Abstract
Glucose (Glc) signaling, along with abscisic acid (ABA) signaling, has been implicated in regulating early plant development in Arabidopsis. It is generally believed that high levels of exogenous Glc cause ABA accumulation, which results in a delay of germination and an inhibition of seedling development—a typical stress response. To test this hypothesis and decipher the complex interactions that occur in the signaling pathways, we determined the effects of sugar and ABA on one developmental event, germination. We show that levels of exogenous Glc lower than previously cited could delay the rate of seed germination in wild-ecotype seeds. Remarkably, this effect could not be mimicked by an osmotic effect, and ABA was still involved. With higher concentrations of Glc, previously known Glc-insensitive mutants gin2 and abi4 exhibited germination kinetics similar to wild type, indicating that Glc-insensitive phenotypes are not the same for all developmental stages of growth and that the signaling properties of Glc vary with concentration. Higher concentrations of Glc were more potent in delaying seed germination. However, Glc-delayed seed germination was not caused by increased cellular ABA concentration, rather Glc appeared to slow down the decline of endogenous ABA. Except for the ABA-insensitive mutants, all tested genotypes appeared to have similar ABA perception during germination, where germination was correlated with the timing of ABA drop to a threshold level. In addition, Glc was found to modulate the transcription of genes involved in ABA biosynthesis and perception only after germination, suggesting a critical role of the developmental program in sugar sensing. On the basis of an extensive phenotypic, biochemical, and molecular analysis, we suggest that exogenous Glc application creates specific signals that vary with concentration and the developmental stage of the plant and that Glc-induced fluctuations in endogenous ABA level generate a different set of signals than those generated by external ABA application.
Because metabolic and structural functions require the proper amount of carbon source, different organisms have developed the ability to sense internal levels of sugar and accordingly adjust their cellular and metabolic activities. These regulatory mechanisms are particularly important for plants, because sugar production, consumption, and storage occur in the same organism. On one hand, plants have developed sophisticated programs in managing sugar production in source tissue and sugar storage in sink tissue; on the other hand, a complex regulatory circuit controlling gene expression has evolved to accommodate constant changes of sugar-dependent cellular activities (Smeekens, 2000; Coruzzi and Bush, 2001; Coruzzi and Zhou, 2001).
Because sugars affect the expression of a diverse array of genes involved in different cellular processes, it is proposed that distinct signaling pathways are employed for the control of these genes. At least three types of Glc signal transduction mechanisms have been found in plants: hexokinase (HXK)-dependent pathways (Jang et al., 1997; Xiao et al., 2000), HXK-independent pathways (Martin et al., 1997; Mita et al., 1997; Roitsch, 1999; Xiao et al., 2000; Ciereszko et al., 2001), and glycolysis-dependent pathways that depend on the catalytic activity of HXK (Xiao et al., 2000). Besides gene regulation, sugars also negatively affect seed germination and early seedling development. This developmental arrest has been used for the genetic selection of mutants with altered response to sugars. For example, Glc-insensitive (gin; Zhou et al., 1998; Arenas-Huertero et al., 2000) and Suc-insensitive (sis; Laby et al., 2000) mutants were isolated using 333 mm Glc and 300 mm Suc, respectively. In contrast, Suc-uncoupled (sun) mutants were identified in a screen on the basis of the expression of a sugar-responsive fusion gene PC: LUC (Huijser et al., 2000). More recently, the impaired Suc induction (isi) mutants were isolated using a reporter gene ApL3:GUS (Rook et al., 2001). Surprisingly, most of these mutants turned out to be abscisic acid (ABA)-related. For instance, gin6/isi3/sis5/san5/sun6 is allelic to ABA-insensitive mutant abi4-1, gin1/isi4/san3/sis4/sre1 is allelic to ABA deficient mutant aba2, and gin5/isi2/sis3 is allelic to aba3 (Rolland et al., 2002). It has been suggested that Glc-induced ABA accumulation is essential for HXK-mediated Glc responses (Arenas-Huertero et al., 2000). However, the role of ABA in sugar signaling, how sugar signaling and ABA signaling crosstalk, and whether ABA-independent sugar-signaling pathways exist are still uncertain (Finkelstein and Gibson, 2001).
The relationship between sugar and ABA in regulating seed germination is unclear. It is presumed that high levels of exogenous Glc cause ABA accumulation, which results in a delay of germination and an inhibition of early seedling development. Arenas-Huertero et al. (2000) showed that ABA accumulated 3- to 6-fold in seedlings when treated with high concentrations of Glc. In contrast, endogenous ABA concentration was not changed in seedlings when treated with low levels (e.g. 27.8 mm) of Glc (Garciarrubio et al., 1997). Because these studies were conducted using seedlings but not imbibed or germinating seeds, it is not known whether exogenous sugar can induce ABA accumulation and consequently inhibit seed germination. Intriguingly, a low to intermediate level (<167 mm) of exogenous Glc was able to relieve the inhibition of seed germination induced by exogenous ABA. It was proposed that ABA inhibits seed germination through the restriction of energy and metabolites, because germination could be restored by the addition of metabolizable sugars and amino acids in the culture medium (Garciarrubio et al., 1997). These results were supported by a more recent study using 15 to 90 mm Glc, Suc, or Fru (Finkelstein and Lynch, 2000). However, multiple lines of evidence from this study indicated that the effect of sugar was likely caused by the change of signaling in addition to metabolic events. A subsequent report demonstrated that the addition of sugar inhibited mobilization of stored lipids during germination (To et al., 2002).
In the past, the sensitivity of a plant to sugar signals was primarily determined by looking at overall plant growth, a process involving germination, cotyledon greening and expansion, hypocotyl elongation, true leaf development, and root growth. Although many mutants have been identified in such way, their responses to sugar in each of these developmental steps are not clear. Here, we use a simple but effective approach to systematically determine sugar sensitivity in each mutant. We have found that some mutants previously known to be sugar insensitive are actually sensitive to the sugar-induced delay of seed germination. We have also provided additional evidence for an intimate relationship between sugar and ABA in controlling seed germination.
RESULTS
To understand the linkages between the sugar- and ABA-signaling pathways, we observed how Glc and ABA affected seed germination. Germination kinetics was chosen for study because germination can be simply and objectively scored for all seeds in a sample and because other developmental events (like hypocotyl elongation or cotyledon greening) appear to involve additional or different signaling mechanisms than those for germination. Seeds, including wild ecotypes (WT) and various mutants insensitive to Glc or ABA, were plated on Murashige and Skoog plates with added Glc and/or ABA. To ensure uniformity of results, all seeds were harvested and stored identically, seeds used in tests were selected for similar size and color, the Murashige and Skoog plates used contained the same amount of medium and were handled identically, and the seeds were evenly spaced in grids on the plates to avoid spatial variations. Germination experiments were repeated both as simultaneous replicate plates and as separate experiments. The kinetics of germination was determined by measuring the proportion of seeds at different time points in a sample where the radicle had begun to emerge from the seed coat. To interpret the results, we compared the rates of germination between different strains and different treatments throughout the time course. The slope of the curves and the timing of when the germination rates started to change revealed the differences that occurred among the various samples and treatments.
