Abstract
This report describes part of the signaling pathway and some of the molecules involved in the auxin-induced adventitious root formation in cucumber (Cucumis sativus). Previous results showed that nitric oxide (NO) mediates the auxin response during adventitious root formation (Pagnussat et al., 2002). To determine the order of action of indole acetic acid (IAA) and NO within the signal transduction pathway and to elucidate the target molecules that are downstream of NO action, cucumber hypocotyl cuttings were submitted to a pretreatment leading to endogenous auxin depletion. The auxin depletion treatment provoked a 3-fold reduction of the root number in comparison to the nondepleted explants. The NO-donor sodium nitroprusside was able to promote adventitious rooting in auxin-depleted explants, whereas the specific NO scavenger cPTIO prevented the effect of sodium nitroprusside. The endogenous NO level was monitored in both control and auxin-depleted explants using a NO-specific fluorescent probe. The NO level was 3.5-fold higher in control (nondepleted) explants than in auxin-depleted ones. The exogenous application of IAA restored the NO concentration to the level found in nondepleted explants. Because NO activates the enzyme guanylate cyclase (GC), we analyzed the involvement of the messenger cGMP in the adventitious root development mediated by IAA and NO. The GC inhibitor LY83583 reduced root development induced by IAA and NO, whereas the cell-permeable cGMP derivative 8-Br-cGMP reversed this effect. The endogenous level of cGMP is regulated by both the synthesis via GC and its degradation by the phosphodiesterase activity. When assayed, the phosphodiesterase inhibitor sildenafil citrate was able to induce adventitious rooting in both nondepleted and auxin-depleted explants. Results indicate that NO operates downstream of IAA promoting adventitious root development through the GC-catalyzed synthesis of cGMP.
The plant hormone auxin is involved in the regulation of most aspects of plant growth and development, including cell division, elongation, and differentiation. The role of auxins in adventitious rooting is particularly important because the hormone initiates this process—which involves cell division and primordium formation—inducing the dedifferentiation of the cells to form the apical meristem. Although genetic approaches have revealed some discrete aspects of auxin transport, signaling, and response, the understanding of the molecular mechanism of auxin action in the establishment of new root meristems remains preliminary (Doerner, 2000; Berleth and Sachs, 2001).
Nitric oxide (NO) is a diffusible multifunctional molecule involved in numerous physiological processes in phylogenetically distant species (Gow and Ischiropoulos, 2001). It was first described in mammals, where it plays variable functions ranging from dilation of blood vessels to neurotransmission and immune response. In comparison with animal studies, relative little is known about NO biological functions in plants. However, the presence of NO was conclusively demonstrated in plants, and its involvement as a stress signal in adaptive responses and mediating growth regulation processes was also reported (Gouvêa et al., 1997; Huang and Knopp, 1998; Laxalt et al., 1997; Durner et al., 1998; Leshem et al., 1998; Beligni and Lamattina, 2000, 2001a, 2001b). More recently, NO mediation of the adventitious rooting process induced by auxins was described (Pagnussat et al., 2002), even though the target molecules of the NO action are still unknown in this process.
In animal cells, cGMP is an important component of NO signaling (McDonald and Murad, 1995). NO induces the activation of soluble guanylate cyclase (GC) by binding to the heme group of the enzyme, which results in a transient increase of the cellular messenger cGMP (Stamler, 1994). The binding of NO to the ferrous heme group is reversible, and GC turns off immediately after the NO gradient has been dissipated (Beckman, 1996). In plants, the presence of cGMP was shown by mass spectrometry and radio-immune assays (Brown and Newton, 1992). Probably the first evidence for the occurrence of cGMP in plants came from a report that suggested a change in the concentration of this cyclic monophosphate during cell enlargement and division in tobacco (Nicotiana tabacum; Lundeen et al., 1973). A cyclic nucleotide phosphodiesterase (PDE) activity has been also reported in plants (Brown et al., 1975; Reggiani, 1997), although neither GC nor the PDE have been cloned in plants yet. Various stimuli, such as GA in barley (Hordeum vulgare) aleurone (Penson et al., 1996), light stimulation of bean cells (Brown and Newton, 1992), or NO treatment in spruce needles (Pfeiffer et al., 1994) cause a transient increase in cGMP levels. NO treatment was also reported to induce a transient increase of cGMP levels in tobacco (Durner et al., 1998). On the other hand, cGMP was reported to trigger anthocyanins production and, in combination with calcium, to induce the development of mature chloroplasts (Bowler et al., 1994). Moreover, specific GC inhibitors were able to suppress the NO-induced expression of Phe ammonia-lyase genes in tobacco (Durner et al., 1998). In addition, the specific inhibition of GC activity blocked the NO-induced cell death in Arabidopsis cells, and this inhibition is reversed by the cell-permeable cGMP analog, 8-Br-cGMP (Clarke et al., 2000). More recently, it was reported that NO is a signaling component of the abscisic acid (ABA)-induced stomatal closure and that both ABA- and NO-induced closure require the synthesis and action of cGMP (Neill et al., 2002).
