Abstract
Leaves of ground ivy (Glechoma hederacea) contain a lectin (called Gleheda) that is structurally and evolutionary related to the classical legume lectins. Screening of a population of wild plants revealed that Gleheda accounts for more than one-third of the total leaf protein in some clones, whereas it cannot be detected in other clones growing in the same environment. Gleheda is predominantly expressed in the leaves where it accumulates during early leaf maturation. The lectin is not uniformly distributed over the leaves but exhibits a unique localization pattern characterized by an almost exclusive confinement to a single layer of palisade parenchyma cells. Insect feeding trials demonstrated that Gleheda is a potent insecticidal protein for larvae of the Colorado potato beetle (Leptinotarsa decemlineata). Because Gleheda is not cytotoxic, it is suggested that the insecticidal activity is linked to the carbohydrate-binding specificity of the lectin, which as could be demonstrated by agglutination assays with different types of polyagglutinable human erythrocytes is specifically directed against the Tn antigen structure (N-acetylgalactosamine O-linked to serine or threonine residues of proteins).
Recently, a galactoside-binding lectin was isolated and characterized from leaves of ground ivy (Glechoma hederacea), which shares a high sequence similarity with the classical legume lectins and exhibits a very similar overall fold and three-dimensional structure (Wang et al., 2003). The identification of the ground ivy agglutinin (Gleheda) as a fully active ortholog of the classical legume lectins eventually solved the problem of the classification of the Lamiaceae lectins, but at the same time, it raised several important questions with respect to the evolution and physiological role of the legume lectin family. It is evident that the apparent occurrence of a typical legume lectin outside the family Fabaceae urges revision and refinement of all previously proposed models of the molecular evolution of this lectin family (Van Damme et al., 1998; Barre et al., 2002). In addition, the discovery of Gleheda puts the physiological role of the classical and novel legume lectins in a new perspective because one cannot take it for granted that the Lamiaceae lectins have been designed for the same activities/functions as the Fabaceae lectins. For example, the proposed involvement of—at least some—classical legume lectins in the specific interaction between legumes and their symbiotic nitrogen-fixing Rhizobia sp. (Diaz et al., 1989; Brewin and Kardailsky, 1997) cannot be extrapolated to the Lamiaceae lectins because members of this plant family do not establish a symbiotic relationship with nitrogen-fixing bacteria. In contrast, other roles proposed for the classical legume lectins like defense against herbivorous animals and/or phytophagous invertebrates possibly combined with a role as a storage protein (Peumans and Van Damme, 1995), may well apply to Gleheda or other Lamiaceae lectins. However, because at present virtually no information is available about the temporal and spatial regulation of the expression of Gleheda and the possible effects of the lectin on foreign organisms, it remains speculative to attribute a comparable physiological function to the Lamiaceae and the classical Fabaceae lectins. For the same reason, it cannot be precluded that the Lamiaceae lectins fulfill a yet unidentified role differing from all functions previously attributed to the legume lectins. Therefore, it seemed worthwhile to study some basic physiological aspects of the newly discovered ground ivy lectin to find clues about the role of this lectin and to establish possible functional relationships—or the absence thereof—to the previously studied classical legume lectins.
This paper gives an overview of the occurrence of Gleheda in a population of ground ivy clones and the temporal and spatial regulation of the expression of the lectin and presents evidence that Gleheda is well different from all previously described legume lectins. Gleheda is not only unique for what concerns the extremely high expression level in certain clones but is also the first documented example of a protein that is predominantly expressed in a single layer of palisade parenchyma cells. In addition, Gleheda exhibits insecticidal activity toward larvae of the Colorado potato beetle (Leptinotarsa decemlineata), indicating that the lectin may be involved in plant defense against insects.
RESULTS
Evidence for Dramatic Differences in the Gleheda Content between Individual Ground Ivy Clones
A preliminary screening revealed that some ground ivy plants exhibit a reasonably high agglutination activity, whereas others are apparently completely devoid of lectin. To corroborate the obvious differences in lectin content between individual plants, a more extended screening was set up. Ground ivy is a perennial weed with creeping stems that root at the nodes. Flowers develop only during spring on erect inflorescences, but the creeping offshoots keep growing throughout the entire growing season. As a result, ground ivy often forms a dense, rapidly expanding mat of vegetation. Although many patches of ground ivy consist of a single clone, others are a composite of multiple individuals. To ensure that individual plants were sampled, single offshoots were collected and analyzed. A total number of 41 ground ivy clones were sampled from six different locations (Table I). To avoid multiple sampling of a single genotype, plants were collected from patches separated from each other by at least 50 m. Because the lectin content of the leaves increases as a function of age (see below) care was taken to use fully expanded mature leaves of a comparable age for the preparation of the extracts used for the estimation of the lectin content.
Table I.
Lectin content in the leaves of different G. hederacea clones from six locations
Samples were collected in Eeghenhoven forest (EF 1-8), on KULeuven campus Arenberg (CA 1 and 2), on KULeuven Campus Gasthuisberg (GH 1), in Langdorp (Aarschot; LD 1-7), in Heverlee forest (Zoete waters; Vaalbeek; HF 1-11), and in Meerdaal forest (Mollendaal; MD 1-12)
| Clone No. | Total Leaf Lectin Content | Total Protein Represented by the Lectin | Clone No. | Total Leaf Lectin Content | Total Protein Represented by the Lectin |
|---|---|---|---|---|---|
| μg g-1 fresh wt | % | μg g-1 fresh wt | % | ||
| CA 1 | <0.5a | <0.01 | EF 1 | 135 | 6.4 |
| CA 2 | 270 | 3.3 | EF 2 | 270 | 6.4 |
| GH 1 | <0.5a | <0.01 | EF 3 | 810 | 13.0 |
| LD 1 | 270 | 5.0 | EF 4 | 540 | 8.4 |
| LD 2 | 405 | 3.4 | EF 5 | 2,700 | 32.5 |
| LD 3 | 202 | 2.6 | EF 6 | 540 | 5.4 |
| LD 4 | 0.9 | 0.01 | EF 7 | 945 | 21.3 |
| LD 5 | <0.5a | <0.01 | EF 8 | 135 | 2.6 |
| LD 6 | <0.5a | <0.01 | MD 1 | 540 | 11.1 |
| LD 7 | 0.9 | 0.01 | MD 2 | 540 | 6.8 |
| HF 1 | 202 | 4.8 | MD 3 | 405 | 4.3 |
| HF 2 | 2,550 | 35.7 | MD 4 | 405 | 11.9 |
| HF 3 | 102 | 8.9 | MD 5 | 202 | 2.7 |
| HF 4 | 405 | 5.0 | MD 6 | 540 | 15.2 |
| HF 5 | 54 | 0.6 | MD 7 | 125 | 1.6 |
| HF 6 | 405 | 4.5 | MD 8 | 2,000 | 30.6 |
| HF 7 | 54 | 0.4 | MD 9 | 8 | 0.3 |
| HF 8 | 54 | 0.5 | MD 10 | 250 | 3.7 |
| HF 9 | 1,020 | 11.2 | MD 11 | 405 | 7.4 |
| HF 10 | 135 | 3.3 | MD 12 | 405 | 11.9 |
| HF 11 | <0.5a | <0.01 |
The agglutination test does not allow detecting lectin concentrations below a threshold value of 0.5 μg g-1 fresh wt.
Semiquantitative agglutination assays revealed dramatic differences between the lectin levels of the individual clones. Some plants contained more than 2.5 mg lectin g–1 leaf tissue (fresh weight), whereas in others, the threshold level for detection (0.5 μg g–1) was not surpassed (Table I). When calculated on a protein basis, Gleheda represented more than one-third of the total protein in some plants, whereas the lectin accounted for less than 0.01% of the total protein in others. An overview of the data shown in Table I indicates that Gleheda contributes for <0.01%, 0.01% to 0.1%, 0.1% to 1%, 1% to 10%, and >10% of the total soluble leaf protein in 5, 2, 4, 20, and 10 plants, respectively.