Arabidopsis Seed Germination Is Sensitive to Low Levels of Glc
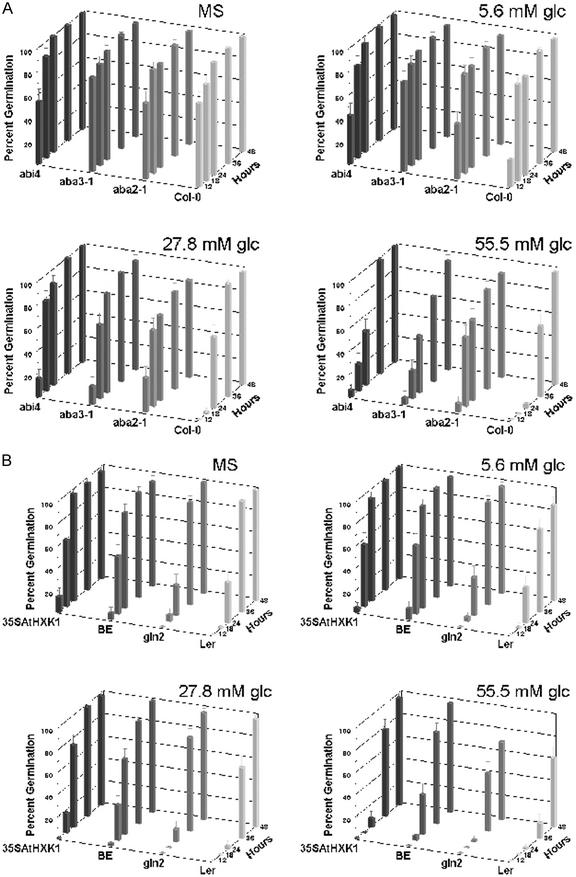
To assess the amount of Glc required to transmit a signal during germination, seeds were grown in the presence of low Glc levels, and germination kinetics was determined (Fig. 1). WTs Columbia (Col-0), Landsberg erecta (Ler), and Bensheim (BE) all showed a delay in germination in the presence of exogenous Glc. Delay of germination was evident even at 27.8 mm Glc in Col-0, which is considered well below the level (333 mm) repressive for cotyledon greening and early seedling development (Jang et al., 1997) or ABA accumulation (Garciarrubio et al., 1997). Compared with other tested ecotypes, Ler exhibited an overall shift toward later germination, whereas BE was less sensitive to low exogenous Glc (Fig. 1B). In all ecotypes, the sensing of the low Glc levels occurred very early in the germination process, where germination delay was evident as early as 12 h after transfer to 24°C in the light.
Figure 1.
Arabidopsis can sense a low level of exogenous Glc during seed germination. Arabidopsis seeds were surface-sterilized and water-imbibed in the dark for 3 d at 4°C. Seeds were transferred to 1× Murashige and Skoog plates containing B5 vitamins, 0.05% (w/v) MES (pH 5.7), and 0.7% (w/v) phytagar (Invitrogen) without sugar (Murashige and Skoog) or with Glc (concentration indicated in the figure). The plant material was incubated in the dark at 4°C for 3 d to break dormancy and was then transferred to light at 24°C. Germination kinetics was determined by measuring the time of radicle emergence after transfer to constant light and 24°C. A, ABA biosynthetic mutants aba2-1 and aba3-1 and ABA perception mutant abi4 are less sensitive to Glc than their background ecotype Col-0. B, Compared with the background ecotype, there were no apparent changes in Glc sensitivity in either HXK (AtHXK1) loss-of-function mutant gin2 (in Ler background) or AtHXK1 overexpresser 35SAtHXK1 (in BE background).
The initial delay of germination was largely relieved in the ABA biosynthetic mutant aba2-1, indicating that somehow, ABA is involved in transmitting the signal triggered by low-level Glc. The ABA-insensitive mutant abi4-1, which has been reported to be a transcriptional regulator affecting ABA signaling, also reduced this delay but to a lesser extent than did aba2-1. Another ABA biosynthetic mutant aba3-1 also reduced this delay but was not as effective as aba2-1 (Fig. 1A). Unlike aba2-1, aba3-1, and abi4-1, the dominant ABA-insensitive mutant abi1-1 appeared to be sensitive to Glc. It had germination kinetics similar to Ler, thus ABI1 is not likely to be an important component in transmitting a sugar signal (data not shown). The mutant strain gin2, which is defective in AtHXK1 (Rolland et al., 2002), had slightly quicker germination kinetics in the presence of Glc when compared with its parental ecotype Ler (Fig. 1B), more notable at 55.5 mm Glc. In contrast, a Glc-hypersensitive transgenic line overexpressing AtHXK1, CaMV35S:AtHXK1, exhibited a somewhat enhanced Glc-induced delay of germination when compared with parental ecotype BE. Although both results were reproducible, the subtle difference suggests that AtHXK1 may play only an indirect role in sensing low levels of Glc during germination.
Arabidopsis Seed Germination Is Not Affected by Low Levels of Mannitol
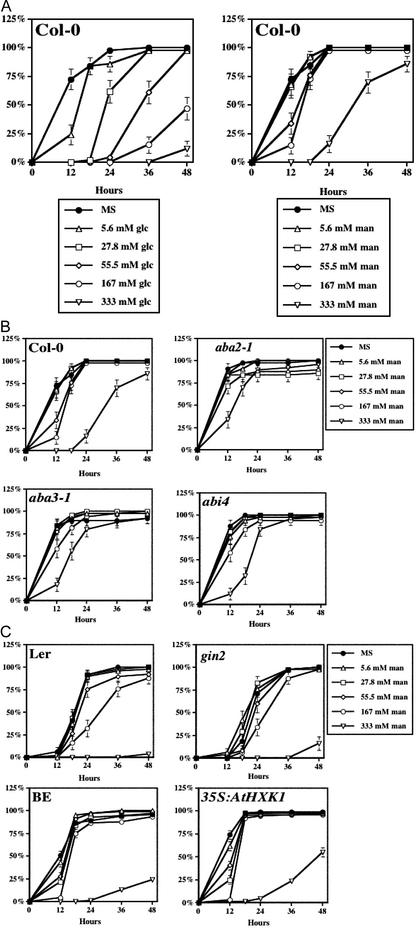
To test whether Glc-induced delay of seed germination was an osmotic effect, germination kinetics was also determined for seeds plated on medium containing mannitol, a non-metabolizable sugar. As shown in Figure 2, a delay in germination still occurred, but only when mannitol was at a high concentration (333 mm), and the delay was much less effective when compared with that caused by Glc (Fig. 2A). This suggests that the delay in kinetics caused by low Glc (shown in Fig. 1) is not a pure osmotic response. The delay caused by 333 mm mannitol may be due to osmotic stress, because aba2-1, aba3, and abi4-1 were less sensitive to this treatment (Fig. 2B).
Figure 2.
Osmotic effect does not mimic the inhibitory effect of Glc on seed germination. Plant material was prepared as described in Figure 1, except that no sugar (Murashige and Skoog) or various concentrations of either mannitol or Glc (indicated in the legend) were added to the Murashige and Skoog plates. Germination kinetics was determined by measuring the time of radicle emergence after transfer cultures to constant light, 24°C. A, Compared with mannitol, Glc is more effective in delaying seed germination. B, ABA biosynthetic mutants aba2-1 and aba3-1 and ABA perception mutant abi4 are less sensitive to 333 mm mannitol than the background ecotype Col-0. C, HXK does not appear to be involved in osmotic sensing. Compared with the background ecotype, there is no apparent difference in germination kinetics in response to mannitol in either HXK (AtHXK1) loss-of-function mutant gin2 (in Ler background) or AtHXK1 overexpresser 35SAtHXK1 (in BE background).
Similar to Glc response, different ecotypes exhibited varied sensitivity to mannitol. Ler and BE ecotypes turned out to be hypersensitive to 333 mm mannitol when compared with the Col-0. In contrast to ABA mutants, HXK did not seem to be involved in sensing low concentrations of mannitol because gin2 and CaMV35S:AtHXK1 displayed a similar pattern to Ler and BE, respectively (Fig. 2C).
Do Glc and ABA Inhibit Seed Germination through the Same Mechanism?
