. To test this hypothesis, we applied cytokinins as well as other hormones to developing kernels, seedling roots, and leaf discs to determine whether they could induce Ckx1 expression.
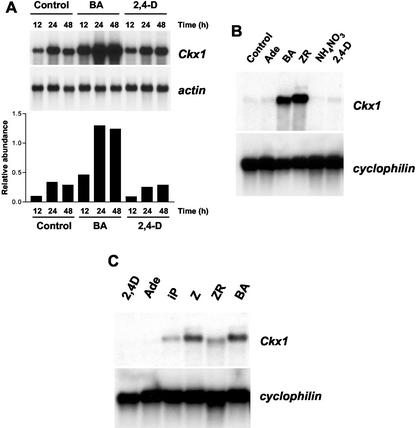
We incubated 4-DAP kernels (which have inherently low Ckx1 transcript levels) in a solution containing Suc and Gln supplemented with water (control), benzyladenine (BA), or 2,4-dichlorphenoxy acidic acid (2,4-D). Ckx1 expression was increased 5-fold after 12 h of incubation in BA compared with controls, whereas no increase was detected with 2,4-D-treated samples (Fig. 7A). After 24 h, Ckx1 transcript levels were increased 4-fold in the BA-treated samples but were still unchanged in 2,4-D-treated kernels compared with controls. The same pattern was observed after 48 h of treatment.
Figure 7.
Effect of exogenous application of cytokinins on Ckx1 transcript levels in different organs. A, Expression of Ckx1 in B73 4-DAP kernels after incubation for 12, 24, or 48 h in a solution containing water (control) or in water containing 10 μm BA or 2,4-D and quantification of transcript abundance compared with actin transcript levels (arbitrary units). Five micrograms of poly(A) was used for this experiment. B, Effect of exogenous application of cytokinins on Ckx1 transcript levels in roots. The root systems of 2-week-old seedlings were wrapped in germ paper soaked with deionized water (Control), 10 μm Ade, BA, ZR, 10 mm ammonium nitrate (NH4NO3), or 10 μm 2,4-D. Root systems were collected after 48 h of incubation, and 3 μg of poly(A) RNA was used for northern-blot quantification of Ckx1 transcripts. C, Ckx1 transcript levels in leaf discs incubated in the presence of 2,4-D, Ade, isopentenyladenine (iP), Z, ZR, or BA at 10 μm. Three micrograms of poly(A) RNA was used. Cyclophilin was used as a loading control.
To determine whether a similar response could be observed in roots, different hormones and ammonium nitrate were applied to the root system of 2-week-old seedlings, by incubating them for 48 h with 10 μm Ade, BA, ZR, or 2,4-D or 10 mm ammonium nitrate. As shown in Figure 7B, strong induction was observed when either BA or ZR was used, but no detectable induction was observed with 2,4-D, Ade, or ammonium nitrate.
In a separate experiment, leaf discs punched from fully expanded leaves were floated on either deionized water or deionized water with different compounds for 16 h. As shown in Figure 7C, induction of the gene was observed with all cytokinins. The strongest induction was detected with discs treated with Z or BA compared with samples treated with ZR or N6-[2-isopentenyl]adenine (iP). No induction was found with either the synthetic auxin hormone 2,4-D or Ade. From these results, we conclude that Ckx1 gene expression is inducible by cytokinins in different plant organs.
Time Course and Dose-Response Induction of Ckx1 by BA
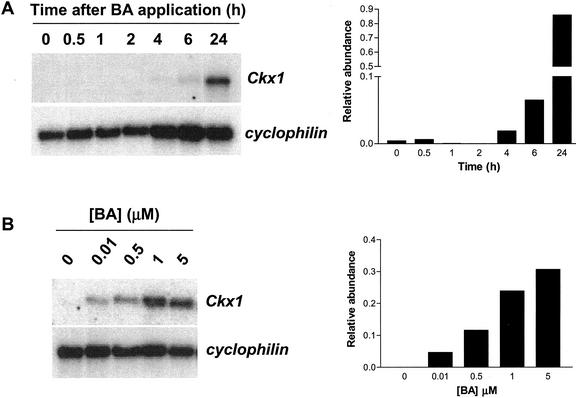
We studied the time course induction of Ckx1 expression by incubating leaf discs with 10 μm BA and following Ckx1 expression over 24 h. Results are presented in Figure 8A and show that an increase in Ckx1 transcripts relative to cyclophilin transcript levels can be observed after 4 to 6 h. In a separate experiment, we monitored induction after 24 h in response to different concentrations of BA (Fig. 8B). Induction was observed for the lowest concentration used, 0.01 μm, and the amount of transcripts detected was dependent on the concentration of BA in the solution. All together, these data show that maize leaves can sense cytokinin concentrations and adjust transcript levels of cytokinin oxidase accordingly over a relatively short period of time. This suggests that a cytokinin sensing and signal transduction system may be involved in the control of Ckx1 gene expression.
Figure 8.