Previous results in our laboratory showed that NO mediates the auxin response during the adventitious rooting process in cucumber (Cucumis sativus; Pagnussat et al., 2002). However, although a role for NO in root development was proposed, the cross talk between indole acetic acid (IAA) and NO still remains to be elucidated. In the presence of endogenous auxins, a serial linkage IAA → NO → rooting, cannot be distinguished from a situation in which both IAA and NO are needed to act in parallel on a third intermediate. Therefore, the depletion of endogenous auxins appears to be a useful tool to study the auxin signal transduction pathway. This experimental strategy was previously used to investigate Aux/IAA mRNA accumulation during seedling development and gravity response in cucumber (Fuji et al., 2000) and to study the regulation of lateral and adventitious root formation in Arabidopsis (Casimiro et al., 2001; Díaz-Sala et al., 2002). Using this experimental approach, we provide evidence here that NO acts as a second messenger in the IAA-mediated pathway that induces adventitious root formation through the activation of the GC-catalyzed synthesis of cGMP.
RESULTS
NO Mediates Adventitious Root Development Induced by IAA
To clarify the mechanism(s) through which IAA and NO promotes adventitious rooting, apical IAA production was interrupted by decapitation of the explants, a treatment that was reported to reduce basal IAA concentration and to inhibit adventitious rooting (Nordström and Eliasson, 1991). Intact (non-depleted) or decapitated explants were respectively pre-incubated in water or in the presence of the inhibitor of the basipetal auxin efflux 1-naphthylphtalamic acid (NPA; IAA-depleted) for 48 h before primary root removal. NPA was used at 10 μm, a concentration that prevents both adventitious and lateral root formation in Arabidopsis (Casimiro et al., 2001; Díaz-Sala et al., 2002).
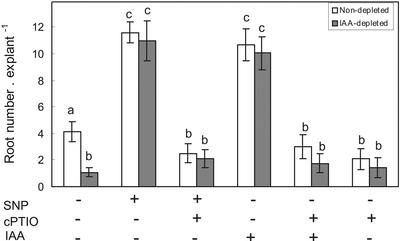
As was previously reported (Pagnussat et al., 2002), the NO-donor sodium nitroprusside (SNP) applied to nondepleted cucumber explants was able to induce de novo root organogenesis mimicking the effect of the auxin IAA (Fig. 1). Both length (not shown) and number of adventitious roots exhibit similar behavior in either IAA or SNP treatments (Fig. 1, t test, P < 0.05). Nondepleted explants displayed 3-fold more roots than the auxin-depleted ones (Fig. 1, t test, P < 0.05). SNP and IAA treatments were able to reverse the reduction of adventitious root development in auxin-depleted explants. The NO scavenger cPTIO prevented the effect of both SNP and IAA on the IAA-depleted explants (Fig. 1). When cPTIO was administered alone to nondepleted explants, it resulted in a significant reduction of adventitious root formation (Fig. 1).
Figure 1.
Adventitious root development in cucumber explants. Explants without any pretreatment (nondepleted, white bars) or with an auxin depletion pretreatment (IAA-depleted, dark bars) were incubated as indicated for 7 d. Root number values are expressed as mean ± se (n = 20 explants from at least three independent experiments). SNP and IAA were used at 10 μm and the NO scavenger cPTIO was used at 200 μm. Bars with different letters are significantly different with P < 0.05 (t test).