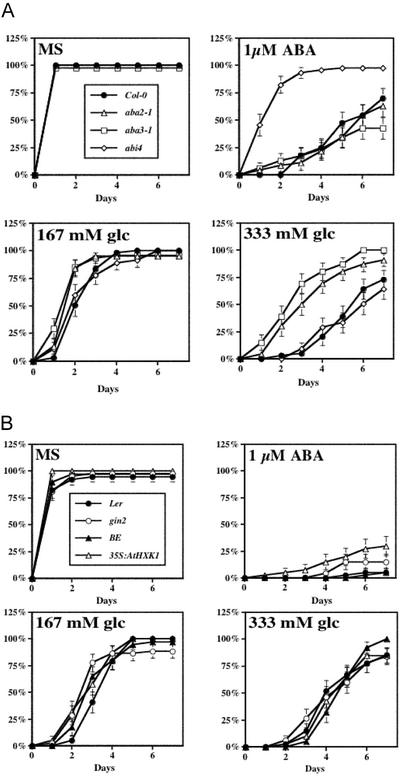
It is generally accepted that high levels of Glc induce ABA accumulation, which causes delays in development (Arenas-Huertero et al., 2000). However, there has not been a systematic investigation during germination using various WT ecotypes and mutants to confirm this notion. To do this, we compare germination kinetics between intermediate (167 mm) and high (333 mm) levels of Glc and 1 μm exogenous ABA (Fig. 3A). Surprisingly, neither aba2-1/aba3-1 nor abi4-1 showed significant difference in germination kinetics caused by 167 mm Glc when compared with the WT. However, whereas aba2-1 and aba3-1 showed resistance to 333 mm Glc, abi4-1 exhibited a response similar to the WT. By contrast, the abi4-1 mutant displayed a robust resistance to 1 μm ABA that was not observed in aba2-1, aba3-1, or WT. We also determined the role of HXK in sensing high levels of Glc and ABA (Fig. 3B). Similar to ABA mutants, neither gin2 nor 35S:AtHXK1 displayed altered sensitivity to 167 mm Glc. Surprisingly, neither of them was significantly different from the WT in the response to 333 mm Glc. Together, these results raise the possibility that neither ABI4 nor AtHXK1 is involved in germination controlled by high levels of Glc. The results also indicate that a “Glc-insensitive” phenotype may not be universal to all developmental stages of plant growth, because although these mutants appear to be sensitive to high Glc levels during germination, abi4 (Arenas-Huertero et al., 2000; Finkelstein and Lynch, 2000; Huijser et al., 2000; Laby et al., 2000; Rook et al., 2001) and plants with reduced HXK levels (Jang et al., 1997) are less sensitive to high levels of Glc during early seedling development.
Figure 3.
Sugar sensing is affected by developmental program, because previously known sugar-insensitive mutants abi4 and gin2 are sensitive to a high level of Glc during germination. Plant material was prepared as described in Figure 1, except that no sugar (Murashige and Skoog), or Glc (concentration indicated in the figure) or 1 μmABA was added to the Murashige and Skoog plates. Germination kinetics was determined by measuring the time of radicle emergence after transfer cultures to constant light and 24°C. A, Compared with the background ecotype Col-0, whereas ABA biosynthetic mutants aba2-1 and aba3-1 are less sensitive to Glc, abi4 is less sensitive to ABA. B, HXK AtHXK1 is not involved in sensing either Glc or ABA during seed germination. Compared with the background ecotype, there is no apparent difference in germination kinetics in either HXK (AtHXK1) loss-of-function mutant gin2 (in Ler background) or AtHXK1 overexpresser 35SAtHXK1 (in BE background).
Glc Modulates ABA-Induced Delay of Seed Germination
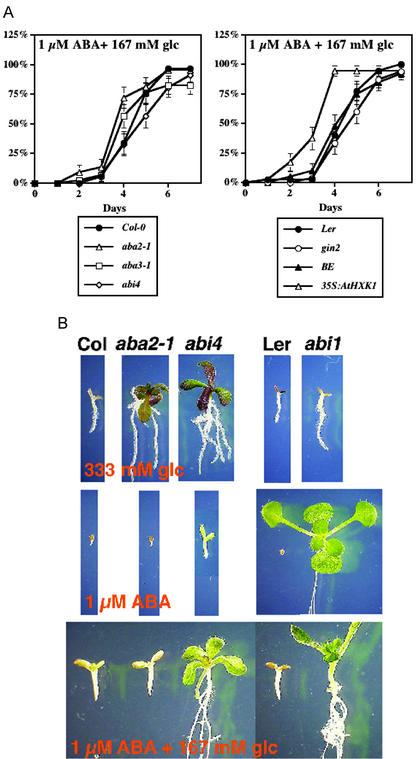
Germination kinetics provided information on another developmental effect that we observed, partial relief of ABA-induced delay of germination in the presence of exogenous Glc. This effect was first noted in WT seeds (Garciarrubio et al., 1997); we further investigated the kinetics of this relief in various Glc- and ABA-insensitive strains. In the presence of exogenous ABA, seed germination was usually repressed (Fig. 3A). This repression was diminished when 167 mm Glc, a level repressive for germination but not for subsequent overall seedling development, was also added to the medium (Fig. 4A). Lower levels of exogenous Glc, e.g. 16.7 mm, were also effective in diminishing the delay in germination caused by ABA (data not shown). Interestingly, the rate of germination was not statistically different in aba2-1, aba3-1, abi4-1, and their parental ecotype Col-0, suggesting that the presence of both Glc and ABA overrides the normal ABA-responsive pathways. However, 35S: AtHXK1 did show quicker relief of repression than the parental ecotype BE in the rescue experiment (Fig. 4A), suggesting that HXK is likely a positive factor for Glc effect antagonistic to ABA. In a previous report, sugar was shown in WT to suppress ABA inhibition of radicle emergence but not seedling growth (Finkelstein and Lynch, 2000), although the genetic components involved were not identified. We were surprised to find that except for the ABA-insensitive mutants, seedlings that germinated with Glc and ABA did not develop further after germination (Fig. 4B). We noted that plants did not develop uniformly in the absence of exogenous sugar. Although abi4 could germinate without delay and turned green in the presence of 1 μm ABA, less than 50% of the seedlings developed further to form true leaves. This is in contrast to abi1, in which more than 50% of the seedlings could form true leaves under the same condition. The phenotypic variation between abi4 and abi1 might be due to the different background ecotype, or it is possible that ABI4 and ABI1 differ in their temporal expression or their developmental roles. This response of abi4 may also be due to the specific characteristics of the abi4-1 allele, because differences in phenotypic expression were apparent in abi4-3 and abi4-4 plants treated with 3 μm ABA after 3 d of growth (Huijser et al., 2000). Together, these results suggest that the signaling events occurring during germination are distinct from those affecting post-germination events and that ABA can have important regulatory effects in post-germination growth.
Figure 4.
Glc partially relieves ABA-induced inhibition of seed germination but not subsequent seedling development. A, Compared with the background ecotype Col-0, Glc rescue of ABA-induced inhibition of seed germination is not altered in either ABA biosynthetic mutants aba2-1 and aba3-1 or ABA perception mutant abi4. However, HXK seems to be a positive regulator for this rescue, because faster germination kinetics was observed in AtHXK1 overexpresser 35SAtHXK1 (in BE background). Plant material was prepared as described in Figure 1, except that 167 mm Glc and 1 μm ABA were added to the Murashige and Skoog plates. Germination kinetics was determined by measuring the time of radicle emergence after transfer cultures to constant light and 24°C. B, Although Glc can rescue the inhibition of seed germination induced by ABA, subsequent seedling development only occurred in ABA-insensitive mutants (abi1 and abi4). Seedlings of aba2-1 (Col-0 background), abi4 (Col-0 background), and abi1 (Ler background) were grown for 21 d in constant white light (90 μE m–2 s–1) at 25°C. Plant material was prepared as described in Figure 1, except that either 333 mm Glc, 1 μm ABA, or a combination of 167 mm Glc and 1 μm ABA was added to the Murashige and Skoog plates.