The apparent high incidence of lectin-negative ground ivy clones raised the question of the sensitivity of the detection method. Taking into consideration the minimal concentration required for the agglutination of trypsin-treated rabbit erythrocytes (0.1 μg mL–1), the level of detection in a crude extract is approximately 0.5 μg mL–1 when tested according to the procedure described in “Materials and Methods” (i.e. in a reaction mixture consisting of 1 volume of extract and 4 volumes of a 1% [v/v] suspension of red blood cells). To lower the level of detection, the extracts that yielded negative results in the standard assay were dialyzed against 0.5 m ammonium sulfate and assayed by mixing 45 μL with 5 μL of a 20% (v/v) suspension of red blood cells (which allowed detection of Gleheda levels of 0.1 μg mL–1 in the extracts, provided that the extracts are dialyzed and incubation lasts for at least 6 h). Taking into consideration that the extracts were made by grinding the leaf tissue in 4 volumes of buffer, the overall detection limit corresponded to roughly 0.5 μg Gleheda g–1 leaf tissue (on a fresh weight basis). According to the results summarized in Table I, the concentration of Gleheda remained below this level of detection in five clones. Attempts to use serological techniques to reduce the detection level were unsuccessful because western-blot analysis of extracts was less sensitive than the agglutination assays and the specificity of an ELISA could not be guaranteed at low concentrations of the lectin. In an alternative approach, 500-g samples of leaves of two lectin-negative clones (CA 1 and LD 5) were extracted and processed as described for the large scale preparation of Gleheda. The fractions (25 mL in total) desorbed from the column (2 × 2.6 cm; approximately 10-mL bed volume) of Gal-Sepharose 4B after the first affinity chromatography step were checked for the presence of Gleheda. No agglutination activity could be detected, indicating that the affinity-purified fractions contained <0.1 μg lectin mL–1, which implies that the total lectin content of the leaves was less than 2.5 μg 500 g–1 (or <0.005 μg g–1 fresh weight).
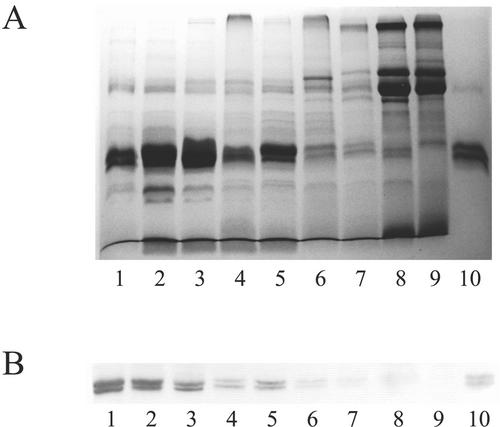
To check whether the extremely high agglutination activities of the leaf extracts from some plants were really due to a correspondingly high level of Gleheda, the extracts were also analyzed by SDS-PAGE and western blot. As shown in Figure 1A, the intensity of the (Coomassie Blue-stained) polypeptides corresponding to the 28- and 26-kD Gleheda subunits was in good accordance with the results of the semiquantitative agglutination assays. Two prominent bands comigrating with the 28- and 26-kD Gleheda subunits can be distinguished in the lanes loaded with extracts from plants with a high agglutination activity (lanes 1–3). Both polypeptide bands are far less prominent (although still distinguishable) in the extracts from plants with a lower agglutination activity (lanes 4–6) and are apparently absent in the lanes loaded with extracts from the nonagglutinating clones. Western-blot analysis of a comparable gel with antibodies raised against Gleheda confirmed that the polypeptides comigrating with the 28- and 26-kD Gleheda subunits correspond to the respective lectin subunits (Fig. 1B). Moreover, because direct sequencing of the 28- and 26-kD polypeptides present in the extract from plant EF 5 (corresponding to lane 1 of Fig. 1, A and B) yielded a single sequence (KTTHF AVPPA LTFQG DAFDP NDTSF IRLT) identical to the N terminus of purified Gleheda, there is no doubt that Gleheda is the most abundant protein in the leaves of some ground ivy clones. Densitometric analysis of the gel shown in Figure 1A indicated that the 28- and 26-kD Gleheda subunits together account for approximately 35% of the total protein in lanes 1 to 3, which is in good accordance with the values calculated on the basis of the results of the semiquantitative agglutination assays (shown in Table I).
Figure 1.
SDS-PAGE and western-blot analysis of crude extracts from leaves of different ground ivy clones. A, Crude extracts from leaves of clones EF 5, HF 2, MD 8, HF 9, MD 1, MD 10, MD 7, LD 4, and CA 1 were loaded in lanes 1 through 9, respectively. Lane 10 was loaded with 2 μg of purified Gleheda. B, Western-blot analysis with anti-Gleheda antibody. Samples were loaded as in A except that only 0.2 μg of purified Gleheda was loaded in lane 10. All samples were reduced with 2% (v/v) β-mercaptoethanol.
To corroborate whether the apparent absence of Gleheda in lectin-negative plants was possibly due to some unknown environmental and/or biological factors, an apparent lectin-negative clone (CA 1) growing in its natural habitat was regularly checked for the presence of lectin. In addition, clone CA 1 was also grown in the greenhouse for 6 months and was regularly checked for the possible presence of lectin. No lectin activity could be detected at any time. Similar analyses of a high-lectin clone (EF 3) growing under the same conditions (both in the field and in the greenhouse) consistently yielded a very high agglutinating activity. Although not conclusive, these observations strongly indicate that the expression level of Gleheda is genetically determined.
The Lectin Concentration and Content Changes as a Function of Leaf Age
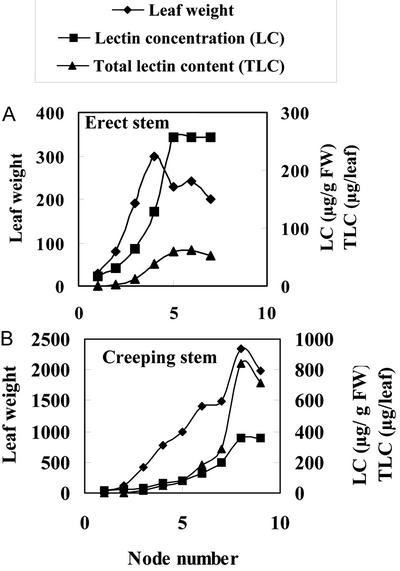
Preliminary experiments indicated that extracts from old leaves exhibited a much stronger agglutination activity than extracts from young leaves of the same plant. To check whether there is a possible relationship between leaf development/age and lectin concentration/content, all pairs of leaves from a single offshoot were collected separately and extracted, and their agglutinating activity was determined. For this experiment, both an erect (flowering) stem and a (vegetatively growing) creeping stem from clone EF 3 were used. As shown in Figure 2, the lectin concentration strongly increases during early leaf formation/maturation. Starting from the distal end (youngest leaves) to the proximal end (older leaves), the lectin concentration increases approximately 10-fold within the first four and six pairs of leaves of the erect and creeping stem, respectively. Thereafter, the increase becomes less dramatic and eventually comes to a halt. When the total lectin content is considered, the increase is even more dramatic. Fully expanded leaves of the erect flowering stem contained approximately 100 times more lectin than the youngest leaves, and in leaves of the creeping stem, the total lectin content increased more than 2,000-fold. To check whether the observed increase in agglutinating activity was really due to a corresponding increase in Gleheda concentration, extracts from the different pairs of leaves were also analyzed by SDS-PAGE and western blot. As shown in Figure 3, the intensity of the polypeptides reacting with antibodies against Gleheda dramatically increases from the distal to the proximal leaves. It can be concluded therefore that Gleheda is present in relatively small quantities in the youngest leaves but accumulates very rapidly during early leaf formation and maturation. Once the leaves are fully expanded (which takes approximately 20–25 d), no further accumulation of the lectin occurs. It should be emphasized here that the maximal lectin content is reached long before the onset of senescence. Ground ivy leaves have a long lifetime that spans almost the entire growth season.
Figure 2.
Lectin concentration and total lectin content in different leaves from single offshoots of a ground ivy plant (clone EF 3). A, Leaves from an erect (flowering) stem; B, leaves from a creeping stem. Leaf numbers refer to the number of the node on which the pair of leaves were attached (starting from the distal end toward the proximal end). The lectin concentration (micrograms per gram fresh weight) was determined by agglutination assays; the total lectin content (micrograms per leaf) is the product of the lectin concentration and leaf weight.
Figure 3.
