Abstract
The CDSP32 protein (chloroplastic drought-induced stress protein of 32 kD) is a thioredoxin participating in the defense against oxidative damage. We recently have identified in vitro the BAS1 2-Cys peroxiredoxin, a peroxide-detoxifying enzyme, as a target for CDSP32. Here, we report the characterization under stress conditions of transgenic potato (Solanum tuberosum) plants lacking CDSP32 with regard to the BAS1 redox state and the level of lipid peroxidation. Under control conditions, BAS1 is present at similar levels both in wild-type (WT) and transgenic plants. Under drought and methyl viologen treatment, CDSP32-lacking plants display, compared with WT, an increased proportion of BAS1 monomer corresponding to an overoxidized form of the protein. Leaf discs from transgenic plants treated with methyl viologen exhibit earlier degradation of BAS1 than WT plants do. Using several approaches, i.e. a probe emitting fluorescence when reacting with peroxides, high-performance liquid chromatography determination of lipid hydroxy fatty acid content, and measurement of chlorophyll thermoluminescence, we show a higher lipid peroxidation level under methyl viologen treatment in thylakoids from CDSP32-lacking plants compared with WT. These data show that CDSP32 is a critical component in the defense system against lipid peroxidation in photosynthetic membranes, likely as a physiological electron donor to the BAS1 peroxiredoxin.
Thioredoxins are proteins present in all organisms from bacteria to human (Homo sapiens). Despite different amino acid sequences, they display a typical folding and are characterized by a conserved redox active center, Cys-Gly-Pro-Cys, able to reduce disulfide bridges of target proteins (Eklund et al., 1991). Initially described as electron carriers in ribonucleotide reduction in Escherichia coli, thioredoxins serve as redox carriers in a wide variety of physiological processes (Arnér and Holmgren, 2000). In bacteria, yeast (Saccharomyces cerevisiae), and animal cells, most thioredoxin genes are induced by oxidative conditions (Kuge and Jones, 1994; Paget et al., 1998; Ritz et al., 2000), and numerous reports have shown the participation of these proteins in oxidative stress responses (Muller, 1991; Takemoto et al., 1998). In various organisms, thioredoxins are involved particularly in the protection against oxidative stress as electron donors for thioredoxin peroxidases (or peroxiredoxins), which detoxify hydrogen peroxide (H2O2) and alkyl hydroperoxides (Chae et al., 1994a, 1994b; Poole et al., 2000; Goyer et al., 2002).
Three main types of thioredoxins with multiple isoforms have been described in plants. The h-type is located in cytosol and likely participates in different processes such as seed germination, plant reproduction, cell communication, and cell division (Mouaheb et al., 1998; Meyer et al., 1999). The plastidic f- and m-types are involved in the control of the enzyme activities of CO2 fixation cycle in relation to light intensity (Schürmann and Jacquot, 2000). In contrast to other organisms, the participation of plant thioredoxins in oxidative stress responses has only emerged recently. Verdoucq et al. (1999) showed that Arabidopsis h3 thioredoxin was able to interact with a yeast peroxiredoxin. Mouhaeb et al. (1998) and Issakidis-Bourguet et al. (2001) reported that thioredoxins h3 and m from Arabidopsis could complement the H2O2 hypersensitivity of a yeast thioredoxin-deficient mutant. In the past few years, we identified another type of plastidic thioredoxin, induced by drought and oxidative stress conditions (Pruvot et al., 1996; Rey et al., 1998; Broin et al., 2000). The protein, designated CDSP32 (chloroplastic drought-induced stress protein of 32 kD), is composed of two thioredoxin domains with only one active redox disulfide center in the C-terminal part (Rey et al., 1998). Transgenic potato (Solanum tuberosum) plants lacking the CDSP32 protein due to a cosuppression phenomenon displayed an increased susceptibility to photooxidative stress conditions (Broin et al., 2002). In vitro incubation assays and affinity chromatography indicated that the 2-Cys peroxiredoxin BAS1 is likely a main target for CDSP32 (Broin et al., 2002). In plant cells, the 2-Cys peroxiredoxin type is localized in chloroplasts where it participates in the antioxidant defense system (Baier and Dietz, 1997, 1999; Baier et al., 2000). Based on in vitro assays, the protein, which is functional as a homodimer, has been shown to reduce H2O2 and alkyl hydroperoxides (König et al., 2002).
In the present study, we further characterized transgenic potato plants lacking the CDSP32 thioredoxin to gain insight about its function in the protection against oxidative damage. We report that, compared with wild type (WT), transgenic plants displayed under stress conditions substantial changes with regards to the redox state of the BAS1 peroxiredoxin and to the level of lipid peroxidation. The participation of CDSP32 in the detoxification of lipid hydroperoxides is discussed in relation to a function of electron donor to the BAS1 peroxiredoxin.
RESULTS
BAS1 Abundance in the Different Organs of WT and CDSP32-Lacking Plants
In WT plants grown under control conditions, the CDSP32 thioredoxin was present in leaves and not detected in other organs (flowers, stems, tubers, and roots; Fig. 1A). In the transgenic lines previously shown to lack the thioredoxin in leaves (Broin et al., 2002), the protein was also found to be absent in other organs (Fig. 1A), even when loading larger amounts of protein (data not shown). The BAS1 peroxiredoxin abundance was compared in proteins of WT and transgenic lines separated under reducing conditions. A high abundance of the BAS1 23-kD monomer was observed in all organs of both plant types (Fig. 1B). Note that WT and transgenic plants displayed a similar pattern with regard to BAS1 abundance in the different organs, the highest level being observed in leaves.
Figure 1.
Immunoblot analysis of CDSP32 and BAS1 abundances in the different organs of WT and CDSP32-lacking potato lines. Soluble proteins were separated by SDS-PAGE under reducing conditions. Proteins (10 μg) from WT potato and a CDSP32-lacking (CDSP32–) plant (D4 line) were analyzed using the CDSP32 (A) and BAS1 (B) antisera diluted 1:1,000 (v/v) and 1:10,000 (v/v), respectively. The bands corresponding to CDSP32 and BAS1 were revealed at 32 and 23 kD, respectively. WF, Whole flowers; L, leaves; S, stems; T, tubers; R, roots.