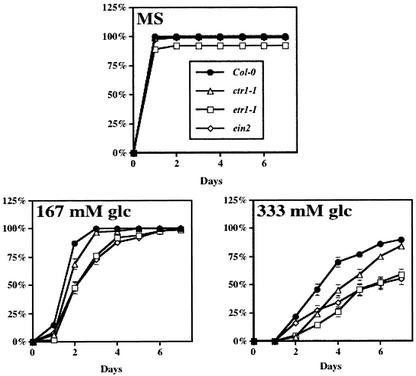
The Role of Ethylene in Glc Signaling
On the basis of seedling development, sugar signaling has been shown to interact with ethylene-signaling pathway (Zhou et al., 1998; Gazzarrini and McCourt, 2001). Although ethylene acts as a negative regulator of ABA action during germination and CTR1, EIN2, and ETR1 are involved in this interaction (Beaudoin et al., 2000; Ghassemian et al., 2000; Gazzarrini and McCourt, 2001), it is not clear whether these components are involved in sugar response during germination. We determined the effect of exogenous Glc on the germination kinetics of ethylene-signaling mutants. Similar to the abi4-1 mutant, ctr1-1 was as sensitive to high concentrations of Glc (167 or 333 mm) as its parental ecotype (Fig. 5), indicating either that high Glc may have a repressive effect downstream of CTR1 in the ethylene-signaling pathway or that high Glc represses seed germination independent of CTR1. It is noted that the germination kinetics of Col-0 with 333 mm Glc was somewhat different in Figures 3A and 5; this is likely due to the use of two different WT seed lots for the two sets of experiments. Ethylene-insensitive mutants etr1-1 and ein2-1 appeared to have a delay of germination kinetics even greater than that of ctr1-1 or WT in the presence of high concentrations of Glc (Fig. 5). Consistent with the idea that CTR1 is a negative regulator and EIN2 is a positive regulator of ethylene response, we have found that whereas ctr1-1 is less sensitive, ein2-1 is more sensitive to exogenous ABA (1 μm) during germination (data not shown). Collectively, our results suggest that ethylene signaling antagonizes the inhibitory effect of Glc on seed germination. ABA is likely involved in transmitting the sugar signal because our results resemble a model showing that ethylene plays a role opposing ABA during seed germination (Beaudoin et al., 2000).
Figure 5.
Ethylene-signaling mutants have altered Glc response during germination. Germination kinetics was determined in Col-0 or ethylene-signaling mutants ctr1-1, etr1-1, or ein2. Plant material was prepared as described in Figure 1, except that no sugar (Murashige and Skoog) or high concentration of Glc (indicated in the figure) was added to the Murashige and Skoog plate. Germination kinetics was determined by measuring the time of radicle emergence after transfer to constant light and 24°C.
Exogenous Glc Delays Decline in Endogenous ABA Concentration during Seed Germination
To better understand how endogenous ABA affects germination, we observed how endogenous ABA levels fluctuate over time with and without the presence of exogenous Glc or ABA. ABA levels were measured in plant material of various time points and treatments using the Phytodetek ABA kit (Agdia Inc., Elkhart, IN). To minimize variation in the results, different lots of kit reagent were tested for consistency before obtaining the final results, the most consistent reagent lot (no. 38, mfg. date 2/99) was used for all experiments, duplicate samples were prepared in each experiment, and each experiment was repeated. Consistent with previous reports (Leon-Kloosterziel et al., 1996), the endogenous ABA concentration was significantly lower in ABA biosynthetic mutants aba2-1 and aba3-1 than that in the WT (Table I). This reduction was also evident in gin1, a mutant allelic to aba2-1 (Cheng et al., 2002), compared with its WT WS (data not shown). In contrast, the transcriptional regulator mutant abi4-1 had endogenous ABA levels in imbibed seeds similar to WT, indicating that ABI4 does not affect internal ABA levels in the seeds (Table I). Cellular ABA concentration went down in WT seeds after a 3-d incubation in 4°C water. Similar changes in ABA concentration were observed in aba2-1 and aba3-1. Surprisingly, the concentration of ABA in abi4-1 did not decrease after 3 d in 4°C water, indicating that unlike aba2-1 and aba3-1, this mutant may not have responded normally to the cold treatment. The endogenous ABA concentration continued to drop after the seeds were transferred to Murashige and Skoog medium containing Glc during a 3-d incubation at 4°C in the dark. It appeared that the onset of maximal germination occurred when endogenous ABA levels decreased to 2 ng ABA g–1 fresh weight. Some minor variations to this trend were noted in our data; we suspect that this is due to the limited number of replicate experiments that we could conduct because the availability of lot 38 reagent was limited. In Col-0, internal ABA levels dropped from 5.4 (Table I) to 1.9, 2.9, and 4.2 ng ABA g–1 fresh weight with a treatment of 0, 167, and 333 mm Glc, respectively (d 0 data in Table II). A concentration-dependent Glc inhibition of ABA level decline was also observed in abi4-1 (compare data in Table I with d 0 data in Table III), aba2-1, and aba3-1 (data not shown). Another trend that became apparent in both WTs and mutant strains was that in the absence or presence of 167 mm Glc, ABA levels continued to decline both before and after the onset of germination in the light at 25°C. Endogenous ABA concentrations in aba2-1 and aba3-1 had already dropped below the threshold level on d 0, and they continued to decline during germination (data not shown). In contrast, internal ABA levels began to rise after reaching a threshold low level in the WT and abi4-1 treated with 333 mm Glc (Tables II and III). Together, these results indicate that ABA levels decline as part of the normal germination process, and this can be perturbed by high concentrations of exogenous Glc. The rebound of ABA concentration in samples treated with 333 mm Glc on d 6 is likely due to ABA accumulation in a portion of germinated seeds (64.4% in WT; 51% in abi4). ABA levels declined more rapidly and stayed low in the presence of low-level Glc, whereas high exogenous Glc reduced the rate of the ABA decline. Additionally, the timing of germination appeared to be correlated with the decline of ABA concentration. Germination appeared to reach maximal levels as the ABA concentration declined past a threshold value of approximately 2 ng ABA g–1 fresh weight.
Table I.
Cellular ABA contents in imbibed and cold-treated seeds
| Treatment | Col-0 | aba2-1 | aba3-1 | abi4-1 |
|---|---|---|---|---|
| Imbibitiona | 8.3 ± 1.2b | 3.9 ± 0.6 | 3.6 ± 0.2 | 7.0 ± 0.8 |
| 3 d in 4°C water | 5.4 ± 0.2 | 2.4 ± 0.2 | 2.2 ± 0.4 | 8.1 ± 1.0 |
Seeds were collected after surface sterilized with 50% commercial bleach for 10 min and rinsed with sterile distilled water for four times at 10-min intervals. b Values are the mean ± SD of ng ABA g-1 fresh weight.
Table II.