The IAA Depletion Treatment Diminished the Endogenous Level of NO
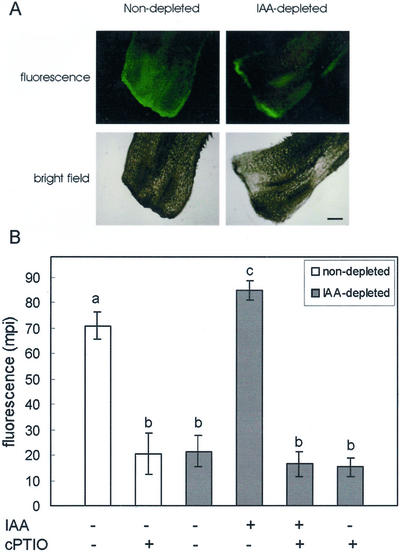
Because a transient increase of endogenous NO was measured in cucumber explants as a response to exogenous IAA (Pagnussat et al., 2002), the NO level was monitored in nondepleted and in IAA-depleted explants without any treatment or treated with exogenous IAA or with IAA plus the NO-scavenger cPTIO, or with cPTIO alone. Longitudinal sections of hypocotyls were incubated with the fluorophore 4,5-diamino-fluorescein diacetate (DAF-2 DA), which allows the detection of NO presence in both animal and plant cells (Kojima et al., 1998; Foissner et al., 2000). The compound 4-aminofluorescein diacetate (4 AF DA) was used as a negative control of DAF-2 DA to assure that the green fluorescence detected corresponds to an accumulation of endogenous NO and not to unspecific reactions of the probe. The green fluorescence was quantified by measuring the medium green pixel intensity (mpi). NO was detected in nondepleted control explants where it was mainly visible in the basal region of the hypocotyl (mpi, 70.8 ± 5.4; Fig. 2, A and B). When cPTIO was added to nondepleted explants, the signal was significantly reduced (mpi, 20.5 ± 8.2, t test, P < 0.05; Fig. 2B). In explants submitted to the auxin depletion treatment, the signal was highly and significantly reduced in comparison with nondepleted explants (mpi, 21.5 ± 6.1, t test, P < 0.05). This could be reversed when the auxin-depleted explants were treated with exogenous IAA (mpi, 84.8 ± 4.7), whereas a slight signal was detected in explants treated with IAA plus the NO scavenger cPTIO (mpi, 16.5 ± 4.8; Fig. 2B), in explants treated with cPTIO alone (mpi, 15.2 ± 3.5; Fig. 2B), or in negative controls (hypocotyls sections treated with the negative probe 4 AF DA, not shown).
Figure 2.
Endogenous NO level in nondepleted and IAA-depleted cucumber explants. A, The specific fluorescent probe DAF-2 DA was used to monitor NO in longitudinal sections of hypocotyl cutting tips from nondepleted and IAA-depleted explants. IAA and cPTIO were respectively used at 10 and 200 μm. B, The fluorescence was quantified by measuring the medium pixel intensity (mpi) as described. Bar indicates 0.5 mm. Bars with different letters are significantly different with P < 0.05 (t test).
NO Acts Downstream of IAA through a Cyclic GMP-Dependent Pathway
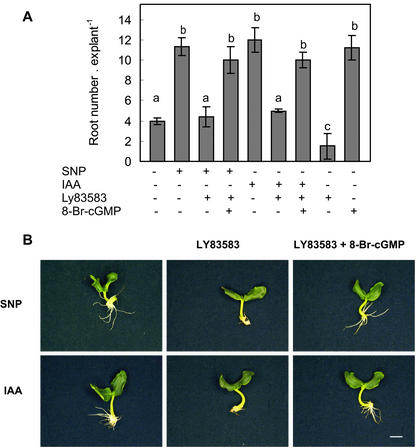
Because cGMP is an important component of NO-mediated signaling pathways (McDonald and Murad, 1995), we determined root development in non-depleted explants treated with either SNP or IAA in the presence of the GC inhibitor 6-anilino-5,8-quinolinedione (LY83583). The treatment of explants with SNP plus LY83583 produced a delay in root emergence and a significant reduction in both the root length (not shown) and root number (Fig. 3, A and B, t test, P < 0.05). The cell-permeable cGMP derivative 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP) reversed the effect of LY83583 on adventitious root development, showing explants with similar response to the SNP-treated ones (Fig. 3, A and B). In addition, LY83583 produced a significant decrease in the root number of the IAA-treated explants (Fig. 3, A and B, t test, P < 0.05). This effect could be also reversed when 8-Br-cGMP was added together with IAA and LY83583 (Fig. 3, A and B). Moreover, 8-Br-cGMP alone was able to induce adventitious root development at a similar extent than those displayed in explants treated with IAA or SNP (Fig. 3A). On the contrary, when LY83583 was administered alone, a significant reduction of root development was shown (Fig. 3A, t test, P < 0.05).
Figure 3.