Abundance and Redox State of BAS1 in Leaves under Drought Stress
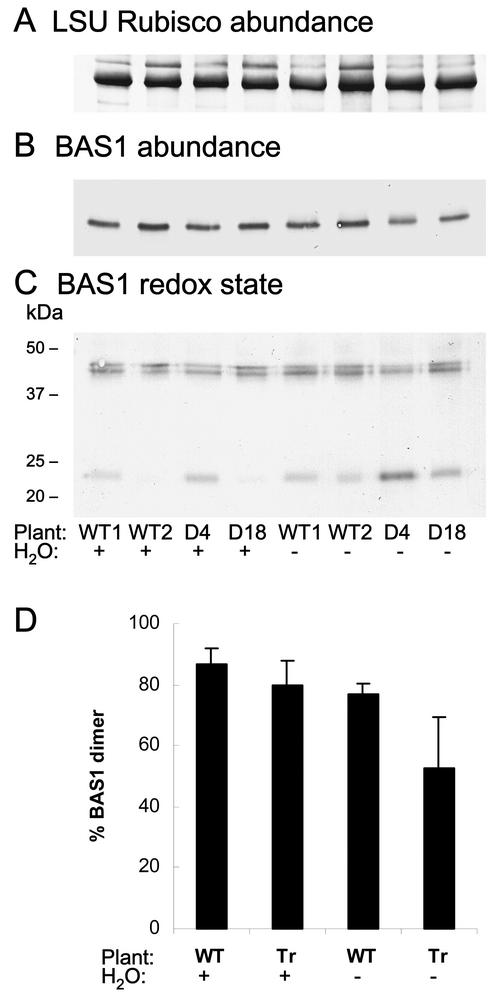
We investigated the BAS1 abundance and redox state in whole potato plants subjected to water deficit, down to a leaf relative water content of around 65%. Under these conditions, the CDSP32 amount increased in leaves of WT compared with control conditions as previously reported (Rey et al., 1998), but the protein was not detected in transgenic lines (data not shown). In leaf proteins from well-watered plants separated under reducing conditions, a similar abundance of BAS1 peroxiredoxin was noticed both in WT and in CDSP32-lacking lines (Fig. 2B). Under drought conditions, no change in BAS1 abundance was noticed in WT, whereas a level reduced by around one-third (image analysis performed using Genetools) was observed in the two lines deprived of CDSP32 (Fig. 2B). Proteins were separated in the absence of reducing agent to quantify the proportion of BAS1 dimer, which corresponds to the functional form of the protein (Dietz et al., 2002). Under well-watered conditions, BAS1 was found mainly as a dimer at around 43 to 45 kD (around 87% and 80% in WT and transgenic lines, respectively; Fig. 2, C and D). Due to the presence of one or two disulfide bridges, two dimer forms with distinct electrophoretic mobility were revealed as reported by Dietz et al. (2002). Although a slight decrease in the proportion of BAS1 dimer (down to 77%) was noticed in WT under drought, the proportion of this form was strongly reduced (around 53%) in water-stressed transgenic plants, particularly in the D4 line (Fig. 2, C and D). Note that in our experiments, no BAS1 oligomerization was observed under stress, conversely to the data reported by König et al. (2002) in barley (Hordeum vulgare). In thylakoids of wilted potato plants, BAS1 was found only as a dimer, and its relative abundance was much lower compared with that observed in soluble proteins (data not shown).
Figure 2.
Immunoblot analysis of the BAS1 abundance and redox state in leaves from WT and CDSP32-lacking potato lines under drought stress. Leaf soluble proteins from whole plants (A–C) were separated by SDS-PAGE under reducing (A and B) or nonreducing (C) conditions. Proteins (5 and 15 μg in reducing and nonreducing conditions, respectively) were analyzed using the Rubisco (A) and BAS1 (B and C) antisera diluted 1:4,000 (v/v) and 1:10,000 (v/v), respectively. The Rubisco immunoblot is presented to show homogeneous loading of lanes. The bands corresponding to large Rubisco subunit and BAS1 dimer and monomer were revealed at around 50, 43 to 45, and 23 kD, respectively. Two WT plants and two CDSP32-lacking (D4 and D18) plants were either grown under control conditions (H2O+) or subjected to water shortage for 10 d (H2O–). D, Percentage of BAS1 protein present as a dimer in leaf-soluble proteins. The data were obtained by image analysis, using Genetools (Syngene, Cambridge, UK), of immunoblot of proteins separated under nonreducing conditions (as in C). The values presented were calculated from data originating from five independent plants per treatment. Tr, D4 and D18 plants.
Abundance and Redox State of BAS1 in Leaf Discs Subjected to Photooxidative Stress
We reported previously that leaf discs from CDSP32-lacking plants exposed to light in the presence of the photooxidizing PSI acceptor, methyl viologen (Babbs et al., 1989), exhibit compared with WT increased damage within the photosynthetic apparatus (Broin et al., 2002). Western analyses were carried out to investigate the abundance and redox state of the BAS1 peroxiredoxin after a 4-h period of light exposure in the presence of methyl viologen. At this stage, leaf discs from transgenic plants started to exhibit photooxidative damage, whereas WT discs displayed almost no stress symptoms (data not shown). When proteins were separated under reducing conditions, a similar BAS1 abundance was revealed both in WT and in transgenic discs incubated on water (Fig. 3A). Under nonreducing conditions, much lower percentages of BAS1 dimer (less than 45%) were revealed in discs incubated on water (Fig. 3, B and C) compared with those of control whole plants (more than 80%, Fig. 2D). Note that WT discs were found to display a significantly higher percentage of BAS1 dimer than discs from transgenic plants (41% and 25%, respectively). In methyl viologen-treated discs, blots from gels run in the presence of dithiothreitol revealed a lower BAS1 level in both plant types (around 25% less in comparison with control discs incubated on water, Fig. 3A). A band with a lower size compared with that of the BAS1 monomer was revealed using the BAS1 antiserum only in the two CDSP32-lacking lines. In the D4 line, the relative abundance of this band was estimated, using Genetools, to be around 30% of that of the BAS1 monomer. Under nonreducing conditions during migration, the proportion of BAS1 dimer fell down to less than 20% in both disc types treated with methyl viologen (Fig. 3, B and C). Note also that only one dimer form was detected in the presence of the photooxidizing compound (Fig. 3B), likely indicating substantial changes in the redox state of the BAS1 dimer under stress.