The relationship between germination and cellular ABA contents in the WT Col-0
| Timea
|
Treatment
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 mM Glc | 167 mM Glc | 333 mM Glc | 1 μM ABA | |||||
| d 0 | 0b | (1.9 ± 0.6)c | 0 | (2.9 ± 1.2) | 0 | (4.2 ± 1.8) | 0 | (5.0 ± 0.6) |
| d 2 | 100 | (2.0 ± 0.0) | 50.9 ± 10 | (1.8 ± 0.0) | 3.4 ± 3.6 | (4.7 ± 0.2) | 0 | (9.2 ± 2.4) |
| d 4 | 100 | (1.8 ± 0.2) | 98.4 ± 2.5 | (2.1 ± 1.0) | 20.4 ± 8.1 | (2.9 ± 0.0) | 24.6 ± 8.6 | (13.2 ± 3.4) |
| d 6 | 100 | (1.6 ± 1.0) | 100 | (1.0 ± 0.4) | 64.4 ± 9.6 | (4.2 ± 1.0) | 54.6 ± 10 | (15.0 ± 3.0) |
Time after seeds had been surface sterilized, incubated in 4°C water in the dark for 3 d, incubated in 4°C Murashige and Skoog in the dark for 3 d, and transferred to 25°C in the light. b Values are the mean ± SD of percentage of germination as shown in Figure 3A. c Values are the mean ± SD of ng ABA g-1 fresh weight.
Table III.
The relationship between germination and cellular ABA contents in abi4-1 mutant
| Timea
|
Treatment
|
|||||
|---|---|---|---|---|---|---|
| 0 mM Glc | 167 mM Glc | 333 mM Glc | ||||
| d 0 | 0b | (4.5 ± 0.8)c | 0 | (7.8 ± 1.4) | 0 | (7.3 ± 2.2) |
| d 2 | 100 | (2.0 ± 0.0) | 60.3 ± 9.8 | (3.3 ± 0.4) | 0 | (4.1 ± 0.2) |
| d 4 | 100 | (1.1 ± 0.0) | 89.0 ± 6.3 | (2.0 ± 0.4) | 28.9 ± 9.1 | (3.1 ± 0.2) |
| d 6 | 100 | (0.8 ± 0.2) | 100 | (0.7 ± 0.4) | 51.0 ± 10 | (5.2 ± 0.4) |
Time after seeds had been surface sterilized, incubated in 4°C water in the dark for 3 d, incubated in 4°C Murashige and Skoog in the dark for 3 d, and transferred to 25°C in the light. b Values are the mean ± SD of percentage of germination derived from Figure 3A. c Values are the mean ± SD of ng ABA g-1 fresh weight.
To further understand the mechanisms controlling germination, we compared the endogenous levels of ABA present in germinating seeds treated with exogenous Glc or ABA. In previous studies, aba2-1 was shown to be insensitive to Glc, so we anticipated that Glc-repressed germination in WT seeds would occur through increased biosynthesis of endogenous ABA. Germination kinetics with 333 mm Glc and 1 μm ABA (Figs. 3A and 5) were quite similar in Col-0, making it seem reasonable that the two treatments repressed through a similar mechanism. When endogenous ABA levels were measured in similarly treated Col-0 seeds, we were surprised to find that whereas ABA levels in 333 mm Glc-treated seeds declined during germination, they accumulated to a very high level in seeds treated with 1 μm exogenous ABA (Table II). These results suggest that the onset of germination may be determined by factors other than overall ABA concentration. We cannot rule out the possibility that Glc repression causes a localized increase of ABA in target tissues or that the levels of ABA present in Glc-treated seeds are sufficient to cause repression. However, because the germination kinetics of Col-0 was similar in both 333 mm Glc and 1 μm ABA, endogenous ABA concentration alone is not sufficient to explain why these treatments had resulted in similar germination kinetics.
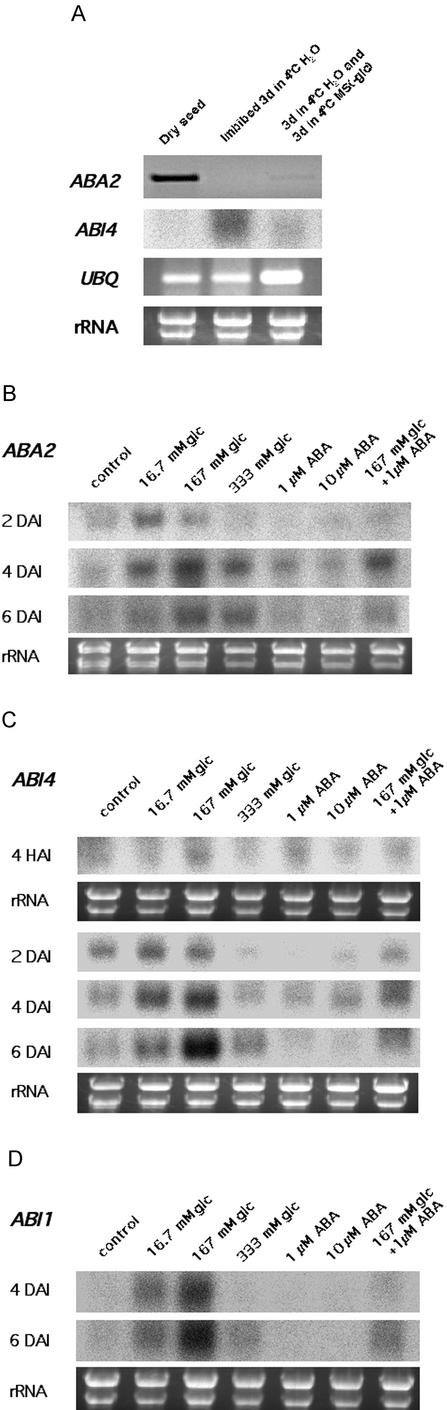
Glc Affects the Expression of ABI1, ABA2, and ABI4
Our results suggest that ABI4 is involved in plant response to a low concentration of Glc (Fig. 1A) and exogenous ABA (Fig. 3A). By contrast, ABA2 is involved in plant response to both low and high concentrations of Glc (Figs. 1A and 3A) but not to exogenous ABA (Fig. 3A). Because the effects of high Glc and exogenous ABA were not identical, we hypothesized that genes involved in ABA biosynthesis and signaling might be regulated by Glc during germination and early seedling development. To compare gene expression profiles with the kinetics of germination rate and cellular ABA contents, we followed the same protocol in preparing plant material. Ecotype Col-0 dry seeds were surface-sterilized and incubated in 4°C water for 3 d. Cold-imbibed seeds were then transferred to a flask containing Murashige and Skoog liquid and incubated for additional 3 d at 4°C to ensure a complete removal of seed dormancy in the control (Murashige and Skoog without Glc or ABA). Cultures with various treatments were then allowed to germinate and grow in the white light at 24°C on a shaker (140 rpm). Consistent with the role of ABA in seed dormancy, ABA2 was present in dry seeds, although the expression level was very low and could only be detected by reverse transcription (RT)-PCR but not by standard RNA gel-blot analysis. The low level of ABA2 expression disappeared after seeds were imbibed in 4°C water for 3 d (Fig. 6A). In contrast, ABI4 was not detectable in WT dry seeds but was induced after imbibition in 4°C water for 3 d. The expression of ABI4 decreased significantly when seeds were transferred from water to Glc-free Murashige and Skoog medium, suggesting that certain components in Murashige and Skoog medium or developmental factors negatively regulated the expression of ABI4, even though metabolic activities of the seeds were relatively low when kept at 4°C (Fig. 6A).
Figure 6.