Cyclic GMP is required for IAA- and NO-mediated adventitious root formation. Non-depleted explants were treated as indicated. A, Root number values are expressed as mean ± se (n = 10 explants from at least three independent experiments). Bars with different letters are significantly different with P < 0.05 (t test). The GC inhibitor LY 83583 was used at 50 μm, 8-Br-cGMP was used at 1 μm, and SNP and IAA were used at 10 μm. B, Photographs were taken after 5 d of treatment. Bar = 5 mm.
PDE Activity Regulates the Formation of Adventitious Roots
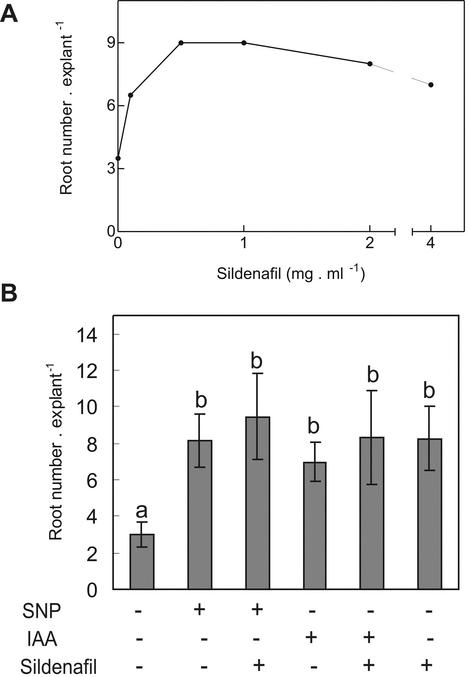
Additionally, we studied how the modulation of the cGMP levels through the inhibition of the PDE activity affects the adventitious root development. Nondepleted explants were treated with different concentrations of the PDE inhibitor sildenafil citrate. Figure 4A shows a 300% increase of root number in cucumber explants treated with 0.5 and 1 mg mL–1 of sildenafil citrate in comparison with control explants. The explants treated with IAA or SNP plus sildenafil presented a similar adventitious root number than explants treated either with the NO donor or IAA alone (Fig. 4B). On the other hand, explants treated with sildenafil alone also exhibited a similar response to those explants treated with IAA or SNP (Fig. 4B).
Figure 4.
The inhibition of PDE activity promotes adventitious root development. A, Explants were treated with different concentrations of the PDE inhibitor sildenafil citrate, and root number per explant was determined after 5 d of treatment. B, Root number was also determined in explants treated with 10 μm IAA, 10 μm SNP, or 1 mg mL–1 sildenafil citrate or in combined treatments. Root number values are expressed as mean ± se (n = 10 explants from at least three independent experiments). Bars with different letters are significantly different with P < 0.05 (t test).
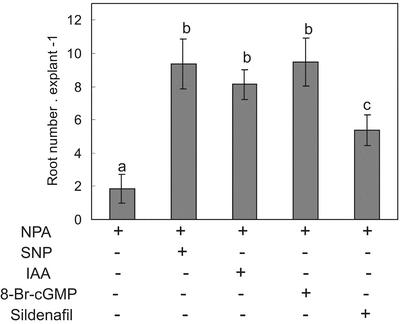
One intriguing question directed to elucidate the mechanism(s) through which cGMP mediates the rooting process activated by IAA and NO is whether cGMP is sufficient to activate adventitious root development. Thus, the effect of the cell-permeable analog 8-Br-cGMP and sildenafil on adventitious rooting was investigated in IAA-depleted explants. When these IAA-depleted explants were treated with 8-Br-cGMP, a similar root number was detected in comparison with IAA- and NO-treated explants (Fig. 5). However, when the IAA-depleted explants were treated with sildenafil, a lower response was obtained. The sildenafil-treated explants displayed in average only a 60% of the root number obtained from the NO-, the IAA- or the 8-Br-cGMP-treated explants (Fig. 5).
Figure 5.
Cyclic GMP activates the rooting process in IAA-depleted explants. Explants with an auxin depletion pretreatment were incubated as described for 7 d. Root number values are expressed as mean ± se (n = 20 explants from at least three independent experiments). SNP and IAA were used at 10 μm, 8 Br-cGMP was used at 1 μm, and sildenafil was used at 1 mg mL–1. Bars with different letters are significantly different with P < 0.05 (t test).