Figure 3.
Immunoblot analysis of the BAS1 abundance and redox state in leaf discs from WT and CDSP32-lacking potato lines under photooxidative stress conditions. Soluble proteins from leaf discs (A and B) were separated by SDS-PAGE under reducing (A) or nonreducing (B) conditions. Proteins were analyzed using the BAS1 antiserum as described in Figure 2. Leaf discs from two independent WT plants or two CDSP32-lacking (D4 and D18) plants were incubated either on water (MV–) or on 1 μm methyl viologen (MV+) for 4 h in the light with a 12-h dark break. C, Percentage of BAS1 protein present as a dimer in leaf discs. The data were obtained by image analysis, using Genetools, of immunoblot of proteins separated under nonreducing conditions (as in B). The values presented were calculated from data originating from six independent plants per treatment. Tr, D4 and D18 plants.
Analysis of the Production of Peroxides in Methyl Viologen-Treated Protoplasts Using a Fluorescent Probe
We further characterized CDSP32-lacking plants by investigating the localization of peroxides under photooxidative stress generated using methyl viologen. For this purpose, we used 2′, 7′-dichlorofluorescein diacetate (H2DCF-DA), a chemical probe emitting fluorescence when reacting with organic peroxides and H2O2 but not with the superoxide anion (Zhu et al., 1994). In plants, H2DCF-DA has been used to monitor the H2O2 release in soybean (Glycine max) after a pathogen attack (Levine et al., 1994) and in mitochondria of tobacco (Nicotiana tabacum) plants modified in alternative oxidase level (Maxwell et al., 1999). Investigations were carried out on protoplasts using laser-scanning confocal microscopy. In non-treated protoplasts both from WT and from CDSP32-deprived lines, the dichlorofluorescein (DCF) fluorescence was hardly detectable (data not shown). Note that no DCF fluorescence was observed in non-fully digested cells, characterized by a noncircular shape, due to H2DCF-DA inability to cross the cell wall. In methyl viologen-treated protoplasts, a much higher DCF green fluorescence signal was recorded in CDSP32-lacking lines than in WT (Fig. 4, right). The DCF fluorescence pattern was found to be close to that of the natural chlorophyll fluorescence (Fig. 4, left), strongly suggesting the occurrence of a higher production of peroxides in plastids of transgenic plants.
Figure 4.
Analysis of the production of peroxides using H2DCF-DA in protoplasts from WT and CDSP32-lacking potato lines under methyl viologen treatment. Laser-scanning confocal microscope images of protoplasts from WT and CDSP32-lacking (CDSP32–) potato lines labeled with H2DCF-DA and treated with 10 μm methyl viologen under light for 5 min. Left, Natural chlorophyll (Chl) fluorescence. Right, DCF fluorescence. The same magnification (×350) was used for all images. The experiment was repeated three times with similar fluorescence intensity signals in D4 and D18 CDSP32-lacking lines.
Analysis of the Production of Lipid Hydroperoxydes in Methyl Viologen-Treated Leaf Discs
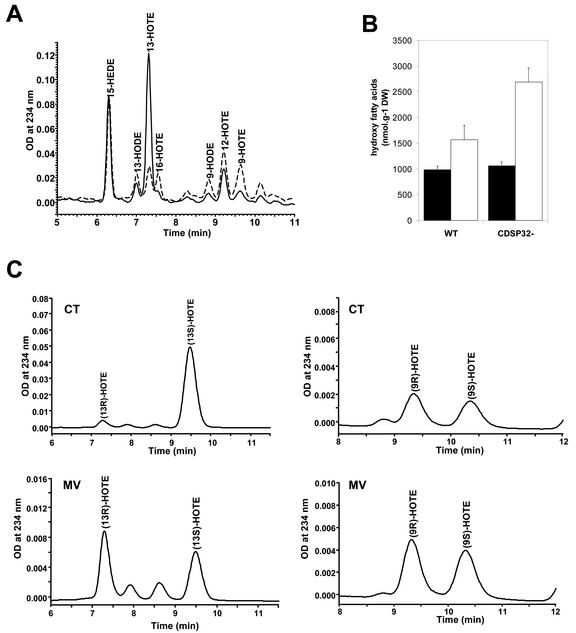
The amount of lipid hydroperoxides in control and methyl viologen-treated leaf discs was measured after reduction using HPLC analysis of hydroxy fatty acids. The level of most hydroxy fatty acids was found to be higher in discs subjected to methyl viologen for 4 h 30 min under light than in discs incubated on water under the same conditions (Fig. 5A; data not shown). However, the 13-HOTE amount, which was very high both in WT and in transgenic discs under control conditions, was found to be much lower in the presence of methyl viologen (Fig. 5A; data not shown). We investigated 13-HOTE chirality to verify whether the high content of the fatty acid in control discs was due to chemical oxidation or to the activity of a specific lipoxygenase induced during the preparation of leaf discs. Royo et al. (1996) reported the activation of a specific lipoxygenase by wounding in potato plants, leading to the generation of 13-HOTE, a precursor of jasmonic acid, a molecule involved in defense reactions (Blechert et al., 1995). After the first HPLC, the peak of 13-HOTE was taken and analyzed by chiral chromatography, as described in “Materials and Methods,” to determine its enantiomer composition. As shown in Figure 5C (upper), 95% 13-HOTE was found chiral (13S-HOTE) in control discs, in contrast to 9-HOTE, which appeared to be racemic. These data indicate that the high 13-HOTE level in discs incubated on water very likely originates from the activity of a specific lipoxygenase induced by wounding. In discs treated with methyl viologen, both 13-HOTE and 9-HOTE were found to be racemic, thus reflecting a membrane autoxidization process (Fig. 5C, lower). The much lower 13-HOTE content during methyl viologen treatment indicates that the lipoxygenase is very likely inactivated under severe photooxidative stress.