Developmental program-dependent induction of ABA2 and ABI4 by Glc. A, Imbibition exerts an opposing effect on the expression of ABA2 and ABI4. Col-0 seeds were surface disinfected and immediately collected (Dry seed), imbibed for 3 d in water at 4°C and collected, or imbibed at 4°C water for 3 d and incubated in 4°C Glc-free 1× Murashige and Skoog medium for another 3 d and then collected. ABA2 expression was determined by RT-PCR following the manufacturer's protocol (Panvera, Madison, WI), and ABI4 was determined by RNA gel-blot analysis. Five micrograms of RNA was loaded in each lane. RT-PCR of ubiquitin expression and ethidium bromide staining of rRNA bands are shown for quality and loading controls, respectively. B, RNA gel-blot analysis of ABA2 expression during germination. Col-0 seeds were surface disinfected, imbibed 3 d in water at 4°C and another 3 d at 4°C in Glc-free 1× Murashige and Skoog liquid medium containing B5 vitamins and MES (pH 5.7) without sugar (control) or with Glc and/or ABA (concentration indicated in the figure). After transferring cultures to constant light at 24°C, samples were collected 2, 4, or 6 d after incubation (DAI), and total RNA was isolated. Five micrograms of RNA was loaded in each lane. Ethidium bromide staining of rRNA bands for 2-DAI samples is shown for loading control. RNA quality and loading are similar for 4- and 6-DAI samples. C, RNA gel-blot analysis of ABI4 expression during germination. Plant material was prepared identically to the procedure in Figure 6B, except that samples were collected 4 h after incubation (HAI) in addition to 2, 4, and 6 DAI. Five micrograms of RNA was loaded in each lane. Ethidium bromide staining of rRNA bands for 4-HAI and 2-DAI samples is shown for loading control. RNA quality and loading are similar for 4- and 6-DAI samples. D, RNA gel-blot analysis of ABI1 expression during germination. Plant material was prepared identically to the procedure in Figure 6B, except that samples were collected 4 and 6 DAI. Five micrograms of RNA was loaded in each lane. Ethidium bromide staining of rRNA bands for 4-DAI samples is shown for loading control. RNA quality and loading are similar for the 6-DAI samples.
Later in the germination and early seedling development, the expression of ABA2, ABI4, and ABI1 was enhanced by Glc but was relatively unaffected by ABA (Fig. 6, B–D). It is possible that the effect of Glc or ABA was limited by the developmental stage of the plants. For instance, the ABA2 induction appeared to be correlated with the onset of post-germination events such as hypocotyl and cotyledon development, i.e. when WT seed germination approached 100% with each Glc treatment (Table II), ABA2 expression reached a maximal level at the same time. Sugar concentration also had an effect independent of germination status, because although the seeds treated with 16.7 and 167 mm Glc were both germinated by d 4, induction of ABA2 was clearly higher with 167 mm Glc. We were not able to detect the presence of ABA2 transcript by either RNA gel-blot analysis or RT-PCR in any sample 4 HAI in the light at 25°C, where no seeds had been germinated. Compared with 167 mm Glc, 333 mm Glc was relatively ineffective in ABA2 induction, presumably because seed germination has yet to occur (Table II). The expression pattern of ABI1 or ABI4 was similar to that of ABA2 except that ABI1 was not expressed in the early stage of germination (4 HAI and 2 DAI; Fig. 6, C and D). This contrasts with seedlings grown 2 or more weeks, where transcription of ABI4 is induced by 333 or 389 mm Glc but not 111 mm Glc (Arenas-Huertero et al., 2000; Cheng et al., 2002). These results indicate that the developmental programs of the seeds tightly control the expression of these genes but that these programs can be modified at specific developmental stages by the presence of sugar. Sugar acts to stimulate ABA biosynthesis in germinated seedlings by up-regulating genes involved in ABA biosynthesis, such as ABA2. On the other hand, sugar also activates the expression of both ABI1 and ABI4, both of which are implicated in ABA signaling. Nevertheless, it is still not clear why 333 mm Glc has such a potent negative effect on germination and early seedling development, because expression of ABA2, ABI1, and ABI4 is only slightly affected by high Glc levels. One possible explanation is that 333 mm Glc may affect other genes critical for ABA metabolism, because high Glc concentration slowed down the decline of endogenous ABA during germination (Tables I,II,III). This could include genes specific to the developmental stage of the plant, because only approximately 50% of the seeds are germinated with high-level Glc compared with nearly 100% germination with 167 mm Glc. Another possibility is that the response to 333 mm Glc is actually a combined response to both a sugar-specific signal and an osmotic signal, because an equivalent concentration of mannitol clearly affected germination in WT (Fig. 2A).
DISCUSSION
A primary role of sugar in regulating seed germination is to modulate both cellular ABA concentration and ABA response. Germination kinetics has assisted in discerning which genes may be involved in the transmission of the sugar or ABA signal. Previous studies have shown that gin6, isi3, sis5, and sun6 are allelic to abi4-1, which confer resistance to growth-repressive levels of sugars. On the basis of these reports, we anticipated that abi4-1 would also confer resistance to Glc during germination. However, abi4-1 showed sensitivity to 333 mm Glc similar to its parental ecotype Col-0. The lack of resistance to sugar in abi4-1 indicates that ABI4 either is not essential or is redundant in the transmission of sugar signal during germination, even though it is involved in osmotic sensing, because abi4-1 showed reduced sensitivity to 333 mm mannitol. Nevertheless, ABI4 is clearly involved in Glc response after seed germination, because there is no developmental arrest observed in abi4-1 mutant (Fig. 4B).
This finding is consistent with studies of ABI5, in which ABI5 was shown to be a regulator required for maintaining a germinated embryo in a quiescent state in a narrow developmental window but not directly involved in the control of germination (Lopez-Molina et al., 2001). A number of other ABA-responsive elements binding bZIP factors such as ABF3 and ABF4 (Kang et al., 2002) also control ABA response in vegetative stage. Overexpression of either ABI5 or ABF3/ABF4 leads to an ABA-hypersensitive phenotype, i.e. an arrest of early seedling development under the level of ABA non-inhibitory to the WT plants. Intriguingly, these plants also showed longer delay of seed germination than the WT when treated with exogenous ABA. This suggests that an unknown negative regulator of seed germination can be trans-activated by excessive amount of either ABI5 or ABF3/ABF4, although their default function is to control vegetative development but not seed germination. It will be interesting to find out whether overexpression of the ABI4 type of AP2/EREBP transcription factors will result in similar phenotypes.
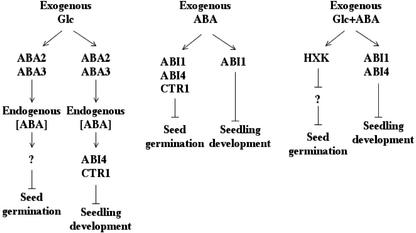
Because abi4-1 (Fig. 3A), ctr1-1 (Beaudoin et al., 2000), and abi1-1 (Gosti et al., 1999) are insensitive to exogenous ABA but sensitive to 333 mm Glc (Figs. 3A and 5) during seed germination, we propose that there are different ABA-sensing mechanisms in controlling seed germination and seedling development (Fig. 7). Sensing may involve changes in expression levels driven by the concentration of signal molecule (Fig. 6) as well as temporal effects of different genes on the developmental program. The latter is evident when comparing ABI4 expression during germination and seedling development, where ABI4 is not induced by 333 mm Glc before germination (Fig. 6C) but is induced by the same treatment during later growth (Arenas-Huertero et al., 2000; Cheng et al., 2002). Unlike aba2-1 and aba3-1, the dominant ABA-insensitive mutant abi1-1 is sensitive to Glc (Arenas-Huertero et al., 2000; Huijser et al., 2000; Laby et al., 2000). We have found that abi1-1 has germination kinetics similar to WT (data not shown), thus ABI1 is not likely an important component in transmitting sugar signals during seed germination. Similar to abi4, the ethylene-signaling mutant ctr1 was sensitive to Glc during seed germination (Fig. 5) but not during subsequent seedling development (Zhou et al., 1998). Although it has been proposed that sugar- and ethylene-signaling pathways may interact and that ctr1 is important for both pathways (Zhou et al., 1998; Gibson et al., 2001), the involvement of CTR1 is apparently restricted to certain but not all developmental processes. Ethylene-signaling mutants etr1 and ein2 were more sensitive to Glc during germination than the WT (Fig. 5), suggesting that the ethylene signal partially reduces the germination repression caused by Glc. Exogenous ABA appears to have an effect on germination different from the effect caused by high Glc treatment, perhaps because one treatment creates additional signaling events that are not replicated in the other treatment. Evidence for this possibility is apparent when one considers the response of abi4 to 1 μm ABA and 333 mm Glc. If a decline in ABA concentration were the sole signal for germination, abi4 would be expected to behave similarly whether a high concentration of Glc or ABA is present. However, abi4 germinated robustly on ABA but behaved like WT with high-level Glc, suggesting that something other than just ABA concentration controls the germination rates. In addition, application of exogenous ABA causes the rise of cellular ABA concentration to a constant high level (Table II) that is different from an ABA-declining curve (Table II) created by Glc treatment. In contrast to abi4, ctr1, and abi1, the germination kinetics of ABA biosynthetic mutants aba2-1 and aba3-1 showed greater resistance to Glc but were sensitive to exogenous ABA.