DISCUSSION
The results presented in this work are significant for both fundamental and applied plant biology. They strongly argue that both NO and cGMP are downstream messengers in the IAA-signaling pathway that promotes adventitious root development. NO treatments were able to induce adventitious rooting in cucumber explants to the same extent as the plant hormone IAA. Because the specific NO-scavenger cPTIO causes a significant reduction of adventitious root number in IAA-treated explants, endogenous NO appears to play a key role in the auxin-mediated adventitious rooting process (Fig. 1). The endogenous IAA is synthesized in the apical bud, above cotyledons, and is basipetally transported down the plant stem via the polar transport system (Ford et al., 2001). The involvement of polar auxin transport in supplying the auxin for rooting was clearly established in carnation (Dianthus caryophyllus) cuttings (Guerrero et al., 1999). To clarify the nature of the IAA-NO communication, a pretreatment leading to depletion of endogenous auxins was carried out before exposing the cucumber explants to the NO donor. Thus, apical IAA production was disrupted by decapitation of the explants, and basipetal transport of auxins was inhibited by NPA. These treatments were able to significantly reduce adventitious root formation. Interestingly, this result could be reversed by NO (Fig. 1), suggesting that IAA and NO might be acting through a serial signaling pathway.
In mammalian systems, one of the most studied targets of NO is the enzyme GC. GC, together with PDE, regulates the endogenous level of the cellular messenger cGMP. cGMP is an important signaling molecule present in both eukaryote and prokaryote cells, typically involved in sensing extracellular stimuli and in the transduction of the signal into metabolic responses (Reggiani, 1997). In the last years, mounting evidence supports the existence of an NO-inducible GC in plants (Pfeiffer et al., 1994; Durner et al., 1998). To determine the putative involvement of cGMP in our system, the adventitious root formation capacity of both SNP- and IAA-treated explants was assayed in the presence of the GC inhibitor LY83583. Clearly, LY83583 was able to reduce adventitious root number in both IAA- and SNP-treated explants. This inhibition could be reversed when 8-Br-cGMP was added together with LY83583 and SNP or IAA (Fig. 3). Moreover, the treatment with 8-Br-cGMP alone did not show differences with the SNP- or IAA-treated explants. As well, when LY83583 was added alone to nondepleted explants (Fig. 3A), they displayed a number of adventitious roots comparable with the number observed in IAA-depleted explants (Fig. 1). Additionally, the inhibition of cGMP degradation by the PDE inhibitor sildenafil citrate also induced adventitious root development. Together, these results strengthen the hypothesis that cGMP synthesis is required and might be sufficient for adventitious rooting. To test that hypothesis, adventitious rooting was assayed in IAA-depleted explants. The treatment of IAA-depleted explants with 8-Br-cGMP was sufficient to induce rooting to a similar extent than IAA and SNP. However, the explants treated with the PDE inhibitor sildenafil reached only a 60% of the root number displayed by the 8-Br-cGMP-treated ones. This behavior could be explained if we consider the low levels of cGMP that should be synthesized in IAA-depleted explants, where no NO is present to activate the GC enzyme. Thus, although the inhibition of PDE activity in these explants could result in an increased level of cGMP, its level would not reach those of IAA- or SNP-treated ones. Some reports have described how small concentrations of sildenafil can double the life span of cut flowers delaying their natural senescence process (Leshem et al., 1998; Siegel-Itzkovich, 1999). On the other hand, light and Ca 2+-calmodulin were also identified as plant PDE effectors (Brown et al., 1989). Thus, the production of cGMP in plant appears to be stimulated by the action of phytochrome and might be part of the molecular events that control development throughout the plant life cycle. It would be interesting, therefore, to explore this scenario and to integrate the cGMP targets that are operating to affect biological processes such as chlorophyll biosynthesis, root development, and cell division and differentiation.