Western-blot analysis of crude extracts from different leaves of a single offshoot from clone EF 3. A, Extracts from leaf pairs 1 to 7 from an erect (flowering) stem were loaded in lanes 2 to 8, respectively. Lane 1 was loaded with 0.2 μg of purified Gleheda. B, Extracts from leaf pairs 1 to 9 from a (vegetatively growing) creeping offshoot were loaded in lanes 2 through 10, respectively. Lane 1 was loaded with 0.2 μg of purified Gleheda. All samples were reduced with 2% (v/v) β-mercaptoethanol. The oldest leaves of both the erect and creeping stem were approximately 25 d old.
The final lectin concentration in leaves from erect and creeping stems is comparable. However, due to their much larger size, leaves from creeping stems contain approximately 10 times more lectin compared with leaves from the erect stems.
Gleheda Can Only Be Detected in Leaves and Calyces
Gleheda was originally discovered and isolated from leaves. To address the question of the possible tissue-specific expression of Gleheda, extracts were made from leaves, petioles, stems, roots, calyces, petals, and ovary tissues from a single ground ivy plant (EF 3) and were tested for the presence of lectin by agglutination assays and western-blot analysis. Agglutination assays yielded positive results only for extracts from leaves and calyces. No activity could be detected in the extracts from the other tissues. Western-blot analysis of the extracts from the different tissues confirmed that Gleheda occurs only in leaves and calyces (data not shown).
Gleheda Is Not Induced by Phytohormones, Wounding, Insect Feeding, or Senescence
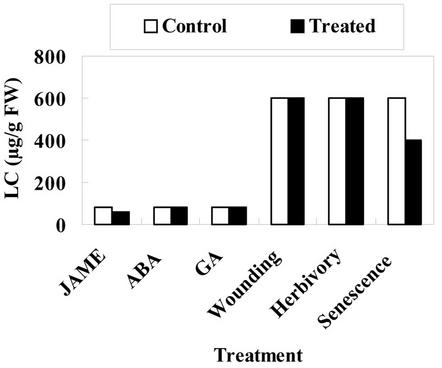
To check the possible induction of Gleheda in a normally lectin-negative clone, isolated leaves were treated with the phytohormones methyl jasmonate, abscisic acid, and GA3. No lectin could be induced by these phytohormones in the lectin-negative clone CA 1. Wounding of leaves of intact plants of clone CA 1 did not result in the expression of detectable amounts of Gleheda neither in the wounded leaves nor in other leaves of the plant. Similarly, no agglutination activity could be detected in intact and insect-affected leaves of CA 1 and LD 5 plants grown in the field. Extensive checking of senescing leaves sampled from clones CA 1 and LD 5 at the end of the growing season (i.e. in October) yielded no positive agglutination results, suggesting that senescence does not induce the expression of Gleheda in lectin-negative plants.
Similar experiments were done with leaves and intact plants of the lectin-positive clone EF 3. As far as could be concluded from semiquantitative agglutination assays, neither the phytohormones nor wounding or insect attack provoked an increased expression of Gleheda. Moreover, senescence also was not accompanied with an increased level of Gleheda (Fig. 4).
Figure 4.
Effect of phytohormones, wounding, insect herbivory, and senescence on the lectin concentration in leaves from ground ivy plant clone EF 3. Developing leaves (located at the fifth internode) were excised from greenhouse-grown plants and treated in vitro with jasmonate methyl ester (JAME), abscisic acid, or GA3. Wounding, herbivory, and senescence experiments were done with fully mature leaves attached to plants grown in their natural habitat. The lectin content of lectin concentration (micrograms per gram fresh weight) was determined by agglutination assays. Note that the lower lectin content of the in vitro assayed leaves is due to their early developmental stage.
Gleheda Is Predominantly Located in a Single Layer of Palisade Parenchyma Cells
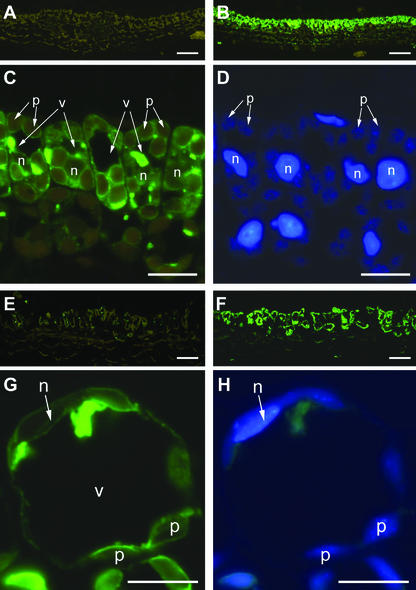
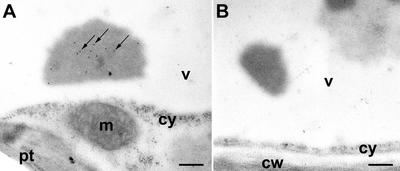
Because at present, no data have been published on the cellular and subcellular location of a lectin in a species of the family Lamiaceae, it seemed worthwhile to localize Gleheda by immunocytochemical techniques. In addition, it was expected that immunolocalization studies could provide additional information about the dramatic increase in lectin content during leaf development. Both young and mature leaves were investigated to analyze possible differences in tissue and cell specificity of the occurrence of Gleheda in high-lectin and no-lectin plants. Staining of cross sections from young leaves (diameter, <1 cm; corresponding to node 2) with anti-Gleheda antibodies yielded no signal for the no-lectin plant (CA 1; Fig. 5A) but resulted in a heavy labeling of the palisade parenchyma of the high-lectin clone (EF 5; Fig. 5B), suggesting that Gleheda is confined to a single cell layer. A closer examination of the high-magnification micrographs of the palisade cells indicates that the cytoplasm (around the plastids) exhibits some label (Fig. 5C). However, the label is predominantly associated with “inclusions” that are located within the vacuole near to the cytoplasm and possibly correspond to a sort of protein bodies (Fig. 5C). Microscopic analysis of cross sections from older leaves (diameter, 5 cm; corresponding to node 5) yielded similar results as for young leaves for what concerns the cellular distribution of Gleheda. No or virtually no lectin could be detected in leaves from the no-lectin plant, whereas a very strong labeling was observed in the palisade parenchyma cells of the high-lectin clone (Fig. 5, E versus F). Examination of the high-magnification pictures further indicates that in the older leaves, Gleheda is predominantly located in the vacuole and is only to a small extent associated with the cytoplasm (Fig. 5G). This apparent vacuolar location of Gleheda is in good agreement with the results of the molecular cloning because the presence of a signal peptide in the primary translation product suggested that Gleheda is targeted to the secretory pathway (Wang et al., 2003). To refine the results obtained by light microscopy, the intracellular location of Gleheda was also studied by electron microscopy. According to the results of these studies, Gleheda is confined to the vacuoles where the lectin is apparently concentrated in electron-dense inclusions (Fig. 6). These inclusions were specifically labeled by the anti-Gleheda antibodies (Fig. 6A).
Figure 5.
Immunofluorescence labeling of Gleheda in leaves of ground ivy. Developing leaves (A–D) and fully developed leaves (E–H) were used for embedding. Cross sections of leaves of a no-lectin (clone CA 1; A and E) and a highlectin plant (clone EF 5; B–D and F–H) were probed with anti-Gleheda-antibody, followed by a labeling with fluorescence-labeled secondary antibody. Labeling in the sections of the low-lectin clone exhibits only the yellow-brown autofluorescence of chloroplasts (A and E), whereas the strong green fluorescence label within the palisade parenchyma in the sections of the high-lectin clone (B and F) indicates the location of the lectin. Higher magnifications of B and F are shown in C and G, respectively, to visualize the subcellular location of Gleheda. Note the high amount of fluorescence-labeled Gleheda within the vacuoles (v). DNA-containing organelles (n, nucleus; p, plastids) were identified by concomitant 4,6-diamidino-2-phenylindole (DAPI) staining (shown in D and H). Bars = 50 μm in A, B, E, and F; and 10 μm in C, D, G, and H, respectively.
Figure 6.
Immunogold labeling localizes Gleheda in vacuolar located inclusions. A, Labeling is exclusively present in electron dense inclusions located within the vacuole (arrows). B, Controls performed by omitting the primary antibody did not exhibit label. cw, Cell wall; cy, cytoplasm; m, mitochondrion; pt, chloroplast; v, vacuole. Bars = 250 nm in both micrographs.