Figure 7.
Proposed models for Glc-, ABA-, and Glc/ABA-signaling pathways in controlling seed germination and seedling development. ABA2 and ABA3 affect the plant response to Glc during both germination and seedling development due to their effects on cellular ABA concentration. Both ABI4 and CTR1 are involved in plant response to high levels of exogenous Glc during seedling development but not during seed germination. In contrast, ABI1, ABI4, and CTR1 are involved in plant response to exogenous ABA during seed germination but not during seedling development (except ABI1). HXK appears to be a positive regulator for Glc-induced rescue of seed germination inhibited by ABA. However, plants do not develop further unless either ABI1 or ABI4 is defective.
Our finding that decreased ABA levels coincide with the onset of germination is consistent with a report showing precocious germination of transgenic tobacco (Nicotiana tabacum) expressing an ABA-modulating antibody (Phillips et al., 1997). In this study, the ABA-modulating antibody gene was placed under control of a seed-specific promoter, which allowed the accumulation of antibody before seed development and reduced the concentration of free ABA. In maturing seeds with high antibody concentration, the embryo within the seed had green cotyledons. When the seed coat was removed, only antibody-expressing seeds could germinate without cold treatment. These results suggest that a reduction of free ABA in seeds triggers the developmental programs normally associated with germination. This is also consistent with some vp mutants in maize (Zea mays), whose seeds germinate precociously due to a deficiency in ABA (Robertson, 1955; Neill and Parry, 1986). In our study, we show that a reduction of ABA occurs during germination in WT Arabidopsis seeds and that Glc can modulate the rate at which the ABA decline occurs.
Exogenously applied Glc appeared either to increase the rate of ABA synthesis or to reduce the rate of ABA decay during germination. Much of the findings suggests the latter alternative. Because aba2-1 and aba3-1 are insensitive to Glc during germination and ABA2 and ABA3 are biosynthetic enzymes in the final stages of ABA biosynthesis, it seemed reasonable to expect Glc would delay germination through induction of ABA biosynthesis. However, from the results in Tables I, II, III, it is clear that ABA concentrations decline in germinating WT seeds even in the presence of Glc. In addition, ABA2 transcription was not induced quickly by Glc during early germination (Fig. 6B). The earliest ABA2 induction was detected 2 DAI, and the induction rate correlated with the onset of germination but not with Glc concentration. This indicates that Glc does not increase ABA biosynthesis during germination by transcriptional up-regulation. Also, early steps of ABA synthesis have been shown occur in the plastids (Seo and Koshiba, 2002). If Glc increased ABA levels in seeds, it would suggest that proplastids are active in synthesizing ABA, which is currently unknown. It still may be possible that Glc stimulates ABA production locally and transiently in the seed, however, the rate of loss/decay of ABA would have to be faster than the rate of Glc-induced synthesis.
Then why are aba2-1 and aba3-1 insensitive to Glc? The most likely explanation is that the endogenous ABA level in aba2-1 and aba3-1 had already dropped near the threshold level (2 ng g–1 fresh weight) after cold treatment at 4°C (Table I). Germination cannot be suppressed even in the presence of high levels of exogenous Glc because ABA biosynthesis is blocked in these mutants. However, similar to the WT, the endogenous ABA level continued to decline when these cold-treated aba2-1 or aba3-1 seeds were transferred and incubated at 4°C in the Murashige and Skoog medium containing Glc. For example, ABA levels in aba3-1 dropped from 2.2 to 1.1, 1.5, and 1.8 ng g–1 fresh weight in the medium containing 0, 167, and 333 mm Glc, respectively. These results clearly indicate that although both aba2-1 and aba3-1 are less sensitive to Glc-induced inhibition of seed germination, their Glc-sensing mechanism is still operational, as evidenced by the differential decline of the endogenous ABA concentration. Their “Glc insensitivity” is most likely due to the block of ABA accumulation beyond the threshold low level that is required for the inhibition of seed germination. However, this does not necessarily mean that aba2-1 and aba3-1 affect germination identically. Both aba2-1 and aba3-1 had similar declines in endogenous ABA concentration, however, aba2-1 appeared to be more insensitive to low levels of Glc during germination than aba3-1. Both aba2-1 and aba3-1 are mutations resulting in a single amino acid substitution (aba2-1, S264N; aba3-1, G469E; Xiong et al., 2001; González-Guzmán et al., 2002). It is possible that mutation of ABA2 causes greater reduction in ABA biosynthesis than mutation of ABA3 because an alternate ABA biosynthetic pathway can substitute for the loss of ABA3 but not ABA2 (Seo and Koshiba, 2002). Because both aba2-1 and aba3-1 are not null mutations, another possibility is that the aba2-1 allele causes a more severe disruption in ABA biosynthesis than the aba3-1 allele. We cannot rule out the existence of factors other than ABA concentration that are important for determining the onset of germination. Our results also suggest that there is not a linear relationship between Glc concentration and the expression of genes involved in ABA biosynthesis, because ABA2 transcription was activated to different extents by low (16.7 mm) and medium (167 mm) levels of Glc independent of germination status. On the basis of these observations, we propose that sugar modulates cellular ABA concentration, but the effect of sugar on seed germination is mediated through a combinatory effect of both sugar and ABA. Although the modulation of ABA level by Glc appears to be modest, this modulation might be sufficient to cause significant delay of seed germination, which resembles the effect of exogenous ABA.
The components that transmit the Glc signal are still unknown, because we have not found any mutants except aba2-1 and aba3-1 that are less sensitive to both low and high concentration of Glc during seed germination. The physiological relevance of different responses to low and high sugar concentrations is also uncertain, however, there are some indications that high sugar levels are associated with biotic stresses. Sugars have been found to accumulate in Arabidopsis at the site of nematode attack (Bockenhoff et al., 1996), and sugar transporters are up-regulated when exposed to pathogens such as bacteria, fungi, or nematodes (Truernit et al., 1996; Juergensen et al., 2003). The AtHXK1 mutant gin2, considered to be sugar insensitive for overall growth, appeared to be slightly more sensitive to 333 mm Glc during germination than its parental ecotype, Ler, again indicating that it may not be critical for transmitting a sugar signal during germination. Because mutation of HXK does confer resistance to high levels of sugar in subsequent growth (Arenas-Huertero et al., 2000; Rolland et al., 2002), this suggests that the signaling functions of HXK may be active only during certain stages of development. However, the subtle delay of seed germination does correlate with the level of HXK when low levels of Glc were used (Fig. 1B). This indicates that the signaling mechanisms of HXK are also affected by sugar concentration and that in the presence of low Glc levels, HXK might play an indirect role in controlling seed germination. The former idea is further supported by abi4 being sensitive to 333 mm but not <55 mm Glc.