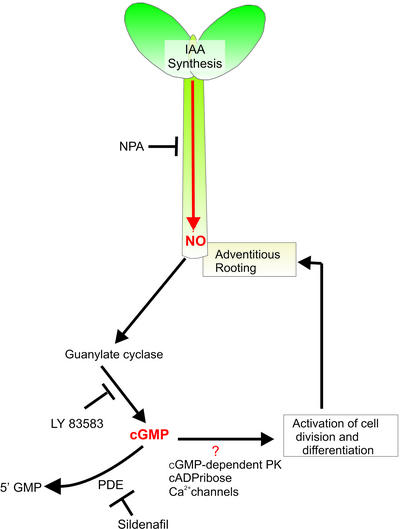
Collectively, our results present strong evidence to unravel part of the signaling cascade that operates downstream of IAA to trigger adventitious root development. This pathway is an NO-induced process mediated by the GC-catalyzed synthesis of cGMP. A schematic model of the suggested pathway involving IAA, NO, and cGMP is summarized in Figure 6. IAA, synthesized in the apical part of the seedling, is basipetally transported and accumulated at the base of the hypocotyl, where triggers a local and transient generation of NO in an as yet unknown manner (Fig. 2; Pagnussat et al., 2002). Two possible sources of NO in plants are the enzymes NO synthase (NOS) and nitrate reductase. NOS-like activity has been proposed in plants by using both biochemical and immunological approaches (Wendehenne et al., 2001). However, to date, neither cDNA nor any NOS-like protein has been found in plants. In addition, no NOS-like gene was found in the Arabidopsis genome (Arabidopsis Genome Initiative, 2000). Recently, pharmacological, physiological, and genetic evidence was provided to support the nitrate reductase-mediated NO generation for ABA-induced stomatal closure (Desikan et al., 2002). Furthermore, several convergent lines of evidence were reported that strengthen those experimental results (García-Mata and Lamattina, 2003). This NO production might activate a cGMP-dependent transduction pathway, where cGMP formation is driven by the nitrosylation activation of GC. Although our results and those previously published (Durner et al., 1998) suggest the existence of a NO-inducible GC in cucumber, tobacco, and Arabidopsis, in a very recently report, Ludidi and Gehring (2003) reported the existence of a NO-insensitive GC in Arabidopsis. The cloned GC protein from Arabidopsis has not a heme-group, and that is consistent with its insensitivity to NO. However, the GC activity was measured on a GS-GC recombinant protein in vitro, which could not reflect of GC activity and regulation in vivo. Additionally, a more complete in vitro assay than that presented by Ludidi and Gehring (2003) should be necessary to confirm that the recombinant GS-GC protein is a NO-insensitive enzyme. The NO donor used by Ludidi and Gehring (2003) to activate the recombinant enzyme in Escherichia coli cells was SNP, which releases the nitrosonium cation NO+. Due to the presence of ascorbate, the nitrosonium cation NO+ is rapidly converted to the NO radical NO+ in planta. Thus, the use of another NO donor like S-nitroso, N-acetyl penicillamine, which releases the NO radical NO+, is advisable to test the NO insensitivity of the recombinant protein. Moreover, only one concentration of NO donor (1 mm) and only one time (10 min) were analyzed to test the activity of GS-GC recombinant protein.
Figure 6.
Schematic illustration of a proposed model for the control of adventitious root formation in cucumber. IAA is synthesized in the apical bud and basipetally transported to the base of the hypocotyl. There, IAA triggers a transient NO accumulation, which activates a cGMP-dependent pathway leading to adventitious root formation. Blue arrow, IAA transport, inhibition.
A potential target for cGMP could be a cGMP-dependent protein kinase, although no cGMP-dependent protein kinase has yet been isolated and cloned in plants. cGMP can also act via cADP Rib (cADPR), as occur in animal cells (Gow and Ischiropoulos, 2001). In plants, it was reported that cADPR is involved in the NO-induced activation of defense genes in tobacco (Durner et al., 1998). Moreover, it was also recently shown that NO is involved in the signaling cascade triggered by the stress hormone ABA (García-Mata and Lamattina, 2002; Neill et al., 2002) and that cADPR takes part in this pathway (Neill et al., 2002). The cADPR elevates cytosolic-free Ca2+ in plants and thereby plays a central role in signal transduction pathways (Navazio et al., 2001). Finally, Ca2+ might be involved in a cascade that leads the activation of cell division and elongation as was suggested by Kiegle et al. (2000).
Because adventitious root development is a key step in vegetative propagation, the study of NO involvement in the rooting process opens a wide field of research important not only for understand the intricate network operating in root development and auxin signal transduction, but also it will be relevant for agricultural and biotechnological procedures.
MATERIAL AND METHODS
Plant Material
Cucumber (Cucumis sativus) seeds were germinated into petri dishes on filter papers embedded in distilled water and maintained at 25°C for 5 d with a 14-h photoperiod (photosynthetically active radiation = 200 μmol m–2 s–1). Cucumber seedlings were used either: (a) intact (nondepleted) or (b) decapitated by excising the apical bud immediately above the cotyledons and incubated in the presence of 10 μm NPA (Chem Service, New Orleans) for 48 h (IAA-depleted), before removing primary root. Cucumber explants were then maintained under the same conditions of temperature and photoperiod for another 5 or 7 d in the presence of different media as indicated below.