Gleheda Inhibits the Feeding and Growth of Colorado Potato Beetle
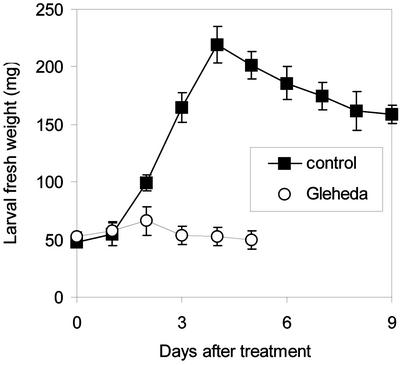
Oral feeding of potato (Solanum tuberosum) leaves dipped in a 2% (w/v) solution of Gleheda in distilled water caused dramatic effects on the growth and survival of the potato beetle larvae. Soon after the onset of the experiment, larvae feeding on Gleheda-treated leaves showed a dramatic inhibition of feeding and weight gain (Fig. 7). None of the Gleheda-fed larvae reached the pupal instar, indicating that the lectin caused complete mortality at the dose administered in this experiment.
Figure 7.
Effect of Gleheda on the growth of last-instar (fourth) larvae of potato beetle. Selected larvae were transferred into petri dishes and fed on control leaves or leaves dipped in a 2% (w/v) solution of Gleheda. Data are expressed as fresh weight means (± se); n = 20.
Gleheda Exhibits a Strong Preference for Tn Cells
It was demonstrated previously that Gleheda is a Gal/N-acetylgalactosamine (GalNAc)-binding lectin with a slight preference for type A over type B human erythrocytes (Wang et al., 2003). Because the overall specificity of Gleheda as well as its molecular structure are reminiscent of that of the lectins from Salvia sclarea (Piller et al., 1986; Medeiros et al., 2000) and Clerodendrum trichotomum (Kitagaki-Ogawa et al., 1986), which both have a high affinity for GalNAc α-linked to Ser or Thr (i.e. Tn antigen), the blood group specificity of Gleheda toward normal and polyagglutinable human red blood cells was studied in some detail. As shown in Table II, Gleheda reacted most strongly, although not exclusively, with Tn cells. Reactions with native non-polyagglutinable red cells, requiring a minimum of 5.3 μg mL–1 for agglutination, were removed or reduced by papain treatment of the cells. This is consistent with a preference for GalNAc α-O-linked to Ser or Thr, because almost all of these determinants are removed with the papain-labile glycophorins. Enhanced reactions with some Cad+ and T polyagglutinable cells, with a minimum concentration of 0.01 to 0.16 μg mL–1 required for agglutination, also fit this specificity. Cad+ cells have more MN glycophorins than normal cells, which may partly explain the higher titer of the lectin against these cells than normal cells. It has also been suggested (Issitt and Anstee, 1998) that cells from Cad+ individuals represent a sliding scale of antigen level, with Cad1 being the strongest and Cad4 weakest. However, the strong enhancement of the reaction with two of three Cad+ cells, requiring a minimum of 0.16 to 1.3 μg mL–1, suggests that the additional GalNAc leads to a conformational change in the pentasaccharide with increased accessibility to the internal GalNAc α-O-linked to Ser/Thr.
Table II.
Agglutination activity of Gleheda towards normal and polyagglutinable human erythrocytes
| Cells
|
Agglutination Activity
|
Significant Structures Associated with Blood Group
|
|
|---|---|---|---|
| MCAa | RAb | ||
| μg mL-1 | |||
| O | 5.3 | 1 | Fuc α(1,2) Gal β(1,4) GlcNAc β(1,3) Gal-R |
| O papain treated | >168 | <0.03 | Fuc α(1,2) Gal β(1,4) GlcNAc β(1,3) Gal-R |
| A1 | 5.3 | 1 | GalNAc α(1,3) [Fuc α(1,2)] Gal β(1,4) GlcNAc β(1,3) Gal-R |
| GalNAc α(1,3) [Fuc α(1,2)] Gal β(1,3) GalNAc α(1,3) [Fuc α(1,2)]Gal-β(1,4) GlcNAc-R | |||
| A2 | 5.3 | 1 | GalNAc α(1,3) [Fuc α(1,2)] Gal β(1,4) GlcNAc β(1,3) Gal-R |
| B | 10.6 | 0.5 | Gal α(1,3) [Fuc α(1,2)] Gal β(1,4) GlcNAc β(1,3) Gal-R |
| Tn (n = 4) | 0.01-0.16 | 32-512 | GalNAc α-Ser/Thr |
| Tn papain treated | 2.6 | 2 | Greatly reduced amounts of GalNAc α-Ser/Thr |
| Cad (n = 3) | 0.16-10.6 | 0.5-32 | Neu5Ac α(2,3) [GalNAc β(1,4)] Gal β(1,3) [Neu5Ac α(2,6)]GalNAc α-Ser/Thr |
| Cad neuraminidase (n = 2) | 0.66-1.3 | 4-8 | GalNAc β(1,4) Gal β(1,3) GalNAc α-Ser/Thr |
| Sd(a++) (n = 7) | 10.6-168 | 0.03-0.5 | Neu5Ac α(2,3) [GalNAc β(1,4)]Gal β(1,4) GlcNAc β(1,3)Gal |
| T | 0.16 | 32 | Gal β(1,3) GalNAc α-Ser/Thr |
| T papain treated | >168 | <0.03 | Greatly reduced amounts of Gal β(1,3) GalNAc α-Ser/Thr |
| T + Tk | 2.6 | 2 | Gal β(1,3) GalNAc α-Ser/Thr & GlcNAc β(1,3) Gal-R |
| Tk (O, enzyme modified) | > 168 | <0.03 | GlcNAc β(1,3) Gal-R |
| Th | 1.3 | 4 | Neu5Ac α(2,3) Gal β(1,3) [Neu5Ac α(2,6)]GalNAc α-Ser/Thr (Partially reduced sialic acid) |
| Acquired B (n = 2) | 5.3-42.4 | 0.12-1 | GalN α(1,3) [Fuc α(1,2)] Gal β(1,4) GlcNAc β(1,3) Gal-R |
Minimal concentration required for agglutination. b Relative agglutinability” of cells (compared with that of type O cells).
Gleheda Is Not Cytotoxic toward Human and Murine Cells and Exhibits No Antifungal Activity
To check whether the observed insecticidal activity of Gleheda could be due to a general cytotoxic activity of the lectin, two human and two murine cell lines were challenged in vitro with increasing concentrations of purified Gleheda. L1210 and CEM cells were not affected by Gleheda at concentrations below 200 μg mL–1. For FM3A and Molt4CI8 cells, the concentration required to obtain 50% inhibition was 155 (±37) and 188 (±16) μg mL–1, respectively. These experiments indicate that Gleheda exhibits little or no general cytotoxic activity toward human and murine cells. To assess the possible antiretroviral activity of Gleheda, the inhibition of the infection of CEM cells by HIV-1 and HIV-2 was evaluated. No effect was observed at concentrations up to 100 μg mL–1, indicating that Gleheda is devoid of antiretroviral activity.
Gleheda also failed to affect the in vitro growth and development of Neurospora crassa and Botrytis cinerea, suggesting that the lectin does not act as an antifungal protein (data not shown).