Figure 5.
HPLC analysis of the hydroxy fatty acid content in leaf discs from WT and CDSP32-lacking potato lines treated with methyl viologen. Leaf discs from WT and CDSP32-lacking (CDSP32–) lines were floated on water (CT) or 1 μm methyl viologen (MV) and exposed to light for 2 h, then to dark for 16 h, and to light for 2 h 30 min, before analysis. Hydroperoxy fatty acids were extracted and analyzed by HPLC after reduction. A, Chromatograms of hydroxy fatty acids from CDSP32– leaf discs, CT (—), and MV (---). B, Amount of hydroxy fatty acids (9-, 12-, and 16-hydroxy-octadecatrienoic acid [HOTE] and 9- and 13-hydroxy-octadecadienoic acid) in WT and CDSP32– leaf discs incubated on water (▪) or on 1 μm methyl viologen (□). Values are means (±sd) of three determinations on 1 g of leaf discs from two plants per type (two WT, one D4, and one D18). The experiment was repeated twice with similar results. C, Chromatograms of chiral HPLC analysis of 13-HOTE (left) and 9-HOTE (right) from CDSP32– leaf discs under control conditions (CT, upper) and methyl viologen treatment (MV, lower).
In discs floated on water, the content of all hydroxy fatty acids (9-, 12-, and 16-HOTE and 9- and 13-hydroxy-octadecadienoic acid, except 13-HOTE) was around 1,000 nmol g–1 dry weight both in WT and CDSP32-lacking lines (Fig. 5B). The content was significantly higher under methyl viologen treatment, reaching 1,500 nmol g–1 dry weight in WT and around 2,700 nmol g–1 dry weight in transgenic lines (Fig. 5B). These data clearly reveal a higher production of lipid hydroperoxydes due to membrane autoxidization in leaf discs from transgenic lines under photooxidative stress.
Analysis of Lipid Peroxidation in Thylakoids of Methyl Viologen-Treated Leaf Discs
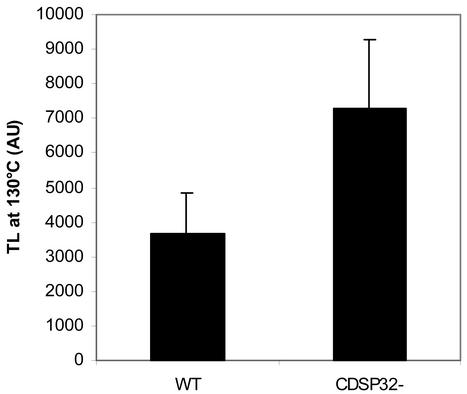
The level of chlorophyll thermoluminescence (TL) signal, which has been correlated to the level of lipid peroxidation in thylakoids (Vavilin and Ducruet, 1999), was measured in discs subjected either to control conditions or to photooxidative stress generated by 1 μm methyl viologen for 270 min under light. In non-treated discs from WT and CDSP32-lacking lines, the peak of chlorophyll TL at 130°C was not detected (data not shown). In methyl viologen-treated discs, a high TL signal at 130°C was recorded (Fig. 6), indicating the production of lipid hydroperoxides during the treatment. The signal was 2-fold higher in transgenic lines than in WT, showing increased lipid peroxidation in the thylakoids of plants lacking CDSP32.
Figure 6.
Analysis of the thylakoid lipid peroxidation level by measuring the chlorophyll TL signal in leaf discs from WT and CDSP32-lacking potato lines treated with methyl viologen. Leaf discs from WT and CDSP32-lacking (CDSP32–) lines were floated on water containing 1 μm methyl viologen and exposed to light for 2 h, then to dark for 16 h, and to light for 2 h 30 min, before analysis. The level of chlorophyll TL signal at 130°C was recorded. Values are means (±sd) from 24 discs originating from three independent experiments with two plants per type (two WT, one D4, and one D18) in each experiment. AU, Arbitrary unit.
DISCUSSION
Modifications in the Abundance and Redox State of the BAS1 Peroxiredoxin in Plants Lacking the CDSP32 Thioredoxin
This paper first documents the characteristics of WT and CDSP32-deprived plants with regard to the abundance and redox state of BAS1, a plastidic peroxide-detoxifying enzyme, which has been presumed to be a target in planta for the CDSP32 thioredoxin (Broin et al., 2002). A substantially decreased proportion of BAS1 dimer, the functional form of the protein, was revealed under drought stress in transgenic plants and in discs of both plant types under methyl viologen treatment. In in vitro assays, oxidizing stress conditions have been reported to provoke overoxidation and monomerization of 2-Cys and type II peroxiredoxins such as plant BAS1 (König et al., 2002) or yeast Ahp1 (Prouzet-Mauléon et al., 2002), respectively. Based on these reports and on the fact that a very high percentage of BAS1 monomer was found in leaf discs subjected to a severe photooxidizing treatment generated by methyl viologen, we conclude that the stress-induced BAS1 monomer observed in our experiments corresponds to an overoxidized form of the protein. These findings corroborate those of Rabilloud et al. (2002), who concluded that mammal 2-Cys peroxiredoxins constitute targets for peroxides, and indicate that overoxidation of such peroxiredoxins also occurs in plants under stress conditions. It is worth mentioning that, in comparison with well-watered whole plants, leaf discs incubated on water for a few hours in the light displayed substantial changes with regard to the BAS1 redox state. We conclude from this observation that, even in the absence of methyl viologen, discs are subjected to rather stressful conditions leading to changes in the plastidic redox state and in the photosynthetic activity, as reported by Kato et al. (2002). Altogether, these data, showing a significantly higher percentage of BAS1 monomer in transgenic plants than in WT under drought and in leaf discs floated on water, indicate that CDSP32-lacking lines display increased overoxidation of BAS1 under stress. Further, despite no variation in the BAS1 level under control conditions, transgenic plants have a lower 2-Cys peroxiredoxin abundance than WT under water deficit, likely indicating accelerated protein degradation. In other respects, under methyl viologen treatment, up to 30% of BAS1 monomer was found with a lower molecular mass in discs from CDSP32-deprived plants. It is noteworthy that BAS1 monomer has a higher propensity to partial proteolysis (König et al., 2002) and that overoxidation of a 2-Cys mammal peroxiredoxin leads to inactivation and accelerated degradation of the protein (Rabilloud et al., 2002). Following on from this, we propose that the BAS1 form displaying a lower molecular mass in transgenic lines under methyl viologen treatment originates from a degradation process, which would be accelerated as a result of earlier overoxidation of the protein.