Time course and dose responsiveness of Ckx1 transcript accumulation to BA application. A, Ckx1 transcript levels in leaf discs (B73) incubated in the presence of 10 μm BA for the indicated time period. B, Ckx1 transcript levels in leaf discs (B73) incubated with different concentrations of BA for 24 h. Three micrograms of poly(A) RNA was used. Cyclophilin was used as a loading control. The relative abundance of transcripts (arbitrary units) is indicated in the graph.
Induction of Ckx1 Expression by Abiotic Stress and ABA
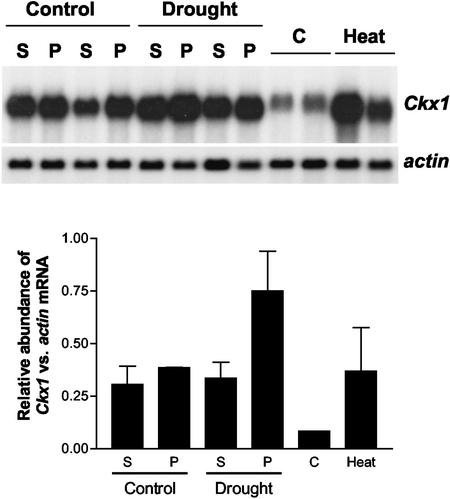
Evidence in the literature (Cheikh and Jones, 1994) demonstrates that cytokinins can buffer the negative effects of abiotic stress on kernel growth. To determine what role, if any, cytokinin oxidase could play in this response, we investigated the effect of drought and heat stress on the expression of cytokinin oxidase during kernel development. Growth chamber-grown plants were subjected to a controlled water stress starting at 4 DAP, and kernels from stressed and well-watered plants were harvested at 8 DAP. The drought stress treatment was sufficient to reduce leaf photosynthesis of 8-DAP plants to near zero (data not shown). Kernels were dissected into pedicel/placento-chalazal region and the rest of the kernel. Pedicel samples showed a significant increase of approximately 2-fold in Ckx1 mRNA relative to actin transcripts, whereas no difference could be detected in the rest of the kernel (Fig. 9). When Ckx1 transcripts were measured in field-grown plants whose ears had been heat stressed at 35°C from 4 to 8 DAP, a strong 4-fold increase in cytokinin oxidase transcripts was observed. These results suggest that the expression of Ckx1 in the developing kernel is responsive to abiotic stresses.
Figure 9.
Effect of abiotic stress on Ckx1 expression in developing kernels. A drought stress was applied to growth chamber-grown plants (hybrid 3732) at 4 DAP by withholding water until 8 DAP. At that point, glumes were removed from developing seeds, and the pedicel region (P) was separated from the rest of the kernel (S). For the heat stress experiment, field-grown Mo17 plants ears were heat stressed, and transcript levels were measured in kernels of which the pedicel had been removed. Transcripts levels were measured using Ckx1 or actin as a probe and the relative abundance of transcripts is indicated in the graph (arbitrary units). Error bars represent the se. Four micrograms of poly(A) RNA was used.
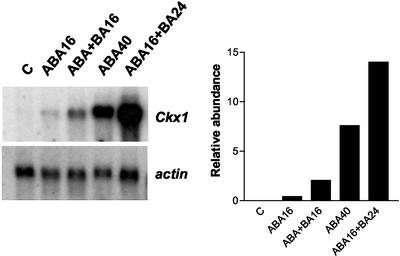
An increase in ABA concentration is a common feature of abiotically stressed organs; therefore, we also measured the effect of ABA application on Ckx1 expression. When leaf discs were incubated for 16 h on a solution containing 10 μm of ABA, a weak induction of Ckx1 expression was observed; however, this response was enhanced by a combined treatment with BA (Fig. 10). After treatment with ABA for 40 h, accumulation of Ckx1 transcripts was more evident, and like that of the 16-h treatment, this effect was enhanced by the addition of BA. Therefore, we hypothesize that the induction of cytokinin oxidase transcripts during abiotic stress might be in part mediated by an increase in ABA concentration and that cytokinins and ABA treatments are additive.
Figure 10.
Effect of ABA on accumulation of Ckx1 in leaf discs. Leaf discs were floated either for 40 h on distilled water (control) or for 16 h (ABA16) or 40 h (ABA40) on 10 μm ABA. Effect of ABA and BA cotreatment was studied by incubating leaf discs with ABA + BA (10 μm each) for 16 h (ABA+BA16) or with 10 μm BA for 24 h after pre-incubation with ABA for 16 h (ABA16+BA24). The graph to the right shows the relative abundance of Ckx1 and actin transcripts (arbitrary units). Three micrograms of poly(A) RNA was used.
DISCUSSION
Ckx1 Gene Expression in Vegetative Organs
We measured relatively high levels of Ckx1 mRNA in roots and developing kernels of maize. These two organs were previously shown to have high cytokinin oxidase activity (Jones and Schreiber, 1997; Bilyeu et al., 2001). In maize seedlings, northern analysis indicated that transcript levels where detectable at high levels in roots and to a much lesser extent in the mesocotyl, whereas no signal was detected in leaves (N. Brugière, unpublished data). Expression levels in developing kernels were approximately three times greater than those in seedling roots (data not shown). These data correlate with the fact that in vitro cytokinin oxidase activity is several fold greater in roots relative to shoots (Jones and Schreiber, 1997; Jones and Setter, 2000).