DISCUSSION
The present study of the physiology and biological activities indicates that Gleheda differs from all previously described classical legume lectins for what concerns its “general biology” and in several aspects, can be considered a unique lectin. A first remarkable observation is the apparent huge difference in lectin content between individual ground ivy plants and the high incidence of plants without any detectable lectin activity. In this respect, ground ivy resembles some legume species in which lectin-negative genotypes have been identified. It should be mentioned, however, that the lectin-negative genotypes identified in collections of soybean (Glycine max; Pull et al., 1978; Goldberg et al., 1983; Vodkin and Raikhel, 1986), common bean (Phaseolus vulgaris; Staswick and Chrispeels, 1984), and other legumes (Pull et al., 1978) were only tested for the presence of the seed lectin(s), and therefore are not necessarily devoid of lectins in their vegetative tissues. On the contrary, some seed lectin-deficient soybean varieties have been shown to express a lectin in roots (Vodkin and Raikhel, 1986). A second striking observation is the extremely high expression level of Gleheda in leaves of some ground ivy clones (up to 35% of the total soluble protein), which is far superior to that of any other documented leaf lectin. Most other leaf lectins are minor proteins representing less than 1% or maximally a few percent of the total leaf protein (Kitagaki-Ogawa et al., 1986; Hankins et al., 1987; Van Damme and Peumans, 1990; Smeets et al., 1997). A third particularity concerns the exclusive expression of Gleheda in leaves and to a lesser extent in calyces. In general, plant lectins that are found in leaves occur in all vegetative tissues (e.g. petioles, roots, and petals) and are usually far more prominent in vegetative storage tissues like bark, tubers, rhizomes, and bulbs (Van Damme et al., 1998). A fourth striking observation concerns the regulation of the expression of Gleheda in leaves, which is characterized by a dramatic increase during leaf expansion/maturation. No comparable increase in lectin content has been reported for any other plant species. In general, leaf lectins accumulate only during the earliest phases of leaf development. Thereafter, the total lectin content remains more or less constant but due to the increase of the leaf size the concentration, progressively decreases as a result of the dilution of the lectin in the leaf. The fifth, and probably the most spectacular feature of Gleheda, is the predominant location of the lectin in a single layer of palisade parenchyma cells. To the best of our knowledge, such a specific location pattern has not been reported for any plant lectin (or plant protein in general). Detailed information about the distribution of a typical legume lectin over the different leaf cells has been reported for the so-called soybean vegetative lectin. This lectin, which is related to but not identical with the soybean seed lectin, occurs in leaves, stems, petioles, and cotyledons of seedlings but not in seeds (Spilatro et al., 1996). Within the leaves, the soybean vegetative lectin was found in bundle sheath and paraveinal mesophyll cells but could not be detected in the palisade mesophyll or spongy mesophyll. It is evident, therefore, that the cellular location of Gleheda in the leaves is completely different from that of the vegetative soybean lectin. In addition to the exclusive location in the palisade parenchyma cells, the apparent concentration of Gleheda in inclusion bodies within the vacuole also is rather unusual because other presumed vacuolar leaf lectins are more or less evenly distributed over the protein-storage vacuoles, as has been demonstrated for the leaf lectin of Sophora japonica (Herman et al., 1988) and Dolichos biflorus (Bunker and Etzler, 1994). These observations leave no doubt that the temporal and spatial regulation of the expression and the cellular and subcellular location of Gleheda differ from that of the classical legume lectins and, as a matter of fact, of all other known plant lectins. It is unlikely, therefore, that the ground ivy lectin fulfills the same function as its orthologs in legume species. Accordingly, the search for the physiological role of Gleheda can hardly be supported by extrapolations from functional research with other legume lectins or members from unrelated lectin families, but it should be focused on the specific expression pattern of the ground ivy lectin and its biological activities.
Although only preliminary, the results of the insect feeding trials may give an important clue to the unraveling of the role of Gleheda because they demonstrate that the lectin concentration in the leaves of most ground ivy clones is sufficiently high to exert a noxious effect on at least some insects. At present, the mode of action of Gleheda on the herbivorous pest insect potato beetle is still unknown, but it is unlikely that the observed insecticidal activity can be ascribed to an aspecific cytotoxicity because the lectin does not affect the viability of human and murine cells in vitro. Most probably, the observed adverse effects on the potato beetle larvae are somehow related to the pronounced specificity of Gleheda toward the Tn antigen (α-GalNAc O-linked to Ser or Thr residues from polypeptide chains). This Tn antigen, which is formed as a first step in mucin-type O-glycosylation (through the transfer of GalNAc to Ser and Thr residues on proteins by a UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase) corresponds to the simplest mucin-type O-glycans. Possibly, some insects express Tn structures on the mucins or other O-glycosylated glycoproteins in their digestive tract that act as specific receptors for a Tn antigen-binding dietary lectin. If such a lectin-glycan interaction provokes deleterious effects on the cells carrying the Tn structures, the digestive system of the insect may be impaired, which in turn may lead to a more or less general toxic effect of the lectin. Irrespective of the exact mode of action, the observed noxious effects suggest that Gleheda may be exploited as a biological insecticide to control the Colorado potato beetle, which is not only a major pest insect of potato and eggplant (Solanum melongena) crops in Europe, Russia, and northern America, but also exhibits resistance against classical and novel groups of insecticides (Oerke et al., 1994).
Although Gleheda is certainly not the first member of the family of legume lectins and related proteins that is found to exhibit anti-insect activity, it was not obvious to assume a priori that Gleheda was an insecticidal protein. There have been several reports that some legume lectins inhibit the growth and/or development of some insects and in some cases even exert lethal effects. However, there are several caveats. First, in some instances, the presumed anti-insect activity was not due to the lectin but to contaminating insecticidal proteins (e.g. it has been shown that the toxicity of phytohemagglutinin preparations toward insects was due to contaminating α-amylase inhibitor; Murdock et al., 1990). Second, in some cases, the doses required for activity were extremely high, which indicates that the observed effects of the lectins are most probably aspecific. Third, there are a lot of contradictory data published. Fourth, for some lectins the anti-insect activity has been described only once, which implies that the results remain to be confirmed. To the best of our knowledge, firm evidence for genuine anti-insect activity has been provided for only a few legume lectins. Activity against chewing insects has been demonstrated for the Bauhinia purpurea seed lectin (Czapla and Lang, 1990), the chitin-binding lectin from Griffonia simplicifolia (called GS-II; Zhu et al., 1996), the peanut (Arachis hypogaea) seed lectin (Habibi et al., 1993), and the yam bean (Sphenostylis stenocarpa) seed lectin (Machuka et al., 2000). For sucking insects, inhibitory activity has only been reported for the Man-specific lectins from jack bean (Canavalia ensiformis), lentil (Lens culinaris), and pea (Pisum sativum; Rahbé et al., 1995). Even if this list can be extended with some other legume lectins, the total number of putative anti-insect legume lectins (<10) still represents only a minor fraction of the currently known genuine legume lectins (>200). It is also important to emphasize that the most potent anti-insect proteins that have been identified within the family of the legume lectins and related proteins (namely the α-amylase inhibitors and the arcelins) are not genuine lectins but are proteins that are devoid of carbohydrate-binding activity and hence are by definition not lectins (Cardona et al., 1989; Shade et al., 1994).
Evidently, after it turned out that Gleheda was capable of killing larvae of the Colorado potato beetle, it became clear that there might be some analogy to the insecticidal or entomotoxic properties of some legume lectins. However, to the best of our knowledge, no legume lectin has ever been reported to exert a toxic effect on this insect, which indicates that Gleheda is quite different from the legume lectins with respect to its entomotoxic properties. Most probably, this difference is intimately linked to the differences in carbohydrate-binding specificity between Gleheda and the legume lectins. It should be emphasized that the extremely high specificity toward the Tn antigen is unique for Gleheda and has not been observed for any legume lectin. As already mentioned above, the high affinity for the Tn antigen may explain the potent toxicity of Gleheda toward insects. This structurally simple O-linked glycan is quite common in lower animals but is normally not present in higher vertebrates because higher organisms possess glycosyltransfereases that extend the Tn structure with additional sugar residues (e.g. Gal and sialic acid). For example, in humans, the Tn antigen is diagnostic for a genetic disorder or a pathological condition. On the basis of the particular specificity, it is tempting to speculate that Gleheda (and the other Lamiaceae lectins) are directed against lower animals (insects and other invertebrates), whereas the legume lectins, which preferentially bind to complex N- and O-glycans that typically occur in higher animals, are believed to play a role in plant defense against vertebrate herbivores (Peumans and Van Damme, 1995).
According to the results of the induction experiments, Gleheda is not an inducible protein but is constitutively expressed. However, the level of expression strongly differs among individual genotypes. Southern-blot and PCR experiments indicated that lectin-negative clones do not possess the Gleheda gene (data not shown). Although the results from these experiments have to be interpreted with some care, they may explain why no Gleheda can be detected in the lectin-negative clones. The apparent absence of the Gleheda gene in some individuals has important consequences for what concerns the possible defensive role of the lectin. It is evident that Gleheda is not essential for the survival of the plant because a wild population comprises a reasonable fraction of lectin-negative individuals. As far as can be concluded from a visual inspection in the field, there is no apparent phenotypical difference between lectin-negative and -positive clones, and both types of clones show a similar insect damage. However, it is risky to draw conclusions from such observations because there is always a possibility that the target insect(s) (or other pests) were not present at the time of the observations. In this respect, we would like to draw the attention to the analogy of the arcelins in bean. Although there is firm experimental evidence that arcelins act as anti-insect proteins in planta, it is also well known that not all bean accessions express these proteins, which implies that the arcelins are not essential for survival of these lines (Mirkov et al., 1994). Accordingly, arcelins are not considered the anti-insect protein of beans but rather one particular type of anti-insect compound. The same applies to Gleheda. One can reasonably expect that ground ivy developed a wide array of defensive compounds. In this respect, it worth mentioning that leaves of ground ivy contain not only toxic proteins but also a wide range of toxic secondary metabolites (which renders the plant poisonous to higher animals).