Explant Treatments
After primary roots were removed, cucumber explants were put into petri dishes containing water (control), 10 μm of the auxin IAA (Fluka, Buchs, Switzerland), or 10 μm of the NO donor SNP (Merck, Darmstadt, Germany) and kept at 25°C for different periods according to the experiment. As a control, 200 μm 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, potassium salt (c-PTIO, Molecular Probes, Eugene, OR) was added together with SNP or IAA. Where indicated, explants were incubated with 50 μm of the GC inhibitor 6-anilino-5,8-quinolinedione (LY83583, Sigma-Aldrich, St. Louis) or with 1 μm of the cell-permeable cGMP derivative 8-Br-cGMP (Sigma-Aldrich) or with different concentrations of the PDE inhibitor sildenafil citrate (1-[[3-(6,7-dihydro-1-methyl-7-oxo-3 propyl-1 H-pyrazolo [4,3-d] pyrimidin-5-yl′)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate), Viagra, Pfizer, New York) as indicated. For preparation of sildenafil citrate, tablets of Viagra were ground up and dissolved in distilled water. The mix was shaken for at least 5 min and gently centrifuged to remove all the insoluble material. The concentrations of the compounds used for experiments were supported by published results (Durner et al., 1998; Pagnussat et al., 2002).
Endogenous NO Detection
Endogenous NO was monitored by incubating 200- to 250-μm longitudinal hypocotyl sections from cucumber explants with either 7.5 μm of the positive fluorescent probe DAF-2 DA (Calbiochem, San Diego) or with 7.5 μm of the negative probe 4 AF DA (Calbiochem) in 20 mm HEPES-NaOH pH 7.5 (buffer A). Thereafter, the sections were washed three times for 15 min with buffer A and examined by fluorescence (excitation 490 nm; emission 525 nm) and bright field microscopy in a Eclipse E 200 microscope (Nikon, Tokyo). Green fluorescence quantification was performed using the Matrox Inspector v3.1 computer program (Matrox Electronic Systems, Dorval, Quebec).
Data Analysis
Where indicated, values were expressed as means ± se of at least three independent experiments. For statistical analysis, t test was used as appropriate, after testing for data normality. A value of P < 0.05 was considered significant for mean differences.
Acknowledgments
We thank Dr. Ana M. Laxalt and Lic. Magdalena Graziano for critical reading of the manuscript and helpful discussions.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.022228.
This work was supported by grants to L.L. from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), from Agencia Nacional de Promoción Científica y Tecnológica, from Fundación Antorchas and from Universidad Nacional de Mar del Plata (UNMdP), Argentina. L.L. is a career member and G.C.P. is a research postdoctoral fellow from CONICET Argentina. M.L.L. is a student fellow from UNMdP Argentina.
References
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Beckman JS (1996) The physiological and pathological chemistry of nitric oxide. In J Lancaster, ed, Nitric Oxide: Principles and Actions. Academic Press, New York, pp 1–83
- Beligni MV, Lamattina L (2000) Nitric oxide induces seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 210: 215–221 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (2001a) Nitric oxide: a non traditional regulator of plant growth. Trends Plant Sci 6: 508–509 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (2001b) Nitric oxide in plants: The history is just beginning. Plant Cell Environ 24: 267–278 [Google Scholar]
- Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4: 57–62 [DOI] [PubMed] [Google Scholar]
- Bowler C, Neuhaus G, Yamagata H, Chua NH (1994) Cyclic GMP and calcium mediate phytochrome phototransduction. Cell 77: 73–81 [DOI] [PubMed] [Google Scholar]
- Brown EG, Al-Najafi T, Newton RP (1975) Partial purification of adenosine 3′:5′-cyclic monophosphate phosphodiesterase from Phaseolus vulgaris L.: associated activator and inhibitors. Biochem Soc Trans 3: 393–395 [DOI] [PubMed] [Google Scholar]
- Brown EG, Newton RP (1992) Analytical procedures for cyclic nucleotides and their associated enzymes in plant tissue. Phytochem Anal 3: 1–3 [Google Scholar]
- Brown EG, Newton RP, Evans DE, Walton TJ, Younis LM, Vaughan JM (1989) Influence of light on cyclic nucleotide metabolism in plants: effect of dibutyryl cyclic nucleotides on chloroplast components. Phytochemistry 28: 2559–2563 [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ (2000) NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J 24: 667–677 [DOI] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S (2002) A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA 99: 16314–16318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Sala C, Garrido G, Sabater B (2002) Age-related loss of rooting capability in Arabidopsis thaliana and its reversal by peptides containing the Arg-Gly-Asp (RGD) motif. Physiol Plant 114: 601–607 [DOI] [PubMed] [Google Scholar]