Ckx1 Gene Expression Is Regulated by Cytokinins
Exogenous application of cytokinins has previously been shown to stimulate cytokinin oxidase activity in cultured tobacco cells (Terrine and Laloue, 1980) and in callus culture of several plants (Chatfield and Armstrong, 1986; Palmer and Palni, 1987; Motyka and Kamínek, 1992). Transcript accumulation during kernel development correlates with cytokinin oxidase activity, which suggests a transcriptional control of cytokinin oxidase activity. Induction of Ckx1 expression in the kernel is initiated at the same time that cytokinin levels begin to increase i.e. around 6 DAP (Jones et al., 1992; Dietrich at al., 1995). We detected two peaks of Ckx1 transcript accumulation in developing kernels that were associated with two peaks in concentration of different cytokinins: ZR and iPAR in the pedicel and ZR and Z in the rest of the seed (compare Figs. 2 and 3). On the basis of these elements, our results showing accumulation of Ckx1 transcripts in 4-DAP kernels after exogenous application of BA (Fig. 7A), and our demonstration that cytokinins but not Ade or 2,4-D induce cytokinin oxidase gene expression in leaf discs, we hypothesize that endogenous cytokinin levels dictate Ckx1 expression. Our results also suggest that cytokinin oxidase activity is controlled at the transcriptional level. It is interesting to note that in wheat grains, a peak in concentration of an aromatic cytokinin occurs during the late phase of seed maturation that subsequently declines after seed germination (Kamínek et al., 2000). Very little data are available regarding cytokinin levels (especially aromatic forms) and cytokinin oxidase activity at later stages of seed development. Such analysis may help decipher the interplay between enzyme activity and cytokinin levels at this particular stage in kernel development.
A Role for Cytokinin Oxidase in Controlling Xylem Differentiation and Cytokinin Flux
Both in situ data and Ckx1::GUS transgenic plant analysis show strong expression of the gene in the vasculature of different organs and more specifically in differentiating xylem tissues (Figs. 4, 5, 6). On the basis of their abundance in xylem sap of several species, iPAR, Z, and ZR are generally thought to be the translocated forms of cytokinin (Wagner and Beck, 1993; Beveridge et al., 1997; Takei et al., 2001b). The importance of cytokinin sensing in the differentiation of xylem cells has been the subject of a recent report (Mähönen et al., 2000). The WOL gene encodes a two-component signal transducer that is expressed specifically in the root vasculature and is needed for vascular asymmetric divisions in the cambium and vascular morphogenesis (Mähönen et al., 2000). WOL was shown to be identical to CRE1 whose function in cytokinin sensing was recently demonstrated using heterologous complementation (Inoue et al., 2001). The data also suggest that cytokinin oxidase could have a role in vascular morphogenesis. Our hypothesis is that cytokinin levels in the xylem, or other tissues, could be sensed by the CRE1 protein, which in turn would trigger a signal transduction cascade involving the cytokinin response regulator pathway (Sakakiban et al., 1999; Deji et al., 2000; Hwang and Sheen, 2001), resulting in the regulation of genes involved in cytokinin biosynthesis, conjugation, or degradation. The recent report that the cytokinin oxidase promoter of orchid (Dendrobium sp.) directs GUS expression in leaf veins of Arabidopsis transgenics supports the idea of an important role of cytokinin oxidase in regulating cytokinin levels transiting the plant vasculature (Yang et al., 2002).
Evidence suggests that endogenous cytokinins may be protected from the action of cytokinin oxidase through compartmentation, and that the target of the enzyme is apoplastic cytokinins. It is possible that cytokinin oxidase may be involved in differentiation of tissues or organs. In Ckx1::GUS plants, Ckx1 expression was detected at the base of spikelets and in the vasculature of the glumes (X. Niu and N. Brugière, unpublished data). In Dendrobium sp., cytokinin oxidase is also expressed at the base of the flower of mature plants (Yang et al., 2002). In Arabidopsis, one of the newly identified isopentenyladenine transferase genes (AtIPT5; Kakimoto, 2001; Takei et al., 2001a) is also expressed at the base of siliques (T. Kakimoto, personal communication). Collectively, these results suggest that the base of reproductive organs is a site of cytokinin synthesis. Changes in cytokinin oxidase activity may result in changes in cytokinin levels possibly determining organ development, i.e. secondary root initiation or formation of glumes. Inhibition of the formation and growth of lateral roots by enhanced concentrations of cytokinins has been previously reported (Stenlid, 1982; Bertell and Eliasson, 1992; McKenzie et al., 1998). Constitutive expression of Arabidopsis cytokinin oxidase genes in tobacco also results in a dramatic phenotype, i.e. enhanced root biomass, reduced leaf size, and delayed flowering (Werner et al., 2001). One means to test the role of the base of maize reproductive structures in controlling cytokinin metabolism would be to create transgenic plants that have targeted tissue expression of various cytokinin metabolic genes.