Participation of the CDSP32 Thioredoxin in Detoxification of Lipid Hydroperoxides
In comparison with WT, CDSP32-lacking plants exhibited a higher level of DCF fluorescence signal in protoplasts subjected to methyl viologen treatment. Because close fluorescence patterns were observed for DCF and chlorophyll, we concluded that a more elevated production of H2O2 and/or organic peroxides occurs in plastids in the absence of the thioredoxin. In other respects, we noticed approximately 2-fold higher levels of hydroxy fatty acid content and chlorophyll TL signal in leaf discs from transgenic lines treated with methyl viologen than in WT discs. Taken collectively, these data show that plants without CDSP32 are characterized by an increased production of lipid hydroperoxides in thylakoids under photooxidative stress. Based on the results obtained using DCF, we propose that the higher level of lipid peroxidation in transgenic plants under stress originates either from direct oxidation of fatty acids or from an increase in H2O2 content. These findings are a substantial contribution concerning the function of the thioredoxin, which appears to be an essential component for limiting lipid peroxidation in the photosynthetic membranes.
CDSP32. A Physiological Electron Donor to the BAS1 Peroxiredoxin?
In chloroplasts, thylakoid membranes are particularly rich in polyunsaturated fatty acids prone to peroxidation (Browse et al., 1994). Detoxification of lipid hydroperoxides is of peculiar importance because these compounds drive autocatalytic chain reactions and can provoke severe damage in the photosynthetic membranes (Asada, 1996; Havaux and Niyogi, 1999). Besides low-Mr compounds, two main enzymes, the phospholipid hydroperoxide glutathione peroxidase (Mullineaux et al., 1998) and the 2-Cys peroxiredoxin (Dietz et al., 2002), are likely to participate in reduction of plastidic lipid hydroperoxides. On the basis of its abundance and localization, BAS1 is presumed to be the essential enzymatic component for detoxifying hydroperoxides within thylakoid membranes and for maintaining integrity of the photosynthetic apparatus (Dietz et al., 2002). Accordingly, antisense down-regulation of BAS1 expression has been reported to lead to impairment in PSII efficiency in young Arabidopsis leaves (Baier and Dietz, 1999), but the effect was slight probably due to a still substantial protein amount. Similarly, Klughammer et al. (1998) reported that disruption of the 2-Cys peroxiredoxin gene in Synechocystis sp. PCC 6803 results in increased stress sensitivity of the photosynthetic metabolism. In other respects, yeast strains lacking or overexpressing the Ahp1p 2-Cys peroxiredoxin exhibit increased or decreased susceptibility, respectively, to an alkyl hydroperoxide, tertbutyl hydroperoxide (Lee et al., 1999). Lee et al. (1999) also reported that the Ahp1p peroxiredoxin requires the presence of a thioredoxin to perform its antioxidant function because yeast mutants deprived of thioredoxin were found to be highly sensitive to tert-butyl hydroperoxide, even when Ahp1p was overexpressed. These data indicate that, in various organisms, the 2-Cys peroxiredoxin activity level is correlated with the level of stress sensitivity. Previously, we showed that CDSP32-lacking lines display, compared with WT, a lower photosynthetic activity and a reduced chlorophyll content under photooxidative stress conditions (Broin et al., 2002). The data presented in this study indicate that under stress, these lines concomitantly exhibit an increased proportion of inactivated BAS1 protein due to overoxidation and a higher level of lipid peroxidation. As a consequence, we conclude that the increased damage in the photosynthetic membranes of CDSP32-lacking lines originates from a reduced detoxification capacity of H2O2 and/or of hydroperoxides due to accelerated inactivation of BAS1. We propose that this inactivation results from the absence of the CDSP32 thioredoxin, which would normally reduce the peroxiredoxin. To unambiguously show that BAS1 constitutes a target for CDSP32 in planta, it would be worth investigating the presence of the heterodimeric complex formed of the two proteins in plants overexpressing the CDSP32 thioredoxin mutated at its active site.
In contrast to CDSP32, BAS1 is present at high levels in all organs of control WT plants. However, in Chinese cabbage (Brassica campestris L. subsp. pekinensis), Cheong et al. (1999) reported substantially lower levels of the 2-Cys peroxiredoxin in roots and flowers compared with that observed in leaves. These data raise the question, at least in potato, of the role of BAS1 in roots and flowers where plastids are not photosynthetic and not as developed as in leaves. Further, they suggest that electron donors to BAS1 different from CDSP32 exist in these organs and also in leaves in the absence of stress. König et al. (2002) showed that spinach (Spinacia oleracea) thioredoxins f and m reduce oxidized BAS1 in in vitro assays. Motohashi et al. (2001) identified more than 14 targets for spinach thioredoxin m, including the 2-Cys BAS1 peroxiredoxin. However, in these experiments, BAS1 was found to interact with thioredoxin m in a much less specific manner compared with the results published for CDSP32 (Broin et al., 2002). CDSP32 could constitute an electron donor to BAS1 more particularly in leaves under stress conditions when other antioxidant enzymes such as ascorbate peroxidase are inactivated (Dietz et al., 2002). Note also that very recent data reveal that CDSP32 is more abundant in young developing leaves in potato, likely indicating that the protein plays a protective function also during leaf development (Broin et al., 2003). In Arabidopsis plants antisense for the BAS1 gene, an enhancement of the enzyme activities associated with ascorbate metabolism was noticed, but no change in glutathione metabolism was observed (Baier et al., 2000). Further work is needed to characterize plants either lacking or overexpressing CDSP32 to investigate how the thioredoxin is integrated in the antioxidant network during stress and development.