In summary, our simple but effective analyses have provided insights into the complicated effects of sugar in plant development. Plants do not normally encounter either high ABA or high sugar condition except under stress. However, varied levels of exogenous Glc can modulate internal ABA concentration by increasing synthesis or inhibiting degradation, allowing us to see how small fluctuations of internal ABA concentration affect the seed germination process. This modulating effect of Glc cannot be recreated using exogenous ABA. The process of identifying additional sugar-signaling components remains a challenge, no matter whether they will again turn out to be related to ABA-signaling or other hormone-signaling cascades. Global gene expression analysis may help to distinguish the effects of sugar, ABA, and osmotic stress, and new specific marker genes may prove to be more effective in the genetic dissection of sugar-responsive pathways.
MATERIALS AND METHODS
Growth of Plant Material
Arabidopsis seeds were surface-sterilized and water-imbibed in the dark for 3 d at 4°C. Seeds were transferred to 1× Murashige and Skoog basal salt mixture with B5 vitamins and 0.05% (w/v) MES (pH 5.7); Glc, ABA, and/or 0.7% (w/v) phytagar (Invitrogen, Carlsbad, CA) were also added where indicated. For Murashige and Skoog with phytagar, plates were air-dried to remove excess surface moisture, and seeds were individually spotted using a 1-μL sterile loop. The plant material was incubated in the dark at 4°C for 3 d to break dormancy and then was transferred to light at 24°C. Cultures without phytagar were shaken at 140 rpm using an orbital platform shaker (New Brunswick, Edison, NJ) under continuous light; all other cultures were grown with a photoperiod of 16 h of light and 8 h of dark. Germination kinetics was determined by measuring the time of radicle emergence from repeated experiments with duplicate plates of approximately 25 seeds each.
Quantitation of ABA Contents
ABA content was determined in seeds or germinated plants immediately after surface sterilization, imbibition, and at various time points after light treatment. ABA extraction and determination were performed as previously described (Arenas-Huertero et al., 2000). In brief, 30 to 1,000 mg of plant material was washed at least seven times with sterile distilled water before being homogenized in 1 to 2 mL of ABA extraction buffer (10 mm HCl and 1% [w/v] polyvinylpolypyrrolidone in methanol). The extract was mixed overnight at 4°C; the supernatant was collected, measured, and neutralized with 15 μL of 1 m NaOH mL–1 extract. ABA content was quantified using the Phytodetek-ABA kit (AGDIA Inc.) following the manufacturer's protocol. Raw values for ABA content were standardized to compensate for the variations in plant mass and extraction volume.
Gene Expression Analysis
Seedlings were grown in liquid cultures containing various amounts of Glc and/or ABA as indicated and were collected for RNA extraction at various time points after light treatment. Total RNA was isolated from plant material using either a standard protocol (Ausubel et al., 1987) for non-germinating seeds or the plant RNeasy kit (Qiagen USA, Valencia, CA) for germinated material. RNA gel-blot analysis was performed with 5 μg of total RNA per lane using standard protocols (Xiao et al., 2000). The primer pairs used for the synthesis of the probes, the size of the probes, and the accession numbers are as follows: ABI1, 5′-TTTCACCGGGATCAGATT-3′ and 5′-TAGTTCGCTACCTGAGAA-3′ (497 bp; accession no. U12856); ABA2, 5′-AAAGTGGCATTGATCACT-3′ and 5′-TCCTAGTCAAGCCTAGA-3′ (495 bp; accession no. AC037424.10; Jen Sheen, personal communication); and ABI4, 5′-CACCGACTCATCAACTT-3′ and 5′-CATCTGGACCATCTGAT-3′ (508 bp; accession no. AF040959).
Acknowledgments
We thank Arabidopsis Biological Resource Center for providing seed stocks, Dong-mei Li for assisting in germination assays, and Dietz Bauer and Jim Metzger for critical reading of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.020347.
This work was supported by the Ohio Agricultural Research and Development Center and Plant Molecular Biology and Biotechnology Program at the Ohio State University (to J.C.J.). Salaries and research support were provided by the state and federal funds appropriated to the Ohio Agricultural Research and Development Center, the Ohio State University. This is manuscript number HCS02-35.
References
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14: 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, eds (1987) Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-Interscience, New York
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenhoff A, Prior DA, Grundler FM, Oparka KJ (1996) Induction of phloem unloading in Arabidopsis thaliana roots by the parasitic nematode Heterodera schachtii. Plant Physiol 112: 1421–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M et al. (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14: 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciereszko I, Johansson H, Kleczkowski LA (2001) Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem J 354: 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G, Bush DR (2001) Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol 125: 61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi GM, Zhou L (2001) Carbon and nitrogen sensing and signaling in plants: emerging “matrix effects.” Curr Opin Plant Biol 4: 247–253 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gibson SI (2001) ABA and sugar interactions regulating development: cross-talk or voices in a crowd? Curr Opin Plant Biol 5: 26–32 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Lynch TJ (2000) Abscisic acid inhibition of radicle emergence but not seedling growth is suppressed by sugars. Plant Physiol 122: 1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarrubio A, Legaria JP, Covarrubias AA (1997) Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta 203: 182–187 [DOI] [PubMed] [Google Scholar]
- Gazzarrini S, McCourt P (2001) Genetic interactions between ABA, ethylene and sugar signaling pathways. 4: 387–391 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI, Laby RJ, Kim D (2001) The sugar-insensitive1 (sis1) mutant of Arabidopsis is allelic to ctr1. Biochem Biophys Res Commun 280: 196–203 [DOI] [PubMed] [Google Scholar]
- González-Guzmán M, Apostolova N, Belles JM, Barrero JM, Piqueras P, Ponce MR, Micol JL, Serrano R, Rodriguez L (2002) The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 14: 1833–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti F, Beaudoin N, Serizet C, Webb AA, Vartanian N, Giraudat J (1999) ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11: 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser C, Kortstee A, Pego J, Weisbeek P, Wisman E, Smeekens S (2000) The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. Plant J 23: 577–585 [DOI] [PubMed] [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergensen K, Scholz-Starke J, Sauer N, Hess P, Van Bel AJ, Grundler FM (2003) The companion cell-specific Arabidopsis disaccharide carrier At-SUC2 is expressed in nematode-induced syncytia. Plant Physiol 131: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Choi HI, Im MY, Kim SY (2002) Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14: 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23: 587–596 [DOI] [PubMed] [Google Scholar]
- Leon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Hellman H, Schmidt R, Willmitzer L, Frommer WB (1997) Identification of mutants in metabolically regulated gene expression. Plant J 11: 53–62 [DOI] [PubMed] [Google Scholar]
- Mita S, Murano N, Akaike M, Nakamura K (1997) Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J 11: 841–851 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Hogan R, Parry AD (1986) The carotenoid and abscisic acid content of viviparous kernels and seedlings of Zea mays L. Planta 169: 87–96 [DOI] [PubMed] [Google Scholar]
- Phillips J, Artsaenko O, Fiedler U, Horstmann C, Mock HP, Muntz K, Conrad U (1997) Seed-specific immunomodulation of abscisic acid activity induces a developmental switch. EMBO J 16: 4489–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DS (1955) The genetics of vivipary in maize. Genetics 40: 745–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2: 198–206 [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell Suppl 14: S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW (2001) Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J 26: 421–433 [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Trends Plant Sci 7: 41–48 [DOI] [PubMed] [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51: 49–81 [DOI] [PubMed] [Google Scholar]
- To JP, Reiter WD, Gibson SI (2002) Mobilization of seed storage lipid by Arabidopsis seedlings is retarded in the presence of exogenous sugars. BMC Plant Biol 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8: 2169–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Sheen J, Jang JC (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44: 451–461 [DOI] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis los5/aba3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13: 2063–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]