The apparent analogy to the arcelins implies that there is no reason to preclude an in planta defensive role of Gleheda. On the contrary, this observation cannot but stimulate further research aimed to corroborate the role of this novel insecticidal lectin in ground ivy itself and exploit its possible use as an insect resistance factor in transgenic plants.
MATERIALS AND METHODS
Plant Materials
Collection of Individual Ground Ivy (Glechoma hederacea) Clones
A total of 41 ground ivy plants were sampled at random from six different locations (see legend to Table I). Single offshoots were removed. Leaves of comparable age were collected for estimation of total protein and lectin content. The rest of the offshoot was planted in pots in pot soil and was transferred to the greenhouse to maintain the different genotypes for further research.
Mass Propagation of a High-Lectin Clone
Clone EF 3 was propagated on a large scale to produce the starting material for the purification of large quantities of Gleheda. After an initial propagation in a pot for approximately 4 weeks, offshoots were removed and cut into small pieces (corresponding to two internodes). The fragmented offshoots were transferred onto a mat soaked in a nutrient solution (standard medium) and covered with a film of transparent plastic to stimulate root formation. As soon as the newly formed roots had penetrated the mat (after approximately 3 weeks), the plastic film was removed and the plants were grown in hydroponics in a greenhouse under a day/night regime of 16/8 h. The temperature was kept at 25°C and 18°C during the light and night period, respectively, and the relative humidity was maintained at 55%. After 3 months, leaves were collected, and the plants were allowed to regrow for another 3 months for a second crop of leaves.
Large-Scale Isolation of Gleheda
Freshly harvested leaves (5 kg) were extracted in 20 L of 20 mm unbuffered 1,3-diaminopropane containing 0.01% (w/v) thiourea using a Waring blender. The homogenate was filtered through glass wool, solid CaCl2 was added to a final concentration of 20 mm, and the extract was centrifuged at 3,000g for 10 min. After adjusting the pH to 7.5 with 1 n H3PO4, the crude extract was kept overnight in the cold room at 2°C and centrifuged (8,000g for 10 min). The resulting supernatant was brought at 1 m ammonium sulfate, centrifuged (8,000g for 10 min), and filtered through filter paper (3MM, Whatman, Beverly, MA). The cleared extract was loaded onto a column (5 × 5 cm; approximately 100-mL bed volume) of Gal-Sepharose 4B equilibrated with 1 m ammonium sulfate. Binding of the lectin was monitored by regular checking of the agglutination activity of the eluate. After loading the extract, the column was washed with 1 m ammonium sulfate until the A280 fell below 0.01 and the bound lectin eluted with 500 mL of 20 mm Tris-HCl (pH 10). The pH of the affinity-purified lectin fraction was adjusted to 7.5 with 1 n acetic acid, solid NaCl was added to a final concentration of 0.2 m, and the solution was centrifuged (8,000g for 10 min). The supernatant was loaded onto a column (20 × 2.6 cm; approximately 100-mL bed volume) of Gal-Sepharose 4B equilibrated with 20 mm Tris-HCl (pH 7.8) containing 0.2 m NaCl. After extensive washing with the same Tris buffer, the bound lectin was eluted with 500 mL of 0.1 m Gal in 0.2 m NaCl. The affinity-purified lectin fraction was brought at 1 m ammonium sulfate and loaded onto a column (10 × 2.6 cm; approximately 50-mL bed volume) of phenyl-Sepharose equilibrated with 1 m ammonium sulfate. After loading the lectin solution, the column was washed with 500 mL of 1 m ammonium sulfate (to remove any traces of Gal), and the lectin was eluted with 75 mL of 20 mm Tris-HCl (pH 10). The lectin solution was dialyzed against water and lyophilized. Using this procedure, approximately 500 mg of Gleheda was obtained from 5 kg of leaves.
Preparation of Crude Extracts
Tissue samples were extracted with mortar and pestle in 4 volumes (v/w) of 20 mm unbuffered 1,3-diaminopropane. The homogenates were transferred to Eppendorf tubes and centrifuged (12,000g for 10 min).
Induction Experiments
Treatment of Excised Leaves with Phytohormones
Leaves of both a lectin-negative clone (CA 1) and a lectin-positive clone (EF 3) grown under greenhouse conditions (in March) were cut with a scalpel blade and transferred to petri dishes (15 cm in diameter) filled with 80 mL of water or test solutions and incubated for 4 d under constant light (100 W m–2). For each treatment, lots of five leaves from the fifth node (starting from the distal end) of five different creeping stems were combined. The five opposite leaves from the same nodes were used as a control (and incubated in water). The test solutions contained 100 μm jasmonate methyl ester, 25 μm abscisic acid, or 25 μm GA3. At the end of the experiment, all leaves of CA 1 were individually assayed for lectin activity by a simplified agglutination assay on microscope glass slides. For clone EF 3, individual leaves were pooled and extracted, and the lectin content was estimated by a semiquantitative agglutination assay. All experiments were done with leaves from plants grown under greenhouse conditions.
Wounding and Insect Herbivory
Wounding experiments were done with leaves attached to CA 1 and EF 3 plants growing in their natural environment. Five fully mature leaves (attached to the plants) from five different nodes were squeezed with a pair of tweezers twice a day for 4 consecutive d. At the end of the experiment, the wounded leaves were collected and assayed for agglutination activity. Untreated leaves from the same nodes were used as a control. For the clone CA 1, all leaves were individually assayed for lectin activity by a simplified agglutination assay on microscope glass slides. For the lectin-positive clone EF 3, the five intact and five damaged leaves were pooled and extracted, and the lectin content was estimated by a semiquantitative agglutination assay. To check the possible effect of insect attack on the Gleheda content, both intact and damaged leaves were sampled from plants grown in their natural environment. For the lectin-negative clones CA 1 and LD 5, 20 intact leaves and 20 damaged leaves were checked for the presence of lectin by a simple agglutination test on a glass slide. For the lectin-positive clone EF 3, the 20 intact and 20 damaged leaves were pooled and extracted, and the lectin content estimated by a semiquantitative agglutination assay. All wounding and insect damage experiments were carried out during early summer (first half of July).
Senescence
To check the possible effect of senescence on the expression of Gleheda, 20 leaves were sampled at random from CA 1 and LD 5 plants growing in their natural habitat at the end of the growing season (i.e. in October) and were individually assayed for lectin activity by a simple agglutination test on a glass slide. A similar experiment was set up with EF 3 plants, but in this case, the leaves were pooled and extracted, and the lectin content was estimated by a semiquantitative agglutination assay (and compared with that of leaves collected in early summer).
Agglutination Assays and Estimation of Lectin Content
Agglutination assays were carried out in small glass tubes or in the wells of 96 U-welled microtiter plates in a final volume of 50 μL containing 40 μL of a 1% (v/v) suspension of trypsin-treated rabbit erythrocytes and 10 μL of extracts or lectin solutions. Agglutination was monitored visually after 1 h of incubation at room temperature. To quantify the lectin activity, crude extracts were serially diluted with 2-fold increments, and the dilution endpoint was determined. The absolute lectin content of the extracts was calculated by comparison with the agglutination activity of a dilution series of a lectin solution with known concentration.
To check a large number of leaf samples for the presence of Gleheda, a simplified detection method was used that is based on an agglutination assay on microscope glass slides. A piece of leaf tissue was squeezed between two glass slides and to the resulting sap a small droplet (50–100 μL) of a 2% (v/v) suspension of trypsin-treated rabbit erythrocytes in 1 m ammonium sulfate was added. The sample was gently shaken, and the agglutination was visually inspected after 10 min of incubation at room temperature.
Analytical Methods
Proteins were separated by SDS-PAGE using 15% (w/v) acrylamide gels, as described by Laemmli (1970). The protein content of the crude extracts was estimated according to the method described by Bradford (1976) using purified Gleheda as a standard.