MATERIALS AND METHODS
Plant Material, Drought, and Photooxidative Treatments
Potato (Solanum tuberosum cv Désirée) WT and transgenic plants were propagated in vitro and transferred for growth in vivo in a phytotron (photosynthetic photon flux density = 200 μmol m–2 s–1, 12-h night, 23°C/19°C day/night). Two independent lines, designated D4 and D18 (Broin et al., 2002) and cosuppressed for CDSP32 expression, were used. A gradually increasing water deficit was applied on 3-week-old plants by withholding watering for around 10 d. Relative water content was determined on leaf pieces as previously described (Rey et al., 1998). For incubation experiments, 1.4-cm diameter discs were excised from young well-expanded leaflets of 3-week-old plants. Discs were incubated in the phytotron on water containing 0.1% (v/v) Tween 20 with or without 1 μm methyl viologen (Sigma, St. Louis) in the light (375 μmol photons m–2 s–1) for 2 h, then in the dark for 12 h and in the light for 2 to 2 h 30.
Protein Extraction, SDS-PAGE, and Western Analysis
For preparing leaf-soluble proteins, leaflet or disc samples were blended in liquid N2, and the powder was resuspended in 50 mm Tris-HCl (pH 8.0) and 1 mm phenylmethylsulfonyl fluoride, with or without reducing agent (50 mm β-mercaptoethanol), and centrifuged (10000g, 4°C, 10 min). The soluble proteins were precipitated at –20°C by the addition of 1 volume of acetone to the supernatant. The soluble proteins from other organs (whole flowers, stems, tubers, and roots from 6-to 8-week-old plants) were prepared similarly using 50 mm β-mercaptoethanol in the extraction buffer.
Protein content was determined using a modified Lowry method (Sigma). One-dimensional electrophoresis (Laemmli, 1970) was performed with 13% (w/v) acrylamide gels with or without dithiothreitol (0.1 m) in the solubilization buffer and using an Mr electrophoresis calibration kit (BioRad Laboratories, Hercules, CA). After SDS-PAGE, proteins were electroblotted onto 0.45 μm nitrocellulose (Pall Gelman Sciences, Pall Corporation, Ann Arbor, MI) for western analysis. The sera raised against the potato CDSP32 N-terminal region (Rey et al., 1998) and the Arabidopsis BAS1 protein (Broin et al., 2002) were used diluted 1:1,000 (v/v) and 1:10,000 (v/v), respectively. Bound antibodies were detected using an anti-rabbit IgG alkaline phosphatase conjugate diluted 1:10,000 (v/v; Sigma). Image analysis and estimation of the intensity of bands were carried out using Genetools.
Preparation of Protoplasts
Protoplasts were prepared from young fully expanded leaves from 3-week-old plants, cut into slices, and incubated for 16 h (at 28°C in the dark) on a maceration fluid containing 0.45 m mannitol, the macro- and micro-elements of Murashige and Skoog (1962), 1 mm CaCl2 (pH 5.8; washing medium) supplemented with 0.5% (w/v) cellulase O-R-10 (Yakult Honsha Co., Tokyo) and 0.5% (w/v) macerozyme R-10 (Yakult Honsha Co.). Protoplasts were purified using a Percoll gradient procedure (Mills and Joy, 1980) and resuspended to 500 μg chlorophyll mL–1 in washing medium.
Confocal Microscopy
The intracellular production of H2O2 and organic peroxides was investigated using H2DCF-DA (Molecular Probes, Eugene, OR), a compound converted, when taken up by cells, to the membrane-impermeant derivative H2DCF by esterases. H2DCF is nonfluorescent but rapidly oxidized to the highly fluorescent DCF by reaction with the intracellular peroxides (Zhu et al., 1994).
Fresh protoplasts were diluted to 200 μg chlorophyll mL–1 in 50 μL of washing medium containing 100 mm Tricine (pH 7.5). After simultaneous addition of methyl viologen (10 μm final concentration) and H2DCF-DA (5 μm final concentration), the reaction mix was illuminated for 5 min (photosynthetic photon flux density = 300 μmol m–2 s–1) and analyzed using a Fluoview krypton-argon laser scanning confocal microscope (Olympus, Tokyo). DCF and chlorophyll were excited at 488 nm, and the emitted fluorescence was detected through 530-/20-nm bandpass and >660-nm longpass filters, respectively. The laser intensity was identical in all experiments. Data were computerized and imported into Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA) for the preparation of figures.
Hydroxy Fatty Acid and Hydroperoxy Fatty Acid Analysis
Free and bonded hydroxy and hydroperoxy fatty acids were analyzed using HPLC, as free hydroxy fatty acids, after NaBH4 reduction and hydrolysis. Control and methyl viologen-treated leaf discs (1 g fresh weight) were frozen in liquid nitrogen and homogenized in 0.2 m NaOH and 5% (w/v) NaBH4 in the presence of an internal reference, 15-hydroxy-11, 13(Z,E)-eicosadienoic acid (40 nmol g–1 fresh weight). Extraction and HPLC analysis were performed as described by Degousée et al. (1994) and Rustérucci et al. (1999), using a Zorbax RX-SIL silica column (Interchim, Montluçon, France). Hydroxy fatty acids isomers were detected at 234 nm and identified using standards (Degousée et al., 1995). Quantification was performed with reference to 15-hydroxy-11, 13(Z,E)-eicosadienoic acid, assuming a similar extinction coefficient at 234 nm for all hydroxy fatty acids.
For chirality analysis of 13-HOTE and 9-HOTE, the corresponding hydroxy fatty acid was taken after HPLC analysis through a Zorbax RX-SIL silica column (Interchim). The hydroxy fatty acids were then analyzed by HPLC using a Chiralcel OD-H silica column (Interchim), and the isomers were eluted with a hexane:isopropanol:acetic acid (95:5:0.1 [v/v]) isocratic flux (1 mL min–1) and detected at 234 nm.