A Role for Cytokinin Oxidase during Abiotic Stresses
The positive effect of ABA on Ckx1 transcript levels (Fig. 10) suggests a role for this hormone in modulating cytokinin concentrations under different abiotic stresses. Results were recently reported showing an increase of cytokinin oxidase activity in root tips and shoot tips of maize when seedlings were cold stressed at 4°C for 3 d (Li et al., 2000). In addition, the influence of a drought-stress at flowering time upon kernel development has been well documented. For example, Setter et al. (2001) showed that ABA levels increase dramatically in kernels subjected to a water stress during early development. Interestingly, they observed a concomitant decrease in Z-like cytokinins in pedicels of water-stressed kernels compared with unstressed controls. Furthermore, in another study, it was shown that exogenous application of ABA inhibits maize endosperm cell division and endoreduplication (Mambelli and Setter, 1998). Seemingly, levels of Z and ZR are greatly reduced under short- or long-term heat stress (Cheikh and Jones, 1994). Although the exact mechanism of this reduction is not clear, preliminary data indicated that this was the result of an increase in cytokinin oxidase activity (Cheikh and Jones, 1994). The present study supports this idea because a strong increase in Ckx1 transcripts can be detected in heat-stressed kernels. We also provide evidence that an increase in ABA concentration, which typically occurs with environmental stresses, could trigger a premature increase of cytokinin oxidase transcripts (Fig. 10) and activity (Cheikh and Jones, 1994). In stressed kernels, precocious expression of Ckx1 before the natural cytokinin peak could prevent the accumulation of cytokinin, causing a reduction in peak intensity and inhibition of kernel development. Such low levels of cytokinin were previously described in heat-stressed kernels (Cheikh and Jones, 1994) and in drought-stressed pedicels (Setter et al., 2001); and consequently, we believe that an increase in cytokinin oxidase activity could play a role in the reduction of endosperm cell division, endoreduplication, and/or starch granule numbers observed under stress (Commuri and Jones, 2001; Engelen-Eigles et al., 2001; Setter and Flannigan, 2001). In general, it appears that cytokinin degradation is a means by which maize controls growth and development of organs under abiotic stress conditions.
Under cold, drought, or heat stress conditions, the action of ABA on cytokinin oxidase expression in organs such as leaves would result in a reduction of cytokinin levels, causing less cell division and therefore limiting growth under unfavorable conditions. During kernel maturation, as ABA accumulates, it might also be an important factor regulating cytokinin oxidase transcript levels and activity, in conjunction with cytokinins. The second peak in Ckx1 transcript levels seen during the maturation phase of kernel development (Fig. 2, 34 and 42 DAP) could also be linked to the ABA accumulation occurring at this developmental stage. The high cytokinin oxidase activity found in mature kernels could provide the plant with a means to prevent precocious germination. One possible way to test this hypothesis would be to measure levels of cytokinin oxidase activity in viviparous maize mutants.
In an associated experiment, Hoth et al. (2002) used massively parallel signature sequencing gene expression technology to identify genes up- or down-regulated by ABA in Arabidopsis seedlings. Analysis of their results (data not shown) showed that of the seven different Arabidopsis cytokinin oxidase genes, only one, AtCkx7, was significantly induced (17-fold) by ABA application. This indicates that there is a differential response of cytokinin oxidase genes to ABA, which might correspond to unique roles for individual genes. Whether ABA differentially controls the different cytokinin oxidase genes in maize (see below), especially under abiotic stress conditions, remains to be determined.
Different Cytokinin Oxidases for Different Roles?
Cytokinin oxidase might play a role as a “detoxifier” of cytokinins in plants. In roots, expression of Ckx1 in the epidermal cell layer might prevent cytokinins in the rhizosphere from perturbing root growth or cell division. This role of “gate-keeper” could also apply to the kernel, because it has been shown that the kernel is able to synthesize cytokinin de novo (Schreiber, 1990; Cheikh and Jones, 1994). By extension, cytokinin oxidase could act as a “gate keeper” responsible for cytokinin degradation around the sites of cytokinin biosynthesis, therefore modulating the amount of hormone reaching a developing organ.
Cytokinin oxidase may have different regulating roles depending on the organ considered, one for cytokinins transiting in the xylem stream and another one for regulating cytokinin levels in primordia (root) or meristems during organ differentiation and environmental stresses. Consequently, two different sets of enzymes may be involved in each role. Several related cytokinin oxidase sequences from maize can be found in the public database. Moreover, a search of the Pioneer/DuPont database revealed the existence of several additional genes whose products have homology with CKX1. The partial amino acid sequence of these gene products show approximately 40% identity with the same region of CKX1 and a very low homology at the nucleotide level (N. Brugière, unpublished data). Together with two-dimensional gel analysis of kernel protein extracts (data not shown), these results suggest that several genes might encode different cytokinin oxidases in maize. Recently, Bilyeu et al. (2001) reported the existence of seven genes in the Arabidopsis genome whose products have only 40% to 50% identity with the maize CKX1. The biochemical characterization of the different Arabidopsis and maize isoforms will help us to understand whether they have different physiological functions.
Future work in our lab will aim at studying the expression of the putative cytokinin oxidase genes during maize development, as well as substrate specificity and activity of the corresponding enzymes. We will also study the expression of these genes under different abiotic stresses to examine their possible role in kernel sink-strength and cell division.