Western-Blot Analysis
Proteins were separated by SDS-PAGE and electroblotted on an Immobilon P membrane (Millipore, Bedford, MA). Before immunodetection, the free binding sites on the membrane were blocked with 5% (w/v) bovine serum albumin (BSA) in 10 mm Tris, 150 mm NaCl, and 0.1% (v/v) Triton X-100, pH 7.6 (TSB), for 1 h at room temperature. After washing the membrane with TSB for 5 min, the membrane was consecutively treated with primary antibody (overnight incubation at room temperature), goat-anti-rabbit antibody (1 h incubation at room temperature), and peroxidase-anti-peroxidase-complex (1 h incubation at room temperature). After every treatment, the membrane was washed three times with TSB for 5 min. Before the immunodetection, the membrane was washed for 5 min with 0.1 m Tris-HCl (pH 7.6). The peroxidase reaction was carried out in a fresh solution of 0.1 m Tris-HCl (pH 7.6) containing 0.7 mm 3,3′-diaminobenzidine tetrahydrochloride and 0.01% (v/v) H2O2. The reaction was stopped by washing the membrane in distilled water.
Preparation of Monospecific Antibodies
Polyclonal antibodies were raised against Gleheda in a male New Zealand white rabbit. The animal was injected subcutaneously with 1 mg of purified Gleheda dissolved in phosphate-buffered saline (PBS; 1.5 mm KH2PO4, 10 mm Na2HPO4, 3 mm KCl, and 140 mm NaCl, pH 7.4) and emulsified in 1 mL of Freund's complete adjuvant. Four booster injections with 1 mg of Gleheda in 1 mL of PBS were given with 10-d intervals. Ten days after the final injection, blood was collected from an ear marginal vein. After clotting, the crude serum was prepared by centrifugation. Because western-blot analysis of crude extracts from ground ivy demonstrated that the crude antiserum reacted not only with the lectin polypeptides but also with several other proteins, the antiserum was further purified. Affinity chromatography of the crude antiserum on immobilized Gleheda did not markedly improve the specificity of the antiserum. Even after an additional affinity chromatography on immobilized Robinia pseudoacacia lectin (which removes most of antibodies that are directed against N-glycans; Desmyter et al., 2001), the antibody fraction still showed a strong cross-reaction with several proteins from the crude extract. Therefore, the antibody fraction that was not retained on the column of immobilized R. pseudoacacia lectin was dialyzed against 0.1 m NaCl in 20 mm Tris-HCl (pH 8.7) and was loaded onto a column of Q Fast Flow (2 × 1.5 cm; approximately 4-mL bed volume; Amersham Biosciences, Uppsala) equilibrated with the same buffer. After loading, the column was eluted with the same buffer until the A280 fell below 0.01. Western-blot analysis showed that the unretained fraction reacted exclusively with the lectin polypeptides in the crude extracts and hence can be considered monospecific. This fraction of purified monospecific antibodies was used for the immunocytochemical experiments.
Immunocytochemistry
Small pieces of leaves were fixed with 4% (w/v) paraformaldehyde/0.1% (v/v) Triton X-100 in PBS, embedded in polyethylene glycol, and cut as described (Hause et al., 1996). Cross sections (2 μm thick) were immunolabeled by incubation with purified primary antibodies against Gleheda (diluted 1:2,000 in PBS containing 2% [w/v] acetylated BSA and 1 mg mL–1 goat IgG) followed by a goat-anti-rabbit-IgG antibody conjugated with Alexa488 (Molecular Probes, Eugene, OR). After immunolabeling, sections were stained with 0.1 μg mL–1 DAPI for 15 min and mounted in citifluor/glycerol. Control experiments were performed by omitting the primary antibody and revealed no signal. The fluorescence of immunolabeled Gleheda and of DAPI-stained nuclei and plastids was visualized with an epifluorescence microscope (Axioskop, Zeiss, Jena, Germany) using the proper filter combinations. Micrographs were taken by a CCD camera (Sony, Tokyo) and processed through the Adobe Photoshop (Adobe Systems, Mountain View, CA).
For electron microscopy, leaf material was fixed with 3% (w/v) paraformaldehyde/0.2% (v/v) glutaraldehyde in PBS and dehydrated in a graded ethanol series. Ethanol of specimens was substituted by LR White (Polysciences, Warrington, PA). Immunolabeling of ultrathin sections was carried out with purified primary antibodies against Gleheda (diluted 1:500 in PBS containing 1% [w/v] acetylated BSA and 0.1% [v/v] Tween 20) and a goat anti-rabbit IgG conjugated with 10 nm of colloidal gold (Sigma-Aldrich, St. Louis). After immunolabeling, sections were post-stained with uranyl acetate and lead citrate. Sections were visualized with a Zeiss TEM 900 electron microscope.
Serological Reactions of Gleheda toward Polyagglutinable Human Red Blood Cells
The agglutination activity of Gleheda toward normal and polyagglutinable human erythrocytes was estimated by determining the minimal concentration required to agglutinate the different cells. The polyagglutinable cells used were recovered from liquid nitrogen storage, except Tk, which was prepared by endo-β-galactosidase treatment of papain-modified group O red cells (Meichenin et al., 2000). The normal cells used were control cells supplied by Diagnostics Scotland (Edinburgh, UK).
Insect Feeding Bioassay
Purified Gleheda was tested for insecticidal activity against larvae of the Colorado potato beetle (Leptinotarsa decemlineata Say; [Coleoptera; Chrysomelidae]). Newly moulted (0–12 h) last (fourth)-instar larvae were selected from a continuous culture that was maintained under standard conditions (23°C ± 2°C; 65% ± 5% relative humidity; photoperiod, 16-h light and 8-h dark) and provided fresh potato (Solanum tuberosum cv Bintje) foliage ad libitum (Smagghe and Degheele, 1994). Four groups of five larvae were placed in a 9-cm diameter petri dish and provided with fresh potato leaves treated with Gleheda. For treatment with the lectin, freshly harvested leaf discs were dipped for 10 s in a 2% (w/v) solution of Gleheda in distilled water and kept in a fume hood for 15 to 20 min to allow the lectin solution to drain off (so that only a film of the protein remained on the leaves). In the control experiments, leaf discs were dipped in distilled water only. Control and Gleheda-treated leaves were offered ad libitum. At daily intervals, fresh weight gain of larvae was followed, and abnormalities and mortality were scored at the moment of metamorphosis into the pupal instar in the control groups (after 10 d; Smagghe and Degheele, 1994).
Cytotoxicity and Antiviral Activity
Gleheda was evaluated for cytotoxic/cytostatic activity against murine leukemia L1210, murine mammary carcinoma FM3A, human T-lymphocyte Molt 4/clone 8, and human CD4-positive T lymphocytes (CEM cells). Cells were seeded in 96-well microtiter plates at approximately 50,000 to 75,000 cells per 200 μL well in RPMI-1640 cell culture medium in the presence of serial dilutions of Gleheda, the highest concentration tested being 400 μg mL–1. After 2 d (L1210 and FM3A) or 3 d (Molt 4/clone 8 and CEM) incubation at 37°C, cell numbers were determined. Data are expressed as 50% inhibitory concentration or compound concentration required to inhibit cell proliferation by 50%. The methodology for testing the antiviral activity of Gleheda toward human immunodeficiency virus has been described previously (Balzarini et al., 1991).
Assays for Antifungal Activity
In vitro microtiter plate assays (according to Broekaert et al., 1990) were used to check the possible antifungal activity of Gleheda. Each well contained 2-fold serial dilutions of 20 μL of filter-sterilized Gleheda and 80 μL of potato dextrose broth (12 g L–1; Difco, Detroit) containing 2 × 10–4 fungal spores mL–1, with or without addition of extra salts (final concentration: 1 mm CaCl2 and 50 mm KCl). Neurospora crassa strain FGSC 2489 and Botrytis cinerea were used as test fungi. The plate was incubated at 25°C in the dark, and fungal growth was monitored by microspectrometry and microscopy after 24 and 48 h.
Acknowledgments
We thank Prof. J. Balzarini and L. Van Berckelaer (Rega Institute for Medical Research, Katholieke Universiteit Leuven, Belgium) for testing the cytotoxicity and antiviral activity of Gleheda.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.023853.
This work was supported in part by the Fund for Scientific Research-Flanders (Belgium; grant no. G.0113.01). W.W. received scholarships from the Education Ministry of China, the Flemish Community, the Catholic University of Leuven, and Ghent University to finish her PhD research.