Chlorophyll TL
The excited forms of lipid peroxides are able to transfer their energy to chlorophyll, and heating a leaf sample results in the desexcitation of chlorophyll through photon emission. This process, termed chlorophyll TL, is used to estimate the level of lipid peroxidation within thylakoids (Stallaert et al., 1995). Measurements were carried out on 1.4-cm-diameter leaf discs, treated (or not) with 1 μm methyl viologen as described above, according to Ducruet and Miranda (1992) and Broin et al. (2000).
Acknowledgments
We are very grateful to Dr. Jean-Luc Montillet (Laboratoire de Radiobiologie Végétale, DEVM, CEA/Cadarache, Saint-Paul-lez-Durance, France) for valuable advice on HPLC analysis of hydroxy fatty acids and for critical reading of the manuscript. We are also grateful to Dr. Michel Havaux (Laboratoire d'Ecophysiologie de la Photosynthèse, DEVM, CEA/Cadarache) for helpful advice in chlorophyll TL experiments. We wish to thank Jean-Pierre Agnel (Laboratoire de Radiobiologie Végétale, Département d'Ecophysiologie Végétale et de Microbiologie, CEA/Cadarache) for help in analysis of hydroxy fatty acids and Jacqueline Massimino, Françoise Eymery, and Véronique Cardettini (Laboratoire d'Ecophysiologie de la Photosynthèse, Département d'Ecophysiologie Végétale et de Microbiologie, CEA/Cadarache) for assistance in growing plant material.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021626.
References
- Arnér ESJ, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267: 6102–6109 [DOI] [PubMed] [Google Scholar]
- Asada K (1996) Radical production and scavenging in the chloroplast. In NR Baker, ed, Advances in Photosynthesis, Vol 5, Photosynthesis and the Environment. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 123–150
- Babbs CF, Pham JA, Coolbaugh RC (1989) Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol 90: 1267–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Dietz KJ (1997) The plant 2-Cys peroxiredoxin BAS1 is a nuclear-encoded chloroplast protein: its expressional regulation, phylogenetic origin, and implications for its specific physiological function in plants. Plant J 12: 179–190 [DOI] [PubMed] [Google Scholar]
- Baier M, Dietz KJ (1999) Protective function of chloroplast 2-cysteine peroxiredoxin in photosynthesis: evidence from transgenic Arabidopsis. Plant Physiol 119: 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier M, Noctor G, Foyer C, Dietz KJ (2000) Antisense suppression of 2-cysteine peroxiredoxin in Arabidopsis specifically enhances the activities and expression of enzymes associated with ascorbate metabolism but not glutathione metabolism. Plant Physiol 124: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert S, Brodschelm W, Holder S, Kammerer L, Kutchan TM, Mueller MJ, Xia ZQ, Zenk MH (1995) Proc Natl Acad Sci USA 92: 4099–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broin M, Besse I, Rey P (2003) Evidence for post-translational control in the expression of a gene encoding a plastidic thioredoxin during leaf development in Solanum tuberosum plants. Plant Physiol Biochem 41: 303–308 [Google Scholar]
- Broin M, Cuiné S, Eymery F, Rey P (2002) The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. Plant Cell 14: 1417–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broin M, Cuiné S, Peltier G, Rey P (2000) Involvement of CDSP32, a drought-induced thioredoxin, in the response to oxidative stress in potato plants. FEBS Lett 467: 245–248 [DOI] [PubMed] [Google Scholar]
- Browse J, Miquel M, McConn M, Wu J (1994) Arabidopsis mutants and genetic approaches to the control of lipid composition. In AR Cossins, ed, Temperature Adaptation of Biological Membranes. Portland Press, London, pp 141–154
- Chae HZ, Chung SJ, Rhee SG (1994a) Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem 269: 27670–27678 [PubMed] [Google Scholar]
- Chae HZ, Robinson K, Poole LB, Church G, Storz G, Rhee SG (1994b) Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci USA 91: 7017–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong NE, Choi YO, Lee KO, Kim WY, Jung BG, Chi YH, Jeong JS, Kim K, Cho MJ, Lee SY (1999) The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redoxhierarchy of photosynthetic electron flux. Plant Mol Biol 40: 825–83410487217 [Google Scholar]
- Degousée N, Triantaphylidès C, Montillet JL (1994) Involvement of oxidative processes in the signaling mechanisms leading to the activation of glyceollin synthesis in soybean (Glycine max). Plant Physiol: 104: 945–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degousée N, Triantaphylidès C, Starek S, Iacazio G, Martini D, Bladier C, Voisine R, Montillet JL (1995) Measurement of thermally produced volatile alkanes: an assay for plant hydroperoxy fatty acid evaluation. Anal Biochem 224: 524–531 [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Horling F, König J, Baier M (2002) The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. J Exp Bot 53: 1321–1329 [PubMed] [Google Scholar]
- Ducruet JM, Miranda T (1992) Graphical and numerical analysis of the thermoluminescence and fluorescence F0 emission in photosynthetic material. Photosynth Res 33: 15–27 [DOI] [PubMed] [Google Scholar]
- Eklund H, Gleason FK, Holmgren A (1991) Structural and functional relations among thioredoxins of different species. Prot Struct Funct Genet 11: 13–28 [DOI] [PubMed] [Google Scholar]
- Goyer A, Haslekas C, Miginiac-Maslow M, Klein U, Le Marechal P, Jacquot JP, Decottignies P (2002) Isolation and characterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. Eur J Biochem 269: 272–282 [DOI] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96: 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issakidis-Bourguet E, Mouaheb N, Meyer Y, Miginiac-Maslow M (2001) Heterologous complementation of yeast reveals a new putative function for chloroplast m-type thioredoxin. Plant J 25: 127–135 [DOI] [PubMed] [Google Scholar]