MATERIALS AND METHODS
Plant Materials, Transformation, and Abiotic Stresses
For the northern analysis of Ckx1 expression, maize (Zea mays) B73 plants were grown in the field, and ears were covered with glassine bags before silk emergence. Plants were self-pollinated and harvested from 0 to 42 DAP. For each time point, duplicate ears were taken from each of four field replicates. Samples were collected between 10 am and 12 pm. Ovules and developing kernels were separated from the glumes. From 6 to 42 DAP, the pedicel region was separated from the rest of the kernel.
In situ hybridizations were performed on immature ear (7 weeks after planting; genotype An1Bz2), ovaries at silking (9 weeks after planting; genotype An1Bz2), 8-DAP kernels (Pioneer inbred N46), and primary root tips (V6 stage; inbred B73). A 1-kb EcoRV/EcoRI fragment, corresponding to the 3′ region of the amplified coding region, was subcloned into pBluescript to generate sense and antisense RNA probes.
All transformations were performed as previously described (Zhao et al., 1998). Root and coleoptile tissue were harvested from 3-week-old T1 seedlings of Ckx1::GUS plants. Samples were stained overnight for GUS activity and analyzed with a dissecting scope.
For the drought stress experiment, maize (cv Pioneer Hi-Bred 3732) plants were grown in 22-L pots containing a mixture of soil:sand (1:1, w/w). Pots were placed in a controlled-environment chamber with day/night temperatures and relative humidity of 30°C/20°C ±1°C and 40%/95% ± 5%, respectively, and were saturated with water each day. Cool-white fluorescent lamps provided a 14-h photoperiod with an irradiance of 850 to 1,000 μmol photosynthetically active radiation m–2 s–1 throughout the day at the top of the canopy. The drought stress was applied at 4 DAP by withholding water until 8 DAP. At that point, glumes were removed from developing seeds, and the pedicel region was separated from the rest of the kernel. Ears of field-grown Mo17 plants were heat stressed by subjecting them to a 35°C temperature as previously described (Commuri and Jones, 2001) from 4 to 8 DAP. Kernels were harvested from four ears at 8 DAP.
Distribution of Materials
Novel materials described in this publication may be available for noncommercial research purposes upon acceptance and signing of a material transfer agreement. In some cases, such materials may contain or be derived from materials obtained from a third party. In such cases, distribution of material will be subject to the requisite permission from any third-party owners, licensors, or controllers of all or parts of the material. Obtaining any permission will be the sole responsibility of the requestor. Plant germplasm and transgenic material will not be made available except at the discretion of the owner and then only in accordance with all applicable governmental regulations.
RT-PCR, RNA Extraction, Northern-Blot, and in Situ Hybridization
The Ckx1 coding region was PCR amplified from a reverse transcribed mRNA sample from 18-DAP embryos of Pioneer inbred N46, using the following primers: 5′-CGGGATCCTCATCATCAGTTGAAGATGTCCT-3′ and 5′-CATGCCATGGCGGTGGTTTATTACCTGCT-3′. The amplification product was cloned, sequenced, and used as a probe for northern-blot experiments.
For the kernel development study, poly(A) RNA was extracted from a mix of eight samples collected on each date. For each time point, total RNA was prepared from 1 g of material using a hot phenol extraction procedure as previously described (Brugière et al., 1999). Poly(A) was prepared from total RNA (400 μg) using Oligotex poly(A) purification kit (Qiagen USA, Valencia, CA). Probes were labeled with [α-32P]dCTP using random priming (Rediprime, Amersham Biosciences, Little Chalfont, Buckinghamshire, UK). Hybridizations were performed overnight at 42°C using the procedure described by Brugière et al. (2001). Successive washes were performed as follows: twice at 25°C for 10 min each with 2 × SSC; 0.1% (w/v) SDS (1 × SSC is 150 mm NaCl and 15 mm sodium citrate), once at 65°C for 10 min with 2 × SSC; 0.1% (w/v) SDS and twice for 20 min at 65°C with 0.1 × SSC; 0.1% (w/v) SDS. Relative mRNA abundance was quantified using a phosphor imager (MD860, Molecular Dynamics, Sunnyvale, CA) with an imaging software (ImageQuant, Molecular Dynamics).
A 1-kb subclone of Ckx1 was used to produce digoxigenin-labeled sense and antisense ribonucleotide probes using T3 and T7 RNA polymerases (Roche Diagnostics, Mannheim, Germany). Tissue was fixed and embedded, and hybridizations were carried out according to Jackson (1991) with modifications according to Bradley et al. (1993).
Promoter Isolation and Vector Construction
The Ckx1 promoter, consisting of 1,470 bp downstream of the deduced transcription start, was isolated by PCR amplification using B73 genomic DNA. Primers were designed based on the sequence published by Morris et al. (1999; accession no. AF04460), and their sequences are as follows: 5′-ATGTGTGAGTGTTTGGTAACTAGGTGA-3′ and 5′-GCATGCTTGAGTCATATCTTGGAAA-3′. PCR conditions were seven cycles of 94°C for 2 s and 66°C for 3 min, followed by 35 cycles of 94°C for 2 s and 61°C for 3 min, followed by 10 min at 61°C.