- Doerner P (2000) Plant stem cells: The only constant thing is change. Curr Biol 10: 201–203 [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig D (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP-ribose. Proc Natl Acad Sci USA 95: 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23: 817–824 [DOI] [PubMed] [Google Scholar]
- Ford YY, Bonham EE, Cameron RWF, Blake PS, Judd HL, Harrison-Murray RS (2001) Adventitious rooting: examining the role of auxin in an easy- and a difficult-to-root plant. Plant Growth Regul 00: 1–11 [Google Scholar]
- Fujii N, Kamada M, Yamasaki S, Takahashi H (2000) Differential accumulation of Aux/IAA mRNA during seedling development and gravity response in cucumber (Cucumis sativus). Plant Mol Biol 42: 731–740 [DOI] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128: 790–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2003) Abscisic acid, nitric oxide and stomatal closure: Is nitrate reductase one of the missing links? Trends Plant Sci 8: 20–26 [DOI] [PubMed] [Google Scholar]
- Gow AJ, Ischiropoulos HJ (2001) Nitric oxide chemistry and cellular signaling. J Cell Physiol 187: 277–282 [DOI] [PubMed] [Google Scholar]
- Guerrero JR, Garrido G, Acosta M, Sanchez-Bravo J (1999) Influence of 2,3,5-triiodobenzoic acid and 1-N-naphthylphthalamic acid on indoleacetic acid transport in carnation cuttings: relationship with rooting. Plant Growth Regul 18: 183–190 [DOI] [PubMed] [Google Scholar]
- Huang JS, Knopp JA (1998) Involvement of nitric oxide in Ralstonia solanacearum-induced hypersensitive reaction in tobacco. In P Prior, J Elphinstone, C Allen, eds, Bacterial Wilt Disease: Molecular and Ecological Aspects. Institut National de la Recherche Agronomique and Springer Editions, Berlin, pp 218–224
- Kiegle E, Gilliham M, Haseloff J, Tester M (2000) Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J 21: 225–229 [DOI] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Tanaka J, Kudo Y, Nagano T (1998) Direct evidence of NO production in rat hippocampus and cortex using a new fluorescent indicator: DAF-2 DA. Neuroreport 9: 3345–3348 [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Beligni MV, Lamattina L (1997) Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. Eur J Plant Pathol 73: 643–651 [Google Scholar]
- Leshem YY, Wills RBH, Ku VVV (1998) Evidence for the function of the free radical gas—nitric oxide (NO)—as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem 36: 825–833 [Google Scholar]
- Ludidi N, Gehring C (2002) Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J Biol Chem 278: 6490–6494 (Papers in Press, December 12, Manuscript M210983200) [DOI] [PubMed] [Google Scholar]
- Lundeen CV, Wood NH, Braun AC (1973) Intracellular levels of cyclic nucleotides during cell enlargement and cell division in excised tobacco piths. Differentiation 1: 225–260 [Google Scholar]
- McDonald LJ, Murad F (1995) Nitric oxide and cGMP signaling. Adv Pharmacol 34: 263–276 [DOI] [PubMed] [Google Scholar]
- Navazio L, Mariani P, Sanders D (2001) Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiol 125: 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128: 13–16 [PMC free article] [PubMed] [Google Scholar]
- Nordström AC, Eliasson L (1991) Levels of endogenous indole-3-acetic acid and indole-3-acetylaspartic acid during adventitious root formation in pea cuttings. Physiol Plant 82: 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L (2002) Nitric oxide is required for root organogenesis. Plant Physiol 129: 954–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penson SP, Schuurink RC, Fath A, Gubler F, Jacobsen JV, Jones RL (1996) cGMP is required for gibberellic acid-induced gene expression in barley aleurone. Plant Cell 8: 2325–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer S, Janistyn B, Jessner G, Pichorner H, Eberman RE (1994) Gaseous nitric oxide stimulates guanosine 3′, 5′-cyclic monophosphate formation in spruce needles. Phytochemistry 36: 259–262 [Google Scholar]
- Reggiani R (1997) Alteration of levels of cyclic nucleotides in response to anaerobiosis in rice seedlings. Plant Cell Physiol 38: 740–742 [Google Scholar]
- Siegel-Itzkovich J (1999) Viagra makes flowers stand up straight. Br Med J 319: 274. [DOI] [PubMed] [Google Scholar]
- Stamler JS (1994) Redox signaling: nitrosylation and related target interaction of nitric oxide. Cell 78: 931–936 [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6: 177–183 [DOI] [PubMed] [Google Scholar]