References
- Balzarini J, Naesens L, Slachmuylders J, Niphuis H, Rosenberg I, Holy A, Schellekens H, De Clercq E (1991) 9-(2-Phosphonylmethoxyethyl) adenine (PMEA) effectively inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in rhesus monkeys. AIDS 5: 21–28 [DOI] [PubMed] [Google Scholar]
- Barre A, Hervé C, Lescure B, Rougé P (2002) Lectin receptor kinases in plants. Crit Rev Plant Sci 21: 379–399 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brewin NJ, Kardailsky IV (1997) Legume lectins and nodulation by Rhizobium. Trends Plant Sci 2: 92–98 [Google Scholar]
- Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J (1990) An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett 69: 55–60 [Google Scholar]
- Bunker TW, Etzler ME (1994) The stem and leaf lectin of Dolichos biflorus L., previously thought to be cell wall associated, is sequestered in vacuoles. Planta 192: 144–147 [Google Scholar]
- Cardona C, Posso CE, Kornegay J, Valor J, Serrano M (1989) Antibiosis effects of wild dry bean accessions on the Mexican bean weevil and the bean weevil (Coleoptera, Bruchidae). J Econ Entomol 82: 310–315 [Google Scholar]
- Czapla TH, Lang BA (1990) Effect of plant lectins on the larval development of European corn borer (Lepidoptera: Pyralidae) and southern corn root-worm (Coleoptera:Chrysomelidae). J Econ Entomol 83: 2480–2485 [Google Scholar]
- Desmyter S, Vandenbussche F, Van Damme EJM, Peumans WJ (2001) Preparation of monospecific polyclonal antibodies against Sambucus nigra lectin related protein, a glycosylated plant protein. Prep Biochem Biotechnol 31: 209–216 [DOI] [PubMed] [Google Scholar]
- Diaz C, Melchers LS, Hooykaas PJJ, Lugtenberg BJJ, Kijne JW (1989) Root lectin as a determinant of host-plant specificity in the Rhizobium-legume symbiosis. Nature 338: 579–581 [Google Scholar]
- Goldberg RB, Hoschek G, Vodkin LO (1983) An insertion sequence blocks the expression of a soybean lectin gene. Cell 33: 465–475 [DOI] [PubMed] [Google Scholar]
- Habibi J, Backus EA, Czapla TH (1993) Plant lectins affect survival of the potato leafhopper (Homoptera: Cicadellidae). J Econ Entomol 86: 945–951 [Google Scholar]
- Hankins CN, Kindinger J, Shannon LM (1987) The lectins of Sophora japonica: I. Purification, properties, and N-terminal amino acid sequences of two lectins from leaves. Plant Physiol 83: 825–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Demus U, Teichmann C, Parthier B, Wasternack C (1996) Developmental and tissue-specific expression of JIP-23, a jasmonate-inducible protein of barley. Plant Cell Physiol 37: 641–649 [DOI] [PubMed] [Google Scholar]
- Herman EM, Hankins CN, Shannon LM (1988) Bark and leaf lectins of Sophora japonica are sequestered in protein-storage vacuoles. Plant Physiol 86: 1027–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issitt PD, Anstee DJ (1998) Applied Blood Group Serology, Ed 4. Montgomery Scientific Publications, Durham, NC
- Kitagaki-Ogawa H, Matsumoto I, Seno N, Takahashi N, Endo S, Arata Y (1986) Characterization of the carbohydrate moiety of Clerodendrum trichotomum lectins: its structure and reactivity toward plant lectins. Eur J Biochem 161: 779–785 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Machuka JS, Okeola OG, Chrispeels MJ, Jackai LEN (2000) The African yam bean seed lectin affects the development of the cowpea weevil but does not affect the development of larvae of the legume pod borer. Phytochemistry 53: 667–674 [DOI] [PubMed] [Google Scholar]
- Medeiros A, Bianchi S, Calvete JJ, Balter H, Bay S, Robles A, Cantacuzène D, Nimtz M, Alzari PM, Osinaga E (2000) Biochemical and functional characterization of the Tn-specific lectin from Salvia sclarea seeds. Eur J Biochem 267: 1434–1440 [DOI] [PubMed] [Google Scholar]
- Meichenin M, Rocher J, Galanina O, Bovin N, Nifant'ev N, Sherman A, Cassagnau E, Heymann MF, Bara J, Fraser RH et al. (2000) Tk, a new colon tumor-associated antigen resulting from altered O-glycosylation. Cancer Res 60: 5499–5507 [PubMed] [Google Scholar]
- Mirkov TE, Wahlstrom JM, Hagiwara K, Finardi-Filho F, Kjemtrup S, Chrispeels MJ (1994) Evolutionary relationships among proteins in the phyto-hemagglutinin-arcelin-alpha-amylase inhibitor family of the common bean and its relatives. Plant Mol Biol 26: 1103–1113 [DOI] [PubMed] [Google Scholar]
- Murdock LL, Huesing JE, Nielsen SS, Pratt RC, Shade RE (1990) Biological effects of plant lectins on the cowpea weevil. Phytochemistry 29: 85–89 [Google Scholar]
- Oerke A-C, Dehne DW, Schönbeck F, Weber A (1994) Crop Production and Crop Protection. Estimated Losses in Major Food and Cash Crops. Elsevier Science BV, Amsterdam
- Peumans WJ, Van Damme EJM (1995) Lectins as plant defense proteins. Plant Physiol 109: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller V, Piller F, Carton J-P (1986) Isolation and characterization of an N-acetylgalactosamine-specific lectin from Salvia sclarea seeds. J Biol Chem 261: 14069–14075 [PubMed] [Google Scholar]
- Pull SP, Pueppke SG, Hymowitz JH, Orf JH (1978) Soybean lines lacking the 120,000-dalton seed lectin. Science 200: 1277–1279 [DOI] [PubMed] [Google Scholar]
- Rahbé Y, Sauvion N, Febvay G, Peumans WJ, Gatehouse AMR (1995) Toxicity of lectins and processing of ingested protein in the pea aphid Acyrthosiphon pisum. Entomol Exp Appl 76: 143–155 [Google Scholar]
- Shade RE, Schroeder HE, Pueyo JJ, Tabe LM, Murdock LL, Higgins TJV, Chrispeels MJ (1994) Transgenic pea seeds expressing the α-amylase inhibitor of the common bean are resistant to bruchid beetles. Bio/Technology 12: 793–796 [Google Scholar]
- Smagghe G, Degheele D (1994) Action of a novel nonsteroidal ecdysteroid mimic, tebufenozide (RH-5992), on insects of different orders. Pestic Sci 42: 85–92 [Google Scholar]
- Smeets K, Van Damme E, Peumans WJ (1997) Developmental regulation of lectin and alliinase synthesis in garlic bulbs and leaves. Plant Physiol 113: 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilatro RS, Cochran GR, Walker RE, Kablish KL, Bittner CC (1996) Characterization of a new lectin of soybean vegetative tissues. Plant Physiol 110: 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P, Chrispeels MJ (1984) Expression of lectin genes during seed development in normal and phytohemagglutinin-deficient cultivars of Phaseolus vulgaris. J Mol Appl Genet 2: 525–535 [PubMed] [Google Scholar]
- Van Damme EJM, Peumans WJ (1990) Developmental changes and tissue distribution of lectin in Galanthus nivalis L. and Narcissus cv. Carlton. Planta 182: 605–609 [DOI] [PubMed] [Google Scholar]
- Van Damme EJM, Peumans WJ, Barre A, Rougé P (1998) Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit Rev Plant Sci 17: 575–692 [Google Scholar]
- Vodkin LO, Raikhel NV (1986) Soybean lectin and related proteins in seeds and roots of Le+ and Le– soybean varieties. Plant Physiol 81: 558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Peumans WJ, Rougé P, Rossi C, Proost P, Chen J, Van Damme EJM (2003) Leaves of the Lamiaceae species Glechoma hederacea (ground ivy) contain a lectin that is structurally and evolutionary related to legume lectins. Plant J 33: 293–304 [DOI] [PubMed] [Google Scholar]
- Zhu K, Huesing JE, Shade RE, Bressan RA, Hasegawa PM, Murdock LL (1996) An insecticidal N-acetylglucosamine-specific lectin gene from Griffonia simplicifolia (Leguminosae). Plant Physiol 110: 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]