The Ckx1::GUS (PHP17468) construct was created by fusing the 1.47-kb Ckx1 promoter to the Escherichia coli GUS gene. This construct uses the BAR gene as a selectable marker. The gene fusion was terminated with the poly(A) addition site from the potato (Solanum tuberosum) PinII gene (Unger et al., 1993).
Cytokinin Level and Cytokinin Oxidase Activity Measurements
Endogenous cytokinins were extracted from 0.6- to 1.0-g tissue samples using cold (–80°C) methanol:water:acetic acid (70:30:3, v/v) containing 10 mg L–1 butylated hydroxytoluene. In addition, approximately 10,000 dpm of [3H]cytokinin internal standards was added to each sample before extraction to facilitate determination of sample recovery. Samples were then passed through an anion-exchange column (DEAE-Sephadex:DEAE-Cellulose [2:1]), purified using immunoaffinity chromatography, and quantified by HPLC diode array detection (MacDonald and Morris, 1985; Schreiber, 1990; Nicander et al., 1993). Verification of authenticity and purity of cytokinins was achieved by comparing the absorption spectra from each peak (obtained form UV/VIS diode array detection) with the absorption spectra of authentic cytokinin standards. This method of cytokinin purification and detection has been reported to be accurate (Nicander et al., 1993) and yields rapid, quantitative data allowing analysis of a large number of samples. Mass spectrometric analysis indicated that cytokinin fractions identified by UV/VIS diode array detection are authentic and do not contain other UV-absorbing compounds (data not shown).
Cytokinin oxidase activity measurements were made using a radioactive procedure. Samples were ground in liquid nitrogen, and approximately 0.5 g of frozen powder was homogenized using a ground glass thistle-tube hand homogenizer containing 0.1 m phosphate buffer (pH 6.5). After centrifugation, an aliquot of the extract was desalted on a Bio-Gel P-6 column using 0.1 m immidazole buffer at pH 6.5. An aliquot of the extract was assayed for cytokinin oxidase activity using 14C-labeled Z (Sigma-Aldrich, St. Louis) and separation of the reaction product 14C-Ade from this substrate by HPLC according to Schreiber (1990). se measured between identical samples was less than 10%.
Exogenous Application of Cytokinins and ABA
Ovules of Pioneer inbred P38 were collected at 4 DAP. Subtending tissue was removed, and approximately 40 kernels from three to five different ears were incubated in a scintillation vial containing 10 mL of incubation solution (100 mm Suc, 100 mm mannitol, 25 mm Gln, 10 mm MES adjusted to pH 5.5 with 1,2-bis(tris-[hydroxymethyl methylamino] propane); Schussler and Westgate, 1991). Kernels were incubated at 25°C on a platform rotating at 250 rpm. A pre-incubation of 30 min in the incubation solution preceded the addition of the hormone.
To study the effect of different substances on Ckx1 root expression, kernels were soaked for 16 h in tap water and germinated on filter paper. After 2 weeks, seedlings were removed, and the root systems were wrapped in filter paper soaked with the appropriate substance. Root systems were collected after 48 h of incubation.
Leaf discs (5 mm in diameter) were collected from fully expanded leaves of 8-week-old plants (inbred B73) and were incubated in petri dishes containing water or water supplemented with hormones. Approximately 100 discs per sample collected from three different leaves were used for each treatment, and discs were incubated at 25°C for 16 h. For ABA application, leaf discs were floated for 40 h on either distilled water (control), or on 10 μm ABA for 16 h (ABA16) or 40 h (ABA40). To show cumulative effect of ABA and BA treatments, leaf discs were also treated with ABA + BA (10 μm each) for 16 h (ABA + BA16) or incubated with 10 μm BA for 24 h after pre-incubation with ABA for 16 h (ABA16 + BA24).
Acknowledgments
We acknowledge the help provided by Xiaolan Duan and Deping Xu with the transgenic plants, Tim Helentjaris for his mapping work, Sharon Cerwick for her field support, and Mark Chamberlain for his help with the microscopy. We also thank Mike Muszynski and Nic Bate for their contribution to scientific discussions and reviewing the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.017707.