- Kato MC, Hikosaka K, Hirose T (2002) Leaf discs floated on water are different from intact leaves in photosynthesis and photoinhibition. Photosynth Res 72: 65–70 [DOI] [PubMed] [Google Scholar]
- Klughammer B, Baier M, Dietz KJ (1998) Inactivation by gene disruption of 2-cysteine peroxiredoxin in Synechocystis sp. PCC 6803 leads to increased stress sensitivity. Physiol Plant 104: 699–706 [Google Scholar]
- König J, Baier M, Horling F, Kahmann U, Harris G, Schürmann P, Dietz KJ (2002) The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redox-hierarchy of photosynthetic electron flux. Proc Natl Acad Sci USA 99: 5738–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S, Jones N (1994) YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J 13: 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lee J, Spector D, Godon C, Labarre J, Toledano MB (1999) A new antioxidant with alkyl hydroperoxide defense properties in yeast. J Biol Chem 27: 4537–4544 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96: 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y, Verdoucq L, Vignols F (1999) Plant thioredoxins and glutaredoxins: identity and putative roles. Trends Plant Sci 4: 388–394 [DOI] [PubMed] [Google Scholar]
- Mills WR, Joy KW (1980) A rapid method for isolation of purified physiologically active chloroplasts used to study the intracellular distribution of amino acids in pea leaves. Planta 148: 75–83 [DOI] [PubMed] [Google Scholar]
- Motohashi K, Kondoh A, Stumpp MT, Hisabori T (2001) Comprehensive survey of proteins target by chloroplast thioredoxin. Proc Natl Acad Sci USA 98: 11224–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouaheb N, Thomas D, Verdoucq L, Montfort P, Meyer Y (1998) In vivo functional discrimination between plant thioredoxins by heterologous expression in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA 95: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller EGD (1991) Thioredoxin deficiency in yeast prolongs S phase and shortens G1 interval of the cell cycle. J Biol Chem 266: 9194–9202 [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Jiménez A, Cleary SP, Robinson C, Creissen GP (1998) Identification of cDNAs encoding plastid-targeted glutathione peroxidase. Plant J 13: 375–379 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Paget MSB, Kang JG, Roe JH, Buttner MJ (1998) σR, an RNA polymerase sigma factor that modulates expression of the thioredoxin system in response to oxidative stress in Streptomyces coelicolor A3(2). EMBO J 17: 5776–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LB, Godzik A, Nayeem A, Schmitt JD (2000) AhpF can be dissected into two functional units: tandem repeats of two thioredoxin-like folds in the N-terminus mediate electron transfer from the thioredoxin reductase-like C-terminus to AhpC. Biochemistry 39: 6602–6615 [DOI] [PubMed] [Google Scholar]
- Prouzet-Mauléon V, Monribot-Espagne C, Boucherie H, Lagniel G, Lopez S, Labarre J, Garin J, Lauquin GJM (2002) Identification in Saccharomyces cerevisiae of a new stable variant of alkyl hydroperoxide reductase 1 (Ahp1) induced by oxidative stress. J Biol Chem 277: 4823–4830 [DOI] [PubMed] [Google Scholar]
- Pruvot G, Massimino J, Peltier G, Rey P (1996) Effects of low temperature, high salinity and exogenous ABA on the synthesis of two chloroplastic drought-induced proteins in Solanum tuberosum. Physiol Plant 97: 123–131 [Google Scholar]
- Rabilloud T, Heller M, Gasnier F, Luche S, Rey C, Aebersold R, Benahmed M, Louisot P, Lunardi J (2002) Proteomics analysis of cellular response to oxidative stress: evidence for in vivo overoxidation of peroxiredoxins at their active site. J Biol Chem 277: 19396–19401 [DOI] [PubMed] [Google Scholar]
- Rey P, Pruvot G, Becuwe N, Eymery F, Rumeau D, Peltier G (1998) A novel thioredoxin-like protein located in the chloroplast is induced by water deficit in Solanum tuberosum L. plants. Plant J 13: 97–107 [DOI] [PubMed] [Google Scholar]
- Ritz D, Patel H, Doan B, Zheng M, Aslund F, Storz G, Beckwith J (2000) Thioredoxin 2 is involved in the oxidative stress response in Escherichia coli. J Biol Chem 275: 2505–2512 [DOI] [PubMed] [Google Scholar]
- Royo J, Vancanneyt G, Perez AG, Sanz C, Stormann K, Rosahl S, Sanchez-Serrano JJ (1996) Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. J Biol Chem 271: 21012–21019 [DOI] [PubMed] [Google Scholar]
- Rustérucci C, Montillet JL, Agnel JP, Battesti C, Alonso B, Knoll A, Bessoule JJ, Etienne P, Suty L, Blein JP et al. (1999) Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. J Biol Chem 274: 36446–36455 [DOI] [PubMed] [Google Scholar]
- Schürmann P, Jacquot JP (2000) Plant thioredoxin systems revisited. Annu Rev Plant Physiol Plant Mol Biol 51: 371–400 [DOI] [PubMed] [Google Scholar]
- Stallaert VM, Ducruet JM, Tavernier E, Blein JP (1995) Lipid peroxidation in tobacco leaves treated with the elicitor cryptogein: evaluation by high temperature thermoluminescence emission and chlorophyll fluorescence. Biochim Biophys Acta 1229: 290–295 [Google Scholar]
- Takemoto T, Zhang QM, Yonei S (1998) Different mechanisms of thioredoxin in its reduced and oxidized forms in defense against hydrogen peroxide in Escherichia coli. Free Rad Biol Med 24: 556–562 [DOI] [PubMed] [Google Scholar]
- Vavilin D, Ducruet JM (1999) The origin of 115–130°C thermoluminescence bands in chlorophyll containing material. Photochem Photobiol 68: 191–198 [Google Scholar]
- Verdoucq L, Vignols F, Jacquot JP, Chartier Y, Meyer Y (1999) In vivo characterisation of a thioredoxin h target protein defines a new peroxiredoxin family. J Biol Chem 274: 19714–19722 [DOI] [PubMed] [Google Scholar]
- Zhu H, Bannenberg GH, Moldeus P, Shertzer HG (1994) Oxidation pathways for the intracellular probe 2′, 7′-dichlorofluorescein. Arch Toxicol 68: 582–587 [DOI] [PubMed] [Google Scholar]