References
- Armstrong DJ (1994) Cytokinin oxidase and the regulation of cytokinin degradation. In DWS Mok, MC Mok, eds, Cytokinins, Chemistry, Activity and Function. CRC Press, Boca Raton, FL, pp 139–154
- Bertell G, Eliasson L (1992) Cytokinin effects on root growth and possible interactions with ethylene and indol-3-acetic acid. Physiol Plant 84: 255–261 [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C (1997) The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J 11: 339–345 [Google Scholar]
- Bilyeu KD, Cole JL, Laskey JG, Riekhof WR, Esparza TJ, Kramer MD, Morris RO (2001) Molecular and biochemical characterization of a cytokinin oxidase from maize. Plant Physiol 125: 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Carpenter R, Sommer H, Hartley N, Coen E (1993) Complementary floral homeoetic phenotypes result from opposite orientation of a transposon at the plena locus of Antirrhinum. Cell 72: 85–95 [DOI] [PubMed] [Google Scholar]
- Brugière N, Cui Y, Bi YM, Rothstein SJ (2001) The AtPP gene of the Brassica napus S locus region is specially expressed in the stigma and encodes a protein similar to a methyltransferase involved in plant defense. Sex Plant Reprod 13: 309–314 [Google Scholar]
- Brugière N, Dubois F, Limami AM, Lelandais M, Roux Y, Sangwan RS, Hirel B (1999) Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell 11: 1995–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield JM, Armstrong DJ (1986) Regulation of cytokinin oxidase activity in callus tissue of Phaseolus vulgaris L. cv. Great Northern. Plant Physiol 80: 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheikh N, Jones RJ (1994) Disruption of maize kernel growth and development by heat stress. Plant Physiol 106: 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commuri PD, Jones RJ (2001) High temperatures during endosperm cell division in maize: a genotypic comparison under in vitro and field conditions. Crop Sci 41: 1122–1130 [Google Scholar]
- Deji A, Sakakibara H, Ishida Y, Yamada S, Komari T, Kubo T, Sugiyama T (2000) Genomic organization and transcriptional regulation of maize ZmRR1 and ZmRR2 encoding cytokinin-inducible response regulators. Biochim Biophys Acta 1492: 216–220 [DOI] [PubMed] [Google Scholar]
- Dietrich JT, Kamínek M, Blevins DG, Reinbott TM, Morris RO (1995) Changes in cytokinins and cytokinin oxidase activity in developing kernels and the effects of exogenous cytokinin on kernel development. Plant Physiol Biochem 33: 327–336 [Google Scholar]
- Engelen-Eigles G, Jones RJ, Phillips RL (2001) DNA endoreduplication in maize endosperm cells is reduced by high temperature during the mitotic phase. Crop Sci 41: 1114–1121 [Google Scholar]
- Gavel Y, von Heijne G (1990) Sequence differences between glycosylated and non-glycosylated Asn-Thr/Ser acceptor sites: implication for protein engineering. Protein Eng 3: 433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare PD, van Staden J (1994) Cytokinin oxidase: biochemical features and physiological significance. Physiol Plant 91: 128–136 [Google Scholar]
- Hoth S, Morgante M, Sanchez J-P, Hanafey MK, Tingey SV, Chua N-H (2002) Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J Cell Sci 115: 4891–4900 [DOI] [PubMed] [Google Scholar]
- Houba-Hérin N, Pethe C, d'Alayer J, Laloue M (1999) Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts. Plant J 17: 615–626 [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413: 383–389 [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kat T, Tabata S, Shinozaki K, Kakimoto T (2001) Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: 1060–1063 [DOI] [PubMed] [Google Scholar]
- Jackson DP (1991) In situ hybridization in plants. In DJ Bowles, SJ Gurr, M McPhereson, eds, Molecular Plant Pathology: A Practical Approach. Oxford University Press, Oxford, pp 163–174
- Jones RJ, Schreiber BM, McNeil K, Brenner ML, Foxon G (1992) Cytokinin levels and oxidase activity during kernel development. In M Kamínek, DWS Mok, E Zažímalová, eds, Physiology and Biochemistry of Cytokinins in Plants. SPB Academic Publishing, The Hague, The Netherlands, pp 235–239
- Jones RJ, Schreiber BMN (1997) Role and function of cytokinin oxidase in plants. Plant Growth Regul 23: 123–134 [Google Scholar]
- Jones RJ, Setter TL (2000) Hormonal regulation of early kernel development. In M Westgate, K Boote, eds, Physiology and Modeling Kernel Set in Maize. Special Publication 29. Crop Science Society of America, Madison, WI, pp 25–42
- Kakimoto T (2001) Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol 42: 677–685 [DOI] [PubMed] [Google Scholar]
- Kamínek M, Dobrev P, Gaudinová A, Motyka V, Malbeck J, Trávníc̀ková A, Trc̀ková M (2000) Potential physiological function of cytokinin-binding proteins in seeds of cereals. Abstract of the 12th Congress of the Federation of European Societies of Plant Physiology (Budapest, Hungary). Plant Physiol Biochem Suppl 38 79 [Google Scholar]
- Laloue M, Fox JE (1989) Cytokinin oxidase from wheat: partial purification and general properties. Plant Physiol 90: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Sosa J, del Barrio MA, Zavala ME (2000) Accumulation of cytokinin oxidase in Zea mays under cold stress. Proc Am Soc Plant Physiol 1: 107–108 [Google Scholar]
- MacDonald EMS, Morris RO (1985) Isolation of cytokinins by immunoaffinity chromotography and analysis by high-performance liquid chromatography radioimmunoassay. Methods Enzymol 110: 347–358 [Google Scholar]
- Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y (2000) A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Gene Dev 14: 2938–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambelli S, Setter TL (1998) Inhibition of maize endosperm cell division and endoreduplication by exogenously applied abscisic acid. Physiol Plant 104: 266–277 [Google Scholar]
- Marivet J, Frendo P, Burkard G (1995) DNA sequence analysis of a cyclophilin gene from maize: developmental expression and regulation by salicylic acid. Mol Gen Genet 247: 222–228 [DOI] [PubMed] [Google Scholar]
- McCalla DR, More DJ, Osborne DJ (1962) The metabolism of a kinin, benzyladenine. Biochim Biophys Acta 55: 522–528 [Google Scholar]
- McKenzie MJ, Mett V, Reynolds PHS, Jameson PE (1998) Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter. Plant Physiol 116: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DWS, Martin RC (1994) Cytokinin metabolic enzymes. In DWS Mok, MC Mok, eds, Cytokinins, Chemistry, Activity and Function. CRC Press, Boca Raton, FL, pp 139–154
- Morris R, Bilyeu K, Laskey J, Cheikh N (1999) Isolation of a gene encoding a glycosylated cytokinin oxidase from maize. Biochem Biophys Res Commun 225: 328–333 [DOI] [PubMed] [Google Scholar]
- Motyka V, Kamínek M (1992) Characteristic of cytokinin oxidase from tobacco and poplar callus cultures. In M Kamínek, DWS Mok, E Zažímalová, eds, Physiology and Biochemistry of Cytokinins in Plants. SPB Academic Publishing, The Hague, The Netherlands, pp 33–39
- Nicander B, Ståhl U, Björkman P-O, Tillberg E (1993) Immunoaffinity co-purification of cytokinins and analysis by high-performance liquid chromatography with ultraviolet spectrum detection. Planta 189: 312–320 [DOI] [PubMed] [Google Scholar]
- Pac̀es V, Werstiuk E, Hall RH (1971) Conversion of N6-(Δ2-isopentenyl) adenosine to adenosine by enzyme activity in tobacco tissue. Plant Physiol 48: 775–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M, Palni MS (1987) Substrate effects on cytokinin metabolism in soybean callus culture. J Plant Physiol 126: 365–371 [Google Scholar]
- Sakakibara H, Hayakawa A, Deji A, Gawronski SW, Sugiyama T (1999) His-Asp phosphotransfer possibly involved in the nitrogen signal transduction mediated by cytokinin in maize: molecular cloning of cDNAs for two-component regulatory factors and demonstration of phosphotransfer activity in vitro. Plant Mol Biol 41: 563–573 [DOI] [PubMed] [Google Scholar]
- Schreiber BMN (1990) The role of cytokinins in maize kernel development. PhD thesis. University of Minnesota, St. Paul
- Schussler JR, Westgate ME (1991) Maize kernel set at low water potential: II. Sensitivity to reduced assimilates at pollination. Crop Sci 31: 1196–1203 [Google Scholar]
- Setter TL, Flannigan BA (2001) Water deficit inhibits cell division and expression of transcripts involved in cell proliferation and endoreduplication in maize endosperm. J Exp Bot 52: 1401–1408 [DOI] [PubMed] [Google Scholar]
- Setter TL, Flannigan BA, Melkonian J (2001) Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Sci 41: 1530–1540 [Google Scholar]
- Stenlid G (1982) Cytokinins as inhibitors of root growth. Physiol Plant 56: 500–506 [Google Scholar]
- Takei K, Sakakibara H, Sugiyama T (2001a) Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J Biol Chem 276: 26405–26410 [DOI] [PubMed] [Google Scholar]
- Takei K, Sakakibara H, Tanigguchi M, Sugiyama T (2001b) Nitrogen-dependent accumulation of cytokinins in root and translocation to leaf: implication of cytokinin species that induces gene expression of maize response regulator. Plant Cell Physiol 42: 85–93 [DOI] [PubMed] [Google Scholar]
- Terrine C, Laloue M (1980) Kinetics of N6-(D2-isopentenyl) adenosine degradation in tobacco cells: evidence of regulatory mechanism under the control of cytokinins. Plant Physiol 65: 1090P–1095P [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger E, Parsons RL, Schmidt RJ, Bowen B, Roth BA (1993) Dominant negative mutants of opaque2 suppress transactivation of a 22-kD zein promoter by opaque2 in maize endosperm cells. Plant Cell 5: 831–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BM, Beck E (1993) Cytokinins in the perennial herb Urtica dioica L. as influenced by its nitrogen status. Planta 190: 511–518 [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T (2001) Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA 98: 10487–10492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty CD, Hall RH (1974) A cytokinin oxidase in Zea mays. Can J Biochem 52: 789–799 [DOI] [PubMed] [Google Scholar]
- Yang SH, Yu H, Goh CJ (2002) Isolation and characterization of the orchid cytokinin oxidase DSCKX1 promoter. J Exp Bot 53: 1899–1907 [DOI] [PubMed] [Google Scholar]
- Zažímalová E, Kamínek M, Br̀ezinová A, Motyka V (1999) Control of cytokinin biosynthesis and metabolism. In PJJ Hooykaas, MA Hall, KR Libbenga, eds, Biochemistry and Molecular Biology of Plant Hormones. Elsevier Science, Amsterdam, pp 141–160
- Zhao Z, Gu W, Tagliani L, Hondred D, Bond D, Krell S, Rudert M, Bruce W, Pierce D (1998) Molecular analysis of T0 plants transformed by Agrobacterium and comparison of Agrobacterium-mediated transformation and bombardment transformation in maize. Maize Genetics Corporation Newsletter 72: 34–37 [Google Scholar]